Abstract
Purpose
To evaluate the safety and tolerability of a microsphere thermo-responsive hydrogel drug delivery system (DDS) loaded with aflibercept in a nonhuman primate model.
Methods
A sterile 50 µL of aflibercept-loaded microsphere thermo-responsive hydrogel-DDS (aflibercept-DDS) was injected intravitreally into the right eye of 10 healthy rhesus macaques. A complete ophthalmic examination, intraocular pressure (IOP) measurement, fundus photography, spectral-domain optical coherence tomography (SD-OCT), and electroretinogram were performed monthly for 6 months. One macaque was euthanized monthly, and the enucleated eyes were submitted for measurement of bioactive aflibercept concentrations. Four eyes were submitted for histopathology.
Results
Injected aflibercept-DDS was visualized in the vitreous until 6 months postinjection. No abnormalities were observed in the anterior segment, and IOP remained within normal range during the study period. A small number of cells were observed in the vitreous of some macaques, but otherwise the remainder of the posterior segment examination was normal. No significant changes in retinal architecture or function as assessed by SD-OCT and histology or full-field electroretinography, respectively, were observed. A mild, focal foreign body reaction around the injectate was observed with histology at 6 months postinjection. A mean of 2.1 ng/µL of aflibercept was measured in the vitreous.
Conclusions
Intravitreally injected aflibercept-DDS achieved controlled, sustained release of aflibercept with no adverse effects for up to 6 months in the eyes of healthy rhesus macaques.
Translational Relevance
Aflibercept-DDS may be a more effective method to deliver bioactive antivascular endothelial growth factor agents than current practice by reducing the frequency of intravitreal injections and providing controlled drug release.
Keywords: anti-VEGF, thermo-responsive hydrogel, aflibercept, nonhuman primate, intravitreal injection, controlled drug delivery
Introduction
Intravitreal (IVT) injection of anti-vascular endothelial growth factor (VEGF) drugs is the current gold standard treatment for several vision threatening retinal conditions, including exudative age-related macular degeneration (AMD),1 diabetic macular edema,2 diabetic retinopathy,3 retinal vein occlusions,4 and other diseases resulting in retinal vascular leakage or choroidal neovascularization.5,6 In the past decade, multiple clinical trials have confirmed the efficacy of anti-VEGF agents, such as aflibercept, ranibizumab, and off-label use bevacizumab, for the aforementioned diseases.7–9 Nevertheless, a major limitation of current treatment is that repeated injection is required every 4 to 8 weeks because of the rapid clearance and short half-life of these drugs.10 Repeated injections carry a risk of serious potential complications, including IVT hemorrhage, cataracts, endophthalmitis, and retinal detachment.11,12 Furthermore, the human health care burden of these frequent injections is immense with approximately 6 million injections performed in the United States alone in 2016—a number that is expected to increase annually.13,14 Therefore it is critical to find a therapy that is efficacious but requires less frequent administration to minimize the socioeconomic impact associated with recurrent injections and to lower the risk of associated potential complications.
To address this clinical need, an effective drug delivery platform would achieve sustained drug release with high encapsulation efficiency. So far, only a few sustained ocular drug delivery systems (DDS) are commercially available and most of these devices are solid implants that are unsuitable for delivery of delicate antibody-based agents, such as anti-VEGF agents.15,16 Therefore we developed a DDS consisting of biodegradable poly lactic-co-glycolic acid (PLGA) microspheres suspended within a thermo-responsive, injectable hydrogel to address this clinical requirement.17,18 Thermo-responsive hydrogel has demonstrated great promise in drug delivery owing to their ability to change physical state rapidly and is reversible. Below the transition temperature (∼32°C) the hydrogel is swollen in liquid-like form, and above the transition temperature the hydrogel collapses to solidified form.19 The thermo-responsive hydrogel used as the basis of our DDS is easy to inject intravitreally and undergoes rapid transform into a viscoelastic solid upon reaching physiological temperatures in the vitreous.20 As a bioerodible and biocompatible copolymer, PLGA has been used extensively in many US Food and Drug Administration (FDA)-approved therapeutic devices.16 Numerous drugs have been delivered using PLGA for a duration ranging from weeks to months depending on the formulation and agent.18,21,22 In addition, sustained release of drugs with tailored characteristics can be achieved by encapsulating drugs within PLGA microspheres.17 Moreover, our previous studies have demonstrated controlled release of bioactive anti-VEGF therapeutics from PLGA microspheres for approximately 200 days.17,20
To further investigate the potential clinical usage of anti-VEGF-loaded DDS, in vivo studies of the safety and biocompatibility of the device is required, preferentially in the eyes of an animal model that has similar eye size and retinal structure as a human. Therefore the purpose of this study was to evaluate the safety and preliminary bioactive aflibercept concentrations in the vitreous from the aflibercept encapsulated microsphere-hydrogel DDS using a nonhuman primate model.
Methods
Fabrication of Aflibercept-Loaded PLGA Microsphere Thermo-Responsive Hydrogel (Aflibercept-DDS)
Aflibercept-DDS was synthesized as previously described.17,18,23 Briefly, aflibercept stock solution (40 mg/mL) was used as an inner water phase (w1) to make aflibercept-loaded ∼7 µm PLGA microspheres. The primary water-in-oil emulsion (w1/o) was created by vortex; this first emulsion was immediately added to the outer aqueous phase (w2) containing 10% polyvinyl alcohol to create a (water-in-oil)-in-water (w1/o/w2) double emulsion by vortex. Several excipients were used to stabilize and protect the aflibercept during fabrication, storage, and release: bovine serum albumin (BSA), linear polyethylene glycol (PEG; MW 8 kDa), and sucrose were added in the w1 phase, whereas Mg(OH)2 was added as a buffering salt in the oil phase (o). After solvent evaporation, microspheres were harvested by centrifugation, washed three times in deionized (DI) water, and lyophilized to a dry powder.
Nondegradable thermo-responsive hydrogels composed of poly (ethylene glycol) diacrylate (PEG-DA) and N-isopropylacrylamide (NIPAAm) were synthesized by free-radical polymerization method as described elsewhere.20,24 Briefly, hydrogels precursors were prepared by dissolving 350 mM NIPAAm, 50 mM N-tert-butylacrylamide, 2 mM PEG-DA, and 13 mM ammonium persulfate in pH 7.4 1x phosphate buffered saline (PBS). To make microsphere-hydrogel DDS, aflibercept-loaded PLGA microspheres were placed under ultraviolet light for 30 minutes, and then 20 mg/mL PLGA microspheres were suspended in the aforementioned hydrogel precursors. Under sterile conditions, the hydrogel precursor and initiator were filtered through a sterile 13-mm syringe filter (0.22 µm; Fisherbrand, Thermo Fisher Scientific, Waltham, MA) before polymerization. Polymerization of the hydrogel was initiated by mixing 168 mM N,N,N’,N’-tetramethylethylenediamine (pH 7.4) into the hydrogel precursors; the reaction was allowed to proceed on ice for 30 minutes to form the microsphere-hydrogel DDS. After polymerization, the microsphere-hydrogel DDS were collected and washed at least three times in DI water. The aflibercept-DDS was loaded into a 1 cc insulin syringe and stored at 4°C in the refrigerator until use.
