Graphical abstract
Keywords: Supercritical carbon dioxide (scCO2), N-halamine, Polyester, Antibacterial surface modification
Highlights
-
•
Sustainable approach was investigated on antibacterial functionalization of PET.
-
•
Hydantoin acrylamide was synthesized by free-radical polymerization.
-
•
The best processing parameters were achieved at 120 °C, 25 MPa for 6 h.
-
•
The treated PET demonstrated good color, mechanical, and abrasion performance.
-
•
Stability, rechargeability, and durability were remarkably good after 50 washes.
Abstract
Biocidal functionalization in polyester fibers is a really tough challenge because of the lack of tethering groups. This study indicated supercritical carbon dioxide application using N-halamine would be an alternative solution for obtaining antibacterial function on the polyester surface. Firstly, N-(2-methyl-1-(4-methyl-2,5-dioxo-imidazolidin-4 yl)propan-2 yl)acrylamide was synthesized and applied to the polyester in supercritical carbon dioxide medium, at 120 °C, 30 MPa for different processing times. The addition of N-halamine on the surface significantly brought antibacterial activity against E. coli. The chlorine loadings showed that 6 -h exposure time was critical to obtain sufficient antibacterial activity. This treatment caused a reasonable and tolerable loss in color and mechanical properties. But, the durability to abrasion, stability, and rechargeability of oxidative chlorine, and the durability of N-halamine on the surface were remarkably good. Conclusively, it can be available to work on polyester surfaces with resource-efficient and eco-friendly supercritical carbon dioxide technique for getting more functionalization and modification.
1. Introduction
In recent years, there has been a lot of news about outbreaks and diseases in the world, which negatively affect human life, including coronavirus, influenza, hepatitis, Salmonella and E. coli infections [1]. Therefore, consumers focused extremely on medical products, and then as a natural consequence, the use of textiles on medical, hygiene, and health care fields has become significantly widespread with new antimicrobials, fibers, and technologies. Medical Textiles Market is strongly growing due to the rising both U.S. healthcare expenditure and demand from the Asia Pacific region under the COVID-19 outbreak. For example, The U.S. healthcare expenditure has increased from around US$ 1.1 trillion in 2010 to about US$ 1.8 trillion in 2020. As a result of all these, Medical Textiles Market is estimated to will reach US$ 16.8 billion by 2024 from US$ 12.2 billion in 2019, with a growth rate of more than 5% every year [2,3].
Biocidal functionalization on textiles have also been studied extensively, and many chemicals have been explored and developed for inhibiting the growth of bacteria and preventing infections and diseases in medical applications. Many antimicrobials such as organometallics and metal ions (especially silver ions) [[4], [5], [6], [7]], phenols [[8], [9], [10], [11], [12], [13]], biguanides, quaternary ammonium salts [[14], [15], [16]], organo-silicones and N-halamines [[17], [18], [19]] have been used in textile industry for decades. N-halamines can have the forms of amine, amide, and imide as a biocidal agent. They offer many advantages and benefits over other biocides, such as they have potent, broad-spectrum, generally non-leaching, rechargeable, relatively inexpensive, and hydrophobic character. Their lethal action occurs with the transfer of the oxidative chlorine to the cell membrane of the microorganisms. Their most distinctive property is that they can be recharged again (by converting N—H bond to N-Cl) by aqueous chlorine exposure when oxidative halogen ion (chlorine, bromine, or iodine) inactivates the microorganisms and then is vanished. They can easily be polymerized on the surfaces, be modified to produce desirable functional groups for attaching to surfaces, be impregnated into polymeric materials, and be incorporated on surfaces, as a biocide [[20], [21], [22], [23]].
For medical textiles, modification and functionalization are also significant to obtain active groups on the surface using textile-wet processes. However, attachments of any chemical and surface treatment to inert polymers such as polyester and polypropylene are complicated and a big challenge due to lack of surface tethering (functional) groups [24,25]. While there are methods such as spin coating and layer by layer assembly to impart functionality to inert substrates, these methods are not universally applicable as they only provide stable functionality on high surface tension substrates. Therefore, pretreatment is generally required to increase the surface tension of the inert surface or to form reactive groups on the surface. Unfortunately, the formation of functional properties on the surface was proven to be unsuccessful for polyester in conventional textile applications [24,26]. At the same time, the growth of consumer’s demand for innovative technologies and value-added textile materials, Governmental rules, and increasing environmental concerns force the textile industry to develop green technologies [[27], [28], [29], [30]].
Considering all of the above, modification, functionalization, and processing of materials could be easily carried out through supercritical fluids. They offer several specific physical, chemical, and toxicological advantages to reduce the consumption of energy, water (wastewater), chemicals, and also get functional properties on the polymer surfaces [26,31,32]. Since the late 1990s, supercritical fluids related studies have increased exponentially, and carbon dioxide (CO2) is the most desirable among them. Besides being non-flammable and non-toxic, due to its cheapness, CO2 is widely used as the supercritical fluid. Its critical coordinates are low conditions (Tc = 31 °C and Pc = 7.38 MPa), and it also becomes gaseous and is spontaneously separated from the processed products when pressure is decreased to 60 bar or less. So, it can be industrialized without high equipment and operating costs [31,33,34]. In a supercritical state, CO2 shows low viscosity, high permeation and diffusion abilities like that of gases combined with high density, and has a solvating power similar to liquid solvents. Therefore, CO2 in the supercritical state has both high solvating power to chemicals and swelling and plasticizing efficiency to hydrophobic polymers [26,[35], [36], [37], [38], [39], [40], [41]]. For example, polyester can be easily functionalized in supercritical CO2 (scCO2) state. However, medium and small molecular weight compounds (monomers, cross-liners, initiators, etc.) can dissolve and quickly diffuse into polyester in scCO2 [33,37,42]. Many functional chemicals such as polysiloxanes, N-halamines, and fluoropolymers are soluble in scCO2 and served as the anchoring tool to attach functional groups to the surface of the substrate without the need of polymerization since this application does not require reactive sites on substrates, as well [43,44]. As a result, scCO2 is highly suited to textile processes such as pre-treatment, dyeing, and finishing for modification and functionalization of surfaces.
In this study, we focused on the antibacterial functionalization of polyester with N-halamine in scCO2. Firstly, N-(2-methyl-1-(4-methyl-2,5-dioxo-imidazolidin-4 yl)propan-2 yl)acrylamide (HA) was synthesized and polymerized by free radical polymerization, and then their structures were confirmed by NMR and FTIR, respectively. The presence of N-halamine on the polyester surface was also investigated by FTIR. The properties of color, mechanical, and durability to abrasion were measured, and also stability, rechargeability, and durability to washings of the antibacterial properties were examined after scCO2 treatments.
