Abstract
Purpose
Dupilumab, a monoclonal antibody directed against the interleukin-4 receptor subunit α (IL-4Rα) of IL-4 and IL-13, is increasingly being used to control atopic disease. Dupilumab use has been associated with a poorly understood conjunctivitis. In this study, we sought to investigate the hypothesis that dupilumab use and the associated IL-13 blockade causes a relative ocular mucin deficiency.
Methods
Tear levels of mucin 5ac (Muc5AC) and total tear protein levels were evaluated from 28 eyes of 14 patients. Bilateral tear samples were acquired from seven patients on dupilumab and seven patients with no exposure to dupilumab. Study subjects were age and gender matched. In addition to tear samples, photographic documentation of ocular surface findings and a questionnaire of ocular surface symptoms were obtained. Between-group mean differences were calculated.
Results
Compared with control, ocular Muc5AC levels normalized to total tear protein was statistically significantly lower. The average Muc5AC levels for persons on dupilumab was 1.54 ± 0.58 ng/mg and that of controls was 7.99 ± 1.16 ng/ mg. Persons on dupilumab reported a statistically increased occurrence of ocular fatigue/eye strain, uncomfortable sensation, pain, red eye, and itching.
Conclusions
This study demonstrates for the first time, a relative deficiency of Muc5AC in patients on dupilumab.
Translational Relevance
The results of this study support the previously reported role of IL-13 in increasing goblet cell density and associated Muc5AC production. Further efforts are underway to better understand the relative contribution of Muc5AC deficiency in the overall presentation of conjunctivitis associated with dupilumab use.
Keywords: dupilumab, mucin, Muc5AC, goblet cells, conjunctivitis
Introduction
Dupilumab, trade name Dupixent (Sanofi and Regeneron Pharmaceuticals), is a monoclonal antibody that acts as an interleukin-4 (IL-4) receptor alpha antagonist that blocks the signaling of IL-4 and IL-13.1,2 It is increasingly being used clinically to control over expression of such Th2 cytokines found in a variety of allergic diseases.3,4 Dupilumab was first approved by the US Food and Drug Administration for eczema in March 2017 followed by approval for moderate to severe asthma in October 2018. Dupilumab was then approved for moderate to severe atopic dermatitis (AD) in March 2019 and chronic rhinosinusitis with nasal polyposis in June 2019.5 Similar approval has been achieved outside the United States, with the European Medicine Agency authorizing dupilumab for AD September 2017 with numerous other approval under way.1,6,7
Throughout the various trials for approval of dupilumab, conjunctivitis has been identified. In various phase 3 clinical trials, patients with AD treated with dupilumab had a 14% to 19% rate of conjunctivitis, whereas patients with AD treated with a placebo had an 8% rate of conjunctivitis.5,8–10 Although the increased rate of conjunctivitis was reported, its underlying etiology was not explored. In the setting of AD, it is not uncommon for patients to have a comorbidity of allergic conjunctivitis.11 How might a therapeutic that is so powerful in suppressing atopic disease of the skin cause worsening of allergic conjunctivitis?1,5,12,13 Dermatologists prescribed topical steroid eye drops for persons with conjunctivitis.1,5 In the setting of introduction of topical steroids, in these trials only 1 of 425 patients discontinued dupilumab because of conjunctivitis.5,10,14,15
As with many debilitating conditions, patients are willing to withstand significant side effects if they perceive the overall benefit of the medication results in positive outcomes. Although limited, current studies by ophthalmologists have reported a rate of ocular surface disease as high as 23% for patients on dupilumab.1,8,12,14–16 Of concern, some of these cases involve cicatrizing disease.14,17 With the varied specialty focus of dermatology and ophthalmology, it is not surprising that a higher incidence of ocular surface disease associated with dupilumab use has been identified in the ophthalmology setting compared to dermatology centered trials. Therefore, it is essential to understand the mechanisms underlying dupilumab conjunctivitis and the apparent paradox in worsening ocular symptoms with improving systemic symptoms. As one patient stated, “How could something so good for my skin be so bad for my eyes?”
Pflugfelder and colleagues have reported on the direct correlation of IL-13 on goblet cell production and mucin levels.18 It stands to reason that blockade of IL-13 may lead to a mucin deficiency. The purpose of this study was to investigate the tear levels of Mucin 5AC (Muc5AC) in the presence or absence of dupilumab treatment. A secondary aim was to assess self-reported ocular surface symptoms of persons on dupilumab, as well as document clinical findings associated with dupilumab-associated mucin deficiency (DAMD) and the overall dupilumab-related ocular surface disease.
