Abstract
Introduction
Rapid descent in bone mineral density (BMD) and ascent in bone turnover marker (BTM) occur within the short period following denosumab (Dmab) discontinuation. In addition, the incidence of vertebral fracture also rises within the short period. The purpose of this study is to investigate the effects of sequential therapy using zoledronic acid (ZOL) on any adverse events after Dmab discontinuation.
Materials and methods
This study was a multicenter retrospective observational study, and the subjects were osteoporosis patients who visited our institutions between 2013 and 2018. We performed sequential therapy using ZOL for 30 patients who had difficulty continuing Dmab, due to physical or social reasons, and investigated the fracture incidence and BMD/BTM changes at 4 time points (at the start of Dmab, the start of ZOL, 6 months after ZOL and 12 months after ZOL).
Results
No new vertebral/nonvertebral fractures were observed at each time point after switching from Dmab to ZOL in any of the 30 patients. The BMD/BTM changes were evaluated in 18 of the 30 cases, since all data of lumbar/femoral neck BMDs and TRACP-5b at 4 time points was only available in 18 cases. BMDs significantly increased at each time point compared with that at the start of Dmab. Serum TRACP-5b significantly decreased at each time point compared with that at the start of Dmab.
Conclusion
It was suggested that sequential therapy using ZOL could suppress the decrease of BMD, and increase of BTM, if the period of Dmab administration was less than 3 years.
Electronic supplementary material
The online version of this article (10.1007/s00774-020-01126-w) contains supplementary material, which is available to authorized users.
Keywords: Denosumab, Zoledronic acid, Sequential therapy, Bone mineral density, Bone turnover marker
Introduction
It is important for us to continue the osteoporosis treatment to increase bone mineral density (BMD) and suppress the incidence of fragile fractures. The previous study indicated that adherence to bisphosphonate (BP) therapy was associated with significantly fewer fractures at 24 months and increasing refill compliance levels were associated with progressively lower fracture rates [1]. In general, reducing the amount of medicine taken improves medication adherence [2], and the treatment persistence rate of monthly BP is higher than daily or weekly BP [3]. In addition, the treatment persistence rate of intravenous BP is higher than that of oral administration [4]. However, the compliance of osteoporosis treatment is not always sufficient in real-world daily clinical practice in spite of the development of various drugs [5].
Denosumab (Dmab) is a human IgG2 monoclonal antibody that targets RANKL (receptor activator for nuclear factor-κB ligand). It inhibits bone resorption by specifically inhibiting RANKL by suppressing osteoclast formation [6, 7]. Dmab is a useful agent in treating osteoporosis, which decreases fracture risk with sustained increase in BMD [8]. Dmab was approved as an indication of osteoporosis in May 2013 in Japan, and since then has been clinically used. Although it was expected that Dmab could simply be continued with a single subcutaneous injection every 6 months, the treatment persistence rate of Dmab for 24 months was 58% in the United States and Canada, and 11.7% in Japan [9, 10]. In real-world daily clinical practice, we often experience that elderly osteoporosis patients cannot go to hospital due to poor physical condition or lack of caregivers. Alternatively, Dmab treatment is discontinued due to a patient being entered into a nursing home or moving location. In addition, many patients stop going to the hospital on their own accord without recognizing the significance of continuing Dmab. Therefore, it is often difficult to continue Dmab semi-permanently.
The effect of Dmab is reversible after Dmab discontinuation. The suppressed effect of bone resorption is not persistent, and the value of bone turnover marker (BTM) rises above the value before the start of Dmab administration. Furthermore, it has been reported that a rapid decrease in BMD and multiple vertebral fractures occur [11, 12]. Multiple vertebral fractures that occurred within the short period after Dmab discontinuation can rapidly worsen the patient’s Activities of Daily Life (ADL) and Quality of Life (QOL).
The task force of the American Society for Bone and Mineral Research (ASBMR) and the United States National Osteoporosis Foundation (NOF) in 2017 reported on the policy of “Goal-directed treatment”, as follows [13]. The concept of a “drug holiday” applies only to patients taking BPs. A drug holiday is not appropriate for non-BPs as the patient’s BMD rapidly decreases after treatment discontinuation. Therefore, after a T-score goal is achieved with a non-BP, treatment should generally be continued with an agent that maintains BMD, possibly a BP. “Drug holiday” is not applied to Dmab, which is a non-BP, and sequential therapy is required. The European Calcified Tissue Society (ECTS) states that Dmab should not be discontinued without considering alternative treatments [14]. According to the Swiss Association Against Osteoporosis (SVGO/ASCO) position statement, it is recommended to switch to non-reversible antiresorptive drugs if the risk of fracture is low at 4–5 years after administration of Dmab [15]. Non-reversible antiresorptive drugs like BPs are taken into the bone matrix. After discontinuing Dmab administration, sequential therapy with BP is considered desirable.
There is little evidence that showed the effect of sequential therapy by zoledronic acid (ZOL) on the adverse events after Dmab discontinuation, such as the increased incidence of vertebral fractures, the reduction of BMD and the elevation of BTM. In this study, we retrospectively investigated the occurrence of new vertebral/nonvertebral fractures and changes in BMD and BTM in patients who switched from Dmab to ZOL in real-world daily clinical practice, and examined the effects of switching from Dmab to ZOL for osteoporosis patients.
