Abstract
In India, many people living with HIV (PLHIV) do not successfully initiate antiretroviral therapy (ART) after diagnosis. We conducted a clinic-based qualitative study at the Y.R. Gaitonde Centre for AIDS Research in Chennai, Tamil Nadu to explore factors that influence ART non-initiation. We interviewed 22 men and 15 women; median age was 42 (IQR, 36–48) and median CD4+ was 395 (IQR, 227–601). Participants were distrustful of HIV care freely available at nearby government facilities. Faced with the perceived need to access the private sector and therefore pay for medications and transportation costs, non-initiators with high CD4+ counts often decided to postpone ART until they experienced symptoms whereas non-initiators with low CD4+ counts often started ART but defaulted quickly after experiencing financial stressors or side effects. Improving perceptions of quality of care in the public sector, encouraging safe serostatus disclosure to facilitate stronger social support, and alleviating economic hardship may be important in encouraging ART initiation in India.
Keywords: antiretroviral therapy, initiation, India, poverty
Introduction
India, which has the third-largest population of people living with HIV (PLHIV) at 2.1 million persons (National AIDS Control Organisation, 2018), features a concentrated epidemic with higher HIV prevalence among female sex workers, men who have sex with men, and injection drug users (World Bank, 2012). In 2017, India introduced a “Test and Treat” policy ensuring that all PLHIV are ART-eligible. Yet only 49% of all Indian PLHIV, and only 63% of Indian PLHIV who are aware of their serostatus, are estimated to be on ART (UNAIDS, 2017).
The success of “Test and Treat” in achieving virologic suppression for all Indian PLHIV will depend upon universal ART initiation at the point of testing. Failure to successfully initiate ART may result from patients declining to start ART or patients agreeing to start but stopping ART shortly thereafter (Katz et al., 2015; 2011). In studies in sub-Saharan Africa, factors associated with unsuccessful ART initiation include feeling too healthy, disclosure concerns, financial burdens, lack of social support, and presence of maladaptive coping strategies (Katz et al., 2011; 2015). However, there have been no such studies in India, which features a markedly different socio-cultural environment and epidemic distribution.
A better understanding of the factors that contribute to ART decision-making in India would help identify individuals at risk for unsuccessful ART initiation and lay the groundwork for interventions. To that end, we conducted a qualitative study at an ART care center in southern India, using inductive reasoning to identify themes around failed ART initiation and to create a conceptual framework of why PLHIV are unsuccessful in initiating ART.
Methods
We conducted this patient-focused qualitative study at the Y.R. Gaitonde Centre for AIDS Research and Education (YRG CARE), Chennai, one of the largest private HIV providers in India. While there are no publicly available numbers on the proportion of PLHIV in India who seek care in the private sector, the Indian National Strategic Plan for HIV/AIDS and STI 2017–2024 notes that the private sector is a “major” component of the effort against HIV and that private sector facilities comprise 3,500 of the 22,000 HIV Counselling and Testing Centres in the country. YRG CARE serves patients who are newly diagnosed with HIV as well as patients referred from other HIV providers (including the public sector). Historically, most patients (>95%) at YRG CARE have self-identified as heterosexual, as there are other local organizations perceived as specializing in care for sexual and gender minorities. YRG CARE charges modest fees for treatment and care (unlike the public sector, where care is free). At the time of the study, typical first-line treatment (efavirenz-based ART) was the same as in the public sector.
We conducted semi-structured interviews with adult PLHIV who had previously established care at YRG CARE and presented for care between June-August 2017. Using maximum variation sampling, we purposively sampled participants based on ART initiation vs. non-initiation, sex, and CD4+ count (≥ or <500 cells/mm3). We defined non-initiators as people who refused ART at time of diagnosis or who accepted ART but stopped for any reason within six months. Participants were offered Rs. 250 (approximately 3.50 USD at an exchange rate of 71:1) and a lunch token worth Rs. 30 (approximately 0.50 USD) as compensation for their time. We obtained informed consent from all participants. Ethical approval was obtained from the Institutional Review Boards of Partners Healthcare and YRG CARE as well as the Health Ministry Screening Committee of India. Study procedures were approved by the YRG CARE Community Advisory Board.