Animals
Ten healthy rhesus macaques (Macaca mulatta) were used in this study; 4 of them were male and 6 were female with a mean ± standard deviation age and body weight of 7.6 ± 2.6 years and 9.3 ± 3.3 kg, respectively (Supplementary material 2). All macaques were colony members housed at the California National Primate Research Center (CNPRC), which is accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All guidelines of the Association for Research in Vision and Ophthalmology Statement for the Use of Animal in Ophthalmic and Vision Research were followed. All studies were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (National Academies Press, 2011). Ophthalmic examination and injection of aflibercept-DDS was performed according to an animal protocol approved by the Institutional Animal Care and Use Committee at the University of California-Davis.
Six animals were allocated to the measurement of bioactive aflibercept concentrations in the vitreous and four were used for the safety study arm. Macaques were always sedated with intramuscular ketamine hydrochloride (5–30 mg/kg) and dexmedetomidine (0.0075–0.15 mg/kg) for all procedures and monitored by a trained technician and CNPRC veterinarian. Ophthalmic examinations were performed immediately prior to and following IVT injection then biweekly for up to 6 months; advanced ocular imaging was performed monthly. For the bioactive aflibercept concentration measurement, one macaque was euthanized every month after the IVT injection and eyes were enucleated.
IVT Injection of the Aflibercept-DDS
IVT injection of the aflibercept-DDS was performed by a board-certified veterinary ophthalmologist (SMT). Following sedation, the corneal and conjunctival surfaces were rinsed with 1% povidone iodine and sterile saline solution, and the conjunctival sac fornixes were cleaned with sterile cotton tip applicators wetted with the 1% povidone iodine solution. An eyelid speculum was placed, and the injection site was swabbed with a proparacaine soaked cotton tip applicator to achieve local anesthesia prior to the injection. Then a sterile aliquot of 50 µL aflibercept-DDS containing 0.0282 µg/µL aflibercept was administered by IVT injection to the right eye at 2.5 mm posterior to the superior limbus using a 22-gauge (G) needle. On removing the needle, the injection site was compressed with forceps and a cotton tip swab to prevent reflux. Neomycin-polymixin-bacitracin ophthalmic ointment was placed after the injection.
Ophthalmic Examination and Advanced Ocular Imaging
Complete ophthalmic examination with the semiquantitative preclinical ocular toxicology scoring (SPOTS) system25 was performed using a handheld slit lamp (Kowa SL-17; Kowa Optimed, Tokyo, Japan) and indirect ophthalmoscopy (Keeler VANTAGE Plus; Keeler Inc., Broomall, PA) with a 28 and 2.2 D indirect lens (Volk Optical, Inc., Mentor, OH) before IVT injection (baseline) and biweekly thereafter for up to 6 months. Intraocular pressure (IOP) was measured with rebound tonometry (TonoVet; Icare, Helsinki, Finland) and eyes were photographed with a digital color camera (Canon EOS5D; flash 1/64. ios 200, F16) at each examination. Pupil dilation was achieved with instillation of 1% tropicamide (Bausch & Lomb Inc., Tampa, FL) and 2.5% phenylephrine hydrochloride (Paragon BioTeck, Inc., Portland, OR) before evaluating the posterior segment.
Advanced ocular imaging was performed monthly for up to 6 months. Color fundus photography was performed using a CF-1 Retinal Camera with a 50° wide-angle lens (Canon, Tokyo, Japan). Infrared reflectance (IR) and blue-peak fundus autofluorescence imaging was performed with spectral-domain optical coherence tomography (SD-OCT) using the Spectralis device (HRT+OCT platform; Heidelberg Engineering, Heidelberg, Germany), followed by fluorescein angiography using the same instrument. The IR images were captured using an 820 nm diode laser, along with a 30° x 5° SD-OCT horizontal raster scan with 1536 A-scans per B-scan and 234 µm spacing between B-scans in high-resolution mode. Confocal scanning laser ophthalmoscopy was used to capture 30° x 30° autofluorescence images using an excitation light of 488 nm and long-pass barrier filter starting at 500 nm, along with a 20° x 20° SD-OCT volume scan with 1024 A-scan per B-scan and raster line spacing of 51 µm, centered on the fovea, in high-resolution mode. The Heidelberg eye tracking Automatic Real-Time (ART) software was set at 25 scans for each B-scan.
Retinal Thickness Measurement with OCT Images
Quantitative measurement of retinal layers of the SD-OCT images was obtained using Heidelberg Explorer software (version 1.8.6.0; Heidelberg Engineering) at 3 mm laterally away from the center of the fovea. Measured layers include total retinal thickness (from the internal limiting membrane to Brush's membrane), inner retinal layer (from the internal limiting membrane to the external limiting membrane), outer retinal layer (from the external limiting membrane to Brush's membrane), nerve fiber layer, ganglion cell/inner plexiform layers (GCL/IPL), inner nuclear layer, outer plexiform layer, outer nuclear layer, and retinal pigment epithelium. The GCL/IPL were measured as a single complex owing to difficulty in distinguishing between the two layers on SD-OCT.
Electroretinography (ERG)
ERG was performed monthly for 6 months in three macaques. While sedated, macaques were dilated and dark-adapted for 30 minutes. The RETevet device (LKC Technologies, Gaithersburg, MD) and an ERG-Jet electrode (LKC Technologies) were used to perform a standard flash ERG according to the approved protocol of the International Society for Clinical Electrophysiology of Vision.26 This test consisted of ERG using flash stimuli at 0.01, 3.0 and 10.0 cd·s/m2 all in the dark-adapted state. In addition, flash stimuli of 3.0 cd·s/m2 intensity were used after 10 minutes in the light-adapted state, as well as a 30 Hz photopic flicker. Measurements were recorded and displayed using the manufacturer's software.
