Abstract
We investigated the perception of two mechanoreceptive modalities alone and in combination: main effects and interaction between auditory and somatosensory stimulation in mice. Fifteen C57/BL6J mice between the ages of 1 and 6 months were tested three times each. Experimental design roughly followed published procedures using pre-pulse inhibition (PPI) of the acoustic startle response, except pre-pulses included vibration of the test chamber as well as soft sounds. Auditory pre-pulses were 80 dB broadband noises of 4, 9, 25, or 45 ms duration. Vibrations were of the same duration but of different frequencies (500, 460, 360, and 220 Hz). Pre-pulse inhibition increased with duration of the auditory pre-pulses, as expected. There was significant PPI to some but not all vibrotactile pre-pulses. Multimodal PPI was approximately additive (no significant auditory-by-somatosensory interaction). PPI increased more with age to somatosensory than to auditory pre-pulses. Future studies of multi-modal psychophysics in various mouse mutants could lend support to more mechanistic studies of neural specificity and possibly autism, tinnitus, and PTSD.
Keywords: acoustic startle response, multi-modal, sensory binding, vibrotactile
1. Introduction
The startle reflex is a motor response to an unexpected, intense stimulus [1]. The present experiment used a sudden, loud, acoustic stimulus to elicit a full-body startle in mice. A pre-pulse is a stimulus that does not elicit a startle, but if presented approximately 30–500 ms before the startle-eliciting stimulus can reduce the startle [2]. This is known as pre-pulse inhibition, or PPI. A pre-pulse can be an auditory or somatosensory [1]. In the present study auditory and vibratory pre-pulses are studied together and separately as mice age.
Many of the factors of interest in this paper have been studied in humans, including developmental changes in PPI [3], the use of vibrotactile pre-pulses with acoustic startle stimuli [4], and the dependence of PPI on pre-pulse intensity [5]. There is, to our knowledge, no previous report of simultaneous non-startling auditory and somatosensory pre-pulses interacting to inhibit an auditory startle response in either humans or mice. Behavioral analyses of multi-modal reflex modification in mice can be combined with anatomical and physiological studies to better understand both normal and abnormal development of neural specificity, and could become a useful animal model of disorders such as tinnitus, PTSD, autism, and schizophrenia.
2. Methods
2.1. Subjects
C57BL/6J mice (n=15) in three age groups were tested three times each. The groups include four ‘old’ (166–181 days), five ‘middle-aged’ (108–125 days), and six ‘young’ (30–67 days) mice. All young mice were males. Middle-aged mice were of both sexes. Sexes of the ‘old’ group unfortunately were not tracked. Apart from the one hour testing session, mice had access to food and water, and were housed with their same sex in BioZone Inc. MiniRack™ individually HEPA filtered cages. All procedures were approved by the James Madison University Institutional Animal Care and Use Committee (IACUC).
2.2. Apparatus and stimuli
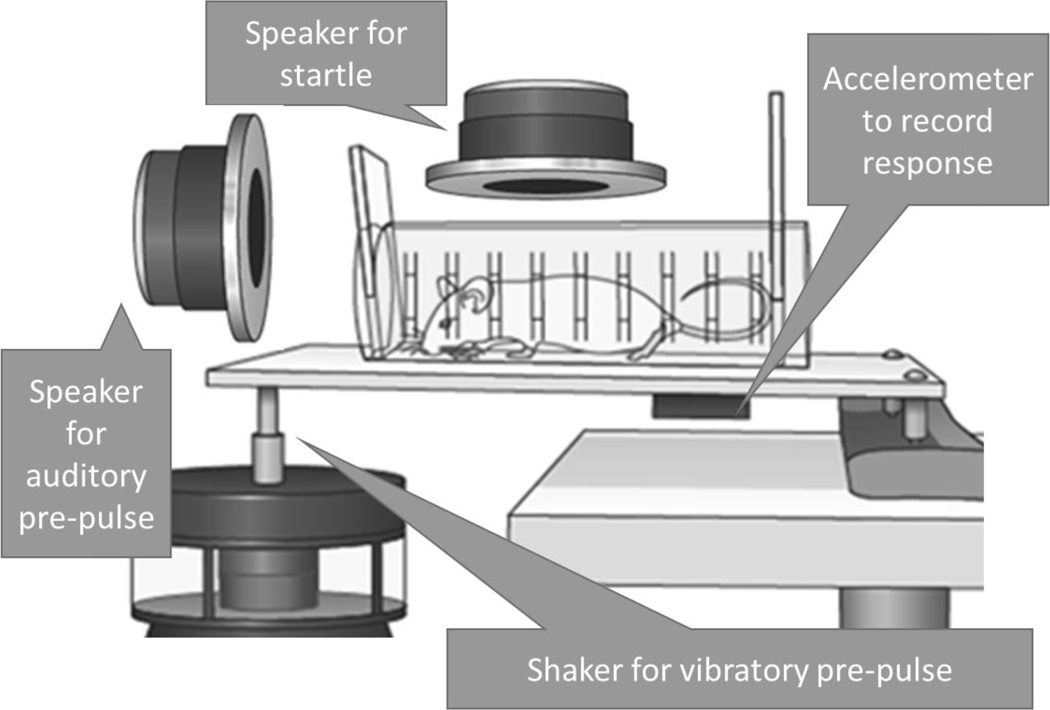
All testing took place within a 2.13 m2 Industrial Acoustics Inc., double-walled, double-floored, sound-attenuating booth. Mice were tested in a Plexiglas tube with an inside diameter of 5 cm and a length of 12.5 cm, glued to a 124 by 200 by 4.5 mm horizontal plastic plate, with an accelerometer beneath the plate: the inside part of a SR-Lab, Small Animal test chamber [6]. Figure 1 shows a schematic of the multi-modal testing equipment. Two modalities of pre-pulses, somatosensory and auditory, were used, both with an auditory startle.
