Abstract
Purpose:
Studies demonstrating mortality benefit of beta blockers (BB) after myocardial infarction (MI) were conducted before the era of percutaneous intervention and widespread use of statins. Recent retrospective studies show inconsistent results regarding which subgroups of coronary artery disease (CAD) patients benefit. Most studies did not account for medication changes over time. We evaluated the association of time-varying BB exposure with death in CAD patients with or without a history of MI.
Methods:
This retrospective cohort study included all patients with MI and those with coronary disease but no MI at a single health care system who also had health insurance from January 1, 1997 to June 30, 2011. Pharmacy claims data were used to estimate BB exposure over 6-month rolling windows. The primary endpoint was all-cause death. The effect of BB exposure was tested using time-updated Cox proportional hazards models.
Results:
We identified 6220 patients with MI and 21,285 patients with CAD but no MI. Among patients who suffered MI, BB exposure was associated with a 31% relative risk reduction in all-cause death (hazard ratio [HR] 0.69, P = 0.001). Among subjects who survived 3 years after MI, BB retained a protective association (HR 0.71, P = 0.001). Among CAD only patients BB exposure was also associated with risk reduction (HR 0.85, P = 0.001).
Conclusion:
Among patients with CAD, BB exposure is associated with reduced risk of death. The association is strongest among those who have suffered MI. This favorable association appears durable beyond 3 years.
Keywords: Heart failure, coronary artery disease, Beta Blocker, survival
INTRODUCTION
Current practice guidelines recommend beta blockers (BB) for patients with coronary artery disease (CAD) and at least 3 years after myocardial infarction (MI) (1,2). However, these recommendations are based primarily upon randomized clinical trials conducted mostly in the 1980s (3,4). Subsequently, clinical trials have demonstrated the benefit of statins (for CAD in 1994 (5) and for acute coronary syndrome in 2001 (6)), adenosine diphosphate (ADP) antagonists (7) and coronary revascularization using stents, each of which is also now the standard of care in acute coronary syndrome. The most recent randomized trial of BB in MI was the COMMIT Collaborative Group’s evaluation of metoprolol in patients with ST elevation MI (recruitment from 1999–2005) (8). That trial found that early intravenous metoprolol followed by oral metoprolol was associated with reduced reinfarction and ventricular tachycardia/ventricular fibrillation, but increased risk of cardiogenic shock. Additionally, the study’s short overall follow up of 28 days leaves unclear the long-term effect of BB use as secondary prevention. Add to this the concern surrounding BB use in the perioperative setting (9) and other possibly adverse impacts of BB and it becomes clear that there could be doubt about the incremental benefit of BB in the setting of modern care, which has not been reassessed in randomized clinical trials.
There have been a number of studies attempting to shed light on this question. Bangalore et al explored this within a longitudinal observational study (the REACH registry) (10), and did not demonstrate a benefit in terms of composite cardiovascular events (cardiovascular death, nonfatal MI, or nonfatal stroke) associated with BB. Subsequent works have shown inconsistent result. A post hoc analysis of the CHARIMA trial found that BB were beneficial for patients with a prior MI (11) but were not protective in CAD patients without prior MI, similar to the findings of another study (12). Puymirat et al examined the role of BB and mortality after MI in patients without heart failure and found a 30-day morality benefit that did not continue out to 1 year (13), but Dondo et al performing a similar analysis found no benefit at all(14). In a meta-analysis of the relationship between BB and outcomes in patients who had an MI, Huang et al found a limited benefit in the subset of patients with low left ventricular systolic function, low adherence to other secondary prevention therapies, and in those who had a non-ST-elevation MI (15). Thus, there remains uncertainty about which subgroups of CAD patients are likely to gain benefit from BB exposure and the duration of that benefit. Importantly, most of these studies rely on discharge BB status and there remains potential confounding issues that may not have been completely accounted for, specifically the impact of differing doses of BB, changes in therapy over time, and the impact of patient adherence behaviors. In this context we sought to assess the clinical outcomes associated with BB exposure in a modern cohort of CAD patients with and without a history of MI and taking into account quantified medication exposure over time and patient adherence.
