Abstract
Objective
to compare the glycemic threshold for pharmacotherapy initiation in women with gestational diabetes (GDM) based on maternal race/ethnicity.
Methods
A retrospective cohort study of women with GDM who received pharmacotherapy during pregnancy, in addition to diet and exercise, between 2015 and 2019 in a university center. The primary outcome was percent of elevated capillary blood glucoses (CBGs) prior to pharmacotherapy initiation. This was compared between four maternal racial and ethnic groups: non-Hispanic white (NHW), non-Hispanic black (NHB), Hispanic and other race and ethnicity group that included Asian, American Indian and Alaskan Native. Univariable and multivariable analyses were done to estimate whether there was an independent association between maternal race and ethnicity and the percent of elevated CBGs prior to pharmacotherapy initiation.
Results
A total of 440 women met inclusion criteria. In univariable analysis, NHB women, Hispanic, and women of other race and ethnicity had higher percent of elevated CBGs prior to pharmacotherapy initiation, compared to NHW women (45.5% ± 22.5% for NHW, 65.2% ± 25.4% for NHB, 58.3% ± 21.7% for Hispanic and 51.6% ± 26.8% for other race and ethnicity, respectively, p<0.001). After the adjustment for maternal demographic and clinical factors, maternal race and ethnicity remained to be significantly associated with timing of pharmacotherapy initiation, with women of racial and ethnic minority having a higher percent of elevated CBGs prior to pharmacotherapy initiation (18.1%, 95% CI 11.3 – 25.0 for NHB, 13.2%, 95% CI 5.0 – 21.4 for Hispanic, 9.8%, 95% CI 2.6 – 16.9 for women of other race and ethnicity).
Conclusion
A significant variation was identified in glycemic threshold for pharmacotherapy initiation in women with GDM across different maternal racial and ethnic groups with minority women starting pharmacotherapy at higher percent of elevated CBGs.
Keywords: gestational diabetes, racial disparity, pharmacotherapy, glycemic threshold
Introduction
Gestational diabetes mellitus (GDM) is defined as abnormal glucose tolerance in pregnancy and is a product of heightened insulin resistance due to the physiologic changes of pregnancy [1]. GDM affects 7% of pregnant women and is associated with several adverse maternal and perinatal outcomes [2–10]. Racial and ethnic differences in GDM prevalence and GDM-related adverse perinatal outcomes are well documented [11–16]. Interestingly, even though the prevalence of GDM is higher among Hispanic and Asian women, non-Hispanic black women have the highest rate of GDM-related adverse outcomes, including preeclampsia, preterm delivery and neonatal hypoglycemia [15–16]. These differences are not explained by demographic, anthropometric, and socioeconomic factors [15].
Monitoring and treating GDM reduces diabetes-related adverse pregnancy outcomes [4–6]. Nearly 90% of women diagnosed with GDM will fail the initial trial of prescribed diet and exercise [2]. Following diet and exercise, prescription of medication is the second line of treatment [2]. It is crucial to note, however, that the definition of what constitutes an unsuccessful attempt at diet and exercise has not been established [11,17–19]. Consequently, the need to start insulin or oral hypoglycemic agent is at a provider’s discretion with wide variability in practice.
In search for an alternative explanation for racial and ethnic disparities in GDM-related adverse outcomes, we thought to investigate variation in GDM management across different racial and ethnic groups. Given the lack of consensus regarding the glycemic threshold for conversion from dietary to medical treatment for GDM, the aim of this study was to compare timing of pharmacotherapy initiation for women with GDM based on race and ethnicity. We hypothesize that there will be a significant variation in timing of pharmacotherapy initiation across maternal racial and ethnic groups.
Methods
Sample and Population
This was a retrospective chart review of women with GDM who received medical treatment/pharmacotherapy during pregnancy between 2015 and 2019 at Froedtert Memorial Lutheran Hospital and the Medical College of Wisconsin. Institutional review board approval was obtained prior to initiation of this study. Women were included in this analysis if they were older than 18 years of age, had a singleton non-anomalous gestation, were diagnosed with GDM any time during the pregnancy and started on pharmacotherapy during pregnancy for blood glucose control. Women were excluded if their GDM remained diet-controlled, defined as not requiring prescription of insulin or oral hypoglycemic agent throughout the pregnancy, or if the variable of maternal race and ethnicity was missing in the medical chart. In addition, women who required pharmacotherapy initiation at the first visit after a trial of diet and exercise were excluded as they may have had a more severe phenotype of GDM that required treatment initiation early.
