Abstract
Fasting induces profound changes in the hypothalamus-pituitary-thyroid axis and peripheral thyroid hormone (TH) metabolism, ultimately leading to lower serum thyroid hormone (TH) concentrations. In the present study, we aimed to investigate the regulation of type 3 deiodinase (D3) during fasting in two metabolic tissues: liver and white adipose tissue (WAT). To this end, we studied the effect of modulation of the mammalian target of rapamycin (mTOR) and hypoxia inducible factor 1α (HIF1α) on D3 expression in primary rat hepatocytes and in 3T3-L1 adipocytes. In addition, we studied the role of the constitutive androstane receptor (CAR) on liver TH metabolism using primary hepatocytes and CAR-/- mice. Twenty-four-hour fasting increased liver Dio3 expression in mice. Inhibition of mTOR using mTOR inhibitors markedly induced Dio3 mRNA expression in primary hepatocytes; this increase was accompanied by a small increase in D3 activity. Stimulation of these cells with a CAR agonist induced both Dio3 mRNA expression and activity. Fasting increased hepatic D3 expression in WT but not in CAR-/- mice. In WAT, Dio3 mRNA expression increased five-fold after 48-h fasting. Treatment of 3T3-L1 adipocytes with mTOR inhibitors induced Dio3 mRNA expression, whereas stimulation of these cells with cobalt chloride, a compound that mimics hypoxia and stabilizes HIF1α, did not induce Dio3 mRNA expression. In conclusion, our results indicate an important role of mTOR in the upregulation of D3 in WAT and liver during fasting. Furthermore, CAR plays a role in the fasting induced D3 increase in the liver.
Keywords: fasting, type 3 deiodinase, white adipose tissue, liver
Introduction
Fasting has profound effects on the hypothalamus-pituitary-thyroid (HPT) axis, probably in order to save energy when nutrient supply is low. Fasting decreases serum triiodothyronine (T3) and thyroxine (T4) concentrations, whereas thyroid-stimulating hormone (TSH) secretion from the pituitary and thyrotropin-releasing hormone (TRH) secretion from the hypothalamus remains low (1), pointing to an altered hypothalamic setpoint of the HPT axis. In addition to altered central regulation, fasting changes peripheral thyroid hormone (TH) metabolism, thereby contributing to the low serum TH concentrations (2, 3, 4), and also to low tissue TH concentrations (5).
In the liver, TH can be metabolized via different pathways: deiodination, sulfation and glucuronidation. Type 3 deiodinase (D3) is regarded as the major TH-inactivating enzyme as it catalyzes inner-ring deiodination of both T4 and T3, exclusively resulting in the production of biologically inactive rT3 and reverse diiodothyronine (T2). In addition to D3, hepatic sulfotransferases (Sults) and UDP-glucuronosyltranferases (UGTs) enhance the clearance of T3 and T4 by increasing the affinity for type 1 deiodinase and by promoting the clearance of TH via the bile and urine (6).
Fasting markedly affects hepatic thyroid hormone metabolism; liver D3 (expressed at low levels in the fed condition) increases during fasting in mice and rats and this increase is dependent on the drop of serum leptin (7). In addition, fasting results in changes in Sults and UGTs and in their upstream activator, the constitutive androstane receptor (CAR). This receptor, which is expressed predominantly in the liver, is thereby involved in hepatic clearance of T3. Furthermore, CAR is important for endo- and exobiotic sensing (8). The synthetic CAR agonists phenobarbital and TCPOBOP reduce T4 serum levels in rats and mice, respectively (9, 10), supposedly via effects on hepatic TH metabolism. In addition, in CAR-knockout mice, the decrease in serum TH concentrations upon fasting is less pronounced (9). Since fasting markedly increases CAR expression, we aimed to determine if CAR activation by itself is able to increase D3 expression and activity.
White adipose tissue (WAT) is another important metabolic organ responsive to TH. TH counterintuitively stimulates both fatty acid synthesis and oxidation in WAT (11) and is also important for adipocyte differentiation (12). In addition, T3 stimulates UCP2 expression in WAT, thereby affecting basal metabolic rate (13). Both D2 and D3 are expressed in WAT, as well as the thyroid hormone receptors TRβ1 and TRα1 (14). During fasting, lipid metabolism in WAT changes dramatically aimed at the release of free fatty acids as energy source. Although TH is known to regulate lipogenesis and lipolysis, there are no data available on the effects of fasting on TH metabolism in WAT.
Thus, both liver and WAT contain the molecular machinery for TH metabolism and action. Previous studies suggest a role for TH in energy metabolism during starvation (1) probably partly via local TH metabolism in liver and adipose tissue. D3 is the main T3-inactivating enzyme and affected during starvation in the liver (7). We hypothesize that starvation regulates D3 mRNA expression and activity in liver and white adipose tissue, the mechanisms involved are incompletely understood. To this end, we studied the effect of a variety of molecular determinants involved in nutrient sensing (including the mammalian target of rapamycin (mTOR)) on Dio3 expression and D3 activity in a primary rat hepatocyte culture and 3T3-L1 adipocytes. Furthermore, the role of HIF1α was investigated in 3T3-L1 adipocytes. HIF1α is a potent inducer of D3; under normal conditions, HIF1α is hydroxylated by prolyl hydroxylase 2 (PHD2) which makes it prone for proteasomal degradation (15). Hypoxia inhibits PHD activity and thus stabilizes HIF1α. However, this process also requires 2-oxoglutarate (2-OG), a substrate that is limited during starvation resulting in decreased PHD2 activity (16). In addition, we studied the role of CAR on liver TH metabolism using primary rat hepatocytes and CAR-/- mice as CAR increases markedly in liver during starvation.
Materials and methods
Animal experiments
We performed two experiments: (1) 48-h fasting: Male B6129S6F1 mice (approx. 22 g, used at the age of 12 weeks, n = 6 per group) were either starved for 48 h or received food and water ad lib. After 48 h, blood was taken by cardiac puncture under isoflurane anesthesia and mice were killed by cervical dislocation. White adipose tissue was isolated and snap frozen in liquid nitrogen and stored until further analysis. This experiment was approved by the Local Animal Welfare Committee of the University of Leiden, the Netherlands; (2) 24-h fasting: Female CAR-/- mice and WT controls were purchased from Taconic (Hudson, NY, USA) and were studied at 7–13 weeks of age. CAR-/- mice and WT controls (n = 6) were either starved for 24 h or received food and water ad lib. After 24 h, blood was taken by cardiac puncture under isoflurane anesthesia and mice were killed by cervical dislocation. Blood samples were spun down and serum was stored at −20°C. The liver was dissected, snap-frozen in liquid nitrogen and stored at −80°C. This experiment was approved by the Animal Welfare Committee of the Academic Medical Center (AMC) of the University of Amsterdam. During both experiments, the animals were kept under a 12/12 h light/dark cycle in a temperature controlled room (23°C). Water and food were available ad libitum before the experiments. Starvation results in marked alterations in TH concentrations in both male and female mice; we therefore used the tissues of male mice that were made available to us and did not decide the repeat the experiment in female mice as 48-h starvation results in severe distress in mice.
