Abstract
Diffusion-weighted magnetic resonance spectroscopy (DW-MRS) investigates noninvasively microstructural properties of tissue by probing metabolite diffusion in vivo. Despite the growing interest in DW-MRS for clinical applications, little has been published on the reproducibility of this technique. In this study, we explored the optimization of a single-voxel DW-semi-LASER sequence for clinical applications at 3 T, and evaluated the reproducibility of the method under different experimental conditions. DW-MRS measurements were carried out in ten healthy participants and repeated across three sessions. Metabolite apparent diffusion coefficients (ADCs) were calculated from mono-exponential fits (ADCexp) up to b-value = 3300 s/mm2, and from the diffusional kurtosis approach (ADCK) up to b-value = 7300 s/mm2. The inter-subject variabilities of ADCs of N-acetylaspartate + N-acetylaspartylglutamate (tNAA), creatine + phosphocreatine (tCr), choline containing compounds (tCho), and myo-inositol (mIns) were calculated in the posterior cingulate cortex (PCC) and in the corona radiata (CR). We explored the effect of physiological motion on the DW-MRS signal and the importance of cardiac gating and peak-thresholding to account for signal amplitude fluctuations. Additionally, we investigated the dependence of the intra-subject variability on the acquisition scheme using a bootstrapping resampling method. Coefficients of variation were lower in PCC than CR, likely due to the different sensitivity to motion artifacts of the two regions. Finally, we computed coefficients of repeatability for ADCexp and performed power calculations needed for designing clinical studies. Power calculation for ADCexp of tNAA showed that in the PCC 7 subjects per group are sufficient to detect a difference of 5% between two groups with an acquisition time of 4 minutes, suggesting that ADCexp of tNAA is a suitable marker for disease-related intracellular alteration even in small case-control studies. In the CR, further work is needed to evaluate voxel size and location that minimize motion artifacts and variability of the ADC measurements.
Keywords: diffusion, metabolites, optimization, reproducibility, repeatability, power calculation
1. Introduction
Diffusion-weighted magnetic resonance spectroscopy (DW-MRS) explores metabolic and microstructural properties of healthy and diseased brains by probing the diffusion of several metabolites in vivo1–4. Similarly to conventional MRS, DW-MRS exploits the specific compartmentalization of metabolites in different cell types, thus enabling differentiation between different physiological or pathological mechanisms affecting brain tissue. The addition of magnetic field gradient pulses to MRS sequences allows sensitization of the NMR signal to diffusion, and quantification of metabolite displacement in tissue at a given time-scale. From metabolite diffusion measures, it is possible to derive information on cell size and morphology5–8, as well as on the properties of the intracellular environment, such as viscosity and molecular crowding9. When combined with diffusion-weighted imaging techniques, DW-MRS provides a unique way to differentiate between axonal degeneration, glial activation, and demyelination10–13. Although very promising, the application of DW-MRS techniques in clinical studies is challenging due to the intrinsic low signal-to-noise ratio (SNR) of metabolites, especially when the spectra are acquired at high diffusion-weightings. To overcome the issue of low SNR at high b-values, DW-MRS often requires long acquisition times, which are not always feasible in a clinical setting. In addition, obtaining robust and reproducible DW-MRS data is hampered by the high sensitivity of this technique to bulk and physiological motion, affecting both the phase and the amplitude of individual DW-MRS acquisitions. These deleterious effects increase dramatically the variance in DW-MRS calculated measures, resulting in low reproducibility and in significantly over-estimated diffusion coefficients4,14. All these factors point towards the need for accurate post-processing procedures, in addition to effective acquisition strategies.
Currently, the most commonly used techniques for DW-MRS are based on stimulated echo acquisition mode (STEAM)15, point-resolved spectroscopy (PRESS)16, and localization by adiabatic selective refocusing (LASER)17, with magnetic field gradient pulses added in variable configurations for diffusion sensitization4. DW-STEAM allows for long diffusion times keeping short echo time (TE), and is thus ideal for exploration of the time dependence of metabolite diffusion5,9,18,19. DW-STEAM has also been shown to better quantify diffusion of J-coupled metabolites compared to DW-PRESS20. Spin-echo sequences provide higher SNR for a given TE, and, when equipped with a full bipolar diffusion gradient scheme, they allow maximization of the achievable b-value. These characteristics are highly beneficial, especially for applications on clinical scanners, where the maximum available gradient strength is limited by hardware constraints. Fully adiabatic LASER17 or partially adiabatic semi-LASER21 coupled with diffusion gradients (DW-semi-LASER) have the additional advantages of reducing signal losses related to B1 field inhomogeneities and lower chemical shift displacement error compared to STEAM and PRESS sequences with standard radiofrequency pulses22. Despite the increasing number of studies reporting different applications of DW-MRS to investigate brain tissue, and the importance of the evaluation of the reliability of the method for a study design, to our knowledge only two reports so far tested the reproducibility of DW-MRS methods. Differently from our study, one of these reports focused on a specific model of metabolite diffusion in the human corpus callosum employing a DW-PRESS sequence at both 3 T and 7 T23. In the second report, a DW-STEAM sequence was employed to measure metabolite apparent diffusion coefficients (ADCs) at 3 T, and the reproducibility of the method was tested in the subcortical white matter in a small group of three subjects24. Yet, the long acquisition time employed in this study is not suitable for clinical applications.
The goal of the present study was to optimize the acquisition and post-processing procedures for single-voxel DW-MRS experiments, and to evaluate the feasibility of clinical studies using a DW-semi-LASER sequence at 3 T. To this aim, we report the variability of ADCs of N-acetylaspartate + N-acetylaspartylglutamate (tNAA), creatine + phosphocreatine (tCr), choline containing compounds (tCho), and myo-inositol (mIns), measured using DW-semi-LASER in two brain regions containing mostly grey matter (GM) or white matter (WM). In order to explore the impact of a series of methodological issues on the variability of metabolite ADCs, the reproducibility of the diffusion measures was evaluated for different experimental conditions across repeated measurements of the same subject, and across subjects. In particular, the effect of physiological motion on the DW-MRS signal and the importance of cardiac gating and peak-thresholding to account for signal amplitude fluctuations were investigated. The ADCs were calculated using mono-exponential functions up to moderately high b-values (b = 3300 s/mm2), as well as using kurtosis model for measurements up to high b-values (b = 7300 s/mm2). Finally, based on the variance of the metabolite ADCs, we provided power calculations that can be used for planning clinical studies, and discussed the suitability of DW-MRS for case-control studies in disease populations.
2. Material and Methods
2.1. Human subjects
Ten healthy volunteers (seven males, three females; mean age ± standard deviation: 25 ± 3 years, range: 20–29 years) participated in this study. Each subject underwent the same MRI/MRS examination during three different sessions (S1, S2, and S3), each on a different day, with a maximum delay between sessions of three weeks. All subjects provided informed consent according to local procedures prior to the study. The study was approved by the local ethics committee.
2.2. MRI hardware
All subjects were scanned on a 3 T whole-body Siemens MAGNETOM Prisma Fit MRI scanner (Siemens Medical Solutions, Erlangen, Germany). The scanner was equipped with gradient coils capable of reaching 80 mT/m on each of the three orthogonal axes. The standard radio-frequency body-coil was used for excitation and a 64-channel receive-only head coil for reception.
2.3. MRI/DW-MRS protocol
At the beginning of each scan, three-dimensional (3D) T1-weighted magnetization-prepared rapid gradient echo (MPRAGE) images (field of view, 256 (anterior-posterior) × 256 (foot-head) × 231 (right-left) mm3; isotropic resolution, 0.9 mm; TR/TE, 2300/2.08 ms; total acquisition time, 5 min 17 s) were acquired to position the spectroscopic volumes of interest (VOIs) and to perform tissue segmentation.
The DW-MRS acquisitions were performed using a single-voxel semi-LASER sequence with diffusion gradients added in a bipolar configuration, as shown in Figure 1. Using the bipolar configuration of the diffusion gradients minimizes eddy currents as well as cross terms between the diffusion gradients and gradients rising from inhomogeneities of the B0 field25. DW-MRS data were acquired in two VOIs of 20 × 20 × 20 mm3 located in the posterior cingulate cortex (PCC), from here on referred to as VOIPCC (Figure 2A), and in the corona radiata (CR), from here on referred to as VOICR, (Figure 2C). In the CR, data were acquired in eight subjects also in a smaller VOI (VOI’CR) of 15 (foot-head) × 20 (anterior-posterior) × 15 (right-left) mm3, with the same center as VOICR (in two subjects it was not possible to perform the measurement in VOI’CR due to technical reasons). For all VOIs, sequence parameters were: TE = 120 ms, spectral width = 3 kHz and number of complex points = 2048. All resonances were excited using a slice selective 90° pulse (pulse length of 2.52 ms) followed by two pairs of slice-selective adiabatic refocusing pulses in the other two dimensions (HS1, R = 24, pulse length of 7 ms). The 64-channels signals were combined on-line using a reference water scan, after appropriate phase adjustment and amplitude weighting of each channel for optimal SNR combination. All acquisitions were synchronized with cardiac cycle using a pulse-oximeter device, in order to start each acquisition every 3 heart beats, while keeping a minimum TR of 2.5 s. To verify the effect of cardiac gating on signal fluctuations, an additional acquisition was performed in one subject without pulse-oximeter device and TR = 2.5 s. Diffusion-weighting was applied in three orthogonal directions (dir1 = [1, 1, −0.5], dir2 = [1, −0.5, 1], dir3 = [−0.5, 1, 1] in the VOI coordinate system) with diffusion gradient duration (δ) = 18 ms, diffusion time (td) = 60 ms and three increasing gradient strengths resulting in the b-values b1 = 850, b2 = 3300 and b3 = 7300 s/mm2. The b-values were calculated using the chronograms of the pulse sequence, and therefore accounted for all gradients present in the sequence, including slice selective and crusher gradients. A non-diffusion-weighted condition with diffusion gradient amplitude set to zero was also acquired: b0 = 11 s/mm2, where the small b-value originates from the slice selective and crushers gradients. Forty averages were collected for each diffusion-weighting condition and saved as individual free induction decays (FID) for further post-processing. Water suppression was performed using variable power with optimized relaxation delays (VAPOR) and outer volume suppression26. The delays used for VAPOR were 150, 100, 146, 105, 106, 68, 80, and 22 ms27. Unsuppressed water reference scans were acquired from the same VOIs using the same parameters as water suppressed spectra for eddy current corrections. B0 shimming was performed using a fast automatic shimming technique with echo-planar signal trains utilizing mapping along projections, FAST(EST)MAP28.
Figure 1: Schematic of the DW-semi-LASER sequence.
