Abstract
Faithful transmission of genetic information is only possible with the structural and functional integrity of the genome. PTEN has been recognized as a guardian of the genome since the identification of its noncanonical localization and function in the nucleus. Yet, the role of PTEN in guarding the genome relies on integration of diverse mechanisms elicited by its canonical activity in antagonizing PI3K as well as emerging noncanonical functions. In the nucleus, PTEN maintains the structural integrity of chromosomes and the architecture of heterochromatin by physically interacting with chromosomal and nucleosomal components. PTEN also controls the functional integrity of key genetic transmission machineries by promoting proper assembly of the replisome and mitotic spindles. Deregulation of PTEN signaling impairs genome integrity, leading to spontaneous replication/mitotic stress and subsequent stress tolerance. Identification of novel targets of PTEN signaling and illumination of the interplay of diverse PTEN pathways in genome maintenance will help us better understand mechanisms underlying tumor evolution and therapeutic resistance.
Cancer is a convergent consequence of structural and functional impairment at multiple omic levels including genome, epigenome, metabolome, and immunome. Among these multifarious aberrant events, genetic and epigenetic alterations generate combined forces to drive transformation, whereas metabolic and immune responses provide selective pressures leading to clonal evolution of cancer. Although one gene mutation is usually insufficient to trigger tumorigenesis, single Pten mutations in mice lead to a variety of spontaneous tumors that mimic the spectrum of human cancers harboring PTEN mutations (Wang et al. 2010; Papa et al. 2014; Sun et al. 2014; Caserta et al. 2015). More intriguingly, studies using Pten models have uncovered its essential roles in governing the genome (Puc et al. 2005; Maser et al. 2007; Shen et al. 2007; Bassi et al. 2013; Sun et al. 2014), the epigenome (Chen et al. 2014; Gong et al. 2015), metabolism (Garcia-Cao et al. 2012; Ortega-Molina et al. 2012; Shinde and Maddika 2017), and immunity (Di Cristofano et al. 1999; Suzuki et al. 2001, 2003; Anzelon et al. 2003; Walsh et al. 2006; Heit et al. 2008; Leong et al. 2015). These studies demonstrate the diverse functions of PTEN in regulating nearly all the fundamental processes related to cancer evolution.
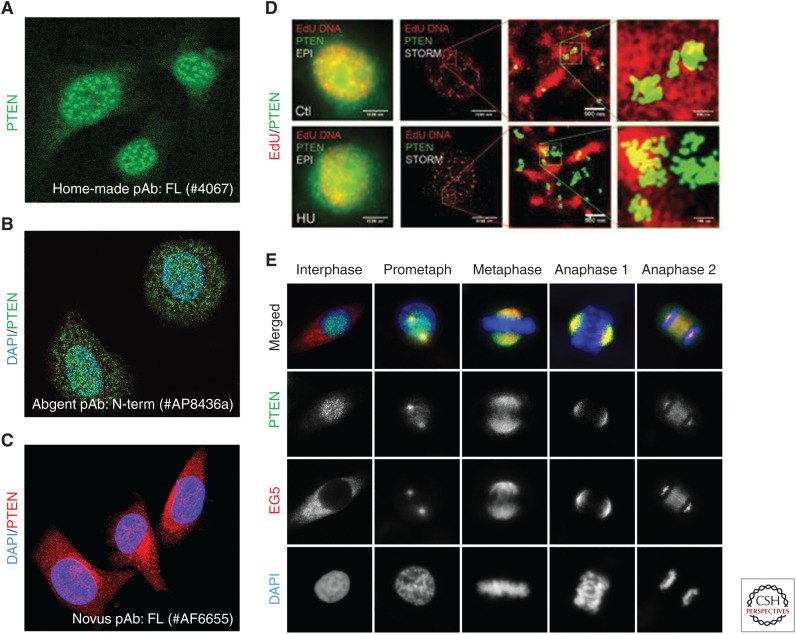
PTEN is well characterized as a lipid phosphatase that antagonizes PI3K signaling (Maehama and Dixon 1998; Chalhoub and Baker 2009). Although this canonical activity of PTEN is insufficient to justify its functional diversity and potency in tumor suppression, the PTEN–PI3K pathway plays an important part in noncanonical PTEN functions such as DNA repair. For example, the role of PTEN in maintaining a functional DNA damage checkpoint relies on its lipid phosphatase activity that suppresses PI3K-dependent phosphorylation of CHK1 (Puc et al. 2005). As a bona fide phosphatase, dephosphorylation of target molecules along a signaling cascade is expected to be the default means of guarding the genome for PTEN. Nevertheless, such “remote” regulation through a multistep pathway is rather uncommon. Instead, PTEN often uses an “adjoining” mode of regulation via physical association with multicomponent machinery to govern the integrity and functionality of the assemblage. The on-site involvement in multiple regulatory systems requires PTEN to be physically available with sufficient quantity and motility. This demand is well accommodated by the basal abundance of wild-type PTEN and its variable distribution patterns in different cell types at distinctive phases of the cell cycle (Fig. 1). These features are in stark contrast with the expression profile of p53, another well-characterized guardian of the genome and tumor suppressor. Wild-type p53 has a low basal expression level but possesses the ability to rapidly increase its expression in response to genotoxic stress (Oren 2003; Meek 2009). As a transcription factor and a stress-responsive molecule, p53 is predominantly localized in the nucleus and rapidly reactive to intracellular stress conditions and environmental stimuli by regulating gene transcription (Kastenhuber and Lowe 2017). Interestingly, PTEN depletion can activate p53 (Chen et al. 2005; Sun et al. 2014), likely by generating replication stress and mitotic stress at the genome level (collectively referred to as genome stress). These findings suggest that PTEN is a suppressor of genome stress. Clearly, these two guardians of the genome constitute a two-phase stress-management system—constitutively present PTEN prevents stress buildup, and if this fails, p53 induction and activation will elicit timely cellular stress responses.
Figure 1.
Diverse patterns of PTEN localization. (A) Predominant nuclear localization of PTEN as punctate centromere-like foci (mouse embryonic fibroblast cells, unpubl. data), shown by immunofluorescence of PTEN using a home-made rabbit polyclonal antibody against full-length PTEN (#6047, antibody reported in Shen et al. 2007). (B) PTEN in both the nucleus and the cytoplasm (MCF-7 cells), shown by confocal immunofluorescent analysis using a rabbit polyclonal antibody against an amino-terminal region of PTEN (#AP8436a). (Panel B reprinted, with permission, from Abgent © 2019.) For more information, see www.abgent.com/products/AP8436a-PTEN-Antibody-N-term. (C) PTEN in both the cytoplasm and the nucleus (Neuro-2A mouse neuroblastoma cells), shown by immunofluorescence of PTEN using a goat polyclonal antibody against full-length PTEN (#AF6655). (Panel C reprinted, with permission, from Novus Biologicals.) For more information, see www.novusbio.com/products/pten-antibody_af6655. (D) Colocalization of DNA replication sites (red) and PTEN (green) with or without hydroxyurea (HU) treatment (HCT116 cells), shown by stochastic optical reconstruction microscopy (STORM). (Panel D reprinted from Wang et al. 2015, with permission, from Nature Publishing © 2015.) (E) Colocalization between PTEN and EG5 during mitosis (HeLa cells), shown by confocal immunofluorescence using the rabbit anti-PTEN polyclonal antibody (#6067, as in A, green) in overlay with EG5 (red). DNA was counterstained with DAPI. (Panel E reprinted from He et al. 2016, with permission, from Nature Publishing © 2016.)
