Abstract
Rationale:
MK801, like other NMDA receptor open channel blockers (e.g., ketamine and phencyclidine), increases the locomotor activity of rats and mice. Whether this behavioral effect ultimately relies on monoamine neurotransmission is of dispute.
Objective:
The purpose of this study was to determine whether these psychopharmacological effects and underlying neural mechanisms vary according to sex and age.
Methods:
Across four experiments, male and female preweanling and adolescent rats were pretreated with vehicle, the monoamine depleting agent reserpine (1 or 5 mg/kg), the dopamine (DA) synthesis inhibitor α-methyl-dl-p-tyrosine (AMPT), the serotonin (5-HT) synthesis inhibitor 4-chloro-dl-phenylalanine methyl ester hydrochloride (PCPA), or both AMPT and PCPA. The locomotor activity of preweanling and adolescent rats was then measured after saline or MK801 (0.3 mg/kg) treatment.
Results:
As expected, MK801 increased the locomotor activity of all age groups and both sexes, but the stimulatory effects were significantly less pronounced in male adolescent rats. Preweanling rats and adolescent female rats were more sensitive to the effects of DA and 5-HT synthesis inhibitors, as AMPT and PCPA caused only small reductions in the MK801-induced locomotor activity of male adolescent rats. Co-administration of AMPT+PCPA or high-dose reserpine (5 mg/kg) treatment substantially reduced MK801-induced locomotor activity in both age groups and across both sexes.
Conclusions:
These results, when combined with other recent studies, show that NMDA receptor open channel blockers cause pronounced age-dependent behavioral effects that can vary according to sex. The neural changes underlying these sex and age differences appear to involve monoamine neurotransmission.
Keywords: MK801, Reserpine, α-Methyl-dl-p-tyrosine (AMPT), 4-Chloro-dl-phenylalanine methyl ester hydrochloride (PCPA), Locomotor activity, Ontogeny
Introduction
MK801 (dizocilpine), phencyclidine (PCP), and ketamine are NMDA receptor open channel blockers that produce a pronounced trapping block (Huettner and Bean 1988; MacDonald et al. 1991; Bolshakov et al. 2003; Fernandes et al. 2015; Glasgow et al. 2018). Not surprisingly, MK801, PCP, and ketamine share many of the same behavioral actions, as all three compounds increase the locomotor activity of adult male rats and mice (Castellani and Adams 1981; Ford et al. 1989; Liljequist 1991; Usun et al. 2013; Yamamoto et al. 2016). Important sex and age differences are evident, since MK801 causes relatively greater amounts of locomotion in female rats than male rats (Blanchard et al. 1992; Criswell et al. 1993; Hönack and Löscher 1993; Frantz and Van Hartesveldt 1999b; Giordano and Mejia-Vigevano 2001; Feinstein and Kritzer 2013). In addition, preweanling and adolescent rats exhibit more MK801-induced locomotor activity than adult rats (Frantz and Van Hartesveldt 1999a, 1999b; Jacobs et al. 2000; Pesić et al. 2010).
In terms of the neural mechanisms mediating these behavioral actions, there is conflicting evidence that MK801 increases locomotor activity by either: (a) directly or indirectly modifying monoamine neurotransmission, or (b) activating neural circuity that is independent of monoamine systems. Although initial studies suggested that MK801 and other NMDA receptor open channel blockers directly modulate monoamine release and reuptake at the presynaptic terminal (Hiramatsu et al. 1989; Jackisch et al. 1992; Kiss et al. 1994; Hancock and Stamford 1999, but see Mele et al. 1998; Can et al. 2016), it appears more likely that NMDA open channel blockers indirectly increase locomotor activity by altering the firing rate of DA and 5-HT neurons in the ventral tegmental area, substantia nigra, raphe, and/or striatum (French and Cici 1990; Overton and Clark 1992; Gartside et al. 2007; Witkin et al. 2016; but see Mele et al. 1997; Adams and Moghaddam 1998). Alternatively, Mele et al. (1998) have hypothesized that DA neurotransmission is not directly involved, and that MK801 increases locomotor activity through “global” actions affecting multiple subcortical structures.