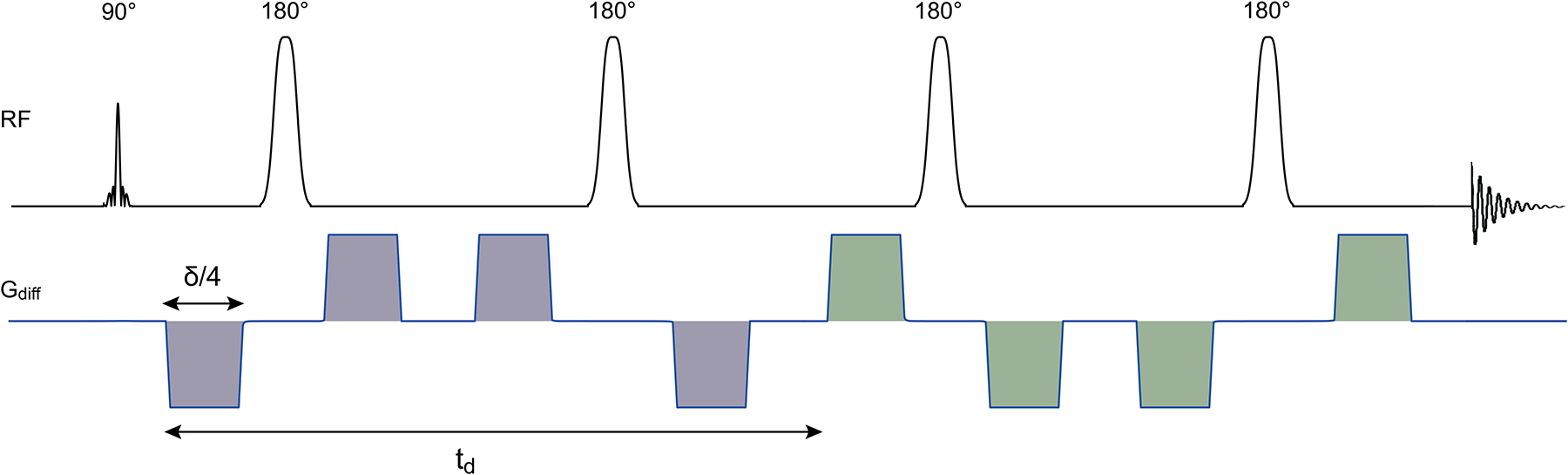
The RF pulses are shown together with the diffusion-weighted gradients, but without slice-selective and crusher gradients. The diffusion time td is the time between the first lobe of the de-phasing diffusion gradient group (in grey) and the first lobe of the re-phasing diffusion gradient group (in green). The total gradient duration δ corresponds to the sum of the durations of four lobes, and is identical for de-phasing and re-phasing groups.
Figure 2: Diffusion-weighted spectra and VOIs.
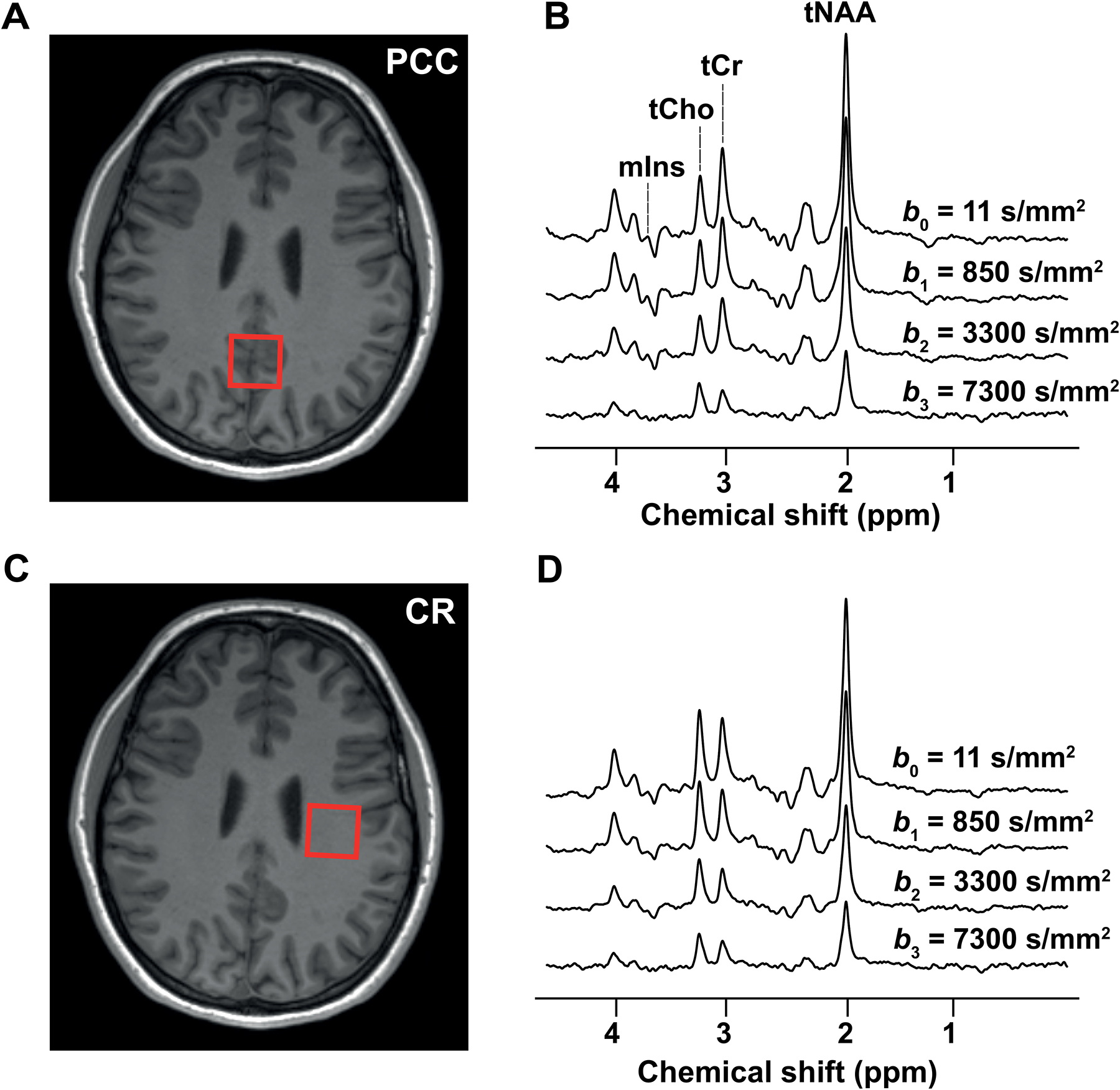
The locations of the VOIs in (A) the PCC and (C) the CR are shown on T1-weighted images together with examples of diffusion-weighted spectra acquired at different b-values in (B) VOIPCC and (D) VOICR
The total DW-MRS scan time for VOIPCC and VOICR was about 17 minutes for each VOI. In VOI’CR, an additional shorter acquisition was performed using only b0 and b2 (the latest applied in the three orthogonal directions) and 24 averages per diffusion condition (about 4 minutes), to evaluate, at these experimental conditions, the effect of voxel size on data variability.
2.4. Spectral processing
All spectra were processed with in-house written routine in MATLAB release R2016b (Mathworks, Natick, MA, USA). DW-MRS data were first corrected for eddy currents using water reference scans. Zero-order phase fluctuations and frequency drifts were corrected on single averages before summation using an area minimization and penalty algorithm and a cross-correlation algorithm, respectively29. A peak thresholding procedure was applied, for each diffusion condition, to discard the single averages with artifactual low SNR caused by non-translational tissue motion, and which is not justified by gaussian noise alone (see Supplementary material S1). The remaining spectra, for each condition, were averaged. Finally, the averaged spectra were analyzed with LCModel30 for metabolite quantification. The basis set was simulated with an in-house written routine in MATLAB based on the density matrix formalism31 and using previously reported chemical shifts and J-couplings32,33. The basis set included alanine, ascorbate, aspartate, creatine (Cr), γ-aminobutyric acid, glucose, glutamate, glutamine, glutathione, glycerophosphorylcholine, mIns, lactate, N-acetylaspartate (NAA), N-acetylaspartylglutamate, phosphocreatine (PCr), phosphorylcholine, phosphorylethanolamine, scyllo-inositol, and taurine. Independent spectra for the CH3 and CH2 groups of NAA, Cr and PCr were simulated and included in the basis set.
2.5. Metabolite diffusion measures
Based on the LCModel data, metabolite diffusivity properties for tNAA, tCr, tCho and mIns were calculated in each VOI. ADCs were computed assuming a mono-exponential decay of the signal up to b2 (Figure 3) in each diffusion direction:
| (1) |
where Si(b) is the signal measured at a given b-value in direction i, S0 is the signal measured at b0, and ADCiexp is the corresponding apparent diffusion coefficient estimated in direction i. Since the signal decay obtained up to b3 was not mono-exponential, the signal decay up to this b-value was evaluated using the kurtosis approach34,35 (Figure 3):
| (2) |
where ADCiK is the apparent diffusion coefficient for the direction i and Ki is the kurtosis parameter in the same direction. The quality of both mono-exponential and kurtosis fits up to b3 was assessed using a chi-square (χ2) goodness-of-fit test for the residuals, using the Cramér-Rao lower bounds (CRLBs) provided by LCModel as standard deviations for the metabolite signal amplitudes.
Figure 3: tNAA attenuation curves.
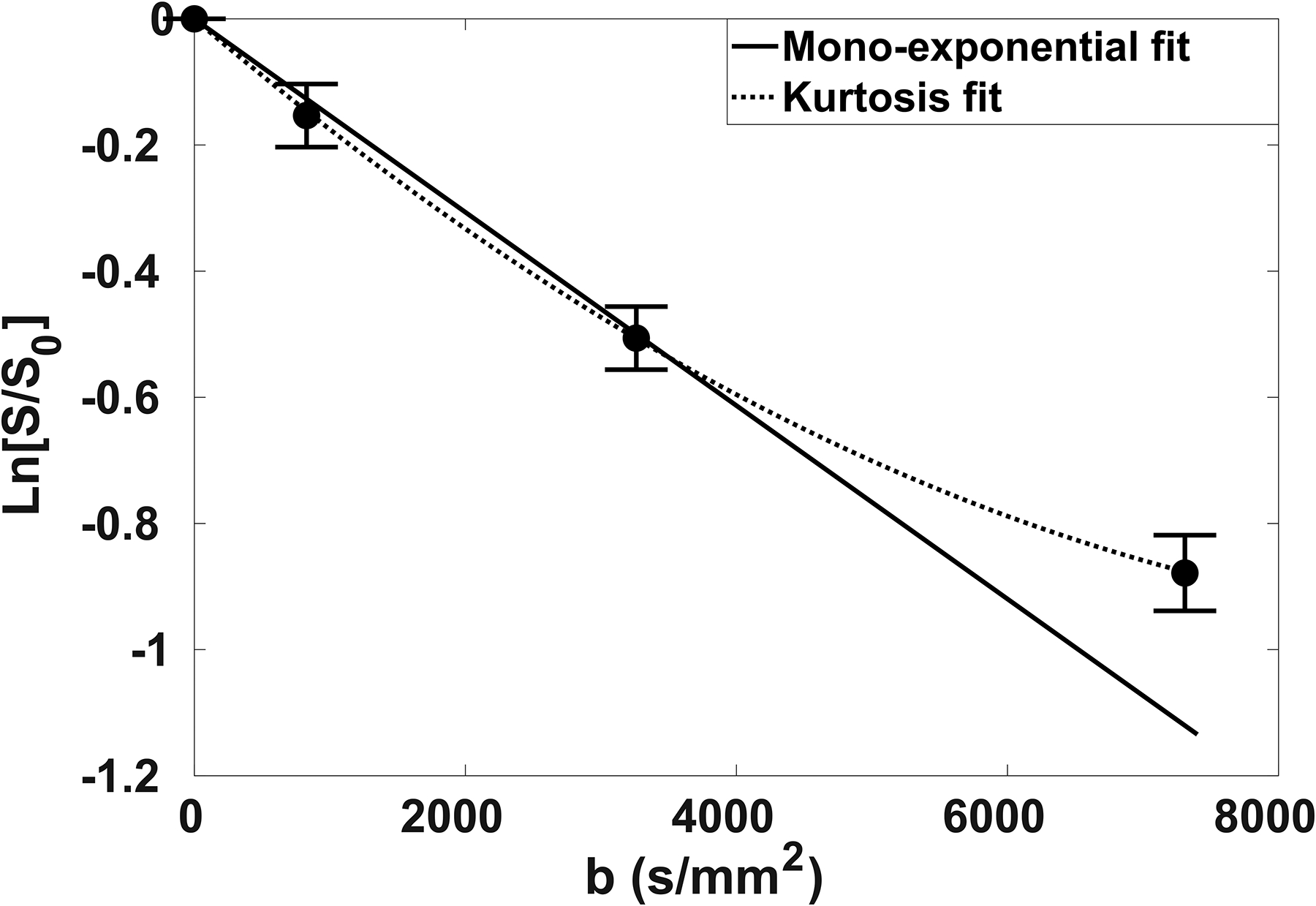
Natural logarithm of tNAA normalized signal decay plotted as a function of b-value. The attenuation curve was fitted to a mono-exponential function (solid line) up to b2 = 3300 s/mm2 and to a kurtosis model (dashed line) up to b3 = 7300 s/mm2.