2. Experimental
2.1. Materials
N-(2-methyl-1-(4-methyl-2,5-dioxo-imidazolidin-4 yl)propan-2-yl)acrylamide (hydantoin acrylamide, HA) was synthesized by using N-(1,1-Dimethyl-3-oxobutyl)acrylamide (DA, Aldrich), ammonium carbonate (AC, Sigma-Aldrich), potassium cyanide (KCN, Merck) and potassium persulfate (PPS, Acros) as an initiator for polymerizing HA. The woven polyethylene terephthalate (PET) fabric (120 g/m2, Tg = 75.8 °C, Tm = 256.3 °C, ΔHm (J/g) 50.8, 72.34 % crystallinity) as polyester was used for this study. The contaminants on the fabric were removed by washing it in a domestic laundry machine at 60 °C for 30 min by using a standard detergent. For antibacterial tests, Tryptic soy broth (TSB), Nutrient Broth (NB), and Nutrient Agar (NA) for supporting growth and cultivation of bacteria were supplied by Becton Dickinson. For preparing a buffer solution, NaH2PO4.2H2O (sodium dihydrogen phosphate) and Na2HPO4.12H2O (disodium hydrogen phosphate dodecahydrate) were purchased from Sigma Aldrich. Escherichia coli (E. coli ATCC 25922) was only used as test strains according to ASTM E2149 because Gram-negative bacteria are usually more resistant than Gram- positive. The 99.5 % purity CO2 used in all experiments was purchased from AGA Industrial gases (Lidingo, Sweden).
2.2. Synthesis of hydantoin acrylamide
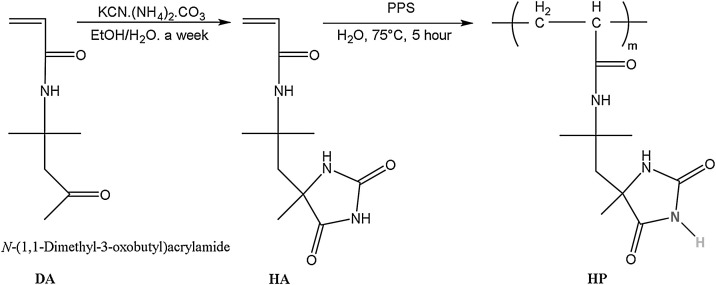
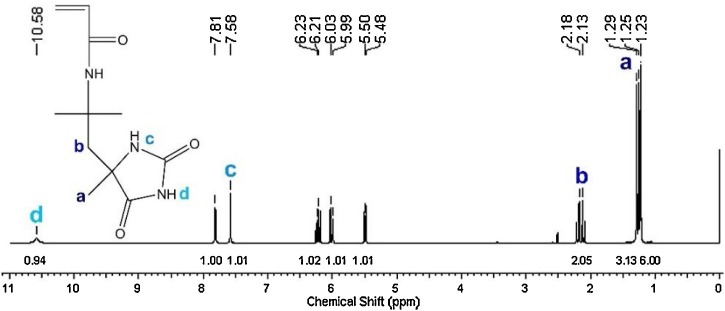
HA was synthesized according to the procedure described in Kocer et al. [45]. The synthesis and polymerization reactions of HA were given in Fig. 1 . 100 mmol DA, 200 mmol KCN, and 600 mmol AC were reacted in water/ethanol mixture (1:1 by volume) at room temperature for one week. After evaporation of ethanol, the products were isolated by treating with dilute HCl and filtrated. Then, homopolymer of HA (HP) was polymerized by free radical polymerization. The HA (8.4 mmol) was dissolved in water with 0.004 g of PPS. By nitrogen bubbling for 15 min dissolved oxygen was removed and the reaction was continued under nitrogen atmosphere by stirring at 75 °C for 5 h. The obtained polymer was filtered as white powder form. Its structure was confirmed by NMR and FTIR spectroscopies. 1H NMR (DMSO-d6, 400 MHz) δ 1.23 (3 H), 1.25 (3 H), 1.29 (3 H), 2.15 (2 H), 5.49 (1 H), 6.01 (1 H), 6.22 (1 H), 7.58 (1 H), 7.81 (1 H), 10.58 (1 H) ppm. FTIR 3353, 3216, 3203, 3076, 2977, 1764, 1720, 1710, 1665, 1651, 1532, 1254, 765, 636, 608 cm−1.
Fig. 1.
Synthesis of hydantoin acrylamide (HA) and its polymer (HP).
2.3. scCO2 treatment
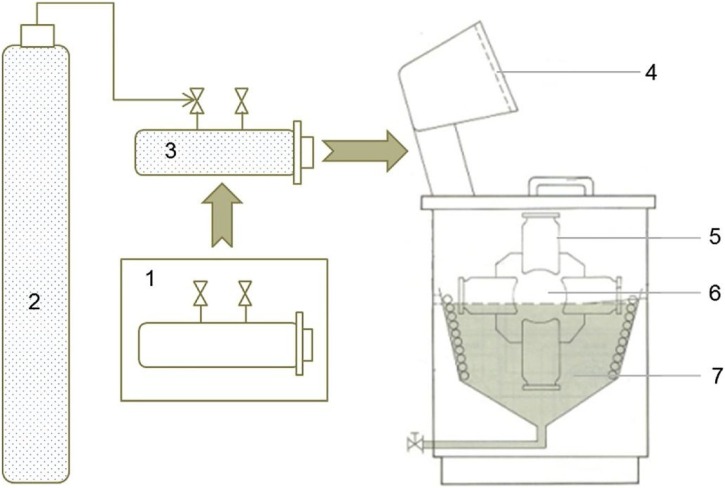
The schematic description of the apparatus used for scCO2 applications was described in Fig. 2 [35]. This apparatus was equipped with motor, temperature and time controllers, heating and cooling systems, a rotary pattern where the vessels are mounted, and a high-temperature oil bath. The vessels are stainless steel cylinders (internal volume = 290 mL, Pmax = 30 MPa, and Tmax = 130 °C) and equipped with closure with Teflon seal, safety valve, and needle valve used to fill and vent the CO2. The PET sample (16 × 24 cm, ca. 10 g) was wrapped around a stainless-steel perforated beam, and 5 % of HA homopolymer (HP) (owing to fabric weight, ca 0.5 g) was placed at the bottom of the vessel which was then filled with CO2 up to predetermined temperature and pressure during durations from 1 to 24 h. For easy filling of CO2, the vessels were cooled in a separate freezer for 15 min. Then, the vessels were mounted on the pattern rotating inside the oil bath. The experiments have been done considering poor solubility, and it was decided to accomplish at different durations; 1, 3, 6, 12, and 24 h, while keeping pressure and temperature constant (at 120 °C, 25 MPa). After treatments, samples were removed from vessels, rinsed with acetone to remove loosely attached polymers on the surface, and dried at ambient temperature.
Fig. 2.
Schematic of apparatus; (1) Freezer, (2) CO2 gas tank, (3) Vessel, (4) Control board, (5) Vessels mounted on the shaft, (6), Rotating pattern, (7) Oil bath.
