Introduction
Coronavirus disease 2019 (COVID-19) is a global health problem, but optimal treatment modality is unclear. The treatment protocol for patients with COVID-19 consists of azithromycin, hydroxychloroquine (HCQ), and low-molecular-weight heparin in our center. Also, we prescribe oseltamivir until the return of influenza test results. Initially, HCQ was administered to all our patients for 5 days to 10 days (400 mg twice a day as a loading dose on the first day and then 200 mg twice a day as a maintenance dose for the next 4 to 9 days), without renal dose adjustments, according to the insufficient information at drug manufacturer's recommendations for short-term treatment.
HCQ is an immunomodulatory medication that has been used for decades to treat autoimmune disorders. Rheumatology guidelines recommend adjusting the daily dose according to body weight or serum drug levels for long-term therapies.1 The efficiency of HCQ in COVID-19 is controversial, but many centers frequently used it as a treatment during the pandemic. Also, the optimal dosage and dose alterations of HCQ in kidney failure remain uncertain due to scant data for COVID-19 therapy (Table 1). Here, we report 3 nondiabetic hemodialysis patients who developed hypoglycemia after HCQ treatment for COVID-19.
Table 1.
Teaching points
| 1. Hypoglycemia is a rare side effect of hydroxychloroquine. |
| 2. Hydroxychloroquine dosage must be reduced in hemodialysis patients, even for short-term treatment protocols. |
| 3. The optimal treatment of COVID-19 in patients with chronic kidney disease remains unknown. |
| 4. New well-designed and qualified trials for the treatment of COVID-19 are needed. |
COVID-19, coronavirus disease 2019.
Case Presentation
Case 1
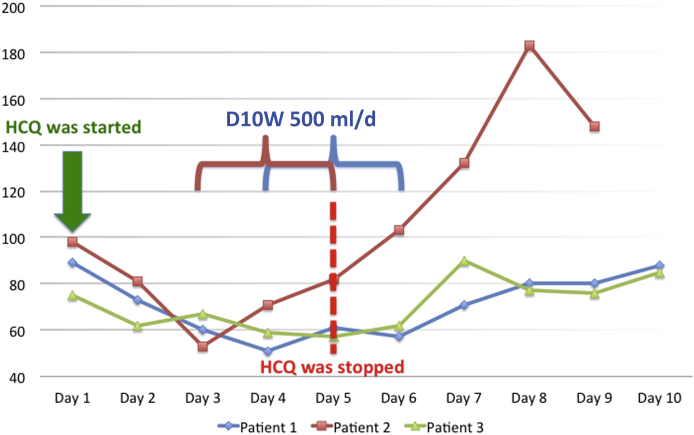
A 64-year-old woman with end-stage kidney disease due to hypertensive nephrosclerosis and a failed kidney transplantation recently on hemodialysis was admitted with fever. Her medications were amlodipine 10 mg/d, aspirin 100 mg/d, and prednisone 5 mg/d. The patient was not previously diagnosed with diabetes or had no history of a hypoglycemic event; the last HbA1c level was measured 15 days previously and was 5.4%. Laboratory tests showed lymphopenia and elevated C-reactive protein levels (Table 2). Chest computed tomography scan was normal. Real-time reverse transcriptase-polymerase chain reaction of nasopharyngeal swab confirmed the COVID-19 diagnosis. Hence, our treatment protocol was initiated. On the fourth day of treatment, fasting hypoglycemia was noted with serum glucose level 50 mg/dl (Figure 1). The patient was diagnosed with stress-induced hypocortisolism, and the patient was treated with 500 ml/d of 10% dextrose infusion and 20 mg/d i.v. methylprednisolone. Despite these treatments, hypoglycemia persisted, and HCQ treatment was stopped on the fifth day of the treatment, but she required dextrose infusion from the fourth to sixth day of hospitalization. Enteral or parenteral support was not added to the patient's nutritional program because of adequate oral calorie intake until the hypoglycemic period. Eventually, she was discharged on the 10th day of hospitalization without any other complications.
Table 2.
Clinical features, laboratory characteristics, and outcomes of the patients
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Gender | Female | Male | Male |
| Age, yr | 64 | 61 | 74 |
| Symptoms and findings | |||
| Fever | Yes | No | No |
| Pneumonia | No | Yes (severe) | Yes (severe) |
| Diarrhea | Yes | No | No |
| Initial laboratory results | |||
| Lymphocyte count, /mm3 | 850 | 450 | 280 |
| Serum CRP levels, mg/dl | 99 | 182 | 170 |
| D-Dimer levels, ng/ml | 11,990 | 4420 | 3020 |
| Serum ferritin levels, ng/ml | 817 | 7412 | 3800 |
| Serum LDH levels, U/l | 165 | 508 | 456 |
| Serum AST levels, U/l | 9 | 14 | 11 |
| Serum ALT levels, U/l | 3 | 6 | 6 |
| Serum ALP levels, U/l | 56 | 81 | 69 |
| Serum GGT levels, U/l | 11 | 10 | 38 |
| Prothrombin time, s | 11.4 | 8.7 | 9.2 |
| Partial thromboplastin time, s | 36 | 34 | 35 |
| INR | 1.1 | 1.09 | 1.06 |
| Events | |||
| Time of hypoglycemia | Day 4 | Day 3 | Day 2 |
| Symptomatic hypoglycemia | Yes | Yes | No |
| Outcome | Discharged | Death | Discharged |
ALP, alkaline phosphatase; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CRP, C-reactive protein; GGT, gamma-glutamyl transferase; INR, international normalized ratio; LDH, lactate dehydrogenase.