The ubiquity and general availability of PTEN allow it to play multifaceted roles in maintaining genomic integrity. In the absence of PTEN, the structural and functional organization of the genome is impaired, demonstrated by chromosomal breakage and chromatin decondensation (Shen et al. 2007; Chen et al. 2014; Sun et al. 2014; Gong et al. 2015), as well as incomplete DNA replication and erroneous chromosome segregation (Feng et al. 2015; He et al. 2015, 2016; Wang et al. 2015; van Ree et al. 2016; Zhang et al. 2016). These spontaneous genomic lesions are a manifestation of endogenous genome stress, and their accumulation may directly lead to tumor development. In addition to the tumorigenic role, these cell-intrinsic lesions may impact cellular fitness and response to exogenous genome-threatening catalysts such as environmental stimuli and anticancer therapies. For example, PTEN-deficient cells often escape from the cell-cycle checkpoint surveillance system and exhibit resistance or unresponsiveness to cancer therapy (Puc et al. 2005; He et al. 2015; Liu et al. 2017). Identification of the signaling events on specific PTEN pathways that cause endogenous genotoxic stress or exogenous stress tolerance will help develop novel preventive or therapeutic strategies to selectively target PTEN-deficient tumors.
ROLE OF PTEN IN MAINTAINING STRUCTURAL INTEGRITY OF THE GENOME
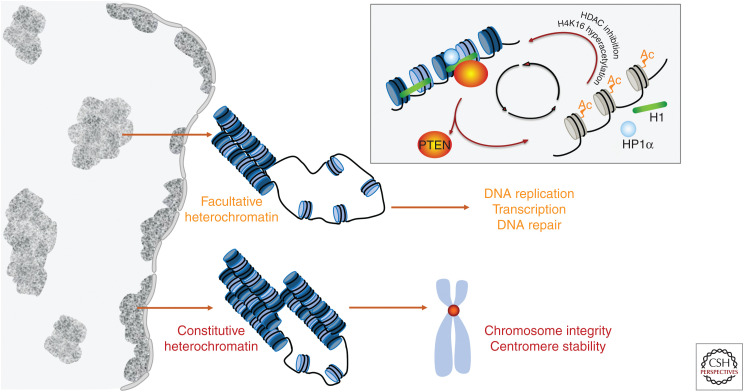
The genome comprises genetic information stored in DNA and the epigenetic machinery that facilitates all DNA-templated processes including replication, transcription, and repair. The genome and epigenome are spatiotemporally inseparable and coexist interwoven into a compact chromatin fiber loaded with different densities of structural units known as nucleosomes. Dynamic positioning and clustering of nucleosomes give rise to a specific chromatin landscape that shapes the transcriptome and genetic transmission. Tightly packed nucleosomes constitute heterochromatin that limits superfluous accessibility of regulatory factors and protects the intrinsic stability of the genome. Heterochromatin may be classified as constitutive heterochromatin or facultative (Fig. 2). Whereas constitutive heterochromatin consistently maintains its condensed state and is typically found in centromeres, facultative heterochromatin is reversibly formed from euchromatin and may lose its compaction resulting in deregulation of the transcriptome (Wang et al. 2016). The loss of PTEN disrupts the structure of both constitutive and facultative heterochromatin, which leads to centromere instability (Shen et al. 2007; Sun et al. 2014) and aberrant regulation of the transcriptome (Fig. 2; Chen et al. 2014; Gong et al. 2015). These observations suggest that PTEN guards both the core structure of chromosomes and the overall chromatin environment for genome maintenance.
Figure 2.
PTEN in the maintenance of structural integrity of the genome. An epigenetic pathway of PTEN plays a critical role in maintaining the architecture of both constitutive and facultative heterochromatin to ensure the structural integrity of chromosomes and proper function of gene transcription, DNA replication, and repair. As summarized in the diagram (upper right corner), PTEN forms a complex with HP-1α and histone H1 on the nucleosome to promote chromatin condensation. Loss of PTEN results in dissociation of both HP-1α and histone H1 from chromatin, as well as hyperacetylation of histone H4 at lysine 16. The physical association of PTEN with HP-1α and histone H1 can also be disrupted by hyperacetylated lysine 16 of histone H4 or inhibition of histone deacetylases (HDACs). As such, PTEN deficiency triggers a feedforward loop between hyperacetylation of histone H4 and unloading of heterochromatin proteins, leading to epigenome stress, and genome instability.
Maintenance of Genome Architecture: PTEN in Guarding Chromatin Compaction
The discovery of the PTEN gene that is localized within a frequently deleted chromosome region portended its life as a tumor suppressor (Li et al. 1997; Simpson and Parsons 2001). As the second most frequently mutated tumor suppressor gene after p53, PTEN may perform similar functions to p53 by inducing apoptosis and cell arrest (Davies et al. 1999; Weng et al. 1999, 2001). Moreover, PTEN reportedly modulates the transcription of individual genes (Freeman et al. 2003; Chang et al. 2004), implying that PTEN might be directly involved in transcription regulation. Later studies suggest that PTEN acts indirectly to modulate the function of genuine transcription factors such as E2F-1 (Shen et al. 2007; Malaney et al. 2018) or to regulate gene transcription profiles on a larger scale (Carver et al. 2011; Mulholland et al. 2012). It is now clear that PTEN guards the epigenome by maintaining heterochromatin architecture and promoting nucleosome compaction (Chen et al. 2014; Gong et al. 2015). These findings not only help interrogate previously observed transcriptome alterations in response to PTEN loss but also suggest that PTEN may use the same mechanism to regulate other DNA-templated processes such as genome duplication and DNA repair (Fig. 2).