Diagnosis and Treatment of GDM within Health System
In our institution the diagnosis of GDM is made by a two-step approach testing that includes: 1) a first screening step with 50-g oral glucose solution followed by a 1-hour venous glucose determination; 2) a second step of a 100-g, 3-hour diagnostic oral glucose tolerance test (OGTT) using Carpenter-Coustan criteria for women whose glucose levels meet or exceed 140mg/dl in the first step. Women are diagnosed with GDM if they have two or more abnormal values on the 3-hour OGTT. Institutional guidelines for women diagnosed with GDM include nutritional therapy and dispense of a glucometer. Women record their home capillary blood glucose (CBG) values four times daily on average (one fasting and three postprandial values) for 1–2 weeks intervals. The cutoffs for elevated values are fasting venous blood glucoses of at least 95 mg/dL or a one - or 2- hour post-prandial venous blood glucose of at least 140 or 120 mg/dL, respectively. The provider then assesses the glucose log and decides if adjuvant medication initiation, with insulin typically being first line, is appropriate at that time. The woman continues to record CBG values for 1–2 weeks between visits throughout the pregnancy, and the provider again assesses treatment or no treatment at each of those visits or telephone encounters. Per our clinic protocol, women that do not record their CBG values cannot be started on pharmacotherapy. Fetal growth assessment is performed at 32 and 36 weeks with weekly antenatal testing starting 32 weeks for all women with GDM requiring pharmacotherapy until delivery during the 39th week. There were no changes to the institutional guidelines in terms of GDM diagnosis or management throughout the course of the study except Metformin becoming a more prevalent second line therapy after insulin following recent ACOG practice bulletin publication [2].
Primary Outcome
The primary outcome of interest was the percent of elevated CBG values prior to initiation of pharmacotherapy. This was calculated over 1- or 2-week intervals between the clinic visits. The CBG log for that time period was reviewed and percent of CBG values above 95 mg/dL for fasting and above 140 or 120 mg/dL for postprandial was calculated. We then reviewed whether pharmacotherapy was initiated at the end of that 1–2 weeks interval. Both abnormal fasting and postprandial CBG values were used to calculate the total percent of abnormal CBG values when pharmacotherapy was initiated. Separate calculations for fasting values only or postprandial values only was not done as in our practice, the decision to start pharmacotherapy is based on the review of the entire weekly CBG log and calculation of the overall percent of abnormal CBG values during that week. The outcome was analyzed as a continuous variable. Weekly logs of CBG values were reviewed until the week during which pharmacotherapy was initiated. The percent of abnormal CBG values from the last week prior to treatment was the primary outcome.
Primary Predictor, Covariates and Secondary Outcomes
The main predictor for this analysis was self-reported maternal race and ethnicity as captured in the medical record, grouped into non-Hispanic white (NHW), non-Hispanic black (NHB), Hispanic and other race and ethnicity. Other race and ethnicity category included the following minorities: American Indian, Alaskan Native, Asian and “other” as was self-identified by the patient. Women of multiple racial and ethnic background were part of the “other” race and ethnicity category.
The medical records were also abstracted for sociodemographic and clinical characteristics, including maternal age, body mass index (BMI) at first pregnancy visit and at the time of delivery, obstetric history, prior medical history and obstetric and perinatal outcomes. GDM diagnosis was abstracted from the chart using ICD10-CM-Diagnosis code 0.99.810, standing for “abnormal glucose complication pregnancy”. Each chart was reviewed to confirm presence of GDM based on the diagnostic criteria described above. Information about characteristics of GDM were recorded, including gestational age at the time of diagnosis, glucose levels 1 hour after 50-g and fasting, 1-,2- and 3-hours after 100-g glucose loading tests, gestational age at the time of pharmacotherapy initiation and weeks from diagnosis to initiation of pharmacotherapy. The following maternal outcomes were collected: gestational age at delivery, preterm birth (<37 weeks), preeclampsia, cesarean delivery, third or fourth degree perineal laceration, postpartum hemorrhage (defined as estimated blood loss ≥1000ml), neonatal birth weight, small- and large-for-gestational age (defined as a birth weight less than the 10th percentile or greater than the 90th percentile for gestational age and gender, respectively) [20], shoulder dystocia, Apgar<7 at 5 minutes, neonatal intensive care unit (NICU) admission, neonatal hypoglycemia (CBG<70mg/dL) and neonatal jaundice requiring phototherapy.