Materials and methods
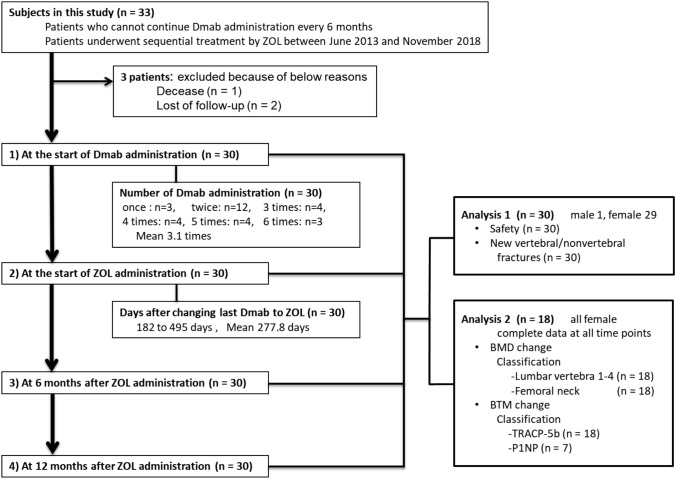
Study design (Fig. 1)
Fig. 1.
Flow diagram of subjects. Dmab denosumab, ZOL zoledronic acid, BMD bone mineral density, BTM bone turnover maker, TRACP-5b Tartrate Resistant Acid Phosphatase 5b, P1NP total type I procollagen N-terminal propeptide
This study was a multicenter, retrospective observational study conducted at eight separate institutions, and we evaluated the safety, new vertebral/nonvertebral fractures, BMD and BTM after switching from Dmab to ZOL as sequential therapy at 4 time points (1: at the start of Dmab administration, 2: at the start of ZOL administration, 3: at 6 months after ZOL administration and 4: 12 months after ZOL administration) (Fig. 1). This clinical study was started after deliberation and approval by the “Medical Corporation Ouryokukai Nihonbashi Sakura Clinic Ethics Review Committee”, including conflicts of interest of the principal investigator. In addition, subject consent forms were processed using the Opt-out system.
Subjects (patient disposition) (Figs. 1, 2)
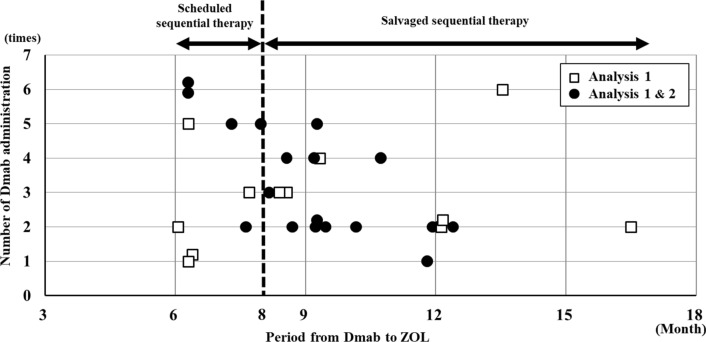
Fig. 2.
The scatter diagrams showing the transitional period from Dmab to ZOL and number of Dmab administration. Dmab denosumab, ZOL zoledronic acid. A square mark indicates the case of analysis 1 and a closed circle mark indicates the case of analysis 1 & 2. Switching to ZOL with ≤ 8 months or > 8 months after the last administration of Dmab was defined as scheduled sequential therapy or salvaged sequential therapy, respectively, and each case was classified based on the period of transition from Dmab to ZOL
In real-world daily clinical practice, it is difficult for Dmab treatment to be continued due to nursing home admission or lack of family support, even if the attending physician explained the necessity of continuing Dmab. The subjects in this study were 33 patients who received sequential therapy by ZOL between June 2013 and November 2018 but who as mentioned previously had difficulty in long-term continuation of Dmab for the above reasons. In addition, 3 of 33 patients withdrew (1 patient deceased and 2 patients stopped visiting the hospital) (Fig. 1). All subjects met the diagnostic criteria of osteoporosis in Japan Osteoporosis Society, and underwent a radiograph, DXA and blood test at each time point when possible. A breakdown of each case is shown in Fig. 1. At all institutions, patients who did not go to each of their respective hospitals on the scheduled Dmab administration date have been instructed by the osteoporosis coordinators to continue with their treatment. In general, the incidence of vertebral fractures increase from 8 months after the discontinuation of Dmab [16–18]. Thus, switching to ZOL with ≤ 8 months or > 8 months after the last administration of Dmab was defined as scheduled sequential therapy or salvaged sequential therapy, respectively. Additionally, each case was classified based on the period of transition from Dmab to ZOL (Fig. 2).
Safety and new fragile fractures (Fig. 1, analysis 1)
Safety and new vertebral/nonvertebral fractures were assessed based on medical records. The adverse events by Dmab and ZOL administration are as follows: hypocalcemia, antiresorption-related osteonecrosis/osteomyelitis of the jaw (ARONJ) and atypical femoral fracture (AFF). In particular, the acute phase reactions (APRs), increased body temperature (≥ 1 °C above 37.5 °C), muscle pain, joint pain, and headache, are the adverse events from ZOL treatment, and APRs were defined by Okimoto et al. [19]. Morphological vertebral fractures at the thoracic and lumbar spine were evaluated using X-ray images. Vertebral bodies of T4–L4 were independently measured by each investigator. Vertebral fractures were defined according to Genant [20] (Fig. 1, analysis 1).
Bone mineral density and bone turnover markers (Fig. 1, analysis 2)
QDR (Hologic, Marlborough, MA, USA) or DPX (GE, Madison, WI, USA) devices were used for measuring BMD. BMD at lumbar vertebra (L1–L4) and femoral neck were measured using DXA at the each time point. BTMs were carried out on serum Tartrate Resistant Acid Phosphatase 5b (TRACP-5b/EIA method, normal range, male; 170–590, female; 120–420) and/or serum total Type I procollagen N-terminal propeptide (P1NP/ECLIA method, normal range, male; 18.1–74.1, female; 26.4–98.2) at each of the 4 time points. Of the 30 cases, 12 cases were excluded due to incomplete lumbar/femoral neck BMD or serum TRACP-5b data, and the remaining 18 cases (all females) were analyzed using all data of BMD and serum TRACP-5b at each time point (Fig. 1, analysis 2).