2.6. Inter-subject variability
The inter-subject variability of the diffusion measures was evaluated for all four metabolites and all VOIs. Coefficients of variation (CV) were calculated as ratios between standard deviations (SD) and mean values of the diffusion parameters. SD and mean values were estimated across subjects for each session separately, as well as across subjects and sessions, and were consequently used for the CV evaluations.
At first, the effects of peak thresholding, diffusion-weighting direction and VOI size on the mean values and variability of tNAA diffusion measures were evaluated. Subsequently, the CV were calculated for metabolite ADCexp, ADCK and K values estimated using peak-thresholding and averaged over three diffusion directions.
2.7. Intra-subject variability
The intra-subject variability analysis was carried out on ADCexp, ADCK and K of tNAA and tCho derived from VOIPCC and VOICR. The dependence of the intra-subject variability of the diffusion measures on the acquisition time was evaluated by computing the diffusivity parameters for each subject, considering different diffusion-weighting schemes and different numbers of spectral averages per diffusion condition. For this purpose, a bootstrapping subsampling procedure was used: the datasets from each session were randomly resampled with replacement prior to averaging. Each subset consisted respectively of 10, 15, 20, 25, 30, 35 and 40 averages per diffusion condition (e.g., per b-value and diffusion direction). In addition, ADCexp and their variabilities were estimated from different diffusion-weighting schemes: scheme b[0,1,2] employs b0, b1, and b2; scheme b[0,2] employs b0 and b2; scheme b[0,1] employs b0 and b1. K were estimated using all b-values b0, b1, b2 and b3 (scheme b[0–3]).
For each resampled subset, the averaged spectra needed for calculation of the diffusion metrics were obtained. The bootstrapping procedure was repeated 200 times for each subset size to obtain a bootstrap population. From the bootstrap populations, mean values and SDs of the diffusivity parameters were obtained and utilized for intra-subject CV calculations.
2.8. Reproducibility and sample size analysis
A one-way repeated analysis of variance (rANOVA) model (MATLAB release R2016b) was performed to evaluate the within-subject variability (σ) of metabolite ADCexp averaged over the diffusion directions. σ was used for repeatability coefficient (CR) and power/sample size calculations. CR within the 95% confidence interval was defined as 36.
Power calculations were performed to estimate the necessary sample size to detect a difference (Δ) in tNAA ADCexp and K between two groups. These were based on a two-sided test with significance level α = 0.05 (z1-α/2 = 1.96), and a power of 80% (1-β = 0.80, z1-β = 0.84):
| (4) |
It was assumed that the means were normally distributed and the variances of the two groups were the same for both groups (σ1 = σ2 = σ).
3. Results
3.1. Diffusion-weighted spectra quality
Representative diffusion-weighted spectra acquired in VOIPCC and VOICR of one subject at all b-values applied in one diffusion gradient direction (dir1) are shown in Figure 2B and 2D, respectively. The CRLBs of tNAA calculated from all subjects, all b-values, all directions and all VOIs ranged from 2 to 7%, while the CRLBs of tCr and of tCho ranged from 3 to 12%. The CRLBs of mIns were lower than 20% for all b-values in VOIPCC and lower than 35% for all b-values up to b2 in VOICR, whereas for b3 in VOICR they were higher than 50% for 5 out of 30 datasets, and were therefore excluded from the kurtosis analysis. In VOI’CR, the CRLBs of mIns at b2 were > 35 % for more than half of the datasets, therefore these signals were not considered for further analysis. The mean SNR, based on the tNAA peak (averaged over all sessions and DW directions), was 24 for b0 and 14 for b3 in VOIPCC. In VOICR, the mean SNR was 23 for b0 and 9 for b3. Both in VOIPCC and in VOICR, no differences in SNR were observed between different DW directions. The SNRs in VOI’CR were considerably lower than in VOICR for each direction and both b-values: SNR = 14 for b0 and SNR = 8 for b2.
The linearity of the signal logarithm attenuation for the acquisition scheme b[0–2] was very good for all metabolites in both GM and WM (R2 > 0.9 for all fits). In contrast, for the acquisition scheme b[0–3] a significant deviation from linearity was observed in about 30% of the fits (p < 0.05 for the χ2 goodness-of-fit test for the residuals), and the signal decay was better fitted by the kurtosis model: for all fits, the null hypothesis of the χ2 goodness-of-fit test was accepted with p > 0.9.
Tissue segmentation results showed that the average WM fraction was 88 ± 4% in VOICR and 94 ± 4% in VOI’CR, respectively, while the average grey matter fraction in VOIPCC was 72 ± 5%.
3.2. Effect of acquisition strategy and post-processing on the variability of the diffusion measures
3.2.1. Cardiac gating
Figure 4A and 4B show SNR of tNAA for single averages (40 averages for each diffusion condition) with and without cardiac gating, respectively. Mean SNRs derived with and without cardiac gating at b1, b2 and b3 were plotted for each diffusion direction (Figure 4C). The SNRs were normalized to the SNR calculated at b0 = 11 s/mm2 (SNR0), in order to remove effects due to fluctuations in TR in the acquisitions with cardiac gating. The mean SNR of the spectra acquired with cardiac gating were higher for each b-value and diffusion condition. The associated standard deviations were slightly lower with heartbeat trigger, except for b1 in dir2.
Figure 4: Effect of cardiac gating on tNAA SNR.
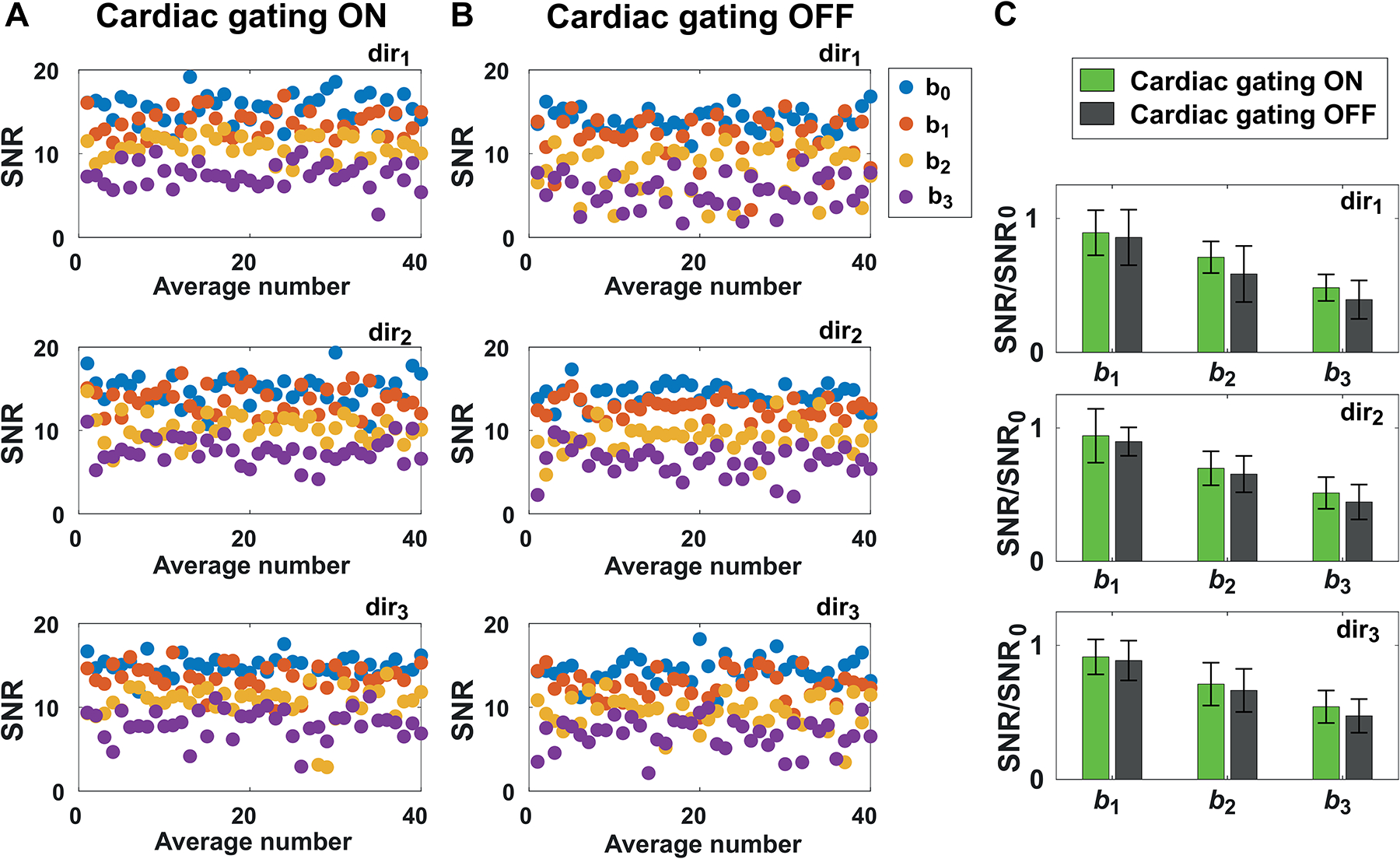
tNAA SNR calculated for single averages in one subject at all b-values and diffusion-weighted directions (A) with and (B) without cardiac gating. (C) Comparison of average SNR at different b-values (b1, b2 and b3) and directions (dir1, dir2, dir3) obtained with and without cardiac gating. The reported SNR were normalized to the SNR calculated at b0 (SNR0), in order to remove effects due to fluctuations in TR in the acquisitions with cardiac gating. Error bars represent standard deviations.
3.2.2. Peak thresholding
The average number of spectra rejected after peak thresholding were 3 for b0 and b1, 4 for b2, 5 for b3 in VOIPCC, and 2, 6, 10, 12, respectively, in VOICR. In VOI’CR, 1 and 2 spectra on average were rejected for b0 and b2, respectively.
Figure 5 shows an example of 40 averages acquired at b3 in VOICR, plotted without post-processing (5A), after eddy current, phase and frequency corrections (5B), and after peak thresholding (5C).