Indian research staff, sometimes with US research staff present, conducted the participant interviews in a private setting in Tamil or Telugu. All interviews were recorded, de-identified, transcribed, and translated into English. We used a multi-step qualitative data analysis approach based on grounded theory (Corbin & Strauss, 2008) and content analysis (Creswell, 2009; Smith, 1992) and supported by Dedoose software. First, we used “open-coding” to inductively draw out themes around important factors in ART decision-making. Next, we created a codebook, listing each theme accompanied by a detailed description, inclusion/exclusion criteria for each code, and examples of text. We then finalized and applied the codebook to the entire sample, using axial coding to examine relationships within and among categories. We assessed intercoder reliability using Cohen’s Kappa. We then agreed upon illustrative quotations that best described factors that compromise or enable ART initiation. Finally, we integrated the key conclusions into a conceptual model of psychosocial, structural, and clinic-based factors affecting ART decision-making and initiation success.
Results
We enrolled 37 participants including 15 women and 22 men (Table 1). The Cohen’s Kappa for intercoder reliability was 87%. We identified key themes that influence patient decision-making and integrated our core findings into a conceptual model (Figure; Table 2). Outright refusal of ART was rare, as participants reported trust in the benefits of ART and lack of trust in alternative treatments. Yet because of perceptions of poor quality of care, lack of respect, and disclosure concerns, participants opted not to seek HIV care freely available at nearby government facilities, leading to increased expenditures associated with accessing care. Relatively well-off participants and participants who could leverage social support, including financial assistance, from friends or family felt prepared to initiate ART and manage obstacles to adherence. Faced with many potential expenditures related to HIV care, non-initiators with high CD4+ counts often decided to postpone ART until they experienced symptoms. Non-initiators with low CD4+ counts, who were usually feeling sick, often started ART but defaulted quickly after experiencing financial stressors or side effects that required the expense of another clinic visit to address. Persistent depressive symptoms, problem alcohol use, and illicit substance use were infrequently reported as reasons for ART non-initiation.
Table I:
Characteristics of study participants
| Characteristic | n=37 |
|---|---|
| Female | 115 (41%) |
| Age, median (IQR), y | 42 (36–48) |
| Language | |
| Tamil | 8 (22%) |
| Telugu | 29 (78%) |
| CD4+ count, median (IQR), cells/mm3 | 395 (230–601) |
| CD4+ ≥ 500 only (n=17), median (IQR), cells/mm3 | 642 (563–756) |
| CD4+ < 500 only (n=20), median (IQR), cells/mm3 | 249 (182–337) |
| ART initiation status | |
| ART initiators | 18 (49%) |
| ART non-initiators | 19 (51%) |
Figure:
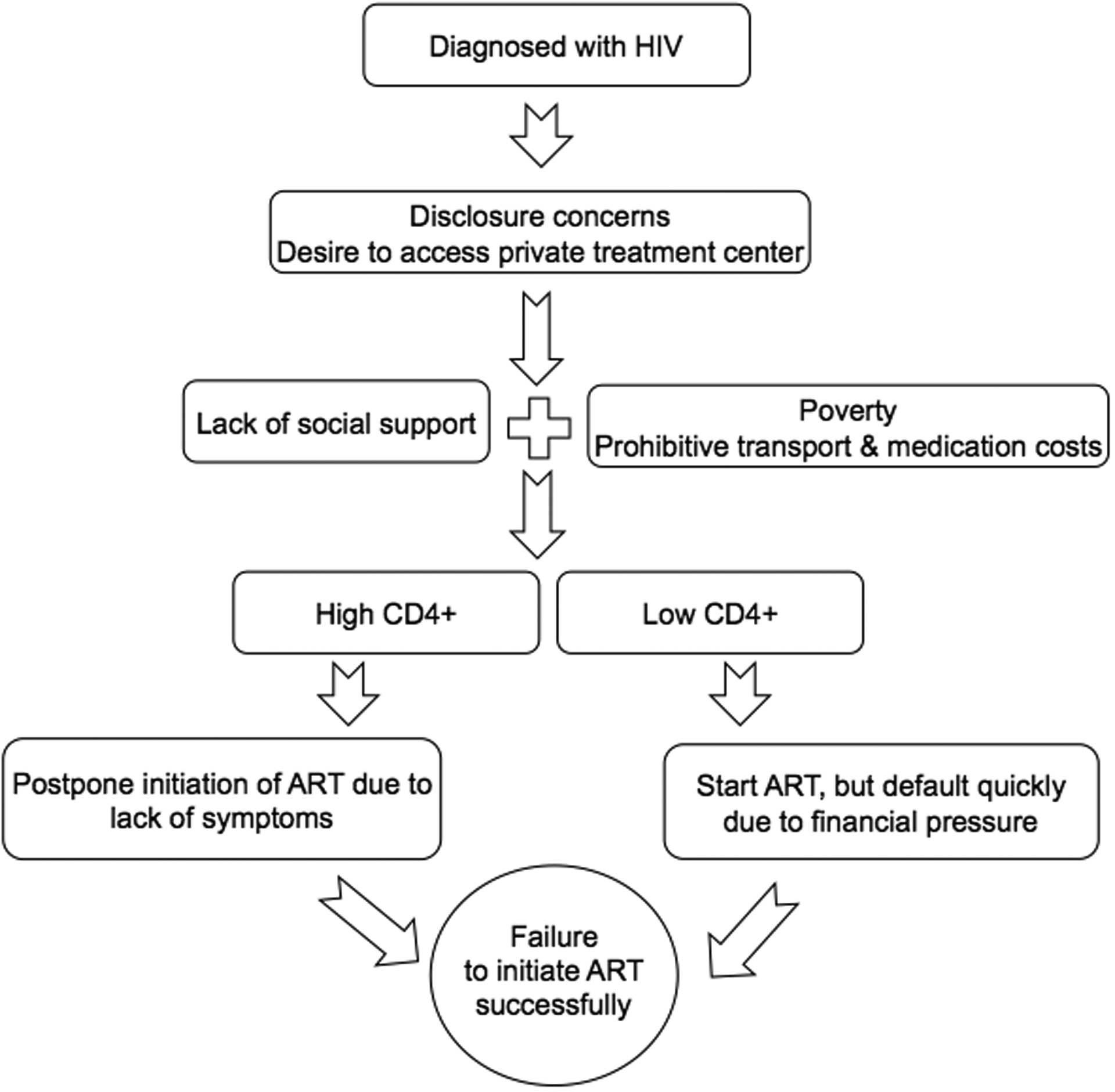
Conceptual model of economic vulnerability and non-initiation of antiretroviral therapy in southern India
Table 2:
Key themes and illustrative quotes related to success of antiretroviral therapy initiation in southern India
| Key theme | Illustrative quotes |
|---|---|
|
Trust in benefits of ART Almost all participants felt that ART would sustain or improve health, thereby allowing the maintenance or restoration of social standing and economic viability |