MATERIALS AND METHODS
Study Population
Subjects were identified from patients receiving health care through the Henry Ford Health System, a vertically integrated health system serving the primary and specialty health care needs of individuals in southeastern Michigan, as well as an affiliated health maintenance organization (HMO). The system maintains a central repository of administrative data that was queried for this study. For the subset of patients enrolled in the HMO, the data include insurance claims information as well as enrollment and disenrollment dates. The study population was limited to individuals who were continuously enrolled in the HMO for at least 1 year before the index date and received care through system physicians. Therefore, the study team had electronic information available for all health care visits and prescription fills, both within and outside of the health system. Using automated data sources, we identified all patients ≥ 18 years of age with a diagnosis of MI from January 1, 1997 to June 30, 2011, as well as patients with a diagnosis of CAD but without a diagnosis of MI. Patients were followed until they reached the study primary endpoint (i.e., death) or were censored at the earlier of either disenrollment from the health plan or final follow-up on June 30, 2011. The study was approved by the Institutional Review Board at Henry Ford Hospital. Vital status was established using the Social Security Death Master File (available through the National Technical Information Service) and the Michigan State Division of Vital Records and Health Statistics.
Pharmacy Claims and Estimation of BB Exposure
Using methodology previously employed by our group (16), we used pharmacy claims to estimate BB exposure. To facilitate analysis of medication exposure across the BB class (i.e., include all BB agents in the exposure estimate), equivalent doses across agents were established. This was based on the proportion of a target/maximal dose for each specific agent. These target doses were adopted from the target dose for MI used in clinical trials, or the maximum daily dose for BB agents that were not specifically studied in clinical trials of patients with MI. For example, 25 mg of metoprolol per day (i.e., 12.5 mg twice a day) was given a 0.125 BB dose equivalent based upon a 200 mg per day (100 mg twice a day) target dose.
Exposure to BB was then calculated as the drug equivalent strength (described above) multiplied by the quantity of medication dispensed in a 6-month time block, divided by the total number of days in the 6-month time block. A specific BB exposure estimate was calculated for each patient for every day of observation, starting 6 months after index date. Thus, all patients had a quantitative estimate of their last 6 months of BB exposure for each day of follow-up (i.e., exposure over the preceding 6 months). Individual exposure measures could range from 0 to 1, vary daily and include periods of no exposure. Therefore, this method accounts for both dose and adherence over a rolling period of time (in this case 6 months), which we have demonstrated is strongly correlated to clinical outcomes (16).
Statistical Analysis
The primary endpoint was the time to death from any cause. We also include a secondary endpoint of non-fatal MI or death, similarly modeled as time-to-event. Because the exposure metric required a 6-month observation window, the first day of observation for outcomes was 6 months after the index date. Thus, patients who died in the first 6 months after the index date were not included in the study cohort.
Baseline variables were compared by means of either x2 tests for categorical variables or 2-sample Student t tests for continuous variables. Those variables that were not distributed normally were compared using a 2-sample Mann-Whitney test. Proportional hazards regression models were used to assess the relationship of BB exposure with the primary endpoint of mortality, with adjustment for all baseline covariates. The BB exposure estimates, generated as described above, were then tested for association with the combined event of death or hospitalization. A proportional hazards regression analysis with time-dependent covariates was used to evaluate this relationship. Models using all the data, as well as stratified by race, were developed. Multivariable models were adjusted for age, sex, race, comorbidities (e.g., atrial fibrillation, hypertension, preexisting heart failure, cerebrovascular disease, peripheral vascular disease, diabetes mellitus, renal dysfunction, and end-stage renal disease on dialysis), as well as ACEI/ARB, statin and ADP-antagonist exposure (calculated similarly to the BB exposure variable). For Cohort 2, as additional protection from incomplete follow-up (in this cohort that was based upon diagnosis of CAD rather than a hospitalization for acute MI), we excluded patients who had no fills of any cardiac medicine (i.e., ACE, ARB, BB, statin, or ADP-antagonist). For primary effects, P values < 0.05 were considered statistically significant. All analyses were performed in SAS version 9.1.3 (SAS Institute, Cary, NC).