2.4. Chlorination treatment
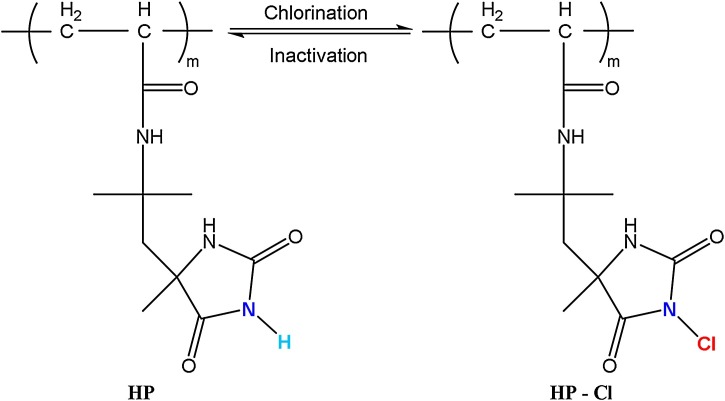
Chlorination was applied after scCO2 treatments for activating N-halamines with halogen ions. For chlorination, 10 % aqueous solution at pH 7 was prepared from household bleach (6 % sodium hypochlorite). After samples were chlorinated for 1 h, the unbonded chlorine on samples was removed by washing with water, and by drying at 45 °C for 1 h. The chlorination mechanism was provided in Fig. 3 .
Fig. 3.
Chlorination of hydantoin acrylamide (HP).
2.5. Characterizations
1H (400 MHz) NMR spectra were recorded with 16 scans on a Bruker spectrometer equipped with SampleXPress autosampler, using the Bruker TopSpin 2.1 software. The sample was prepared by dissolving 5 mg of monomer in 1 mL deuterated dimethyl sulfoxide (DMSO-d6). The spectra were collected at ambient temperature at 30 °C and chemical shift values were given in parts per million (ppm).
FTIR spectrum was obtained using Thermo Nicolet iS50 FTIR spectrometer attenuated total reflectance (ATR) accessory to characterize monomer, polymers, and fabrics. While recording spectra, 32 scans were made at 2 cm−1 spectral resolution, and scanning range was from 400 to 4000 cm−1.
Color values were determined using Datacolour CHECK II Plus spectrophotometer, and the measurements were taken according to AATCC Test Method 173. The samples were folded twice and measurement was conducted in reflectance mode under the condition of D65 illuminantand 10° standard observer. The averages of four measurements were reported as color values (L*, a*,b*) and color difference (ΔE).
The samples were conditioned for 24 h at 20 ± 2 °C and 65 ± 4% RH before tensile tests, and then they were subjected to tensile testing only in accordance with the warp direction, using ISO 13934-1 test method on Instron test machine. Briefly, the strips in 25 × 150 mm size were tested using a 50 kN of the load cell with 100 mm of the gauge length, and 10 cm/min of speed, at room temperature. The mean value and standard deviation (SD) were calculated using SPSS.V25 software and the averages of three measurements were reported as breaking strength (MPa) and breaking extension (%).
The determination of loaded chlorine on the surfaces was made with iodometric/thiosulfate titration [46]. The ethanol with 0.1 N acetic acid (90/10; v/v) was prepared and 0.25 g potassium iodide was dissolved in this solution, then the samples were added to the final solution. Titration was made with 0.005 N sodium thiosulfate solution until the color of the solution is yellow to clear. The weight percent of loaded chlorine (Cl+%) on samples were calculated according to Eq. 1 given below:
| (1) |
where V and N are the volume (L) and concentration (mol/L) and of titrant sodium thiosulfate, respectively, and W is the weight percentage of the sample (g). The mean values and standard deviations of five measurements were reported as the loaded chlorine.
The durability of scCO2 treatments against abrasion was measured for 9000 cycles, according to ASTM D4966 (Martindale Abrasion Tester Method). An untreated PET swatch was used as an abrasive material. The determination of loaded chlorine remaining on the surface (Cl+%) was made with iodometric/thiosulfate titration again.
Antibacterial activity of samples were evaluated against E. coli according to ASTM 2149. The bacterial culture concentration of about 105 CFU/mL was applied to samples (1 g) for testing. Samples were placed in 50 mL bacterial suspension and were shaken for different contact times; 10, 20, and 30 min. Then, solutions were spread onto Muller-Hilton II agar media, and plates were incubated for 24 h at 37 °C. The viable colonies were counted (in CFU/mL), and the bacterial reduction was determined. Reduction rate (R%) of bacteria was calculated using the formula (Eq. 2):
| (2) |
where A is the number of bacteria recovered from inoculated treated test sample in jar incubated for 24 h, and B is the number of bacteria recovered from inoculated treated test sample at ‘0’ contact time.
AATCC Test Method 61 was used to evaluate the stability, rechargeability, and durability of HA on the PET surface against repeated washing cycles. Repeated laundry washes were carried out inside stainless steel canisters, which contain 50 stainless steel balls and 150 mL of 0.15 % AATCC detergent water solution. A washing cycle corresponding to five machine washes (mw) was applied by rotating canisters at 42 rpm and 49 °C for 45 min. After washings, samples were rinsed with distilled water and then dried at ambient temperature. Chlorine loadings on a sample before and after washing cycles were determined by the titration procedure mentioned above. To evaluate the durability of biocidal function and rechargeability of chlorine, samples were titrated immediately before and then after rechlorination. All characterization measurements were repeated three times, and the arithmetic mean and standard deviation were calculated for each variable.
3. Results and discussion
3.1. Characterization of hydantoin acrylamide
The hydantoin ring configuration of HA monomer was obtained by Bucherer-Bergs reaction and polymerized according to the procedure described in Kocer et al. [45]. 1H NMR spectrum of HA monomer was given in Fig. 4 . There were additional signals which could be assigned to forming hydantoin ring, methyl group (a) at 1.23 ppm, methylene group (b) at 2.15 ppm, amide (c) at 7.58 ppm, and imide (d) at 10.58 ppm, respectively. NMR signals were in complete accord with that of literature [[47], [48], [49]].
Fig. 4.
NMR spectrum of HA monomer (solvent: DMSO-d6).
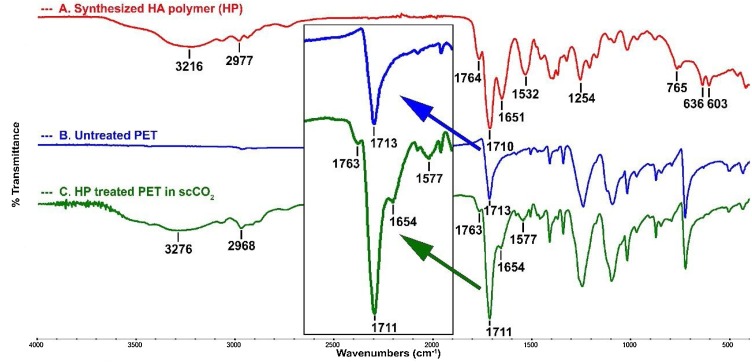
FTIR spectrum of the synthesized HA polymer (HP) was shown in Fig. 5 . The absence of the band at 1630 cm−1 coming from the vinyl bond stretching and the broad bands in the spectrum indicated the formation of polymerization. The bands at 3216, 1764, and 1710 cm−1 have confirmed the presence of the hydantoin ring in the polymer structure. These bands were corresponding to N—H, amide, and imide carbonyl stretching on the hydantoin ring, respectively [50,51].
Fig. 5.
FTIR spectrum of (A) synthesized HA polymer (HP), (B) Untreated PET, (C) HA treated PET in scCO2 for 6 h.