Statistical analysis
Comparison of BMD values and TRACP-5b values were measured at 2 sites of the lumbar vertebrae and femoral neck at the start of Dmab administration, at the start of ZOL administration, at 6 months after ZOL administration, and at 12 months after ZOL administration. Statistical analysis was conducted using a paired t test, and the significance level was 5% on both sides. Data management and statistical analysis were performed by “Pharmaco Basic, Scientist Press Co. Ltd., Tokyo., Japan”.
Results
Patient disposition and characteristics
Patient disposition and characteristics are shown in Table 1. The number of subjects in this study was 30 (1 male, 29 female) with an average age of 78.1 years (61–89 years). The subjects had not undergone the osteoporosis treatment before Dmab administration, except for one female case who had been treated with minodronic acid for 3 months. 21 of 30 cases (70%) had existing vertebral fractures. The average number of existing vertebral fractures per patient was 2.7 (range 1–10). T-score at the start of Dmab administration is as follows: Lumbar vertebrae; − 2.8 SD, femoral neck; − 3.2 SD. The average of serum TRACP-5b levels at the start of Dmab administration was 551.4 mU/dL, and 20 of 28 cases (71%) had higher levels of serum TRACP-5b, which were more than the normal range. The average number of Dmab administration in 30 cases was 3.1 times (once: 3 patients, twice: 12 patients, three times: 4 patients, 4 times: 4 patients, 5 times: 4 patients, and 6 times: 3 patients). The average period from the last administration of Dmab to the start of ZOL administration in 30 cases was 277.8 days (range 182–495 days).
Table 1.
Demographic background of enrolled patients
| Item | Unit | N | Mean | ±SD |
|---|---|---|---|---|
| Sex | ||||
| Male | Number | 1 | – | – |
| Female | Number | 29 | – | – |
| Age | Years old | 30 | 78.1 | 7.2 |
| Body weight | kg | 30 | 53.2 | 10.9 |
| Patient: previously treated with BP | Number | 1 | – | – |
| Patient: prevalent vertebral fractures at baseline | Number | 21 | – | – |
| T-score at the start of Dmab administration | ||||
| Lumbar vertebra | SD | 22 | − 2.8 | 0.9 |
| Femoral neck | SD | 24 | − 3.2 | 0.8 |
| TRACP-5b at the start of Dmab administration | mU/dL | 28 | 551.4 | 261.2 |
| P1NP at the start of Dmab administration | ng/mL | 17 | 70.7 | 35.5 |
| Number of Dmab administration | Times | 30 | 3.1 | 1.6 |
| Days after changing Dmab to ZOL | Days | 30 | 277.8 | 75.0 |
Data are presented as mean ± SD
BP bisphosphonate, Dmab denosumab, P1NP total Type I procollagen N-terminal propeptide, TRACP-5b Tartrate Resistant Acid Phosphatase 5b, ZOL zoledronic acid
Patient disposition and characteristics of the 18 patients who were evaluated for BMD and BTM (analysis 2) are shown in Supplementary Table 1. All 18 patients were female, with an average age of 75.7 years (64–84 years). One female had been treated with minodronic acid for 3 months before Dmab administration. 10 of 18 cases (56%) had existing vertebral fractures. The average number of existing vertebral fractures per patient was 1.8 (range 1–4). T-score at the start of Dmab administration is as follows: Lumbar vertebrae; − 2.7 SD, femoral neck; − 3.1 SD. The average of serum TRACP-5b levels at the start of Dmab administration was 562.3 mU/dL, and 12 of 18 cases (67%) had higher levels of serum TRACP-5b, which were more than the normal range. The average number of Dmab administration in 18 cases was 3.3 times (once: 1 patient, twice: 8 patients, three times: 1 patient, 4 times: 3 patients, 5 times: 3 patients, and 6 times: 2 patients). The average period from the last administration of Dmab to the start of ZOL administration in 18 cases was 274.0 days (range 189–372 days).
The period of transition from the administration of Dmab to ZOL, and the number of Dmab administration in thirty cases are shown in Fig. 2. Ten of the thirty cases (33%) underwent the scheduled sequential therapy, whereas the other twenty cases (66%) underwent the salvaged sequential therapy. The average number of Dmab administration in patients of scheduled- or salvaged-sequential therapy was 3.6 and 2.9 times, respectively. The attending physician explained the necessity of continuing Dmab, however, all subjects had difficulty in the long-term continuation of Dmab administration due to nursing home admission or a lack of family support, and consequently wished to switch from Dmab to ZOL.
Safety and new fragility fractures
During the Dmab administration, there was no clinically significant adverse events, such as hypocalcemia, ARONJ or AFF observed in any of the 30 subjects evaluated. While APRs by sequential ZOL treatment existed 14 of 30 cases (46.7%). These adverse events improved using NSAIDs treatment within a week.
Neither clinical vertebral fractures nor X-ray morphological new vertebral fractures occurred in any of the 30 patients evaluated for fractures at 6 months and 12 months after ZOL administration. None of the cases experienced any nonvertebral fracture during the follow-up period (analysis 1).
Bone mineral density (Fig. 3)
Fig. 3.