Results from psychopharmacological studies are generally consistent with monoaminergic involvement, because selective D1 and D2 receptor antagonists, as well as serotonin 5-HT1A and 5-HT2A receptor antagonists, reduce the MK801-induced locomotor activity of adult rodents (Maj et al. 1991; Hoffman 1992; Löscher and Hönack 1992; Kuribara and Uchihashi 1993; Ouagazzal et al. 1993; Martin et al. 1997). Studies using monoamine depleting agents and synthesis inhibitors provide a more complex pattern of results. Reserpine, which depletes DA and 5-HT in vesicular storage pools, significantly reduces the MK801-induced locomotor activity of adult male rodents (Maj et al. 1991; Kuribara et al. 1992; Starr and Starr 1994; Narayayan et al. 1996). Conversely, the DA synthesis inhibitor α-methyl-dl-p-tyrosine (AMPT) has alternately been reported to either decrease MK801’s locomotor activating effects in adult male rats (Maj et al. 1991) or leave the MK801-induced locomotor activity of adult male mice unaffected (Kuribara et al. 1992; Lapin and Rogawski 1995). Although the effect is small (i.e., about a 17% reduction), the 5-HT synthesis inhibitor 4-chloro-dl-phenylalanine methyl ester hydrochloride (PCPA) also causes significant reductions in the MK801-induced locomotor activity of adult male mice (Martin et al. 1998).
Although these results suggest that monoamine neurotransmission is at least partially responsible for mediating the locomotor activity caused by MK801, the bulk of these findings are based on adult male rats and mice. The almost exclusive use of these animal models is unfortunate because adult males, when compared to younger rats and females, are substantially less sensitive to the locomotor activating effects of MK801 and other NMDA receptor open channel blockers (Nabeshima et al. 1984; Hönack and Löscher 1993; McDougall et al. 2017). This differential sensitivity also provides evidence that the neural mechanisms mediating MK801-induced locomotor activity varies between male and female rats and across ontogeny.
The purpose of the present study was to determine if DA and 5-HT neurotransmission is responsible for mediating the MK801-induced locomotor activity of preweanling and adolescent male and female rats. Whether MK801’s locomotor activating effects are differentially sensitive to the depletion of stored vs. newly synthesized DA and 5-HT was also examined. To accomplish these goals, male and female preweanling and adolescent rats were pretreated with vehicle, reserpine, or AMPT and PCPA (either alone or in combination) and locomotor activity was assessed after an injection of saline or MK801 (0.3 mg/kg). Previous research has shown that the effects of AMPT (2 × 200 mg/kg), PCPA (3 × 200 mg/kg), and high-dose reserpine (5 mg/kg) treatment on dorsal striatal DA and 5-HT content does not differ according to age or sex (Crawford et al. 2020; McDougall et al. 2020). Thus, any age- or sex-related behavioral effects shown by MK801-treated rats cannot be ascribed to differences in the effectiveness of the depleting agents at reducing DA or 5-HT.
Materials and methods
Animals
Subjects were 104 preweanling and 208 adolescent male and female Sprague-Dawley rats that were born and raised at California State University, San Bernardino (CSUSB). Litters were culled to 10 pups on postnatal day (PD) 3. Preweanling rats were kept with the dam and littermates, whereas adolescent rats were weaned at PD 21 and group housed with same-sex littermates. All rats were housed in large polycarbonate maternity cages (30.5 × 43 × 19 cm) on ventilated racks. Food and water were freely available. The colony room was maintained at 22–23 °C and kept under a 12:12 light-dark cycle. Testing was done in a separate experimental room and was conducted during the light phase of the cycle. Subjects were cared for according to the “Guide for the Care and Use of Laboratory Animals” (National Research Council 2010) under a research protocol approved by the Institutional Animal Care and Use Committee of CSUSB.
Apparatus
Behavioral testing was done in activity monitoring chambers that have a plastic floor, acrylic walls, and an open top (Coulbourn Instruments, Whitehall, PA). Each chamber includes an X–Y photobeam array, with 16 photocells and detectors, that measures distance traveled (cm). In order to equate for differences in body size (Campbell et al. 1969; Shalaby and Spear 1980), preweanling rats were tested in smaller chambers (26 × 26 × 41 cm) than adolescent rats (41 × 41 × 41 cm).