As described earlier, the diverse positioning patterns (Fig. 1) suggest that PTEN may physically involve multiple regulatory functions at different subcellular localizations. To maintain chromatin condensation, nuclear PTEN uses its carboxy-terminal region to form a complex with heterochromatin proteins such as HP-1α and histone components of the nucleosome such as the linker histone H1 (Chen et al. 2014; Gong et al. 2015). In addition to maintaining anchorage of these essential molecules on heterochromatin, PTEN also regulates histone modification. PTEN maintains histone H4 in a hypoacetylated state to accommodate intrinsic chromatin compaction and to suppress oncogenic transcriptome alterations (Chen et al. 2014). These findings led to the identification of an epigenetic pathway by which PTEN controls nucleosome compaction. A chemical inhibitor of histone deacetylase (HDAC) or a hyperacetylation-mimicking histone H4 mutant can recapitulate chromatin decondensation and H1/HP-1α dissociation as observed in PTEN-deficient cells (Fig. 2; Chen et al. 2014). These observations support the notion that histone H4 hyperacetylation plays a driving role in disrupting the PTEN–H1–HP-1α complex on heterochromatin and in disassembling facultative heterochromatin. PTEN's function in maintaining heterochromatin stability relies on its carboxy-terminal region, likely independent of the phosphatase activity (Chen et al. 2014; Gong et al. 2015). Spontaneous histone hyperacetylation in Pten-deficient cells indicates elevated endogenous stress at the epigenomic level, which may be further reprogrammed by environmental stimuli. Indeed, exposure of PTEN-deficient cells to reactive oxygen species (ROS)-producing agents further promotes histone acetylation and transcription of stress-responsive genes (Sakamoto et al. 2009). Collectively, these findings suggest that histone acetylation resulting from PTEN dysfunction may serve as a biomarker and druggable target for anticancer therapeutics.
Epigenetic mechanisms have attracted substantial research interest in developing novel anticancer strategies (Fardi et al. 2018). HDACs are deregulated in many cancers and thus many HDAC inhibitors have been developed and used to treat cancer patients as single agents or in combination with other therapies (Li and Seto 2016; Suraweera et al. 2018). Although specific epigenetic mechanisms may vary, the PTEN status appears to be a determining factor for the clinical suitability for HDAC inhibitors. Preclinical and clinical studies suggest that tumors with PTEN expression or activation may favorably respond to HDAC inhibitors (Min et al. 2015; Meng et al. 2016; Zhang and Gan 2017). In contrast, PTEN-deficient tumors may be resistant to such treatment. Using a thyroid cancer model, Zhu et al. (2016) reported that Pten-deficient tumors not only fail to respond to suberoylanilide hydroxamic acid (SAHA), an HDAC inhibitor, but also exhibit aggressive tumor progression with accelerated occurrence of vascular invasion, anaplastic foci, and lung metastasis following treatment with the HDAC inhibitor. These results suggest that PTEN plays a critical role in mediating tumor sensitivity to HDAC inhibitors and that heterochromatin instability in PTEN-deficient tumors confers tolerance and resistance to HDAC inhibition.
Maintenance of Chromosome Integrity: PTEN in Guarding the Core Structure of Chromosomes
In eukaryotic cells, the centromere is a key structural element of chromosomes that plays a vital role in directing chromosomal behavior to achieve accurate segregation of sister chromatids during cell division. Centromeric DNA in mammals is characterized by large arrays of tandemly repeated DNA sequences known as satellite repeats. In the absence of PTEN, heterochromatin disruption results in a reduced association of HP-1α with major repetitive DNA sequences (Chen et al. 2014). These results suggest that PTEN is required for maintaining the structural integrity of centromeres and constitutive heterochromatin. Indeed, PTEN physically interacts with centromere proteins such as CENP-C to protect centromere stability and chromosome integrity (Shen et al. 2007). Consistently, the carboxy-terminal region of PTEN is essential in mediating its physical association with heterochromatic centromeres, suggesting that PTEN relies on its interaction with histones and nonhistone heterochromatin proteins to maintain constitutive heterochromatin compaction and to protect centromere stability.
Cancer-associated germline or somatic PTEN mutations identified in its carboxyl terminus are often nonsense or frameshift mutations leading to protein truncations (cancer .sanger.ac.uk/cosmic/gene/analysis?coords=AA%3AAA&wgs=off&id=15&ln=PTEN&start=185&end=404#distribution), that is, partial or complete loss of the PTEN carboxy-terminal region. Carboxy-terminal truncation of PTEN significantly reduces the protein stability and thus is expected to phenocopy complete deletion of PTEN. However, loss of the PTEN carboxyl terminus confers both loss-of-function and gain-of-function phenotypes. PTENΔC, the short form of PTEN lacking the entire carboxyl terminus, loses the ability to physically associate with centromeres. Ectopic expression of PTENΔC in wild-type cells results in massive chromosome aberrations, including new forms of centromere instability never observed in PTEN null cells (Shen et al. 2007). These observations suggest that PTENΔC may have acquired new adverse functionality that impairs the integrity of constitutive heterochromatin, leading to centromere instability.
The combination of loss-of-function and gain-of-function for PTEN carboxy-terminal truncation can be recapitulated in vivo to cause new tumor phenotypes, as shown in the PtenΔC mouse model (Sun et al. 2014). Knock-in of PtenΔC in the mouse genome causes tumor development in multiple tissues, among which B-cell lymphoma is an unusual tumor type when compared to the typical tumor spectrum reported in other Pten-deficient mouse models. Although it is unclear how PtenΔC causes this specific tumor type, these observations demonstrate the acquired ability of the carboxyl terminus-truncated PTEN mutant to confer specific tissue vulnerability during tumor development. This particular mutant also causes unloading of both histone H1 and HP1α from chromatin and a reduction of nucleosome occupancy (Chen et al. 2014). These in vitro and in vivo findings highlight the importance of PTEN carboxy-terminal function and the epigenetic pathway in the maintenance of genome architecture and suppression of tumorigenesis.
ROLE OF PTEN IN MAINTAINING FUNCTIONAL INTEGRITY OF THE GENOME
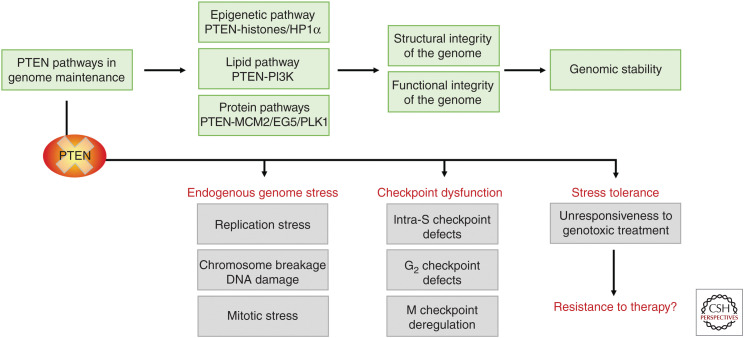
Faithful transmission of genetic material relies on both the structural integrity of the genome and spatiotemporal orchestration of genetic transmission. PTEN performs multifarious tasks in protecting the chromosome/chromatin structure and ensures proper function of major genetic transmission machineries such as DNA replication and chromosome segregation (Hou et al. 2017). Structural aberrations may directly cause functional deregulation. Given the fact that PTEN deficiency results in massive structural alterations including chromosome breakage and chromatin disorganization, one may predict the concurrence of abnormal chromosome behavior and erroneous genome duplication/segregation. Indeed, loss of PTEN leads to functional defects of both DNA replication (Feng et al. 2015; He et al. 2015; Wang et al. 2015) and chromosome segregation (Leonard et al. 2013; Shinde et al. 2013; He et al. 2016; van Ree et al. 2016; Zhang et al. 2016; Choi et al. 2017). As discussed above, the carboxyl terminus of PTEN plays a vital role in maintaining the structural integrity of chromosomes/chromatin through a phosphatase-independent epigenetic pathway. Although the same mechanism may play a critical part in maintaining the functional integrity of the genome, multiple PTEN pathways are likely required to orchestrate the delicate and complex functions of the replisome and the mitotic apparatus (Fig. 3).