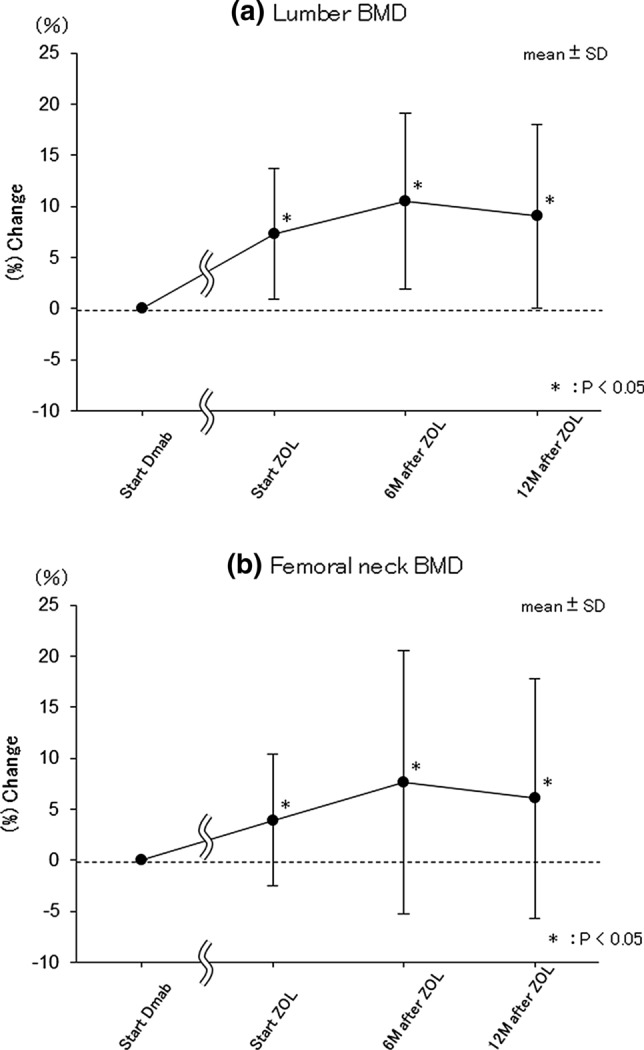
The sequential changes in BMD. Percent changes in a Lumbar-BMD and b Femoral neck-BMD from at the start of ZOL administration. Start Dmab at the start of Dmab administration, Start ZOL at the start of ZOL administration, 6 M after ZOL at 6 months after ZOL administration, 12 M after ZOL at 12 months after ZOL administration. Data are the mean ± SD. *p < 0.05 vs. Start Dmab
Eighteen cases (all females) were analyzed using all data of lumbar/femoral neck BMD at each of the 4 time points (analysis 2).
Average lumbar vertebra T-scores was − 2.7 SD at the start of Dmab administration, − 2.3 SD at the start of ZOL administration, − 2.1 SD at 6 months after ZOL administration, and − 2.2 SD at 12 months after ZOL administration. Compared to pre-Dmab administration, there was a significant increase in BMD change rate at lumbar vertebrae at the start of ZOL administration (p < 0.001; mean 7.327%; SD 6.440), at 6 months after ZOL administration (p < 0.001; mean 10.501%; SD 8.583), and at 12 months after ZOL administration (p < 0.001; mean 9.067%; SD 8.977) (Fig. 3a).
T-scores of the femoral neck was − 3.1 SD at the start of Dmab administration, − 2.9 SD at the start of ZOL administration, − 2.7 SD after 6 months of ZOL administration, and − 2.8 SD after 12 months of administration of ZOL. Compared with the start of Dmab administration, significant increases of BMD change rate at femoral neck were observed at the start of ZOL administration (p = 0.024; mean 3.947%; SD 6.447), 6 months after ZOL administration (p = 0.022; mean 7.674%; SD 12.909) and 12 months after ZOL administration (p = 0.038; mean 6.089%; SD 11.722) (Fig. 3b).
Bone turnover markers (Fig. 4)
Fig. 4.
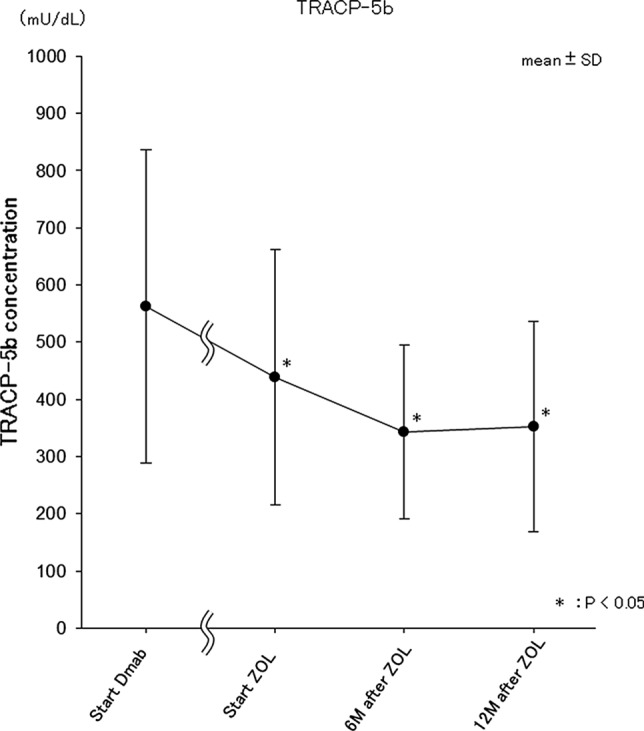
The sequential changes in serum TRACP-5b. Value changes of serum TRACP-5b at 4 time points. Start Dmab at the start of Dmab administration, Start ZOL at the start of ZOL administration, 6 M after ZOL at 6 months after ZOL administration, 12 M after ZOL at 12 months after ZOL administration. Data are the mean ± SD. *p < 0.05 vs. Start Dmab
Eighteen cases (all females) were analyzed using all data of serum TRACP-5b at each 4 time points (analysis 2). Average serum TRACP-5b levels were 562.3 mU/dL at the start of Dmab administration, 438.4 mU/dL at the start of ZOL administration, 342.6 mU/dL 6 months after ZOL administration, and 351.8 mU/dL 12 months after ZOL administration. Compared with at the start of Dmab administration, a significant decrease was observed at the start of ZOL administration (p = 0.029; mean 438.4 mU/dL; SD 223.5), 6 months after ZOL administration (p = 0.003; mean 342.6 mU/dL; SD 152.3) and 12 months after ZOL administration (p = 0.007; mean 351.8 mU/dL; SD 184.0) (Fig. 4).