Subjects and Methods
Subjects
This study protocol to compare Muc5AC levels in the tears of persons on dupilumab and normal control subjects was approved by the University of California San Diego College of Medicine institutional review board. The study was compliant with the Health Insurance Portability and Accountability Act as well as the tenets of the Declaration of Helsinki for clinical research. All participants provided written informed consent after the purpose and associated possible consequences of the study were explained. This study was a prospective observational study at a single institution. Seven persons using dupilumab and seven healthy age- and gender-matched control subjects were used. All persons were asked to refrain from contact lens use or topical ocular lubrication or medication on the day of tear collection. All subjects completed questionnaires about their frequency of ocular symptoms.
Tear Sample Collection
A total of 28 eyes from 14 subjects were analyzed in the study. Tear samples were collected sequentially from the right and then left eye of each subject using a Weck-Cel cellulose eye spear (Beaver-Visitec International, Inc., Waltham, MA). The day of tear collection, subjects were instructed to disuse contact lens use. Tears were collected by gently collecting tears with Weck-Cel spears that absorb tears in the inferior cul-de-sac. Tear samples were collected over a period of 3 minutes in each eye with a 5-minute interval between tear collection between eyes. Tears were eluted from Weck-Cel by incubating the spear in 30 µL phosphate-buffered saline for 20 minutes. The samples were then spun down at 4400 rpm for 10 minutes using an Eppendorf Centrifuge (Westbury, NY). Samples were then stored at 37°F until analysis.
MuC5AC and Tear Protein Measurement
All tear samples were assayed in triplicate. The concentration of MUC5AC in the tear samples was quantified by enzyme-linked immunoassay using the Human MUC5AC ELISA Kit (LifeSpan Biosciences, Seattle, WA) according to the manufacturer’s guidelines. The tear samples, as well as the recombinant human MUC5AC standard solutions provided with the kit were measured in triplicate at 450 nm. Next, a standard microwell Bradford assay using the manufacturer’s protocol was performed (Bio-Rad, Hercules, CA) to determine the total tear protein for each sample using bovine serum albumin as a protein standard. For each MUC5AC sample, the concentration was normalized to total tear protein and expressed as MUC5AC protein (nanograms) per TPC (milligrams).
Ocular Symptom Questionnaire
To assess ocular surface disease symptoms in each of the subjects and controls, a questionnaire was provided to each subject. No clarification or coaching on how to answer questions was provided to the subjects beyond simply telling them to circle the answer that best described their symptoms. The particular questionnaire we used has been previously validated in trials of ocular surface disease with mucin deficiency in particular.19 The questionnaire included 12 questions pertaining to the symptoms of ocular surface disease. For each of the 12 questions, an answer of always, often, sometimes, or never was circled (Supplementary Material).
Statistical Analysis
Data are presented as the mean ± standard deviation. We used Student t-test with a significance level of P < 0.05. We performed all analyses using the software R.
Results
Clinical Features
All patients on dupilumab referred to our ophthalmology clinic had ocular symptoms from dupilumab. Ophthalmologist referral before starting dupilumab did not occur at our institution; therefore, no comment on prevalence of side effects or findings in cases that had mild enough ocular side effects that did not result in referral cannot be assessed. All subjects from this study had some degree of conjunctivitis, keratitis, and blepharitis. In certain persons, the degree of ocular surface inflammation was quite severe (Fig. 1). For most subjects, dupilumab resulted in a bilateral moderate conjunctivitis with limbal hyperemia (Figs. 2–3). Because this was not a longitudinal study, the absence of these findings could only be confirmed in one patient who had been seen in our clinic for unrelated causes before starting dupilumab (Fig. 2C). One patient had what clinically appeared to be a keratinized area of conjunctiva thatstained with Lissamine Green (Fig. 3D).
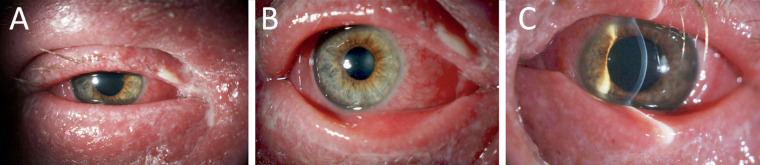
Figure 1.
Severe presentation of DAMD. Slit lamp photos of a patient with DAMD demonstrating ocular surface disease including (A) blepharoconjunctivitis with mucoid discharge, madarosis, and periocular dermatitis; (B) diffuse bulbar and palpebral conjunctivitis; and (C) moderate keratitis with absence of stromal infiltrates, and minimal anterior chamber inflammation.