3.2. FTIR analysis after scCO2 treatment with hydantoin acrylamide
Surface characterization of before and after scCO2 treatment was performed by FTIR to determine the chemical changes and confirm the presence of treatment on the PET. It was first observed the most obvious HA presence in the surface for 6 h, comparing 1 and 3 h, and therefore, this spectrum was provided in Fig. 5 with spectra of HA polymer and untreated PET fabric together. As seen in Figure, the HA treated PET gave a broad band at 1711 cm−1 formed by the overlapping of the imide carbonyl groups of hydantoin ring (at 1710 cm−1) and ester carbonyl of PET (at 1713 cm−1). On the other hand, the presence of the N—H stretching (at 3276 cm−1) and amide carbonyl stretching (at 1763 cm−1) of the hydantoin ring of HA in modified PET (spectrum C) demonstrated that HA polymer has been successfully incorporated on PET structure. This confirmed the presence of HA polymer on PET surface [38].
3.3. Color evaluation
For textile materials, color values and color change are one of the most important fabric quality characteristics, and it is a significant aspect to take into consideration after any surface treatments. The color values were measured after scCO2 treatments with and without HA, and the results were presented in Table 1 . The sample had negligible color values (95.05, −0.20, 1.46, 0.94, and 90.07) after scCO2 treatments without HA for 6 h. Increasing processing time from 1 to 6 h caused a gradual change in fabric color from white to yellow (b* values and Stensby Whiteness Index), increased color difference (ΔE) values, and changed the shades of fabric colors. PET-HA treatments had a lower L* value with enhanced a* and b* values in comparison to untreated PET. These changes in color values might be explained by interpenetrating the HA on the surface [38,52]. The improvement in color values of PET-HA-Cl (96.04, −0.21, 0.99, 0.38, and 92.44) was from the effect of chlorine after chlorination. It is well known that halogens could lead to color changes [53].
Table 1.
Color indices of fabrics treated in scCO2.
| Sample Name | L* | a* | b* | ΔE | Stensby Whiteness Index |
|---|---|---|---|---|---|
| Untreated PET | 95.98 | −0.23 | 1.36 | – | 91.21 |
| PET (6 h) | 95.05 | −0.20 | 1.46 | 0.94 | 90.07 |
| PET-HA (1 h) | 94.91 | −0.21 | 1.44 | 1.07 | 89.96 |
| PET-HA (3 h) | 94.42 | −0.17 | 1.52 | 1.57 | 89.35 |
| PET-HA (6 h) | 93.26 | −0.10 | 1.88 | 2.77 | 87.32 |
| PET-HA (6 h)-Cl | 96.04 | −0.21 | 0.99 | 0.38 | 92.44 |
L: Lightness indicates, a: Red/green coordinate, b: Yellow/blue coordinate, ΔE: Total color difference.
3.4. Tensile strength
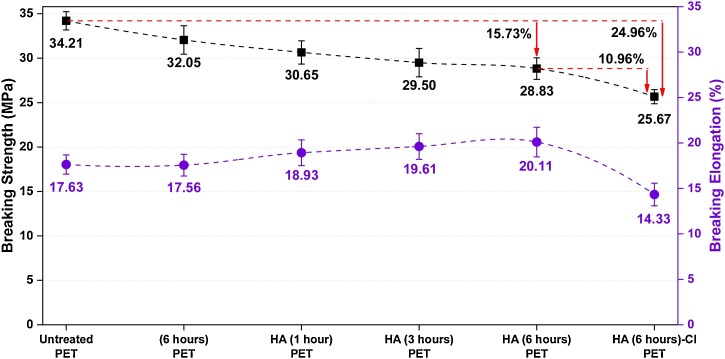
Fabrics were subjected to tensile testing with the warp directions, and test results were displayed in Fig. 6 . No significant change in tensile properties (32.05 MPa and −6.31 %) was obtained since the processes were not so severe after scCO2 treatments without HA for 6 h. Breaking strengths of PET-HA treatments decreased in an acceptable range, 30.65, 29.50, and 28.83 MPa (from −10.41 % to −15.73 %), respectively, while the breaking elongations were slightly increased with an increase in processing times. This reduction in breaking strengths might be explained by the fact that HA acted as a bonding agent on the surface and then led to an increase of intramolecular crosslinking in the fibers, ultimately affecting the overall structure of the surface [54,55]. The breaking strength of PET-HA-Cl was 25.65 MPa; this corresponds to −10.96 and −24,96 % of change after chlorination, comparing untreated PET and HA for 6 h. The chlorination treatment caused a reasonable and tolerable loss in breaking strength, and the reduction of up to 30 % after treatments is acceptable in practical textile applications for PET surfaces [[56], [57], [58]]. Moreover, it was reported volume expansion values between 1.8 % and 2.4 % for PET at 40−50 °C and pressure between 10 and 30 MPa, respectively [59]. It was also recently investigated that the CO2 sorption and polymer swelling of PET under high-pressure CO2 (2−15 MPa) at 40 °C. It was found that both sorption and swelling increase with pressure, and that higher values are reached when specific CO2/polymer interactions can occur and when the mobility of the polymeric chains is higher (or the crystallinity is lower) [60]. As a result, the swelling and CO2/polymer interactions are both enhanced by increasing CO2 density (via pressure increase or temperature decrease). In turn, density improves solute loading, by polymer plasticization and increase of internal diffusion coefficients [60] swelling). As a general conclusion of these works, the processing conditions can be optimized by getting change the temperature and/or time to reduce this loss further because the running with accurate processing time and temperature is critical knowledge for not getting worse the mechanical properties of PET. These results suggested that scCO2 treatments did not affect the mechanical properties of PET fabrics dramatically and exhibited satisfactory results.
Fig. 6.
Breaking strengths of fabrics treated in scCO2.
3.5. Chlorine loading and antibacterial property
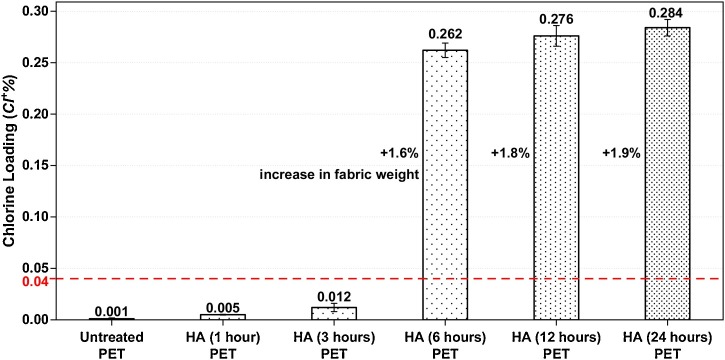
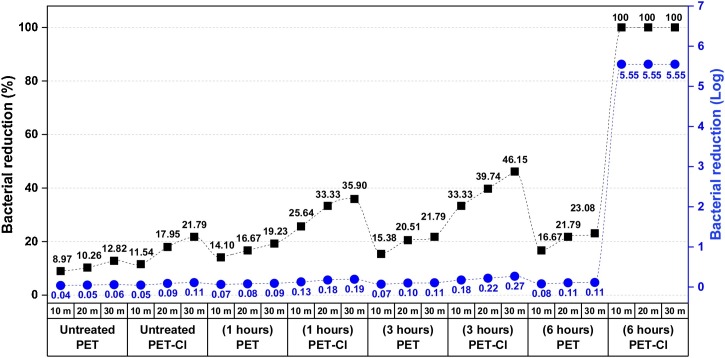
For scCO2 studies, the treatments with 5 % HA were carried out at 120 °C, 25 MPa for different processing times considering chemical solubility in scCO2. The increases of 1.6, 1.8, and 1.9 % on fabric weights were observed in Fig. 7 , considering 6, 12, and 24 h processing times. However, hydantoin acrylamide on the surface must be activated by any aqueous treatment using halogen ions (converting the N—H bond to N-Cl). After chlorination treatment, the presence of oxidative chlorine (Cl+) bonded to HA on the PET was measured by analytical titration of treated samples, and chlorine loadings (Cl+%) concerning process time were shown in Fig. 7. Although the chlorine loading is relatively low comparing previously described studies [38,51,61,62], the treated PET samples provided fast inactivation against E. coli as shown in the antibacterial tests (Fig. 8 ) and the effects of these treatments were not surprising. Earlier works have reported that with a chlorine loading of 0.04 % would be sufficient for getting antibacterial activity [61,63], hence, PET-HA (6 h), PET-HA (12 h), and PET-HA (24 h) samples would still provide antibacterial efficacy. As seen in Fig., in our study the critical time is determined to be 6 h since when the application duration was increased from 3 h to 6 h, chlorine loading was an approximately 20-fold increase, and the chlorine loading exceeds the critical value in literature, whereas the increase of duration after 6 h did not cause a significant increase on chlorine loading.