Statistical Analyses
All analyses were performed with Stata version 14.0 (StataCorp College Station, TX). Univariate summary results are reported in means ± standard deviations (SD), with categorical data presented in percentages. and p <.05 was used to define significance. For univariate analyses, we compared maternal and GDM characteristics by maternal race /ethnicity using χ2 statistics or Fisher’s exact test for categorical variables and one-way ANOVA for continuous variables. Similarly, we compared pregnancy outcomes across maternal racial and ethnic groups. Next, we ran unadjusted and adjusted linear regression models to examine associations between maternal race/ethnicity and percent of abnormal CBG values after controlling for potential demographic and clinical confounding factors. Additionally, we conducted the following sensitivity analyses: 1) adding the additional variable of glucose levels at 50-g glucose loading test to the adjusted linear regression model; and 2) excluding women diagnosed with GDM in the first trimester from the analysis.
Results
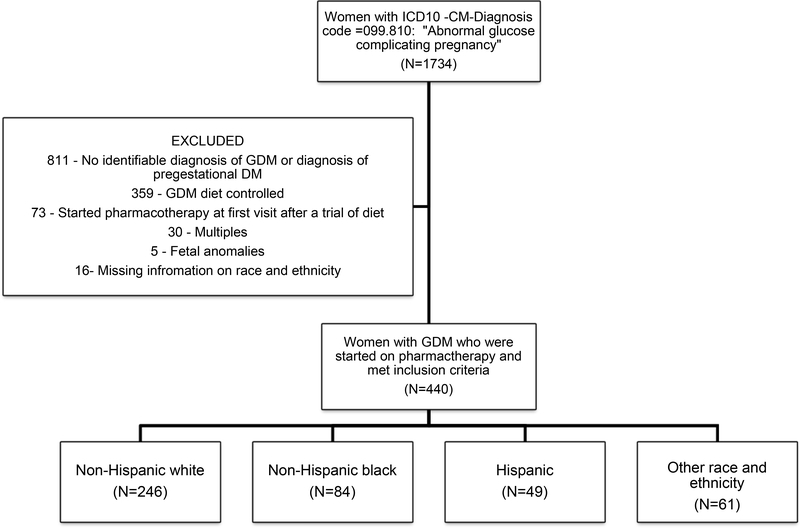
From over 1,734 women with a diagnosis of abnormal glucose tolerance in pregnancy during that time period, 440 women met inclusion criteria (Figure 1).
Figure 1.
Flowchart describing exclusion criteria and study population
Maternal characteristics and GDM features, stratified by the maternal race and ethnicity groups are shown in Table 1. Four racial groups differed in the following characteristics: maternal age, early-pregnancy BMI, marital status and insurance. When comparing the mean CBG value after 50-g glucose loading test, NHB women had the highest abnormal value, followed by other race and ethnicity group and Hispanic women. There was no difference in the type of provider managing the GDM between the four racial and ethnic groups. The primary outcome, percent of elevated CBG values prior to pharmacotherapy initiation was significantly different between all four groups, with NHB women having the highest percent of elevated CBGs values prior to pharmacotherapy initiation (45.5% ± 22.5% for NHW, 65.2% ± 25.4% for NHB, 58.3% ± 21.7% for Hispanic and 51.6% ± 26.8% for other race and ethnicity, respectively, p<0.001). The lowest and the highest percent of elevated CBG values that triggered pharmacotherapy initiation were 12% and 95%, respectively.
Table 1.
