Abstract
We report a case of a kidney transplant recipient who presented with acute kidney injury and nephrotic-range proteinuria in a context of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Kidney biopsy revealed collapsing glomerulopathy. Droplet-based digital polymerase chain reaction did not detect the presence of SARS-CoV-2 RNA in the biopsy fragment, and the virus was barely detectable in plasma at the time of the biopsy. SARS-CoV-2 RNAemia peaked several days later, followed by a seroconversion despite the absence of circulating CD19-positive lymphocytes at admission due to rituximab-based treatment of antibody-mediated rejection 3 months earlier. Genotyping for the 2 risk alleles of the apolipoprotein L1 (APOL1) gene revealed that the donor carried the low-risk G0/G2 genotype. This case illustrates that coronavirus disease 2019 infection may promote a collapsing glomerulopathy in kidney allografts with a low-risk APOL1 genotype in the absence of detectable SARS-CoV-2 RNA in the kidney and that podocyte injury may precede SARS-CoV-2 RNAemia.
Index Words: Kidney transplantation, collapsing glomerulopathy, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), coronavirus 2019 (COVID-19), acute kidney injury (AKI), allograft biopsy, case report
Introduction
Kidney injury is frequent in patients with novel coronavirus disease 2019 (COVID-19).1 It is proposed that kidney cells are targeted by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), thereby causing kidney lesions; viral RNA has been detected in various kidney compartments of patients who died of COVID-19, including glomeruli.2 , 3 Critically, podocytes express membrane proteins such as angiotensin-converting enzyme 2 (ACE2) that are considered to facilitate SARS-CoV-2 entry within the cell.4 Consequently, SARS-CoV-2 could have a preferential tropism for glomerular cells, and podocyte injury occurring in SARS-CoV-2 infection may result in collapsing glomerulopathy in native kidneys. However, the presence of the virus in glomerular cells has never been formally demonstrated in living patients.5, 6, 7 Thus, the mechanism by which SARS-CoV-2 infection promotes glomerular injury is an unresolved issue.
According to the “second-hit hypothesis,“8 these forms of acute glomerulopathy may be predisposed to occur with a genetic background of high-risk apolipoprotein L1 (APOL1) allelic variants. Two cases of COVID-19–associated collapsing glomerulopathy were tested and found to carry 2 high-risk APOL1 genetic variants (G1 or G2),6 , 7 suggesting that SARS-CoV-2 infection may act as a trigger promoting collapsing glomerulopathy lesions in at-risk patients with COVID-19.9 We describe a kidney transplant recipient who, 3 months after an episode of acute antibody-mediated rejection (ABMR), had COVID-19 diagnosed, at which time he was found to have collapsing glomerulopathy in the absence of detectable SARS-CoV-2 RNA in the kidney. In addition, the donor APOL1 genotype was “low risk“ (G0/G2). The onset of glomerular injury was dissociated from SARS-CoV-2 viremia because it preceded it. Viremia occurred secondarily and resolved with seroconversion despite the absence of circulating CD19-positive lymphocytes at admission.
Case Report
A 29-year-old-man of sub-Saharan origin who had kidney failure due to urinary schistosomiasis received a kidney transplant from a deceased donor in 2015 (the ethnicity of the donor is unknown). The immunosuppression regimen was prednisone, tacrolimus, and mycophenolate mofetil. His baseline serum creatinine level was 135 μmol/L. A biopsy-proven ABMR episode was diagnosed in January 2020 (Fig 1 A-C). At the time of ABMR diagnosis, serum creatinine level was 289 μmol/L (estimated glomerular filtration rate was 28 mL/min/1.73 m2 and urinary albumin-creatinine ratio was 3.7 mg/mmol). The patient had the following donor-specific antibodies: anti-DQ5 (mean fluorescence intensity [MFI], 23412), anti-DQ8 (MFI, 8299), and anti-DP∗03 (MFI, 4975). Treatment consisted of high-dose corticosteroids (methylprednisolone, 500 mg/d, for 3 days), rituximab (375 mg/m2), 5 plasma exchanges, and a high dose of intravenous immunoglobulins (2 g/kg). Maintenance immunosuppression consisted of prednisone, 10 mg/d; tacrolimus, 6 mg/d; and mycophenolate mofetil, 500 mg, twice a day. Kidney function did not fully recover (Fig 2 ).