Figure 1. Illustration of the multimodal testing system.
Not to scale; side speaker behind tube, 90o from that shown.
A Tucker Davis Technology Real-Time Processor (TDT RP2.1, running at 50 kHz) formed the SES with amplification by a Crown XLS202 amplifier. The SES for all testing was 110 dB SPL, 15 ms broadband noise, high-pass filtered at 8 kHz with 10 μs linear on/off gates. A Ross Audio Systems TW 30 compression tweeter, 15 cm above the mouse, produced the startle-eliciting stimulus (SES).
The auditory prepulses were 80 dB SPL broad-band noises presented for 4, 9, 25 or 45 ms. A second Ross TW30, 38 cm to one side of the tube, produced the broadband auditory pre-pulse stimuli. Auditory pre-pulse stimuli were 4, 9, 25, or 45 ms in duration; all high-pass filtered at 4 kHz, with instantaneous rise/decay times.
The vibratory stimuli were sine waves (1.5V p-p) from an Agilent 33220A Function Generator into a Pasco Mechanical Wave Vibrator (SF-9324). The single, 6 mm diameter piston at the top of the vibrator fit into a clip in the middle of one short side of the SR-Lab plate. The two stand-offs on the opposite short side of the plate were placed on foam to reduce sound produced by the vibration. Four stimuli of different frequency were selected after an automated search of 900 combinations of frequencies, intensities, durations and inter-stimulus intervals (ISIs) to optimize a combination of significant vibration during the pre-pulse, minimal residual vibration during the 100ms after the ISI, and inaudible sound.
During multimodal pre-pulses the auditory and somatosensory stimuli were simultaneous and (the voltages sent to the two transducers) were of the same duration. Characteristics of the four selected stimuli are seen in Table 1.
Table 1. Characteristics of the four somatosensory and auditory pre-pulses.
The first column, MS, shows the label of the four selected vibratory and/or auditory stimuli used in the graphs and text below; this is also the duration in milliseconds of voltages to the shaker and duration of the broad-band noises in the uni- and multi-modal conditions. The 2nd-4th columns, Cycles, Hz, and ISI) show the programmed characteristics of the vibrations. The 5th and 6th columns, μV and μG, show the magnitude of the pre-pulse, in terms of voltage from the accelerometer and also in calibrated acceleration (where 1μG = 9.8μm/s2). The 7th column, Resonance, shows the amount of any residual vibration persisting beyond the ISI and into the 100ms response period. The 8th column, dB(ML), estimates the level of sound produced by the vibrating platform relative to the mouse audiogram by showing the maximum difference in dB between the power spectrum of sound recorded from a calibrated microphone in the center of the testing tube during each vibration minus an estimate of the C57/BL6J mouse audiogram: 0.00073*kHz3 – 0.06*kHz2 + 55.38*log10Hz2 – 449.4*log10Hz + 1.63*kHz + 918. These 7th and 8th columns are described below in the discussion of ‘controls’. The last column, dB Leq, shows the sound-pressure level of the acoustic prepulses integrated over the ISI [7]; spectrum level with no stimulus was 6 dB SPL leading to 52 dB in the 4 to 44 kHz bandwidth which is added in linear units to the pre-pulse and divided by the ISI to determine the amount of constant sound that would deliver the same total energy over the ISI as the auditory pre-pulse.
| Ms | Cycles | Hz | ISI ms | μV rms | μG | Resonance μV rms | “dB(ML)” | dB (Leq) |
|---|---|---|---|---|---|---|---|---|
| 4 | 2 | 500 | 200 | 49 | 2.5 | 0 | −3 | 55 |
| 9 | 4 | 460 | 200 | 55 | 2.9 | 0 | −2 | 5B |
| 25 | 9 | 360 | 200 | 11B | 6.1 | .37 | −1 | 64 |
| 45 | 10 | 220 | 150 | ss1 | 17.2 | .76 | 5 | 70 |
Auditory calibrations used a B&K4939 3/8-inch, high-frequency microphone (with flat frequency response to ~100 kHz), Listen Inc. SoundConnect Amp, and an Agilent 35670A Dynamic Signal Analyzer. The microphone was clamped in the middle of the testing tube during calibration. Calibration of the SES and of the auditory pre-pulses revealed a flat frequency band from the high-pass limit to 25 kHz, with a gradual high-frequency roll-off.
Vibratory calibrations used the Sensor Kinetics Pro (V 3.1.2) Android app on an LG-P659 cell phone [8]. The cell phone was placed on the horizontal SR-Lab plate beside the tube that typically holds the mouse, and the SR-Lab small-animal test system with the phone upon it were vibrated by the calibrator supplied with the SR-lab system [6]. During those vibrations the app recorded acceleration (in m/s/s), and the computer recorded voltages from the SR Lab system. Analysis of these two simultaneous signals determined that 1 mV from the SR lab accelerometer equals 52 microG or ~.5m/s/s. Magnitude of the somatosensory stimuli (Column 5 in Table 1) were determined from the output of the SR-Lab accelerometer during each stimulus with a 30 g fake mouse in the testing tube.