Demographic and clinical characteristics of women with Gestational Diabetes stratified by maternal race and ethnicity (N=440)
| Non-Hispanic White (N=246) | Non-Hispanic Black (N=84) | Hispanic (N=49) | Other (N=61) | p- value | |
|---|---|---|---|---|---|
| Maternal age (years) | 32.3 ± 4.9 | 29.4 ± 5.1 | 32.7 ± 5.8 | 32.2 ± 4.1 | <0.001 |
| Body mass index in early pregnancy (kg/m2) | 34.1 ± 8.3 | 36.8 ± 10.9 | 35.0 ± 8.0 | 28.9 ± 7.4 | <0.001 |
| BMI≥30 (kg/m2) | 159 (64.6) | 62 (73.8) | 34 (69.4) | 20 (32.8) | <0.001 |
| Marital status | |||||
| Single | 55 (22.4) | 47 (55.9) | 17 (34.7) | 8 (13.1) | <0.001 |
| Married | 173 (70.3) | 25 (29.8) | 24 (48.9) | 45 (73.8) | |
| Divorced | 7 (2.9) | 2 (2.4) | 2 (4.1) | 2 (3.3) | |
| Missing | 11 (4.5) | 10 (11.9) | 6 (12.2) | 6 (9.8) | |
| Insurance | |||||
| Private | 173 (70.3) | 26 (30.9) | 12 (24.5) | 40 (65.6) | <0.001 |
| Public | 51 (20.7) | 43 (51.2) | 30 (61.2) | 12 (19.7) | |
| None | 17 (6.9) | 15 (17.9) | 6 (12.2) | 9 (14.8) | |
| Missing | 5 (2.0) | 0 (0.0) | 1 (2.0) | 0 (0.0) | |
| Chronic hypertension | 10 (4.1) | 8 (9.5) | 2 (4.1) | 1 (1.64) | 0.151 |
| Asthma | 13 (5.3) | 1 (1.2) | 2 (4.1) | 1 (1.6) | 0.336 |
| Nulliparity | 91 (36.9) | 28 (33.3) | 13 (26.5) | 24 (39.3) | 0.47 |
| Prior cesarean delivery | 54 (21.9) | 22 (26.2) | 8 (16.3) | 15 (24.6) | 0.512 |
| GDM characteristics | |||||
| Gestational age at GDM diagnosis (wks) | 25.3 ± 6.2 | 26.4 ± 5.9 | 26.9 ± 5.6 | 24.5 ± 6.5 | 0.100 |
| GDM diagnosis in the first trimester | 26 (10.6) | 10 (11.9) | 3 (6.1) | 8 (13.1) | 0.500 |
| Glucose level after 50-g glucose loading test (mg/dl) | 169.8 ± 25.8 | 185.3 ± 46.3 | 173.6 ± 22.2 | 181.3 ± 38.2 | 0.001 |
| Provider type | 0.052 | ||||
| General obstetrician | 32 (13.1) | 15 (18.1) | 13 (26.5) | 6 (9.8) | |
| Maternal-Fetal-Medicine | 133 (54.5) | 48 (57.8) | 29 (59.2) | 38 (62.3) | |
| Endocrinology | 79 (32.4) | 20 (24.1) | 7 (14.3) | 17 (27.9) | |
| Percent of abnormal blood glucose level prior to pharmacotherapy initiation | 45.5 ± 22.5 | 65.2 ± 25.4 | 58.3 ± 21.7 | 51.6 ± 26.8 | <0.001 |
| Gestational age at medical treatment initiation (wks) | 28.7 ± 6.2 | 30.1 ± 5.4 | 30.4 ± 5.7 | 28.6 ± 5.4 | 0.107 |
All data presented as mean ± standard deviation or N (%)
Bold indicates significant p-values
Maternal and neonatal outcomes are depicted in Table 2. NHB women had significantly higher rates of preeclampsia compared to the other three groups (p=0.005). In addition, neonates of NHB and mothers of other race and ethnicity had lower birth weight compared to NHW and Hispanic groups (p=0.005). Finally, NHB and Hispanic women had significantly higher rates of Apgar score <7 at 5 minutes (p=0.008).
Table 2.
Maternal outcomes for women with Gestational Diabetes stratified by maternal race and ethnicity
| Non-Hispanic White (N=246) | Non-Hispanic Black (N=84) | Hispanic (N=49) | Other (N=61) | p- value | |
|---|---|---|---|---|---|
| Gestational age at delivery (wks) | 38.3 ± 1.5 | 37.9 ± 1.8 | 38.1 ± 1.3 | 38.2 ± 2.5 | 0.512 |
| Preterm delivery < 37wks | 26 (10.6) | 12 (14.3) | 10 (20.4) | 6 (9.8) | 0.228 |
| Preeclampsia | 32 (13.0) | 15 (17.9) | 5 (10.2) | 3 (4.9) | 0.005 |
| Mode of Delivery | |||||
| Vaginal | 142 (57.7) | 47 (55.9) | 35 (71.4) | 35 (57.4) | 0.299 |
| Cesarean | 104 (42.3) | 37 (44.1) | 14 (28.6) | 26 (42.6) | |
| 3rd and 4th degree laceration | 3 (1.2) | 2 (2.4) | 0 | 3 (4.9) | 0.145 |
| Postpartum hemorrhage | 14 (5.7) | 7 (8.3) | 3 (6.1) | 3 (4.9) | 0.895 |
| Birth weight (grams) | 3416 ± 484 | 3330 ± 731 | 3459 ± 593 | 3143 ± 616 | 0.005 |
| Large for gestational age | 41 (16.6) | 17 (20.2) | 8 (16.3) | 5 (8.2) | 0.444 |
| Small for gestational age | 8 (3.3) | 7 (8.3) | 2 (4.1) | 6 (9.8) | 0.172 |
| Shoulder dystocia | 7 (2.9) | 5 (5.9) | 2 (4.1) | 1 (1.6) | 0.176 |