Cell experiments and reagents
Male and female Wistar rats weighing 250–450 g were used for primary hepatocyte isolation. The procedure was carried out under Temgesic (0.1 mL/100 g BW, 0.03 mg/mL) and isoflurane anesthesia. A heparin solution (0.2 mL, 5000 i.e./mL, LEO Pharma, Ballerup, Denmark) was injected in the vena cava caudalis. After 5 min, a cannula was placed in the liver via the vena porta. The liver was perfused with Ca2+-free solution (142 mM NaCL, 6.7 mM KCL, 3.36 mM Hepes, pH 7.4) at 37°C, with a flow rate of 30 mL/min, followed by a deep incision in the vena cava caudalis and complete dissection of the liver. The perfusion with Ca2+-free solution was followed by perfusion with collagenase buffer (66.7 mM NaCL, 6.7 mM KCL, 4.8 mM CaCl2·2H2O, 67.1 mM, 0.3% BSA, pH 7.6) with 0.05% collagenase from clostridium and 0.01% hyaluronidase type I-s (both from Sigma Chemicals Co.) at a flow rate of 13 mL/min. All solutions were sterile filtered with a 0.22 µm filter. After perfusion the liver was dissociated and cells were suspended in Williams E medium (Westburg, Leusden, The Netherlands) with 10% heat-shocked fetal bovine serum (FBS, Bodinco, Alkmaar, The Netherlands), 1% penicillin, streptomycin and Amphotericin B (PSF, Lonza, Basel, Switzerland) and 1% L-glutamine (Lonza). Cells were washed three times by centrifugation at 50 g at 4°C without brake. After isolation cells were plated on six-well Primaria plates (Corning Life Sciences, Tewksbury, MA, USA) in a concentration of 1 × 106/well. Further experiments were started within 36 h after isolation. Cells were incubated during 8 h, 16 h or 24 h with 200 nM Torin (Tocris Bioscience, Bristol, UK) dissolved in DMSO, 200 nM Rapamycine (LC labs, Woburn, MA, USA) dissolved in DMSO, 100 nM TPD (a rat-specific CAR agonist, (2a,4a,6a)-2,4,6-triphenyl-1,3-dioxane, Sigma), 1 μM GW7647 (a PPARα agonist, Tocris Bioscience).
3T3-L1 pre-adipocytes (ATCC: CL-173) were derived from ATCC. Cells were kept in Dulbecco’s modified Eagle’s medium (4.5 g/L glucose) medium supplemented with 10% fetal calf serum (Invitrogen), penicillin (200 U/mL) and streptomycin (200 µg/mL) in a humidified incubator with 10% CO2. Adipocyte differentiation was initiated 3 days post confluence by exposing cells to 100 nM insulin, 1 µM dexamethasone and 0.5 mM 1-methyl-3-isobutylxanthine (DMI) for 48 h, after which cells were switched to medium containing 100 nM insulin for 4 days. Thereafter, cells were kept in maintenance medium containing 10% FCS. Rosiglitazone (1 µM) was added during the first 4 days of differentiation. Media were replenished every other day, and experiments were routinely performed on day 8 after induction of differentiation.
In order to mimic starvation, 3T3-L1 adipocytes were cultured in HBSS (PAA Laboratories, GmBH, Pasching, Austria) for 6 h in a 37°C incubator without CO2 regulation and compared to 3T3-L1 adipocytes cultured in normal culture medium. For all other experiments, 3T3-L1 adipocytes were cultured in a 37°C incubator with 5% CO2. Cells were treated during 24 h with 200 nM torin, 200 nM rapamycine, 2 mM AICAR dissolved in PBS (AMPK activator, Sigma) or with 100 μM cobalt chloride (CoCl2, mimicking hypoxia and thereby inducing HIF1α (17)) dissolved in culture medium. Cell experiments were carried out several times, see legends. For mRNA expression, cells were lysed in 250 µL lysis buffer supplied by the Magna Pure HP total RNA kit (Roche Molecular Biochemicals), and 125 µL PBS.
RNA isolation and qPCR
RNA was isolated using the Magna Pure apparatus (Roche Molecular Biochemicals) and the Magna pure tissue III total RNA kit (for tissue) and the Magna Pure HP total RNA kit (for cells) (Roche Molecular Biochemicals). RNA yield was determined by spectrophotometric analysis using the Nano drop (Nanodrop) and cDNA was synthesized with equal RNA input with the First strand cDNA synthesis kit for qPCR with oligo d(T) primers (Roche Molecular Biochemicals). As a control for genomic DNA contamination, we included a cDNA synthesis reaction without reverse transcriptase. Quantitative PCR was performed using the Lightcycler 480 and Sensifast-SYBR No-ROX mastermix (Bioline, London, UK). Quantification was performed using the LinReg software. PCR efficiency of each sample was calculated and samples that had a deviation of more than 5% of the mean efficiency value of the assay were excluded. Calculated values were normalized by the geometric mean of expression of four reference genes (Cyclophilin, EF1a1, HPRT and Ubiquitin, see Table 1).
Table 1.
Primer sequences, gene names and symbols used for qPCR.
| Gene | Primer sequences |
|---|---|
| HPRT (hprt) | Forward 5′-GCA GTA CAG CCC CAA AAT GG-3′ Reverse 5′-AAC AAA GTC TGG CCT GTA TCC AA-3′ |
| Ubiquitin (Ubc) | Forward 5′-AGC CCA GTG TTA CCA CCA AG-3′ Reverse 5′-CTA AGA CAC CTC CCC CAT CA-3′ |
| EF1a1 (Eef1a1) | Forward 5′-AGT CGC CTT GGA CGT TCT T-3′ Reverse 5′-ATT TGT AGA TCA GGT GGC CG-3′ |
| Cyclophilin (Ppia) | Forward 5′-ATGTGGTCTTTGGGAAGGTG-3′ Reverse 5′-GAAGGAATGGTTTGATGGGT-3′ |
| TBP (Tbp) | Forward 5′-TTCGTGCCAGAAATGCTGAA-3′ Reverse 5′-TGCACACCATTTTCCCAGAAC-3′ |
| D3 (Dio3) | Forward 5′-CTA CGT CAT CCA GAG TGG CA-3′ Reverse 5′-CTG TTC ATC ATA GCG CTC CA-3′ |
| CAR (Nr1i3) | Forward 5′-CGCAGTCCATGCAGGGTTCCA-3′ Reverse 5′-ACTCCGGGTCTGTCAGGGGA-3′ |
| D1 (Dio1) | Forward 5′-GAG CAG CCA GCT CTA CGC GG-3′ Reverse 5′-TGG GGA GCC TTC CTG CTG GT-3′ |
| S1a1 (Sult1a1) | Forward 5′-CTGAAAGAGACACCAGCCCCACG-3′ Reverse 5′-CTCCTCAGGTAGAGAGCGCCCCA-3′ |
| S1d1 (Sult1d1) | Forward 5′-TGCTGAACAACATGCCGTCTCCT-3′ Reverse 5′-TGCTGAACAACATGCCGTCTCCT-3′ |
| U1a1 (Ugt1a1) | Forward 5′-GCCTTCAGAAAAAGCCCCTATCCCA-3′ Reverse 5′-GCCCGAGTCTTTGGATGACCAAGC-3′ |
| MCT8 (Slc16a2) | Forward 5′-GGGGCCCTGTCAGGAGGCAA-3′ Reverse 5′-TTTCCACAG TGGGCGTGGGC-3′ |
| MCT10 (Slc16a10) | Forward 5′-TGATTCCCCTGTGCAGCGCC-3′ Reverse 5′-CCACGTCGTAGGTGCCCAGC-3′ |
| CYP2B2 (Cyp2b2) | Forward 5′-GTCCTGCATGGATGAGAGAGG-3′ Reverse 5′-ATCATCAAGGGATGGTGGCCT-3′ |
| Glut1 | Forward 5′-TTCCTTGCCTGAGACCAGTT-3′ Reverse 5′-CGAGGTCCTTCTCATGGTGT-3′ |
Deiodinase activity
D3 activity was measured using HPLC. Liver samples were homogenized on ice in 10 volumes of PED50 (0.1 M sodium phosphate, 2 mM EDTA pH 7.2, 50 mM DTT) using a polytron (Kinematica, Luzern, Switzerland). Primary hepatocytes were washed, scraped in ice cold PBS, spun down, and the pellet was homogenized in PED50. Homogenates were snap frozen in aliquots and stored in −80°C until further use. Protein concentration was measured with the Bio-Rad protein assay using BSA as the standard following the manufacturer’s instructions (Bio-Rad Laboratories). Deiodinase activity was measured as described before (18).