“Before when I was working, especially in the hot sun, I used to get very tired… After taking meds, for about the first three months, I could see some improvement—I could work in the sun.” (40-year-old man) “… [My mother] is very supportive, she tells me to take tablets. ‘You have to take tablets, otherwise who will take care of your children?’” (39-year-old woman) |
|
Mistrust in alternative treatments
Alternative treatments such as ayurveda and siddha were not perceived as a credible alternatives, with few exceptions. |
“They [traditional practitioners] will just take your money. That is all.” (51-year-old man) “I have not taken [alternative treatments], but yes, I am considering it… I’ll take these meds but I am also looking at other avenues to find a complete cure.” (47-year-old man) |
|
Mistrust of government ART facilities Participants were distrustful of HIV care freely available at nearby government facilities; many thought the ART given at these facilities was different and inferior. |
“If you go to the government hospital, you won’t get treated well, not like here at YRG. They don’t give us respect there. We would rather come here… They are very careless there.” (34-year-old woman) “… I had treatment from the government hospital for two or three years. I had allergy and side effects like pimples so I stopped. Then two years later I came to YRG… The treatment here is better.” (34-year-old man) |
|
Social support
Participants who could leverage social support, including financial assistance, from friends and family felt prepared to initiate ART and manage obstacles to adherence. |
“Maybe [I felt depressed] in the beginning for a month or so, but I got over it. I have a very good support network… They tell me I can manage it.” (30-year-old man) “There is so much good in telling people [my serostatus]. Some people will help us. I told my friend. He has given me some money and told me to go anywhere [for treatment].” (39-year-old man) |
|
Economic vulnerability, patients with high CD4+ counts Faced with the perceived need to pay for medications and prohibitive transportation costs, non-initiators with high CD4+ counts often decided to postpone ART until they experienced symptoms. |
“For the first 8 years I did not want to start because of financial problems. But at the present time my CD4+ is decreasing. In 2016, it was 761. Until then, I did not know my health was decreasing. Then I found out about CD4+ and that it decreasing is bad. I want to improve my health and reduce my viral load so I can arrange another marriage and have one or two children. So I will start ART.” (34-year-old man) |
|
Economic vulnerability, patients with low CD4+ counts Non-initiators with low CD4+ counts often started ART but defaulted quickly after experiencing financial stressors or side effects that required the expense of another clinic visit to address. |
“[ART] started in 2016, I used it for six months. And then my budget was very tight. Then I stopped it.” (30-year-old man) “They told me [about side effects] but my body was not cooperating, so I stopped [ART}… They told me but I did not come because I had some financial difficulties.” (31-year-old woman) |
ART: antiretroviral therapy.
Discussion
In our conceptual model, economic vulnerability, exacerbated by disclosure concerns and distrust of HIV care freely available at nearby government facilities, forced PLHIV in southern India to make difficult choices that resulted in failure to initiate ART. Economically secure PLHIV and PLHIV with good social support networks were often able to overcome obstacles to ART initiation, whereas PLHIV living on the margins with poor social support often postponed ART or defaulted quickly due in large part to financial pressure.