Figure 3.
A summary flow chart of PTEN pathways in genome maintenance and dysfunction in stress management upon PTEN loss. PTEN maintains genome stability and epigenome architecture by regulating distinct but interrelated signaling pathways, including the canonical lipid phosphatase pathway, multiple protein phosphatase-dependent pathways, and an epigenetic pathway. These pathways run awry upon the loss or inactivation of PTEN, leading to the spontaneous generation of genome stress, dysfunction of multiple checkpoints, and tolerance to exogenous stimuli. More studies are needed to illuminate whether loss-of-PTEN-related stress tolerance may be translatable to resistance of PTEN-deficient tumors to anticancer therapies, and how to overcome it.
Functional Balance during Mitotic Spindle Assembly: PTEN in Guarding Chromosome Segregation
The centromere is the major point where PTEN acts to maintain the structural integrity of chromosomes (Shen et al. 2007), and it is also the key structure where mitotic spindles attach to pull sister chromatids apart during cell division (Bloom and Costanzo 2017). PTEN loss-related chromosomal structural instability such as centromere breakage may directly generate mitotic stress. PTEN-null cells exhibit various mitotic errors including irregular mitotic timing, misalignment, atypical spindle geometry, spindle pole fragmentation, abnormal checkpoint activities, and mitotic catastrophe (Liu et al. 2011, 2017; Shinde et al. 2013; Choi et al. 2014, 2017; He et al. 2016; van Ree et al. 2016; Javadi et al. 2017). These diverse manifestations cannot be attributed solely to structural chromosome aberrations but instead may result from combined centromere instability and deregulation of multiple distinct signaling pathways related to mitotic control. Interestingly, the physical interaction between PTEN and the centromere is mainly observed in interphase cells (Fig. 1A; Shen et al. 2007). However, when the cell undergoes mitosis, the centromere is no longer the primary localization site of PTEN. Instead, mitotic PTEN is found mainly in the spindle apparatus, ranging from the separating centrosomes to the spindle fibers or matrix (Fig. 1E; He et al. 2016). This unique feature of task-driven multisite translocation shows that PTEN has the functional flexibility to guard multiple major processes critical to genetic transmission in different phases of the cell cycle.
The mitotic spindle uses microtubule-based motor proteins to generate the force that drives segregation of sister chromatids. EG5 is a plus-end directed motor protein that mediates spindle–kinetochore interaction and by cross-linking microtubules, generates force to drive their relative sliding during spindle assembly, maintenance, and elongation (Sawin et al. 1992; Kashina et al. 1996; Kapitein et al. 2005; Kaseda et al. 2009). The motor activity of EG5 must be precisely regulated as either excessive or insufficient activity may impair chromosome congression and segregation. PTEN has been shown to harness the force-generating activity of EG5 through its phosphatase activity (He et al. 2016). Mitosis is a phosphorylation-enriched regulatory machinery (Medema and Lindqvist 2011) and PTEN-EG5 signaling thus represents a force-balancing mechanism conferred by an equilibrium of mitotic phosphorylation for proper spindle assembly. In addition to this phosphatase-dependent pathway, PTEN may use phosphatase-independent mechanisms to promote precise and punctual assembly of the mitotic apparatus (van Ree et al. 2016; Javadi et al. 2017; Liu et al. 2017). To better understand how multiple distinct PTEN pathways are integrated to refine the complex and error-prone process of chromosome segregation and to ensure faithful delivery of genetic information to daughter cells, further studies are required to uncover novel pathways and targets of PTEN in mitotic control.
Functional Coordination during Replisome Assembly: PTEN in Guarding Genome Duplication
The study of PTEN function in mitotic control reveals chromosome segregation errors that originate from premitotic defects such as DNA replication stress following PTEN depletion (He et al. 2015). Further investigation leads to the identification of multiple distinct pathways of PTEN, both phosphatase-dependent and -independent, in promoting replisome assembly and preventing defective DNA replication (Feng et al. 2015; He et al. 2015; Wang et al. 2015; Hou et al. 2017). Specifically, a protein phosphatase-dependent PTEN–MCM2 pathway suppresses premature strand unwinding to prevent uncoupling of the DNA helicase and polymerase (Feng et al. 2015), whereas a phosphatase-independent PTEN–RPA1 pathway protects nascent DNA strands (Wang et al. 2015). Moreover, PTEN is required for the maintenance of replisome function by preserving the chromatin association with multiple critical regulators such as PCNA, Chk1, and Rad51. PTEN depletion induces chromatin dissociation of these regulatory factors, which can be reproduced by chemical inhibition of HDAC (He et al. 2015). These results suggest that chromatin condensation maintained by the PTEN epigenetic pathway (Chen et al. 2014) plays an essential role in promoting replisome assembly and function.
DNA replication is an intricate operation orchestrated by various essential regulatory factors that are recruited sequentially and selectively to the replisome during unperturbed fork progression or upon DNA damage-induced fork stalling. PTEN has been shown to physically colocalize with replicating DNA in the presence and absence of hydroxyurea treatment (Fig. 1D; Wang et al. 2015), suggesting that PTEN functions to promote not only the basal level of fork progression but also restoration of stalled forks. Indeed, PTEN is required for replisome assembly in both situations, which is demonstrated by the fact that cells lacking PTEN fail to recruit both basal replisome factors such as PCNA during unperturbed DNA replication and fork repair factors such as Rad51 in response to exogenous replication stress (He et al. 2015). As a consequence of replisome assembly defects following PTEN depletion, fork slowing/stalling occurs spontaneously and stalled DNA forks fail to restart even after the stress factors are removed (He et al. 2015). These functional defects of replisome assembly may be directly associated with PTEN deficiency in maintaining chromatin architecture (Chen et al. 2014; Gong et al. 2015) because abnormal chromatin decondensation inevitably affects all chromatin-based DNA-templated processes including DNA replication. Altogether, it is evident that the structural integrity of the epigenome and functional coordination of diverse replisome components require multiple PTEN pathways, and the orchestration of all these pathways is required for accurate and efficient genome duplication (Fig. 3).