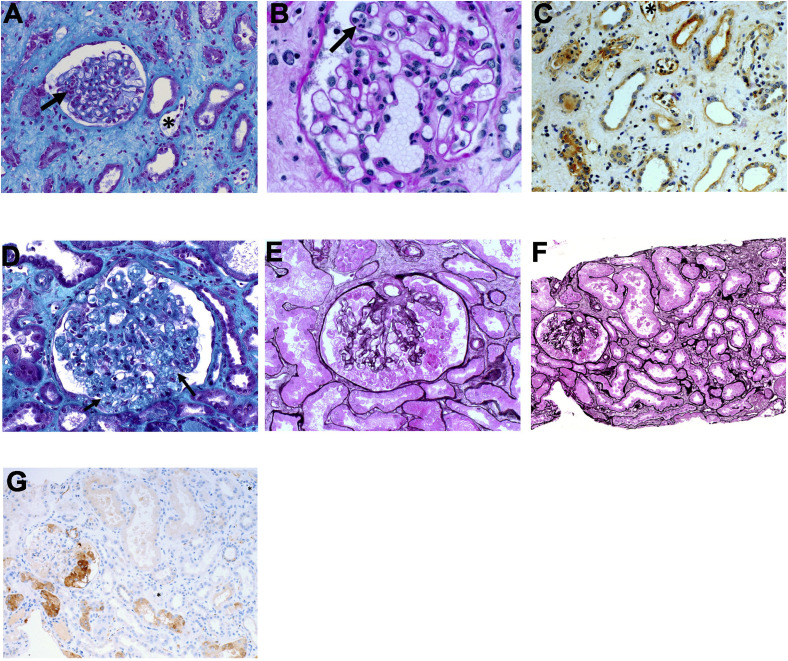
Figure 1.
Kidney allograft pathology findings. (A-C) Kidney histology of the first kidney allograft biopsy with (A) Masson trichrome staining showing patterns of acute antibody-mediated rejection with glomerulitis (arrow) and peritubular capillaritis (∗), (B) periodic acid–Schiff staining showing glomerulitis (arrow), and (C) immunohistochemistry displaying positive staining for C4d on peritubular capillaries (∗) (brown). (D-G) Kidney histology of the second graft biopsy with (D) Masson trichrome staining showing collapsing glomerulopathy with podocyte hypertrophy and hyperplasia and collapse of the glomerular tuft (arrow), (E, F) Jones methenamine silver staining showing (E) collapsing glomerulopathy with podocyte hypertrophy and hyperplasia and collapse of the glomerular tuft and (F) tubular necrosis, and (G) immunohistochemistry displaying negative staining for C4d on peritubular capillaries (∗).
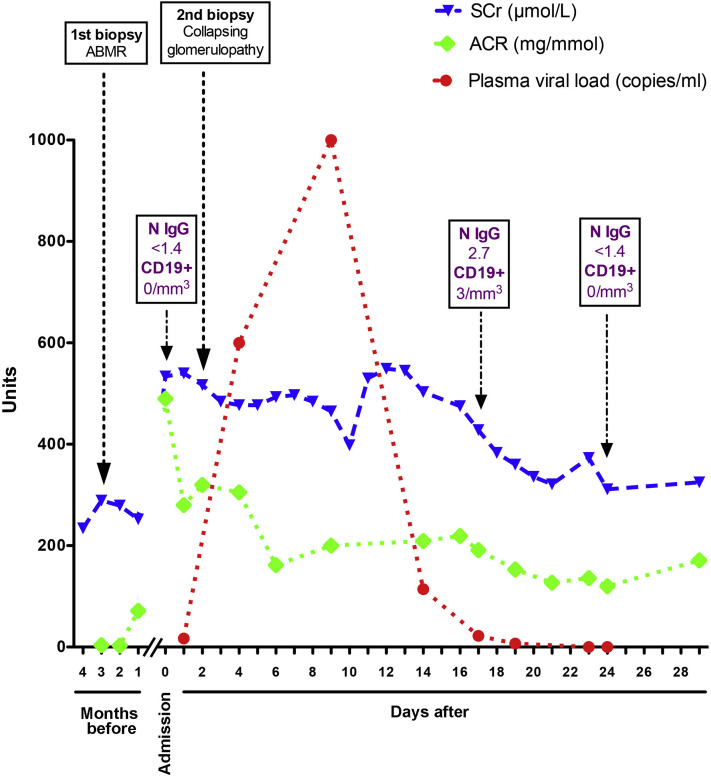
Figure 2.
Evolution of biological parameters during follow-up. Serum creatinine (SCr), urinary albumin-creatinine ratio (ACR), and SARS-CoV-2 RNA in plasma were sequentially measured. Kidney biopsies are indicated, as well as serologic test and B-cell counts. Conversion factor for SCr in μmol/L to mg/dL, ×0.0113. N IgG are immunoglobulin G antibodies against SARS-CoV-2 nucleocapsid. Abbreviation: ABMR, antibody-mediated rejection.
