Abstract
Purpose
Peroxisome proliferator-activated receptor gamma (PPAR-γ) has a key role in hepatic fibrogenesis by virtue of its effect on the hepatic stellate cells (HSCs). Although many studies have shown that PPAR-γ agonists inhibit liver fibrosis, the mechanism remains largely unclear, especially regarding the cross-talk between PPAR-γ and other potent fibrogenic factors.
Methods
This experimental study involved 25 male Wistar rats. Twenty rats were subjected to bile duct ligation (BDL) to induce liver fibrosis, further divided into an untreated group (BDL; n=10) and a group treated with the PPAR-γ agonist thiazolidinedione (TZD), at 14 days post-operation (BDL+TZD; n=10). The remaining 5 rats had a sham operation (sham; n=5). The effect of PPAR-γ agonist on liver fibrosis was evaluated by histopathology, protein immunohistochemistry, and mRNA expression quantitative polymerase chain reaction.
Results
Histology and immunostaining showed markedly reduced collagen deposition, bile duct proliferation, and HSCs in the BDL+TZD group compared to those in the BDL group (p<0.001). Similarly, significantly lower mRNA expression of collagen α-1(I), matrix metalloproteinase-2, platelet-derived growth factor (PDGF)-B chain, and connective tissue growth factor (CTGF) were evident in the BDL+TZD group compared to those in the BDL group (p=0.0002, p<0.035, p<0.0001, and p=0.0123 respectively). Moreover, expression of the transforming growth factor beta1 (TGF-β1) was also downregulated in the BDL+TZD group (p=0.0087).
Conclusion
The PPAR-γ agonist inhibits HSC activation in vivo and attenuates liver fibrosis through several fibrogenic pathways. Potent fibrogenic factors such as PDGF, CTGF, and TGF-β1 were downregulated by the PPAR-γ agonist. Targeting PPAR-γ activity may be a potential strategy to control liver fibrosis.
Keywords: Peroxisome proliferator-activator receptor gamma, Liver cirrhosis, Hepatic stellate cell, Connective tissue growth factor, Platelet-derived growth factor, Transforming growth factor beta1
INTRODUCTION
Fibrosis occurs as a consequence of chronic liver disease [1,2]. Cirrhosis is the end-stage of liver fibrosis that is marked by a distortion in the liver architecture and vasculature resulting from an imbalance of fibrogenesis over fibrolysis, often as a result of an incurable insult [2,3]. Liver injury activates the hepatic stellate cells (HSCs) that initiate perpetuating signals of migration and proliferation at the injury site [3,4].
The available treatments for liver fibrosis focus only on the etiologies of hepatic insult, while patients with incurable liver diseases require fibrosis-specific therapies [3]. Animal models and human clinical trials have identified several mechanisms that decrease fibrogenesis, including interferon-γ, angiotensin II antagonists, and the nuclear receptor peroxisome proliferator-activated receptor gamma (PPAR-γ). Targets of anti-fibrotic therapies can be (1) fibrogenic growth factor, cytokines, and their mediators; (2) intracellular profibrogenic pathways in HSCs and cells upstream of their activation; or (3) stimulation of the fibrolytic process to reverse existing fibrosis [2,5,6].
PPAR-γ is a nuclear receptor expressed in vascular smooth muscle cells and HSCs. It plays a role in the transcriptional control of cell growth, differentiation, and liver fibrosis. Previous studies have reported a decrease in PPAR-γ expression during HSC activation. Conversely, overexpression or modulation of PPAR-γ has been proven to attenuate HSC activation and reduce liver fibrosis [7,8,9]. Existing studies prove that PPAR-γ agonists inhibit HSC activation, thereby reducing the expression of α-smooth muscle actin (α-SMA), collagen, and transforming growth factor beta1 (TGF-β1). This ultimately reduces cell proliferation and the development of fibrosis. Cross-regulation by PPAR-γ of key fibrogenic factors such as TGF-β1, platelet-derived growth factor (PDGF), and hepatocyte growth factor (HGF) signaling, and farnesoid X receptor have been identified as mechanisms by which PPAR-γ inhibits liver fibrosis [10,11,12,13].
The precise molecular mechanism underlying the anti-fibrotic effect of PPAR-γ in liver fibrosis remains largely unknown. This study aimed to investigate the effect of PPAR-γ agonists in liver fibrosis, using thiazolidinedione (TZD) in an animal model of cholestatic liver fibrosis. We hypothesize that, apart from TGF-β1 and PDGF, fibrosis inhibition by PPAR-γ agonists is also mediated by connective tissue growth factor (CTGF), a potent fibrogenic factor.