No extramural funding was used to support this work. The authors are solely responsible for the design and conduct of this study, all study analyses, the drafting and editing of the paper and its final contents.
RESULTS
Our study included 2 cohorts: patients with a known MI and those with established CAD but no known history of MI. Baseline characteristics for both cohorts are summarized in Table 1. The MI group (Cohort 1) included a total of 6220 subjects with history of MI, of which 40.7% were African American. There were a total of 1751 (28.1%) deaths during a median follow-up of 52 months; 527 deaths occurred after the last follow-up (32 within the first 30 days). The mean BB exposure was 0.41 ± 0.53 and 477 (7.7%) subjects were not on a BB during the observation period. The CAD only group (Cohort 2) included a total of 21,285 subjects with CAD but without history of MI, of which 35.4% were African American. There were a total of 3564 deaths (16.7%) during the median follow-up period of 71 months; 1314 deaths occurred after the last follow-up (80 within the first 30 days). Mean BB exposure was 0.28 ± 0.46 and 5797 (27.2%) subjects were not on BB during the observation period. Comparison of patients in with and without any BB in Cohorts 1 and 2 are summarized in Supplemental Tables 1 and 2, respectively. BB use was associated with several coexisting conditions and therapies including vascular disease, kidney disease, statins and ADP-antagonists.
Table 1.
Baseline Characteristics of Patients
| Characteristic | Cohort 1 MI Group (n = 6220) | Cohort 2 No MI Group (n =21285) |
|---|---|---|
| Age, years | 67.6 ± 12.8 | 63.2 ± 12.9 |
| Female, n (%) | 2775 (44.3) | 10509 (50.0) |
| African American, n (%) | 2248 (40.7) | 6431 (35.4) |
| Hypertension, n (%) | 6163 (99.1) | 19917 (94.7) |
| Atrial fibrillation, n (%) | 2419 (38.9) | 5348 (25.4) |
| Heart failure, n (%) | 2730 (43.9) | 4993 (23.7) |
| CVD-TIA, n (%) | 2351 (37.8) | 6113 (29.1) |
| Peripheral vascular disease, n (%) | 2837 (42.4) | 5369 (28.5) |
| Diabetes mellitus, n (%) | 3271 (52.6) | 6631 (31.5) |
| Chronic kidney disease, n (%) | 1623 (26.1) | 3163 (15.0) |
| Beta blockers, n (%) | 5743 (92.3) | 15488 (72.8) |
| ACEI/ARB, n (%) | 5405 (86.9) | 15496 (72.8) |
| ADP-antagonist, n (%) | 2979 (47.9) | 3979 (18.7) |
| Statin, n (%) | 5349 (86.0) | 15577 (73.2) |
ACEI/ARB, angiotensin converting enzyme inhibitors/angiotensin receptor blockers; ADP, adenosine diphosphate; CVD-TIA, cerebrovascular disease-transient ischemic attack; MI, myocardial infarction.
In the fully adjusted analyses (summarized in Table 2), BB exposure was associated with a 31% lower risk for all-cause death in Cohort 1 (hazard ratio [HR] 0.69; 95% CI 0.66, 0.77; P <0.001). ACEI/ARB (HR 0.76; 95% CI 0.17, 0.89; P < 0.001), and statins (HR 0.55; 95% CI 0.49, 0.62; P <0.001), were also significantly associated with a lower hazard of all-cause death. Survival curves of Cohort 1 stratified by any BB treatment vs. no BB at any point is shown in Figure 1. Since BB therapy has been repeatedly shown to have survival benefit in the setting of heart failure, we also tested the association excluding subjects with any heart failure diagnosis. In this subset (n = 3490, 56.1%) BB exposure continued to be associated with benefit (HR 0.65; 95% CI 0.53, 0.79; P <0.001); the full model results are shown in supplemental Table 3.
Table 2.