Thyroid hormones
Serum T3 and T4 levels were measured with in-house RIA’s (19). All samples of one experiment were measured within the same run (intra-assay variability T3: 3,6% and T4: 6,6%). Liver concentrations of T3 and T4 (expressed as pmol/g wet weight) were measured as described before (18).
Statistics
Differences between groups in the in vivo experiment were evaluated using two-way ANOVA with two grouping factors (genotype and fasting). Normal distribution of the data was tested using the Shapiro–Wilk test on the residues of the ANOVA. If not normally distributed, data were ranked before performing ANOVA. P values in the figures represent the significant effects of genotype (pgenotype) or the significant interaction term (pi). Differences between groups in the hepatocyte experiments were tested by Student’s t-test or by one-way ANOVA. For pair-wise comparisons, ANOVA was followed by Student’s t-test if data was normally distributed, or by Mann–Whitney U tests if not normally distributed. Symbols in the figures represent the pair-wise P values. P values of <0.025 were considered statistically significant to correct for the two performed t-tests. For the in vitro experiments, we used the pooled relative data of at least 2–4 separate experiments. All tests were performed using SPSS or IBM SPSS statistics 20 software.
Results
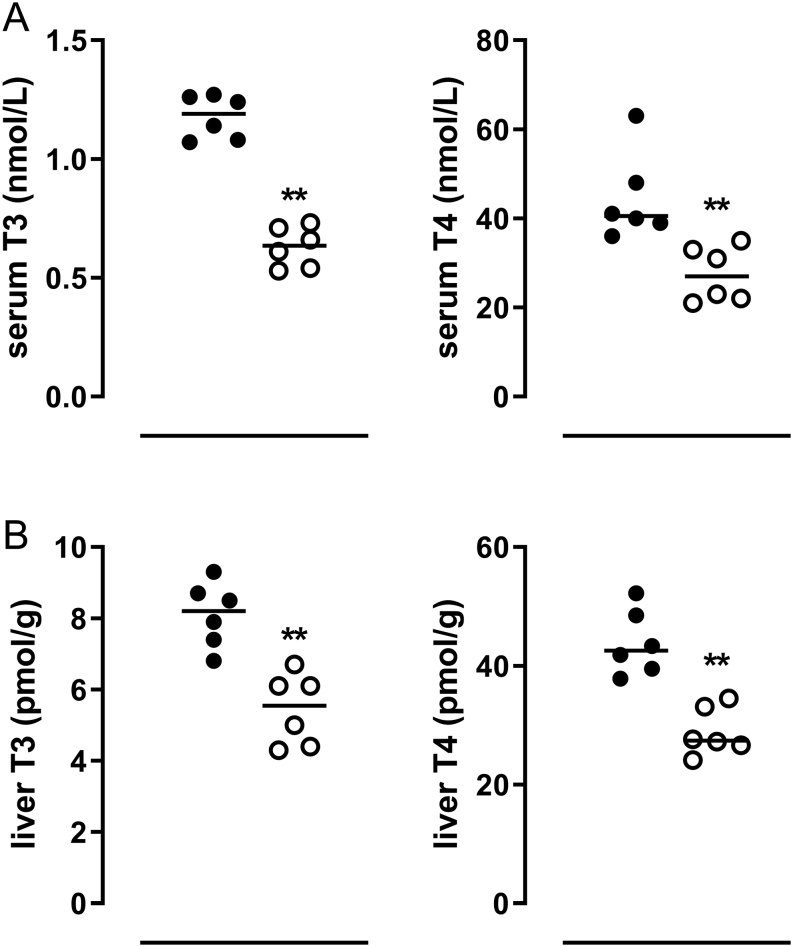
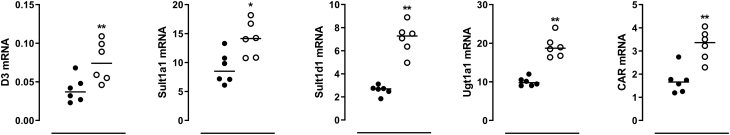
Fasting resulted in markedly decreased serum T4 and T3 concentrations in mice (Fig. 1A). The decrease in serum TH concentrations was accompanied by a marked decrease in liver T4 and T3 concentrations (Fig. 1B) and also by significant increases in mRNA expression of TH metabolizing enzymes like liver Dio3, Ugt1a1, S1a1, and S1d1. Fasting increased hepatic Car mRNA expression (Fig. 2). TH transporter expression appeared to respond differentially to fasting as liver Mct10 mRNA expression increased, whereas liver Mct8 expression decreased (data not shown).
Figure 1.
(A) Serum T4 and T3 concentrations (nmol/L) and B) hepatic T4 and T3 concentrations (pmol/gram) in ad libitum fed (black dots) and 24-h fasted (open circles) mice. Individual values and median are shown. Symbols indicate differences between fed and fasted groups (**P ≤ 0.001) as evaluated by Student’s t-test.
Figure 2.
Relative mRNA expression of Dio3, Sulfotransferase 1a1 (S1a1), Sulfotransferase 1d1 (S1d1), UDP- glucuronosyltranferases 1a1 (Ugt1a1) and constitutive androstane receptor (Car) in liver of ad libitum fed (black dots) and 24-h fasted (open circles) mice. Individual values and median are shown. Symbols indicate differences between fed and fasted groups (*P ≤ 0.025, **P ≤ 0.001).
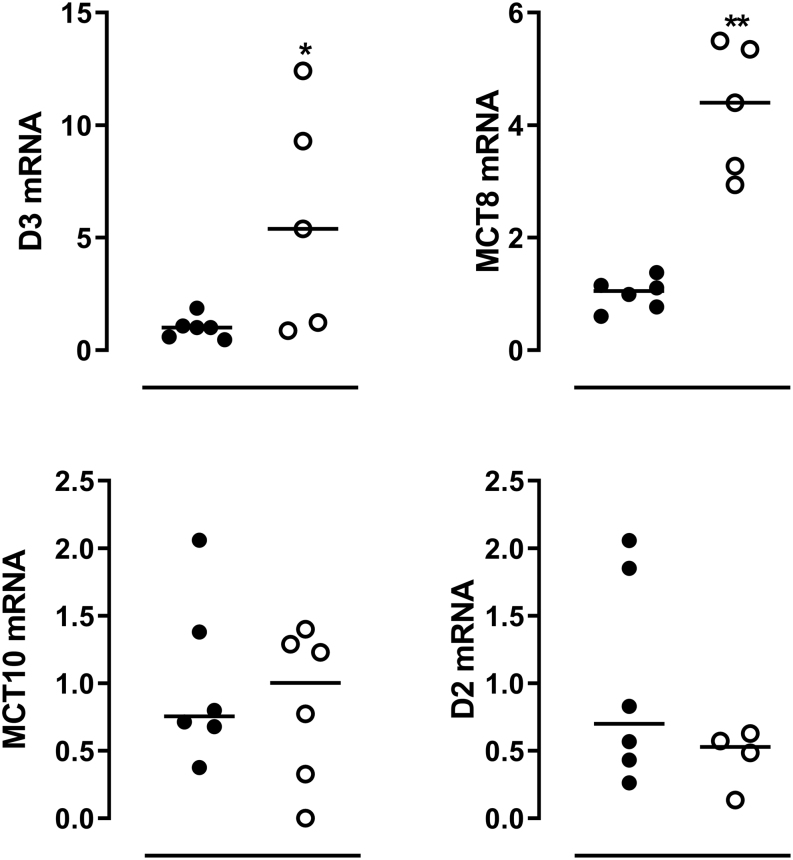
The availability of WAT samples of mice that were fasted for 48 h allowed us to study the effects of severe starvation on adipose tissue. WAT of mice that were fasted for 48 h showed 5- and 4-fold increased Dio3 and Mct8 mRNA expression, respectively, compared to WAT of ad lib fed mice. Dio2 and Mct10 mRNA expression in WAT were not affected by fasting (Fig. 3).