MATERIALS AND METHODS
Animal model of liver fibrosis and treatment protocol
The induction of liver injury and fibrosis was initiated by common bile duct ligation (BDL) and sectioning between ligations using aseptic techniques as per previously described methods [10]. Animal care and surgical procedures were approved by Kyushu University Animal Ethics Committee and were performed according to the guidelines and regulatory details regarding the use of laboratory animals (Approval No. A23-108-0). Rats were fed with a standard commercial rat diet ad libitum, with free access to drinking water. The PPAR-γ agonist TZD (10 mg/kg) was administered once daily via gavage as the treatment drug.
Twenty normal male Wistar rats aged 7–8 weeks were subjected to BDL, before being randomly divided into two groups, BDL without TZD (BDL; n=10) and BDL receiving TZD (BDL+TZD; n=10). Five additional rats underwent laparotomy without the common BDL procedure and were used as a control group (sham; n=5). Starting on day 14, all BDL+TZD rats received 10 mg/kg of TZD once daily via gavage.
All animals were sacrificed 4 weeks post-operation under pentobarbital sodium anesthesia (40 mg/kg, intraperitoneally). Blood samples and a wedge biopsy of the liver were collected from each rat. Liver samples were fixed in 10% formaldehyde for a period of at least 24 hours before being embedded in paraffin and sectioned at 5-μm thickness. Tissue sections were stained with hematoxylin and eosin (H&E) for fibrosis grading and Sirius Red for evaluation of collagen deposition.
Immunohistochemical staining
The primary antibodies used were mouse monoclonal anti-α-SMA (clone IA4, diluted 1:5000; Sigma Immunochemicals, St. Louis, MO, USA), rabbit polyclonal anti-PDGF-BB (diluted 1:500; Abcam, Cambridge, MA, USA), and rabbit polyclonal anti-CTGF (diluted 1:2000; Abcam).
All specimens were immunohistochemically stained using the DAKO EnVision System (Dako Ltd., High Wycombe, Bucks, UK) according to the manufacturer's instructions. Specimens were incubated with the peroxidase-labeled polymer conjugated to goat anti-mouse or anti-rabbit immunoglobulins for 40 minutes, and visualized using diaminobenzidine solution. The tissue sections were then counterstained with Mayers Hematoxylin (Muto, Tokyo, Japan) before mounting onto a cover slip.
Detection of mRNA expression using quantitative real-time polymerase chain reaction
The following rat primer sequences were used: collagen α-1(I): sense: 5′-CTCCCAGCGGTGGTTATGAC-3′, antisense: 5′-TGCTGGCTCAGGCTCTTGA-3′; matrix metalloproteinase 2 (MMP-2): sense: 5′-ACCGTCGCCCATCATCAA-3′, antisense: 5′TTGCACTGCCAACTCTTTGTCT-3′; PDGF-B chain: sense: 5′-CCGCTCCTTTGATGACCTTC-3′, antisense: 5′-GCTCAGCCCCATCTTCGTC-3′; CTGF: sense: 5′-CAATACCTTCTGCAGGCTGGA-3′, antisense: 5′-TTAGCCCGGTAGGTCTTCACA-3′.
Total RNA was extracted from the livers using TRIzol (Invitrogen, Carlsbad, CA, USA), followed by DNase treatment (Promega, Madison, WA, USA), then RNA purification using the Qiagen RNeasy Mini kit (Qiagen Group, Valencia, CA, USA). One microgram of the purified RNA was reverse transcribed using the ReverTra Ace quantitative polymerase chain reaction (qPCR) RT kit (Toyobo Co. LTD., Osaka, Japan). Diluted cDNA samples (1:5 dilution) were amplified by real-time RT PCR using the SYBR Premix Ex Taq II (RR081A; Takara Bio, Shiga, Japan) on a Light Cycler (Roche Diagnostic) detection PCR system according to the manufacturer's instructions. Quantitatively, the expression of each mRNA was compared to that of beta-actin, a housekeeping protein, as a reference value.
Enzyme-linked immunosorbent assay
The concentrations of serum TGF-β1 and interleukin-6 (IL-6) were determined using enzyme-linked immunosorbent assay (ELISA) kits for rat TGF-β1 and IL-6 (Quantikine; R&D systems, Minneapolis, MN, USA) according to the manufacturer's instructions.