Only 7 cases of serum P1NP were measured at all time points, and these data were not analyzed. Average serum P1NP levels were 72.1 ng/mL at the start of Dmab administration, 37.7 ng/mL at the start of ZOL administration, 28.6 ng/mL 6 months after ZOL administration, and 36.8 ng/mL at 12 months after ZOL administration, respectively.
Discussion
There is little evidence that showed any effect of sequential therapy by ZOL after Dmab discontinuation, and this article is the first report in Japan that demonstrated the effect of ZOL on the adverse events after Dmab discontinuation. In our study, we retrospectively investigated new fragility fracture incidence, safety, and changes of BMD and BTM in osteoporosis patients who switched from Dmab to ZOL. As the results of this survey show, no new fragile fractures in any subjects were identified, and it was suggested that ZOL sequential therapy after administering Dmab could suppress the increase of BTM and the decrease of BMD, if the periods of Dmab administration was less than 3 years. Our consideration is described below:
It was reported that Dmab treatment suppresses high bone turnover and the incidence of vertebral/nonvertebral fractures and increases BMD through more than 10 years. In addition, there was no adverse events during the period [8]. In our study, no new vertebral/nonvertebral fractures occurred, BMD significantly increased and serum TRACP-5b was significantly suppressed after Dmab administration. Hypocalcemia was not observed after the administration of calcium agents or active vitamin D3 agents as concomitant medication. Other adverse events such as ARONJ and AFF were not recognized.
The discontinuation of Dmab treatment caused rapidly high bone turnover and bone density loss within a short period, followed by vertebral fractures. In the FREEDOM trial and its extension, a majority of participants who sustained a vertebral fracture after discontinuing denosumab had multiple vertebral fractures, with the greatest risk to participants with a prior vertebral fracture [12]. Although 70% of the subjects had a prior vertebral fracture in our present study, no new vertebral fractures occurred during the follow-up period. It is possible that sequential therapy with ZOL can control multiple vertebral fractures after Dmab discontinuation.
Several reports showed that multiple vertebral fractures occurred from 8 to 20 months after Dmab discontinuation [16–18]. Despite twenty (66%) of thirty cases in our study were involved in salvaged sequential therapy of > 8 months after the last Dmab administration (Fig. 2), no vertebral fractures were indicated between Dmab and ZOL administration. Based on the FREEDOM trial data available, the incidence of vertebral fractures after Dmab discontinuation was 7.1% [12], and it is no surprise even if vertebral fractures occurred in two of thirty patients in our study. The absence of vertebral fractures may be a coincidence, but it is possible that the low number of Dmab administration is a factor. In the report that Dmab was discontinued without sequential therapy after the FREEDOM extension study, the longer the period of Dmab administration was, the lower the BMD became, and it was suggested that the treatment duration of Dmab may be able to predict the change rate of BMD [21]. In our study, the average number of Dmab administration in patients undergoing salvaged sequential therapy was 2.9 times, suggesting that no fractures may have occurred due to the low number of Dmab administration.
Several studies have reported on sequential therapy with BP after Dmab administration. Sequential therapy with Alendronate (ALN) maintained BMD, if the period of Dmab administration was 1 year [22]. Sequential therapy with ZOL maintained BMD, if the periods of Dmab administration were 2, 2.2 and 3 years, respectively [23–25]. However, sequential therapy with ALN reduced BMD, if the period of Dmab administration was 3.5 years [26]. Reid et al. reported that switching to ZOL after Dmab administration over 7 years significantly reduced BMD after 2 years [27]. We switched to ZOL after the start of Dmab treatment within 3 years, and could maintain BMD at 12 months after ZOL. Therefore, we believe that BMD can be maintained by sequential therapy with BP, especially ZOL, if the period of Dmab administration is up to 3 years. However, it is possible that BMD cannot be maintained if the period of Dmab administration is over 3 years. We recommend the cyclic therapy of ZOL after administrating Dmab during 3 years as one way to prepare for any unexpected Dmab discontinuation. At the same time, further strengthening of our recommendations will require comparison with patients using Dmab for more than 3 years.
There are a number of reports related to drug selection in sequential therapy after discontinuation of Dmab. The antiresorptive effect of raloxifene is probably not strong enough to counter the severe rebound effect associated with Dmab discontinuation. Therefore, it seems desirable to prescribe a more potent bone resorption inhibitor, alendronate or zoledronate to minimize high bone turnover at Dmab discontinuation [28]. Another case series evaluated the effect of zoledronate or risedronate administered sequentially after 2 years of Dmab [23]. In the zoledronate group, BMD gained by Dmab administration was retained at 73% at the lumbar spine and 87% at the hip joint, and in the risedronate group, 41% at the lumbar spine and 64% at the hip joint. Thus, at both the spine and hip, there was a substantial retention of the benefits accrued during the trial period in the women subsequently receiving zoledronate. The doses of BP preparation that can be used in Japan is 50% of the overseas dose for ALN and Risedronate, on the other hand, the same dose of ZOL can be used as with the overseas dose. We, therefore, selected ZOL as the therapeutic agent for sequential therapy in this study.