Drugs
(+)-MK801 hydrogen maleate, AMPT (α-methyl-dl-p-tyrosine), and PCPA (4-chloro-dl-phenylalanine methyl ester hydrochloride) were dissolved in saline, while reserpine was dissolved in a minimal amount of glacial acetic acid and diluted with saline. All drugs were purchased from Millipore-Sigma (St. Louis, MO) and injected intraperitoneally (IP).
Procedure
Experiment 1: Effects of reserpine, AMPT, or PCPA on the MK801-induced locomotor activity of male and female preweanling rats
On PD 20, preweanling rats were injected with saline and habituated to the activity chambers for 30 min. Prior to habituation, rats were randomly divided into one of five pretreatment groups (the injection time-line can be seen in Fig. 1). Rats in the PCPA group were injected with PCPA (3 × 200 mg/kg) on PD 18, PD 19, and PD 20 (30 min after habituation). Rats in the high-dose reserpine group were injected with 5 mg/kg reserpine 24 h before testing (i.e., 30 min after completion of habituation on PD 20). Rats in the low-dose reserpine group were injected with 1 mg/kg reserpine 4 h before behavioral testing on PD 21. Rats in the AMPT group were injected with AMPT (2 × 200 mg/kg) 4 h and 2 h before behavioral testing on PD 21. Rats in the vehicle group were divided into four subsets of two rats and injected with vehicle at the same time points as the four other pretreatment groups (the data provided by these separate vehicle groups did not differ and was combined into a single “vehicle” group). The procedure for administering the two doses of reserpine (1 and 5 mg/kg) differed because high-dose reserpine treatment can have acute locomotor-debilitating effects. Therefore, we used previously established protocols for administering 1 mg/kg (Uchihashi et al. 1992) and 5 mg/kg (Niddam et al. 1985; Thomas et al. 1992) reserpine. On PD 21 (24 h after the final injection of PCPA or 5 mg/kg reserpine, 4 h after the injection of 1 mg/kg reserpine, and 2 h after the second injection of AMPT), preweanling rats (n = 4 male and 4 female subjects per group) were injected with saline or 0.3 mg/kg MK801 and immediately placed in activity monitoring chambers where distance traveled was measured for 120 min. We used a 0.3 mg/kg dose of MK801 (see also Duke et al. 1997), because only a narrow dose range of MK801 (0.1–0.5 mg/kg, but not 0.01 or 1 mg/kg) significantly increases the locomotor activity of preweanling and adolescent rats (Frantz and Van Hartesveldt 1999b).
Fig. 1.
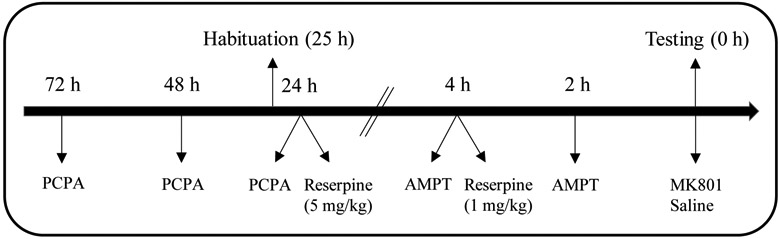
Schematic diagram of the injection time-line for Experiment 1 (preweanling rats) and Experiment 3 (adolescent rats).
Experiment 2: Effects of combined AMPT and PCPA treatment on the MK801-induced locomotor activity of male and female preweanling rats
On PD 20, rats were injected with saline and habituated to the activity chambers for 30 min. Prior to habituation, rats were randomly divided into one of three pretreatment groups (the injection time-line can be seen in Fig. 2). Preweanling rats in the PCPA+AMPT group were injected with PCPA (3 × 200 mg/kg) and AMPT (2 × 200 mg/kg) using the same drug administration regimens as described in Experiment 1. Rats in the high-dose reserpine group were injected with 5 mg/kg reserpine 30 min after completion of habituation testing. Rats in the vehicle group were divided into two subsets of four rats and injected with vehicle at the same time points as the two other pretreatment groups (the data provided by these separate vehicle groups did not differ and was combined into a single “vehicle” group). On PD 21, preweanling rats (n = 4 male and 4 female subjects per group) were injected with 0.3 mg/kg MK801 and immediately placed in the activity monitoring chambers. Distance traveled was measured for 120 min.