Figure 5. Post-processing.
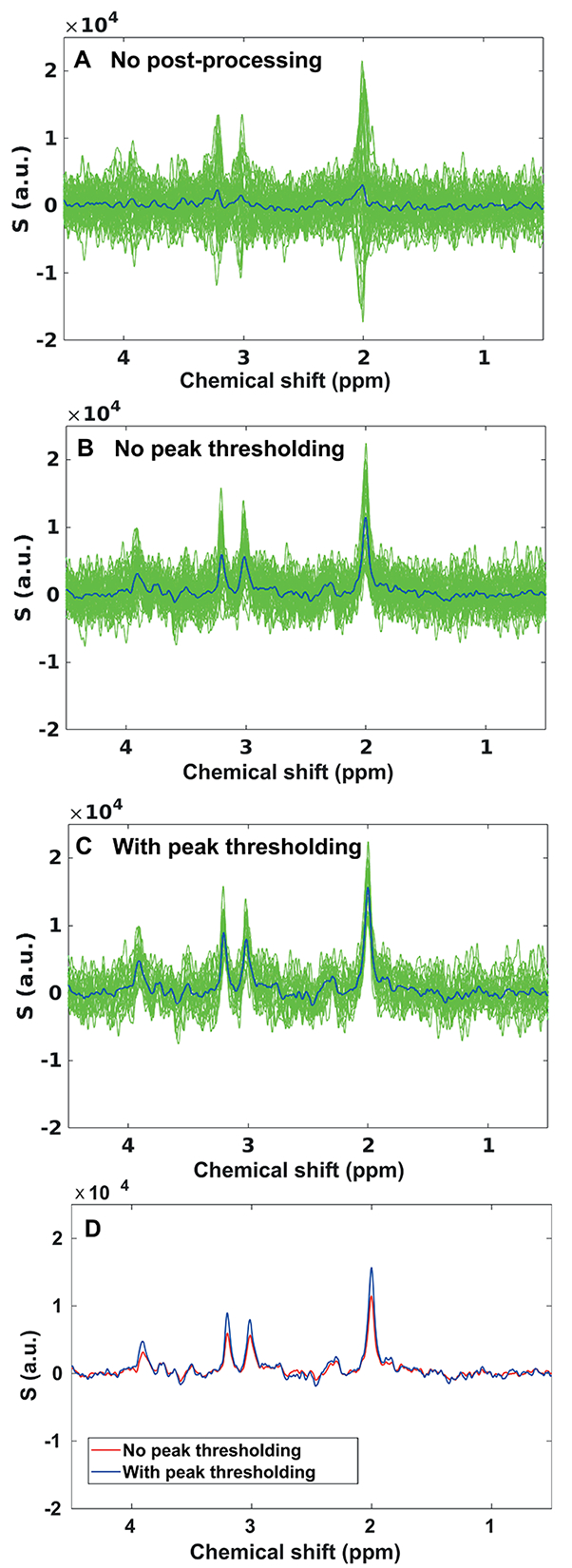
Example of 40 averages acquired with cardiac gating at b3 from VOICR, plotted (A) without post-processing, (B) after post-processing without peak thresholding (eddy current, phase and frequency corrections), and (C) after post-processing with peak thresholding. Blue lines correspond to the average spectra. In this example, fourteen spectra had artifactual low SNR and were discarded. (D) Comparison of the average spectra obtained with (blue line) and without (red line) peak thresholding.
Figure 6 shows ADCexp (6A, 6B), ADCK (6C, 6D) and K (6E, 6F) of tNAA measured in VOIPCC and VOICR for each diffusion direction, from all subjects and all sessions, with and without peak thresholding. In VOIPCC, decreases in both the mean values and the variability of ADCexp were observed when peak thresholding was applied (Figure 6A). The CV of ADCexp decreased after peak thresholding by 26% in dir1, 14% in dir2 and 35% in dir3, respectively. Instead, no differences in the CV of ADCK were observed in any of three directions when peak thresholding was applied. In VOICR the mean values and the CV of ADCexp decreased strongly in each direction when peak thresholding was applied: 33% in dir1, 41% in dir2 and 39% in dir3 (Figure 6B). Similarly, in this region the CV of ADCK decreased by 25%, 36% and 21% in dir1, dir2 and dir3, respectively (Figure 6D). In both VOIs, no differences in the variability of K were observed when peak thresholding was applied (Figure 6E and 6F). The diffusion metrics and their CV reported from here on were calculated with peak thresholding.
Figure 6: Effect of peak thresholding on the variability of tNAA diffusion measures.
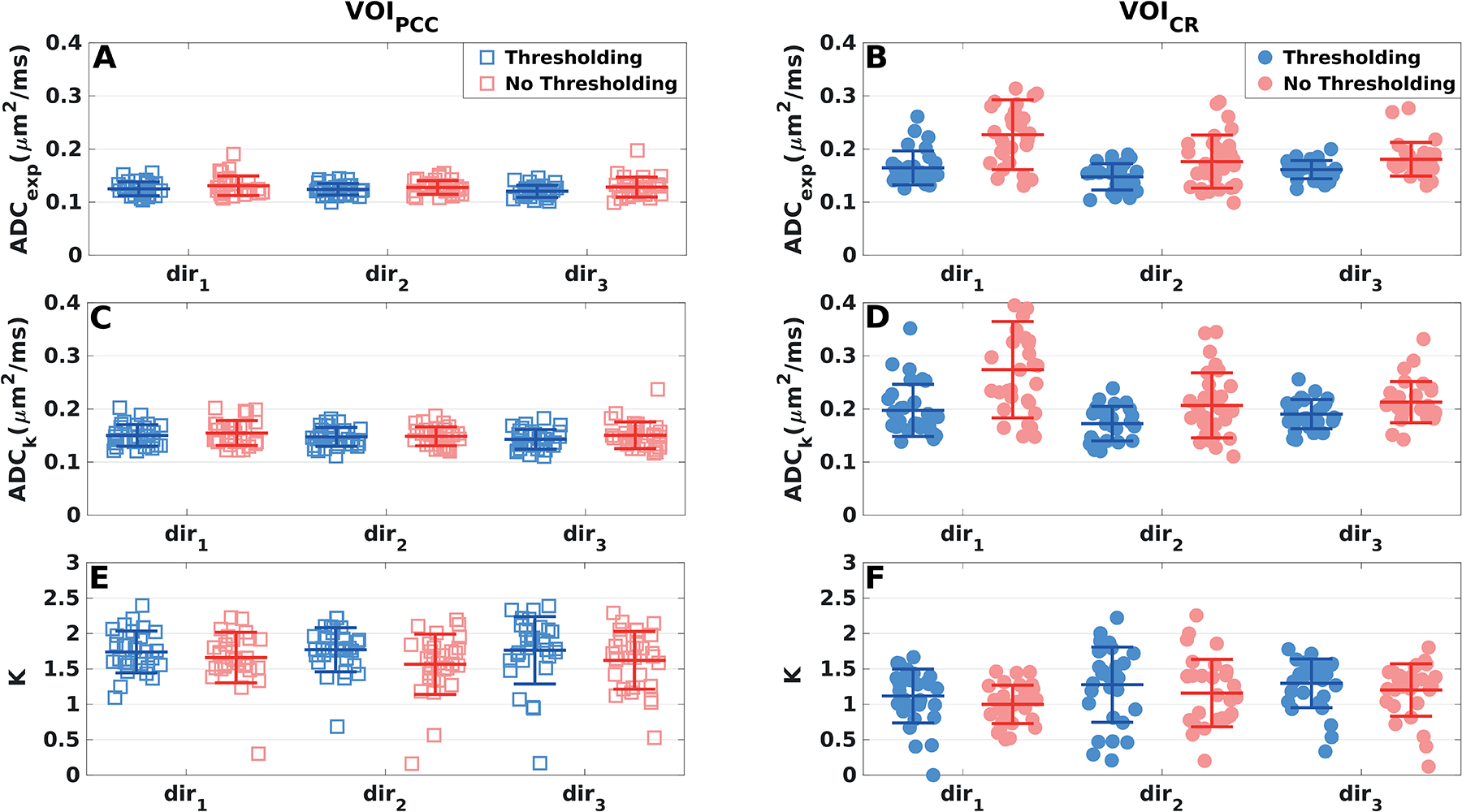
Diffusivity measures of tNAA derived in (A, C, E) VOIPCC (squares) and (B, C, D) VOICR (dots) from all subjects and sessions, displayed separately for each diffusion direction. Mean values (central bars) and SDs (edge bars) are plotted for each parameter and direction. Blue markers: peak thresholding applied; red markers: no peak thresholding.
3.2.3. DW direction
In VOIPCC, the variability of ADCexp and ADCK of tNAA was very similar for the three directions: mean CV = 9% and 12% for the two metrics, respectively (Figure 6A, 6C). In contrast, in VOICR, the variabilities of ADCexp, ADCK and K of tNAA (CV = 10%, 14% and 27%, respectively) were lower in dir3 with respect to the other two directions (CV = 18%, 22% and 38% for both dir1 and dir2) (Figures 6B, 6D and 6F).
3.2.4. Voxel size
Figure 7 shows the ADCexp of tNAA derived in VOICR and VOI’CR, plotted for all subjects and all sessions, and for each DW direction. In both voxels, ADCexp values were estimated using scheme b[0,2] and 24 averages per diffusion condition. Differently from VOICR, the variability estimated from the VOI’CR did not present any significant dependence on the diffusion-weighting direction (averaged CV = 16%). The CV values calculated in VOI’CR in dir1 and dir2 dropped by 19% with respect to those derived from VOICR in the same directions, while they were comparable in dir3. Similar behavior with respect to peak thresholding, DW direction and VOI size was observed for the variability of tCr, tCho and mIns diffusion measures (data not shown).
Figure 7: Effect of the voxel size on the variability of tNAA diffusion measures.