Regarding the timing of ZOL administration, it has been reported that after Dmab administration over 2 years, ZOL was administered after 191–353 days of the last Dmab administration and BMD obtained with Dmab could be maintained 1 year later [23]. In addition, after Dmab administration over 3 years, ZOL was administered after 6 months of the last Dmab administration, and BMD obtained with Dmab could be maintained 2.5 years later [25]. In our investigation, the transitional period from the last Dmab administration to the start of the ZOL administration was 277.8 days (from 182 to 495 days), according to the patients’ convenience, and the timing of ZOL administration may have been delayed. Regarding this point, it is possible that the suitable starting time of BP varies depending on the formulation and administration interval, so further study is necessary. However, since the risk of vertebral fracture increases from 8 months after Dmab administration, we unfortunately regret that the twenty (66%) cases of salvaged sequential therapy with more than 8 months after Dmab administration existed in our study. How to avoid discontinuation of Dmab treatment is an issue for the future, and we aim to improve the treatment continuation rate using liaison services such as sufficient patient education and regular contact with patients who do not go to the hospital on their scheduled date.
We observed APRs at 46.7% after ZOL administration. In the study population, almost all patients had not received any prior BP preparation, except for one patient who had received minodronic acid for 3 months. Symptoms of BP-induced APRs can be well managed by antipyretic and antipyretic analgesic medications, such as paracetamol/acetaminophen and ibuprofen [29–31]. In our study, symptoms improved using NSAIDs within 1 week, and we consider that APRs expression can be suppressed by the prophylactic administration of NSAIDs, as reported by Okimoto et al. [19].
There are, however, several limitations. This study is a case series retrospective study and there are no subjects available to compare, with only as few as thirty cases to be evaluated. The number of administrations and the period until the start of sequential therapy are also different. Also, as issues regarding compliance remain unresolved, it is necessary to conduct an epidemiological survey on compliance of osteoporosis therapeutic agents including Dmab among nursing home residents, to study the continuation rate of ZOL sequential therapy for cases in which Dmab cannot be continued. In addition, we will also need to examine the effect of ZOL on cases of Dmab administration over more than 3 years to obtain evidence regarding the cyclic therapy of ZOL after the administrating Dmab over a 3 year period. Although there are several limitations, we believe that this is an important report that accurately captures the problems of real-world daily clinical practice in Japan. The guidance of ASBMR, Japanese Society for Bone and Mineral Research (JSBMR) and Japan Osteoporosis Society (JOS) under the global pandemic of COVID-19 make us recognize the importance of continuing treatment for osteoporosis [32]. Especially for Dmab, switching to bisphosphonate has been recommended for cases in which Dmab cannot be continued. We hope that this report will be helpful to doctors and patients who are in the same situation, while involved in real-world daily clinical practice.
In conclusion, we demonstrated the effect of sequential therapy of ZOL after administrating Dmab within less than 3 years. No vertebral/nonvertebral fractures in any cases were identified. In addition, sequential therapy using ZOL could maintain the BMD and serum TRACP-5b levels, that were obtained by Dmab administration. Dmab is a very beneficial treatment if it can be continued. However, in real-world daily clinical osteoporotic practice, there are patients who require sequential therapy, especially among the elderly, due to the difficulty of continuing the administration of Dmab in Japan. We believe that switching to ZOL after Dmab discontinuation is an effective means of sequential therapy for them if the period of Dmab administration is less than 3 years. Evidence-based sequential therapy after Dmab discontinuation remains limited, and therefore, deserves an urgent and unaddressed medical need. Further investigations will be required regarding various aspect.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
HK, NO, TY and SA designed the study. NO, TY, SA, YF, TO, YO and YK performed data acquisition. HK, NO, TY, SA, MT, YY, MK and AS were involved in drafting and critical revision of the manuscript for intellectual content. All authors critically reviewed the paper for intellectual content and approved the final version. All authors agree to be accountable for their work and to ensure that any questions related to the accuracy and integrity of this paper will be investigated and properly resolved.
