Abstract
The optimal approach to osteoporosis screening and treatment in postmenopausal women is unclear. We compared 1) the United States Preventive Services Task Force (USPSTF) and Osteoporosis Canada osteoporosis screening strategies; and 2) the National Osteoporosis Foundation (NOF) and Canadian treatment strategies. We used data from the prospective Women’s Health Initiative Observational Study and Clinical Trials of women aged 50–79 years at baseline (n=117,707 followed for self-reported fractures; n = 8,134 in bone mineral density [BMD] subset). We determined the yield of the screening and treatment strategies in identifying women who experienced major osteoporotic fractures (MOF) during 10-year follow-up. Among women aged 50–64 years, 23.1% of women were identified for BMD testing under the USPSTF strategy and 52.3% under the Canadian strategy. For women ≥65 years, 100% were identified for testing under the USPSTF and Canadian strategies, 35–74% were identified for treatment under NOF, and 16–37% were identified for treatment under CAROC (range among 5-year age subgroups). Among women who experienced MOF during follow-up, the USPSTF strategy identified 6.7% of women 50–54 years-old and 49.5% of women 60–64 years-old for BMD testing (versus 54.4% and 60.6% for the Canadian strategy, respectively). However, specificity of the USPSTF strategy was higher than that of the Canadian strategy among women 50–64 years-old. Among women who experienced MOF during follow-up, sensitivity for identifying women as treatment candidates was lowest for both strategies in women aged 50–64 (NOF 10–38%; CAROC 1–15%) and maximal in 75- to 79-year-old women (NOF 82.8%; 51.6% CAROC); specificity declined with advancing age and was lower with the NOF than the CAROC strategy. Among women aged 50–64 years, the screening and treatment strategies examined had low sensitivity for identifying those who subsequently experience MOF; sensitivity was higher among women ≥65 years than among younger women. New screening and treatment algorithms are needed.
Keywords: osteoporosis, fracture, bone density, FRAX, United States Preventive Services Task Force, National Osteoporosis Foundation, Canadian Association of Radiologists and Osteoporosis Canada
Introduction
Up to one out of every two postmenopausal women will experience a fragility osteoporosis-related fracture at some point in her lifetime.(1) Low bone mineral density (BMD) is a strong risk factor for fragility fracture.(2) The combination of clinical risk factors and BMD provide higher sensitivity and specificity then either alone for the prediction of osteoporotic fractures.(3) The Fracture Risk Assessment Tool, FRAX, is a web-based clinical tool that uses individual clinical risk factors and femoral neck BMD to predict the 10-year risk of hip fracture and major osteoporotic fracture (MOF; clinical spine, forearm, hip or shoulder fracture).(4) The use of FRAX has been integrated into screening guidelines for younger women, and into treatment guidelines.
In the context of screening, the USPSTF osteoporosis screening guideline recommends the use of a formal clinical risk assessment tool among postmenopausal women aged between 50 and 64 years to identify candidates for receipt of BMD testing.(2) Specifically, the USPSTF states that “one approach is to perform bone measurement testing in postmenopausal women younger than age 65 years who have a 10-year FRAX risk (without BMD) of MOF greater than that of a 65-year-old white woman without major risk factors”-i.e. greater than 8.4%. (Appendix Table 1a).(2) In contrast, among women aged 50–64 years, the Canadian strategy recommends BMD testing in women with specific clinical risk factors (Table 1, Appendix Table 1b).(5) Therefore, unlike USPSTF, Canada does not currently recommend the use of a risk assessment tool for screening decisions for younger postmenopausal women. Both the USPSTF and Canadian screening guidelines recommend BMD testing for women ≥ 65 years, regardless of their FRAX scores.
Table 1.
Criteria for Identifying Women for Bone Mineral Density Testing Under Each Screening Strategy*
| Age group | USPSTF strategy | Canadian strategy |
|---|---|---|
| 50–64 years | FRAX-predicted risk of major osteoporosis fracture ≥8.4% (using FRAX without bone density information) | Based on presence of one or more clinical risk factor:
|
| ≥65 years | Routine screening | Routine screening |
USPSTF denotes United States Preventive Services Task Force; FRAX denotes Fracture Risk Assessment Tool. Strategies are applicable to women without prior fragility fracture.
Any self-reported fracture ≥55 years of age
≥55 years of age
We did not have information regarding participant weight at age 25, so we substituted information regarding >10% weight loss of weight since age 35 years
Disorders include rheumatoid arthritis (self-reported), premature menopause (≤45 years-old), using a special diet for gastrointestinal disorders (malabsorption, celiac sprue, ulcerative colitis, or Crohn’s disease), liver disease, emphysema, or chronic bronchitis
In the context of treatment, the National Osteoporosis Foundation (NOF) approach uses FRAX to guide treatment decisions, but only in the women who have BMD T-scores between −1 and −2.5 (Table 2). Specifically, for women aged ≥50 years with BMD T-score between −1 and −2.5, the NOF guidelines recommend pharmacotherapy if 10-year FRAX-predicted risk is ≥3% for hip fracture or ≥20% for MOF.(1) In contrast, for women over age 50 years who meet guidelines for BMD testing, the Osteoporosis Canada approach endorses a simplified semiquantitative tool derived from FRAX known as Canadian Association of Radiologists and Osteoporosis Canada (CAROC) as the preferred national risk assessment system.(6) CAROC uses age, femoral neck BMD, a history of prior fragility fracture, and a history of prolonged glucocorticoid use to stratify women into three zones of MOF risk within 10 years: low (< 10%), moderate (10%–20%) and high (> 20%).(5) Women in the high-risk CAROC category, those with prior fractures indicating high risk (hip fracture, vertebral fracture, or more than one fragility fracture) and certain women at moderate risk for MOF (depending on certain clinical characteristics), should be considered for pharmacotherapy. Women in the low risk category should not usually be considered for pharmacotherapy.