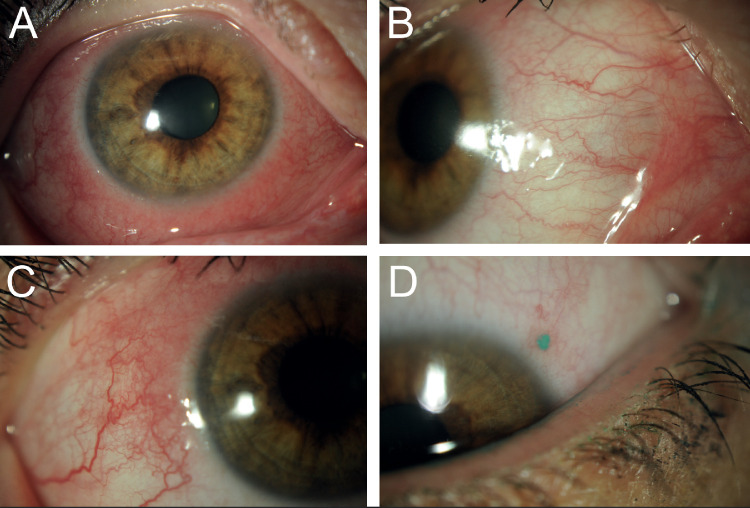
Figure 2.
Moderate presentation of DAMD. Slit-lamp photos of a patient with DAMD demonstrating moderate ocular surface disease including (A) moderate bulbar and palpebral conjunctivitis and (B) mild keratitis. (C) Before initiating dupilumab, the patient had minimal ocular surface disease with no evidence of conjunctivitis, keratitis, or blepharitis.
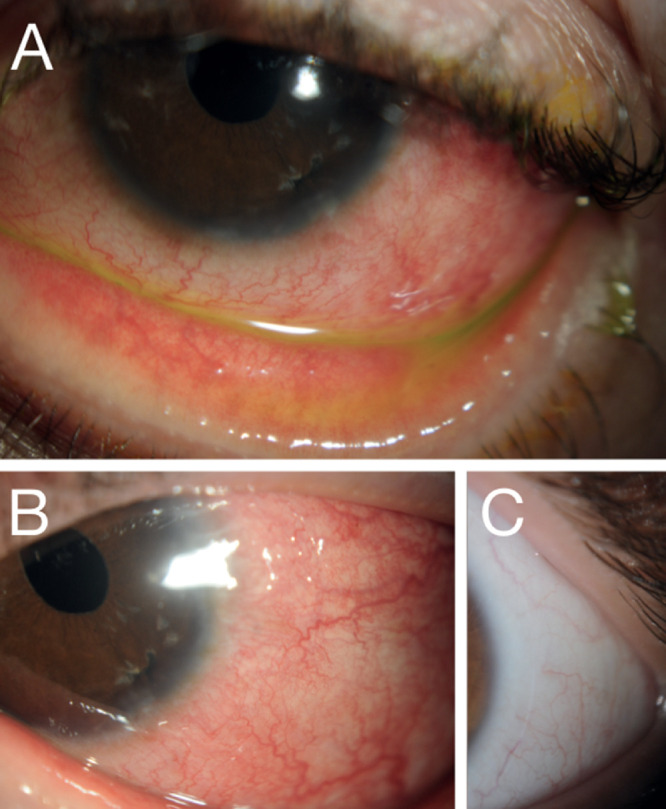
Figure 3.
DAMD response to topical therapy. Slit-lamp photos of a patient with DAMD who improved with topical ocular therapy. (A) Upon presentation, the patient had moderate diffuse bulbar and palpebral conjunctivitis. With the initiation of topical steroids and lifitegrast, the patient had a reduction of ocular surface inflammation but maintained (B) a degree of temporal conjunctival inflammation with (C) fibrotic changes and (D) an area of potential keratinization that stained with Lissamine Green.
MUC5AC Levels
In age- and gender-matched controls recruited from patients and office staff free of ocular surface disease, the ocular level of Muc5AC was reduced in persons on dupilumab. Specifically, compared with control (N = 14), ocular Muc5AC levels normalized to total tear protein in eyes of subjects (N = 14) were found to be reduced (P > 0.05). The average Muc5AC levels for persons on dupilumab was 1.54 ± 0.58 ng/mg and that of controls was 7.99 ± 1.16 ng/mg (Fig. 4).
Figure 4.