Fig. 7.
Chlorine loadings (Cl+%) of fabrics treated in scCO2.
Fig. 8.
Antibacterial activities of fabrics treated in scCO2 against E. colia.
Note: The concentration of bacteria was adjusted to 3.55 × 105 (log 5.55) CFU*/mL for each sample.
* CFU: Colony forming units.
The value of (−) 100 % indicates that all the bacteria on the surface were killed.
The antibacterial activities of chlorinated samples were tested against E. coli, at 3.55 × 105 CFU/mL (log 5.55), for various contact times, according to ASTM 2149. The PET-HA (1 h) and PET-HA (3 h) samples notably exhibited a limited degree of bacterial reduction after 30 min of contact time, which provided only about 0.19 and 0.27 log reductions, respectively, in Fig. 8. It can be concluded from these results that the untreated, PET-HA (1 h), and PET-HA (3 h) did not show inactivation after 30 min (even at the end of the 1 day). PET-HA (6 h) sample with chlorine loadings (Cl+%) of 0.26 % showed rapid inactivation and superior antimicrobial efficacy. They also provided a complete 5.55 log inactivation against E. coli within even a contact time of 10 min, which indicates that all the bacteria were killed. As a result, HA could be effective antimicrobial compounds using in scCO2 treatments for at least 6 h.
3.6. Stability, rechargeability, and durability to washes
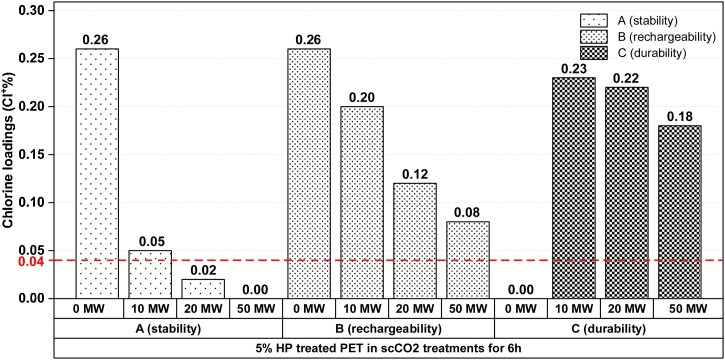
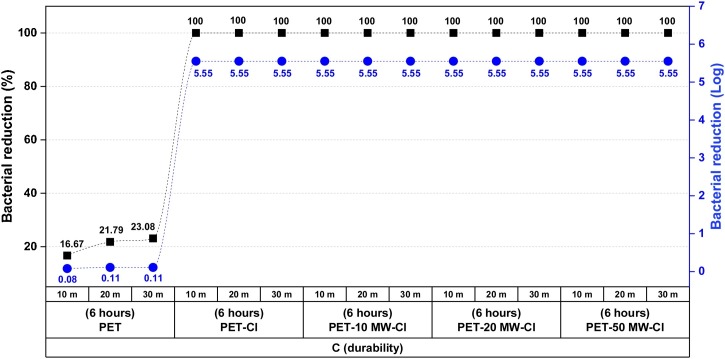
The stability and rechargeability of oxidative chlorine (Cl+), and the durability of HA compound on the PET surface are essential against washes because of the chlorine stability of N-Cl bond and antibacterial property. The tests were applied to PET-HA (6 h) samples, and the treatments were performed on chlorinated samples before washes (A), chlorinated before washes and rechlorinated after washes (B), and unchlorinated before washes, but chlorinated after washes (C), as presented in Fig. 9 . The chlorinated PET samples (A) lost the most of their first chlorine loadings quickly with increasing washes, and the chlorine content decreased from 0.25 to 0.05 % after only 10 washes (MW). This phenomenon is based on N-Cl bond dissociation. Hydrolysis of the N-Cl bond (bond dissociation) resulted in a total loss of chlorine (0.0 %) after 50 washes. It is certainly not linked to the dissociation of tethering groups from PET surface because the rechlorination of PET provided chlorine loadings (B and C) at about their first values (0.20 % and 0.23 %). The chlorine loading of 0.08 % was recharged after 50 washes by the rechlorination. The rechargeability of lost chlorine was excellent and acceptable since the loading of chlorine is still higher than the threshold concentration of 0.04 % for getting antibacterial activity after 50 washes. [61,63,64] The chlorinated samples after washes (C) are also resistant to washes, and 0.18 % of the chlorine was restored after 50 washings. B (rechargability) are worse (lower %Cl- after 10, 20, 50 washing cycles) than those for C (durability). So, polymers might easily lose their chlorine bonded to the surface with weak bonds. The durability of HA on the PET was strong during washes. Consequently, scCO2 treatment with HA exhibited satisfactory stability and durability to washes, and the rechargeability of lost active chlorines was acceptable. The antibacterial results given in Fig. 10 have also confirmed the durability of N-halamine compound on the PET in scCO2 treatments for 6 h.
Fig. 9.
Washing stability of HA treated PET in scCO2 for 6 h.
Fig. 10.
Antibacterial activities of HA treated PET in scCO2 for 6 h against E. colia.