Morphometry evaluation and statistical analysis
Proliferation of HSCs stained with α-SMA antibody and the proportion of collagen deposition by Sirius Red staining were assessed by morphometric analysis using the ImageJ software v.1.46 (National institute of Health, Bethesda, MD, USA). Sections were randomly chosen, with 30 fields assessed from each group (under 40×magnification).
The differences between each group for each parameter were compared using analysis of variance (ANOVA) or the Student's t-test, when appropriate. A p-value <0.05 was considered significant, as analyzed by the PRISM GraphPad Version 6.0a software (La Jolla, CA, USA).
RESULTS
PPAR-γ agonist inhibits α-SMA, collagen deposition, and proliferation of the biliary duct
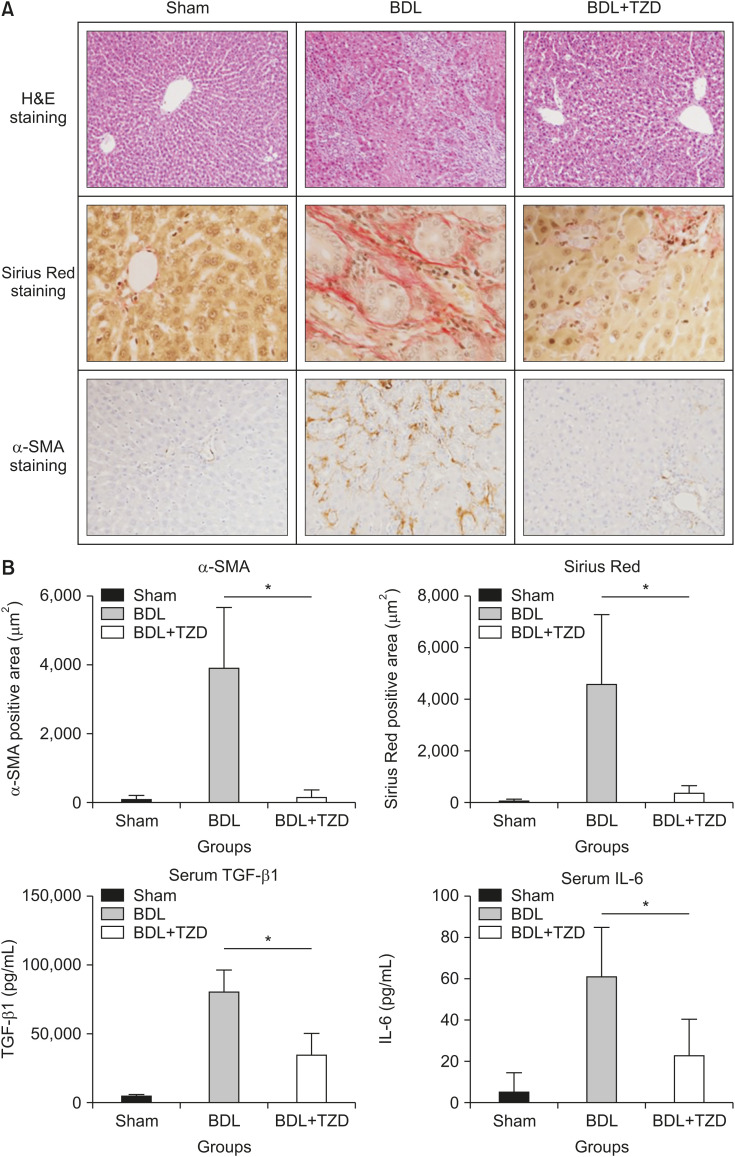
Liver injury was induced by BDL, resulting in proliferation of the biliary duct with matrix deposition in the periductular and lobular area. This has been shown as the area stained by Sirius Red and anti-α-SMA in BDL rats (Fig. 1A). Rats were treated with the widely used PPAR-γ agonist TZD via oral gavage. In contrast, rats administered with a sham operation show neither proliferation of the biliary duct nor matrix deposition (Fig. 1A). Serum alanine aminotransferase (ALT) was significantly lower in the BDL+TZD group (108.6±20.4 U/L) compared to the rats administered with BDL without TZD treatment (373.0±272.4 U/L) (p=0.0123). This confirmed that liver damage was reduced in the TZD treatment-group compared to that in the untreated BDL-group.
Fig. 1. Liver fibrosis following BDL was attenuated in BDL+TZD rats. (A) Representative stained liver sections in sham, BDL, and BDL+TZD groups, stained using H&E (original magnification, ×10) and fibrosis assessment using Sirius Red staining (original magnification, ×40) and α-SMA staining (original magnification, ×20). (B) α-SMA-positive immunostaining area, Sirius Red-positive staining area, and serum levels of TGF-β1 and IL-6 levels in sham, BDL, and BDL+TZD rat groups; all variables are significantly higher in BDL than BDL+TZD groups.