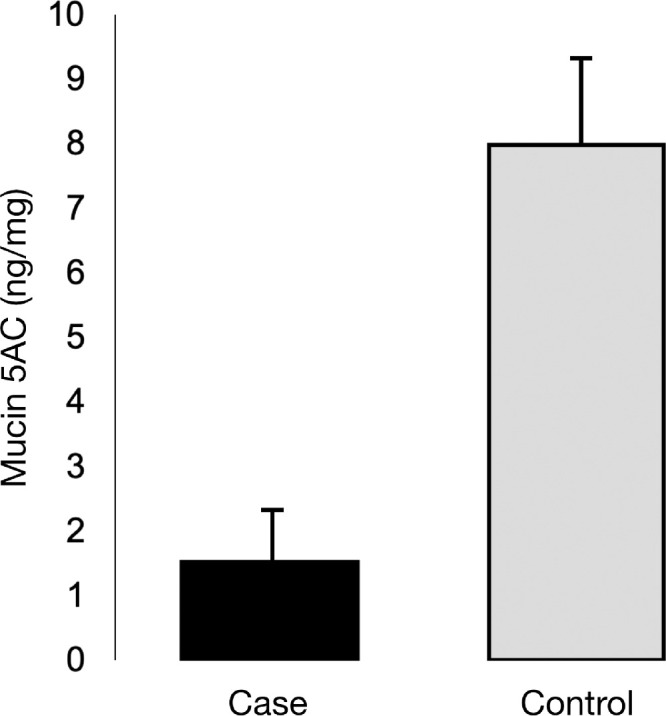
Muc5AC levels with dupilumab and controls. Tear Muc5AC levels normalized to tear total protein in persons on dupilumab are reduced as compared with healthy subjects. The average Muc5AC levels for persons on dupilumab was 1.54 ± 0.58 ng/mg and that of controls was 7.99 ± 1.16 ng/mg.
Questionnaire Results
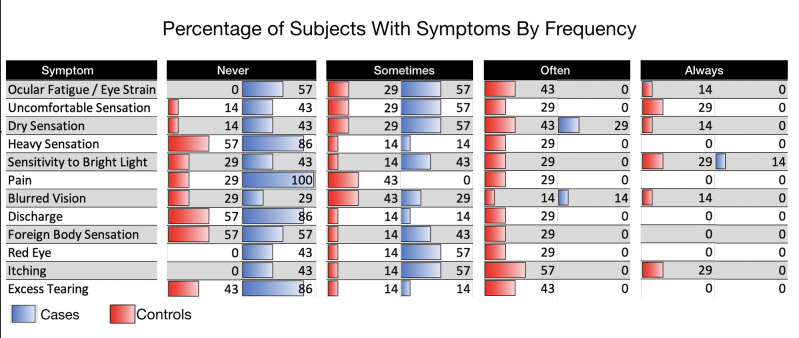
Persons with DAMD displayed a constellation of ocular symptoms including varying degrees of conjunctivitis, blepharitis, and keratitis accompanied by expected ocular symptoms. The result of the questionnaire revealed persons on dupilumab reported a statistically increased occurrence of ocular fatigue/eye strain, uncomfortable sensation, pain, red eye, and itching (Tables 1 and 2). Although not statistically significant because of the small sample size, persons on dupilumab also trended toward increased dry sensation, heavy sensation, sensitivity to bright light, blurred vision, and ocular discharge (Tables 1 and 2).
Table 1.
Ocular Surface Disease Questionnaire
To assess ocular surface disease symptoms in each of the subjects and controls, a questionnaire was provided to each subject. The questionnaire included 12 questions pertaining to the symptoms of ocular surface disease. For each of 12 questions, an answer of always, often, sometimes, or never was circled. Persons on dupilumab reported a statistically increased occurrence of ocular fatigue/eye strain, uncomfortable sensation, pain, red eye, and itching.
Table 2.
Trends in Ocular Surface Disease Symptoms
| Symptom | Case Avg. Score (SD) | Control Avg. Score (SD) | P Value |
|---|---|---|---|
| Ocular fatigue/eye strain | 2. 7 (±0.8) | 1. 4 (±0.5) | 0.0041 |
| Uncomfortable sensation | 2. 7 (±1.1) | 1. 6 (±0.5) | 0.0300 |
| Dry sensation | 2. 6 (±1.0) | 1. 9 (±0.9) | 0.0941 |
| Heavy sensation | 1. 7 (±1.0) | 1. 1 (±0.4) | 0.1030 |
| Sensitivity to bright light | 2. 6 (±1.3) | 1. 9 (±1.1) | 0.3341 |
| Pain | 2. 0 (±0.8) | 1. 0 (±0.0) | 0.0177 |
| Blurred vision | 2. 1 (±1.1) | 1. 6 (±0.8) | 0.2797 |
| Discharge | 1. 7 (±1.0) | 1. 1 (±0.4) | 0.2308 |
| Foreign body sensation | 1. 7 (±1.0) | 1. 4 (±0.5) | 0.4571 |
| Red eye | 2. 6 (±0.8) | 1. 6 (±0.5) | 0.0382 |
| Itching | 3. 1 (±0.7) | 1. 6 (±0.5) | 0.0002 |
| Excess tearing | 2. 0 (±1.0) | 1. 1 (±0.4) | 0.0781 |
Score: 1-Never 2-Sometime 3-Often 4-Always
Persons with DAMD compared with age- and sex-matched controls had an increase incidence of symptoms associated with ocular surface disease. The result of the questionnaire revealed persons on dupilumab reported a statistically increased occurrence of ocular fatigue/eye strain, uncomfortable sensation, pain, red eye, and itching. Although not statistically significant with the small sample size, persons on dupilumab also trended toward increased dry sensation, heavy sensation, sensitivity to bright light, blurred vision, and ocular discharge.