Table 2.
Criteria for Identifying Women for Treatment under Each Strategy*
| NOF strategy | CAROC-based Canadian strategy† |
|---|---|
|
At least one of the following”
|
At least one of the following:
|
NOF denotes National Osteoporosis Foundation; CAROC denotes Canadian Association of Radiologists and Osteoporosis Canada; BMD denotes bone mineral density; FRAX denotes fracture risk assessment tool
Fragility fracture after age 40 or recent prolonged glucocorticoid use increases the CAROC-based risk by one category (i.e. from low to moderate or moderate to high risk).
Major osteoporotic fracture includes clinical spine, forearm, hip or humerus fracture
We operationalized this by using ≥1.0 SD discrepancy.
See the disorders listed in Table 1.
Given the high prevalence of osteoporosis among postmenopausal women and the recent plateau in the decline in hip fracture rates in the U.S.(7,8), it is relevant to examine alternatives to current U.S. osteoporosis screening and treatment strategies. To our knowledge, these screening strategies (USPSTF and Canadian) and treatment strategies (NOF and Canadian) have never been compared in detail in a large prospective cohort of U.S. postmenopausal women. The goals of this project were to compare the proportions of women identified for BMD testing under the USPSTF and Canadian screening strategies, the proportions of women identified for treatment under the NOF and Canadian strategies, and the sensitivity and specificity of the screening and treatment strategies in identifying women who subsequently experienced osteoporotic fractures during follow-up.
Methods
The Women’s Health Initiative (WHI) Study Participants
Between 1993 and 1998, the WHI enrolled 161,808 postmenopausal women aged 50–79 years at 40 Clinical Centers throughout the United States. The WHI study methods have been previously described.(9) Participants were free from serious cardiac, pulmonary, renal, and hepatic conditions and had at least three years’ life expectancy. The WHI Clinical trials evaluated three distinct interventions: a low-fat eating pattern, hormone therapy (HT), and calcium and vitamin D supplementation. The Women’s Health Initiative Observational Study examined the predictors and natural course of important causes of morbidity and mortality in postmenopausal women. To compare the U.S. and Canadian screening strategies, we examined data from all participants of the WHI Clinical Trials and Observational Studies.
To assess treatment strategies, which requires knowledge of BMD values, we used information from a subset of the WHI participants who participated in the WHI Bone Density Substudy. In the WHI Bone Density Substudy, participants at three of the 40 WHI clinical centers (Tucson/Phoenix, Arizona; Pittsburgh, Pennsylvania; and Birmingham, Alabama; n = 10,833) underwent hip and anteroposterior lumbar spine dual-energy x-ray absorptiometry on a Hologic QDR2000 or 4500 W machine (Hologic) at the time of enrollment. Standard protocols were used for positioning and analysis. Quality assurance procedures included cross-clinic hip and spine phantom scans, further evaluation of scans with specific problems, and review of a random sample of all scans.(10) Of these, 8,134 participants provided at least 10 years of follow-up, information required for the NOF algorithm, and were not taking exclusionary medications.
Of the 161,808 participants of the WHI Observational Study (WHI-OS) and WHI Clinical Trials (WHI-CT), we excluded data from 1) participants using osteoporosis medications (bisphosphonates, calcitonin, parathyroid hormone, selective estrogen receptor modulators) or somatostatin agents at baseline (n = 3,660) and 2) participants providing fewer than 10 years of exposure time (without experiencing a fracture event)(n = 40,441)(Supplemental Figure 1). The final study sample consisted of 117,707 women for comparison of the screening strategies and 8,134 (the subset of women for whom BMD information was available, see below) for comparison of the treatment strategies. We did not exclude participants who were HT users, but we accounted for HT use in sensitivity analyses.
Information regarding demographic information, osteoporosis risk factors, fracture prior to WHI enrollment, medical history, reproductive history, and medication use was collected on baseline self-assessment questionnaires. Weight and height were measured at baseline using standardized protocols.(9) Each institution obtained human subjects committee approval. All participants provided written informed consent.
Ascertainment of Incident Fractures
Fractures were self-reported by participants on annual (WHI-OS) and semi-annual (WHI-CT) questionnaires. Participants were asked: “Has a doctor told you for the first time that you have a new broken, crushed, or fractured bone? Which bone did you break?” Response choices included: hip, upper leg (not hip), pelvis, knee (patella), lower leg or ankle, foot (not toe), spine or back (vertebra), lower arm or wrist, hand (not finger), elbow, and upper arm or shoulder.
All hip fractures were subsequently medical record-confirmed by study physicians; other fractures were self-reported. We defined major osteoporotic fracture as clinical spine, hip, lower arm/wrist, or upper arm fracture.