Multivariable Proportional Hazards Model for All-Cause Death
| Cohort 1 (Acute MI patients) |
Cohort 2 (CAD only patients) |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | p | HR (95% CI) | p |
| Beta blockers | 0.69 (0.66, 0.77) | 0.001 | 0.85 (0.79, 0.92) | 0.001 |
| ACEI/ARB | 0.76 (0.67, 0.85) | 0.001 | 0.94 (0.87, 1.02) | 0.128 |
| ADP-antagonist | 1.02 (0.86, 1.22) | 0.791 | 1.34 (1.15, 1.57) | 0.001 |
| Statin | 0.55 (0.49, 0.62) | 0.001 | 0.65 (0.60, 0.71) | 0.001 |
| CVD-TIA | 1.17 (1.06, 1.30) | 0.003 | 1.26 (1.18, 1.35) | 0.001 |
| Chronic kidney disease | 1.79 (1.66, 2.00) | 0.001 | 1.77 (1.64, 1.90) | 0.001 |
| Diabetes mellitus | 1.13 (1.01, 1.26) | 0.027 | 1.14 (1.07, 1.23) | 0.001 |
| Peripheral vascular disease | 0.84 (0.76, 0.93) | 0.001 | 0.99 (0.96, 1.06) | 0.724 |
| Atrial fibrillation | 1.16 (1.04, 1.29) | 0.009 | 1.36 (1.27, 1.47) | 0.001 |
| Heart failure | 1.16 (1.04, 1.30) | 0.008 | 1.19 (1.11, 1.28) | 0.001 |
| Hypertension | 0.70 (0.26, 1.87) | 0.475 | 0.87 (0.66, 1.14) | 0.299 |
| Female | 0.90 (0.82, 1.00) | 0.089 | 0.75 (0.71, 0.81) | 0.001 |
| Age | 1.05 (1.05, 1.06) | 0.001 | 1.07 (1.07, 1.07) | 0.001 |
| Black | 1.11 (1.00, 1.23) | 0.056 | 1.02 (0.95, 1.10) | 0.590 |
ACEI/ARB, angiotensin converting enzyme inhibitors/angiotensin receptor blockers; ADP, adenosine diphosphate; CVD-TIA, cardiovascular disease-transient ischemic attack; HR, hazard ratio. PVD, peripheral vascular disease
Fig. 1.