| Apgar <7 at 5 minutes | 8 (3.3) | 11(13.1) | 5 (10.2) | 1 (1.6) | 0.008 |
| NICU admission | 17 (6.9) | 11 (13.1) | 6 (12.2) | 3 (4.9) | 0.109 |
| Neonatal hypoglycemia | 58 (23.6) | 23 (27.4) | 10 (20.4) | 11 (18.0) | 0.889 |
| Jaundice requiring phototherapy | 13 (5.3) | 14 (16.7) | 2 (4.1) | 5 (8.2) | 0.055 |
All data presented as mean ± standard deviation or N (%)
NICU=neonatal intensive care unit
Bold indicates significant p-value
In unadjusted linear regression, maternal race and ethnicity was significantly associated with higher percent of elevated CBG values prior to initiation of pharmacotherapy: 19.7% (95% CI 13.7– 25.7) higher in NHB women and 12.9.% (95% CI 5.4 – 13.60) higher in Hispanic women compared to NHW women. After adjusting for clinical and demographic confounding factors in multivariable linear regression (Table 3), maternal race and ethnicity remained significantly associated with higher percent of elevated CBG values prior to pharmacotherapy initiation across all ethnic minority groups. The percent of elevated CBG values prior to pharmacotherapy initiation was 18.1% higher (95% CI 11.3 – 25.0) among NHB women compared to NHW women with GDM. Similarly, the percent of abnormal CBG values prior to pharmacotherapy initiation was 13.2% higher (95% CI 5.0 – 21.4) among Hispanic women compared to NHW women. Finally, the percent of abnormal CBG values prior to pharmacotherapy initiation was 9.8% higher (95% CI 2.6 – 16.9) among women of other racial and ethnic group compared to NHW women. Additional variables that remained to be independently associated with the percent of abnormal CBG values prior to pharmacotherapy initiation were maternal BMI and insurance (Table 3).
Table 3.
Linear regression models for the relationship between maternal race and ethnicity and percent of elevated glucoses when pharmacotherapy was initiated
| Percent of abnormal blood glucose values prior to pharmacotherapy initiation | Adjusted linear regression coefficient (95% CI) | p-value |
|---|---|---|
| Race | ||
| Non-Hispanic White | Ref | |
| Non-Hispanic Black | 18.12 (11.26 – 24.98) | <0.001 |
| Hispanic | 13.24 (5.05 – 21.43) | 0.002 |
| Other | 9.76 (2.61 – 16.91) | 0.008 |
| Maternal age | −0.01 (−0.48 – 0.48) | 0.998 |
| Body mass index in early pregnancy(kg/m2) | 0.43 (0.16 – 0.70) | 0.002 |
| Marital Status | ||
| Single | Ref | |
| Married | −2.83 (−8.64 – 2.97) | 0.338 |
| Divorced | 5.42 (−7.92 – 18.76) | 0.425 |
| Insurance Status | ||
| Private | Ref | |
| Public | 6.21 (0.18 – 12.24) | 0.043 |
| None | −5.22 (−13.16 – 2.72) | 0.197 |
| Preexisting hypertension | ||
| No | Ref | |
| Yes | −0.65 (−12.13 – 10.83) | 0.912 |
| Gestational age at GDM diagnosis (wks) | 0.18 (−0.22 – 0.58) | 0.380 |
Bold indicates significant p-value
When performing sensitivity analyses and including in the regression the glucose level at 50-g glucose loading test (available for 359 patients), thus controlling for the severity of the failed GDM screening test, the association between maternal race and ethnicity and the percent of abnormal CBG values prior to pharmacotherapy initiation persisted across all racial groups (data not shown). An additional sensitivity analysis that excluded 44 women diagnosed with GDM in the first trimester was conducted (Tables 4 and 5). It found that NHB women had infants with lower birth weights and higher rates of Apgar <7 at 5 minutes as well as jaundice requiring phototherapy (Table 4). The results of the multivariable linear regression were similar to those of the primary analysis, demonstrating that maternal race and ethnicity, BMI and insurance status were independently associated with the percent of abnormal CBG values prior to pharmacotherapy initiation.
Table 4.