Participants in our study extolled the benefits of ART, in contrast to prior research from sub-Saharan Africa where participants perceived themselves as “too healthy” for ART (Katz et al., 2011). Our results may have differed for several reasons: improvements in knowledge of HIV and ART over time, cultural differences between India and Africa, or our participants being those who were motivated to seek care at a private facility.
Many participants reported poor quality of care in the government sector. Harsh, demeaning, and stigmatizing behavior from health workers has been shown to exacerbate loss from the HIV care continuum (Ware et al., 2013) and delay ART initiation (Maughan-Brown et al., 2018). Poor quality of care and low levels of clinician effort have been shown to be endemic in the Indian public health sector (Das et al., 2012). Thus, suboptimal HIV care in the public sector, whether perceived or experienced, may exacerbate financial stressors on PLHIV by encouraging many to seek care in often-distant private facilities.
The importance of social support concords with findings from sub-Saharan Africa (Katz et al., 2015; 2019). Instrumental support may be particularly important in settings such as India or sub-Saharan Africa where many PLHIV live in poverty and there exists a relatively weak public social safety net. Social support is also critical in helping PLHIV overcome “mental tension,” manage ART side effects, and cope with stigma.
Our findings have important implications. Improving care in the public sector, as well as perceptions of quality (e.g., through media campaigns) would encourage more people to seek care in government facilities, where costs are less of a concern. Interventions that empower PLHIV to safely disclose their serostatus could allow them to build stronger social supports to overcome psychosocial and structural barriers to care. Finally, transportation incentives, food support, and other interventions to alleviate economic hardship may be crucial.
Our study has several limitations. First, as we used a clinic-based sample of participants, our data may not capture reasons for failure to initiate ART among PLHIV unwilling or unable to return to care. For example, mental health concerns and alcohol use were infrequently cited as a barrier to ART initiation; these concerns could be more salient among those unable or unwilling to return to care. Second, our data may not reflect reasons for unsuccessful ART initiation elsewhere within India, including government ART centers and centers that serve greater numbers of sexual and gender minorities. Sampling participants only at a private clinic likely colored our findings on perceptions of quality of care in the public sector. Nevertheless, the fact that a large number of PLHIV “vote with their feet” is a noteworthy finding and speaks to the need to improve perceptions of quality of care in the public sector, if not the quality of care itself. Given these limitations, future research should investigate factors related to ART initiation in a variety of settings throughout India. Lastly, we did not externally verify participants’ financial situations, though we have no reason to believe that participants would exaggerate the financial implications of seeking care.