Figure 3.
Relative mRNA expression of Dio3, Mct8, Mct10 and Dio2 in WAT of ad libitum (black dots) and 24-h fasted (open circles) mice. Expression is shown relative to the fed control group. Individual values and median are shown. Symbols indicate differences between fed and fasted groups (*P ≤ 0.05, **P ≤ 0.01) as evaluated by Student’s t-test.
Possible determinants of the fasting-induced liver D3 increase
Although liver D3 is expressed at a relatively low level in the fed state, Dio3 mRNA expression and D3 activity levels increase during starvation in both rats and mice (7, 18). Leptin administration selectively restores the starvation-induced increase in hepatic Dio3 mRNA expression independently of serum thyroid hormone in mice, suggesting a role for nutrient-sensing pathways (7). In order to study the mechanisms involved in the fasting-induced D3 increase in the liver, we used rat primary hepatocytes. These cells were treated with a variety of possible mediators acting on pathways involved in fasting.
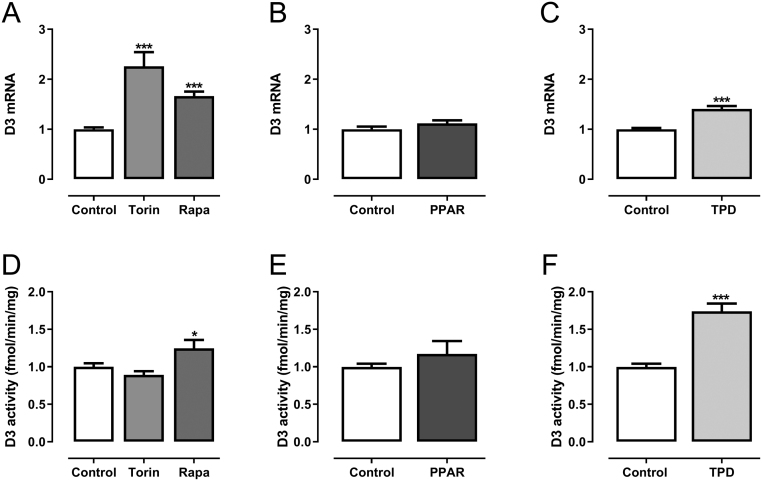
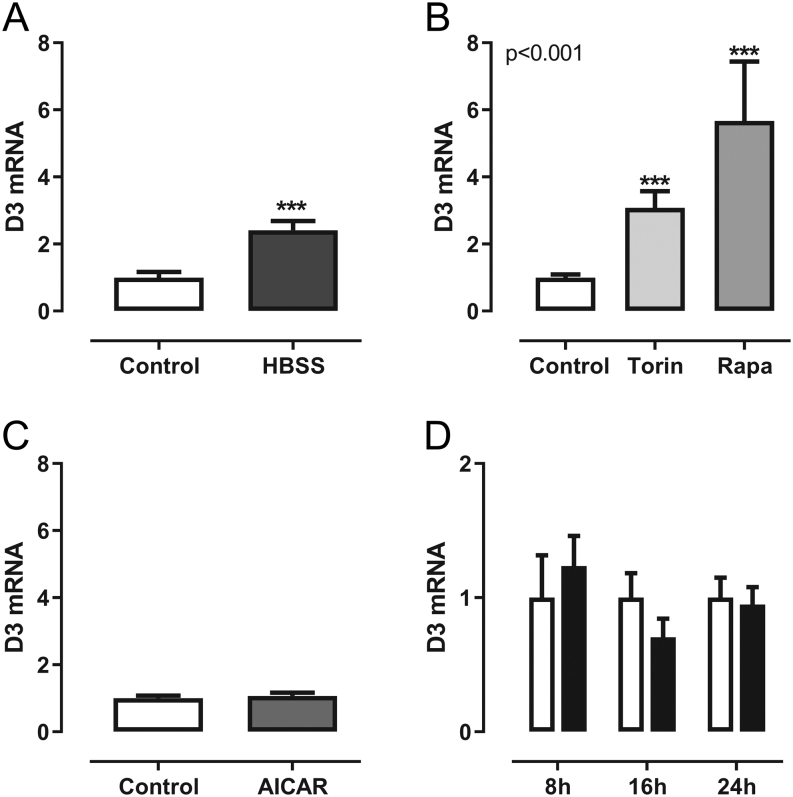
One key player in nutrient sensing is mTOR, which is inhibited in the absence of amino acids, growth factors and feeding-related hormones (20). We, therefore, stimulated the cells with the mTOR inhibitors torin and rapamycin for 16 h, which led to a marked increase in Dio3 mRNA expression (Fig. 4A) in accordance with the in vivo response. The increase in Dio3 mRNA expression after rapamycin, but not torin, was accompanied with a small increase in D3 activity after 24 h (Fig. 4D).
Figure 4.
Relative Dio3 mRNA expression (A/B/C) and D3 activity (D/E/F) in rat primary hepatocytes stimulated for 16 h (mRNA) and 24 h (activity) with 0,025% DMSO (control), 200 nM Torin (n = 3), 200 nM Rapamycin (both mTOR inhibitors, n = 4) or 1 μM GW7647 (PPARα agonist, n = 3) or 100 nM TPD (a rat specific CAR agonist, n = 2). mRNA expression is normalized to the control group. Mean values ± s.e.m. of pooled relative data of independent experiments are shown, n represents the number of independent experiments, each experiment consists of six values per group. P values represent the effect of treatment as analyzed by Mann–Whitney U test or by parametric one-way ANOVA followed by post hoc testing (Dunnet) (*P ≤ 0.025, ***P ≤ 0.001).
Free fatty acids (FFA) in the blood rise during fasting and may bind to PPARα (peroxisome proliferator-activated receptor alpha), a nuclear receptor expressed in liver (21). We therefore stimulated the cells with GW7647, a synthetic PPARα agonist, for 16 h and 24 h. Activation of PPARα, however, did not affect Dio3 mRNA expression and D3 activity (Fig. 4B and E).
CAR is upregulated in both mice and rats (9, 18, 22) during fasting. To investigate whether CAR activation during fasting acts directly on D3, we stimulated rat primary hepatocytes with TPD, a rat-specific synthetic CAR agonist. TPD increased Dio3 mRNA expression and D3 activity after 24 h treatment compared to control hepatocytes (Fig. 4C and F). As a control, mRNA of the known CAR target gene Cyp2b2 was also measured. TPD stimulation for 16 h increased Cyp2b2 mRNA expression by 2–3 fold (data not shown).