SD, standard deviation.
Discussion
This paper demonstrates for the first time DAMD. This finding shows that dupilumab blocks IL-13 and a known correlation between IL-13 and mucin levels exists. Persons with DAMD displayed a constellation of ocular symptoms including varying degrees of conjunctivitis, blepharitis. and keratitis. Most persons with DAMD had bilateral mild-to-moderate conjunctivitis with limbal hyperemia. Compared with control, ocular Muc5AC levels normalized to total tear protein was statistically significantly lower. The average Muc5AC levels for persons on dupilumab was 1.54 ± 0.58 ng/mg and that of controls was 7.99 ± 1.16 ng/mg. Persons on dupilumab reported a statistically increased occurrence of ocular fatigue/eye strain, uncomfortable sensation, pain, red eye, and itching.
In addition to mucin deficiency, histological analysis of conjunctival biopsies from patients on dupilumab has shown marked decrease and, in certain patients, complete absence of goblet cells.8 The median conjunctival intraepithelial goblet cell density was 33 cells/mm2 in persons on dupilumab versus 323 cells/mm2 in healthy controls. In addition to a paucity of goblet cells, persons on dupilumab had a multicellular immune cell stromal infiltrate. This infiltrate comprised primarily T cells (CD3+/CD4+) and eosinophils. In regard to treatment for these patients, would a recombinant topical mucin be sufficient to abrogate dupilumab associated conjunctivitis? The authors suspect the answer may not be so straightforward.
In addition to demonstrating a link between IL-13 and goblet cell and mucin production, Pflugfelder and colleagues have also explored the role of goblet cells in promoting antigen-presenting cell tolerance, thereby suppressing IL-12 production and TH1 polarization. With this in mind, DAMD may be just the tip of the iceberg with goblet cell loss disturbing ocular immune homeostasis. Further studies are critical to not only benefit patients on dupilumab but also to inform the understanding of mucin and goblet cell function in ocular surface disease.
Dupilumab binds to the alpha subunit of the IL-4 receptor, thereby blocking IL-4 and IL-13 signaling.20 In addition to the effect on goblet cells and mucin production, it is feasible some degree of dupilumab's ocular side effects are due to perturbation of these signaling pathways separate and apart from goblet cell homeostasis. For example, a recent paper outside the ocular literature has demonstrated selective suppression of IL-23 by IL-4 and IL-13, which in turn reduces Th17 function.21 From the work of Reza Dana's group, it is known that interferon-γ-expressing Th17 cells are present and are potentially even requisite for severe ocular surface autoimmunity.22
Other hypothesized mechanisms for dupilumab's ocular side effects include worsening of allergic conjunctivitis. One such hypothesis suggests IL-4 and IL-13 signaling inhibition results in increased activity of OX40L and other ligands known to worsen allergic conjunctivitis.5 Another hypothesis is based on the documented transient increase in eosinophils found upon the initiation of dupilumab. It has been hypothesized these increased eosinophils are responsible for ocular side effects.1 These hypotheses may not fully explain the pathogenesis because the ocular side effects of dupilumab tend to not appear acutely when starting the medication, but over a period of weeks or sometimes a month. Further, the phenotype of DAMD patients, at least in the clinical judgment of the authors, appears separate from that of allergic conjunctivitis. In fact, it is this paradox of improving atopic disease and allergic conjunctivitis findings and worsening of a distinct form of ocular inflammation that prompted this study.
The demonstration of goblet cell loss and decreased mucin may be a fruitful area for exploration as the primary cause of the deleterious effects of dupilumab on the ocular surface. Goblet cells have been shown to play a fundamental role in decreasing ocular inflammation, reducing corneal permeability, and decreasing evaporative tear loss.23,24 Goblet cell loss has been implicated in a variety of well-studied ocular surface diseases, including allergic conjunctivitis, ocular cicatricial pemphigoid, and Sjogren syndrome.23,25–29 In seminal work by Argueso et al., it was found that patients with Sjogren syndrome have a concomitant loss of goblet cells and Muc5AC and SPEDF messenger RNA are substantially decreased in the conjunctiva of such patients.28 Moreover, Argueso's group demonstrated that Spdef null mice lack conjunctival goblet cells.26 The patients in this study with DAMD have an ocular surface phenotype that is reminiscent of that in Spdef null mice. Goblet cells are also known to respond to transforming growth factor-beta signaling and indeed appear sensitive to a variety of external and inflammatory stimuli. Accordingly, disruption of transforming growth factor-beta signaling has been shown to improve ocular surface disease symptoms in an experimental model of autoimmune keratoconjunctivitis sicca.30 Another example of goblet cells central role in ocular surface homeostasis is demonstrated by the finding that goblet cell secretion is modulated by leukotrienes and is reduced by resolvins D1 and E1.31 Pflugfelder and colleagues have also demonstrate that neutralization of interferon-gamma decreases loss of goblet cells in an experimental dry eye model.32
Taken together, it appears that the various inflammatory pathways regulate both the number of goblet cells but also function in various pathogenic conditions. Importantly, this relationship appears to be two-way, with goblet cells proving immunomodulatory crosstalk with other cellular mediators. Goblet cells have been shown to act as antigen-presenting cells to dendritic cells in the conjunctival stroma.33 In the same way various inflammatory cytokines control goblet cell number, goblet cells have been shown to maintain dendritic cells in an immature state.34 In the small intestine, goblet cells have been shown to bridge the gap from lumen to the underlying dendritic cells. By sampling the luminal environment and carrying that information to dendritic cells in the underlying epithelium, dendritic cells can sense environmental insults without a break in barrier integrity.6,7 In the authors’ opinion, it is likely that much like goblet cells in the gut, goblet cells in the conjunctiva will emerge as major players in ocular surface immunology. Accordingly, it is conceivable that dupilumab's effects on the ocular surface are entirely the result of mucin and goblet cell deficiency.