BDL: bile duct ligation, TZD: thiazolidinedione, α-SMA: α-smooth muscle actin, TGF-β1: transforming growth factor beta1, IL-6: interleukin-6. *p<0.05.
We used Sirius Red staining to determine the formation of fibrillar collagen as a measure of liver fibrosis, and α-SMA immunostaining as a marker of activated HSCs. From the representative section stained with H&E, Sirius Red, and anti-α-SMA, lower fibrosis formation is seen in the BDL+TZD group compared to that in the BDL group (Fig. 1A). Upon quantification of the positively stained area, Sirius Red staining shows significantly lower fibrosis formation in the BDL+TZD group (357.2±340.4 µm2/field) compared to that in the BDL group (4,593.0±2,757.4 µm2/field) (p<0.0001) (Fig. 1B). Similarly, α-SMA immunostaining is also downregulated in the BDL+TZD group (90.99±171.3 µm2/field) compared to that in the BDL group (3,936.0±184.0 µm2/field) (p<0.0001) (Fig. 1B). Serum TGF-β1, a potent fibrogenic factor measured by ELISA, is also downregulated in the BDL+TZD group (34,718.0±16,554 pg/mL) compared to that in the BDL group (80,233±17,096 pg/mL) (p<0.0087) (Fig. 1B). Moreover, serum IL-6, as a marker of both pro-inflammatory and anti-inflammatory cytokines measured by ELISA, is also significantly lower in the BDL+TZD group (23.1±18.32 pg/mL) compared to that in the BDL group (60.98±24.58 pg/mL) (p<0.0087) (Fig. 1B).
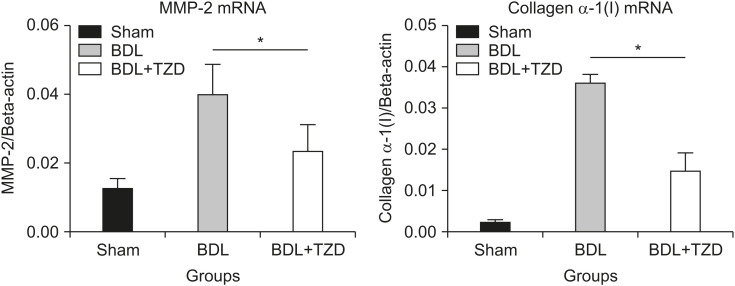
In accordance with the reduction of collagen deposition seen by Sirius Red staining, and the reduction of HSCs seen by α-SMA immunostaining, the hepatic expression of MMP-2 and collagen α-1(I) mRNAs were also significantly lower in the BDL+TZD group. MMP-2 acts as anti-fibrotic enzyme that is increased in the presence of fibrosis. The expression of MMP-2 mRNA relative to beta-actin was 0.024±0.008 in the BDL+TZD group compared to 0.040±0.009 in the BDL group (p=0.035) (Fig. 2). The expression of collagen α-1(I) mRNA relative to beta-actin was 0.015±0.005 in the BDL+TDZ group compared to 0.036±0.002 in the BDL group (p=0.0002), suggesting less fibrosis in the BDL+TZD group.
Fig. 2. Quantification of hepatic MMP-2 and collagen α-1(I) gene expression by qPCR in sham, BDL, and BDL+TZD rat groups; Expression of hepatic MMP-2 and collagen α-1(I) mRNAs are down-regulated in BDL+TZD rats.
MMP-2: matrix metalloproteinase 2, qPCR: quantitative polymerase chain reaction, BDL: bile duct ligation, TZD: thiazolidinedione. *p<0.05.