Tissue Processing for Aflibercept Concentration Measurement and Histology
Euthanasia using sodium pentobarbital (120 mg/kg, intravenous) and enucleation of both eyes was performed by trained staff of the CNPRC. The frozen globe was stored in –80°C after snap freeze with liquid nitrogen and shipped on dry ice to the University of Illinois at Chicago Research Resource Center to measure the bioactive concentration of aflibercept. Four eyes from two macaques (#7 and #8) were placed in 10% buffered formalin and were submitted to the Comparative Ocular Pathology Laboratory of Wisconsin for histopathological evaluation. Briefly, the formalin-fixed eyes were sectioned on the dorsoventral parasagittal plane, to include the injection site. An additional horizontal section of the lateral calotte was obtained to sample the fovea. Both the medial calotte containing the injection site, aligned with a section through the pupillary space and optic nerve head and the additional horizontal section of the lateral calotte containing the fovea were routinely processed and embedded in paraffin. An initial 5-µm-thick section of the tissue blocks was obtained and stained with hematoxylin and eosin (H&E). The blocks were then step sectioned, with additional 5-µm-thick sections obtained every 200 µm step until the fovea and (in the treated eyes) the injected test article were completely sampled, generating an average of 25 slides on the treated and 11 slides on the untreated eyes. All additional slides were H&E-stained and analyzed under light microscopy by a board-certified veterinary pathologist (LBCT).
Measurement of Aflibercept Concentration
Animals received DDS IVT injections in the right eyes; left eyes were uninjected and served as controls. Animals were euthanized at predetermined time points, both eyes enucleated and immediately frozen at –80°C. The frozen eyes were shipped overnight for an analysis of aflibercept concentration. Three sets of globes could not be used for the analysis because of the unexpected temperature change during the shipping (#4–#6; Supplementary material 2). Ten globes from 5 animals at 1, 2, 3, 5, and 6-month post-IVT injection were used for the measurement of aflibercept concentration. The frozen vitreous was isolated from the other parts of the globe and homogenized as described before.27 Dot blot immunoassay was used to measure anti-VEGF drug concentration present in vitreous samples at corresponding time points. The final anti-VEGF concentration was obtained by subtracting the background value from control eyes to eliminate any potential nonspecific binding signal. Briefly, grids indicating blot regions were drawn on the nitrocellulose membrane. Each blot region was coated with 2 µL of 50 ng/µL recombinant human VEGF165 overnight at 4°C. Following × 3 washing with PBS containing 0.05% Tween-20, nonspecific sites were blocked by soaking in 5% BSA in Tris-Buffered Saline with 0.1% Tween-20 (TBS-T) for 30 minutes at 4°C. After × 3 washing with TBS-T, 2µL of vitreous samples were diluted with 0.1% BSA and added at the center of each blot, and incubated for 2.5 hours at 4°C. Following × 3 washing with TBS-T and shaking at 4°C, secondary goat anti-human IgG conjugated with horseradish peroxidase (diluted 1:15,000 in 0.1% BSA) was added to each blot and incubated for 30 minutes at 4°C. The membrane was washed with TBS-T six times, then incubated with enhanced chemiluminescence (ECL) reagent for 30 minutes. Images of blots were taken with a digital camera, and concentrations were quantified using a standard curve.
Statistical Analysis
Statistical analysis was performed with a commercially available statistical software program (GraphPad Prism 7.03; GraphPad Software Inc., San Diego, CA). Repeated measure two-way ANOVA followed by Tukey's multiple comparisons test was performed for comparison of IOP, ERG measurements, and semiautomated segmentation of retinal thickness. Significance was set at P <0.05. Results were expressed as mean ± standard error.
Results
Aflibercept-DDS Could be Delivered by IVT Injection and was Well Tolerated
Delivery of 50 µL aflibercept-DDS was successfully achieved by IVT injection in 9 eyes of 10 rhesus macaques without any complications (Fig. 1). In one macaque (#7), five attempts were made to inject the material using a 25G needle before a 22G needle was successfully used to deliver the material; a retinal tear with IVT hemorrhage occurred, and the macaque was euthanized 1 month postinjection (Supplementary material 1 and 2). On fundus examination immediately post-IVT injection, the retinal hemorrhage measured ∼2 x optic nerve head diameters in length and width and was localized to the medial midperipheral fundus and was still visible but smaller in size at 2 weeks after the injection. At 1 month postinjection, the hemorrhage had nearly resolved with a retinal scar and vitreous liquefaction observed.
Figure 1.
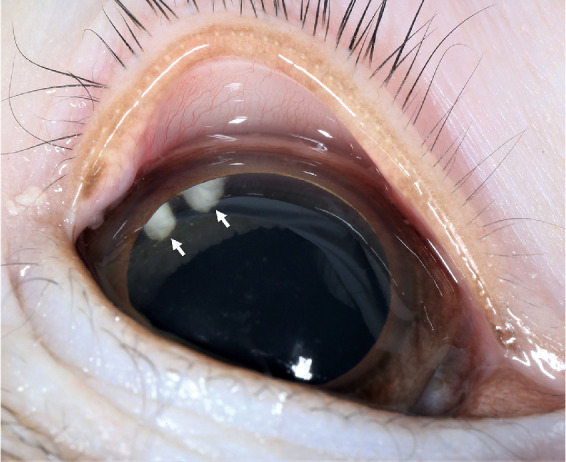
Aflibercept-loaded microsphere thermo-responsive hydrogel DDS (aflibercept-DDS) is visible in the vitreous of a 4-year-old male rhesus macaque at 2 months postinjection. A representative image shows aflibercept-DDS (white arrows) that was delivered by IVT injection in the superotemporal anterior vitreous. The aflibercept-DDS was visible in the vitreous for 6 months postinjection.
Ocular examinations in all macaques revealed symmetric pupillary light reflexes and mostly unremarkable anterior segments. Among 9 injected eyes, 26 to 50 cells predominantly white in color were observed in the slit beam (0.1 mm beam width and 10 mm beam height, 30°–45° beam angle, and 10–16 x magnification) of the anterior chamber at 14 days after the injection in only one eye (#8); at 1-month postinjection no cells were observed. Mean IOP remained in the normal range (10–21 mm Hg)28 after the injection in all groups, and no significant differences were found between groups (P = 0.16) or measurement times (P = 0.66) (Fig. 2).
Figure 2.
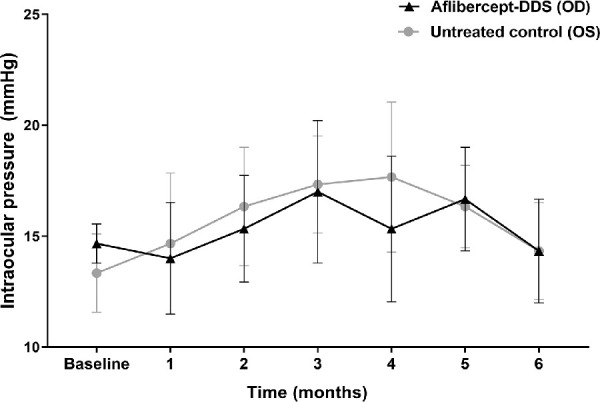
No significant changes in IOP following injection of aflibercept-DDS to the right eye (oculus dexter, OD); the left eye (oculus sinister, OS) served as an untreated control. For the entire experimental timeframe, mean IOP remained in the normal range (10–21 mm Hg)25 without significant differences between groups (P = 0.16) and time points (P = 0.66) by a repeated measure two-way ANOVA followed by Tukey's multiple comparisons test.