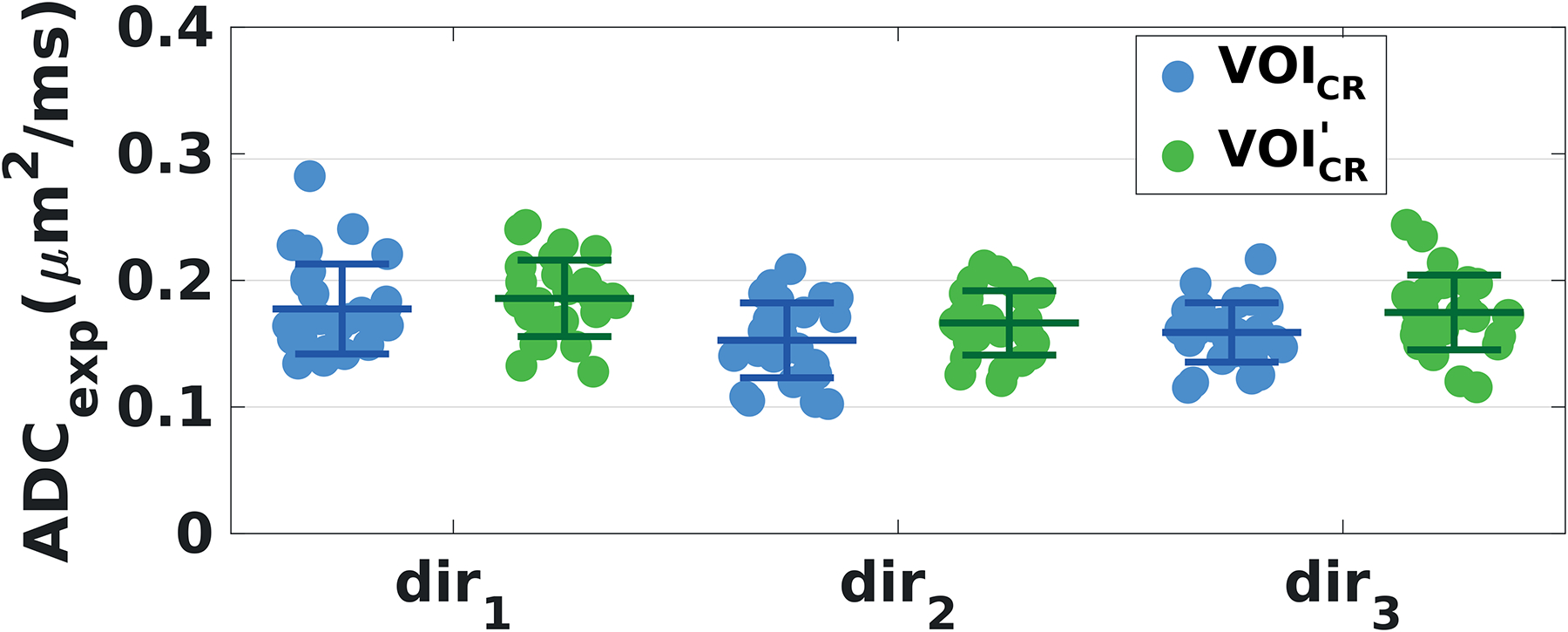
ADCexp derived from all subjects and sessions in VOICR (blue dots) and in VOI’CR (green dots), displayed separately for each diffusion direction. Mean values (central bars) and SDs (edge bars) are plotted for each diffusion direction and VOI. ADCexp values were estimated with scheme b[0,2] and 24 averages per diffusion condition in both voxels. Voxel sizes were: VOICR = 20 × 20 × 20 mm3 and VOI’CR = 15 × 20 × 15 mm3.
3.3. Inter-subject variability
tNAA, tCr, tCho and mIns diffusivity measures averaged across diffusion directions and subjects are reported for each session, with the associated SDs and CV values, in Table 1 and 2, for VOIPCC and VOICR and VOI’CR, respectively. No significant differences in diffusivity measures of the metabolites across the sessions were observed (rANOVA).
Table 1.
Mean standard deviation (SD) and coefficient of variation (CV) of ADCexp, ADCK and K values calculated for each session (S1 to S3) in VOIPCC (scheme b[0,1,2] and 40 averages per diffusion condition).
| VOIPCC | S1 | S2 | S3 | Average | |||||
|---|---|---|---|---|---|---|---|---|---|
| Diffusion Parameter | Metabolite | Mean (SD) | CV (%) | Mean (SD) | CV (%) | Mean (SD) | CV (%) | Mean (SD) | CV (%) |
| ADCexp | tNAA | 0.126 (0.007) | 6 | 0.120 (0.009) | 7 | 0.124 (0.009) | 7 | 0.123 (0.008) | 7 |
| (μm2/ms) | tCr | 0.119 (0.010) | 8 | 0.118 (0.013) | 11 | 0.118 (0.010) | 9 | 0.118 (0.011) | 9 |
| tCho | 0.106 (0.008) | 8 | 0.106 (0.012) | 12 | 0.107 (0.009) | 9 | 0.106 (0.010) | 9 | |
| mIns | 0.107 (0.015) | 14 | 0.106 (0.015) | 14 | 0.112 (0.015) | 14 | 0.108 (0.015) | 14 | |
| ADCK | tNAA | 0.151 (0.011) | 8 | 0.142 (0.014) | 10 | 0.147 (0.014) | 10 | 0.147 (0.013) | 9 |
| (μm2/ms) | tCr | 0.141 (0.015) | 11 | 0.139 (0.024) | 18 | 0.135 (0.016) | 12 | 0.140 (0.019) | 13 |
| tCho | 0.122 (0.012) | 10 | 0.123 (0.020) | 16 | 0.125 (0.013) | 10 | 0.123 (0.015) | 12 | |
| mIns | 0.134 (0.018) | 14 | 0.133 (0.023) | 17 | 0.141 (0.024) | 17 | 0.136 (0.022) | 16 | |
| K | tNAA | 1.808 (0.204) | 11 | 1.712 (0.211) | 12 | 1.744 (0.266) | 15 | 1.757 (0.227) | 15 |
| tCr | 1.689 (0.210) | 12 | 1.692 (0.298) | 18 | 1.552 (0.416) | 27 | 1.645 (0.308) | 19 | |
| tCho | 1.680 (0.486) | 29 | 1.738 (0.262) | 15 | 1.718 (0.291) | 17 | 1.712 (0.346) | 20 | |
| mIns | 1.533 (0.336) | 22 | 1.522 (0.514) | 34 | 1.817 (0.375) | 21 | 1.624 (0.410) | 25 | |
Table 2.
Average, standard deviation (SD) and coefficient of variation (CV) of ADCexp, ADCK and K values calculated for each session (S1 to S3) in VOICR (scheme b[0,1,2] and 40 averages per diffusion condition). Average, SD and CV of ADCexp calculated for each session in VOI’CR (scheme b[0,2] and 24 averages per diffusion condition).
| VOICR | S1 | S2 | S3 | Average | |||||
|---|---|---|---|---|---|---|---|---|---|
| Diffusion Parameter | Metabolite | Mean (SD) | CV (%) | Mean (SD) | CV (%) | Mean (SD) | CV (%) | Mean (SD) | CV (%) |
| ADCexp | tNAA | 0.156 (0.017) | 11 | 0.160 (0.020) | 12 | 0.158 (0.020) | 12 | 0.158 (0.019) | 12 |
| (μm2/ms) | tCr | 0.156 (0.012) | 7 | 0.174 (0.025) | 14 | 0.173 (0.024) | 14 | 0.168 (0.020) | 12 |
| tCho | 0.130 (0.015) | 11 | 0.140 (0.025) | 18 | 0.136 (0.023) | 17 | 0.135 (0.023) | 16 | |
| mIns | 0.126 (0.031) | 22 | 0.156 (0.050) | 32 | 0.130 (0.032) | 24 | 0.137 (0.037) | 27 | |
| ADCK | tNAA | 0.186 (0.029) | 16 | 0.180 (0.018) | 10 | 0.189 (0.022) | 12 | 0.185 (0.022) | 12 |
| (μm2/ms) | tCr | 0.195 (0.025) | 13 | 0.192 (0.031) | 16 | 0.208 (0.026) | 13 | 0.198 (0.031) | 13 |
| tCho | 0.162 (0.027) | 16 | 0.146 (0.021) | 15 | 0.156 (0.026) | 17 | 0.155 (0.023) | 15 | |
| mIns | 0.182 (0.033) | 18 | 0.177 (0.056) | 32 | 0.173 (0.036) | 21 | 0.177 (0.039) | 22 | |
| K | tNAA | 1.308 (0.239) | 18 | 1.238 (0.265) | 21 | 1.190 (0.300) | 25 | 1.246 (0.251) | 20 |
| tCr | 1.283 (0.243) | 19 | 1.280 (0.218) | 17 | 1.305 (0.192) | 15 | 1.289 (0.204) | 16 | |
| tCho | 1.507 (0.293) | 19 | 1.166 (0.376) | 32 | 1.238 (0.473) | 38 | 1.304 (0.357) | 27 | |
| mIns | 1.702 (0.425) | 25 | 1.317 (0.230) | 17 | 1.285 (0.453) | 35 | 1.435 (0.346) | 24 | |
| VOI’CR | S1 | S2 | S3 | Average | |||||
| Diffusion Parameter | Metabolite | Mean (SD) | CV (%) | Mean (SD) | CV (%) | Mean (SD) | CV (%) | Mean (SD) | CV (%) |
| tNAA | 0.185 (0.014) | 8 | 0.179 (0.026) | 15 | 0.163 (0.019) | 12 | 0.176 (0.020) | 11 | |
|
ADCexp (μm2/ms) |
tCr | 0.148 (0.029) | 19 | 0.168 (0.022) | 13 | 0.165 (0.027) | 16 | 0.160 (0.026) | 16 |
| tCho | 0.130 (0.013) | 10 | 0.137 (0.026) | 19 | 0.127 (0.012) | 9 | 0.132 (0.017) | 13 | |
In VOIPCC, the CV values estimated for ADCexp and ADCK of all metabolites ranged from 6% to 14% and from 8% to 18%, respectively, while the CV of K ranged from 11% to 34% (Table 1). In VOICR, the CV of ADCexp and ADCK were higher than those calculated in VOIPCC, ranging from 7% to 32%, while the CV of K ranged from 15% to 38% (Table 2). Finally, in the VOI’CR the CV of tNAA, tCr and tCho ADCexp ranged from 8% to 19% (Table 2). In Figure 8, all diffusivity measures computed for all metabolites from all subjects and all sessions for the two VOIs are shown.
Figure 8. Inter-subject variability.
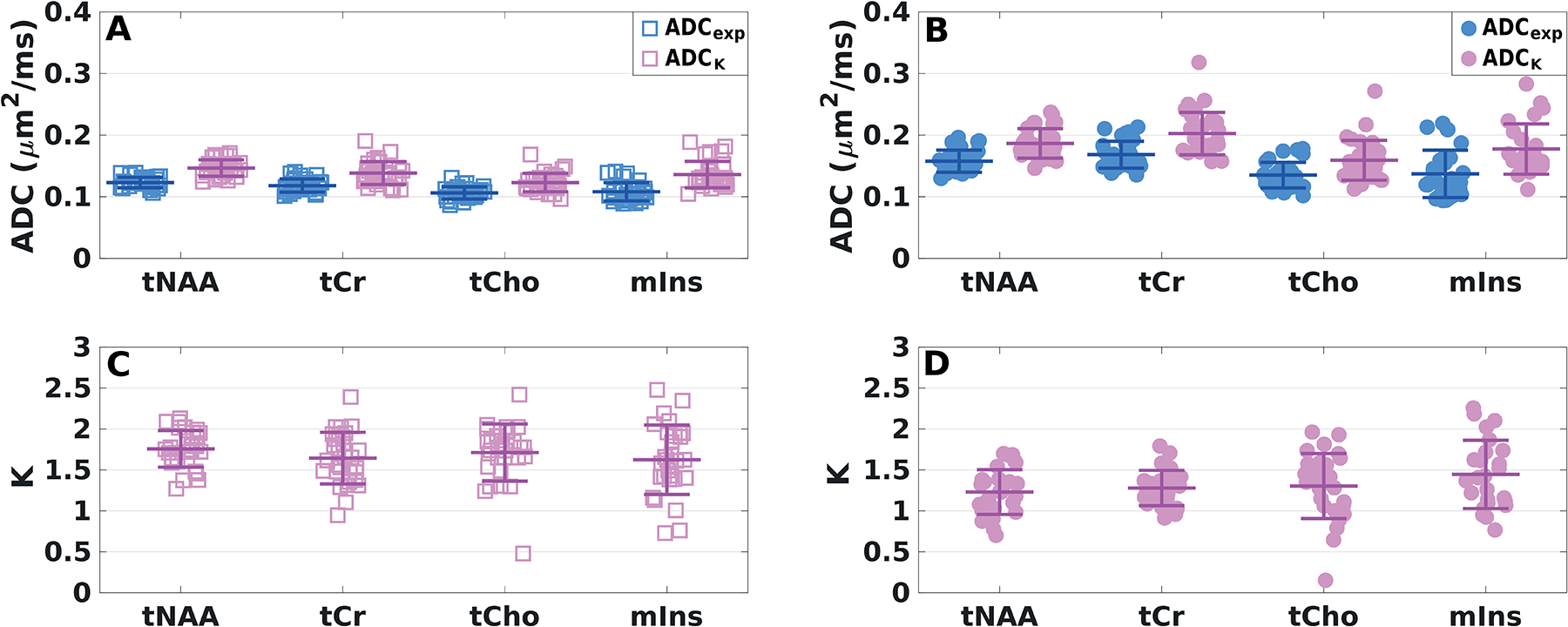
ADCexp, ADCK and K of all metabolites from all subjects and sessions derived (A, C) in VOIPCC (square markers) and (B, D) in VOICR (dot markers) averaged over all diffusion directions. Mean values (central bars) and SDs (edge bars) are displayed for each parameter.