2.3. General Procedures
Each mouse was tested three times, with at least one week separating each testing session. Each testing session consisted of 11 blocks with 15 trials each – 165 trials with an inter-trial interval that varied randomly between 15 and 25 s (uniformly distributed). The 15 trials in each block consisted of the following in random order:
four trials, one with each somatosensory pre-pulse followed by the SES
four trials, one with each auditory pre-pulse followed by the SES
four trials with a simultaneous somatosensory and auditory pre-pulse of identical length (4ms vibration with the 4ms sound … 45ms vibration with 45ms sound) followed by the SES
two control trials in which the SES was presented alone
one trial with no pre-pulse or SES
For each trial, RMS voltage from the SR-Lab accelerometer was recorded for 100 ms from the start of the SES. Pre-pulse inhibition (PPI) was calculated as 1 – (ASRP)/(ASRC) [9]. In this equation ASRP represents the acoustic startle response in the pre-pulse stimulus conditions, and ASRC represents the acoustic startle amplitude in the control condition, without any pre-pulse stimulus before the SES. PPI is thus the reduction in startle amplitude when a pre-pulse was present.
3. Results
An initial repeated-measures test included test-number (the first through third test of each mouse) along with stimulus as within-subjects variables and group (age) as the between-subjects variable. There was no significant main effect nor interaction with test-number (p’s > 0.7). Therefore, the three tests of each mouse were averaged to simplify the analysis (12 PPIs to four durations of three modalities averaged over three tests of 11 blocks). The important repeated-measures ANOVA had two within-subjects factors: modality with three levels (auditory, somatosensory, multi-modal) and duration with four levels (4, 9, 25 and 45 ms), and one between-subjects factor: age with three levels: young, medium, and old. All within-subjects factors met Mauchly’s test of sphericity.
There was a significant effect of modality (F2,24= 43.3, p< .001, pη2=.78).
There was a significant modality-by-age interaction (F4,24= 4.6, p= .007, pη2=.43)
There was a significant effect of duration (F3,36= 23.2, p< .001, pη2=.66), and no duration-by-age interaction (p=.16), but there was a duration-by-modality interaction (F6,72= 6.9, p<.007, pη2=.37)
No duration-by-group interaction; p = .159
No three-way interaction, p = 0.24
An effect of age that approaches significance, p = .08, pη2=.34.
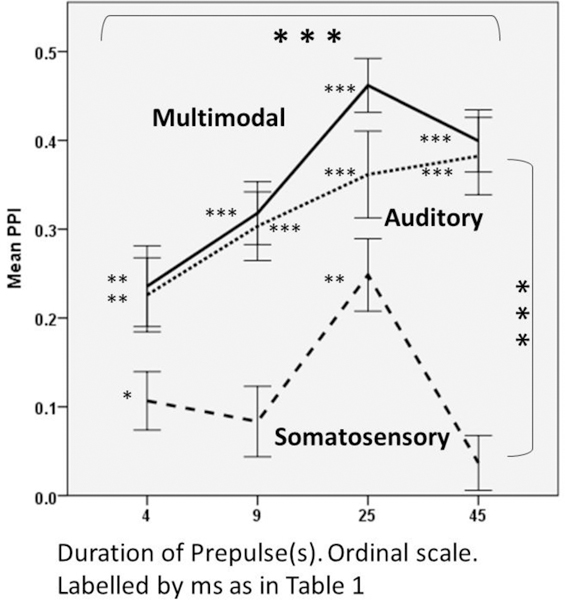
Figure 3 displays the mean PPI of all mice for the auditory, somatosensory, and combined auditory/somatosensory pre-pulses presentation at each stimulus duration, 4, 9, 25, and 45 ms. As stated above, each vibration was a different frequency: 500, 460, 360 and 220 Hz, respectively. Results showed the expected auditory pre-pulse behavior in that sound-elicited PPI increased as the stimulus duration increased. Only two somatosensory stimuli, 2 cycles of 220 Hz (4 ms) and 9 cycles of 360 Hz (25 ms), elicited reliable PPI (p<.05 in single-sample t-tests comparing the PPI to zero). The results also suggest that the 25 ms broadband sound with 9 cycles of 360 Hz vibration resulted in an additive-like effect of multimodal PPI.
Figure 3. Mean PPI of all mice for the auditory (small dashed), somatosensory (large dashed), and auditory and somatosensory (solid) pre-pulses, plotted as a function of duration: 4, 9, 25, and 45 ms.
The large stars inside the top horizontal bracket indicate the significance of duration; large stars inside the vertical bracket indicate the significance of modality, both from the repeated-measures ANOVA presented in bulleted text. The smaller stars beside each mean indicate the univariate, 2-
4. Discussion
4.1. Auditory and somatosensory pre-pulse inhibition
It is well established that PPI can be recorded using an auditory pre-pulse in mice [1, 2, 10–12]. This report shows that somatosensory as well as auditory stimuli, alone and in combination, can elicit PPI in mice.
Reports of somatosensory PPI exist in other species. The marine mollusk, Tritonia diomedea, showed inhibition to a tail shock when a 100 ms vibration was used as a pre-pulse [13]. Pre-pulse inhibition of the acoustic startle response has been found in rats using somatosensory stimulation in the form of electric shock [14]. Researchers have used tactile stimuli such as an air puff as the startle-eliciting response in mice [15]. To our knowledge, the present study is the first to report vibratory pre-pulse inhibition in mice.