A small amount of vitreous cells of brown and white color were observed in all 9 injected eyes for up to 3 months after the injection; median 1.5 (ranging from 0.5–2) at 1 month, 1.5 (ranging from 0.5–2) at 2 months, and 0.5 (ranging from 0.5–1) at 3 months postinjection. Otherwise, the vitreous examination was normal. The injected test article was observed as white sparkly particles in the vitreous throughout the 6 months of examination. At 2 and 4 weeks postinjection, white, sparkly particles consistent with test article were visible on the posterior lens capsule before disappearing by 2 months postinjection.
Normal Retinal Morphology and Function Postinjection of Aflibercept-DDS
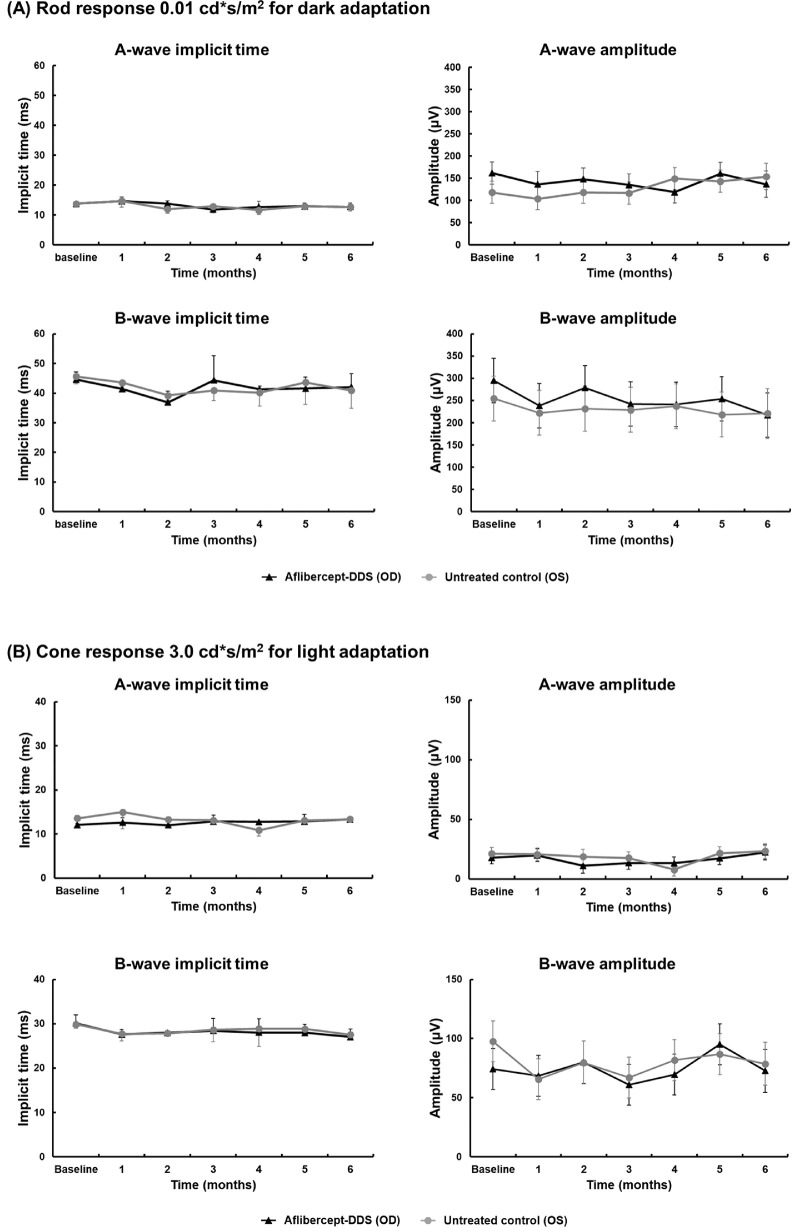
The fundus of all macaques appeared normal at all time points by indirect ophthalmoscopy and fundus photography (Supplementary material 3), except for the previously described findings in the macaque with the retinal tear (#7). Using SD-OCT, no morphologic abnormalities were observed in any of the nine macaques at any time point; all measurements of the retinal layers remained similar to the baseline values (Fig. 3). In addition, there was no significant difference in the thickness of each retinal layer between the treated eye (right) and the control eye (left) (Supplementary material 2). Scotopic and photopic full-field ERGs were performed on both eyes and demonstrated normal rod and cone responses throughout the 6 months postinjection (Fig. 4). There were no significant differences between control and treated eyes or at different time points for all measured values (Supplementary material 4).
Figure 3.
Thickness of the entire retina, as well as individual layers, did not significantly differ over time or between aflibercept-DDS versus untreated eyes. Thickness of the entire retina (A) and its individual layers (B, inner retinal layer; C, outer retinal layer; D, ganglion cell and inner plexiform layer; E, nerve fiber layer; F, inner nuclear layer; G, outer plexiform layer; H, outer nuclear layer; I, retinal pigment epithelium) in the aflibercept-DDS treated right eye (oculus dexter or OD, black line) and untreated left eye (oculus sinister or OS, gray line) were measured monthly with SD-OCT for 6 months. A two-way ANOVA followed by Tukey's multiple comparison test was used to compare groups and time points. The measurements and P values are available for review in Supplementary material 4.
Figure 4.
Retinal function as measured by full-field ERG did not significantly differ over time or between aflibercept-DDS (oculus dexter, OD) versus untreated eyes (oculus sinister, OS). Comparison of A- and B-wave amplitudes and implicit times did not significantly differ between time points or eyes under scotopic (rod response) and photopic (cone response) conditions. (A) Dark adaptation: 0.01 cd·s/m2, chromaticity (0.33, 0.33) at 0.05 Hz; background: 0.0 cd·s/m2; (B) light adaptation: 3.0 cd·s/m2, chromaticity (0.33, 0.33) at 2 Hz, background: 30 cd·s/m2, chromaticity (0.33, 0.33). A two-way ANOVA followed by Tukey's multiple comparison test was used to compare groups and time points. The measurements and P values are available for review in Supplementary material 5.