In the second week of May 2020, the patient was admitted to the hospital because of fever, cough, and vomiting, which had started 5 days earlier. A reverse transcriptase–polymerase chain reaction (PCR) test for SARS-CoV-2 on a nasopharyngeal swab sample was positive at admission. C-Reactive protein level was increased at 92 (reference range, <5) mg/L, as well as levels of fibrinogen, 5.7 (reference range, 1.5-3.5) g/L; D-dimers, 1,050 (reference range, <500) ng/mL; ferritin, 2,672 (reference range, 24-336) μg/L; and lactate dehydrogenase, 477 (reference range, <240) UI/L, reflecting systemic inflammation (Fig S1).
Acute kidney injury was present at admission, with serum creatinine level of 534 μmol/L and nephrotic-range proteinuria (urinary protein-creatinine ratio, 0.8 g/mmol; urinary albumin-creatinine ratio, 490 mg/mmol; and serum albumin, 2.8 g/dL; Figs 2 and S2). Urinary concentrations of the low-molecular-weight proteins retinol binding protein and β2-microglobulin were 100 to 1,000 times the normal value, indicating that the tubular compartment was injured.
Kidney biopsy performed 2 days after admission revealed collapsing glomerulopathy with pronounced podocyte hyperplasia and hypertrophy in absence of evidence of ABMR (Fig 1D and E). Acute proximal tubular injury was severe, with tubular dilatations, flattening of the tubular epithelium, loss of the brush border, and detached tubular epithelial cells in the lumen (Fig 1F). C4D staining of peritubular capillaries was negative (Fig 1 G). There was no evidence of thrombotic microangiopathy. Immunofluorescence assays on glomeruli were negative for immunoglobulin G (IgG), IgM, IgA, C3, C1q, and κ and λ light chains. Electron microscopy of the biopsy specimen was not performed. Serologic tests for human immunodeficiency virus and hepatitis B and C viruses were negative. PCR testing of blood samples for cytomegalovirus, Epstein-Barr virus, parvovirus B19, and BK polyomavirus all gave negative results. Recurrent schistosomiasis was ruled out by the absence of schistosome eggs in urine.
Droplet-based digital PCR was used to detect the presence of SARS-CoV-2 nucleic acids in fluids and tissue (Item S1). This is an ultrasensitive technology with higher efficiency compared with classic quantitative PCR for the detection of rare events.10 , 11 SARS-CoV-2 RNA detected using this method was barely detectable in plasma at the time of the biopsy (17 copies/mL), peaked thereafter at high concentrations (up to 1,000 copies/mL), and rapidly decreased (Fig 2). Clearance of the virus was concomitant with seroconversion. IgG antibodies against SARS-CoV-2 nucleocapsid (assayed using the ARCHITECT i2000 immunoassay analyzer [Abbott]) were absent at admission and were detected 14 days after (with a titer of 2.7 IU/L [the threshold for a positive result was 1.4 IU/L]) despite the absence of circulating CD19-positive cells at admission, and 3/μL 15 days later (Fig 2). Critically, SARS-CoV-2 RNA was not found in the kidney biopsy fragment or in urine (which was also collected before the peak of viremia).
The donor was found to have an APOL1 G0/G2 genotype, which is considered low risk8; the genotype of the recipient was G0/G0. The donor was a carrier of the high-risk HLA-DR4 genotype, and the recipient carried the high-risk HLA-B44 genotype.12 A systematic screen for other genes that are associated with collapsing glomerulopathy was not performed.
Mycophenolate mofetil treatment was discontinued temporarily. The patient did not require intensive care support, but fever and inflammation resolved at the same time that viremia cleared (Figs 2 and S1). Four weeks after the diagnosis of collapsing glomerulopathy, kidney function only partially recovered, with serum creatinine level of 325 μmol/L, severely increased urinary albumin-creatinine ratio (171 mg/mmol), and a normalized serum albumin level (3.7 g/dL; Fig 2). SARS-CoV-2 was no longer detected on a nasopharyngeal swab sample. The immunosuppression regimen at the end of follow-up was prednisone, 10 mg/d, and tacrolimus, 6 mg/d (trough level, 5-8 ng/mL), and mycophenolate mofetil treatment was resumed at 500 mg twice a day.
Discussion
We describe biopsy-proven collapsing glomerulopathy occurring in a kidney transplant recipient with COVID-19 who had a low-risk APOL1 genotype, in the absence of detectable SARS-CoV-2 RNA in the kidney. Histologic findings in the kidney allograft are similar to those observed in native kidneys of patients with COVID-19.3 , 5 , 7 , 13 However, there are many lessons to be learned from this singular case.