Calculation of 10-year predicted absolute risk of fracture using FRAX
The FRAX-predicted 10-year absolute risk of MOF (with and without BMD information) and hip fractures (with BMD information) were calculated for each participant by the World Health Organization (WHO) Collaborating Centre for Metabolic Bone Disease, using the race-specific FRAX tool (U.S. FRAX version 3.0).(11–13)
Assessment of Candidates for BMD testing
Information regarding osteoporosis risk factors (FRAX risk factors and risk factors included in the Canadian screening guideline [Table 1]) was obtained from baseline self-assessment questionnaires.
As recommended by the USPSTF and Canadian guidelines, all women ≥ 65 years were considered candidates for BMD testing. As suggested by the USPSTF screening strategy, women aged 50–64 years with FRAX-predicted 10-year MOF risk ≥ 8.4% (using FRAX without BMD) were classified as candidates for BMD testing.(2) For the Canadian screening strategy, women aged 50– 64 years were classified as candidates for BMD testing if they had any of the clinical risk factors listed in Table 1.(5) Supplemental Tables 1a and 1b provide a detailed description of risk factor variables in the screening strategies. In the WHI study, because of the questionnaire wording, we could only ascertain current glucocorticoid use at baseline rather than use in the past year as stated in the Canadian strategy. Questionnaires asked about self-reported previous fractures since the age of 55, whereas the Canadian strategy ascertains fragility fracture after age 40.
Assessment of Treatment Candidates
For the Canadian strategy, we referenced the CAROC risk table that classifies MOF risk into low-, moderate-, and high-risk categories according to age and femoral neck BMD T-score category (Supplemental Table 2a).(5) The presence of a prior fragility fracture after age 40 or recent prolonged use of systemic glucocorticoids increases fracture risk into the next highest category independent of BMD. Those with a prior hip fracture, vertebral fracture, or more than one fragility fracture are considered to be in the high risk category, regardless of BMD or the presence or absence of other risk factors.(5) All women with high fracture risk were classified as treatment candidates. Women with moderate fracture risk were classified as treatment candidates if they had additional clinical risk factors (Table 2).
For the NOF strategy, participants were classified as pharmacotherapy candidates if they met one of the following criteria: 1) BMD T-score ≤ −2.5 at the lumbar spine, femoral neck, or total hip, or 2) a combination of a BMD T-score between −1 and −2.5 at the lumbar spine, femoral neck, or total hip and a 10-year FRAX-predicted risk ≥3% for hip fracture or ≥20% for MOF.(1) A detailed description of risk factor variables in the treatment strategies is presented in Supplemental Tables 2b and 2c.
Statistical Analysis
For the USPSTF and the Canadian screening strategies, we calculated the proportion of women who were classified as candidates for BMD testing at baseline. Similarly, we calculated the proportion of women who would have been classified as osteoporosis pharmacotherapy candidates under the NOF and the Canadian treatment strategies. Next, in our primary analyses, we determined the yield of each strategy in identifying women for whom BMD testing and consideration of drug therapy may be appropriate. Specifically, among the women who experienced incident MOF during the 10-year follow-up period, we calculated the sensitivity, specificity, and positive predictive value (PPV) of the two strategies for the identification of screening and treatment candidates. Sensitivity of the screening strategies was calculated as the number of women with incident MOF who qualified for BMD testing divided by the total number of women with incident MOF. Specificity of the screening strategies was calculated as the number of women without incident MOF who did not qualify for BMD testing divided by the total number of women without incident MOF. Similar formulas were used for the treatment strategies.
We made the a priori decision to stratify the sensitivity and specificity of the two strategies for the identification of candidates for BMD testing, and treatment candidates, by race/ethnicity and 5-year age group, using chi-square tests to compare the proportions of women identified for BMD testing or treatment within age strata. We also decided a priori to separately examine the sensitivity and specificity of the screening and treatment strategies in the subset of women with BMD T-score ≤ −2.5.
In a sensitivity analysis, we repeated the above-described analyses after excluding data from participants who reported initiating osteoporosis medication any time during study follow-up. In additional sensitivity analyses, we stratified our results according to baseline use vs. non-use of HT. We defined HT users as those who self-reported HT use at baseline or were assigned to receive HT in the WHI Hormone Therapy Trial.
All statistical analyses were performed using SAS for Windows Version 9.4 (SAS Institute Inc., Cary, NC). P values < 0.05 were regarded as statistically significant.
Results
At baseline, mean (standard deviation [SD]) participant age was 62.7 (7.1) years, body mass index was 27.8 (5.8) kg/m2, 86.0% of the 117,707 participants were white, 31.5% had fallen at least once in the year prior to baseline, and 8.6% of participants reported a fracture prior to the baseline WHI study visit (Table 3).
Table 3.