Aflibercept-DDS does not Incite Inflammation
One injected right eye and contralateral noninjected left eye from one macaque (#8) were submitted for histology 6 months postinjection. Histologically, there was a well-delineated accumulation of light basophilic, amorphous to granular material consistent with test article lined by a thin, fibrous membrane containing moderate numbers of macrophages in the dorsal vitreous chamber adjacent to the ciliary body pars plana (Fig. 5). The macrophages presented both epithelioid and multinucleated giant cell phenotype and contained multiple small distinct round intracytoplasmic vacuoles (lipid) or light brown to gray amorphous material in the cytoplasm, morphologically similar to the test article. The cellular reaction associated with the test article material was consistent with a localized foreign body reaction. The foreign body reaction, including the fibrous membranes, was found exclusively around the test article material with no observed extension into the adjacent vitreous or peripheral retina. No retinal or scleral lesions associated with the intraocular injection were identified, including retinal detachment, retinal traction, and scleral fibrosis. Also, no inflammatory reaction was found in the ocular tissue both adjacent or away from the injection site. The remainder of the globe was morphologically normal.
Figure 5.
Histopathology of the right globe of a rhesus macaque demonstrated no inflammatory reaction or pathologic changes 6 months after injection with aflibercept-DDS. (A) Gross photos of the aflibercept-DDS injected eye showed oval white DDS materials (*) in the superotemporal vitreous adjacent to the pars plana of the ciliary body. (B) Well-delineated accumulation of light basophilic, amorphous to granular DDS material (*) lined by a thin, fibrous membrane containing moderate numbers of macrophages. No retinal traction, detachment or thinning was identified in any examined sections.
The histology of the excluded macaque (#7) revealed moderate hemorrhage in the vitreous and a dense accumulation of red blood cells admixed with clusters of macrophages in the anterior vitreous lining the ciliary body pars plana and peripheral retina (Supplementary material 1). Some of the macrophages contained intracytoplasmic red blood cells, whereas others contained a light brown to gray amorphous material consistent with test article. There were no identifiable retinal or scleral lesions within the plane of section examined.
Bioactive Aflibercept was Present Throughout the Study Period
The vitreous concentration of bioactive aflibercept was measured to be 2.19, 1.2, 2.05, 1.31, and 0.6 ng/µL at 1, 2, 3, 5, and 6 months after DDS IVT injection (Fig. 6). The month 4 sample was not analyzed as the sample arrived at room temperature. A steady concentration of aflibercept was detected during the first 5 months, with a decline in concentration (<1 ng/µL) observed at 6 months. This is consistent with a decrease in stability and release as reported in our previous studies.23 However, all the detected vitreous aflibercept concentrations were well above the reported IC50 for human umbilical vein endothelial cells (HUVECs) in vitro (0.003 ng/µL).29
Figure 6.

Vitreous concentrations of aflibercept following aflibercept-DDS IVT injection well exceeds the in vitro half maximal inhibitory concentration (IC50 = 0.003 ng/µL) of HUVECs for 6 months postinjection. One primate was measured at each time point.
Discussion
The current study showed in vivo safety, tolerability, and biocompatibility of the aflibercept-DDS in the eyes of healthy nonhuman primates for up to 6 months after IVT injection. All aflibercept-DDS injected eyes had no morphologic and functional abnormalities for up to 6 months after the IVT injection. The results of the present study using a nonhuman primate model are consistent with our previous studies using aflibercept-DDS in cell-based models and rodents.18,20,23,30,31 In support of the current study, we have done detailed physicochemical characterization of the DDS formulation in our previous publications.17,23,24 We have also previously demonstrated in vitro drug release17,18,20,23,31 and in vivo safety and efficacy of the DDS in a small rodent model.20,30 Specifically, the aflibercept-DDS and its degraded products showed no toxicity to any cells within the eye consistent with the lack of cytotoxicity observed in HUVECs when the aflibercept-DDS was washed for more than three times, identical to the protocol used in this study.23 Also, the injected aflibercept-DDS showed no migration into the anterior chamber and no anterior and posterior uveitis up to 6 months for long-term IVT delivery of VEGF, which contrasts with other recent studies of DDS systems.32–34 A recent study of IVT injection of PLGA microspheres in cynomolgus macaques describes a moderate to severe inflammatory response within 1 month postinjection that included corneal edema, corneal neovascularization, aqueous flare, vitreous cells, iridal hyperemia, posterior synechiae, inflammatory cells on the lens, and cystoid macular edema that necessitated euthanasia for animal welfare purposes.34 Although it was observed in this study that smaller microspheres (20 µm in diameter) caused the most severe reaction, whereas the larger PLGA rods (0.9 × 3.7 mm) were well tolerated,34 we highlight that the encapsulation of the 7 µm PLGA microspheres in the hydrogel is likely responsible for the minimal observed inflammation in the present study. Specifically, the crosslink density of the hydrogel has been optimized to prevent microsphere diffusion out of the hydrogel.17 We have previously demonstrated that the radius of gyration of the hydrogel to be ∼17 nm in swollen state, and this dimension ensures that microspheres will not diffuse out of the hydrogel.17 The lack of anterior and posterior uveitis observed in the present study combined with retention of normal retinal morphology and function suggests that encapsulation of microspheres within a hydrogel composite DDS may be a compelling alternative to IVT drug delivery with PLGA microspheres alone.
In this study, we detected effective release of aflibercept for up to 6 months postinjection in the vitreous. Aflibercept is a 115 kD soluble decoy receptor fusion VEGF that binds to all isoforms of VEGF-A and VEGF-B, as well as placental growth factor. It was FDA-approved for treatment of wet AMD in 2011.35 Excellent treatment outcomes have been demonstrated with aflibercept in human patients.7 However, similar to other anti-VEGF agents, monthly or bimonthly bolus IVT injections are required for aflibercept to treat wet AMD, as well as other neovascular retinal diseases. With each IVT injection, potential complications can occur, including cataract development, retinal detachment, and endophthalmitis.11 The DDS used in the present study has shown sustained drug delivery in vitro17 and in vivo20 Specifically, the drug-coated PLGA microspheres suspended in the thermo-responsive hydrogel DDS used in the present study showed significantly higher encapsulation efficacy and lower initial burst than microspheres alone, resulting in more consistent release of bioactive aflibercept.18 Furthermore, the DDS in this study showed controlled, extended release aflibercept and ranibizumab at bioactive concentrations that was well tolerated in a laser- induced choroidal neovascularization model using rodents.20 In the present study, the bioactive aflibercept concentrations measured in the vitreous well exceed that required to reduce VEGF-A induced vascular endothelial cell proliferation by 50% (1.1 × 10−5 to 2.7 × 10−5 ng/μL)36 throughout the entire study duration. Given the similar anatomy between the macaque and human eye, this suggests that aflibercept-DDS is capable of releasing bioactive aflibercept in human eyes for 6 months as well above the IC50. Although the current study utilized degradable microspheres in a nondegradable thermo-responsive hydrogel to visualize the material during histopathological analysis, we have developed and optimized a fully degradable aflibercept-DDS23,31 and intend to utilize this formulation for future studies in nonhuman primates.