3.7. Durability to abrasion
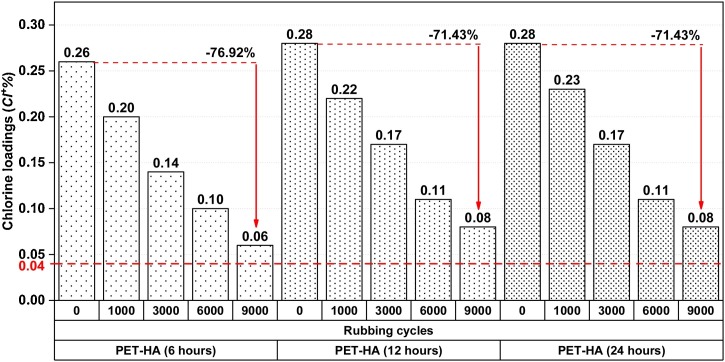
The durability of treatments against abrasion was tested, and the remained chlorine on the surface was determined by titration after defined abrasion cycles. The results were shown in Fig. 11 . The samples had similar values. The chlorine loadings (Cl+%) of PET-HA (6 h) decreased from 0.26 to 0.06 as the abrading cycle increased, and about 23 % of chlorine remained on the surface after the 9000 cycles. This indicated that the polymer was still standing on the surface. Hence, the durability of the treatments against the repeated friction could consider good since they had higher values than the critical value (0.04 %) in literature [39,65].
Fig. 11.
Durability to abrasion of fabrics treated in scCO2.
4. Conclusions
The functionalization of polyester is more challenging than cotton and other related fibers because of the lack of functional groups within the molecular structure. This study investigated the antibacterial functionalization of polyester fabrics with N-halamine in scCO2 medium for different processing times. Therefore, HA was synthesized and then was interpenetrated on the PET surface in scCO2. The measurements and characterizations showed that the presence of HA on PET surface was confirmed by FTIR analysis, and 6 h of exposure time was critical to obtain antibacterial activity against E. coli considering chlorine loadings. The durability to abrasion, stability, and rechargeability of oxidative chlorine (Cl+) and the durability of N-halamine on the surface were superior against repeated washings, although scCO2 treatments caused a reasonable and tolerable loss in color and mechanical properties. This study points out the new application for sustainable fabrication of biocidal polyester using N-halamine by resource-efficient and eco-friendly scCO2 technique. The scCO2 technology can create a new opportunity to focus on nonreactive polymers such as polyester for getting surface functionalization and modification, which would help reduce the cost of production and the environmental pollution with textile finishings.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Scientific and Technological Research Council of Turkey (TUBITAK) under BIDEB 2219. All the Universities collaborating on the program are gratefully acknowledged. We also thank TEKO (Sveriges Textil- och Modeföretag) for invaluable support.
Contributor Information
Mehmet Orhan, Email: morhan@uludag.edu.tr, mehmet.orhan@hb.se.
Fatma Demirci, Email: fatma.demirci@btu.edu.tr.
Hasan B. Kocer, Email: hasan.kocer@btu.edu.tr.
Vincent Nierstrasz, Email: vincent.nierstrasz@hb.se.
References
- 1.WHO, World Health Organization. https://www.who.int, 2020 (12 February 2020).
- 2.MarketsandMarkets, Biomedical Textiles Market. https://www.marketsandmarkets.com/PressReleases/biomedical-textile.asp, 2018 (16 March 2018).
- 3.C. Chantrill, US Health Care Spending for 2020. https://www.usgovernmentspending.com/spending_chart_2010_2025USb_10t, 2020 (26 May 2020).
- 4.Cao Z., Sun X., Yao J., Sun Y. Silver sulfadiazine–Immobilized celluloses as biocompatible polymeric biocides. J. Bioact. Compat. Pol. 2013;28:398–410. doi: 10.1177/0883911513490340. [DOI] [Google Scholar]
- 5.Suleman N., Kalhapure R.S., Mocktar C., Rambharose S., Singh M., Govender T. Silver salts of carboxylic acid terminated generation 1 poly (propyl ether imine)(PETIM) Dendron and dendrimers as antimicrobial agents against S. aureus and MRSA. RSC Adv. 2015;5:34967–34978. doi: 10.1039/c5ra03179f. [DOI] [Google Scholar]
- 6.Erdem R., Rajendran S. Influence of silver loaded antibacterial agent on knitted and nonwoven fabrics and some fabric properties. J. Eng. Fiber. Fabr. 2016;11:38–46. doi: 10.1177/155892501601100107. [DOI] [Google Scholar]
- 7.Butola B., Mohammad F. Silver nanomaterials as future colorants and potential antimicrobial agents for natural and synthetic textile materials. RSC Adv. 2016;6:44232–44247. doi: 10.1039/c6ra05799c. [DOI] [Google Scholar]
- 8.Regös J., Zak O., Solf R., Vischer W.A., Weirich E.G. Antimicrobial Spectrum of triclosan, a broad-spectrum antimicrobial agent for topical application. Dermatology. 1979;158:72–79. doi: 10.1159/000250746. [DOI] [PubMed] [Google Scholar]
- 9.Orhan M., Kut D., Güneşoğlu C. Use of triclosan as antibacterial agent in the textiles. Indian J. Fibre. Text. Res. 2007;32:114–118. [Google Scholar]
- 10.Orhan M., Kut D., Güneşoğlu C. Improving the antibacterial activity of cotton fabrics finished with triclosan by the use of 1, 2, 3, 4‐butanetetracarboxylic acid and citric acid. J. Appl. Polym. Sci. 2009;111:1344–1352. doi: 10.1002/app.25083. [DOI] [Google Scholar]
- 11.Orhan M. Determination and characterization of triclosan on polyethylene terephthalate fibers. Tekstil ve Mühendis. 2012;19:27–30. [Google Scholar]
- 12.Henry N.D., Fair P.A. Comparison of in vitro cytotoxicity, estrogenicity and anti‐estrogenicity of triclosan, perfluorooctane sulfonate and perfluorooctanoic acid. J. Appl. Toxicol. 2013;33:265–272. doi: 10.1002/jat.1736. [DOI] [PubMed] [Google Scholar]
- 13.Wang Z.X., Jiang C.P., Cao Y., Ding Y.T. Systematic review and meta‐analysis of triclosan‐coated sutures for the prevention of surgical‐site infection. Br. J. Surg. 2013;100:465–473. doi: 10.1002/bjs.9062. [DOI] [PubMed] [Google Scholar]
- 14.Zhu P., Sun G. Antimicrobial finishing of wool fabrics using quaternary ammonium salts. J. Appl. Polym. Sci. 2004;93:1037–1041. doi: 10.1002/app.20563. [DOI] [Google Scholar]
- 15.Chen Y., Niu M., Yuan S., Teng H. Durable antimicrobial finishing of cellulose with QSA silicone by supercritical adsorption. Appl. Surf. Sci. 2013;264:171–175. doi: 10.1016/j.apsusc.2012.09.165. [DOI] [Google Scholar]