The mechanisms by which glomeruli are injured in the context of SARS-CoV-2 infection are unknown, and whether the virus promotes podocyte injury directly, indirectly through the activation of antiviral immunity, or both remains to be established. Nevertheless, the SARS-CoV-2 receptor ACE2 is expressed by podocytes, and SARS-CoV-2 nucleic acids and proteins are present in glomerular epithelial cells,2 indicating that the virus can enter the cell and potentially promote damage. Conversely, patients with COVID-19 may have a collapsing glomerulopathy in the absence of SARS-CoV-2 RNA detected in the kidney,6 , 7 supporting a role for indirect mechanisms in the pathogenesis of COVID-19–related kidney injury; for example, a systemic response to SARS-Cov-2 with enhanced type 1 interferon expression.14 However, the detection of virus in kidney tissue may be challenging in terms of sensitivity and specificity.15, 16, 17 Thus, highly sensitive and specific methods are required to detect the presence of the virus in the setting of COVID-19–related kidney diseases. Despite the use of droplet-based digital PCR—an ultrasensitive method to detect nucleic acids—we did not find SARS-CoV-2 RNA in the kidney biopsy fragment. A false-negative finding is unlikely because the endogenous control (RNase P) was correctly amplified. Thus, the temporal sequence of occurrence of a collapsing glomerulopathy negative for SARS-CoV-2 RNA followed by SARS-CoV-2 RNAemia is a strong but not definitive argument for the role of a circulating factor produced on SARS-CoV-2 infection, rather than a direct cytopathic effect of the virus.18
This collapsing glomerulopathy in the setting of SARS-CoV-2 infection occurred in a low-risk APOL1 (G0/G2) donor background.8 APOL1 risk alleles are associated with a higher rate of occurrence for de novo collapsing glomerulopathy12 and there is growing experimental evidence to suggest that kidney-specific expression of the APOL1 G1 and G2 risk variants may interfere with normal podocyte homeostasis.8 APOL1 expression is enhanced in inflammatory settings, being strongly upregulated in response to interferons, lipopolysaccharids, Toll-like receptor agonists, tumor necrosis factor, and other cytokines8; also, type I interferons stimulate APOL1 expression in vitro, causing severe podocyte injury.18 Inheritance of risk for kidney disease conferred by the G1 and G2 APOL1 variants follows a largely recessive pattern, even if a much smaller effect is often observed in G1 heterozygotes that has not been seen in G2 heterozygotes.8 However, one cannot exclude that, in the specific context of infection due to SARS-CoV-2 and in a highly systemic inflammatory burden with activation of type I interferon–mediated signaling pathways, inheriting 1 copy of the G2 allele may sensitize podocytes to injury. Additionally, we did not exclude human immunodeficiency virus and hepatitis C virus viremia, both of which have been associated with collapsing glomerulopathy, although serologic testing was negative and the patient had no other clinical stigmata of these infections.
Finally, we made the observation that SARS-CoV-2 seroconversion can occur even when circulating CD19-positive lymphocytes are virtually nonexistent. Even if correlative, the association of clearance of the virus and the appearance of IgGs targeting the SARS-CoV-2 nucleocapsid is highly suggestive of a role of antibodies in elimination of the virus from the circulation. The origin of the reactive B-cell clone and the mechanism of anti-SARS-CoV-2 IgG production in the context of circulating B-cell depletion remain to be established.
In conclusion, this case illustrates that a high-risk APOL1 donor background is not a prerequisite for severe podocyte injury to occur in kidney allografts in a context of COVID-19. Our findings suggest that a direct cytopathogenic effect of SARS-CoV-2 may not be instrumental for the development of collapsing glomerulopathy.
Article Information
Authors’ Full Names and Academic Degrees
Hélène Lazareth, MD, PhD, Hélène Péré, PharmD, PhD, Yannick Binois, MD, Melchior Chabannes, MD, Juliet Schurder, MD, Thomas Bruneau, BSc, Alexandre Karras, MD, PhD, Eric Thervet, MD, PhD, Marion Rabant, MD, PhD, David Veyer, PharmD, PhD, and Nicolas Pallet, MD, PhD.
Support
None.
Financial Disclosure
The authors declare that they have no relevant financial interests.
Patient Protections
The authors declare that the patient reported here provided consent for use of his medical records for research and that this work was performed under approval of the hospital ethics committee. Further information is available in Item S2.