Ocular delivery of drugs is challenging to pharmaceutical scientists and clinicians given the unique barrier structures in the eye. This is particularly true for delivering therapeutic agents to the posterior segment with IVT injections the currently standard of care in physician-based ophthalmology.37 However, intravitreally injected drugs are rapidly eliminated via anterior and posterior clearance routes with reported half-lives ranging from hours to days based on their particle size or lipophilicity.38,39 Ninety percent of injected anti-VEGF agents are eliminated from the vitreous via the trabecular meshwork because their large molecular size restricts penetration across the blood–retinal barrier,40 with IVT half-lives of ∼1 week.10 Thus IVT injections must be repeated between 4 to 8 weeks with a concomitant health care burden to patients and clinicians, as well as a risk of complications.38 In this study, aflibercept-DDS was observed in the vitreous for up to 6 months postinjection with effective release of bioactive aflibercept. Test article was only observed in the vitreous chamber with no particles visible in the anterior chamber in any macaques throughout the experimental period. The aflibercept-DDS is designed to be retained within the vitreous with microspheres ∼7 µm in diameter17 embedded in hydrogel to limit migration. This is a critical modification as a myriad of DDSs have been shown to migrate into the anterior chamber with resultant anterior uveitis.32,41 In the present study, a nondegradable aflibercept DDS was used to facilitate identification of the material with histopathology. We do acknowledge that this nondegradable DDS material did incite a mild foreign body reaction but highlight that this response was markedly less severe than that observed by Thackaberry et al.34 Furthermore, the aflibercept-DDS did not result in any structural changes to the eye as determined by ocular examination, SD-OCT and histology or functional changes to the retina using electrophysiology. We plan to do further safety studies using a degradable aflibercept-DDS and expect less foreign body reaction in comparison to nondegradable material.
Various delivery systems, such as chemical permeability enhancers,42 prodrugs,43,44 nano-drug carriers,45,46 microneedles,47 and stimuli-responsive in situ gels,48 have been developed to facilitate drug delivery to the posterior segment. Among these systems, in situ gels containing stimuli-responsive polymers (such as pH-, thermo-, or ion- sensitive polymers) are one of the most promising approaches to improve the retention time of drugs.49 Thus far, thermo-responsive polymers have been applied for a variety of ophthalmic applications. For the anterior segment, thermo-responsive in situ forming gels containing antihistamine or anti-inflammatory drugs have been tested to improve transcorneal absorption of drugs.50–52 Reversible thermo-responsive sealants have been applied for temporary closure of surgical incisions or traumatic injuries of the sclera and cornea.53 Moreover, intrascleral delivery of in situ thermo-responsive implants have been tested with a microneedle injection system.54 However, most currently available IVT devices are in solid form.16 In the present study using a nonhuman primate model, the injected aqueous thermo-responsive DDS underwent rapid gelation in the vitreous and achieved sustained release of loaded drugs for at least 6 months. These results show the feasibility of our injectable thermo-responsive DDS for anti-VEGF therapy, as well as a delivery method for other IVT drugs, such as antibiotics or anti-inflammatory medications.
In the present study, a 22G needle was required to deliver the aflibercept-DDS. Previous studies have reported delivery of aflibercept-loaded DDS through a 28G needle in vitro at room temperature,23 as well as in vivo in rat eyes.20 However, the temperature equilibrium between the contiguous environment and the eye is greater in rats versus primates because of the short distance between the ocular surface and uvea in the small eye of rats.55,56 By comparison, the relatively deep anterior chamber of nonhuman primates protects the uvea from fluctuations in ambient temperatures.57 Vitreal temperature of the human eye, which is similar in size to that of rhesus macaques, is ∼33°C to 34°C, and decreased only ∼2.5°C following intraocular surgery.57 Thus we surmise that the aflibercept-DDS gelled rapidly within the small gauge needle from relatively higher temperature in the vitreous of the macaques versus rats. We would thus expect similar challenges when injecting the DDS into human eyes. Although we did not identify any efflux or complications from administration of the aflibercept-DDS through a 22G needle, presumably due to rapid gelation at the needle entry point, either the aflibercept-DDS will need to be modified or a more appropriately sized needle (27G–30G) will need to be designed for IVT injection in human patients.
Conclusions
A major limitation of this study is the small number of animals and future studies are required with larger numbers of nonhuman primates. In the present study, nonhuman primates were utilized as their globe size and retinal structure, particularly the presence of a macula, are most predictive for studying the safety and efficacy of DDSs for anti-VEGF agents.58 In conclusion, the aflibercept-DDS caused no anatomic or functional changes in the eyes of rhesus macaques for up to 6 months after IVT injection other than a mild, focal foreign body reaction around the injectate observed only with histology. Efficacy studies of this system in a nonhuman primate model of choroidal neovascularization are now warranted. Aflibercept-DDS is a promising delivery method for anti-VEGF agents by providing steady, controlled drug release and reducing the frequency of IVT injections.
Supplementary Material
Acknowledgments
The authors thank Bao-Shiang Lee at the University of Illinois at Chicago for measurements of the aflibercept bioactive concentration, and Monica Motta and Ariana Marangakis for excellent technical assistance.
Supported by the National Institutes of Health Office of the Director, OD011107 and P30 EY12576.