Compliance with ethical standards
Conflict of interest
H. Kondo, T. Yoshioka, S. Akahoshi, Y. Fuse, T. Ogawa, Y. Okazaki, Y. Katae, M. Tsukamoto, Y. Yamanaka and M. Kawasaki declare they have no conflict of interest. N. Okimoto has received consulting fees from Asahi-Kasei Pharmaceutical Co., Ltd and Teijin Pharma Ltd, and payments have been made to lectures, including speakers’ bureau fees, from Asahi-Kasei Pharmaceutical Co., Ltd.; Amgen Astellas BioPharma K.K.; Astellas Pharma Inc.; Chugai Pharmaceutical Co.; Daiichi-Sankyo Co. Ltd.; Eisai Co., Ltd.; Eli Lilly Japan K.K.; Mitsubishi-Tanabe Pharma Corp.; Ono Pharmaceutical Co.; Pfizer Japan Inc.; and Teijin Pharma Ltd, for outside the submitted work. A. Sakai has received grants from Asahi-Kasei Pharmaceutical Co., Ltd. and Chugai Pharmaceutical Co., Ltd., during the conduct of this study, and personal lecture fees were received from Asahi-Kasei Pharmaceutical Co., Ltd. and Chugai Pharmaceutical Co., Ltd., for outside the submitted work.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siris ES, Harris ST, Rosen CJ, Barr CE, Arvesen JN, Abbott TA, Silverman S. Adherence to bisphosphonate therapy and fracture rates in osteoporotic women: relationship to vertebral and nonvertebral fractures from 2 US claims databases. Mayo Clin Proc. 2006;81:1013–1022. doi: 10.4065/81.8.1013. [DOI] [PubMed] [Google Scholar]
- 2.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 3.Sakai A, Ikeda S, Okimoto N, Matsumoto H, Teshima K, Okazaki Y, Fukuda F, Arita S, Tsurukami H, Nagashima M, Yoshioka T. Clinical efficacy and treatment persistence of monthly minodronate for osteoporotic patients unsatisfied with, and shifted from, daily or weekly bisphosphonates: the BP-MUSASHI study. Osteoporos Int. 2014;25:2245–2253. doi: 10.1007/s00198-014-2756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durden Emily, Pinto Lionel, Lopez-Gonzalez Lorena, Juneau Paul, Barron Richard. Two-year persistence and compliance with osteoporosis therapies among postmenopausal women in a commercially insured population in the United States. Arch Osteoporos. 2017;12:22. doi: 10.1007/s11657-017-0316-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziller V, Kostev K, Kyvernitakis I, Boeckhoff J, Hadji P. Persistence and compliance of medications used in the treatment of osteoporosis-analysis using a large scale, representative, longitudinal German database. Int J Clin Pharmacol Ther. 2012;50:315–322. doi: 10.5414/CP201632. [DOI] [PubMed] [Google Scholar]
- 6.Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423:337–342. doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 7.Delmas PD. Clinical potential of RANKL inhibition for the management of postmenopausal osteoporosis and other metabolic bone diseases. J Clin Densitom. 2008;11:325–338. doi: 10.1016/j.jocd.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Bone HG, Wagman RB, Brandi ML, Brown JP, Chapurlat R, Cummings SR, Czerwiński E, Fahrleitner-Pammer A, Kendler DL, Lippuner K, Reginster JY, Roux C, Malouf J, Bradley MN, Daizadeh NS, Wang A, Dakin P, Pannacciulli N, Dempster DW, Papapoulos S. 10 years of denosumab treatment in postmenopausal women with osteoporosis: results from the phase 3 randomised FREEDOM trial and open-label extension. Lancet Diabetes Endocrinol. 2017;5:513–523. doi: 10.1016/S2213-8587(17)30138-9. [DOI] [PubMed] [Google Scholar]
- 9.Silverman SL, Siris E, Belazi D. Persistence at 24 months with denosumab among postmenopausal women with osteoporosis: results of a prospective cohort study. Arch Osteoporos. 2018;13:85. doi: 10.1007/s11657-018-0491-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujimori K, Tarasawa K, Nakatoh S. Analysis of the persistence and compliance of medications for osteoporosis using E-claim database. J Jpn Osteoporos Soc. 2019;5:277–285. [Google Scholar]
- 11.Bone HG, Bolognese MA, Yuen CK, Kendler DL, Miller PD, Yang YC, Grazette L, San Martin J, Gallagher JC. Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab. 2011;96:972–980. doi: 10.1210/jc.2010-1502. [DOI] [PubMed] [Google Scholar]
- 12.Cummings SR, Ferrari S, Eastell R, Gilchrist N, Jensen JB, McClung M, Roux C, Törring O, Valter I, Wang AT, Brown JP. Vertebral fractures after discontinuation of denosumab: a post hoc analysis of the randomized placebo-controlled FREEDOM trial and its extension. J Bone Miner Res. 2017;33:190–198. doi: 10.1002/jbmr.3337. [DOI] [PubMed] [Google Scholar]
- 13.Cummings SR, Cosman F, Lewiecki EM, Schousboe JT, Bauer DC, Black DM, Brown TD, Cheung AM, Cody K, Cooper C, Diez-Perez A, Eastell R, Hadji P, Hosoi T, Jan De Beur S, Kagan R, Kiel DP, Reid IR, Solomon DH, Randall S. Goal-directed treatment for osteoporosis: a Progress Report From the ASBMR-NOF Working Group on goal-directed treatment for osteoporosis. J Bone Miner Res. 2017;32:3–10. doi: 10.1002/jbmr.3039. [DOI] [PubMed] [Google Scholar]
- 14.Tsourdi E, Langdahl B, Cohen-Solal M, Aubry-Rozier B, Eriksen EF, Guañabens N, Obermayer-Pietsch B, Ralston SH, Eastell R, Zillikens MC. Discontinuation of Denosumab therapy for osteoporosis: a systematic review and position statement by ECTS. Bone. 2017;105:11–17. doi: 10.1016/j.bone.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Meier C, Uebelhart B, Aubry-Rozier B, Birkhäuser M, Bischoff-Ferrari HA, Frey D, Kressig RW, Lamy O, Lippuner K, Stute P, Suhm N, Ferrari S. Osteoporosis drug treatment: duration and management after discontinuation. A position statement from the SVGO/ASCO. Swiss Med Wkly. 2017;147:w14484. doi: 10.4414/smw.2017.14484. [DOI] [PubMed] [Google Scholar]