4.2. Effects of age.
C57BL/6J mice have progressive age-related hearing loss that starts in the first year. This loss begins at the high frequencies and then includes middle and low frequencies as the animal ages [16]. The broadband auditory stimuli (both the pre-pulse and the SES) in this experiment include frequencies affected by the early high-frequency hearing loss in middle- and old-aged groups.
Mammalian tactile sensation can take weeks or months to mature after birth. In cats, tactile receptors and sensory fiber myelination likely do not reach maturity until one to two months of age, and central pathways may not mature until two to three months of age. At vibratory frequencies above 100 Hz, neonatal response thresholds are five to ten times that of adult cats [17]. No information was found on development of somatosensory afferents in mice.
Pre-pulse inhibition may mature with age, possibly explaining the generally positive slopes in Figure 4. This trend may imply a role of early experience in the facilitation of multimodal circuits. Increasing age, up to postnatal day 35–37, resulted in enhanced gap-detection (an auditory stimulus) PPI in rats [18]. Mice are considered adults at 9 weeks [19]. It may be possible that some or all of the young mice, at 30 – 67 days old, had immature PPI pathways during testing.
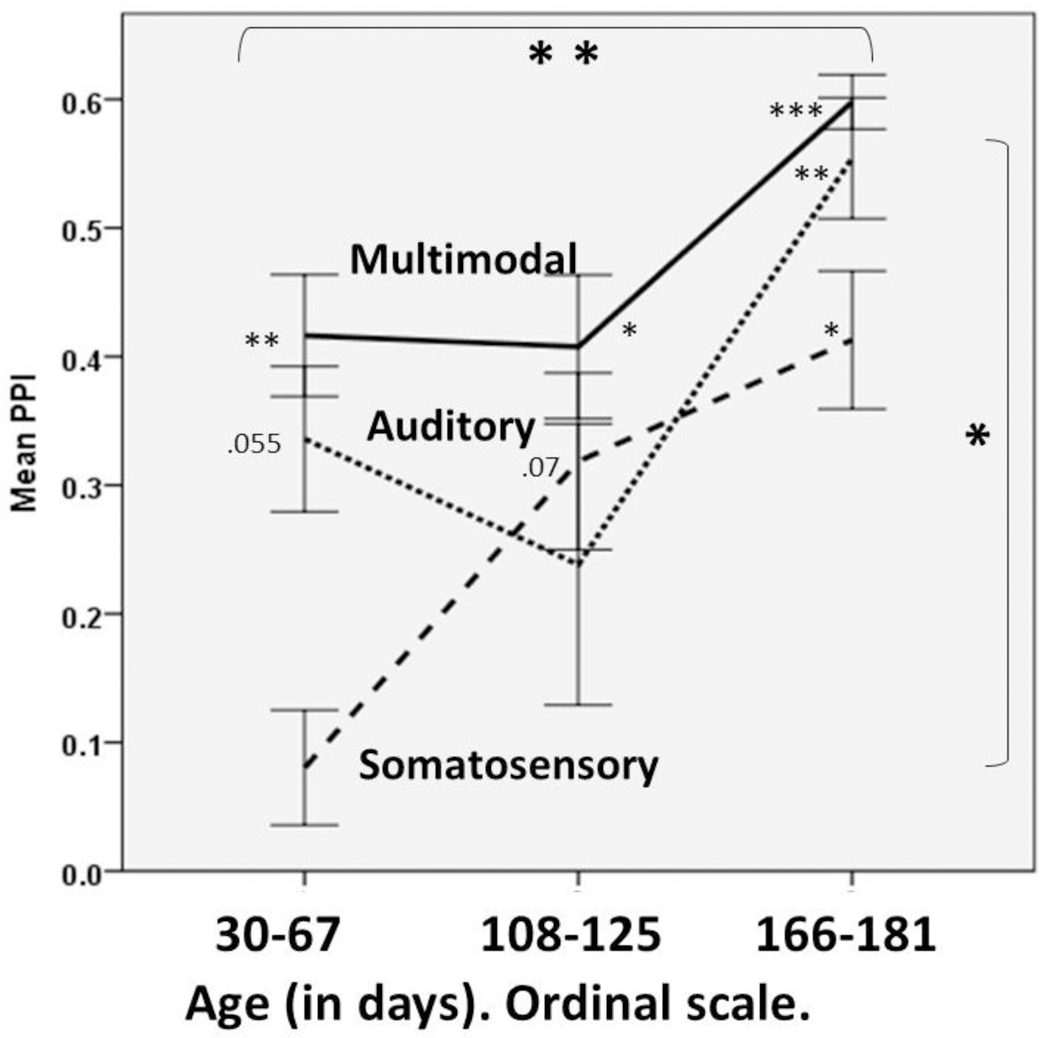
Figure 4. Mean PPI as a function of age (young, middle, and old) for the 25-ms uni- and multi-modal stimuli only.
The large stars inside the top horizontal bracket indicate the significance of age; the large star inside the vertical bracket indicates the significance of modality, both from a repeated-measures ANOVA. The smaller stars beside each mean indicate the univariate, 2-tailed, Bonferroni-corrected significance of each mean response: *<=.05, ** <=.01, ***<=.001. The small p-values where stars might appear show two groups that approach significance. As in Figure 3, PPI is the decrement in the full-body jerk caused by the auditory (small dashed), somatosensory (large dashed), and multimodal (solid line) pre-pulses: PPI= 1 –(ASRP)/(ASRC)where ASRp and ASRc are the responses with and without the pre-pulses respectively. This graph, however, includes only 1 of the 4 different durations– the one with the greatest somatosensory response.