Conclusion
In this qualitative study of PLHIV conducted at a large, private HIV care center in Chennai, we found that economic vulnerability intersected with multiple factors including quality of care, disclosure concerns/anticipated stigma, and social support, forcing PLHIV to make difficult choices that resulted in failure to initiate ART. Improving perceptions of quality of care in the public sector, using better tolerated ART regimens, encouraging safe disclosure of serostatus to enhance social support, and implementing interventions to alleviate economic hardship may be important in encouraging ART initiation in India.
Acknowledgements
We are grateful to the many YRG CARE staff who participated in this project and the participants who shared their time and insights.
Funding: No authors have any conflicts of interest. The authors acknowledge the following sources of support: Harvard Global Health Institute (Colocci, Perlo), Harvard University Center for AIDS Research (Colocci, Perlo), NIH K23MH097667 (Katz), NIH K23MH110338 (Chan).
Footnotes
A preliminary version of this analysis was presented at the 13th International Conference on HIV Treatment and Prevention Adherence, Miami, FL, USA, June 8, 2018.
References
- Corbin J, & Strauss A (2008). Basics of Qualitative Research: Techniques and Procedures for Developing Grounded Theory. Los Angeles: Sage Publications. [Google Scholar]
- Creswell JW (2009). Research design: Qualitative, quantitative, and mixed methods approaches (3rd ed.). Thousand Oaks, CA: Sage. [Google Scholar]
- Das J, Holla A, Das V, Mohanan M, Tabak D, & Chan B (2012). In urban and rural India, a standardized patient study showed low levels of provider training and huge quality gaps. Health Affairs (Project Hope), 31(12), 2774–2784. 10.1377/hlthaff.2011.1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz IT, Bogart LM, Dietrich JJ, Leslie HH, Iyer HS, Leone D, et al. (2019). Understanding the role of resilience resources, antiretroviral therapy initiation, and HIV-1 RNA suppression among people living with HIV in South Africa: a prospective cohort study. AIDS (London, England), 33 Suppl 1, S71–S79. 10.1097/QAD.0000000000002175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz IT, Dietrich JJ, Tshabalala G, Essien T, Rough K, Wright AA, et al. (2015). Understanding treatment refusal among adults presenting for HIV-testing in Soweto, South Africa: a qualitative study. AIDS and Behavior, 19(4), 704–714. 10.1007/s10461-014-0920-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz IT, Essien T, Marinda ET, Gray GE, Bangsberg DR, Martinson NA, & De Bruyn G (2011). Antiretroviral therapy refusal among newly diagnosed HIV-infected adults. AIDS (London, England), 25(17), 2177–2181. 10.1097/QAD.0b013e32834b6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan-Brown B, Kuo C, Galárraga O, Smith P, Lurie MN, Bekker L-G, & Harrison A (2018). Stumbling Blocks at the Clinic: Experiences of Seeking HIV Treatment and Care in South Africa. AIDS and Behavior, 22(3), 765–773. 10.1007/s10461-017-1877-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National AIDS Control Organisation, ICMR-National Institute of Medical Statistics. (2018). India HIV estimations 2017: Technical Report. Retrieved from http://naco.gov.in/sites/default/http://naco.gov.in/sites/default/files/HIV%20Estimations%202017%20Report_1.pdf
- Smith CP (1992). Motivation and personality: Handbook of thematic content analysis. New York: Cambridge University Press. [Google Scholar]
- UNAIDS. (2017). Ending AIDS: progress towards the 90–90–90 targets. Retrieved September 7, 2018, from http://www.unaids.org/sites/default/files/media_asset/Global_AIDS_update_2017_en.pdf
- Ware NC, Wyatt MA, Geng EH, Kaaya SF, Agbaji OO, Muyindike WR, et al. (2013). Toward an understanding of disengagement from HIV treatment and care in sub-Saharan Africa: a qualitative study. PLoS Medicine, 10(1), e1001369–discussion e1001369. 10.1371/journal.pmed.1001369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Bank (Ed.). (2012). HIV/AIDS in India. Retrieved July 27, 2014, from http://www.worldbank.org/en/news/feature/2012/07/10/hiv-aids-india