Despite the pioneering work of many of the groups described previously, therapeutic options in DAMD are limited because of the poorly understood mechanisms regulating goblet cell differentiation in the conjunctiva. For the majority of patients who have been plagued by atopic disease their entire life, the ocular side effects are outweighed by the systemic benefits and thus they may be DAMD if they do. Lifitegrast ophthalmic solution (Xiidra) 5%, cyclosporine ophthalmic emulsion (Restasis) 0.05%, tacrolimus, topical steroids, and topical lubricants have been reported to be effective in this condition.5,8,14,35–38 Tellingly, mast cell stabilizers and antihistamines appear to be of minimal benefit. If primarily a mucin deficiency, it stands to reason that treatment of DAMD should involve mucin replacement. Currently, a recombinant form of mucin is not available. A few therapeutics whose primary mechanism is thought to be increased mucin production are approved outside the United States, including rebamipide in Japan.
This study has a number of limitations. First, the patients referred to the ophthalmology clinic on dupilumab had ocular side effects and thus no comment on prevalence or normal degree of symptoms can be ascertained. As one would expect based upon the symptomatic nature of our patients, the degree of signs and symptoms appeared more than that previously reported in all comers on dupilumab in the dermatology literature. In addition, the sample size in this study was small. Nevertheless, it is telling that in this enriched population, even with small sample size, a significant difference in Muc5AC levels as well as reported ocular surface disease symptoms was found between subjects and controls.
Another significant limitation of this study was the single timepoint when patients were analyzed. Although patients were asked to refrain from topical ocular medications and lubrication on the day of tear collection, the use of topical ocular medication before the day of tear collection was not eliminated as a variable. Because only one healthy control used any ocular medication/lubrication, artificial tears in the one case using drops, the subjects were on a variety of topical ocular medications and lubricants. Because patients were found to clinically and symptomatically improve on these various therapies, it is unlikely that the use of these medications was the cause of the observed decline in tear mucin levels. Nevertheless, a prospective study looking at all patients started on dupilumab with monitoring of Muc5AC as well as corresponding ocular irritation and inflammation would be illustrative.
Although our study does prove that patients on dupilumab with ocular symptoms do have decreased mucin levels, it fails to address three critical questions. Do patients with ocular symptoms from dupilumab introduction already have low Muc5AC levels before starting the medication and dupilumab destroys any Muc5AC reserve? Do all patients that start dupilumab have a decline in Muc5AC levels and, if so, is this percentage decline relatively stable from person to person? Clearly, a prospective study in which Muc5AC and goblet cell density is measured before initiation of dupilumab and followed over time would be of great benefit. Similarly, the evaluation of mucin levels longitudinally after the introduction of topical steroids, cyclosporin, Lifitegrast, rebamipide, or other agents thought to be helpful in DAMD as well as the correlation to clinical signs and ocular surface disease symptoms would be very illustrative. Nevertheless, this study represents an important direction to explore in the better understanding of dupilumab induced ocular surface disease. Moreover, this study further highlights what appears to be a critical role of mucin and goblet cells in a healthy ocular surface.
In conclusion, this study demonstrates for the first time, a relative deficiency of Muc5AC in patients on dupilumab. These results support the previously reported role of IL-13 in increasing goblet cell density and associated Muc5AC production. Persons with DAMD displayed a constellation of ocular symptoms including varying degrees of conjunctivitis, blepharitis, and keratitis. Most persons with DAMD had bilateral, moderate conjunctivitis with limbal hyperemia. Persons on dupilumab reported a statistically increased occurrence of ocular fatigue/eye strain, uncomfortable sensation, pain, red eye, and itching. Further efforts are under way to better understand the relative contribution of Muc5AC deficiency in the overall presentation of ocular surface disease associated with dupilumab use.