3.4. Effect of acquisition time on intra-subject variability
Figure 9 shows the CV of ADCexp and K of tNAA calculated as a function of the number of averages acquired in VOIPCC (Figure 9A and C) and in VOICR (Figure 9B and D), based on data resampling from all subjects and sessions. The CV of ADCexp of tNAA was evaluated for different acquisition schemes: b[0,1,2], b[0,2] and b[0,1]. In both VOIs, CV < 10% for ADCexp of tNAA was observed with more than 60 and 90 averages acquired with schemes b[0,2] and b[0,1,2], respectively, corresponding to scanning times of 3 and 4 minutes. Notably, the CV achieved for b[0,2] with 160 averages (scanning time of 7 minutes) and for b[0,1,2] with 280 total spectra (scanning time of 12 minutes) were comparable (~5% in VOIPCC and ~7% in VOICR). Much higher values of CV (> 15%) were observed for b[0,1] in both VOIs, as expected given the low b-value utilized for this scheme. The CVs of K of tNAA were below 20% when 150 and 250 averages (scanning times of 7 and 11 minutes) were used in VOIPCC and VOICR, respectively. Similar trends were observed for tCr and tCho (data not shown).
Figure 9. Intra-subject variability.
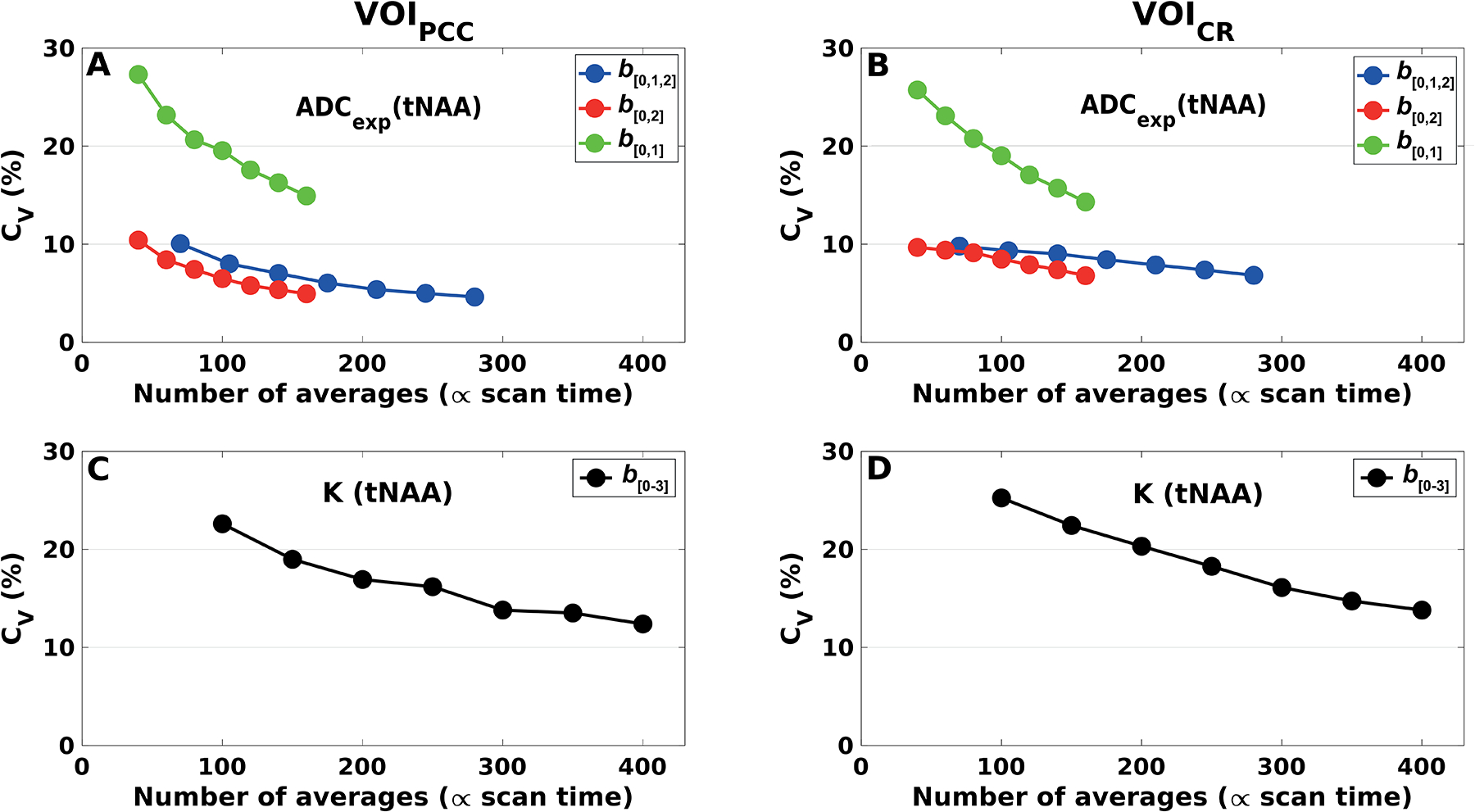
Coefficients of variation for ADCexp and K of tNAA, calculated (A, C) in VOIPCC and (B, D) in VOICR, from a bootstrapping subsampling procedure. The datasets from each subject and session were randomly resampled with replacement prior to averaging. Each subset consisted respectively of 10, 15, 20, 25, 30, 35 and 40 averages per diffusion condition. ADCexp were estimated from different diffusion-weighting schemes: b[0,1,2], b[0, 2] and b[0,1]. K were estimated using all b-values (scheme b[0–3]).
In addition, the diffusion metrics’ variabilities as a function of the SNR estimated at b0 are shown in Figure 4S of the Supplementary material.
3.5. Reproducibility and sample size analysis
CR values for ADCexp of all metabolites were calculated in VOIPCC and VOICR for different acquisition schemes (b[0,1,2] and b[0,2]) and acquisition times (12, 7 and 4 minutes), and reported as percentage of ADCexp mean values (Table 3). The CRs were much lower in VOIPCC than in VOICR, indicating higher repeatability in the first region. Interestingly, while CR values for VOICR gradually increased when reducing the acquisition time, in VOIPCC for all metabolites the CR obtained for scheme b[0,2] and acquisition time of 4 minutes were smaller than those for the same scheme and acquisition time of 7 minutes.
Table 3:
Repeatability coefficient (CR) and variance (σ2) of ADCexp values calculated for different acquisition schemes and acquisition times, in VOIPCC and VOICR. Power analysis can be performed using the σ2 values reported in the table and Equation 4.
| VOI | Metabolite | 12 minute acquisition (scheme b[0,1,2]) | 7 minute acquisition (scheme b[0,2]) | 4 minute acquisition (scheme b[0,2]) | |||
|---|---|---|---|---|---|---|---|
| CR (%mean) (μm2/ms) | σ2 (x10−4) (μm4/ms2) | CR (%mean) (μm2/ms) | σ2 (x10−4) (μm4/ms2) | CR (%mean) (μm2/ms) | σ2 (x10−4) (μm4/ms2) | ||
| VOIPCC | tNAA | 0.015 (12%) | 0.3 | 0.026 (20%) | 0.9 | 0.016 (13%) | 0.3 |
| tCr | 0.023 (20%) | 0.7 | 0.032 (26%) | 1.3 | 0.030 (25%) | 1.1 | |
| tCho | 0.022 (20%) | 0.6 | 0.040 (37%) | 2.1 | 0.025 (23%) | 0.8 | |
| mIns | 0.040 (37%) | 2.1 | 0.066 (59%) | 5.7 | 0.050 (46%) | 3.3 | |
| VOICR | tNAA | 0.042 (27%) | 2.3 | 0.042 (26%) | 2.3 | 0.045 (27%) | 2.6 |
| tCr | 0.040 (24%) | 2.1 | 0.043 (25%) | 2.4 | 0.052 (29%) | 3.5 | |
| tCho | 0.044 (33%) | 2.6 | 0.046 (33%) | 2.7 | 0.050 (34%) | 3.2 | |
| mIns | 0.104 (75%) | 14.1 | 0.085 (62%) | 9.4 | 0.101 (67%) | 13.4 | |
For each of the acquisition schemes reported in Table 3, power calculations were performed for ADCexp of tNAA to reflect the number of subjects per group required to detect a difference between two groups with a power of 80% and significance level of 5% (Figure 10). A 5% difference can be detected with 6 and 29 subjects per group in VOIPCC and VOICR, respectively, with scheme b[0,1,2] and a scanning time of 12 minutes (Figure 10A). With scheme b[0,2] and a scanning time of 7 minutes, a 5% difference can be detected with 17 and 29 subjects per group in VOIPCC and in VOICR, respectively (Figure 10B). With b[0,2] and a reduced scanning time of 4 minutes, a 5% difference can be detected with 7 and 31 subjects per group in VOIPCC and in VOICR, respectively (Figure 10C).
Figure 10. Reproducibility analysis.