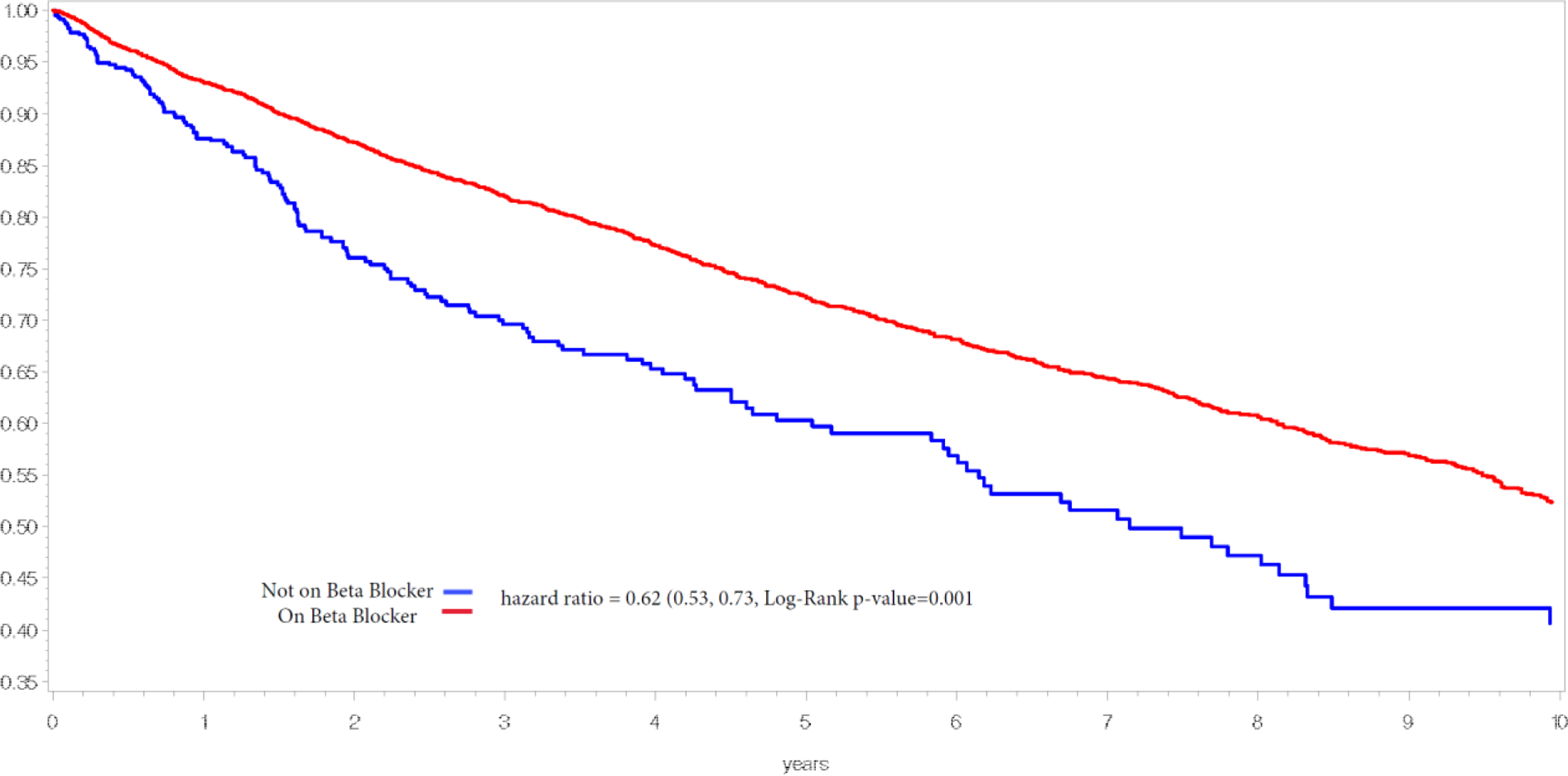
Cohort 1 - Myocardial Infarction Group. Abbreviations: BB, beta blockers
Among patients with known CAD but without a known history of MI (Cohort 2) BB exposure was also associated with a lower risk for all-cause death, though more modest in magnitude. Adjusted Cox models showed a 15% lower risk of death (HR 0.85; 95% CI 0.79, 0.92; P <0.001). Amongst other cardiac medications, statins were also associated with benefit in this group (HR 0.65; 95% CI 0.60, 0.71; P <0.001) but ACEI/ARB impact was not statistically significant (HR 0.94; 95% CI 0.87, 1.02; P = 0.128). As a secondary analysis we also explored possible impact of BB on non-fatal MI in this group of patients, however the association of BB with time to non-fatal MI or death was not significant (HR =1.01, P =0.7).
We then investigated the long term (>3 years) impact of BB therapy in the two cohorts. We repeated our analyses in patients who survived 3 years after their event or index diagnosis date (Cohort 1 n = 3360, deaths = 811; Cohort 2 n=21039, deaths=2284). This analysis is summarized in Table 3 and demonstrated continued favorable association of BB exposure in both Cohort 1 (HR 0.71; 95% CI 0.60, 0.85; P <0.001) and Cohort 2 (HR 0.85; 95% CI 0.80, 0.96; P =0.006). Unadjusted Cohort 1 survival curves for patients beyond 3 years stratified by BB presence or absence (i.e., no BB at any point), is depicted in Figure 2.
Table 3.