Maternal outcomes for women with Gestational Diabetes stratified by maternal race and ethnicity after excluding 44 women diagnosed with GDM in the first trimester
| Non-Hispanic White (N=221) | Non-Hispanic Black (N=75) | Hispanic (N=46) | Other (N=54) | p- value | |
|---|---|---|---|---|---|
| Gestational age at delivery (wks) | 38.4 ± 1.8 | 37.9 ± 1.8 | 38.1 ± 1.4 | 38.3 ± 2.2 | 0.179 |
| Preterm delivery < 37wks | 22 (10.0) | 11 (14.7) | 10 (21.7) | 4 (7.4) | 0.084 |
| Preeclampsia | 28 (12.7) | 13 (18.3) | 4 (8.7) | 3 (5.6) | 0.154 |
| Mode of Delivery | |||||
| Vaginal | 127 (57.5) | 41 (54.7) | 34 (73.9) | 30 (55.6) | 0.155 |
| Cesarean | 94 (42.5) | 34 (45.3) | 12 (26.1) | 24 (44.4) | |
| 3rd and 4th degree laceration | 3 (1.5) | 2 (3.2) | 0 | 3 (6.2) | 0.167 |
| Postpartum hemorrhage | 12 (5.7) | 7 (9.7) | 3 (6.7) | 3 (5.7) | 0.681 |
| Birth weight (grams) | 3428 ± 491 | 3323 ± 715 | 3430 ± 580 | 3208 ± 536 | 0.049 |
| Large for gestational age | 40 (18.2) | 15 (20.0) | 7 (15.2) | 5 (9.3) | 0.377 |
| Small for gestational age | 6 (2.7) | 6 (8.0) | 2 (4.4) | 3 (5.6) | 0.259 |
| Shoulder dystocia | 7 (3.2) | 5 (6.8) | 2 (4.4) | 1 (1.9) | 0.454 |
| Apgar <7 at 5 minutes | 7 (3.2) | 10 (13.3) | 5 (10.9) | 1 (1.9) | 0.003 |
| NICU admission | 14 (6.4) | 10 (13.7) | 6 (13.3) | 2 (3.7) | 0.073 |
| Neonatal hypoglycemia | 53 (24.4) | 19 (26.0) | 9 (20.0) | 8 (14.8) | 0.406 |
| Jaundice requiring phototherapy | 13 (6.0) | 13 (17.8) | 1 (2.3) | 4 (7.6) | 0.006 |
All data presented as mean ± standard deviation or N (%)
NICU=neonatal intensive care unit
Bold indicates significant p-value
Table 5.
Linear regression models for the relationship between maternal race and ethnicity and percent of elevated glucoses when pharmacotherapy was initiated after excluding 44 women diagnosed with GDM in the first trimester
| Percent of abnormal blood glucose values prior to pharmacotherapy initiation | Adjusted linear regression coefficient (95% CI) | p-value |
|---|---|---|
| Race | ||
| Non-Hispanic White | Ref | |
| Non-Hispanic Black | 18.71 (11.63 – 25.79) | <0.001 |
| Hispanic | 11.64 (3.26 – 20.03) | 0.007 |
| Other | 8.53 (0.92 – 16.14) | 0.028 |
| Maternal age | −0.14 (−0.64 – 0.35) | 0.568 |
| Body mass index in early pregnancy(kg/m2) | 0.47 (0.18 – 0.76) | 0.001 |
| Marital Status | ||
| Single | Ref | |
| Married | −1.31 (−7.30 – 4.68) | 0.667 |
| Divorced | 7.27 (−6.97 – 21.51) | 0.316 |
| Insurance Status | ||
| Private | Ref | |
| Public | 7.26 (1.00 – 13.53) | 0.023 |
| None | −4.82 (−12.95 – 3.30) | 0.244 |
| Preexisting hypertension | ||
| No | Ref | |
| Yes | −2.74 (−15.50 – 10.02) | 0.673 |
| Gestational age at GDM diagnosis (wks) | 0.19 (−0.48 – 0.86) | 0.577 |
Bold indicates significant p-value
Discussion
In our study, maternal race and ethnicity was associated with the percent of abnormal CBG values prior to initiation of pharmacotherapy in women with GDM. NHW women with GDM received pharmacotherapy at a lower percent of abnormal CBG values than women of other race and ethnicity groups with GDM. This association persisted when we controlled for demographics, clinical factors including BMI and preexisting hypertension and GDM characteristics, including gestational age at GDM diagnosis and the severity of abnormal CBG values at the time of GDM screening.