- 16.Farah S., Aviv O., Laout N., Ratner S., Beyth N., Domb A.J. Quaternary ammonium poly (diethylaminoethyl methacrylate) possessing antimicrobial activity. Colloids Surf. B Biointerfaces. 2015;128:608–613. doi: 10.1016/j.colsurfb.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 17.Sun Y., Sun G. Novel refreshable N-Halamine polymeric biocides: N-Chlorination of aromatic polyamides. Ind. Eng. Chem. Res. 2004;43:5015–5020. doi: 10.1021/ie030846m. [DOI] [Google Scholar]
- 18.Ren X., Akdag A., Kocer H.B., Worley S., Broughton R., Huang T. N-halamine-Coated cotton for antimicrobial and detoxification applications. Carbohydr. Polym. 2009;78:220–226. doi: 10.1016/j.carbpol.2009.03.029. [DOI] [Google Scholar]
- 19.Demir B., Cerkez I., Worley S., Broughton R., Huang T.-S. N-halamine-Modified antimicrobial polypropylene nonwoven fabrics for use against airborne Bacteria. ACS Appl. Mater. Interfaces. 2015;7:1752–1757. doi: 10.1021/am507329m. [DOI] [PubMed] [Google Scholar]
- 20.Sun G., Wheatley W.B., Worley S.D. A new cyclic N-Halamine biocidal polymer. Ind. Eng. Chem. Res. 1994;33:168–170. doi: 10.1021/ie00025a022. [DOI] [Google Scholar]
- 21.Barnes K., Liang J., Wu R., Worley S., Lee J., Broughton R., Huang T. Synthesis and antimicrobial applications of 5, 5′-ethylenebis [5-methyl-3-(3-triethoxysilylpropyl) hydantoin] Biomaterials. 2006;27:4825–4830. doi: 10.1016/j.biomaterials.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 22.Demir B., Broughton R.M., Qiao M., Huang T.-S., Worley S.D. N-halamine biocidal materials with superior antimicrobial efficacies for wound dressings. Molecules. 2017;22:1582. doi: 10.3390/molecules22101582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Worley S.D., Williams D., Crawford R.A. Halamine water disinfectants. Crit. Rev. Env. Sci. Tec. 1988;18:133–175. doi: 10.1080/10643388809388345. [DOI] [Google Scholar]
- 24.Cerkez I., Kocer H.B., Worley S.D., Broughton R.M., Huang T.S. N-halamine biocidal coatings via a layer-by-Layer assembly technique. Langmuir. 2011;27:4091–4097. doi: 10.1021/la104923x. [DOI] [PubMed] [Google Scholar]
- 25.Jiang L., Lu Y., Liu X., Tu H., Zhang J., Shi X., Deng H., Du Y. Layer-by-layer immobilization of quaternized carboxymethyl chitosan/organic rectorite and alginate onto nanofibrous mats and their antibacterial application. Carbohydr. Polym. 2015;121:428–435. doi: 10.1016/j.carbpol.2014.12.069. [DOI] [PubMed] [Google Scholar]
- 26.Chen Y., Zhang Q., Ma Y., Han Q. Surface-oriented fluorinated pyridinium silicone with enhanced antibacterial activity on cotton via supercritical impregnation. Cellulose. 2018;25:1499–1511. doi: 10.1007/s10570-018-1657-y. [DOI] [Google Scholar]
- 27.Alkaya E., Demirer G.N. Sustainable textile production: a case study from a woven fabric manufacturing mill in Turkey. J. Clean. Prod. 2014;65:595–603. doi: 10.1016/j.jclepro.2013.07.008. [DOI] [Google Scholar]
- 28.Kerr J., Landry J. Global Fashion Agenda. 2017. Pulse of the fashion industry. [Google Scholar]
- 29.Gulzar T., Farooq T., Kiran S., Ahmad I., Hameed A. The Impact and Prospects of Green Chemistry for Textile Technology. Elsevier; 2019. Green chemistry in the wet processing of textiles; pp. 1–20. [DOI] [Google Scholar]
- 30.Yıldırım F.F., Hasçelik B., Yumru Ş., Palamutcu S. Analysis of Water consumption and potential savings in a Cotton textile dye House in Denizli, Turkey. In: Muthu S.S., editor. Water in Textiles and Fashion. Elsevier; 2019. pp. 115–134. [DOI] [Google Scholar]
- 31.Cooper A.I. Porous materials and supercritical fluids. Adv. Mater. 2003;15:1049–1059. doi: 10.1002/adma.200300380. [DOI] [Google Scholar]
- 32.Dyecoo, CO2 Dyeing. http://www.dyecoo.com/co2-dyeing/, 2020 (25 May 2020).
- 33.Cansell F., Aymonier C., Loppinet-Serani A. Review on materials science and supercritical fluids. Curr. Opin. Solid State Mater. Sci. 2003;7:331–340. [Google Scholar]
- 34.Jessop P.G., Hsiao Y., Ikariya T., Noyori R. Homogeneous catalysis in supercritical fluids: hydrogenation of supercritical carbon dioxide to formic acid, alkyl formates, and formamides. J. Am. Chem. Soc. 1996;118:344–355. doi: 10.1021/ja953097b. [DOI] [Google Scholar]
- 35.Abate M.T., Ferri A., Guan J., Chen G., Ferreira J.A., Nierstrasz V. Single-step disperse dyeing and antimicrobial functionalization of polyester fabric with chitosan and derivative in supercritical carbon dioxide. J. Supercrit. Fluids. 2019;147:231–240. doi: 10.1016/j.supflu.2018.11.002. [DOI] [Google Scholar]
- 36.Abate M.T., Zhou Y., Guan J., Chen G., Ferri A., Nierstrasz V. Colouration and bio-activation of polyester fabric with curcumin in supercritical CO2: part II–effect of dye concentration on the colour and functional properties. J. Supercrit. Fluids. 2020;157 doi: 10.1016/j.supflu.2019.104703. [DOI] [Google Scholar]
- 37.Chen Y., He Q., Ren G., Feng C., Li N., Yu H., Han Q. Preparation of biocidal 4‐ethyl‐4‐(hydroxymethyl) oxazolidin‐2‐one‐based N‐halamine polysiloxane for impregnation of polypropylene in supercritical CO2. J. Appl. Polym. Sci. 2018;135 doi: 10.1002/app.46624. [DOI] [Google Scholar]
- 38.Chen Y., Yu P., Ren G., Zhang Q., Han Q., Teng H. Interpenetration of polyethylene terephthalate with biocidal quaternary Ammonium/N-Chloramine polysiloxane in supercritical CO2. Ind. Eng. Chem. Res. 2017;56:9560–9568. doi: 10.1021/acs.iecr.7b02544. [DOI] [Google Scholar]
- 39.Chen Y., Zhong X.-s., Zhang Q. Synthesis of CO2-philic polysiloxane with N-halamine side groups for biocidal coating on cotton. Ind. Eng. Chem. Res. 2012;51:9260–9265. doi: 10.1021/ie300378b. [DOI] [Google Scholar]
- 40.Knittel D., Saus W., Schollmeyer E. Water-free dyeing of textile accessories using supercritical carbon dioxide. Indian J. Fibre. Text. Res. 1997;22:184–189. [Google Scholar]
- 41.Xu W.Z., Yang L., Charpentier P.A. Preparation of antibacterial softwood via chemical attachment of quaternary ammonium compounds using supercritical CO2. ACS Sustain. Chem. Eng. 2016;4:1551–1561. doi: 10.1021/acssuschemeng.5b01488. [DOI] [Google Scholar]