Proportional Hazards Regression Analysis of Patients Surviving More than 3 Years
| Cohort 1 (AMI patients) surviving >3 years |
Cohort 2 (CAD only) patients surviving > 3 years |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| Beta blockers | 0.71 (0.60, 0.85) | 0.001 | 0.85 (0.80, 0.96) | 0.006 |
| ACEI/ARB | 0.79 (0.66, 0.94) | 0.009 | 0.79 (0.71, 0.87) | 0.001 |
| ADP-antagonist | 1.06 (0.81, 1.38) | 0.689 | 1.30 (1.08, 1.58) | 0.007 |
| Statin | 0.54 (0.45, 0.65) | 0.001 | 0.67 (0.60, 0.74) | 0.001 |
| CVD-TIA | 1.32 (1.12, 1.56) | 0.001 | 1.31 (1.20, 1.43) | 0.001 |
| Chronic kidney disease | 1.75 (1.49, 2.06) | 0.001 | 1.85 (1.69, 2.03) | 0.001 |
| Diabetes mellitus | 1.32 (1.11, 1.57) | 0.002 | 1.26 (1.16, 1.38) | 0.001 |
| Peripheral vascular disease | 0.99 (0.84, 1.17) | 0.932 | 1.04 (0.95, 1.14) | 0.405 |
| Atrial fibrillation | 1.28 (1.08, 1.52) | 0.004 | 1.42 (1.29, 1.56) | 0.001 |
| Heart failure | 1.21 (1.01, 1.94) | 0.035 | 1.32 (1.21, 1.45) | 0.001 |
| Hypertension | 0.42 (0.06, 3.01) | 0.384 | 1.24 (0.79, 1.93) | 0.353 |
| Female | 0.83 (0.71, 0.97) | 0.018 | 0.77 (0.71, 0.84) | 0.001 |
| Age | 1.06 (1.05, 1.07) | 0.001 | 1.07 (1.07, 1.07) | 0.001 |
| Black | 1.12 (0.95, 1.32) | 0.183 | 0.95 (0.86, 1.04) | 0.248 |
ACEI/ARB, angiotensin converting enzyme inhibitors/angiotensin receptor blockers; ADP, adenosine diphosphate; AMI, acute myocardial infarction; CVD-TIA, cardiovascular disease-transient ischemic attack.
Fig. 2.

Cohort 1- Myocardial Infarction Group (survived >3 years). Abbreviations: BB, beta blockers
DISCUSSION
Our retrospective data analysis indicates that BB exposure is associated with improved outcomes in all CAD patients. The BB effect is clearly strongest in those with a history of MI, and this appears durable well beyond the 3 years currently recommended in practice guidelines. Our data is the first modern cohort to demonstrate improved outcomes associated with BB therapy in CAD patients without a history of MI, though the magnitude of effect was indeed substantially less compared to patients that have suffered a MI. Our data add to the totality which support continued BB use in CAD patients, supporting the current guideline recommendations and provide some additional reassurance regarding BB use beyond 3 years.
While our results fit well with much of the existing data, they do conflict somewhat with those of Bangalore et al (11) and Andersson et al (12), particularly in regard to the effect of BB in patients with CAD but no history of MI. There are several possible explanations for this. Obviously each of these studies are retrospective in nature, and thus accounting for confounders is critical. A key factor that may be at play is that these did not account for adherence nor for BB dose (i.e., it was dichotomized as present or absent), while evidence indicates that there is a dose response relationship for BB (17), so that missing this information may underestimate the effect or bias towards the null hypothesis. The Banglore study also did not appear to account for how the medication exposure might change over time. While this is typical of many studies because ongoing exposure data can be difficult to gather, it nonetheless could obscure the association of drug exposure with outcomes. On the other hand, the study by Andersson et al (12) did in fact take account of changes in medication over time, utilizing time-varying exposure to BB, though utilized a dichotomized indicator variable, therefore, not accounting for dose and not capturing adherence. This is an important issue because only about one-third of patients with stable ischemic heart disease are on optimum medical therapy at target doses (18). In contrast, our approach has the advantage of capturing dose, adherence and changes over time, and therefore, might be expected to be a more sensitive method to quantify the impact of drug exposure.