Supplementary Material
Acknowledgments
The authors thank Jack Zhao, Ruti Sella, and Derek Welsbie for discussion and kindly loaning equipment.
Supported by an unrestricted grant from Research to Prevent Blindness, New York, New York, United States (NAA).
Disclosure: B.P. Barnett, None; N.A. Afshari, None
References
- 1. Thyssen JP, de Bruin-Weller MS, Paller AS, et al.. Conjunctivitis in atopic dermatitis patients with and without dupilumab therapy - international eczema council survey and opinion. J Eur Acad Dermatol Venereol. 2019; 33: 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seegraber M, Srour J, Walter A, Knop M, Wollenberg A. Dupilumab for treatment of atopic dermatitis. Expert Rev Clin Pharmacol. 2018; 11: 467–474. [DOI] [PubMed] [Google Scholar]
- 3. Sastre J, Davila I. Dupilumab: a new paradigm for the treatment of allergic diseases. J Investig Allergol Clin Immunol. 2018; 28: 139–150. [DOI] [PubMed] [Google Scholar]
- 4. Rabe KF, Nair P, Brusselle G, et al.. Efficacy and safety of dupilumab in glucocorticoid-dependent severe asthma. N Engl J Med. 2018; 378: 2475–2485. [DOI] [PubMed] [Google Scholar]
- 5. Mennini M, Dahdah L, Fiocchi A. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2017; 376: 1090. [DOI] [PubMed] [Google Scholar]
- 6. McDole JR, Wheeler LW, McDonald KG, et al.. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature. 2012; 483: 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Knoop KA, McDonald KG, McCrate S, McDole JR, Newberry RD. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 2015; 8: 198–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bakker DS, Ariens LFM, van Luijk C, et al.. Goblet cell scarcity and conjunctival inflammation during treatment with dupilumab in patients with atopic dermatitis. Br J Dermatol. 2019; 180: 1248–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ou Z, Chen C, Chen A, Yang Y, Zhou W. Adverse events of Dupilumab in adults with moderate-to-severe atopic dermatitis: a meta-analysis. Int Immunopharmacol. 2018; 54: 303–310. [DOI] [PubMed] [Google Scholar]
- 10. Blauvelt A, de Bruin-Weller M, Gooderham M, et al.. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a 1-year, randomised, double-blinded, placebo-controlled, phase 3 trial. Lancet. 2017; 389: 2287–2303. [DOI] [PubMed] [Google Scholar]
- 11. de Bruin-Weller M, Thaci D, Smith CH, et al.. Dupilumab with concomitant topical corticosteroid treatment in adults with atopic dermatitis with an inadequate response or intolerance to ciclosporin A or when this treatment is medically inadvisable: a placebo-controlled, randomized phase III clinical trial (LIBERTY AD CAFE). Br J Dermatol. 2018; 178: 1083–1101. [DOI] [PubMed] [Google Scholar]
- 12. Treister AD, Kraff-Cooper C, Lio PA. Risk factors for dupilumab-associated conjunctivitis in patients with atopic dermatitis. JAMA Dermatol. 2018; 154: 1208–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Rial MJ, Barroso B, Rodriguez-Bermejo C, Sastre J. Letter regarding “Conjunctivitis occurring in atopic dermatitis patients treated with dupilumab-clinical characteristics and treatment”. J Allergy Clin Immunol Pract. 2019; 7: 753. [DOI] [PubMed] [Google Scholar]
- 14. Barnes AC, Blandford AD, Perry JD. Cicatricial ectropion in a patient treated with dupilumab. Am J Ophthalmol Case Rep. 2017; 7: 120–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thomson J, Wernham AGH, Williams HC. Long-term management of moderate-to-severe atopic dermatitis with dupilumab and concomitant topical corticosteroids (LIBERTY AD CHRONOS): a critical appraisal. Br J Dermatol. 2018; 178: 897–902. [DOI] [PubMed] [Google Scholar]
- 16. Simpson EL, Bieber T, Guttman-Yassky E, et al.. Two phase 3 trials of dupilumab versus placebo in atopic dermatitis. N Engl J Med. 2016; 375: 2335–2348. [DOI] [PubMed] [Google Scholar]
- 17. Levine RM, Tattersall IW, Gaudio PA, King BA. Cicatrizing blepharoconjunctivitis occurring during dupilumab treatment and a proposed algorithm for its management. JAMA Dermatol. 2018; 154: 1485–1486. [DOI] [PubMed] [Google Scholar]
- 18. Tukler Henriksson J, Coursey TG, Corry DB, De Paiva CS, Pflugfelder SC. IL-13 stimulates proliferation and expression of mucin and immunomodulatory genes in cultured conjunctival goblet cells. Invest Ophthalmol Vis Sci. 2015; 56: 4186–4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Uchino Y, Uchino M, Yokoi N, et al.. Alteration of tear mucin 5AC in office workers using visual display terminals: The Osaka Study. JAMA Ophthalmol. 2014; 132: 985–992. [DOI] [PubMed] [Google Scholar]