The role of CAR in the fasting-induced D3 increase in liver
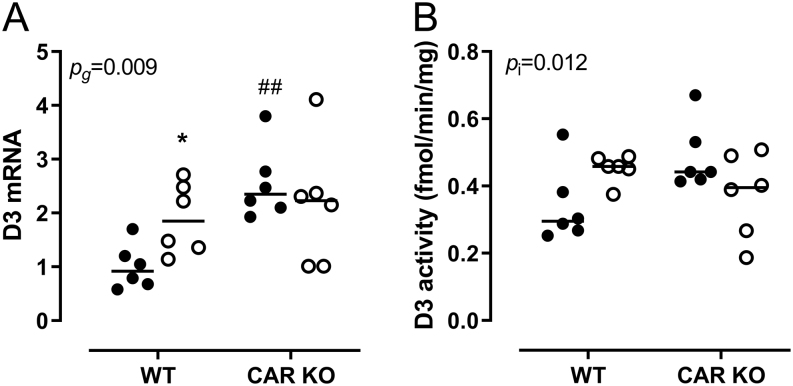
In order to validate the role of CAR in the fasting-induced D3 increase in the liver, we used CAR-/- mice and starved the mice for 24 h. Fasting decreased serum T4 and T3 concentrations to a similar extent in WT and CAR-/- mice (data not shown). The fasting-induced decrease in hepatic T4 and T3 concentrations was also similar between WT and CAR-/- mice. However, basal and fasting-induced hepatic T3 concentrations were lower in CAR-/- mice (data not shown). In CAR-/- mice, Car mRNA expression was reduced by 96% and unresponsive to fasting, while fasting attenuated the fasting induced Ugt1a1, S1a1 and S1d1 increases (data not shown). Interestingly, the fasting-induced increase in Dio3 mRNA expression was absent in CAR-/- mice (Fig. 5A). D3 activity was not significantly affected by either 24 h fasting or genotype. However, statistical analysis showed a significant interaction (Fig. 5B), indicating that the response of D3 activity to fasting was affected in CAR-/- mice compared to WT mice.
Figure 5.
Type 3 deiodinase (Dio3) relative mRNA expression (A) and activity (B) in liver of WT and CAR-knockout mice. Black dots represent ad libitum-fed controls, open circles represent 24-h fasted mice. Individual values and median are shown. P values indicate effects of genotype (pg) or the interaction effect between fasting and genotype (pi) as calculated by parametric two-way ANOVA. Symbols indicate differences between fed and fasted groups (*P ≤ 0.05) or between WT and CAR KO groups (##P ≤ 0.001).
Possible determinants of the fasting-induced D3 increase in adipocytes
In order to mimic starvation, 3T3-L1 adipocytes were cultured in HBSS for 6 h, which resulted in a mild increase in Dio3 mRNA expression (Fig. 6A). To investigate the determinants of the fasting-induced D3 expression in adipose tissue, we stimulated 3T3-L1 adipocytes with the mTOR inhibitors torin and rapamycin for 24 h. Both mTOR inhibitors induced a 2- to 3-fold increase in Dio3 mRNA expression (Fig. 6B). Activation of 5′-AMP protein kinase (AMPK) resulting from low energy status has been implicated in the fasting induced inhibition of mTOR (23). To mimic this, we treated cells with AICAR, a synthetic AMPK activator. However, treatment with AICAR for 24 h did not result in increased Dio3 mRNA expression (Fig. 6C).
Figure 6.
Relative mRNA expression of Dio3 in 3T3-L1 adipocytes. (A) 3T3-L1 adipocytes were cultured in control medium or Hanks Balanced salt solution (HBSS) for 6 h (n = 3). (B) Stimulation of 3T3-L1 adipocytes with 200 nM torin and 200 nM rapamycine for 24 h (n = 3). (C) Stimulation of 3T3-L1 adipocytes with 2 mM AICAR for 24 h (n = 1). (D) Stimulation of 3T3-L1 adipocytes with 100 μM of cobalt chloride (CoCl2) (black bars), compared to control cells (white bars) for 8, 16 or 24 h (n = 3). Mean values ± s.e.m. of pooled relative data of independent experiments are shown, n represents the number of independent experiments, each experiment consists of six values per group. P values represent the effect of treatment as analyzed by one-way ANOVA. Symbols indicate the differences between groups as analyzed by Mann–Whitney U test (***P ≤ 0.001).
HIF1α is a potent inducer of D3; we therefore treated 3T3-L1 adipocytes with cobalt chloride (CoCl2), thereby chemically mimicking hypoxia. CoCl2 is a potent chemical hypoxia inducer; mRNA expression of PGK1, an oxygen-dependent gene, increased markedly after stimulation (controls: 2.19 ± 0,29, CoCl2 10.66 ± 1.56). CoCl2 treatment also induced the expression of the HIF1α target gene Glut1 (data not shown), however, no effect of CoCl2 was seen on Dio3 mRNA expression (Fig. 6D) in adipocytes.
Discussion
The effect of the fasting-induced increase in D3 on systemic TH levels has been studied by Galton et al.; they observed in D3-deficient mice compared to WT a blunted decrease of serum T4 and T3 levels during fasting showing that the systemic changes in thyroid hormone concentrations during food deprivation are indeed party mediated by D3 (24). In the present study, we focus on the mechanisms involved in the fasting-induced D3 increase in both liver and adipose tissue. Using both an in vivo and an in vitro approach, we reveal an important role of mTOR in the upregulation of D3 in WAT and liver during fasting, while CAR plays a role in the fasting-induced D3 increase in the liver.
The mechanism involved in the fasting-induced D3 increase in liver
Fasting affects a variety of humoral factors and metabolic pathways. Secretion of leptin from WAT is decreased, glucose and insulin levels are low, and the concentrations of free fatty acids in the blood rise (25). Changes in circulating factors are sensed by cells, where these inputs converge on mTOR (26) and are integrated to lead to appropriate metabolic responses. As we previously showed that leptin is able to restore fasting-induced increased Dio3 mRNA expression in the liver (7), the involvement of metabolic pathways in D3 regulation is likely. We therefore first evaluated the role of mTOR in Dio3 mRNA expression and activity in primary rat hepatocytes. mTOR is important in many (patho)physiological conditions as it coordinates a variety of mitogenic and metabolic pathways. mTOR is assembled with various other proteins, forming mTORC1 when assembled with raptor and mTORC2 when assembled with rictor. mTORC1 regulates the synthesis of proteins that are essential for cell growth. In order to maintain this function, it is important that mTOR senses available energy to prevent energy demanding processes in the cell during fasting (26). Many energy-sensing mechanisms converge on mTORC1, for example, 5′-AMP protein kinase (AMPK) which is activated when ATP levels are low (27). In addition, mTORC1 responds to nutrient availability by sensing amino acids (28) and mTOR signaling is also intertwined with leptin and insulin signaling since both leptin and insulin have the ability to activate mTOR (29). Taken together, mTOR function is inhibited during shortage of energy and thereby an important integrator of energy-sensing signals.
We observed an increase in Dio3 mRNA expression after 16 h of treatment with torin or rapamycin, two mTOR inhibitors, in primary rat hepatocytes, while D3 activity only marginally increased after 24 h of rapamycin treatment. Although at first sight the marked increase in Dio3 expression may seem difficult to reconcile with the only modest increase in D3 activity, inhibition of mTOR is known to globally downregulate protein synthesis in vitro via inhibition of ribosomal biogenesis and translation (20), which might explain the observed difference.