The abnormal laboratory results are in bold.
Figure 1.
The course of morning fasting blood glucose levels of 3 hypoglycemic patients during coronavirus disease 2019 (COVID-19) treatment. D10W, 10% dextrose solution; HCQ, hydroxychloroquine.
Case 2
A 61-year-old hemodialysis patient was admitted with dyspnea and cough. Outpatient medications were aspirin 100 mg/d, sevelamer 2400 mg/d, and carvedilol 12.5 mg/d. The patient was not previously diagnosed with diabetes. Oxygen saturation was 88% in ambient air, and chest computed tomography scan revealed bilateral ground-glass pneumonic opacities. Laboratory analyses revealed lymphopenia and elevated levels of ferritin and C-reactive protein (Table 2). The nasopharyngeal swab test was positive for SARS-CoV-2. On the third day of the treatment, his clinical condition deteriorated with fluctuating levels of consciousness. The fasting blood glucose level was 52 mg/dl (Figure 1). Hence, dextrose infusion was administered. Any nutritional support was not provided to the patient because of adequate oral calorie intake until the hypoglycemic event. On the fourth day of the treatment, he was transferred to the intensive care unit due to severe hypoxemia. Intermittent dextrose infusion was continued, and HCQ treatment was discontinued due to hypoglycemic episodes. Hypoglycemic events were resolved after the withdrawal of HCQ. Favipiravir was initiated after intubation for severe COVID-19 on the fifth day of hospitalization. Unfortunately, he died as a result of severe SARS-CoV-2 infection on the ninth day of hospitalization.
Case 3
A 74-year-old hemodialysis patient presented to the emergency department with a persistent cough for 2 days. Outpatient medications included carvedilol 6.25 mg/d and sevelamer 2400 mg/d. Similar to other patients, there was no history of diabetes or prediabetes. HbA1c was 5.2% at the time of hospital admission. Oxygen saturation was 95% while breathing ambient air. Laboratory results demonstrated lymphopenia and elevated levels of C-reactive protein, lactate dehydrogenase, and ferritin (Table 2). We observed bilateral ground-glass opacities on the chest computed tomography scan. The nasopharyngeal swab test confirmed the SARS-CoV-2 infection. He was started on the COVID-19 protocol. We noted asymptomatic fasting hypoglycemia on both mornings and evenings after the second day of treatment (Figure 1). The patient developed hypoxemia on the fifth day of the medication. Favipiravir treatment was added to the treatment because of severe clinical status. HCQ was withdrawn due to hypoglycemia, after which we did not observe any hypoglycemic incidents until discharge on the 16th day of hospitalization. Nutritional support and dextrose infusion were not needed due to adequate oral calorie intake. Also, postprandial glucose levels were normal during the hypoglycemic period.
Discussion
HCQ is an antimalarial drug with immunomodulatory effects and widely used for rheumatic diseases. Also, previous studies have shown that HCQ interferes with the glycosylation of angiotensin-converting enzyme-2, the receptor of SARS-CoV-2, and inhibits viral fusion into cells.2,3 Although controversy continues over the effectiveness of HCQ, it is one of the most commonly prescribed drugs in the treatment of COVID-19.
HCQ is mostly metabolized by the liver. However, approximately 25% of the drug is excreted as unchanged into the urine.4 HCQ is not removed by dialysis because of its large molecular size, and it is recommended to give a lower dose after each dialysis session for long term treatments.5,6 However, there is not much data available about the dosage of HCQ for short term COVID-19 treatments in patients with chronic kidney disease.
The common side effects of HCQ are diarrhea, nausea, retinal toxicity, hypoglycemia, myelosuppression, and cardiomyopathy. They are more common in patients with liver or renal impairment and generally associated with cumulative dosage.7 Also, hypoglycemia is a rare side effect of HCQ for both diabetic and nondiabetic patients.8,9,S1 The exact mechanism of HCQ-induced hypoglycemia is not well known. Proposed mechanisms include improving insulin sensitivity, enhancing insulin-dependent glucose transport, and decreasing the degradation of intracellular insulin.S1 A study demonstrated that HCQ could increase insulin secretion and improve blood glucose levels in prediabetic patients.S2 Another trial also showed that chloroquine could lower glucose levels in diabetic patients with a similar mechanism.S3
HCQ-induced hypoglycemia in the nondiabetic population is quite rare. Although the exact mechanism is not well known, a recently published case report showed that HCQ-induced hypoglycemia was associated with increased insulin and C-peptide levels in a nondiabetic patient. Hypoglycemic episodes were observed in our patients, especially during fasting times, similar to this previous case report.S4 Six hemodialysis patients were treated by HCQ without dose adjustments before we changed the treatment protocol. (Three of them experienced hypoglycemia.) None of our 6 hemodialysis patients with or without hypoglycemia had a diagnosis of diabetes; however, we do not know if our patients are prediabetic because of the lack of prior tests. In addition, we cannot determine the exact mechanism of hypoglycemia because of the lack of insulin and C-peptide measurement during hypoglycemic attacks.