PPAR-γ agonist inhibits PDGF and CTGF
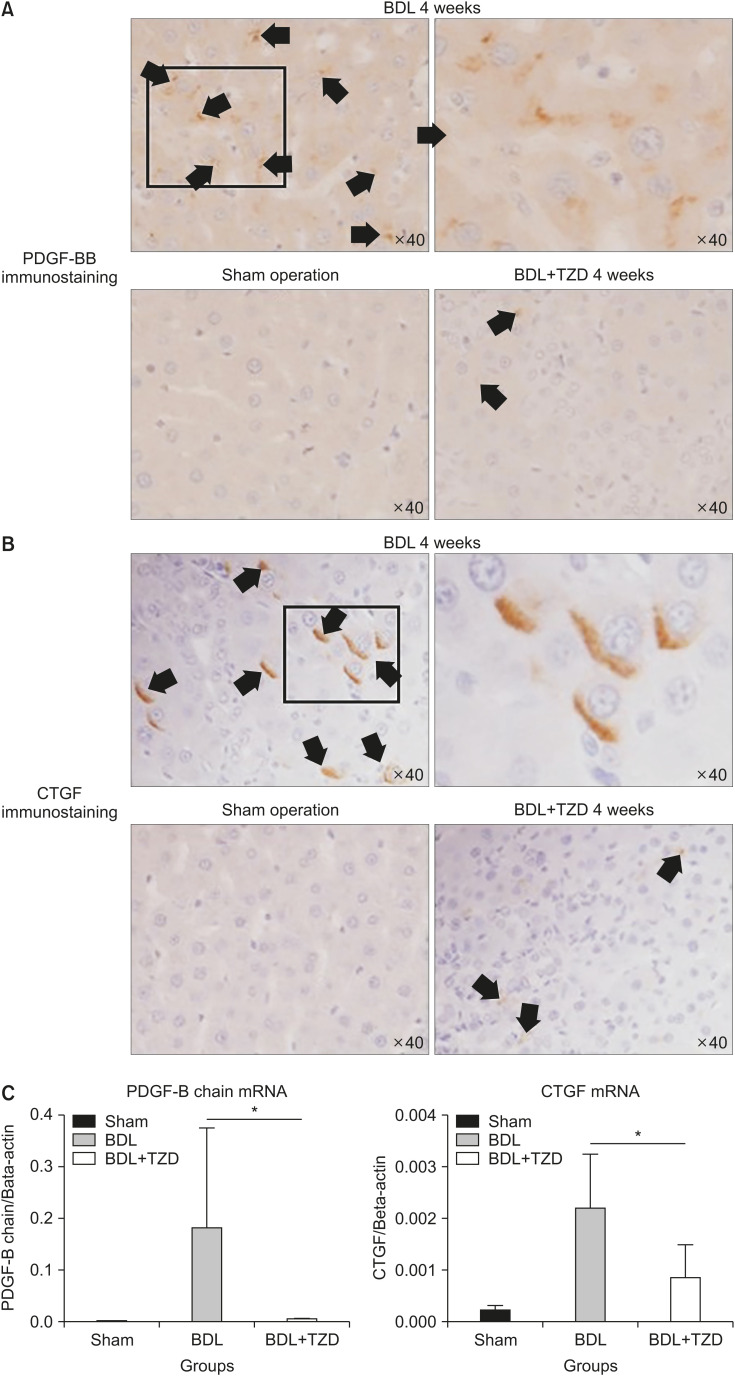
We investigated the cross-talk between fibrogenic growth factors. Following BDL-induced liver injury, some potent fibrogenic factors such as PDGF and CTGF were upregulated. Immunostaining shows high immunoreactivity to both PDGF (Fig. 3A) and CTGF (Fig. 3B) antibodies in the BDL group. In contrast, the sham group does not show reactivity in any part of the liver sections. Conversely, rats that underwent BDL and received PPAR-γ agonist treatment (BDL+TZD) showed only a small number of hepatocytes with positive PDGF and CTGF immunostaining (Fig. 3A, B).
Fig. 3. CTGF & PDGF-BB expressions in liver fibrosis after bile duct ligation were decreased in BDL+TZD rats. Representative PDGF-BB (A) & CTGF (B) immunostaining positive area in sham, BDL and BDL+TZD rats (arrows). Significantly lower PDGF-B chain & CTGF gene expressions in BDL+TZD rats compared to BDL rats upon qPCR assessment (C). CTGF: connective tissue growth factor, PDGF: platelet-derived growth factor, BDL: bile duct ligation, TZD: thiazolidinedione, qPCR: quantitative polymerase chain reaction. (A) PDGF-BB immunostaining left to right, top to bottom: x40, only zoomed in of the square in the ×40 (not via microscope), ×40, and ×40. (B) CTGF immunostaining left to right, top to bottom: ×40, only zoomed in of the square in the x40 (manual zoomed in not via microscope), ×40 and ×40. *p<0.05.