Since fasting markedly increases CAR expression and Maglich et al. showed that the serum TH response to fasting is impaired in CAR-/- mice compared to WT (9), we aimed to determine if CAR activation by itself is able to increase D3 mRNA expression and activity. To this end, we cultured the rat primary hepatocytes in the presence of a rat specific synthetic CAR agonist, TPD. TPD treatment increased both Dio3 mRNA expression and D3 activity after 24 h, indicating that CAR activation by itself upregulates D3. It is unknown at this stage whether the Dio3 promoter has a CAR-responsive element or whether there is an intermediate protein involved. Blanco-Bose et al. identified c-Myc and FoxM1 as genes induced by CAR (30). C-Myc regulates transcription of genes as an heterodimer with the transcription factor MAX via binding to the canonical enhancer box (E-box) CACGTG but also via a variety of non-canonical binding sites like E-box sequence CATGTG and several E-box variants such as CACGCG, CACGAG, CATGCG, and CACGTT (31). We scanned the Dio3 promoter and found the sequences CATGTG, CACGTT and CACGAG suggesting c-Myc as a possible intermediate protein involved. c-Myc is also able to bind to the FoxM1 promoter in a CAR-dependent manner. FoxM1 or Forkhead Box M1 is a member the Forkhead superfamily of transcription factors and binds directly to genes via a consensus DNA-binding site A(T/C)AAA(T/C)AA (32). Scanning the Dio3 promoter did not reveal any of these sequences making a role of FoxM1 in the fasting-induced D3 increase unlikely.
In order to test the relevance of the in vitro results for the in vivo situation, we starved CAR-/- and WT mice for 24 h. The fasting induced increase in liver Dio3 mRNA expression in WT mice was not reflected in D3 activity due to the duration of the fasting period; it is known that 24-h fasting is too short to induce an increase in liver D3 activity in vivo (7). Interestingly, the fasting-induced increase in liver Dio3 expression was absent in CAR-/- mice confirming a role for CAR in D3 regulation during fasting. In addition, basal Dio3 mRNA expression was higher in CAR-/- mice compared to WT mice and we also observed a trend toward increased basal D3 activity. To evaluate whether the increased basal Dio3 expression is a compensation for the lifelong absence of CAR, it would be interesting to investigate conditional CAR knock out mice, but these are not commercially available. A previous study by Maglich et al. showed that the serum TH response to fasting was impaired but not completely absent in CAR-/- mice (9), which is in contrast with our findings. However, it should be noted that Maglich et al. observed only a subtle difference in the fasting induced drop in serum TH concentrations in CAR-/- mice compared to WT mice.
The mechanisms involved in the fasting induced D3 increase in WAT
Adipose tissue is a key metabolic organ sensitive to TH, which may stimulate both local lipogenesis and lipolysis (11). Fasting-induced alterations in TH status are therefore likely to affect lipid metabolism. The expression of fatty acid synthase (Fas, a T3-responsive gene) in WAT decreases upon fasting (33). Thus, low intracellular TH concentrations and subsequent stimulation of lipolysis would be a likely scenario under fasting conditions. In the present study, we investigated deiodinase and TR expression in WAT of mice that were fasted for 48 h, as well as potential molecular determinants of the observed changes using 3T3-L1 adipocytes. Long-term fasting did not result in altered Dio2, Tr α1 or Tr β1 mRNA expression, but it significantly upregulated Mct8 and Dio3 mRNA expression in WAT. Theoretically, the combination of unchanged D2 and stimulated D3 during fasting in WAT would lead to lower intracellular TH concentrations in the adipocyte. However, the observed increased expression of MCT8 may induce enhanced uptake of T3 from the serum into the adipocyte. By inference, the resulting intracellular TH concentrations in WAT during fasting may range from unchanged to lower compared to the fed state.
To investigate the mechanisms involved in the fasting-induced D3 upregulation, we used 3T3-L1 adipocytes. Culturing 3T3-L1 adipocytes for 6h in HBSS, which is a medium deprived of all amino acids and growth factors, strongly induced Dio3 and Dio2 mRNA expression. Since cells only survive these culture conditions for a short period, we aimed to mimic starvation by affecting specific intracellular pathways involved in nutrient sensing. To this end, we inhibited mTOR signaling by using torin and rapamycin. In agreement with the results obtained in liver, inhibition of mTOR increased Dio3 mRNA expression in 3T3-L1 adipocytes. Activation of 5′-AMP protein kinase (AMPK) by treating the cells with AICAR, a synthetic AMPK activator, did not increase Dio3 mRNA expression, indicating that AMPK activation is not crucial for the mTOR-mediated increase in D3.
D3 is known to be regulated by TH itself, but also by numerous other hormones such as estrogen, progesterone, growth hormone and glucocorticoids (34). In addition to these endocrine factors, D3 is positively regulated by HIF1α under hypoxic conditions (35). Under normoxic conditions, prolyl hydroxylases (PHD’s) hydroxylate HIF1α, making it prone to proteosomal degradation. Low oxygen levels inhibit PHD activity, thereby stabilizing HIF1α and enabling translocation to the nucleus and dimerization with HIF1β. Besides oxygen, 2-oxoglutarate (2-OG) is an essential cofactor for PHD activity (36). Hypothetically, fasting might result in decreased 2-OG levels since glucagon (high during fasting) induces the formation of glutamate from 2-OG, thus depleting 2-OG levels (37). However, papers report contradictory results with regard to HIF1α and feeding status; increased expression has been observed in WAT of fasted seals and rats (38, 39), but in WAT of obese subjects with metabolic syndrome HIF1α was also shown to be increased (40). To test the role of HIF1a in the regulation of D3 in 3T3-L1 adipocytes, we treated the cells with CoCl2, which chemically mimics hypoxia (17). We observed increased expression of Glut1 (a HIF1α target gene) and PGK1 (an oxygen-dependent gene) 8 h after CoCl2, confirming the effectivity of the treatment. However, no increase was seen in Dio3 mRNA expression, arguing against a role for HIF1α in D3 regulation during fasting. A limitation of the study is the lack of D3 activity data in 3T3-L1 adipocytes; D3 activity in adipose tissue is generally low and we were not able to measure D3 activity in 3T3-L1 adipocytes with our method. However, previous studies have shown that Dio3 mRNA expression correlates well with D3 activity in liver during regeneration (41) and starvation (5). Furthermore, Kester et al. showed very elegantly in a number of cell lines a strong positive relation between Dio3 mRNA expression and activity levels after a variety of stimuli (42). We are therefore convinced that the observed alterations in Dio3 mRNA expression will be reflected in D3 activity.
In conclusion, Dio3 mRNA expression increased during fasting in both liver and adipose tissue. CAR plays a role in the fasting-induced D3 increase in the liver, as CAR activation in rat primary hepatocytes directly increases D3 expression and activity. mTOR inhibition strongly induced Dio3 expression in liver and WAT but only slightly induced D3 activity in hepatocytes, indicating that additional factors control D3 activity. Dio3 mRNA expression in WAT is responsive to prolonged fasting and is likely to be mediated via inhibition of mTOR activity. A schematic overview of the observed changes and possible mechanisms involved is given in Fig. 7. With these findings, we provide new mechanisms for tissue-specific D3 regulation during fasting.
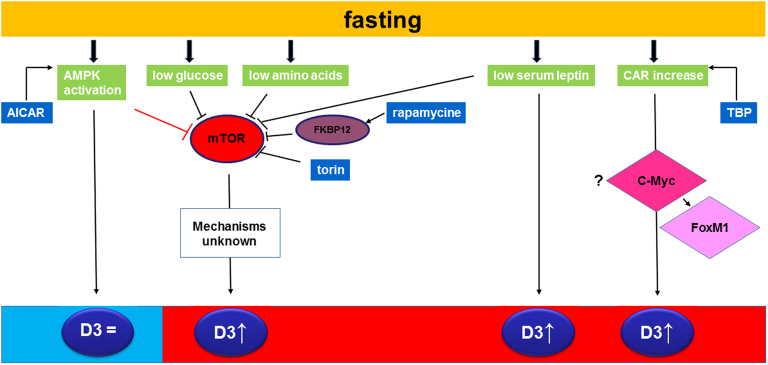
Figure 7.