In terms of clinical application of ours and similar studies, several points should be considered. First, is that BB have a role as antianginal treatment, which is not addressed in our study or in other similar works. Second, while our data supports a beneficial association of BB use in CAD patients (without MI), in weighing this impact it should be noted that the association we describe is for full dose BB exposure (i.e. target dose and good adherence) compared to no BB exposure (i.e. either not taken at all or not prescribed). Thus while convincingly beneficial on average, the BB benefit in this group is of more modest effect size. Coupling this modest average benefit (and the likelihood of variation therein across patients) with concerns that BB therapy can have negative impacts such as on cognitive function, orthostasis, depression, erectile dysfunction and chronic lung disease, it is clear this should ideally to be a nuanced and personalized therapeutic decision.
Limitations
The findings of our study should be interpreted within the context of certain limitations. First, it was based on electronic data sources and therefore some variables may not have been available and/or diagnostic misclassification may have occurred. While we attempted to adjust for confounding variables, residual unmeasured confounding can never be completely ruled out. Related to this, while our methods account for BB dose and adherence we do not have blood pressure or heart rate information to further interrogate optimization of BB therapy. Second, while medications could have been obtained without an insurance claim which would have been missed by our methods, other work from our group suggests that this occurs very infrequently in our HMO population (< 1% of the time) (19). We chose to average medication exposure over rolling 6 month periods because this length of time provides stability to the measure and reflects chronic effect of BB. However, this makes it impossible to examine events within the first 6 months of follow up. As a result we cannot comment on early BB effectiveness (in the first 6 months after diagnosis) and our observations should be interpreted as the longer-term impact of BB in these patients. Another potential limitation is that we did not utilize propensity analysis. Although these methods are commonly used to account for confounders associated with treatment choice (20–22), they do not always adequately account for unmeasured confounders, and our earlier work suggests that propensity scores may not be necessary when the analytic models account for the relevant covariates (23,24). Another concern is that we did not have quantified left ventricular function but our subgroup analysis excluding diagnosed heart failure yielded similar results. We did not attempt to account for revascularization, so cannot assess a potential interaction. Finally, our patients had a high prevalence of hypertension and diabetes so extrapolating our findings to populations with lower prevalence should be done with caution.
CONCLUSIONS
This observational analysis of more than 27,000 patients with CAD and quantified, time updated BB exposure shows a significant association of BB exposure with more favorable survival in all groups. The impact appears greatest amongst those patients that have suffered a MI, in whom it persisted beyond 3 years.
Supplementary Material
Funding:
This study was funded by the NIH grant number R01HL132154.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of Interest: The authors declare that they have no conflict of interest.
Ethical Approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (include name of committee + reference number) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.”
Informed Consent: Informed consent was originally waived for this study.
REFERENCES
- 1.Smith SC Jr., Allen J, Blair SN et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute. Circulation 2006;113:2363–72. [DOI] [PubMed] [Google Scholar]
- 2.Fihn SD, Blankenship JC, Alexander KP et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines, and the American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation 2014;130:1749–67. [DOI] [PubMed] [Google Scholar]
- 3.de Peuter OR, Verberne HJ, Kok WE et al. Differential effects of nonselective versus selective beta-blockers on cardiac sympathetic activity and hemostasis in patients with heart failure. J Nucl Med 2013;54:1733–9. [DOI] [PubMed] [Google Scholar]
- 4.Freemantle N, Cleland J, Young P, Mason J, Harrison J. beta Blockade after myocardial infarction: systematic review and meta regression analysis. Bmj 1999;318:1730–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994;344:1383–9. [PubMed] [Google Scholar]