Baseline Characteristics of both the Overall and BMD samples*
| n | % | n | % | |
|---|---|---|---|---|
| 75–79 | 6197 | 5.3 | 406 | 5.0 |
| Other / unknown | 4343 | 3.7 | 148 | 1.8 |
| ≥ 30 | 33967 | 28.9 | 2514 | 30.9 |
| Yes | 7263 | 6.2 | 575 | 7.1 |
| Yes | 85534 | 72.7 | 5192 | 63.8 |
| Yes | 58316 | 49.5 | 3801 | 46.7 |
| Yes | 14177 | 31.5 | 2416 | 29.7 |
| Yes | 15171 | 12.9 | 986 | 12.1 |
| Yes | 24127 | 20.5 | 1930 | 23.7 |
| Yes | 1094 | 0.9 | 1094 | 13.4 |
| Yes | 1421 | 1.2 | 91 | 1.1 |
| Yes | 10090 | 8.6 | 647 | 8.0 |
| Yes | 5100 | 4.3 | 366 | 4.5 |
| Yes | 376 | 0.3 | 24 | 0.3 |
| Yes | 2790 | 2.4 | 224 | 2.8 |
| Yes | 3575 | 3.0 | 239 | 2.9 |
| Yes | 792 | 0.7 | 57 | 0.7 |
BMD denotes bone mineral density
Self-reported at baseline or assignment to active hormone therapy group in the Women’s Health Initiative Hormone Therapy Trials
Lumbar spine T-score at least 1SD lower than femoral neck T-score
The proportion of women identified for BMD testing by the screening strategies
Among women aged 50–64, the USPSTF strategy identified 23.2% (16,136/69,687) and the Canadian strategy identified 52.5% (36,586 / 69,687) for BMD testing. Both the NOF and Canadian strategies identified 100% of women aged ≥65 years for BMD testing (because both strategies advocate BMD testing in all women aged ≥65 years).
Performance of screening strategies in women with incident MOF
Among women aged 50–64 years who experienced MOF during the 10-year follow-up period, the USPSTF screening strategy would have identified 7% of women 50–54 years-old, and 50% of women 60–64 years-old, for BMD testing at baseline (Table 4).
Table 4.
Proportion of Women with Major Osteoporotic Fractures during 10-year Follow-up who are Identified for Bone Mineral Density Testing under the USPSTF and Canadian Strategies*
| Strategy | Age Group | Total n | Identified for screening, n (%) | MOF Events, n (%) | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) |
|---|---|---|---|---|---|---|---|
| All Participants | 115257 | 62546 (54%) | 14105 (12%) | 72.5 (71.8, 73.3) | 48.3 (48.0, 48.6) | 16.4 (16.1, 16.6) | |
| 75–79 | 5907 | 5907 (100%) | 1732 (29%) | 100.0 (–) | 0.0 (–) | 29.3 (28.2, 30.5) | |
| All Participants | 115257 | 82599 (72%) | 14105 (12%) | 82.9 (82.2, 83.5) | 29.9 (29.6, 30.2) | 14.1 (13.9, 14.4) | |
| 75–79 | 5907 | 5907 (100%) | 1732 (29%) | 100.0 (–) | 0.0 (–) | 29.3 (28.2, 30.5) |
Major osteoporotic fractures (MOF) are defined as adjudicated hip fracture or self-report fracture of lower arm/wrist, spine/back, or upper arm/shoulder. USPSTF denotes United States Preventive Services Task Force
In contrast, among women who experienced MOF during follow-up, the Canadian screening strategy would have identified 54% of women 50–54 years-old, and 61% of women 60–64 years-old, for BMD testing at baseline. For each age subgroup (50–54 years and 60–64 years), the Canadian strategy identified significantly more women who experienced MOF during follow-up than the USPSTF strategy (chi-square p-value <0.001 for both age groups). Conversely, among women 50–64 years-old who experienced MOF during follow-up, the specificity of the USPSTF strategy for identifying BMD testing candidates was consistently higher than that of the Canadian strategy. The PPV of both screening strategies was low, 16% for USPSTF and 14% for the Canadian strategy, and was lowest in the women aged 50–54 years.
In the younger women with BMD T-score ≤ −2.5, the Canadian screening strategy would have identified a much larger proportion for BMD testing than would the USPSTF strategy (Supplemental Table 3). For example, among women aged 50–54 years, the sensitivity was 5% for the USPSTF strategy and 67% for the Canadian strategy for identifying women with T-score ≤ −2.5 as BMD testing candidates (chi-square P value <0.001). Among women aged 50–64 years with a BMD T-score ≤ −2.5, 23% of women were identified for BMD testing under the USPSTF strategy, whereas a higher proportion (52%) were identified for BMD testing under the Canadian strategy.
Among women ≥ 65 years with a BMD T-score ≤ −2.5, 100% of women were identified for BMD testing under both strategies- both strategies advocate routine BMD testing in this age group. As a result, for women aged ≥ 65 years, specificity was 0% for both strategies.
Identification of women with incident MOF as treatment candidates
Among women aged 50–64 years, the NOF treatment strategy identified a larger proportion of women for drug treatment to prevent fractures (16%) than did the Canadian treatment strategy (3%) (chi-square P value <0.001). Among women aged 65 years and over, the NOF strategy also identified a larger proportion of women for drug treatment to prevent fractures 45% than did the Canadian strategy (21.3%) (chi-square P value <0.001).
Among all women aged ≥ 50 years who experienced MOF during the 10-year follow-up period, the NOF strategy had a higher sensitivity for identifying them as treatment candidates at baseline, but the sensitivity was low for both strategies (NOF 50%; Canadian 25%)(Table 5). Specificity was higher for the Canadian strategy than for the NOF strategy.
Table 5.