This schematic overview summarizes fasting induced changes in type 3 deiodinase expression and its putative mechanisms. Fasting results in activation of AMPK, decreased glucose and amino acids concentrations, decreased serum leptin and increased hepatic CAR expression. AMPK activation can be mimicked by AICAR stimulation and does not have an effect on D3 expression despite affecting mTOR. In contrast, low glucose + low amino acids (mimicked by culturing the cells in HBSS) increases D3 expression. Inhibition of mTOR signaling by rapamycin and torin also results in increased D3 expression. The downstream mechanisms are however unknown. Fasting decreases serum leptin concentrations which are known to affect D3 expression as leptin administration restores the fasting-induced increase in D3 (7). The present study shows that hepatic CAR activation by TPD increases D3 expression. Blanco-Bose et al. identified c-Myc and FoxM1 as genes induced by CAR (30). C-Myc regulates transcription of genes as an heterodimer with the transcription factor MAX via binding to the canonical Enhancer box (E-box) CACGTG but also via a variety of non-canonical binding sites like E-box sequence CATGTG and several E-box variants such as CACGCG, CACGAG, CATGCG, and CACGTT (31). We scanned the D3 promoter and found the sequences CATGTG, CACGTT and CACGAG suggesting c-Myc as a possible intermediate protein involved . c-Myc is also able to bind to the FoxM1 promoter in a CAR-dependent manner. FoxM1 or Forkhead Box M1 is a member the Forkhead superfamily of transcription factors and binds directly to genes via a consensus DNA binding site A(T/C)AAA(T/C)AA (32). Scanning the D3 promoter did not reveal any of these sequences making a role of FoxM1 in the fasting-induced D3 increase unlikely.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Acknowledgements
The authors would like to thank Dirk-Jan Saaltink and Erno Vreugdenhil (Medical Pharmacology/LACDR/LUMC, University of Leiden, Leiden, The Netherlands) for providing murine fat tissue, the staff of the Endocrine Laboratory for measuring serum thyroid hormones and Leslie Eggels (Endocrine laboratory) for her expert technical assistance with the animal experiments. They would like to thank Noam Zelcer, Boris Bleijlevens and Marc Tol from the Biochemistry Department for providing us with the PPARα agonist and mTOR inhibitors and Marc Tol also for his expert assistance with the adipocyte differentiation.
References
- 1.Boelen A, Wiersinga WM, Fliers E. Fasting-induced changes in the hypothalamus-pituitary-thyroid axis. Thyroid 2008. 18 123–129. ( 10.1089/thy.2007.0253) [DOI] [PubMed] [Google Scholar]
- 2.Harris ARC, Fang SL, Vagenakis AG, Braverman LE. Effect of starvation, nutriment replacement, and hypo-thyroidism on invitro hepatic T4 to T3 conversion in rat. Metabolism: Clinical and Experimental 1978. 27 1680–1690. ( 10.1016/0026-0495(78)90290-1) [DOI] [PubMed] [Google Scholar]
- 3.Omara BA, Dittrich W, Lauterio TJ, StGermain DL. Pretranslational regulation of type-I 5′-deiodinase by thyroid-hormones and in fasted and diabetic rats. Endocrinology 1993. 133 1715–1723. ( 10.1210/endo.133.4.8404614) [DOI] [PubMed] [Google Scholar]
- 4.Vagenakis AG, Portnay GI, Obrian JT, Rudolph M, Arky RA, Ingbar SH, Braverman LE. Effect of starvation on production and metabolism of thyroxine and triiodothyronine in euthyroid obese patients. Journal of Clinical Endocrinology and Metabolism 1977. 45 1305–1309. ( 10.1210/jcem-45-6-1305) [DOI] [PubMed] [Google Scholar]
- 5.de Vries EM, van Beeren HC, Ackermans MT, Kalsbeek A, Fliers E, Boelen A. Differential effects of fasting vs food restriction on liver thyroid hormone metabolism in male rats. Journal of Endocrinology 2015. 224 25–35. ( 10.1530/JOE-14-0533) [DOI] [PubMed] [Google Scholar]
- 6.Kester MHA, Kaptein E, Roest TJ, van Dijk CH, Tibboel D, Meinl W, Glatt H, Coughtrie MWH, Visser TJ. Characterization of human iodothyronine sulfotransferases. Journal of Clinical Endocrinology and Metabolism 1999. 84 1357–1364. ( 10.1210/jcem.84.4.5590) [DOI] [PubMed] [Google Scholar]
- 7.Boelen A, van Beeren M, Vos X, Surovtseva O, Belegri E, Saaltink DJ, Vreugdenhil E, Kalsbeek A, Kwakkel J, Fliers E. Leptin administration restores the fasting-induced increase of hepatic type 3 deiodinase expression in mice. Thyroid 2012. 22 192–199. ( 10.1089/thy.2011.0289) [DOI] [PubMed] [Google Scholar]
- 8.Wang YM, Ong SS, Chai SC, Chen T. Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opinion on Drug Metabolism and Toxicology 2012. 8 803–817. ( 10.1517/17425255.2012.685237) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maglich JM, Watson J, McMillen PJ, Goodwin B, Willson TM, Moore JT. The nuclear receptor CAR is a regulator of thyroid hormone metabolism during caloric restriction. Journal of Biological Chemistry 2004. 279 19832–19838. ( 10.1074/jbc.M313601200) [DOI] [PubMed] [Google Scholar]
- 10.O’Connor JC, Frame SR, Ladics GS. Evaluation of a 15-day screening assay using intact male rats for identifying steroid biosynthesis inhibitors and thyroid modulators. Toxicological Sciences 2002. 69 79–91. ( 10.1093/toxsci/69.1.79) [DOI] [PubMed] [Google Scholar]
- 11.Williams CM, Ellis R. Thermogenic and metabolic consequences of thyroid hormone treatment in brown and white adipose tissue. Bioscience Reports 1985. 5 175–184. ( 10.1007/BF01117064) [DOI] [PubMed] [Google Scholar]
- 12.Obregon MJ. Thyroid hormone and adipocyte differentiation. Thyroid 2008. 18 185–195. ( 10.1089/thy.2007.0254) [DOI] [PubMed] [Google Scholar]
- 13.Masaki T, Yoshimatsu H, Kakuma T, Hidaka S, Kurokawa M, Sakata T. Enhanced expression of uncoupling protein 2 gene in rat white adipose tissue and skeletal muscle following chronic treatment with thyroid hormone. FEBS Letters 1997. 418 323–326. ( 10.1016/s0014-5793(97)01404-x) [DOI] [PubMed] [Google Scholar]
- 14.Rodriguez-Perez A, Palos-Paz F, Kaptein E, Visser TJ, Dominguez-Gerpe L, varez-Escudero J, Lado-Abeal J. Identification of molecular mechanisms related to nonthyroidal illness syndrome in skeletal muscle and adipose tissue from patients with septic shock. Clinical Endocrinology 2008. 68 821–827. ( 10.1111/j.1365-2265.2007.03102.x) [DOI] [PubMed] [Google Scholar]
- 15.Aragones J, Fraisl P, Baes M, Carmeliet P. Oxygen sensors at the crossroad of metabolism. Cell Metabolism 2009. 9 11–22. ( 10.1016/j.cmet.2008.10.001) [DOI] [PubMed] [Google Scholar]