The elevation of PDGF and CTGF in the BDL group and reduction of these fibrogenic factors in rats administered with the PPAR-γ agonist (BDL+TZD group) shown by immunostaining were supported by the quantification of gene expression by qPCR. The hepatic expression of PDGF mRNA and CTGF mRNA are significantly lower in the BDL+TZD group. PDGF mRNA expression relative to beta-actin is 0.007±0.0008 in the BDL+TZD group compared to 0.181±0.1938 in the BDL group (p<0.0001). CTGF mRNA expression relative to beta-actin is 0.001±0.0006 in the BDL+TZD group compared to 0.002±0.0010 in the BDL group (p=0.0123) (Fig. 3C). These results are consistent with the effect of PPAR-γ ligands on liver fibrosis via the attenuation of HSCs.
DISCUSSION
Liver injury causes a complex distortion of parenchymal and non-parenchymal cell populations, resulting in necrosis, apoptosis, proliferation, and altered cellular functions which lead to scarring, i.e., increased production (fibrogenesis), deposition, and remodeling of the extracellular matrix (ECM) [14]. HSCs are the main fibrogenic cells that have been considered to play a role in liver fibrogenesis. In response to injury, HSCs undergo activation and acquire a myofibroblastic phenotype that is driven by the cytokines TGF-β and PDGF. This process leads to increased expression of contractile filaments, such as α-SMA, and ECM proteins, such as collagen I [15,16]. In order to evaluate the effects of the PPAR-γ agonist on the inhibition of HSC activation and liver fibrosis, we generated liver fibrosis by BDL prior to PPAR-γ agonist administration. We used TZD, which is more commonly known as glitazone, a selective agonist of PPAR-γ. A clinical study in patients with non-alcoholic steatohepatitis (NASH) showed that an administration of a PPAR-γ agonist reduced liver fibrosis in 67% of the patients. Furthermore, their transaminase levels were normalized [17]. This data supports our study, which found significantly lower serum ALT concentrations in the BDL+TZD group compared to those in the BDL group.
In this report, we have demonstrated that a PPAR-γ agonist has potent protective effects against hepatic fibrosis. The PPAR-γ agonist inhibited HSC activation (shown by α-SMA immunostaining) and collagen production (shown by Sirius Red staining). The evidence indicates that PPAR-γ agonists play an important role in fibrogenesis, by virtue of their effects on HSCs. The transcription factor PPAR-γ has been shown to convert activated HSCs to a quiescent HSC phenotype, resulting in the reduction of excessive ECM production [18]. PPAR-γ maintains HSC quiescence by regulating a panel of adipogenic transcription factors which decrease HSC transdifferentiation [11,19]. Experiments have indicated that PPAR-γ ligands inhibit proliferation and activation of HSCs in vitro, and prevent fibrogenesis in experimental liver injury [1,20,21,22].
Apart from HSC inhibition by PPAR-γ agonist administration, markers of fibrogenesis were also significantly reduced (Fig. 1). Total collagen expression was significantly different between the PPAR-γ agonist (BDL+TZD)-group and the untreated BDL-group. The molecular mechanism underlying the anti-fibrotic effects of PPAR-γ remains under intense investigation. Current studies focus on the cross-talk between PPAR-γ and TGF-β signaling as the main regulators of myofibroblast activation. PPAR-γ has been shown to disrupt TGF-β-activated stress-activated protein kinase/c-Jun N-terminal kinase signaling, leading to decreased Smad2/3 activity and myofibroblast activation [12,23]. PPAR-γ may also exert an anti-fibrotic effect via other pathways, such as indirect mediation by the HGF and adiponectin as its target genes [23].
Fibrogenesis depends on both pro- and anti-fibrotic/inflammatory cytokines. Targeting potent pro-fibrogenic growth factors, such as PDGF and TGF-β, may result in significant reduction of liver fibrosis [23,24]. PDGF is the most potent HSC mitogen, and its intracellular signaling pathways participate in HSC proliferation [25]. The mechanism underlying the specific inhibitory effect of the PPAR system may be due to its cross-talk with other systems. This study shows a significant reduction of serum TGF-β1 upon PPAR-γ agonist administration (Fig. 1B). Additionally, immunostaining and gene expression results (Fig. 3) indicate a high expression of PDGF and CTGF in the hepatocytes of the BDL group. In contrast, both immunoreactivity and gene expression were markedly reduced in the PPAR-γ agonist (BDL+TZD) group. This implies that the PPAR-γ system is involved in liver fibrosis not only through TGF-β1 and PDGF signaling, as mentioned in the literature, but also through CTGF signaling.