This study adds important information to the literature on the association between maternal race and ethnicity and GDM management. First, it highlights the lack of consensus in timing of pharmacotherapy initiation for women with GDM. There is no guidance regarding what percent of abnormal CBG values that exceed the recommended GDM targets should trigger initiation of insulin or oral hypoglycemic agent, in addition to diet and exercise [2,11,17,19,21]. Two GDM landmark trials conducted by Crowther et al and Landon et al., comparing pregnancy outcomes in women with treated and untreated GDM, utilized two different thresholds to start pharmacotherapy.4,5 Crowther et al. started pharmacotherapy beyond diet and exercise when two abnormal CBG values were recorded over a 14–day span, with cutoffs of 99 mg/dL for fasting and 126 mg/dL for 2-hour post-prandial values [4]. Landon et al utilized a protocol with initiation of insulin if “the majority” of a patient’s CBG values were elevated with cutoffs above 95 mg/dL for fasting and 120 mg/dL for 2-hour post-prandial levels [5]. A recent review of 15 GDM studies found a wide variation in pharmacotherapy initiation, ranging from one abnormal CBG value over the course of 1–2 weeks to more than 50% of abnormal CBG values per week [11]. The findings of our study confirm this variability in GDM management that exists even within a single medical center. Second, our study suggests that maternal race and ethnicity may affect this variability as NHB women and other racial and ethnic minority women were started on pharmacotherapy at significantly higher percent of abnormal CBG values compared to NHW women. Racial disparities have been shown to exist in GDM prevalence and GDM-related outcomes [12–16]. A study of women with GDM in North Carolina found that GDM prevalence was twice more common among Hispanic compared to NHW and NHB women [16]. In addition, the authors found that Hispanic women with GDM were more likely to receive pharmacotherapy and had lower rates of preterm delivery, hypertensive disorder of pregnancy and NICU admission compared to Caucasian women [16]. Another study of singleton births among women with GDM in California found that NHB women with GDM had a higher risk of preeclampsia, preterm birth, primary cesarean delivery and neonatal hypoglycemia compared to White women [15]. Explanations proposed in these studies for their findings included sociocultural support structures and traditions, health care utilization, patient-provider relations and biases and inherent genetic predisposition. Our study suggests an additional explanation to the disparity seen in GDM-related outcomes and that is that women of racial and ethnic minorities are more likely to have pharmacotherapy for GDM started at a higher glycemic threshold.
Based on our study findings a few targets for intervention and research can be identified. First, the lack of consensus in GDM management provides an opportunity for developing guidelines on when to add pharmacotherapy for GDM treatment. This will provide practices followed across all pregnant women with GDM and will aid in removing the racial and ethnic disparity seen in our study. Second, it points towards a need to design prospective studies that will compare different glycemic thresholds for pharmacotherapy initiation and their effect on maternal and perinatal outcomes in women with GDM.
There are a few limitations of our study that should be noted. First, currently there is no consensus that tighter GDM control and pharmacotherapy initiation at a lower percent of elevated CBG values leads to improved maternal and neonatal outcomes. A trial in New Zealand is being conducted to answer this question [22]. Second, the findings of our study are less applicable to biracial individuals as the medical records did not have data on biracial identity and these women were analyzed together with “other” race and ethnicity group. Future studies should investigate this relationship in larger and more diverse samples to determine if these findings can be generalized beyond our medical center. Another limitation may exist that the GDM phenotype in women of ethnic minorities may be more severe than in NHW women, and this is not related to provider management of pharmacotherapy initiation. In order to address this limitation, we excluded women who were started on pharmacotherapy within the first clinic visit following a week of dietary modifications. Finally, it is prudent to mention that we did not assess women’s adherence to treatment nor the degree of glycemic control after initiation of pharmacotherapy and cannot comment whether the difference seen in maternal outcomes in Tables 2 and 4 was secondary to timing of pharmacotherapy initiation versus adherence to treatment.
Despite the limitations, our study adds to the existing literature by examining maternal clinical and demographic factors that may affect GDM management. It also provides information on GDM characteristics that are associated with differences in glycemic threshold for pharmacotherapy initiation. In conclusion, we identified a significant variation in the percent of elevated glucose levels for pharmacotherapy initiation in women with GDM across different maternal racial and ethnic groups with minority women starting pharmacotherapy at higher glycemic threshold. Future studies are needed to: 1) determine whether this variation contributes to racial and ethnic disparity present in GDM-related outcomes, and 2) provide guidance on timing of pharmacotherapy initiation.
Footnotes
Financial disclosures: The authors did not report any potential conflict of interest.
Each author has indicated that he/she has met the journal’s requirements for authorship.