Stress Suppression and Management in Genome Maintenance: PTEN in Checkpoint Control
Examination of DNA replication defects in PTEN null cells reveals both an enhanced level of spontaneous replication stress and acquired stress tolerance in response to exogenous stimuli that block DNA replication (He et al. 2015). In the absence of external perturbation to the replisome, loss of PTEN is sufficient to induce spontaneous replication fork slowing and stalling, evincing a stress status during baseline fork progression. However, the generation of spontaneous replication stress does not result in cell-cycle arrest of PTEN-null cells in S phase. Even after exogenous challenge with aphidicolin, a DNA polymerase inhibitor, these cells fail to accumulate in S phase while the same treatment successfully induces S phase arrest in control cells with wild-type PTEN (He et al. 2015). These results indicate that the intra-S checkpoint function is compromised in cells lacking PTEN, or alternatively that their response to the S phase checkpoint is impaired, leading to checkpoint bypass and tolerance to DNA replication stress. Therefore, PTEN functions at the replisome to suppress spontaneous DNA lesions and plays an essential role in promoting DNA replication checkpoint activity and response (Fig. 3).
The concurrent elevation of spontaneous stress and stress tolerance seems to be a frequent consequence of PTEN depletion that is not limited to DNA replication but applicable to the entire cell cycle. Depletion of PTEN also induces mitotic stress even in the absence of exogenous perturbation, manifested by spontaneous occurrences of chromosome misalignment, missegregation, and aneuploidy (Liu et al. 2011; He et al. 2016; Choi et al. 2017). In response to spindle toxins, cells deficient for PTEN often exhibit a reduction or failure of mitotic arrest (Gupta et al. 2009; Liu et al. 2017). Similarly, loss of PTEN leads to spontaneous elevation of DNA double-strand breaks (Puc and Parsons 2005; Shen et al. 2007; He et al. 2011; Mukherjee and Karmakar 2013). In response to radiation-induced DNA damage, Pten-null cells display a reduction of G2/M arrest but an increase of mitotic index as compared to wild-type cells, indicating a G2 checkpoint defect and slippage (Puc et al. 2005). These data collectively suggest that PTEN governs the function of multiple critical checkpoints that safeguard genetic transmission (Fig. 3).
Aberrant checkpoint response of PTEN-deficient cells does not always manifest as a reduction or failure of cell-cycle arrest. Loss of PTEN may promote the checkpoint complex formation and checkpoint response, which often occurs at the mitotic checkpoint following aggravation from spindle poisons (Liu et al. 2011; Choi et al. 2017). For example, nocodazole induces more prominent mitotic arrest in PTEN-deficient prostate cancer cells than in PTEN-proficient control cells (Liu et al. 2011). In addition to mitotic spindle disturbance, inhibition of the DNA damage checkpoint can also induce excessive mitotic checkpoint responses, which may confer synthetic lethality upon PTEN-deficient cells. For example, the ATM inhibitor KU-55933 preferentially suppresses PTEN-null tumor cells in colony formation, which may be attributed to enhanced mitotic arrest (McCabe et al. 2015). These data suggest that accumulation of DNA lesions resulting from prior checkpoint bypass may subsequently promote succeeding checkpoint response, leading to the effective elimination of PTEN-deficient cells. Therefore, deregulation of checkpoint response conferred by PTEN deficiency may create novel therapeutic opportunities (Fig. 3). Further investigation is needed to understand how PTEN loss impinges on the cellular stress-management system and how targeting stress-maladaptive signaling events in PTEN-deficient tumors may create clinical advantages.
CONCLUSIONS AND PERSPECTIVES
Since the recognition of PTEN as a guardian of the genome (Kritikou 2007), multiple distinct pathways have been identified that, together with the canonical PTEN–PI3K pathway, are often cooperative and complementary in controlling chromosome stability and the fidelity of genetic transmission (Fig. 3). We now understand that genome maintenance requires PTEN's multifaceted functions in both the nucleus and cytoplasm to coordinate signaling events required to ensure accurate transmission of genetic materials (Hou et al. 2017). Nevertheless, our knowledge of the PTEN signaling network in guarding the genome and coping with genome stress, in the context of epigenome programming, is still limited. Many fundamental questions remain to be answered, such as (1) how PTEN loss results in the dysfunction of multiple cell-cycle checkpoints; (2) whether the elevation of endogenous genome stress in PTEN-deficient cells is intrinsically responsible for the acquisition of stress tolerance in the presence of exogenous genotoxic stimuli; and (3) how the stress-management defects in PTEN-deficient tumor cells may translate to treatment resistance and how to reverse it. Identification of new PTEN pathways and targets in genome maintenance may help address these questions.
Even with limited knowledge, it is clear that PTEN maintains both the structural and functional integrity of the genome (Fig. 3). Functional orchestration of diverse signaling pathways controlled by PTEN is warranted by its task-driven accessibility to distinct machineries of DNA replication, chromosome segregation, and chromatin remodeling. Indeed, PTEN has been found to physically associate with multiple major instruments including DNA replication forks (Wang et al. 2015), DNA damage repair sites (Choi et al. 2013), nucleosomes (Chen et al. 2014), and mitotic spindles (Fig. 1; He et al. 2016). Nevertheless, proteomic analysis of the replisome and mitotic machinery often fails to detect PTEN (Alabert et al. 2014; Dungrawala et al. 2015; Ly et al. 2017; Ginno et al. 2018; Heusel et al. 2019). These seemingly conflicting data suggest that PTEN may serve as a facultative component endowed with spatiotemporal flexibility capable of dynamic translocation for multiple functionality among a diverse array of cellular mechanisms.
PTEN is among the top genes that are highly mutated in human cancer and different PTEN mutations may affect specific PTEN pathways leading to clinically distinct phenotypes. Precise prediction of tumor phenotype and response requires identification of novel PTEN signaling pathways in guarding the genome and further characterization of the signaling connections between the PTEN genotype and the tumor phenotype. Even with the current knowledge, it is time to determine how inactivation of each known PTEN pathway leads to distinct tumor phenotypes and how specific signaling deregulation alters tumor behavior and may create treatment opportunity.
ACKNOWLEDGMENTS
The authors would like to thank the National Institutes of Health Grant (R01GM100478) and the Irma T. Hirschl/Monique Weill-Caulier Trust for funding the research in the Shen laboratory. We would also like to thank all laboratory members and colleagues at Weill Cornell Medicine for critical discussions.