CTGF has been identified as a secreted PDGF-related mitogen that plays a major role in the accumulation of ECM in wound healing and fibrotic disorders [14]. CTGF regulates cell proliferation, differentiation, adhesion, chemotaxis, migration, apoptosis, and ECM production. The normal hepatic CTGF level is low, but elevation is seen in patients with liver fibrosis and in experimental animal models of liver fibrosis [26]. In fibrotic livers, HSCs show sustained CTGF expression as they assume an activated phenotype upon liver injury, thereby becoming responsible for excessive collagen production. Knockdown of CTGF considerably attenuates experimental liver fibrosis [14,26].
There are some limitations of our study that should be noted. We were not able to assess baseline fibrosis grading in the BDL+TZD rats prior to treatment, as this would have required sacrifice of the rats. Hence, there is no comparison of the fibrosis severity before and after intervention with the PPAR-γ agonist in the BDL+TZD treatment-group rats. We assume that fibrosis improves upon administration of TZD by providing a comparison between the BDL+TZD and BDL groups.
In conclusion, this study shows a potential inhibitory effect of the PPAR-γ agonist TZD on liver fibrosis through several fibrogenic mechanisms. Administration of the PPAR-γ agonist downregulates TGF-β1, PDGF, and CTGF. CTGF is a potent fibrogenic factor, and a connection to PPAR-γ agonists has not been described in studies to date. This study is the first to elucidate a connection between CTGF and PPAR-γ. We hope that these data can contribute to further understanding of the inhibition of liver fibrogenesis by PPAR-γ agonists. Targeting PPAR-γ activity may be a potential strategy to control liver fibrosis in cases where disease-specific therapy is not available.
Footnotes
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest: The authors have no financial conflicts of interest.
References
- 1.Iwaisako K, Haimerl M, Paik YH, Taura K, Kodama Y, Sirlin C, et al. Protection from liver fibrosis by a peroxisome proliferator-activated receptor δ agonist. Proc Natl Acad Sci U S A. 2012;109:E1369–E1376. doi: 10.1073/pnas.1202464109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Popov Y, Schuppan D. Targeting liver fibrosis: strategies for development and validation of antifibrotic therapies. Hepatology. 2009;50:1294–1306. doi: 10.1002/hep.23123. [DOI] [PubMed] [Google Scholar]
- 3.Thompson AJ, Patel K. Antifibrotic therapies: will we ever get there? Curr Gastroenterol Rep. 2010;12:23–29. doi: 10.1007/s11894-009-0080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moreira RK. Hepatic stellate cells and liver fibrosis. Arch Pathol Lab Med. 2007;131:1728–1734. doi: 10.5858/2007-131-1728-HSCALF. [DOI] [PubMed] [Google Scholar]
- 5.Rockey DC. Current and future anti-fibrotic therapies for chronic liver disease. Clin Liver Dis. 2008;12:939–962. xi. doi: 10.1016/j.cld.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marra F, Efsen E, Romanelli RG, Caligiuri A, Pastacaldi S, Batignani G, et al. Ligands of peroxisome proliferator-activated receptor gamma modulate profibrogenic and proinflammatory actions in hepatic stellate cells. Gastroenterology. 2000;119:466–478. doi: 10.1053/gast.2000.9365. [DOI] [PubMed] [Google Scholar]
- 7.Wei J, Bhattacharyya S, Jain M, Varga J. Regulation of matrix remodeling by peroxisome proliferator-activated receptor-γ: a novel link between metabolism and fibrogenesis. Open Rheumatol J. 2012;6:103–115. doi: 10.2174/1874312901206010103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang Z, Xu JP, Zheng YC, Chen W, Sun YW, Wu ZY, et al. Peroxisome proliferator-activated receptor gamma inhibits hepatic fibrosis in rats. Hepatobiliary Pancreat Dis Int. 2011;10:64–71. doi: 10.1016/s1499-3872(11)60009-x. [DOI] [PubMed] [Google Scholar]