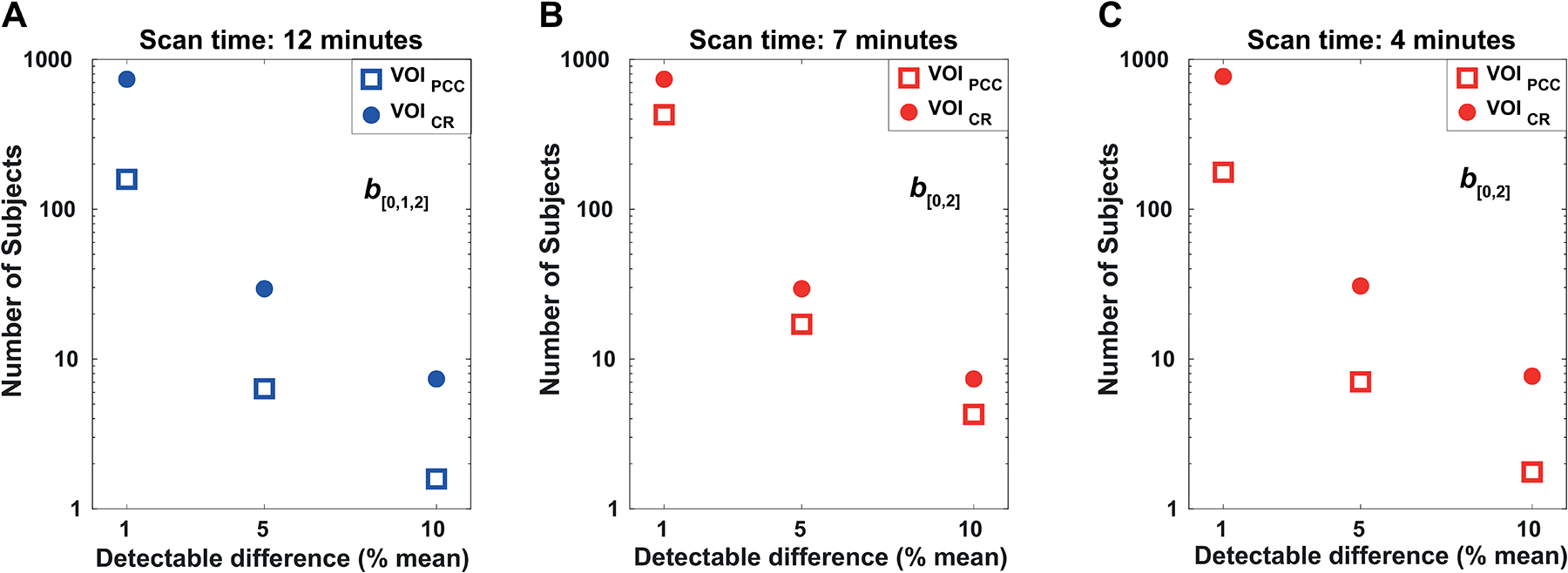
Number of subjects (per group) required to detect a difference in the ADCexp of tNAA (as a percentage of the mean) with significance level α = 0.05 and power of 1-β = 0.80. The power was calculated in VOIPCC (filled dots) and VOICR (empty squares) for (A) the full data-set including all b-values up to b2 = 3300 s/mm2 (scheme b[0,1,2]), corresponding to an acquisition time of 12 minutes; (B, C) sub-sampled data-sets using b0 = 11 s/mm2 and b2 = 3300 s/mm2 (scheme b[0,2]) and different number of averages, corresponding to acquisition times of 7 and 4 minutes, respectively.
In addition, CR values for K of all metabolites were calculated in VOIPCC and VOICR and reported as percentage of K mean values (Table 4S, Supplementary data). The power calculation for tNAA K revealed that a 10% difference can be observed with 11 and 21 subjects per group in VOIPCC and VOICR, respectively.
4. Discussion
The variability of metabolite diffusion parameters measured with DW-semi-LASER across different subjects and sessions was evaluated in two brain regions of interest (PCC and CR) and with different acquisition and post-processing strategies. Our data showed that DW-MRS is feasible at 3 T with an excellent statistical power even employing a short acquisition protocol in small subject populations, providing that the proper choice of b-values, the execution with cardiac triggering, and the post-processing with peak thresholding are all taken carefully into account. The optimized acquisition and analysis pipeline allowed to obtain high quality DW-MR spectra for all subjects, sessions and VOIs, up to the highest b-value of b = 7300 s/mm2 (Figure 2B, D), enabling the utilization of this method in clinically feasible acquisition times.
Nevertheless, the variance of the diffusion measures differed significantly across brain regions (despite the similar SNR at b0), as they are affected by motion artifacts to different extents, and it also depended on the VOI size: while acquisitions in a larger VOI provide higher SNR, a smaller VOI may allow for more robust datasets and lower variability of the diffusion measures.
From power calculations we estimated that, under our experimental conditions, 5% differences in tNAA mean diffusion coefficient can be detected with a sample size of 7 and 31 subjects per group in the PCC and in the CR, respectively, with an acquisition time of 4 minutes. Remarkably, the measure of metabolite diffusion parameters was extremely robust and reproducible in the PCC, while much higher variability was observed in the CR when the VOI was located next to the ventricles.
4.1. Effect of physiological motion on the DW signal
Potential sources of bias in the evaluation of metabolite ADCs were identified, mainly attributed to the effect of physiological motion on the DW signal. Whilst artifacts induced by pure translational motion can be corrected by properly adjusting frequency and phase of the single averages before summation, small rotations or compressive motion due to cardiac and cerebrospinal fluid (CSF) pulsation may induce severe amplitude drops that are more difficult to address4.
To evaluate the effect of physiological motion on the DW signal, in one subject we investigated the influence of cardiac gating on tNAA SNR over time and across different directions. The mean normalized signal was higher when the acquisition was synchronized to the cardiac cycle at all b-values, while the associated standard deviations were always lower except for one condition (b = 850 s/mm2, dir2) (Figure 4C). This result highlights the importance of employing cardiac gating in DW-MRS experiments with high b-values, and the fact that the close proximity of the VOI to the ventricles may be detrimental for the DW signal. However, the assessment of the optimal trigger delay would require a more systematic investigation, since it may depend on the region of interest, DW-MRS sequence parameters, and cardiac gating device utilized. One possible limitation of using cardiac gating is that the effective TR is slightly variable during the acquisition, thus possibly introducing another source of ADC variability. Moreover, it may differ from subject to subject, depending on the individual heart rate. Nevertheless, the intra-subject TR fluctuations induced by irregular heart beatings were source of smaller signal amplitude variability compared to that observed without cardiac synchronization (Figure 4C).
In a previous study, the metabolite-cycling method and the use of the inherent water peak were proposed for compensation of significant signal loss37. Peak thresholding represents an alternative way to overcome the issue of motion-related signal amplitude fluctuations, and becomes crucial especially at high diffusion-weighting values. This procedure avoids the risk of artificial overestimation of ADCs as a consequence of underestimation of the DW signal in the presence of significant drops in the signal amplitude due to non-translational motion. Although the removal of some of the spectra before averaging could introduce a relatively small artificial increase of SNR (see Supplementary material S1), as expected, in both VOIs the variability of ADCs across subjects and sessions was lower when peak thresholding was applied (Figure 6). Notably, the effect of peak thresholding was more pronounced in VOICR than VOIPCC, suggesting that motion affects the diffusion-sensitized signal in the parietal WM more than in the PCC. In addition, the variability of the ADCs was much higher in two of the three DW directions (dir1 and dir2) in VOICR (Figure 6) indicating that motion is associated preferentially with specific directions, while in VOIPCC there was no significant difference among directions. Interestingly, the ADC variability in the smaller voxel VOI’CR did not depend on the diffusion direction (Figure 7), and, despite the intrinsic lower SNR related to voxel size, the ADC variability in VOI’CR was lower than that in VOICR in dir1 and dir2, suggesting that in those directions the effect of motion is dominant with respect to noise, thus affecting to a higher extent the bigger VOI. In addition, the average number of rejected spectra after peak thresholding was smaller in VOI’CR than in VOICR, further corroborating this hypothesis.
4.2. Metabolite kurtosis analysis
Diffusion-attenuation curves were fitted to mono-exponential functions up to b2 = 3300 s/mm2 and to a kurtosis model (Equation 2) up to b3 = 7300 s/mm2. The χ2 goodness-of-fit test revealed that the mono-exponential fit was not able to explain 30% of the metabolite signal decays up to b3, whereas the kurtosis approach was found to be more accurate (the null hypothesis was accepted for all fits with p > 0.9). The non mono-exponential behavior of the signal decay at high b-values can originate from non-gaussian, restricted diffusion within individual compartments, distributions of diffusion coefficients associated with multiple gaussian compartments, fiber dispersion, exchange effects, or a combination of all these processes38. Sampling DW-MRS data at high b-values is necessary to derive information on tissue morphology at the microscopic scale6,39. Although reaching ultra-high b-values > 15,000 s/mm2 would be desirable for accurate modeling of DW-MRS signals and extraction of microstructural parameters, this may be very challenging in a clinical context with stringent limitations in the acquisition times. In the present study, we evaluated the possibility to capture deviations from mono-exponentiality using the kurtosis approach, with a reasonably high b-value of 7300 s/mm2 and a relatively short acquisition protocol. Significantly higher K mean values in GM compared to WM for all metabolites confirmed previous results suggesting greater diffusional heterogeneity in GM40. Coefficients of variation for K were much higher than those obtained for the ADCs.
4.3. Inter-subject and intra-subject variability
The inter-subject variability calculated from full data-sets was less than 16% for ADCexp and ADCK of all metabolites under investigation in VOIPCC (Table 1), while it was higher in VOICR (< 16% for tNAA, tCr and tCho, < 27% for mIns) (Table 2). Instead, the inter-subject variability of K was less than 27% for all metabolites in both VOIs.
The robustness of the DW-MRS acquisition was evaluated by exploring the variability of the diffusion measures associated with different sub-sampling of the data. The procedure was repeated for different number of averages per diffusion-weighted condition and different diffusion-weighting schemes, corresponding to different acquisition times. In both VOIs, the coefficients of variation for tNAA, tCr and tCho ADCs were lower than 10% when 60 or more averages were considered using scheme b[0,2], corresponding to acquisition times of at least 3 minutes (Figure 9A, B). With scheme b[0,1,2], the same variability could be reached with at least 90 averages, indicating that acquiring spectra at low b-value of 850 s/mm2 does not add stability to the ADC calculation. Finally, using scheme b[0, 1] should be avoided, since it does not provide sufficient diffusion-weighting for proper ADC estimations. Figure 4S (Supplementary material) shows the behavior of the diffusion metrics’ variabilities as a function of the SNR estimated at b0. These plots report the non-diffusion-weighted SNR necessary to obtain a certain variability of the diffusion measures in the brain regions under investigation, providing that all acquisition and post-processing steps are properly performed, and can be useful to translate our results to data acquired on different experimental setups. However, depending on the effect of motion and other factors such phase and amplitude fluctuations on the diffusion-weighted signal detected in the region of interest, the SNR at b0 cannot be directly linked to the robustness of the measurements, and these results should be adapted with caution to different experimental conditions.
DW-MRS can be used to characterize microstructural alterations affecting brain tissue in a variety of diseases. Previous clinical and preclinical studies reported differences greater than 20% in the diffusion of several metabolites in patients with ischemic stroke or tumor, compared to healthy subjects41,42. Wood et al. reported differences of almost 20% in the tNAA diffusivity in the corpus callosum of patients with multiple sclerosis13, while differences in tCho and tCr ADCs of more than 15% were observed in systemic lupus erythematosus11. Our results on ADCexp repeatability (Table 3) suggest that for investigating tNAA, tCr, tCho and mIns diffusion abnormalities in pathologies where the expected ADCexp differences are greater than 10%, it is sufficient to keep the acquisition time per region of interest below 5 minutes, with a group size of about 30 subjects. In contrast, to explore more subtle microstructural abnormalities in normal aging or neurological diseases at the very early stage, longer acquisition times are probably desirable, and need to be evaluated case-by-case depending on the location and size of the brain region under investigation and the expected differences in the diffusion metrics.
5. Conclusions
We evaluated the performance of a DW-semi-LASER sequence at 3 T and demonstrated the feasibility of this method in a clinical setting, providing that all procedures from experimental planning (choice of b-values and number of averages), execution (cardiac triggering) and post-processing (peak thresholding in addition to standard phase and frequency corrections) are all carefully performed. Altered metabolite diffusion in tissue has been shown to reflect specific structural damage in disease. In particular, the diffusion of the neuronal marker tNAA has been suggested to reflect pure intra-axonal damage in white matter diseases, while the diffusion of tCho and tCr represent potential markers of inflammation and glial cells alterations. DW-semi-LASER may allow the exploration of microscopic cellular alterations in different pathological conditions, providing useful insights on the pathogenesis and evolution of the disease, and eventually helping to choose the most appropriate temporal window for tailored therapies and to monitor treatment response.