Disclosure: S. Kim, None; J.J. Kang-Mieler, microsphere thermo-responsive drug delivery system (P); W. Liu, None; Z. Wang, None; G. Yiu, None; L.B.C. Teixeira, None; W.F. Mieler, None; S.M. Thomasy, None
References
- 1. Ba J, Peng RS, Xu D, et al.. Intravitreal anti-VEGF injections for treating wet age-related macular degeneration: a systematic review and meta-analysis. Drug Des Devel Ther. 2015; 9: 5397–5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. You G, Manjunath V, Chiu SJ, Farsiu S, Mahmoud TH. Effect of anti-vascular endothelial growth factor therapy on choroidal thickness in diabetic macular edema. Am J Ophthalmol. 2014; 158: 745–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bolinger MT, Antonetti DA. Moving past anti-VEGF: novel therapies for treating diabetic retinopathy. Int J Mol Sci. 2016; 17:pii:E1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiang Y, Mieler WF.. Update on the use of anti-VEGF intravitreal therapies for retinal vein occlusion. Asia-Pac J Ophthalmol. 2017; 6: 546–553. [DOI] [PubMed] [Google Scholar]
- 5. Barth T, Zeman F, Helbig H, Gamulescu MA. Intravitreal anti-VEGF treatment for choroidal neovascularization secondary to punctate inner choroidopathy. Int Ophthalmol. 2018; 38: 923–931. [DOI] [PubMed] [Google Scholar]
- 6. Hua DA, Casella AM, Berrocal MH, et al. Outcomes of anti-VEGF therapy in choroidal neovascularization after macular surgery. Retin Cases Brief Rep. 2018; 12: 359–366. [DOI] [PubMed] [Google Scholar]
- 7. Spooner K, Hong T, Nair R, et al.. Ling-term outcomes of switching to aflibercept for treatment-resistant neovascular age-related macular degeneration. Acta Ophthalmol. 2019; 97: e706–e712. [DOI] [PubMed] [Google Scholar]
- 8. Scott AW, Bressler SB.. Long-term follow-up of vascular endothelial growth factor inhibitor therapy for neovascular age-related macular degeneration. Curr Opin Ophthalmol. 2013; 24: 190–196. [DOI] [PubMed] [Google Scholar]
- 9. Kaiser PK, Singer M, Tolentino M, et al.. Long-term safety and visual outcome of intravitreal aflibercept in neovascular age-related macular degeneration. OphthalmolRetina. 2017; 1: 304–313. [DOI] [PubMed] [Google Scholar]
- 10. Stewart MW, Rosenfeld PJ, Penha FM, et al.. Pharmacokinetic rationale for dosing every 2 weeks versus 4 weeks with intravitreal ranibizumab, bevacizumab, and aflibercept (vascular endothelial growth factor Trap-eye). Retina. 2012; 32: 434–457. [DOI] [PubMed] [Google Scholar]
- 11. Sachdeva MM, Moshiri A, Leder HA, Scott AW. Endophthalmitis following intravitreal injection of anti-VEGF agents: long-term outcomes and the identification of unusual micro-organisms. J Ophthalmic Inflamm Infect. 2016; 6: 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta A, Sun JK, Silva PS. Complications of intravitreous injections in patients with diabetes. Semin Ophthalmol. 2018; 33: 42–50. [DOI] [PubMed] [Google Scholar]
- 13. Grzybowski A, Told R, Sacu S, et al.. 2018 update on intravitreal injections: Euretina expert consensus recommendations. Ophthalmolgica. 2018; 239: 181–193. [DOI] [PubMed] [Google Scholar]
- 14. Patel S. Medicare spending on anti-vascular endothelial growth factor medications. Ophthalmol Retina. 2018; 2: 785–791. [DOI] [PubMed] [Google Scholar]
- 15. Choonara YE, Pillay V, Danckwerts MP, Carmichael TR, du Toit LC. A review of implantable intravitreal drug delivery technologies for the treatment of posterior segment eye diseases. J Pham Sci. 2010; 99: 2219–2239. [DOI] [PubMed] [Google Scholar]
- 16. Kang-Mieler JJ, Osswald CR, Mieler WF. Advances in ocular drug delivery: emphasis on the posterior segment. Expert Opin Drug Deliv. 2014; 11: 1647–1660. [DOI] [PubMed] [Google Scholar]
- 17. Osswald CR, Kang-Mieler JJ.. Controlled and extended release of a model protein from a microsphere-hydrogel drug delivery system. AnnBiomedEng. 2015; 43: 2609–2617. [DOI] [PubMed] [Google Scholar]
- 18. Osswald CR, Kang-Meiler JJ.. Controlled and extended in vitro release of bioactive anti-vascular endothelial growth factors from a microsphere-hydrogel drug delivery system. CurrEye Res. 2016; 41: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 19. Dhara D, Chatterji PR.. Phase transition in linear and cross-linked poly(N-isopropylacrylamide) in water: effect of various types of additives. J Macromol Sci Rev Macromol Chem Phys. 2000; 40: 51–68. [Google Scholar]
- 20. Osswald CR, Guthrie MJ, Avila A, Valio JA Jr, Mieler WF, Kang-Mieler JJ. In vivo efficacy of an injectable microsphere-hydrogel ocular drug delivery system. Curr Eye Res. 2017; 42: 1293–1301. [DOI] [PubMed] [Google Scholar]
- 21. Holmkvist AD, Friberg A, Nilsson UJ, Schouenborg J. Hydrophobic ion pairing of an minocycline/Ca(2+)/AOT complex for preparation of drug-loaded PLGA nanoparticles with improved sustained release. Int J Pharm. 2016; 499: 351–357. [DOI] [PubMed] [Google Scholar]
- 22. Park MH, Jun HS, Jeon JW, et al.. Preparation and characterization of bee venom-loaded PLGA particles for sustained release. Pharm Dev Technol. 2018; 23: 857–864. [DOI] [PubMed] [Google Scholar]
- 23. Liu W, Lee BS, Mieler WF, Kang-Mieler JJ. Biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended release of bioactive aflibercept in vitro. Curr Eye Res. 2019; 44: 264–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Darpala PW, Jiang B, Chiu YC, et al.. The effect of glutathione as chain transfer agent in PNIPAAm-based thermo-responsive hydrogels for controlled release of proteins. Pharm Res. 2014; 31: 742–753. [DOI] [PubMed] [Google Scholar]
- 25. Eaton JS, Miller PE, Bentley E, Thomasy SM, Murphy CJ. The SPOTS system: an ocular scoring system optimized for use in modern preclinical drug development and toxicology. J Ocul Pharmacol Ther. 2017; 33: 718–734. [DOI] [PubMed] [Google Scholar]
- 26. McCulloch DL, Marmor MF, Brigell MG, et al.. ISCEV standard for full-field clinical electroretinography (2015 update). Doc Ophthalmol. 2015; 130: 1–12. [DOI] [PubMed] [Google Scholar]
- 27. Schwartz DM, Shuster S, Jumper MD, Chang A, Stern R. Human vitreous hyaluronidase: isolation and characterization. Curr Eye Res. 1996; 15: 1156–1162. [DOI] [PubMed] [Google Scholar]
- 28. Bito LZ, Merritt SQ, DeRousseau CJ. Intraocular pressure of rhesus monkey (Macaca mulatta). I. An initial survey of two free-breeding colonies. Invest Ophthalmol Vis Sci. 1979; 18: 785–793. [PubMed] [Google Scholar]