References
- 1.Lockwood CJ, Iams JD and Greene MF. Creasy and Resnik’s maternal-fetal medicine: principles and practice. Elsevier Health Sciences, 2013. [Google Scholar]
- 2.ACOG Practice Bulletin No. 190: Gestational Diabetes Mellitus. Obstet Gynecol. 2018;131:e49–e64. [DOI] [PubMed] [Google Scholar]
- 3.Langer O, Yogev Y, Most O, Xenakis EM. Gestational diabetes: the consequences of not treating. Am J Obstet Gynecol. 2005; 192: 989–97. [DOI] [PubMed] [Google Scholar]
- 4.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. NEJM 2005; 352: 2477–2486. [DOI] [PubMed] [Google Scholar]
- 5.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009; 361: 1339–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008; 358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 7.Hartling L, Dryden DM, Guthrie A, Muise M, Vandermeer B, Donovan L. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S. Preventive Services Task Force and the National Institutes of Health Office of Medical Applications of Research. Ann Intern Med. 2013; 159: 123–9. [DOI] [PubMed] [Google Scholar]
- 8.Athukorala C, Crowther CA, Willson K. Women with gestational diabetes mellitus in the ACHOIS trial: risk factors for shoulder dystocia. Aust N Z J Obstet Gynaecol. 2007; 47: 37–41. [DOI] [PubMed] [Google Scholar]
- 9.Gilbert WM, Nesbitt TS, Danielsen B. Associated factors in 1611 cases of brachial plexus injury. Obstet Gynecol. 1999; 93:536–40. [DOI] [PubMed] [Google Scholar]
- 10.Boney CM, Verma A, Tucker R, Vohr BR. Metabolic syndrome in childhood: association with birth weight, maternal obesity, and gestational diabetes mellitus. Pediatrics. 2005; 115: e290–6. [DOI] [PubMed] [Google Scholar]
- 11.Caissutti C, Berghella V. Scientific Evidence for Different Options for GDM Screening and Management: Controversies and Review of the Literature. Biomed Res Int. 2017; 2017:2746471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hedderson MM, Darbinian JA, Ferrara A. Disparities in the risk of gestational diabetes by race-ethnicity and country of birth. Paediatr Perinat Epidemiol. 2010; 24: 441–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pu J, Zhao B, Wang EJ, Nimbal V, Osmundson S, Kunz L, et al. Racial/Ethnic Differences in Gestational Diabetes Prevalence and Contribution of Common Risk Factors. Paediatr Perinat Epidemiol. 2015; 29:436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Silva JK, Kaholokula JK, Ratner R, Mau M Ethnic Differences in perinatal outcome of gestational diabetes mellitus. Diabetes Care. 2006; 29:2058–2063 [DOI] [PubMed] [Google Scholar]
- 15.Nguyen BT, Cheng YW, Snowden JM, Esakoff TF, Frias AE, Caughey AB. The effect of race/ethnicity on adverse perinatal outcomes among patients with gestational diabetes mellitus. Am J Obstet Gynecol. 2012; 207: 322.e1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Berggren EK, Boggess KA, Funk MJ, Stuebe AM. Racial disparities in perinatal outcomes among women with gestational diabetes. J Womens Health (Larchmt). 2012; 21:521–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landon MB, Gabbe SG, Sachs L. Management of diabetes mellitus and pregnancy: a survey of obstetricians and maternal fetal specialists. Obstet Gynecol 1990;75:635e40. [PubMed] [Google Scholar]
- 18.Kjos SL, Schaefer-Graf U, Sardesi S, Peters RK, Buley A, Xiang AH, et al. A randomized controlled trial using glycemic plus fetal ultrasound parameters versus glycemic parameters to determine insulin therapy in gestational diabetes with fasting hyperglycemia. Diabetes Care 2001; 24: 1904e10. [DOI] [PubMed] [Google Scholar]
- 19.Hiersch L, Yogev Y. Management of diabetes and pregnancy--when to start and what pharmacological agent to choose? Best Pract Res Clin Obstet Gynaecol. 2015; 29:225–36. [DOI] [PubMed] [Google Scholar]
- 20.Duryea EL, Hawkins JS, McIntire DD, Casey BM, Leveno KJ. A revised birth weight reference for the United States. Obstet Gynecol 2014;124: 16–22. [DOI] [PubMed] [Google Scholar]
- 21.American Diabetes Association. 13. Management of diabetes in pregnancy. Diabetes Care. 2017; 40(Supplement 1):S114–9. [DOI] [PubMed] [Google Scholar]
- 22.Crowther CA, Alsweiler JM, Hughes R, Brown J; Target Study Group. Tight or less tight glycaemic targets for women with gestational diabetes mellitus for reducing maternal and perinatal morbidity? (TARGET): study protocol for a stepped wedge randomised trial. BMC Pregnancy Childbirth. 2018;18: 425. [DOI] [PMC free article] [PubMed] [Google Scholar]