Footnotes
Editors: Charis Eng, Joanne Ngeow, and Vuk Stambolic
Additional Perspectives on The PTEN Family available at www.perspectivesinmedicine.org
REFERENCES
- Alabert C, Bukowski-Wills JC, Lee SB, Kustatscher G, Nakamura K, de Lima Alves F, Menard P, Mejlvang J, Rappsilber J, Groth A. 2014. Nascent chromatin capture proteomics determines chromatin dynamics during DNA replication and identifies unknown fork components. Nat Cell Biol 16: 281–291. 10.1038/ncb2918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzelon AN, Wu H, Rickert RC. 2003. Pten inactivation alters peripheral B lymphocyte fate and reconstitutes CD19 function. Nat Immunol 4: 287–294. 10.1038/ni892 [DOI] [PubMed] [Google Scholar]
- Bassi C, Ho J, Srikumar T, Dowling RJ, Gorrini C, Miller SJ, Mak TW, Neel BG, Raught B, Stambolic V. 2013. Nuclear PTEN controls DNA repair and sensitivity to genotoxic stress. Science 341: 395–399. 10.1126/science.1236188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom K, Costanzo V. 2017. Centromere structure and function. Prog Mol Subcell Biol 56: 515–539. 10.1007/978-3-319-58592-5_21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, et al. 2011. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19: 575–586. 10.1016/j.ccr.2011.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caserta E, Egriboz O, Wang H, Martin C, Koivisto C, Pecot T, Kladney RD, Shen C, Shim KS, Pham T, et al. 2015. Noncatalytic PTEN missense mutation predisposes to organ-selective cancer development in vivo. Genes Dev 29: 1707–1720. 10.1101/gad.262568.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalhoub N, Baker SJ. 2009. PTEN and the PI3-kinase pathway in cancer. Annu Rev Pathol 4: 127–150. 10.1146/annurev.pathol.4.110807.092311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CJ, Freeman DJ, Wu H. 2004. PTEN regulates Mdm2 expression through the P1 promoter. J Biol Chem 279: 29841–29848. 10.1074/jbc.M401488200 [DOI] [PubMed] [Google Scholar]
- Chen Z, Trotman LC, Shaffer D, Lin HK, Dotan ZA, Niki M, Koutcher JA, Scher HI, Ludwig T, Gerald W, et al. 2005. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature 436: 725–730. 10.1038/nature03918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZH, Zhu M, Yang J, Liang H, He J, He S, Wang P, Kang X, McNutt MA, Yin Y, et al. 2014. PTEN interacts with histone H1 and controls chromatin condensation. Cell Rep 8: 2003–2014. 10.1016/j.celrep.2014.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH, Chen Y, Dai W. 2013. Chromatin PTEN is involved in DNA damage response partly through regulating Rad52 sumoylation. Cell Cycle 12: 3442–3447. 10.4161/cc.26465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH, Pagano M, Dai W. 2014. Plk1 protein phosphorylates phosphatase and tensin homolog (PTEN) and regulates its mitotic activity during the cell cycle. J Biol Chem 289: 14066–14074. 10.1074/jbc.M114.558155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BH, Xie S, Dai W. 2017. PTEN is a negative regulator of mitotic checkpoint complex during the cell cycle. Exp Hematol Oncol 6: 19 10.1186/s40164-017-0079-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MA, Koul D, Dhesi H, Berman R, McDonnell TJ, McConkey D, Yung WK, Steck PA. 1999. Regulation of Akt/PKB activity, cellular growth, and apoptosis in prostate carcinoma cells by MMAC/PTEN. Cancer Res 59: 2551–2556. [PubMed] [Google Scholar]
- Di Cristofano A, Kotsi P, Peng YF, Cordon-Cardo C, Elkon KB, Pandolfi PP. 1999. Impaired Fas response and autoimmunity in Pten+/– mice. Science 285: 2122–2125. 10.1126/science.285.5436.2122 [DOI] [PubMed] [Google Scholar]
- Dungrawala H, Rose KL, Bhat KP, Mohni KN, Glick GG, Couch FB, Cortez D. 2015. The replication checkpoint prevents two types of fork collapse without regulating replisome stability. Mol Cell 59: 998–1010. 10.1016/j.molcel.2015.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fardi M, Solali S, Farshdousti Hagh M. 2018. Epigenetic mechanisms as a new approach in cancer treatment: an updated review. Genes Dis 5: 304–311. 10.1016/j.gendis.2018.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Liang J, Li J, Li Y, Liang H, Zhao X, McNutt MA, Yin Y. 2015. PTEN controls the DNA replication process through MCM2 in response to replicative stress. Cell Rep 13: 1295–1303. 10.1016/j.celrep.2015.10.016 [DOI] [PubMed] [Google Scholar]
- Freeman DJ, Li AG, Wei G, Li HH, Kertesz N, Lesche R, Whale AD, Martinez-Diaz H, Rozengurt N, Cardiff RD, et al. 2003. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3: 117–130. 10.1016/S1535-6108(03)00021-7 [DOI] [PubMed] [Google Scholar]
- Garcia-Cao I, Song MS, Hobbs RM, Laurent G, Giorgi C, de Boer VC, Anastasiou D, Ito K, Sasaki AT, Rameh L, et al. 2012. Systemic elevation of PTEN induces a tumor-suppressive metabolic state. Cell 149: 49–62. 10.1016/j.cell.2012.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginno PA, Burger L, Seebacher J, Iesmantavicius V, Schübeler D. 2018. Cell cycle–resolved chromatin proteomics reveals the extent of mitotic preservation of the genomic regulatory landscape. Nat Commun 9: 4048 10.1038/s41467-018-06007-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong L, Govan JM, Evans EB, Dai H, Wang E, Lee SW, Lin HK, Lazar AJ, Mills GB, Lin SY. 2015. Nuclear PTEN tumor-suppressor functions through maintaining heterochromatin structure. Cell Cycle 14: 2323–2332. 10.1080/15384101.2015.1044174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta A, Yang Q, Pandita RK, Hunt CR, Xiang T, Misri S, Zeng S, Pagan J, Jeffery J, Puc J, et al. 2009. Cell cycle checkpoint defects contribute to genomic instability in PTEN deficient cells independent of DNA DSB repair. Cell Cycle 8: 2198–2210. 10.4161/cc.8.14.8947 [DOI] [PubMed] [Google Scholar]