Although HCQ-induced hypoglycemia is an exception in dialysis patients with rheumatic disease,S5 to the best of our knowledge, it has not been reported before in patients with COVID-19. This side effect could be associated with a second hit mechanism, such as a critical illness, besides the drug toxicity. Increased catabolism, malnutrition, liver failure, and septic status could also contribute to hypoglycemia.S4 The liver function tests of our patients who developed hypoglycemia were in the normal range (Table 2). Also, these patients had adequate nutrient intake. On the other hand, they were frail older adults with multiple comorbidities. Hence, we thought that the critical status associated with COVID-19 might enhance the hypoglycemic side effect of HCQ in our patients. Unfortunately, we were unable to measure HCQ blood levels. For this reason, it is not possible to establish a direct relationship between HCQ blood levels and hypoglycemic events. Interestingly, none of these patients associated with HCQ treatment have other side effects, such as QT prolongation.
During the COVID-19 pandemic, the optimal strategy for the administration of HCQ is unclear. Hence, these protocols showed variability among COVID-19 treatment centers. A previous study has shown that HCQ is more effective than chloroquine if 400 mg twice a day is administered on the first day and 200 mg twice a day for the next 4 days.S6 However, the optimal renal dose adjustment strategy of HCQ for COVID-19 treatment is unknown in patients with chronic kidney disease. Some centers recommend a dose reduction protocol as 200 mg after dialysis sessions (thrice a week) in hemodialysis patients.S7 All of our patients who developed hypoglycemia were oligoanuric, and we assumed that increased exposure to HCQ caused hypoglycemia due to impaired urinary excretion of the drug in these patients. We did not observe any hypoglycemic events in dialysis patients during the pandemic after dose-reduced protocol. On the other hand, although the efficacy of HCQ in COVID-19 treatment is unclear,S8–S10 dose reduction could be associated with the decreased potency of HCQ for COVID-19 treatment; however, there are not sufficient data for this concern.
Conclusion
In conclusion, we suggest reducing the HCQ dosage in hemodialysis patients because it reduces the hypoglycemic side effect. Also, dose adjustment could be considered according to the weight in patients with chronic kidney disease. Screening for the side effects of HCQ is essential in these patients.
Disclosure
All the authors declared no competing interests.
Acknowledgments
Author Contributions
ABD and AT designed the study; experiments were carried out by ED, SS, NG, ARU, OAO, HY, and AT; ABD and ED created the figures; the paper was drafted and revised by ABD, ED, SS, NG, ARU, OAO, HY, AM, MK, FG, SSY, and AT; all authors approved the final version of the manuscript.
Footnotes
Supplementary References.
Supplementary Material
References
- 1.Durcan L., Clarke W.A., Magder L.S. Hydroxychloroquine Blood Levels in SLE: Clarifying dosing controversies and improving adherence. J Rheumatol. 2015;42:2092–2097. doi: 10.3899/jrheum.150379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schrezenmeie E., Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16:155–166. doi: 10.1038/s41584-020-0372-x. [DOI] [PubMed] [Google Scholar]
- 3.Liu J., Cao R., Xu M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tett S.E., Cutler D.J., Ray A.O. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1998;26:303–313. doi: 10.1111/j.1365-2125.1988.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Izzeddine H., Jhaveri K.D., Perazella M.A. COVID-19 therapeutic options for patients with kidney disease. Kidney Int. 2020;97:1297–1298. doi: 10.1016/j.kint.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tett S.E., Cutler D.J., Ray A.O. Bioavailability of hydroxychloroquine tablets in healthy volunteers. Br J Clin Pharmacol. 1989;27:771–779. doi: 10.1111/j.1365-2125.1989.tb03439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Minami J., Hiyama K., Nagasaki Y. A case of administration of hydroxychloroquine in a hemodialysis patient with COVID-19. http://www.kansensho.or.jp/uploads/files/topics/2019ncov/covid19_casereport_en_200413.pdf Available at: Accessed May 7, 2020.
- 8.Shojania K., Koehler B.E., Elliott T. Hypoglycemia induced by hydroxychloroquine in a type II diabetic treated for polyarthritis. J Rheumatol. 1999;26:195–196. [PubMed] [Google Scholar]
- 9.Cansu D.U., Korkmaz C. Hypoglycemia induced by hydroxychloroquine in a non-diabetic patient treated for RA. Rheumatology. 2008;47:378–379. doi: 10.1093/rheumatology/kem378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.