Supplementary Material
Funding information:
This work was supported by the programs ‘Institut des neurosciences translationnelle’ [ANR-10-IAIHU-06] and ‘Infrastructure d’avenir en Biologie Santé’ [ANR-11-INBS-0006]. Małgorzata Marjańska and Edward J. Auerbach acknowledge support of NIH grants [BTRC P41 EB015894 and P30 NS076408].
Abbreviations:
- ADC
apparent diffusion coefficient
- CR
coefficient of repeatability
- CR
corona radiata
- CRLB
Cramér-Rao lower bound
- CV
coefficient of variation
- δ
diffusion gradient duration
- DW
diffusion-weighted
- mIns
myo-inositol
- PCC
posterior cingulate cortex
- SD
standard deviation
- SNR
signal-to-noise ratio
- tCho
choline containing compounds
- tCr
creatine + phosphocreatine
- td
diffusion time
- tNAA
N-acetylaspartate + N-acetylaspartylglutamate
- VOI
volume of interest
References
- 1.Cao P, Wu EX. In vivo diffusion MRS investigation of non-water molecules in biological tissues: Diffusion Spectroscopy in Biological Tissues. NMR Biomed. 2017;30(3):e3481. [DOI] [PubMed] [Google Scholar]
- 2.Nicolay K, Braun KPJ, de Graaf RA, Dijkhuizen RM, Kruiskamp MJ. Diffusion NMR spectroscopy. NMR Biomed. 2001;14(2):94–111. [DOI] [PubMed] [Google Scholar]
- 3.Palombo M, Shemesh N, Ronen I, Valette J. Insights into brain microstructure from in vivo DW-MRS. Neuroimage. 2018;182:97–116. [DOI] [PubMed] [Google Scholar]
- 4.Ronen I, Valette J. Diffusion-weighted magnetic resonance spectroscopy. In: Harris RK, Wasylishen RL, eds. EMagRes. Chichester, UK: John Wiley & Sons, Ltd; 2015:733–750. [Google Scholar]
- 5.Palombo M, Ligneul C, Najac C, et al. New paradigm to assess brain cell morphology by diffusion-weighted MR spectroscopy in vivo. Proc Natl Acad Sci. 2016;113(24):6671–6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Palombo M, Ligneul C, Hernandez-Garzon E, Valette J. Can we detect the effect of spines and leaflets on the diffusion of brain intracellular metabolites? Neuroimage. 2018;182:283–293. [DOI] [PubMed] [Google Scholar]
- 7.Shemesh N, Rosenberg JT, Dumez J-N, Muniz JA, Grant SC, Frydman L. Metabolic properties in stroked rats revealed by relaxation-enhanced magnetic resonance spectroscopy at ultrahigh fields. Nat Commun. 2014;5(1):4958. [DOI] [PubMed] [Google Scholar]
- 8.Shemesh N, Rosenberg JT, Dumez J-N, Grant SC, Frydman L. Distinguishing neuronal from astrocytic subcellular microstructures using in vivo Double Diffusion Encoded 1H MRS at 21.1 T. Motta A, ed. Plos One. 2017;12(10):e0185232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valette J, Ligneul C, Marchadour C, Najac C, Palombo M. Brain metabolite diffusion from ultra-short to ultra-long time scales: what do we learn, where should we go? Front Neurosci. 2018;12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Branzoli F, Ercan E, Valabrègue R, et al. Differentiating between axonal damage and demyelination in healthy aging by combining diffusion-tensor imaging and diffusion-weighted spectroscopy in the human corpus callosum at 7 T. Neurobiol Aging. 2016;47:210–217. [DOI] [PubMed] [Google Scholar]
- 11.Ercan E, Magro-Checa C, Valabregue R, et al. Glial and axonal changes in systemic lupus erythematosus measured with diffusion of intracellular metabolites. Brain. 2016;139(5):1447–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ligneul C, Palombo M, Hernández-Garzón E, et al. Diffusion-weighted magnetic resonance spectroscopy enables cell-specific monitoring of astrocyte reactivity in vivo. NeuroImage. 2019;191:457–469. [DOI] [PubMed] [Google Scholar]
- 13.Wood ET, Ronen I, Techawiboonwong A, et al. Investigating Axonal Damage in Multiple Sclerosis by Diffusion Tensor Spectroscopy. J Neurosci. 2012;32(19):6665–6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Posse S, Cuenod CA, Le Bihan D. Human brain: proton diffusion MR spectroscopy. Radiology 1993;188:719–725. [DOI] [PubMed] [Google Scholar]
- 15.Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hänicke W, Sauter R. Localized high-resolution proton NMR spectroscopy using stimulated echoes: Initial applications to human brainin vivo. Magn Reson Med. 1989;9(1):79–93. [DOI] [PubMed] [Google Scholar]
- 16.Bottomley PA. Spatial localization in NMR spectroscopy in vivo. Ann N Y Acad Sci. 1987;508:333–348. [DOI] [PubMed] [Google Scholar]
- 17.Garwood M, DelaBarre L. The return of the frequency sweep: designing adiabatic pulses for contemporary NMR. J Magn Reson. 2001;153(2):155–177. [DOI] [PubMed] [Google Scholar]
- 18.Branzoli F, Ercan E, Webb A, Ronen I. The interaction between apparent diffusion coefficients and transverse relaxation rates of human brain metabolites and water studied by diffusion-weighted spectroscopy at 7 T. NMR Biomed. 2014;27(5):495–506. [DOI] [PubMed] [Google Scholar]
- 19.Najac C, Branzoli F, Ronen I, Valette J. Brain intracellular metabolites are freely diffusing along cell fibers in grey and white matter, as measured by diffusion-weighted MR spectroscopy in the human brain at 7 T. Brain Struct Funct. 2016;221(3):1245–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deelchand DK, Auerbach EJ, Marjańska M. Apparent diffusion coefficients of the five major metabolites measured in the human brain in vivo at 3T: ADC of human brain metabolites at 3T. Magn Reson Med. 2018;79(6):2896–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scheenen TWJ, Klomp DWJ, Wijnen JP, Heerschap A. Short echo time1H-MRSI of the human brain at 3T with minimal chemical shift displacement errors using adiabatic refocusing pulses. Magn Reson Med. 2008;59(1):1–6. [DOI] [PubMed] [Google Scholar]
- 22.Andronesi OC, Ramadan S, Ratai E-M, Jennings D, Mountford CE, Sorensen AG. Spectroscopic imaging with improved gradient modulated constant adiabaticity pulses on high-field clinical scanners. J Magn Reson. 2010;203(2):283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wood ET, Ercan AE, Branzoli F, et al. Reproducibility and optimization of in vivo human diffusion-weighted MRS of the corpus callosum at 3T and 7T. NMR Biomed. 2015;28(8):976–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ellegood J, Hanstock CC, Beaulieu C. Trace apparent diffusion coefficients of metabolites in human brain using diffusion weighted magnetic resonance spectroscopy. Magn Reson Med. 2005;53(5):1025–1032. [DOI] [PubMed] [Google Scholar]
- 25.Zheng G, Price WS. Suppression of background gradients in (B0 gradient-based) NMR diffusion experiments. Conc Magn Reson Part A. 2007;30A(5):261–277. [Google Scholar]
- 26.Tkac I, Starcuk Z, Choi I-Y, Gruetter R. In vivo 1H NMR spectroscopy of rat brain at 1 ms echo time. Magn Reson Med. 1999;41:649–656. [DOI] [PubMed] [Google Scholar]
- 27.Tkac I, Gruetter R. Methodology of 1H NMR spectroscopy of the human brain at very high magnetic fields. Appl Magn Reson. 2005;29:139–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gruetter R, Tkáč I. Field mapping without reference scan using asymmetric echo-planar techniques. Magn Reson Med. 2000;43(2):319–323. [DOI] [PubMed] [Google Scholar]
- 29.de Brouwer H. Evaluation of algorithms for automated phase correction of NMR spectra. J Magn Reson. 2009;201(2):230–238. [DOI] [PubMed] [Google Scholar]
- 30.Provencher SW. Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med. 1993;30(6):672–679. [DOI] [PubMed] [Google Scholar]
- 31.Henry P-G, Marjanska M, Walls JD, Valette J, Gruetter R, Ugurbil K. Proton-observed carbon-edited NMR spectroscopy in strongly coupled second-order spin systems. Magn Reson Med. 2006;55(2):250–257. [DOI] [PubMed] [Google Scholar]
- 32.Govindaraju V, Young K, Maudsley AA. Proton NMR chemical shifts and coupling constants for brain metabolites. NMR Biomed. 2000;13:129–153. [DOI] [PubMed] [Google Scholar]
- 33.Kaiser LG, Marjańska M, Matson GB, et al. 1H MRS detection of glycine residue of reduced glutathione in vivo. J Magn Reson. 2010;202(2):259–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jensen JH, Helpern JA, Ramani A, Lu H, Kaczynski K. Diffusional kurtosis imaging: The quantification of non-gaussian water diffusion by means of magnetic resonance imaging. Magn Reson Med. 2005;53(6):1432–1440. [DOI] [PubMed] [Google Scholar]
- 35.Yablonskiy DA, Sukstanskii AL. Theoretical models of the diffusion weighted MR signal. NMR Biomed. 2010;23(7):661–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bartlett JW, Frost C. Reliability, repeatability and reproducibility: analysis of measurement errors in continuous variables. Ultrasound Obstet Gynecol. 2008;31(4):466–475. [DOI] [PubMed] [Google Scholar]
- 37.Döring A, Adalid V, Boesch C, Kreis R. Diffusion-weighted magnetic resonance spectroscopy boosted by simultaneously acquired water reference signals. Magn Reson Med. 2018;80(6):2326–2338. [DOI] [PubMed] [Google Scholar]
- 38.Henriques RN, Jespersen SN, Shemesh N. Microscopic anisotropy misestimation in spherical-mean single diffusion encoding MRI. Magn Reson Med. 2019;81(5):3245–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Palombo M, Ligneul C, Valette J. Modeling diffusion of intracellular metabolites in the mouse brain up to very high diffusion-weighting: Diffusion in long fibers (almost) accounts for non-monoexponential attenuation. Magn Reson Med. 2017;77(1):343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ingo C, Brink W, Ercan E, Webb AG, Ronen I. Studying neurons and glia non-invasively via anomalous subdiffusion of intracellular metabolites. Brain Struct Func. 2018;223(8):3841–3854. [DOI] [PubMed] [Google Scholar]
- 41.Harada M, Uno M, Hong F, Hisaoka S, Nishitani H, Matsuda T. Diffusion-weightedin vivo localized proton MR spectroscopy of human cerebral ischemia and tumor. NMR Biomed. 2002;15(1):69–74. [DOI] [PubMed] [Google Scholar]
- 42.Zheng DD, Liu ZH, Fang J, Wang XY, Zhang J. The Effect of Age and Cerebral Ischemia on Diffusion-Weighted Proton MR Spectroscopy of the Human Brain. Am J Neuroradiol. 2012;33(3):563–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