- He X, Ni Y, Wang Y, Romigh T, Eng C. 2011. Naturally occurring germline and tumor-associated mutations within the ATP-binding motifs of PTEN lead to oxidative damage of DNA associated with decreased nuclear p53. Hum Mol Genet 20: 80–89. 10.1093/hmg/ddq434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Kang X, Yin Y, Chao KS, Shen WH. 2015. PTEN regulates DNA replication progression and stalled fork recovery. Nat Commun 6: 7620 10.1038/ncomms8620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J, Zhang Z, Ouyang M, Yang F, Hao H, Lamb KL, Yang J, Yin Y, Shen WH. 2016. PTEN regulates EG5 to control spindle architecture and chromosome congression during mitosis. Nat Commun 7: 12355 10.1038/ncomms12355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heit B, Robbins SM, Downey CM, Guan Z, Colarusso P, Miller BJ, Jirik FR, Kubes P. 2008. PTEN functions to “prioritize” chemotactic cues and prevent “distraction” in migrating neutrophils. Nat Immunol 9: 743–752. 10.1038/ni.1623 [DOI] [PubMed] [Google Scholar]
- Heusel M, Frank M, Köhler M, Amon S, Frommelt S, Rosenberger G, Bludau I, Aulakh S, Linder MI, Liu Y, et al. 2019. A global screen for assembly state changes of the mitotic proteome by SEC-SWATH-MS. bioRxiv 633479 10.1101/633479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou SQ, Ouyang M, Brandmaier A, Hao H, Shen WH. 2017. PTEN in the maintenance of genome integrity: from DNA replication to chromosome segregation. Bioessays 10.1002/bies.201700082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javadi A, Deevi RK, Evergren E, Blondel-Tepaz E, Baillie GS, Scott MG, Campbell FC. 2017. PTEN controls glandular morphogenesis through a juxtamembrane β-Arrestin1/ARHGAP21 scaffolding complex. eLife 6: e24578. 10.7554/eLife.24578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapitein LC, Peterman EJ, Kwok BH, Kim JH, Kapoor TM, Schmidt CF. 2005. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435: 114–118. 10.1038/nature03503 [DOI] [PubMed] [Google Scholar]
- Kaseda K, McAinsh AD, Cross RA. 2009. Walking, hopping, diffusing and braking modes of kinesin-5. Biochem Soc Trans 37: 1045–1049. 10.1042/BST0371045 [DOI] [PubMed] [Google Scholar]
- Kashina AS, Baskin RJ, Cole DG, Wedaman KP, Saxton WM, Scholey JM. 1996. A bipolar kinesin. Nature 379: 270–272. 10.1038/379270a0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenhuber ER, Lowe SW. 2017. Putting p53 in context. Cell 170: 1062–1078. 10.1016/j.cell.2017.08.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kritikou E. 2007. PTEN—a new guardian of the genome. Nat Rev Mol Cell Biol 8: 179 10.1038/nrm2128 [DOI] [Google Scholar]
- Leonard MK, Hill NT, Bubulya PA, Kadakia MP. 2013. The PTEN-Akt pathway impacts the integrity and composition of mitotic centrosomes. Cell Cycle 12: 1406–1415. 10.4161/cc.24516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leong JW, Schneider SE, Sullivan RP, Parikh BA, Anthony BA, Singh A, Jewell BA, Schappe T, Wagner JA, Link DC, et al. 2015. PTEN regulates natural killer cell trafficking in vivo. Proc Natl Acad Sci 112: E700–E709. 10.1073/pnas.1413886112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Seto E. 2016. HDACs and HDAC inhibitors in cancer development and therapy. Cold Spring Harb Perspect Med 6: a026831 10.1101/cshperspect.a026831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. 1997. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275: 1943–1947. 10.1126/science.275.5308.1943 [DOI] [PubMed] [Google Scholar]
- Liu XS, Song B, Elzey BD, Ratliff TL, Konieczny SF, Cheng L, Ahmad N, Liu X. 2011. Polo-like kinase 1 facilitates loss of Pten tumor suppressor-induced prostate cancer formation. J Biol Chem 286: 35795–35800. 10.1074/jbc.C111.269050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Du X, Zhang S, Liu Y, Zhang Q, Yin Q, McNutt MA, Yin Y. 2017. PTEN regulates spindle assembly checkpoint timing through MAD1 in interphase. Oncotarget 8: 98040–98050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly T, Whigham A, Clarke R, Brenes-Murillo AJ, Estes B, Madhessian D, Lundberg E, Wadsworth P, Lamond AI. 2017. Proteomic analysis of cell cycle progression in asynchronous cultures, including mitotic subphases, using PRIMMUS. eLife 6: e27574 10.7554/eLife.27574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maehama T, Dixon JE. 1998. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem 273: 13375–13378. 10.1074/jbc.273.22.13375 [DOI] [PubMed] [Google Scholar]
- Malaney P, Palumbo E, Semidey-Hurtado J, Hardee J, Stanford K, Kathiriya JJ, Patel D, Tian Z, Allen-Gipson D, Davé V. 2018. PTEN physically interacts with and regulates E2F1-mediated transcription in lung cancer. Cell Cycle 17: 947–962. 10.1080/15384101.2017.1388970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maser RS, Choudhury B, Campbell PJ, Feng B, Wong KK, Protopopov A, O'Neil J, Gutierrez A, Ivanova E, Perna I, et al. 2007. Chromosomally unstable mouse tumours have genomic alterations similar to diverse human cancers. Nature 447: 966–971. 10.1038/nature05886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe N, Hanna C, Walker SM, Gonda D, Li J, Wikstrom K, Savage KI, Butterworth KT, Chen C, Harkin DP, et al. 2015. Mechanistic rationale to target PTEN-deficient tumor cells with inhibitors of the DNA damage response kinase ATM. Cancer Res 75: 2159–2165. 10.1158/0008-5472.CAN-14-3502 [DOI] [PubMed] [Google Scholar]
- Medema RH, Lindqvist A. 2011. Boosting and suppressing mitotic phosphorylation. Trends Biochem Sci 36: 578–584. 10.1016/j.tibs.2011.08.006 [DOI] [PubMed] [Google Scholar]
- Meek DW. 2009. Tumour suppression by p53: a role for the DNA damage response? Nat Rev Cancer 9: 714–723. 10.1038/nrc2716 [DOI] [PubMed] [Google Scholar]
- Meng Z, Jia LF, Gan YH. 2016. PTEN activation through K163 acetylation by inhibiting HDAC6 contributes to tumour inhibition. Oncogene 35: 2333–2344. 10.1038/onc.2015.293 [DOI] [PubMed] [Google Scholar]
- Min A, Im SA, Kim DK, Song SH, Kim HJ, Lee KH, Kim TY, Han SW, Oh DY, Kim TY, et al. 2015. Histone deacetylase inhibitor, suberoylanilide hydroxamic acid (SAHA), enhances anti-tumor effects of the poly (ADP-ribose) polymerase (PARP) inhibitor olaparib in triple-negative breast cancer cells. Breast Cancer Res 17: 33 10.1186/s13058-015-0534-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Karmakar P. 2013. Attenuation of PTEN perturbs genomic stability via activation of Akt and down-regulation of Rad51 in human embryonic kidney cells. Mol Carcinog 52: 611–618. 10.1002/mc.21903 [DOI] [PubMed] [Google Scholar]