- 29. Papadopoulos N, Martin J, Ruan Q, et al.. Binding and neutralization of vascular endothelial growth factor (VEGF) and related ligands by VEGF Trap, ranibizumab and bevacizumab. Angiogenesis. 2012; 15: 171–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Turturro SB, Guthrie MJ, Appel AA, et al.. The effects of cross-linked thermo-responsive PNIPAAM-based hydrogel injection on retinal function. Biomaterials. 2011; 32: 3620–3626. [DOI] [PubMed] [Google Scholar]
- 31. Liu W, Borrell MA, Venerus DC, Mieler WF, Kang-Mieler JJ. Characterization of biodegradable microsphere-hydrogel ocular drug delivery system for controlled and extended releases of ranibizumab. Transl Vis Sci Technol. 2019; 8: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adamson P, Wilde T, Dobrzynski E, et al.. Single ocular injection of a sustained-release anti-VEGF delivers 6months pharmacokinetics and efficacy in a primate laser CNV model. J Control Release. 2016; 244: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nayak K, Misra M.. A review on recent drug delivery systems for posterior segment of eye. Biomed Pharmacother. 2018; 107: 1564–1582. [DOI] [PubMed] [Google Scholar]
- 34. Thackaberry EA, Farman C, Zhong F, et al.. Evaluation of the toxicity of intravitreally injected PLGA microspheres and rods in monkeys and rabbits: effects of depot size on inflammatory response. Invest Ophthalmol Vis Sci. 2017; 58: 4274–4285. [DOI] [PubMed] [Google Scholar]
- 35. Food and Drug Administration. Summary of approval–Eylea (aflibercept): proposed indication: treatment of patients with neovascular (wet) age-related macular degeneration (AMD), application number:125387Orig1x000 Kansas: Food and Drug Administration (FDA); 2011. [Google Scholar]
- 36. Xu L, Lu T, Tuomi L, et al.. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci. 2013; 54: 1616–1624. [DOI] [PubMed] [Google Scholar]
- 37. Del Amo EM, Rimpelӓ AM, Heikkinen E, et al.. Pharmacokinetic aspects of retinal drug delivery. Prog Retin Eye Res. 2017; 57: 134–185. [DOI] [PubMed] [Google Scholar]
- 38. Subrizi A, Del Amo EM, Korzhakov-Vlakh V, Tennikova T, Ruponen M, Urtti A. Design principles of ocular drug delivery systems: importance of drug payload, release rate, and material properties. Drug Discov Today. 2019; 24: 1446–1457. [DOI] [PubMed] [Google Scholar]
- 39. Del Amo EM, Vellonen KS, Kidron H, Urtti A. Intravitreal clearance and volume of distribution of compounds in rabbits: in silico prediction and pharmacokinetic simulations for drug development. Eur J Pharm Biopharm. 2015; 95: 215–226. [DOI] [PubMed] [Google Scholar]
- 40. Hutton-Smith LA, Gaffney EA, Byrne HM, Caruso A, Maini PK, Mazer NA. Theoretical insights into the retinal dynamics of vascular endothelial growth factor in patients treated with ranibizumab, based on an ocular pharmacokinetic/pharmacodynamic model. Mol Pharm. 2018; 15: 2770–2784. [DOI] [PubMed] [Google Scholar]
- 41. Mozayan A, Farah S.. Acute anterior uveitis following intravitreal injection of bevacizumab. Ophthalmic Surg Lasers Imaging Retina. 2013; 44: 25–27. [DOI] [PubMed] [Google Scholar]
- 42. Jiang K, Gao X, Shen Q, et al.. Discerning the composition of penetratin for safe penetration from cornea to retina. Acta Biomater. 2017; 63: 123–134. [DOI] [PubMed] [Google Scholar]
- 43. Mandal A, Cholkar K, Khurana V, et al.. Topical formulation of self-assembled antiviral progrug nanomicelles for targeted retinal delivery. Mol Pharm. 2017; 14: 2056–2069. [DOI] [PubMed] [Google Scholar]
- 44. Daull P, Paterson CA, Kuppermann BD, Garrigue JS. A preliminary evaluation of dexamethasone palmitate emulsion: a novel intravitreal sustained delivery of corticosteroid for treatment of macular edema. J Ocul Pharmacol Ther. 2013; 29: 258–269. [DOI] [PubMed] [Google Scholar]
- 45. Del Pozo-Rodriguez A, Delgago D, Gascon AR, Solinis MA. Lipid nanoparticles as drug/gene delivery systems to the retina. J Ocul Pharmacol Ther. 2013; 29: 173–188. [DOI] [PubMed] [Google Scholar]
- 46. Jiang S, Franco YL, Zhou Y, Chen J. Nanotechnology in retinal drug delivery. Int J Ophthalmol. 2018; 11: 1038–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Jung JH, Chiang B, Grossniklaus HE, Prausnitz MR. Ocular drug delivery targeted by iontophoresis in the suprachoroidal space using a microneedle. J Control Release. 2018; 277: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kang Derwent JJ, Mieler WF. Thermoresponsive hydrogels as a new ocular drug delivery platform to the posterior segment of the eye. Trans Am Ophthalmol Soc. 2008; 106: 206–213. [PMC free article] [PubMed] [Google Scholar]
- 49. Wu Y, Liu Y, Li X, et al.. Research progress of in-situ gelling ophthalmic drug delivery system. Asian JPharmSci. 2019; 14: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Iohara D, Okubo M, Anraku M, et al.. Hydrophobically modified polymer/α-cyclodextrin thermoresponsive hydrogels for use in ocular drug delivery. Mol Pharm. 2017; 14: 2740–2748. [DOI] [PubMed] [Google Scholar]
- 51. Sharif Makhmalzadeh B, Salimi A, Niroomand A. Loratadine-loaded thermoresponsive hydrogel: characterization and ex-vivo rabbit cornea permeability studies. Iran J Pharm Res. 2018; 17: 460–469. [PMC free article] [PubMed] [Google Scholar]
- 52. M A Fathalla Z, Vangala A, Longman M, et al.. Poloxamer-based thermoresponsive ketorolac tromethamine in situ gel preparations: design, characterization, toxicity and transcorneal permeation studies. Eur J Pharm Biopharm. 2017; 114: 119–134. [DOI] [PubMed] [Google Scholar]
- 53. Bayat N, Zhang Y, Falabella P, et al.. A reversible thermoresponsive sealant for temporary closure of ocular trauma. Sci Transi Med. 2017; 9:pii:eaan3879. [DOI] [PubMed] [Google Scholar]
- 54. Thakur RR, Fallows SJ, McMillan HL, Donnelly RF, Jones DS. Microneedle-mediated intrascleral delivery of in situ forming thermoresponsive implants for sustained ocular drug delivery. J Pharm Pharmacol. 2014; 66: 584–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Salido EM, Dorfman D, Bordone M, Chianelli M, Gonzalez Fleitas MF, Rosenstein RE. Global and ocular hypothermic preconditioning protect the rat retina from ischemic damage. PLoS One. 2013; 8: 261656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kessel L, Johnson L, Arvidsson H, Larsen M. The relationship between body and ambient temperature and corneal temperature. Invest Ophthalmol Vis Sci. 2010; 51: 6593–6597. [DOI] [PubMed] [Google Scholar]
- 57. Iguchi Y, Asami T, Ueno S, et al.. Changes in vitreous temperature during intravitreal surgery. Invest Ophthalmol Vis Sci. 2014; 55: 2344–2349. [DOI] [PubMed] [Google Scholar]
- 58. Pennesi ME, Neuringer M, Courtney RJ. Animal models of age-related macular degeneration. Mol Aspects Med. 2012; 33: 487–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.