- 16.Duran RV, MacKenzie ED, Boulahbel H, Frezza C, Heiserich L, Tardito S, Bussolati O, Rocha S, Hall MN, Gottlieb E. HIF-independent role of prolyl hydroxylases in the cellular response to amino acids. Oncogene 2013. 32 4549–4556. ( 10.1038/onc.2012.465) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee HR, Leslie F, Azarin SM. A facile in vitro platform to study cancer cell dormancy under hypoxic microenvironments using CoCl2. Journal of Biological Engineering 2018. 12 12 ( 10.1186/s13036-018-0106-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.de Vries EM, Eggels L, van Beeren HC, Ackermans MT, Kalsbeek A, Fliers E, Boelen A. Fasting-induced changes in hepatic thyroid hormone metabolism in male rats are independent of autonomic nervous input to the liver. Endocrinology 2014. 155 5033–5041. ( 10.1210/en.2014-1608) [DOI] [PubMed] [Google Scholar]
- 19.Wiersinga WM, Chopra IJ. Radioimmunoassays of thyroxine. Methods in Enzymology 1982. 84 272–303. ( 10.1016/0076-6879(82)84024-x) [DOI] [PubMed] [Google Scholar]
- 20.Richter JD, Sonenberg N. Regulation of cap-dependent translation by eIF4E inhibitory proteins. Nature 2005. 433 477–480. ( 10.1038/nature03205) [DOI] [PubMed] [Google Scholar]
- 21.Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. Journal of Clinical Investigation 1999. 103 1489–1498. ( 10.1172/JCI6223) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ding X, Lichti K, Kim I, Gonzalez FJ, Staudinger JL. Regulation of constitutive androstane receptor and its target genes by fasting, cAMP, hepatocyte nuclear factor alpha, and the coactivator peroxisome proliferator-activated receptor gamma coactivator-1alpha. Journal of Biological Chemistry 2006. 281 26540–26551. ( 10.1074/jbc.M600931200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell 2003. 115 577–590. ( 10.1016/s0092-8674(03)00929-2) [DOI] [PubMed] [Google Scholar]
- 24.Galton VA, Hernandez A, St Germain DL. The 5-deiodinases are not essential for the fasting-induced decrease in circulating thyroid hormone levels in male mice: possible roles for the type 3 deiodinase and tissue sequestration of hormone. Endocrinology 2014. 155 3172–3181. ( 10.1210/en.2013-1884) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robertson DG, Ruepp SU, Stryker SA, Hnatyshyn SY, Shipkova PA, Aranibar N, McNaney CA, Fiehn O, Reily MD. Metabolomic and transcriptomic changes induced by overnight (16 h) fasting in male and female Sprague-Dawley rats. Chemical Research in Toxicology 2011. 24 481–487. ( 10.1021/tx200074f) [DOI] [PubMed] [Google Scholar]
- 26.Yang X, Yang C, Farberman A, Rideout TC, de Lange CF, France J, Fan MZ. The mammalian target of rapamycin-signaling pathway in regulating metabolism and growth. Journal of Animal Science 2008. 86 (Supplement) E36–E50. ( 10.2527/jas.2007-0567) [DOI] [PubMed] [Google Scholar]
- 27.Hawley SA, Selbert MA, Goldstein EG, Edelman AM, Carling D, Hardie DG. 5′-AMP activates the AMP-activated protein kinase cascade, and Ca2+/calmodulin activates the calmodulin-dependent protein kinase I cascade, via three independent mechanisms. Journal of Biological Chemistry 1995. 270 27186–27191. ( 10.1074/jbc.270.45.27186) [DOI] [PubMed] [Google Scholar]
- 28.Hara K, Yonezawa K, Weng QP, Kozlowski MT, Belham C, Avruch J. Amino acid sufficiency and mTOR regulate p70 S6 kinase and eIF-4E BP1 through a common effector mechanism. Journal of Biological Chemistry 1998. 273 14484–14494. ( 10.1074/jbc.273.23.14484) [DOI] [PubMed] [Google Scholar]
- 29.Fruhbeck G. Intracellular signalling pathways activated by leptin. Biochemical Journal 2006. 393 7–20. ( 10.1042/BJ20051578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Blanco-Bose WE, Murphy MJ, Ehninger A, Offner S, Dubey C, Huang W, Moore DD, Trumpp A. C-Myc and its target FoxM1 are critical downstream effectors of constitutive androstane receptor (CAR) mediated direct liver hyperplasia. Hepatology 2008. 48 1302–1311. ( 10.1002/hep.22475) [DOI] [PubMed] [Google Scholar]
- 31.Allevato M, Bolotin E, Grossman M, Mane-Padros D, Sladek FM, Martinez E. Sequence-specific DNA binding by MYC/MAX to low-affinity non-E-box motifs. PLoS ONE 2017. 12 e0180147 ( 10.1371/journal.pone.0180147) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liao GB, Li XZ, Zeng S, Liu C, Yang SM, Yang L, Hu CJ, Bai JY. Regulation of the master regulator FOXM1 in cancer. Cell Communication and Signaling 2018. 16 57 ( 10.1186/s12964-018-0266-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palou M, Sanchez J, Priego T, Rodriguez AM, Pico C, Palou A. Regional differences in the expression of genes involved in lipid metabolism in adipose tissue in response to short- and medium-term fasting and refeeding. Journal of Nutritional Biochemistry 2010. 21 23–33. ( 10.1016/j.jnutbio.2008.10.001) [DOI] [PubMed] [Google Scholar]
- 34.Hernandez A. Structure and function of the type 3 deiodinase gene. Thyroid 2005. 15 865–874. ( 10.1089/thy.2005.15.865) [DOI] [PubMed] [Google Scholar]
- 35.Simonides WS, Mulcahey MA, Redout EM, Muller A, Zuidwijk MJ, Visser TJ, Wassen FW, Crescenzi A, da-Silva WS, Harney J. et al. Hypoxia-inducible factor induces local thyroid hormone inactivation during hypoxic-ischemic disease in rats. Journal of Clinical Investigation 2008. 118 975–983. ( 10.1172/JCI32824) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webb JD, Coleman ML, Pugh CW. Hypoxia, hypoxia-inducible factors (HIF), HIF hydroxylases and oxygen sensing. Cellular and Molecular Life Sciences 2009. 66 3539–3554. ( 10.1007/s00018-009-0147-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ui M, Exton JH, Park CR. Effects of glucagon on glutamate metabolism in the perfused rat liver. Journal of Biological Chemistry 1973. 248 5350–5359. [PubMed] [Google Scholar]
- 38.Sonanez-Organis JG, Vazquez-Medina JP, Crocker DE, Ortiz RM. Prolonged fasting activates hypoxia inducible factors-1alpha, -2alpha and -3alpha in a tissue-specific manner in northern elephant seal pups. Gene 2013. 526 155–163. ( 10.1016/j.gene.2013.05.004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang X, McCormick K, Mick G. Nutritional regulation of white adipocyte vascular endothelial growth factor (VEGF). Hormone and Metabolic Research 2003. 35 211–216. ( 10.1055/s-2003-39476) [DOI] [PubMed] [Google Scholar]
- 40.He Q, Gao Z, Yin J, Zhang J, Yun Z, Ye J. Regulation of HIF-1{alpha} activity in adipose tissue by obesity-associated factors: adipogenesis, insulin, and hypoxia. American Journal of Physiology: Endocrinology and Metabolism 2011. 300 E877–E885. ( 10.1152/ajpendo.00626.2010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kester MH, Toussaint MJ, Punt CA, Matondo R, Aarnio AM, Darras VM, Everts ME, de Bruin A, Visser TJ. Large induction of type III deiodinase expression after partial hepatectomy in the regenerating mouse and rat liver. Endocrinology 2009. 150 540–545. ( 10.1210/en.2008-0344) [DOI] [PubMed] [Google Scholar]
- 42.Kester MH, Kuiper GG, Versteeg R, Visser TJ. Regulation of type III iodothyronine deiodinase expression in human cell lines. Endocrinology 2006. 147 5845–5854. ( 10.1210/en.2006-0590) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a