Proportion of Women with Major Osteoporotic Fractures during 10-year Follow-up who are Identified for Treatment under the NOF and Canadian Strategies*
| Strategy | Age Group | Total n | Identified for treatment, n (%) | MOF Events, n (%) | Sensitivity (95% CI) | Specificity (95% CI) | Positive Predictive Value (95% CI) |
|---|---|---|---|---|---|---|---|
| All Participants | 7926 | 2184 (28%) | 1034 (13%) | 50.3 (47.2, 53.3) | 75.9 (74.8, 76.9) | 23.8 (22.0, 25.6) | |
| 75–79 | 388 | 288 (74%) | 122 (31%) | 82.8 (76.0, 89.6) | 29.7 (24.2, 35.2) | 35.1 (29.5, 40.6) | |
| All Participants | 7926 | 826 (10%) | 1034 (13%) | 24.7 (22.0, 27.3) | 91.7 (91.1, 92.4) | 30.9 (27.7, 34.0) | |
| 75–79 | 388 | 142 (37%) | 122 (31%) | 51.6 (42.6, 60.6) | 70.3 (64.8, 75.8) | 44.4 (36.1, 52.6) |
Major osteoporotic fractures (MOF) are defined as adjudicated hip fracture or self-report fracture of lower arm/wrist, spine/back, or upper arm/shoulder. NOF denotes National Osteoporosis Foundation
Among 406 women aged 50–64 years who subsequently experienced incident MOF, the NOF strategy identified 119 (29%), and the Canadian strategy identified 35 (9%) women for treatment. The sensitivity of the treatment strategies increased with age to a maximum of 83% for the NOF strategy and 52% for the CAROC-based strategy among women aged ≥ 65 years. Conversely, specificity of both strategies decreased with age.
Of the women aged 50–64 who had BMD T-score between −1 and −2.5, 3% (76/2439) met the NOF criteria for treatment according to FRAX-predicted fracture risk thresholds (≥3% hip fracture risk and/or ≥20% MOF risk).
Sensitivity analyses: Race/ethnicity and HT use
In a sensitivity analysis, the sensitivity of each of the strategies for identifying high-risk screening and treatment candidates (i.e., women who experienced MOF during study follow-up, women with BMD T-score ≤−2.5) was lower in users than in non-users of HT at baseline (Supplemental Table 4). The lower sensitivity of the screening strategies in HT users than in HT nonusers occurred largely because HT use was confounded by age, i.e. HT users were younger than nonusers of HT. Stratification by age subgroup removed the differences in sensitivity of the screening strategies between HT users and nonusers (Supplemental Table 5).
The pattern of lower sensitivity for the USPSTF strategy than for the Canadian strategy for identifying high-risk groups of women to receive BMD testing at baseline was present for both HT users and non-users. In addition, the pattern of higher sensitivity of the NOF strategy than the Canadian strategy in identifying high-risk women as being treatment candidates was present for both users and non-users of HT at baseline.
Figure 1 illustrates the ability of the two strategies to identify for initial BMD testing the women aged 50–64 years who subsequently experienced incident MOF during the 10-year follow-up period, stratified by race/ethnicity. Among women aged 50–64 years, the sensitivity was low for all racial/ethnic groups. The sensitivity of the USPSTF screening strategy was higher (and specificity was lower) for white women than for women in other racial/ethnic groups. In contrast, there were no evident differences in sensitivity or specificity of the Canadian screening strategy across racial/ethnic categories.
Figure 1.
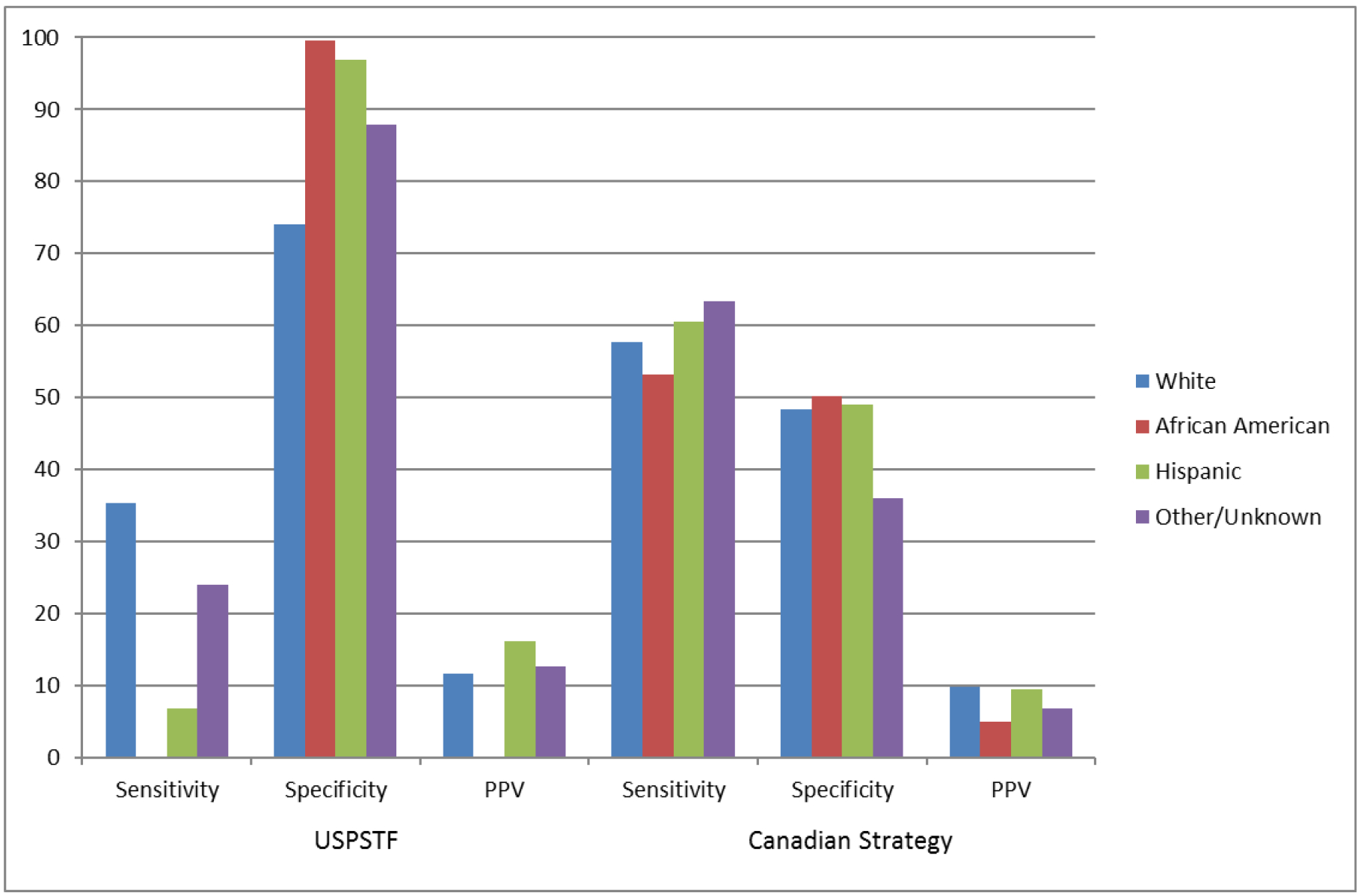
Identification of women aged 50–64 years who experienced major osteoporotic fractures during study follow-up: Sensitivity and specificity of screening strategies by race/ethnicity. USPSTF = United States Preventive Service Task Force; PPV = positive predictive value
For identifying women who experienced incident MOF during the 10-year follow-up period as treatment candidates, although the sensitivity of both strategies was low, the sensitivity of the NOF strategy was lower in Hispanic women than for white or African American women; in contrast, the sensitivity of the Canadian strategy was lower in African American women than in white or Hispanic women, in both the younger (Supplemental Figure 2a) and older (Supplemental Figure 2b) age groups.