- Mulholland DJ, Kobayashi N, Ruscetti M, Zhi A, Tran LM, Huang J, Gleave M, Wu H. 2012. Pten loss and RAS/MAPK activation cooperate to promote EMT and metastasis initiated from prostate cancer stem/progenitor cells. Cancer Res 72: 1878–1889. 10.1158/0008-5472.CAN-11-3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oren M. 2003. Decision making by p53: life, death and cancer. Cell Death Differ 10: 431–442. 10.1038/sj.cdd.4401183 [DOI] [PubMed] [Google Scholar]
- Ortega-Molina A, Efeyan A, Lopez-Guadamillas E, Muñoz-Martin M, Gómez-López G, Cañamero M, Mulero F, Pastor J, Martinez S, Romanos E, et al. 2012. Pten positively regulates brown adipose function, energy expenditure, and longevity. Cell Metab 15: 382–394. 10.1016/j.cmet.2012.02.001 [DOI] [PubMed] [Google Scholar]
- Papa A, Wan L, Bonora M, Salmena L, Song MS, Hobbs RM, Lunardi A, Webster K, Ng C, Newton RH, et al. 2014. Cancer-associated PTEN mutants act in a dominant-negative manner to suppress PTEN protein function. Cell 157: 595–610. 10.1016/j.cell.2014.03.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puc J, Parsons R. 2005. PTEN loss inhibits CHK1 to cause double stranded-DNA breaks in cells. Cell Cycle 4: 927–929. 10.4161/cc.4.7.1795 [DOI] [PubMed] [Google Scholar]
- Puc J, Keniry M, Li HS, Pandita TK, Choudhury AD, Memeo L, Mansukhani M, Murty VV, Gaciong Z, Meek SE, et al. 2005. Lack of PTEN sequesters CHK1 and initiates genetic instability. Cancer Cell 7: 193–204. 10.1016/j.ccr.2005.01.009 [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Iwasaki K, Sugiyama H, Tsuji Y. 2009. Role of the tumor suppressor PTEN in antioxidant responsive element-mediated transcription and associated histone modifications. Mol Biol Cell 20: 1606–1617. 10.1091/mbc.e08-07-0762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin KE, LeGuellec K, Philippe M, Mitchison TJ. 1992. Mitotic spindle organization by a plus-end-directed microtubule motor. Nature 359: 540–543. 10.1038/359540a0 [DOI] [PubMed] [Google Scholar]
- Shen WH, Balajee AS, Wang J, Wu H, Eng C, Pandolfi PP, Yin Y. 2007. Essential role for nuclear PTEN in maintaining chromosomal integrity. Cell 128: 157–170. 10.1016/j.cell.2006.11.042 [DOI] [PubMed] [Google Scholar]
- Shinde SR, Maddika S. 2017. PTEN Regulates glucose transporter recycling by impairing SNX27 retromer assembly. Cell Rep 21: 1655–1666. 10.1016/j.celrep.2017.10.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinde SR, Gangula NR, Kavela S, Pandey V, Maddika S. 2013. TOPK and PTEN participate in CHFR mediated mitotic checkpoint. Cell Signal 25: 2511–2517. 10.1016/j.cellsig.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson L, Parsons R. 2001. PTEN: life as a tumor suppressor. Exp Cell Res 264: 29–41. 10.1006/excr.2000.5130 [DOI] [PubMed] [Google Scholar]
- Sun Z, Huang C, He J, Lamb KL, Kang X, Gu T, Shen WH, Yin Y. 2014. PTEN C-terminal deletion causes genomic instability and tumor development. Cell Rep 6: 844–854. 10.1016/j.celrep.2014.01.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suraweera A, O'Byrne KJ, Richard DJ. 2018. Combination therapy with histone deacetylase inhibitors (HDACi) for the treatment of cancer: achieving the full therapeutic potential of HDACi. Front Oncol 8: 92 10.3389/fonc.2018.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Yamaguchi MT, Ohteki T, Sasaki T, Kaisho T, Kimura Y, Yoshida R, Wakeham A, Higuchi T, Fukumoto M, et al. 2001. T cell-specific loss of Pten leads to defects in central and peripheral tolerance. Immunity 14: 523–534. 10.1016/S1074-7613(01)00134-0 [DOI] [PubMed] [Google Scholar]
- Suzuki A, Kaisho T, Ohishi M, Tsukio-Yamaguchi M, Tsubata T, Koni PA, Sasaki T, Mak TW, Nakano T. 2003. Critical roles of Pten in B cell homeostasis and immunoglobulin class switch recombination. J Exp Med 197: 657–667. 10.1084/jem.20021101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ree JH, Nam HJ, Jeganathan KB, Kanakkanthara A, van Deursen JM. 2016. Pten regulates spindle pole movement through Dlg1-mediated recruitment of Eg5 to centrosomes. Nat Cell Biol 18: 814–821. 10.1038/ncb3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh PT, Buckler JL, Zhang J, Gelman AE, Dalton NM, Taylor DK, Bensinger SJ, Hancock WW, Turka LA. 2006. PTEN inhibits IL-2 receptor-mediated expansion of CD4+ CD25+ Tregs. J Clin Invest 116: 2521–2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Karikomi M, Naidu S, Rajmohan R, Caserta E, Chen HZ, Rawahneh M, Moffitt J, Stephens JA, Fernandez SA, et al. 2010. Allele-specific tumor spectrum in pten knockin mice. Proc Natl Acad Sci 107: 5142–5147. 10.1073/pnas.0912524107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Li Y, Wang P, Liang H, Cui M, Zhu M, Guo L, Su Q, Sun Y, McNutt MA, et al. 2015. PTEN regulates RPA1 and protects DNA replication forks. Cell Res 25: 1189–1204. 10.1038/cr.2015.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jia ST, Jia S. 2016. New insights into the regulation of heterochromatin. Trends Genet 32: 284–294. 10.1016/j.tig.2016.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng LP, Smith WM, Dahia PL, Ziebold U, Gil E, Lees JA, Eng C. 1999. PTEN suppresses breast cancer cell growth by phosphatase activity-dependent G1 arrest followed by cell death. Cancer Res 59: 5808–5814. [PubMed] [Google Scholar]
- Weng L, Brown J, Eng C. 2001. PTEN induces apoptosis and cell cycle arrest through phosphoinositol-3-kinase/Akt-dependent and -independent pathways. Hum Mol Genet 10: 237–242. 10.1093/hmg/10.3.237 [DOI] [PubMed] [Google Scholar]
- Zhang G, Gan YH. 2017. Synergistic antitumor effects of the combined treatment with an HDAC6 inhibitor and a COX-2 inhibitor through activation of PTEN. Oncol Rep 38: 2657–2666. 10.3892/or.2017.5981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Hou SQ, He J, Gu T, Yin Y, Shen WH. 2016. PTEN regulates PLK1 and controls chromosomal stability during cell division. Cell Cycle 15: 2476–2485. 10.1080/15384101.2016.1203493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Kim DW, Zhao L, Willingham MC, Cheng SY. 2016. SAHA-induced loss of tumor suppressor Pten gene promotes thyroid carcinogenesis in a mouse model. Endocr Relat Cancer 23: 521–533. 10.1530/ERC-16-0103 [DOI] [PMC free article] [PubMed] [Google Scholar]