The middle- and old-aged mice were most responsive to 360 Hz vibrations, and not as responsive to the higher and lower frequencies, suggesting a U-shaped curve for vibratory thresholds as a function of frequency. In humans, thresholds of vibration are different across frequencies [20] in a similar U-shaped function.
4.3. Multi-sensory binding
The prepulse combining 9 cycles of 360 Hz vibration with 25 ms broadband sound resulted in an additive-like effect, in that the multimodal PPI was close to the sum of the PPI to each modality alone. This behavioral finding supports the theory that auditory and somatosensory afferent pathways can converge to inhibit responsiveness to startling stimuli. Human studies have shown enhanced startle responses when tactile and auditory stimuli were presented together [21, 22], but the present data are the first, to our knowledge, to show effects of combined non-startling auditory and somatosensory pre-pulses on the inhibition of a startle response.
Mice at 108 days showed significant PPI to the somatosensory pre-pulses; the young mice at 67 days and younger showed no somatosensory PPI. This suggests heightened somatosensory responses possibly co-occurring with age-related decline in hearing. There is increased representation of the somatosensory system with auditory deprivation in another rodent [23]. More specifically, guinea pigs with noise-damaged auditory systems had reduced auditory thresholds and enhanced responses to somatosensory stimulation, with increased numbers of cells showing bimodal integration in the dorsal cochlear nucleus [23].
4.4. Controls showing no unintended effects of the vibratory stimuli
Because the somatosensory stimuli were vibrations of the SR-Lab testing platform, some shaking (resonances) might persist past the ISI. To confirm no significant ‘carry over’ of the stimulus into the response, two control experiments were run. First the exact procedure was rerun with a ‘fake mouse’ (30 g of a viscous gel in a mouse-sized plastic bag). Only the vibrations of the 25 and 45 ms vibrations were greater than the noise floor during the 100ms response period with the fake mouse. The magnitude of these residual resonances are shown in Table 1: 0.37 and 0.76 μV. The average response of the real mice was >100 μ V RMS during response periods with somatosensory stimuli only. Thus, residual resonances from the 25 and 45 ms resonances add less than 1% to the measurements of the startle, thus possibly decreasing PPI by less than 1%, which would not be statistically significant given variability in real startle responses. Five mice were run in a second control to confirm that residual vibrations were not significant. The procedure was rerun but with no SES. With no startle, the RMS recorded during the response period would be due to any residual vibration, plus any startle or excitement produced by the pre-pulse alone. As expected, the vibratory pre-pulses had no effect on the response when there was no SES (p>.5).
Because the somatosensory stimuli were vibrations, they produced sounds. That sound was primarily at the frequency of the vibration, 220–500 Hz, likely well below the mouse audiogram. To confirm no audible auditory artifact from the vibrations, ten mice were tested in a stationary testing tube with a second, vibrating, testing chamber placed as close as possible but not touching the mouse. The vibrations went to a chamber with a ‘fake mouse’, and the real mice heard but did not feel the somatosensory stimuli. Under these conditions, the sounds from the vibrations produced no PPI (t39=−1.2, p>.2). The 70 dB broadband noise produced significant PPI as expected (t39=3.9, p>.001), and the ‘multi-modal’ stimuli (that were in this control a combination of sounds produced by the speaker plus sounds from the adjacent vibrating platform) were no different than the auditory stimuli alone (F1,77=.5; p=.5).
4.5. Effect of Sex
There was an effect of sex (p=.014, in the multivariate duration-by-sex interaction), with the middle-aged males showing a trend toward more PPI with a ‘large’ [24] effect size, Cohen’s d = .73 in a 2-sample t-test of averaged PPI (but with p=.48 in the univariate test). Similar complexities and overall larger PPI in males has been found in other studies [25].
There were 2 males and 3 females in the control for residual vibratory resonances; there was no effect of sex in the RMS during the 100 ms response period, with no startle-eliciting stimulus (p=.7 in repeated measures ANOVA but with larger overall PPI in males in this measure of background activity as in other data [25]). There were 9 females and 1 male in the control test on the stationary platform with no difference between sexes (p>.999).
4.6. Implications for future research
Newly discovered pathways into a midbrain structure involved in PPI (the lateral cortex of the inferior colliculus or LCIC) [26–28] might mediate these multi-modal psychophysical responses. Somatosensory and auditory afferents project to LCIC [29,31]. Recent research has focused on the mechanisms and development of these multimodal projection pathways within the LCIC. It is likely that the LCIC is the area of integration of auditory and somatosensory information [31], but the functional/behavioral consequences of these multi-modal interactions are not fully understood. The present report demonstrates that auditory and somatosensory stimuli, as well as their combinations, can be investigated using PPI in mice. Future experiments might use this method to explore how mutations, known to affect the LCIC, affect behavior. Effects of sex should be carefully considered in such future experiments, with deliberately balanced sex ratios in each condition.
Tactile and auditory stimuli may have different latencies to elicit human startles, seen in analyses of 30, 60, and 90 ms following uni- and multi-modal onsets [21]. Perhaps simultaneous somatosensory and auditory pre-pulses might also have different latencies to inhibit a startle.
Individuals with post-traumatic stress disorder (PTSD) and/or traumatic brain injuries (TBI) have altered responses to non-startling tactile stimulation [32], and often heightened startle responses to auditory stimuli [33]. There are mouse models of PTSD and TBI [34, 35]; PPI has been used to establish behavioral deficits in affected mice, but only using auditory pre-pulses startling stimuli [34]. Studies of auditory/somatosensory psychophysics could reveal the functional consequences of the trauma.