- 20. McCauley HA, Guasch G.. Three cheers for the goblet cell: maintaining homeostasis in mucosal epithelia. Trends Mol Med 2015; 21: 492–503. [DOI] [PubMed] [Google Scholar]
- 21. Guenova E, Skabytska Y, Hoetzenecker W, et al.. IL-4 abrogates T(H)17 cell-mediated inflammation by selective silencing of IL-23 in antigen-presenting cells. Proc Natl Acad Sci USA. 2015; 112: 2163–2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Chen Y, Chauhan SK, Shao C, Omoto M, Inomata T, Dana R. IFN-gamma-expressing Th17 cells are required for development of severe ocular surface autoimmunity. J Immunol. 2017; 199: 1163–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mantelli F, Argueso P. Functions of ocular surface mucins in health and disease. Curr Opin Allergy Clin Immunol. 2008; 8: 477–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li D, Hodges RR, Jiao J, et al.. Resolvin D1 and aspirin-triggered resolvin D1 regulate histamine-stimulated conjunctival goblet cell secretion. Mucosal Immunol. 2013; 6: 1119–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gupta D, Harvey SA, Kaminski N, Swamynathan SK. Mouse conjunctival forniceal gene expression during postnatal development and its regulation by Kruppel-like factor 4. Invest Ophthalmol Vis Sci. 2011; 52: 4951–4962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Marko CK, Menon BB, Chen G, Whitsett JA, Clevers H, Gipson IK. Spdef null mice lack conjunctival goblet cells and provide a model of dry eye. Am J Pathol. 2013; 183: 35–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang Y, Lam O, Nguyen MT, et al.. Mastermind-like transcriptional co-activator-mediated Notch signaling is indispensable for maintaining conjunctival epithelial identity. Development. 2013; 140: 594–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Argueso P, Balaram M, Spurr-Michaud S, Keutmann HT, Dana MR, Gipson IK. Decreased levels of the goblet cell mucin MUC5AC in tears of patients with Sjogren syndrome. Invest Ophthalmol Vis Sci. 2002; 43: 1004–1011. [PubMed] [Google Scholar]
- 29. Vujkovic V, Mikac G, Kozomara R. Distribution and density of conjunctival goblet cells. Med Pregl. 2002; 55: 195–200. [DOI] [PubMed] [Google Scholar]
- 30. De Paiva CS, Volpe EA, Gandhi NB, et al.. Disruption of TGF-beta signaling improves ocular surface epithelial disease in experimental autoimmune keratoconjunctivitis sicca. PLoS One. 2011; 6: e29017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Dartt DA, Hodges RR, Li D, Shatos MA, Lashkari K, Serhan CN. Conjunctival goblet cell secretion stimulated by leukotrienes is reduced by resolvins D1 and E1 to promote resolution of inflammation. J Immunol. 2011; 186: 4455–4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang X, De Paiva CS, Su Z, Volpe EA, Li DQ, Pflugfelder SC. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp Eye Res. 2014; 118: 117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Contreras-Ruiz L, Masli S. Immunomodulatory cross-talk between conjunctival goblet cells and dendritic cells. PLoS One. 2015; 10: e0120284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Contreras-Ruiz L, Ghosh-Mitra A, Shatos MA, Dartt DA, Masli S. Modulation of conjunctival goblet cell function by inflammatory cytokines. Mediators Inflamm. 2013; 2013: 636812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Attas-Fox L, Barkana Y, Iskhakov V, et al.. Topical tacrolimus 0.03% ointment for intractable allergic conjunctivitis: an open-label pilot study. Curr Eye Res. 2008; 33: 545–549. [DOI] [PubMed] [Google Scholar]
- 36. Aszodi N, Thurau S, Seegraber M, de Bruin-Weller M, Wollenberg A. Management of dupilumab-associated conjunctivitis in atopic dermatitis. J Dtsch Dermatol Ges. 2019; 17: 488–491. [DOI] [PubMed] [Google Scholar]
- 37. de Bruin-Weller M, Graham NMH, Pirozzi G, Shumel B. Could conjunctivitis in patients with atopic dermatitis treated with dupilumab be caused by colonization with Demodex and increased interleukin-17 levels? Reply from the authors. Br J Dermatol. 2018; 178: 1220–1221. [DOI] [PubMed] [Google Scholar]
- 38. Shen E, Xie K, Jwo K, Smith J, Mosaed S. Dupilumab-induced follicular conjunctivitis. Ocul Immunol Inflamm. 2018;1–3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.