Peer Review
Received May 28, 2020. Evaluated by 2 external peer reviewers, with direct editorial input from the Pathology Editor, the Education Editor, and a Deputy Editor. Accepted in revised form June 30, 2020.
Footnotes
Complete author and article information provided before references.
Figure S1: Evolution of inflammatory markers during follow-up.
Figure S2: Evolution of serum albumin and protein-creatinine ratio during follow-up.
Item S1: SARS-CoV-2 RNA extraction procedures and quantification.
Item S2: Research ethics approval and informed consent.
Supplementary Material
Figures S1-S2; Items S1-S2.
References
- 1.Hirsch J.S., Ng J.H., Ross D.W. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020;98(1):209–218. doi: 10.1016/j.kint.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Puelles V.G., Lutgehetmann M., Lindenmeyer M.T. Multiorgan and renal tropism of SARS-CoV-2. N Engl J Med. 2020;383(6):590–592. doi: 10.1056/NEJMc2011400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Su H., Yang M., Wan C. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int. 2020;98(1):219–227. doi: 10.1016/j.kint.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sungnak W., Huang N., Becavin C. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kissling S., Rotman S., Gerber C. Collapsing glomerulopathy in a COVID-19 patient. Kidney Int. 2020;98(1):228–231. doi: 10.1016/j.kint.2020.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsen C.P., Bourne T.D., Wilson J.D., Saqqa O., Sharshir M.A. Collapsing glomerulopathy in a patient with coronavirus disease 2019 (COVID-19) Kidney Int Rep. 2020;5(6):935–939. doi: 10.1016/j.ekir.2020.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peleg Y., Kudose S., D'Agati V. Acute kidney injury due to collapsing glomerulopathy following COVID-19 infection. Kidney Int Rep. 2020;5(6):940–945. doi: 10.1016/j.ekir.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Friedman D.J., Pollak M.R. APOL1 and kidney disease: from genetics to biology. Annu Rev Physiol. 2020;82:323–342. doi: 10.1146/annurev-physiol-021119-034345. [DOI] [PubMed] [Google Scholar]
- 9.Nasr S.H., Kopp J.B. COVID-19-associated collapsing glomerulopathy: an emerging entity. Kidney Int Rep. 2020;5(6):759–761. doi: 10.1016/j.ekir.2020.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Veyer D., Wack M., Mandavit M. HPV circulating tumoral DNA quantification by droplet-based digital PCR: a promising predictive and prognostic biomarker for HPV-associated oropharyngeal cancers. Int J Cancer. 2020;147(4):1222–1227. doi: 10.1002/ijc.32804. [DOI] [PubMed] [Google Scholar]
- 11.Sedlak R.H., Kuypers J., Jerome K.R. A multiplexed droplet digital PCR assay performs better than qPCR on inhibition prone samples. Diagn Microbiol Infect Dis. 2014;80(4):285–286. doi: 10.1016/j.diagmicrobio.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 12.Santoriello D., Husain S.A., De Serres S.A. Donor APOL1 high-risk genotypes are associated with increased risk and inferior prognosis of de novo collapsing glomerulopathy in renal allografts. Kidney Int. 2018;94(6):1189–1198. doi: 10.1016/j.kint.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batlle D., Soler M.J., Sparks M.A. Acute kidney injury in COVID-19: emerging evidence of a distinct pathophysiology. J Am Soc Nephrol. 2020;31(7):1380–1383. doi: 10.1681/ASN.2020040419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaillard F., Ismael S., Sannier A. Tubuloreticular inclusions in COVID-19-related collapsing glomerulopathy. Kidney Int. 2020;98(1):241. doi: 10.1016/j.kint.2020.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldsmith C.S., Miller S.E., Martines R.B., Bullock H.A., Zaki S.R. Electron microscopy of SARS-CoV-2: a challenging task. Lancet. 2020;395(10238):e99. doi: 10.1016/S0140-6736(20)31188-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roufosse C., Curtis E., Moran L. Electron microscopic investigations in COVID-19: not all crowns are coronas. Kidney Int. 2020;98(2):505–506. doi: 10.1016/j.kint.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang W., Xu Y., Gao R. Detection of SARS-CoV-2 in different types of clinical specimens. JAMA. 2020;323(18):1843–1844. doi: 10.1001/jama.2020.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang J.H., Husain S.A., Santoriello D. Donor's APOL1 risk genotype and “second hits“ associated with de novo collapsing glomerulopathy in deceased donor kidney transplant recipients: a report of 5 cases. Am J Kidney Dis. 2019;73(1):134–139. doi: 10.1053/j.ajkd.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figures S1-S2; Items S1-S2.