- 9.Fiorucci S, Rizzo G, Antonelli E, Renga B, Mencarelli A, Riccardi L, et al. Cross-talk between farnesoid-X-receptor (FXR) and peroxisome proliferator-activated receptor gamma contributes to the antifibrotic activity of FXR ligands in rodent models of liver cirrhosis. J Pharmacol Exp Ther. 2005;315:58–68. doi: 10.1124/jpet.105.085597. [DOI] [PubMed] [Google Scholar]
- 10.Bae MA, Rhee SD, Jung WH, Ahn JH, Song BJ, Cheon HG. Selective inhibition of activated stellate cells and protection from carbon tetrachloride-induced liver injury in rats by a new PPARgamma agonist KR62776. Arch Pharm Res. 2010;33:433–442. doi: 10.1007/s12272-010-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, Lu Y, Zheng S. Peroxisome proliferator-activated receptor-γ cross-regulation of signaling events implicated in liver fibrogenesis. Cell Signal. 2012;24:596–605. doi: 10.1016/j.cellsig.2011.11.008. [DOI] [PubMed] [Google Scholar]
- 12.Zhao C, Chen W, Yang L, Chen L, Stimpson SA, Diehl AM. PPARgamma agonists prevent TGFbeta1/Smad3-signaling in human hepatic stellate cells. Biochem Biophys Res Commun. 2006;350:385–391. doi: 10.1016/j.bbrc.2006.09.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alatas FS, Masumoto K, Matsuura T, Hayashida M, Saeki I, Kohashi K, et al. Synchronized expressions of hepatic stellate cells and their transactivation and liver regeneration during liver injury in an animal model of cholestasis. J Pediatr Surg. 2011;46:2284–2290. doi: 10.1016/j.jpedsurg.2011.09.016. [DOI] [PubMed] [Google Scholar]
- 14.Gressner OA, Gressner AM. Connective tissue growth factor: a fibrogenic master switch in fibrotic liver diseases. Liver Int. 2008;28:1065–1079. doi: 10.1111/j.1478-3231.2008.01826.x. [DOI] [PubMed] [Google Scholar]
- 15.Troeger JS, Mederacke I, Gwak GY, Dapito DH, Mu X, Hsu CC, et al. Deactivation of hepatic stellate cells during liver fibrosis resolution in mice. Gastroenterology. 2012;143:1073–83.e22. doi: 10.1053/j.gastro.2012.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–218. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reynaert H, Geerts A, Henrion J. Review article: the treatment of non-alcoholic steatohepatitis with thiazolidinediones. Aliment Pharmacol Ther. 2005;22:897–905. doi: 10.1111/j.1365-2036.2005.02682.x. [DOI] [PubMed] [Google Scholar]
- 18.Hazra S, Miyahara T, Rippe RA, Tsukamoto H. PPAR Gamma and Hepatic Stellate Cells. Comp Hepatol. 2004;3(Suppl 1):S7. doi: 10.1186/1476-5926-2-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.She H, Xiong S, Hazra S, Tsukamoto H. Adipogenic transcriptional regulation of hepatic stellate cells. J Biol Chem. 2005;280:4959–4967. doi: 10.1074/jbc.M410078200. [DOI] [PubMed] [Google Scholar]
- 20.Yang L, Stimpson SA, Chen L, Wallace Harrington W, Rockey DC. Effectiveness of the PPARγ agonist, GW570, in liver fibrosis. Inflamm Res. 2010;59:1061–1071. doi: 10.1007/s00011-010-0226-0. [DOI] [PubMed] [Google Scholar]
- 21.Miyahara T, Schrum L, Rippe R, Xiong S, Yee HF, Jr, Motomura K, et al. Peroxisome proliferator-activated receptors and hepatic stellate cell activation. J Biol Chem. 2000;275:35715–35722. doi: 10.1074/jbc.M006577200. [DOI] [PubMed] [Google Scholar]
- 22.Galli A, Crabb DW, Ceni E, Salzano R, Mello T, Svegliati-Baroni G, et al. Antidiabetic thiazolidinediones inhibit collagen synthesis and hepatic stellate cell activation in vivo and in vitro. Gastroenterology. 2002;122:1924–1940. doi: 10.1053/gast.2002.33666. [DOI] [PubMed] [Google Scholar]
- 23.Gressner AM. Mediators of hepatic fibrogenesis. Hepatogastroenterology. 1996;43:92–103. [PubMed] [Google Scholar]
- 24.Pinzani M, Rombouts K. Liver fibrosis: from the bench to clinical targets. Dig Liver Dis. 2004;36:231–242. doi: 10.1016/j.dld.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 25.Parsons CJ, Takashima M, Rippe RA. Molecular mechanisms of hepatic fibrogenesis. J Gastroenterol Hepatol. 2007;22(Suppl 1):S79–S84. doi: 10.1111/j.1440-1746.2006.04659.x. [DOI] [PubMed] [Google Scholar]
- 26.Huang G, Brigstock DR. Regulation of hepatic stellate cells by connective tissue growth factor. Front Biosci (Landmark Ed) 2012;17:2495–2507. doi: 10.2741/4067. [DOI] [PubMed] [Google Scholar]