- 42.Tanaka M., Rastogi A., Toepperwein G.N., Riggleman R.A., Felix N.M., de Pablo J.J., Ober C.K. Fluorinated quaternary ammonium salts As dissolution aids for polar polymers in environmentally benign supercritical carbon dioxide. Chem. Mater. 2009;21:3125–3135. doi: 10.1021/cm900406c. [DOI] [Google Scholar]
- 43.Liu S., Zhao N., Rudenja S. Surface interpenetrating networks of poly (Ethylene terephthalate) and polyamides for effective biocidal properties. Macromol. Chem. Phys. 2010;211:286–296. doi: 10.1002/macp.200900381. [DOI] [Google Scholar]
- 44.O’Neill M.L., Cao Q., Fang M., Johnston K.P., Wilkinson S.P., Smith C.D., Kerschner J.L., Jureller S.H. Solubility of homopolymers and copolymers in carbon dioxide. Ind. Eng. Chem. Res. 1998;37:3067–3079. doi: 10.1021/ie980010x. [DOI] [Google Scholar]
- 45.Kocer H.B., Cerkez I., Worley S.D., Broughton R.M., Huang T.S. Polymeric antimicrobial N-Halamine epoxides. ACS Appl. Mater. Interfaces. 2011;3:2845–2850. doi: 10.1021/am200351w. [DOI] [PubMed] [Google Scholar]
- 46.Kocer H.B., Worley S., Broughton R., Acevedo O., Huang T. Effect of phenyl derivatization on the stabilities of antimicrobial N-chlorohydantoin derivatives. Ind. Eng. Chem. Res. 2010;49:11188–11194. doi: 10.1021/ie101258s. [DOI] [Google Scholar]
- 47.Kocer H.B. Residual disinfection with N-halamine based antimicrobial paints. Prog. Org. Coat. 2012;74:100–105. doi: 10.1016/j.porgcoat.2011.11.022. [DOI] [Google Scholar]
- 48.Cerkez I. Rapid disinfection by N-halamine polyelectrolytes. J. Bioact. Compat. Polym. 2013;28:86–96. https://doi.org/10.1177%2F0883911512470863. [Google Scholar]
- 49.Cerkez I., Worley S., Broughton R., Huang T. Antimicrobial surface coatings for polypropylene nonwoven fabrics. React. Funct. Polym. 2013;73:1412–1419. doi: 10.1016/j.reactfunctpolym.2013.07.016. [DOI] [Google Scholar]
- 50.Cerkez I., Kocer H.B., Worley S., Broughton R., Huang T. N-halamine copolymers for biocidal coatings. React. Funct. Polym. 2012;72:673–679. doi: 10.1016/j.reactfunctpolym.2012.06.018. [DOI] [Google Scholar]
- 51.Hui F., Debiemme-Chouvy C. Antimicrobial N-halamine polymers and coatings: a review of their synthesis, characterization, and applications. Biomacromolecules. 2013;14:585–601. doi: 10.1021/bm301980q. [DOI] [PubMed] [Google Scholar]
- 52.Pan N., Liu Y., Ren X., Huang T.-S. Fabrication of cotton fabrics through in-situ reduction of polymeric N-halamine modified graphene oxide with enhanced ultraviolet-blocking, self-cleaning, and highly efficient, and monitorable antibacterial properties. Colloids Surf. A Physicochem. Eng. Asp. 2018;555:765–771. doi: 10.1016/j.colsurfa.2018.07.056. [DOI] [Google Scholar]
- 53.Si Y., Cossu A., Nitin N., Ma Y., Zhao C., Chiou B.s., Cao T., Wang D., Sun G. Mechanically robust and transparent N‐halamine grafted PVA‐co‐PE films with renewable antimicrobial activity. Macromol. Biosci. 2017;17 doi: 10.1002/mabi.201600304. [DOI] [PubMed] [Google Scholar]
- 54.Cerkez I., Kocer H.B., Worley S., Broughton R., Huang T. Multifunctional cotton fabric: antimicrobial and durable press. J. Appl. Polym. Sci. 2012;124:4230–4238. doi: 10.1002/app.35402. [DOI] [Google Scholar]
- 55.Cheng X., Ma K., Li R., Ren X., Huang T. Antimicrobial coating of modified chitosan onto cotton fabrics. Appl. Surf. Sci. 2014;309:138–143. doi: 10.1016/j.apsusc.2014.04.206. [DOI] [Google Scholar]
- 56.Sun G., Xu X., Bickett J.R., Williams J.F. Durable and regenerable antibacterial finishing of fabrics with a new hydantoin derivative. Ind. Eng. Chem. Res. 2001;40:1016–1021. doi: 10.1021/ie000657t. [DOI] [Google Scholar]
- 57.Zhang S., Li R., Huang D., Ren X., Huang T.-S. Antibacterial modification of pet with quaternary ammonium salt and silver particles via electron-beam irradiation. Mater. Sci. Eng. C. 2018;85:123–129. doi: 10.1016/j.msec.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Zhang S., Ding F., Wang Y., Ren X., Huang T.-S. Antibacterial and hydrophilic modification of PET fabrics by electron beam irradiation process. Fibers Polym. 2020;21:1023–1031. doi: 10.1007/s12221-020-9765-3. [DOI] [Google Scholar]
- 59.Von Schnitzler J., Eggers R. Mass transfer in polymers in a supercritical CO2-atmosphere. J. Supercrit. Fluids. 1999;16:81–92. doi: 10.1016/S0896-8446(99)00020-0. [DOI] [Google Scholar]
- 60.Goñi M.L., Gañán N.A., Strumia M.C., Martini R.E. Eugenol-loaded LLDPE films with antioxidant activity by supercritical carbon dioxide impregnation. J. Supercrit. Fluids. 2016;111:28–35. doi: 10.1016/j.supflu.2016.01.012. [DOI] [Google Scholar]
- 61.Liu Y., Liu Y., Ren X., Huang T.S. Antimicrobial cotton containing N-halamine and quaternary ammonium groups by grafting copolymerization. Appl. Surf. Sci. 2014;296:231–236. doi: 10.1016/j.apsusc.2014.01.106. [DOI] [Google Scholar]
- 62.Chen Y., Zhang Q., Han Q., Mi Y., Sun S., Feng C., Xiao H., Yu P., Yang C. Synthesis of polysiloxane with 5, 5‐dimethylhydantoin‐based N‐halamine pendants for biocidal functionalization of polyethylene by supercritical impregnation. J. Appl. Polym. Sci. 2017;134 doi: 10.1002/app.44721. [DOI] [Google Scholar]
- 63.Liang J., Chen Y., Barnes K., Wu R., Worley S., Huang T.-S. N-halamine/quat siloxane copolymers for use in biocidal coatings. Biomaterials. 2006;27:2495–2501. doi: 10.1016/j.biomaterials.2005.11.020. [DOI] [PubMed] [Google Scholar]
- 64.Worley S.D., Chen Y., Wang J.W., Wu R., Cho U., Broughton R.M., Kim J., Wei C.I., Williams J.F., Chen J. Novel N-halamine siloxane monomers and polymers for preparing biocidal coatings. Surf. Coat. Int. B: Coat. Trans. 2005;88:93–99. doi: 10.1007/bf02699539. [DOI] [Google Scholar]
- 65.Liang J., Wu R., Wang J.-W., Barnes K., Worley S., Cho U., Lee J., Broughton R., Huang T.-S. N-halamine biocidal coatings. J. Ind. Microbiol. Biotechnol. 2007;34:157–163. doi: 10.1007/s10295-006-0181-5. [DOI] [PubMed] [Google Scholar]