Discussion
This study examined two screening and two treatment strategies for osteoporosis in a large prospective cohort of postmenopausal women. The careful assessment of current screening and treatment guidelines is particularly important given the recent plateau in the decline in hip fracture rates in the U.S.(8) Among women aged 50–64 years, the USPSTF osteoporosis screening strategy identified a much lower proportion of women for BMD testing than did the Canadian strategy. Of the women aged 50–64 years who actually experienced incident MOF during the 10-year study follow-up, the Canadian strategy identified a higher proportion for BMD testing than the USPSTF strategy but this greater sensitivity was accompanied by lower specificity. For the identification of treatment candidates among women aged 50–64 years, neither strategy performed well, but the NOF strategy identified a higher proportion for treatment among the women who subsequently experienced incident MOF than did the Canadian strategy. However, the higher sensitivity of the NOF strategy was balanced by its poorer specificity. The strategies performed more favorably in women aged ≥ 65 years.
To our knowledge, no previously published studies in the U.S. have compared the potential implications of applying the U.S. and Canadian osteoporosis screening and treatment guidelines in a large cohort of U.S. young postmenopausal women. Although it did not compare Canadian and U.S. screening or treatment strategies, one Canadian study did examine potential implications of using CAROC versus FRAX in relation to numbers of persons at high-risk for fracture who would be candidates for treatment.(14) In the large Canadian Manitoba Registry Cohort of men and women aged 50 and older (mean follow-up of 9.8 years for incident fractures), FRAX and CAROC were used to classify 10-year major osteoporotic fracture risk into categories (<10%, 10–20%, and >20%) in persons referred for osteoporosis screening.(14) In that study, moving from CAROC to FRAX resulted in a significant improvement in risk classification, i.e. in fracture risk prediction.(14) Although the Manitoba study did not report results for the subgroup of women aged 50–64 separately, the improvement in risk classification in moving from CAROC to FRAX was evident among women and among all persons <65 years old.(14) The same report also examined the potential treatment implications of using FRAX instead of CAROC. On the assumption that only persons at high risk would be treated, the use of FRAX versus CAROC would result in statistically significant improvement in the net reclassification index (i.e. more accurate identification of potential treatment candidates).(14) The Manitoba study focused on MOF probability and did not apply all NOF criteria.
One likely factor that contributes to the observed low positive predictive value of the screening and treatment strategies among women aged 50–64 years in this study is the low absolute incidence of fracture among women aged 50–64 years. Positive predictive value decreases as absolute fracture incidence decreases. Future studies should test whether measurement of trabecular bone score (TBS), a texture measure derived from the lumbar spine DXA image which has been associated with fracture risk independently of BMD in several cohorts, may be beneficial in refining the selection of treatment candidates in this age group.(15)
Our findings have the potential to challenge clinical paradigms and inform clinical guidelines in the field of osteoporosis. The sensitivity of the screening strategies to identify women aged 50–64 years who experienced MOF during the follow-up was low for both the USPSTF and the Canadian screening strategies. Sensitivity was especially low among women between 50 and 54 years-old (7% for USPSTF, 54% for the Canadian strategy), although low sensitivity was counterbalanced by high specificity (96% for USPSTF, 49% for Canadian strategy). Also, both screening strategies had low sensitivity to identify women aged 50–64 years-old who had BMD T-scores ≤ −2.5.