- 16.Anastasilakis AD, Polyzos SA, Makras P, Aubry-Rozier B, Kaouri S, Lamy O. Clinical features of 24 patients with rebound-associated vertebral fractures after denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. 2017;32:1291–1296. doi: 10.1002/jbmr.3110. [DOI] [PubMed] [Google Scholar]
- 17.Florez H, Ramírez J, Monegal A, Guañabens N, Peris P. Spontaneous vertebral fractures after denosumab discontinuation: a case collection and review of the literature. Semin Arthritis Rheum. 2019;49:197–203. doi: 10.1016/j.semarthrit.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Niimi R, Kono T, Nishihara A, Hasegawa M, Kono T, Sudo A. Rebound-associated vertebral fractures after discontinuation of denosumab for the treatment of maxillitis. Osteoporos Int. 2018;29:769–772. doi: 10.1007/s00198-017-4334-3. [DOI] [PubMed] [Google Scholar]
- 19.Okimoto N, Sakai A, Yoshioka T, Kobayashi T, Asano K, Akahoshi S, Ishikura T, Fukuhara S, Fuse Y, Mizuno T, Katae Y, Matsumoto H, Ogawa T, Nishida S, Ikeda S, Menuki K, Saito J, Okazaki Y, Mizuno N, Fujiwara S. Efficacy of non-steroidal anti-inflammatory drugs on zoledronic acid-induced acute-phase reactions: randomized, open-label. Japanese OZ study. J Bone Miner Metab. 2019 doi: 10.1007/s00774-019-01050-8. [DOI] [PubMed] [Google Scholar]
- 20.Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8:1137–1148. doi: 10.1002/jbmr.5650080915. [DOI] [PubMed] [Google Scholar]
- 21.Popp AW, Varathan N, Buffat H, Senn C, Perrelet R, Lippuner K. Bone mineral density changes after 1 year of denosumab discontinuation in postmenopausal women with long-term denosumab treatment for osteoporosis. Calcif tissue Int. 2018;103:50–54. doi: 10.1007/s00223-018-0394-4. [DOI] [PubMed] [Google Scholar]
- 22.Freemantle N, Satram-Hoang S, Tang ET, Kaur P, Macarios D, Siddhanti S, Borenstein J, Kendler DL. Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23:317–326. doi: 10.1007/s00198-011-1780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horne AM, Mihov B, Reid IR. Bone loss after romosozumab/denosumab: effects of bisphosphonates. Calcif Tissue Int. 2018;103:55–61. doi: 10.1007/s00223-018-0404-6. [DOI] [PubMed] [Google Scholar]
- 24.Anastasilakis AD, Papapoulos SE, Polyzos SA, Appelman-Dijkstra NM, Makras P. Zoledronate for the prevention of bone loss in women discontinuing denosumab treatment. a prospective 2-year clinical trial. J Bone Miner Res. 2019;34:2220–2228. doi: 10.1002/jbmr.3853. [DOI] [PubMed] [Google Scholar]
- 25.Everts-Graber J, Reichenbach S, Ziswiler HR, Studer U, Lehmann T. A single infusion of zoledronate in postmenopausal women following denosumab discontinuation results in partial conservation of bone mass gains. J Bone Miner Res. 2020 doi: 10.1002/jbmr.3962. [DOI] [PubMed] [Google Scholar]
- 26.Lamy O, Fernández-Fernández E, Monjo-Henry I, Stoll D, Aubry-Rozier B, Benavent-Núñez D, Aguado P, Gonzalez-Rodriguez E. Alendronate after denosumab discontinuation in women previously exposed to bisphosphonates was not effective in preventing the risk of spontaneous multiple vertebral fractures: two case reports. Osteoporos Int. 2019;30:1111–1115. doi: 10.1007/s00198-018-04820-8. [DOI] [PubMed] [Google Scholar]
- 27.Reid IR, Horne AM, Mihov B, Gamble GD. Bone loss after denosumab: only partial protection with zoledronate. Calcif Tissue Int. 2017;101:371–374. doi: 10.1007/s00223-017-0288-x. [DOI] [PubMed] [Google Scholar]
- 28.Gonzalez-Rodriguez E, Stoll D, Lamy O. Raloxifene has no efficacy in reducing the high bone turnover and the risk of spontaneous vertebral fractures after denosumab discontinuation. Case Rep Rheumatol. 2018;17(2018):5432751. doi: 10.1155/2018/5432751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Black DM, Delmas PD, Eastell R, Reid IR, Boonen S, Cauley JA, Cosman F, Lakatos P, Leung PC, Man Z, Mautalen C, Mesenbrink P, Hu H, Caminis J, Tong K, Rosario-Jansen T, Krasnow J, Hue TF, Sellmeyer D, Eriksen EF, Cummings SR. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med. 2007;356:1809–1822. doi: 10.1056/NEJMoa067312. [DOI] [PubMed] [Google Scholar]
- 30.Silverman SL, Kriegman A, Goncalves J, Kianifard F, Carlson T, Leary E. Effect of acetaminophen and fluvastatin on postdose symptoms following infusion of zoledronic acid. Osteoporos Int. 2011;22:2337–2345. doi: 10.1007/s00198-010-1448-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wark JD, Bensen W, Recknor C, Ryabitseva O, Chiodo J, 3rd, Mesenbrink P, de Villiers TJ. Treatment with acetaminophen/paracetamol or ibuprofen alleviates post-dose symptoms related to intravenous infusion with zoledronic acid 5 mg. Osteoporos Int. 2012;23:503–512. doi: 10.1007/s00198-011-1563-8. [DOI] [PubMed] [Google Scholar]
- 32.The guidance below has been created to assist clinicians in the management of patients with osteoporosis in the era of COVID-19. https://www.asbmr.org/ASBMRStatementsDetail/joint-guidance-on-osteoporosis-management-in-era-o. Accessed 20 May 2020.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.