Somatosensory influence to the cochlear nucleus is heightened after auditory loss. This phenomenon has been speculated to be a mechanism causing tinnitus; therefore, somatosensory-based tinnitus treatments have been developed [36], but require further investigation. The procedures described in this report may be utilized to set up an animal model to study possible cross-modal, auditory-somatosensory therapies for tinnitus [37]. PPI is more effectively elicited by a gap in background noise than by the onset of a sound [9, 12]. Interestingly, gap detection is used as an animal and human model for tinnitus testing [38–41] .
Abnormal PPI across multiple modalities occurs in schizophrenia [42, 43]. Multimodal (auditory and visual) pre-pulses with auditory and tactile SESs are abnormal in a mouse model of schizophrenia [15]. The method in the present paper would allow auditory and somatosensory pre-pulses in addition to tactile (air puff) SESs.
Tactile perceptions are impaired in autism [44, 45], as are multi-modal responses [45–48]. There are mutant mouse models of autism [49–51], and the procedure described in this paper could compare multi-sensory (auditory and somatosensory) ‘binding’ in normal and mutant mice.
5. Summary and Conclusions
A vibratory stimulus can be an effective pre-pulse in mice. A standard commercial mouse testing tube can be vibrated with an inexpensive shaker.
Auditory and somatosensory pre-pulses affect PPI differently across ages (seen in Figure 3).
Mice at 1 to 2 months of age are not responsive to the 25 ms (360 Hz) vibrations, but become responsive to these vibrations at 3.5 – 6 months of age.
The longer the stimulus, the larger the PPI, seen more for the auditory than for the vibrotactile stimuli (Figure 2).
The effects of duration on PPI are the same regardless of age.
The main effect of age approaches significance with a ‘considerable’ effect size, pη2=.34. (pη2 of .14 is considered ‘large’[24])
In conclusion, this paper describes a behavioral paradigm to assess functional consequences of somatosensory/auditory interactions in mice. Further studies of multi-sensory binding can have implications for autism, schizophrenia, tinnitus, PTSD, and functional implications of signaling pathways that affect early neuronal guidance and pruning.
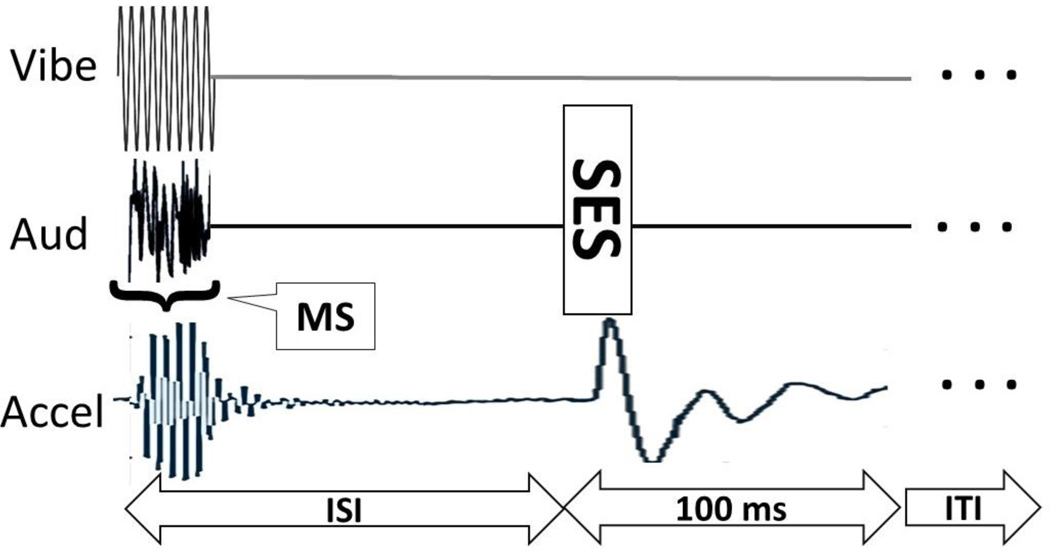
Figure 2. Schematic details of stimulus presentations and response measurement.
The x-axis is time, not to scale. The y-axis is volts, also not to scale, in three different channels. The top trace, ‘Vibe’, is the signal to the shaker, in this case 9 cycles, as in the ‘25ms’, 360 Hz stimulus. The middle trace, ‘Aud’, is the signal to the speakers, first an 80 dB SPL auditory prepulse, followed after an inter-stimulus-interval (ISI, 200 ms for 25ms prepulses) by the startle eliciting stimulus (SES, 110 dB SPL for 15 ms). The horizontal ‘brace’ labelled MS indicates duration of the somatosensory and/or auditory prepulses; MS was either 0, 4, 9, 25 or 45 ms.. The bottom trace, Accel, is input from the accelerometer: first the physical response of vibratory pre-pulse, which has some rise/fall time and resonance, followed by the whole-body jerk of the mouse elicited by the SES. RMS voltage from the accelerometer is calculated for 100 ms starting at the onset of the SES. There is then a random inter-trial-interval (ITI of 15–25 s). This example would be of a multimodal trial because there are simultaneous vibratory and auditory prepulses. There are also unimodal and control trials where various signals are absent, but the response is always measured for 100ms.
Highlights.
This is (to our knowledge) the first report of simultaneous non-startling auditory and somatosensory stimuli inhibiting an acoustic startle response in humans or mice.