In the current study, our results differed across racial/ethnic groups. Among women aged 50–64 years, the sensitivity of the USPSTF screening strategy in identifying for BMD testing the women who subsequently experienced MOF during the 10-year follow-up period was higher, and specificity was lower, for white women than for women in other racial/ethnic groups. The sensitivity of the treatment strategies for identifying women who experienced MOF during follow-up also varied by race/ethnicity, with lower sensitivity in Hispanic women for the NOF strategy and lower sensitivity in African American women for the CAROC treatment strategy.
Current screening strategies for young postmenopausal women do not separately consider HT users, but the lower sensitivity of the screening strategies in HT users than in HT nonusers in the current study indicates that such consideration may be warranted. Although the prevalence of HT use is lower now than at the time WHI was initiated, our results suggest that screening strategies for younger postmenopausal women need reevaluation. The lower sensitivity of the screening strategies in HT users than in HT nonusers occurred largely because HT use was confounded by age. That is, HT users were younger than nonusers of HT. Stratification by age subgroup removed the differences in sensitivity of the screening strategies between HT users and nonusers.
Although we found that the USPSTF and Canadian strategies had low sensitivity for identifying for BMD testing the women who would subsequently experience MOF, there are other currently-available models that predict who has BMD T-score ≤−2.5 or who will fracture. Besides FRAX, the Osteoporosis Self-Assessment Tool (OST) is another tool recommended for osteoporosis risk assessment by the USPSTF guideline.(2) In our prior study, OST score <2 identified 80% of women aged 50–64 years who had femoral neck BMD T-score −2.5.(12) Therefore, the use of OST may be a reasonable strategy for identification of young postmenopausal women with BMD T-score in the osteoporotic range.
Our study has several strengths, including the large number of study participants, the collection of detailed information regarding osteoporosis risk factors, information regarding the anatomical location of fractures, the objective measurements of height and weight, and the prospective study design providing incident fracture data over 10 years of follow-up.
Our study has limitations. Although the study cohort was well-characterized with regard to osteoporosis risk factors, certain information included in the Canadian screening and CAROC-based risk-assessment algorithms was not collected in the WHI. For example, for women aged 50–64 years, the Canadian guideline suggests screening of women with fragility fracture after age 40 years. However, the WHI data only allowed us to ascertain whether fractures prior to baseline occurred < age 55 years or ≥ age 55 years, and the WHI questionnaire items did not differentiate between fragility and non-fragility fractures. Sixteen percent of participants reported fracture (excluding finger and toe fracture) prior to age 55 on their baseline questionnaires. It is difficult to predict the potential influence of the lack of information on fracture between ages 40 and 55 years on our results, but this may have caused our results regarding the sensitivity of the Canadian screening strategy to be falsely lowered. Also, the use of glucocorticoids by WHI participants was only captured if the participants reported currently taking glucocorticoids at the baseline visit, whereas the Canadian guideline considers prolonged current or previous (at least 3 months during the previous year) use of glucocorticoid therapy (daily prednisone-equivalent dose 7.5 mg or greater) as a risk factor. WHI did not collect information regarding hyperparathyroidism, type I diabetes, osteogenesis imperfecta, uncontrolled hyperthyroidism, or Cushing’s disease. We believe that these limitations were not likely to have substantial influence on our results because the overall prevalence of these conditions would be low. Finally, future studies are needed to address the dilemma that initiation of antiresorptive therapy in women aged 50–64 years may result in duration-dependent adverse effects (e.g. atypical femoral fractures) that may lead clinicians to recommend discontinuation of pharmacotherapy as these women reach older age, when they are at especially higher risk of fracture.
In conclusion, current osteoporosis screening and treatment guidelines (USPSTF, NOF, Canadian) have low sensitivity in identifying younger postmenopausal women who subsequently experience MOF. The Canadian screening strategy has a higher sensitivity (but lower specificity) than the USPSTF screening strategy for identifying for BMD testing the women who will subsequently experience MOF. In contrast, the U.S. NOF treatment strategy has a higher sensitivity (but lower specificity) than the Canadian treatment strategy for identifying for therapy the women will subsequently experience MOF. Our findings highlight the difficulty in developing validated osteoporosis screening and treatment strategies that show acceptable performance in women age 50–64 years. New screening and treatment algorithms are needed.
Supplementary Material
Acknowledgements
The authors thank the participants of the Women’s Health Initiative Study for their time and effort.
The WHI program is funded by the National Heart, Lung, and Blood Institute, National Institutes of Health, U.S. Department of Health and Human Services through contracts HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN26820160000 3C, and HHSN268201600004C.
Program Office (National Heart, Lung, and Blood Institute, Bethesda, Maryland):
Jacques Rossouw, Shari Ludlam, Joan McGowan, Leslie Ford, and Nancy Geller
Clinical Coordinating Center (Fred Hutchinson Cancer Research Center, Seattle, WA):
Garnet Anderson, Ross Prentice, Andrea LaCroix, and Charles Kooperberg
Investigators and Academic Centers:
(Brigham and Women’s Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian Limacher; (University of Iowa, Iowa City/Davenport, IA) Jennifer Robinson; (University of Pittsburgh, Pittsburgh, PA) Lewis Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally Shumaker; (University of Nevada, Reno, NV) Robert Brunner; (University of Minnesota,Minneapolis, MN) Karen L. Margolis
Footnotes
Disclosures: All authors have no disclosures relevant to this manuscript.