- 6.Schwartz GG, Olsson AG, Ezekowitz MD et al. Effects of atorvastatin on early recurrent ischemic events in acute coronary syndromes: the MIRACL study: a randomized controlled trial. JAMA 2001;285:1711–8. [DOI] [PubMed] [Google Scholar]
- 7.Yusuf S, Zhao F, Mehta SR et al. Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 2001;345:494–502. [DOI] [PubMed] [Google Scholar]
- 8.Chen ZM, Pan HC, Chen YP et al. Early intravenous then oral metoprolol in 45,852 patients with acute myocardial infarction: randomised placebo-controlled trial. Lancet 2005;366:1622–32. [DOI] [PubMed] [Google Scholar]
- 9.Wijeysundera DN, Duncan D, Nkonde-Price C et al. Perioperative beta blockade in noncardiac surgery: a systematic review for the 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014;64:2406–25. [DOI] [PubMed] [Google Scholar]
- 10.Bangalore S, Steg G, Deedwania P et al. Beta-Blocker use and clinical outcomes in stable outpatients with and without coronary artery disease. JAMA 2012;308:1340–9. [DOI] [PubMed] [Google Scholar]
- 11.Bangalore S, Bhatt DL, Steg PG et al. Beta-blockers and cardiovascular events in patients with and without myocardial infarction: post hoc analysis from the CHARISMA trial. Circ Cardiovasc Qual Outcomes 2014;7:872–81. [DOI] [PubMed] [Google Scholar]
- 12.Andersson C, Shilane D, Go AS et al. Beta-blocker therapy and cardiac events among patients with newly diagnosed coronary heart disease. J Am Coll Cardiol 2014;64:247–52. [DOI] [PubMed] [Google Scholar]
- 13.Puymirat E, Riant E, Aissaoui N et al. Beta blockers and mortality after myocardial infarction in patients without heart failure: multicentre prospective cohort study. BMJ 2016;354:i4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dondo TB, Hall M, West RM et al. Beta-blockers and mortality after acute myocardial infarction in patients without heart failure or ventricular dysfunction. J Am Coll Cardiol 2017;69:2710–2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang BT, Huang FY, Zuo ZL et al. Meta-analysis of relation between oral beta-blocker therapy and outcomes in patients with acute myocardial infarction who underwent percutaneous coronary intervention. Am J Cardiol 2015;115:1529–38. [DOI] [PubMed] [Google Scholar]
- 16.Lanfear DE, Hrobowski TN, Peterson EL et al. Association of beta-blocker exposure with outcomes in heart failure differs between African American and white patients. Circ Heart Fail 2012;5:202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fiuzat M, Wojdyla D, Kitzman D et al. Relationship of beta-blocker dose with outcomes in ambulatory heart failure patients with systolic dysfunction: results from the HF-ACTION (Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training) trial. J Am Coll Cardiol 2012;60:208–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garg P, Wijeysundera HC, Yun L, Cantor WJ, Ko DT. Practice patterns and trends in the use of medical therapy in patients undergoing percutaneous coronary intervention in Ontario. J Am Heart Assoc 2014;3:e000882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williams LK, Joseph CL, Peterson EL et al. Patients with asthma who do not fill their inhaled corticosteroids: a study of primary nonadherence. J Allergy Clin Immunol 2007;120:1153–9. [DOI] [PubMed] [Google Scholar]
- 20.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med 1998;17:2265–81. [DOI] [PubMed] [Google Scholar]
- 21.Glynn RJ, Schneeweiss S, Sturmer T. Indications for propensity scores and review of their use in pharmacoepidemiology. Basic Clin Pharmacol Toxicol 2006;98:253–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seeger JD, Williams PL, Walker AM. An application of propensity score matching using claims data. Pharmacoepidemiol Drug Saf 2005;14:465–76. [DOI] [PubMed] [Google Scholar]
- 23.Alqaisi F, Williams LK, Peterson EL, Lanfear DE. Comparing methods for identifying patients with heart failure using electronic data sources. BMC Health Serv Res 2009;9:237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Joffe MM, Rosenbaum PR. Invited commentary: propensity scores. Am J Epidemiol 1999;150:327–33. [DOI] [PubMed] [Google Scholar]
- 25.Yang JJ, Burchard EG, Choudhry S et al. Differences in allergic sensitization by self-reported race and genetic ancestry. J Allergy Clin Immunol 2008;122:820–827 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Granger BB, Swedberg K, Ekman I et al. Adherence to candesartan and placebo and outcomes in chronic heart failure in the CHARM programme: double-blind, randomised, controlled clinical trial. Lancet 2005;366:2005–11. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