We believe this to be a novel example of multi-modal pre-pulse inhibition (PPI).
Many of the factors of interest in this paper have been studied separately in humans and are here studied together in mice.
A standard, commercial, mouse-testing tube can be vibrated with an inexpensive shaker, and that somatosensory stimulus can be an effective prepulse in mice.
Further studies of ‘multi-sensory binding’ using variations of this novel method in various mouse strains and mutants can have implications for autism, schizophrenia, tinnitus, PTSD, and functional implications of signaling pathways that affect early neuronal guidance and pruning.
Acknowledgements.
This work was supported by NIH R15 grants DC012421 to M.G. and DC015353 to M.G. & L.G. Dr. Christopher Clinard and anonymous reviewers provided helpful comments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Koch M The neurobiology of startle. Prog Neurobiol. 1999, 59:107–28. [DOI] [PubMed] [Google Scholar]
- [2].Hoffman HS, Ison JR Reflex modification in the domain of startle: I. some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980, 87:175–89. [PubMed] [Google Scholar]
- [3].Gebhardt J, Schulz-Juergensen S, Eggert P Maturation of prepulse inhibition (PPI) in childhood. Psychophysiol. 2012, 49:484–8. [DOI] [PubMed] [Google Scholar]
- [4].Hill BD, Blumenthal TD Inhibition of acoustic startle using different mechanoreceptive channels. Percept Psychophys. 2005, 67:741–7. [DOI] [PubMed] [Google Scholar]
- [5].Peterson H, Blumenthal TD Efficacy of stimulus intensity increases and decreases as inhibitors of the acoustic startle response. Psychophysiology. 2018, 55:e13266. [DOI] [PubMed] [Google Scholar]
- [6].[Anonymous]. . SR lab system.
- [7].Gray L, Philbin MK Measuring sound in hospital nurseries. J Perinatol. 2000, 20:100. [DOI] [PubMed] [Google Scholar]
- [8].[Anonymous]. . Sensor kinetics pro.
- [9].Allen PD, Ison JR Sensitivity of the mouse to changes in azimuthal sound location: Angular separation, spectral composition, and sound level. Behav Neurosci. 2010, 124:265–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Fitch RH, Threlkeld SW, McClure MM, Peiffer AM Use of a modified prepulse inhibition paradigm to assess complex auditory discrimination in rodents. Brain Res Bull. 2008, 76:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Parisi T, Ison JR Ontogeny of control over the acoustic startle reflex by visual prestimulation in the rat. Dev Psychobiol. 1981, 14:311–6. [DOI] [PubMed] [Google Scholar]
- [12].Liuzzo A, Gray L, Wallace M, Gabriele ML Erratum to “effects of eph-ephrin mutations on pre-pulse inhibition in mice” [physiology & behavior 135, (2014) 232–236. Physiol Behav. 2017, 171:268. [DOI] [PubMed] [Google Scholar]
- [13].Mongeluzi DL, Hoppe TA, Frost WN Prepulse inhibition of the tritonia escape swim. J Neurosci. 1998, 18:8467–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Pinckney LA Inhibition of the startle reflex in the rat by prior tactile stimulation. Anim Learn Behav. 1976, 4:467–72. [Google Scholar]
- [15].Brody SA, Dulawa SC, Conquet F, Geyer MA Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol Psychiatry. 2004, 9:35–41. [DOI] [PubMed] [Google Scholar]
- [16].Li HS, Borg E Age-related loss of auditory sensitivity in two mouse genotypes. Acta Otolaryngol. 1991, 111:827–34. [DOI] [PubMed] [Google Scholar]
- [17].Rowe MJ Development of mammalian somatosensory pathways. Trends Neurosci. 1982, 5:408–11. [Google Scholar]
- [18].Dean KF, Sheets LP, Crofton KM, Reiter LW The effect of age and experience on inhibition of the acoustic startle response by gaps in background noise. Psychobiology. 1990, 18:89–95. [Google Scholar]
- [19].Kempermann G, Kuhn HG, Gage FH Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997, 94:10409–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Verrillo RT Age related changes in the sensitivity to vibration. J Gerontol. 1980, 35:185–93. [DOI] [PubMed] [Google Scholar]
- [21].Flaten MA, Blumenthal TD Effects of positive and negative stimulus onset asynchronies between weak stimuli and blink reflex eliciting stimuli. Journal of Psychophysiology. 1996, 10:189–97. [Google Scholar]
- [22].Flaten MA, Blumenthal TD A parametric study of the separate contributions of the tactile and acoustic components of airpuffs to the blink reflex. Biol Psychol. 1998, 48:227–34. [DOI] [PubMed] [Google Scholar]
- [23].Shore SE, Koehler S, Oldakowski M, Hughes LF, Syed S Dorsal cochlear nucleus responses to somatosensory stimulation are enhanced after noise-induced hearing loss. Eur J Neurosci. 2008, 27:155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cohen J Statistical power analysis for the behavioral sciences. Hillsdale, N.J.: L. Erlbaum Associates; 1988, p567. [Google Scholar]
- [25].Ison JR, Allen PD Pre- but not post-menopausal female CBA/CaJ mice show less prepulse inhibition than male mice of the same age. Behav Brain Res. 2007, 185:76–81. [DOI] [PubMed] [Google Scholar]