Contributor Information
Carolyn J. Crandall, Dept. of Medicine, David Geffen School of Medicine at University of California, Los Angeles, CA,.
Joseph Larson, Fred Hutchinson Cancer Research Center, Seattle, WA,.
JoAnn E. Manson, Division of Preventive Medicine, Brigham and Women’s Hospital, Harvard Medical School, Boston, MA,.
Jane A. Cauley, Dept. of Epidemiology, Graduate School of Public Health, University of Pittsburgh, Pittsburgh, PA,.
Andrea LaCroix, Dept. of Family and Preventive Medicine, University of California, San Diego, CA,.
Jean Wactawski-Wende, Dept. of Epidemiology and Environmental Health, University at Buffalo, the State University of New York, Buffalo, NY,.
Mridul Datta, Dept. of Food Science and Human Nutrition, Iowa State University, Ames, IA,.
Maryam Sattari, Dept. of Medicine, University of Florida College of Medicine, Gainesville, FL,.
John T. Schousboe, Research Investigator, HealthPartners Institute, Park Nicollet Clinic and University of Minnesota, Minneapolis, MN,.
William D. Leslie, Dept. of Medicine, University of Manitoba, Winnipeg, Canada,.
Kristine E. Ensrud, Division of Epidemiology and Community Health, University of Minnesota, Minneapolis, MN,.
REFERENCES
- 1.National Osteoporosis Foundation. Clinician’s Guide to Prevention and Treatment of Osteoporosis. Washington, DC: National Osteoporosis Foundation; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Force USPST, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, et al. Screening for Osteoporosis to Prevent Fractures: US Preventive Services Task Force Recommendation Statement. JAMA. June 26 2018;319(24):2521–31. Epub 2018/06/28. [DOI] [PubMed] [Google Scholar]
- 3.Kanis JA, Oden A, Johnell O, Johansson H, De Laet C, Brown J, et al. The use of clinical risk factors enhances the performance of BMD in the prediction of hip and osteoporotic fractures in men and women. Osteoporos Int. August 2007;18(8):1033–46. [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization Collaborating Centre for Metabolic Bone Diseases UoS, UK,. FRAX ® WHO Fracture Risk Assessment Tool version 3.8. Sheffield, UK: University of Sheffield, UK,. [Google Scholar]
- 5.Papaioannou A, Morin S, Cheung AM, Atkinson S, Brown JP, Feldman S, et al. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. November 23 2010;182(17):1864–73. Epub 2010/10/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leslie WD, Berger C, Langsetmo L, Lix LM, Adachi JD, Hanley DA, et al. Construction and validation of a simplified fracture risk assessment tool for Canadian women and men: results from the CaMos and Manitoba cohorts. Osteoporos Int. June 2011;22(6):1873–83. Epub 2010/10/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wright NC, Looker AC, Saag KG, Curtis JR, Delzell ES, Randall S, et al. The recent prevalence of osteoporosis and low bone mass in the United States based on bone mineral density at the femoral neck or lumbar spine. J Bone Miner Res. November 2014;29(11):2520–6. Epub 2014/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michael Lewiecki E, Wright NC, Curtis JR, Siris E, Gagel RF, Saag KG, et al. Hip fracture trends in the United States, 2002 to 2015. Osteoporos Int. March 2018;29(3):717–22. Epub 2017/12/29. [DOI] [PubMed] [Google Scholar]
- 9.Design of the Women’s Health Initiative clinical trial and observational study. The Women’s Health Initiative Study Group. Control Clin Trials. February 1998;19(1):61–109. [DOI] [PubMed] [Google Scholar]
- 10.LaCroix AZ, Cauley JA, Pettinger M, Hsia J, Bauer DC, McGowan J, et al. Statin use, clinical fracture, and bone density in postmenopausal women: results from the Women’s Health Initiative Observational Study. Ann Intern Med. July 15 2003;139(2):97–104. [DOI] [PubMed] [Google Scholar]
- 11.Kanis JA, Johnell O, Oden A, Johansson H, McCloskey E. FRAX and the assessment of fracture probability in men and women from the UK. Osteoporos Int. April 2008;19(4):385–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crandall CJ, Larson J, Gourlay ML, Donaldson MG, LaCroix A, Cauley JA, et al. Osteoporosis screening in postmenopausal women 50 to 64 years old: comparison of US Preventive Services Task Force strategy and two traditional strategies in the Women’s Health Initiative. J Bone Miner Res. July 2014;29(7):1661–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crandall CJ, Larson JC, Watts NB, Gourlay ML, Donaldson MG, LaCroix A, et al. Comparison of fracture risk prediction by the US Preventive Services Task Force strategy and two alternative strategies in women 50–64 years old in the Women’s Health Initiative. J Clin Endocrinol Metab. December 2014;99(12):4514–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leslie WD, Majumdar SR, Lix LM, Josse RG, Johansson H, Oden A, et al. Direct comparison of FRAX(R) and a simplified fracture risk assessment tool in routine clinical practice: a registry-based cohort study. Osteoporos Int. September 2016;27(9):2689–95. Epub 2016/04/25. [DOI] [PubMed] [Google Scholar]
- 15.Silva BC, Leslie WD. Trabecular Bone Score: A New DXA-Derived Measurement for Fracture Risk Assessment. Endocrinol Metab Clin North Am. March 2017;46(1):153–80. Epub 2017/01/31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