- [26].Dillingham CH, Gay SM, Behrooz R, Gabriele ML Modular-extramodular organization in developing multisensory shell regions of the mouse inferior colliculus. J Comp Neurol. 2017, 525:3742–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wallace MM, Harris JA, Brubaker DQ, Klotz CA, Gabriele ML Graded and discontinuous EphA-ephrinB expression patterns in the developing auditory brainstem. Hear Res. 2016, 335:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Cramer KS, Gabriele ML Axon guidance in the auditory system: Multiple functions of eph receptors. Neuroscience. 2014, 277:152–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Gruters KG, Groh JM Sounds and beyond: Multisensory and other non-auditory signals in the inferior colliculus. Front Neural Circuits. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Loftus WC, Malmierca MS, Bishop DC, Oliver DL The cytoarchitecture of the inferior colliculus revisited: A common organization of the lateral cortex in rat and cat. Neuroscience. 2008, 154:196–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Aitkin LM, Dickhaus H, Schult W, Zimmermann M External nucleus of inferior colliculus: Auditory and spinal somatosensory afferents and their interactions. J Neurophysiol. 1978, 41:837–47. [DOI] [PubMed] [Google Scholar]
- [32].Badura-Brack AS, Becker KM, McDermott TJ, Ryan TJ, Becker MM, Hearley AR, Heinrichs-Graham E, Wilson TW Decreased somatosensory activity to nonthreatening touch in combat veterans with posttraumatic stress disorder. Psychiatry Res. 2015, 233:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Rouw R, Erfanian M A large-scale study of misophonia. J Clin Psychol 2018, 74:453–79. [DOI] [PubMed] [Google Scholar]
- [34].Teutsch P, Jones CE, Kaiser ME, Avalon Gardner N, Lim MM Gait and conditioned fear impairments in a mouse model of comorbid TBI and PTSD. Behav Neurol. 2018, 2018:6037015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ratliff WA, Mervis RF, Citron BA, Schwartz B, Rubovitch V, Schreiber S, Pick CG Mild blast-related TBI in a mouse model alters amygdalar neurostructure and circuitry. Exp Neurol. 2019, 315:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Marks KL, Martel DT, Wu C, Basura GJ, Roberts LE, Schvartz-Leyzac KC, Shore SE Auditory-somatosensory bimodal stimulation desynchronizes brain circuitry to reduce tinnitus in guinea pigs and humans. Sci Transl Med. 2018, 10: 10.1126/scitranslmed.aal3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dehmel S, Cui YL, Shore SE Cross-modal interactions of auditory and somatic inputs in the brainstem and midbrain and their imbalance in tinnitus and deafness. Am J Audiol. 2008, 17:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Berger JI, Coomber B, Shackleton TM, Palmer AR, Wallace MN A novel behavioural approach to detecting tinnitus in the guinea pig. J Neurosci Methods. 2013, 213:188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Fournier P, Hebert S Gap detection deficits in humans with tinnitus as assessed with the acoustic startle paradigm: Does tinnitus fill in the gap?. Hear Res. 2013, 295:16–23. [DOI] [PubMed] [Google Scholar]
- [40].Longenecker RJ, Chonko KT, Maricich SM, Galazyuk AV Age effects on tinnitus and hearing loss in CBA/CaJ mice following sound exposure. Springerplus. 2014, 3:542,542. eCollection 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Turner JG, Brozoski TJ, Bauer CA, Parrish JL, Myers K, Hughes LF, Caspary DM Gap detection deficits in rats with tinnitus: A potential novel screening tool. Behav Neurosci. 2006, 120:188–95. [DOI] [PubMed] [Google Scholar]
- [42].Braff DL, Grillon C, Geyer MA Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992, 49:206–15. [DOI] [PubMed] [Google Scholar]
- [43].Grillon C, Ameli R, Charney DS, Krystal J, Braff D Startle gating deficits occur across prepulse intensities in schizophrenic patients. Biol Psychiatry. 1992, 32:939–43. [DOI] [PubMed] [Google Scholar]
- [44].Puts NA, Wodka EL, Tommerdahl M, Mostofsky SH, Edden RA Impaired tactile processing in children with autism spectrum disorder. J Neurophysiol. 2014, 111:1803–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Robertson CE, Baron-Cohen S Sensory perception in autism. Nat Rev Neurosci. 2017, 18:671–84. [DOI] [PubMed] [Google Scholar]
- [46].Collignon O, Charbonneau G, Peters F, Nassim M, Lassonde M, Lepore F, Mottron L, Bertone A Reduced multisensory facilitation in persons with autism. Cortex. 2013, 49:1704–10. [DOI] [PubMed] [Google Scholar]
- [47].Stevenson RA, Segers M, Ncube BL, Black KR, Bebko JM, Ferber S, Barense MD The cascading influence of multisensory processing on speech perception in autism. Autism. 2018, 22:609–24. [DOI] [PubMed] [Google Scholar]
- [48].Ostrolenk A, Bao VA, Mottron L, Collignon O, Bertone A Reduced multisensory facilitation in adolescents and adults on the autism spectrum. Sci Rep. 2019, 9:11965–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Gogolla N, Takesian AE, Feng G, Fagiolini M, Hensch TK Sensory integration in mouse insular cortex reflects GABA circuit maturation. Neuron. 2014, 83:894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kumar A, Wadhawan R, Swanwick CC, Kollu R, Basu SN, Banerjee-Basu S Animal model integration to AutDB, a genetic database for autism. BMC Med Genomics. 2011, 4:15-. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Rendall AR, Truong DT, Fitch RH Learning delays in a mouse model of autism spectrum disorder. Behav Brain Res. 2016, 303:201–7. [DOI] [PMC free article] [PubMed] [Google Scholar]