Fig. 2.
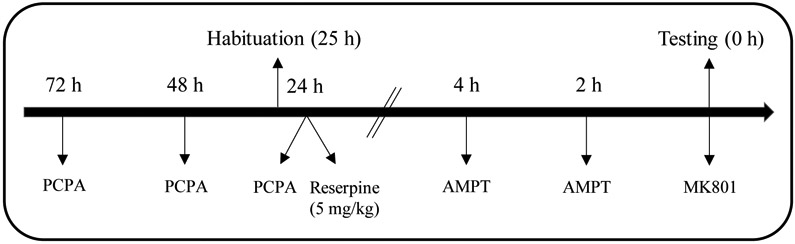
Schematic diagram of the injection time-line for Experiment 2 (preweanling rats) and Experiment 4 (adolescent rats).
Experiment 3: Effects of reserpine, AMPT, or PCPA on the MK801-induced locomotor activity of male and female adolescent rats
The procedures and injection time-line were the same as described for Experiment 1 (see Fig. 1), with two exceptions: (1) rats were habituated on PD 40 and tested on PD 41, and (2) twice as many male and female rats were used. Although sex differences are typically not reported in prepubertal groups, NMDA receptor open channel blockers often affect the locomotor activity of male and female adolescent rats differently (Frantz and Van Hartesveldt 1999a; Wilson et al. 2007; McDougall et al. 2017, 2019, 2020; Crawford et al. 2020). Therefore, to enhance statistical power, 8 male and 8 female adolescent rats were assigned to each group.
Experiment 4: Effects of combined AMPT and PCPA treatment on the MK801-induced locomotor activity of male and female adolescent rats
The procedures and injection time-line were the same as described for Experiment 2 (see Fig. 2), with the exceptions that rats were habituated on PD 40 and tested on PD 41, and eight male and eight female rats were randomly assigned to each group.
Statistics
Distance traveled data were analyzed using repeated measures analyses of variance (ANOVAs). When the assumption of sphericity was violated, as determined by Mauchly’s test, the Huynh-Feldt epsilon statistic was used to adjust degrees of freedom (Huynh and Feldt 1976). Corrected degrees of freedom were rounded to the nearest whole number and are indicated by a superscripted “a” in the parenthetical statistical reports. Post hoc analysis of distance traveled data was done using Tukey tests. When interpreting statistically significant higher order interactions, the mean square error terms (i.e., MSerror) used for the Tukey calculations were based on separate one- or two-way ANOVAs at each time block. Main effects, interactions, and post hoc tests were considered significant at P<0.05. For both preweanling and adolescent rats, litter effects were controlled by assigning no more than one male and one female rat per litter per group (Holson and Pearce 1992). Unlike for the statistical analyses involving distance traveled data, analysis of body weight data required that a single litter mean be calculated from multiple littermates assigned to the same pretreatment condition. The reason for this statistically conservative approach is that multiple subjects from each litter were pretreated with vehicle, reserpine, AMPT, or PCPA, thus the unit of analysis for the body weight ANOVA was the mean score of identically treated littermates (Holson and Pearce 1992; Zorrilla 1997).
Results
Experiment 1: Effects of reserpine, AMPT, or PCPA on the MK801-induced locomotor activity of male and female preweanling rats
MK801 significantly enhanced the locomotor activity of preweanling rats [Drug main effect, F1,70 =144.42, P<0.001], but this effect varied according to pretreatment condition [Pretreatment main effect, F4,70 =57.40, P<0.001]. Among saline-treated rats (Fig. 3, upper graph, right panel), minimal locomotor activity was evident and it was not significantly affected by drug pretreatment. Among MK801-treated rats (Fig. 3, lower graph, right panel), reserpine (1 and 5 mg/kg) fully attenuated distance traveled scores, while AMPT and PCPA partially attenuated MK801-induced locomotion [aPretreatment × Drug interaction, F4,70 =46.52, P<0.001]. More specifically, MK801-treated rats injected with vehicle, AMPT, or PCPA exhibited more distance traveled than saline-treated rats injected with the same pretreatment compound (Tukey tests). In contrast, preweanling rats pretreated with reserpine (1 or 5 mg/kg) showed the same low level of locomotion regardless of whether they were injected with saline or MK801.
Fig. 3.
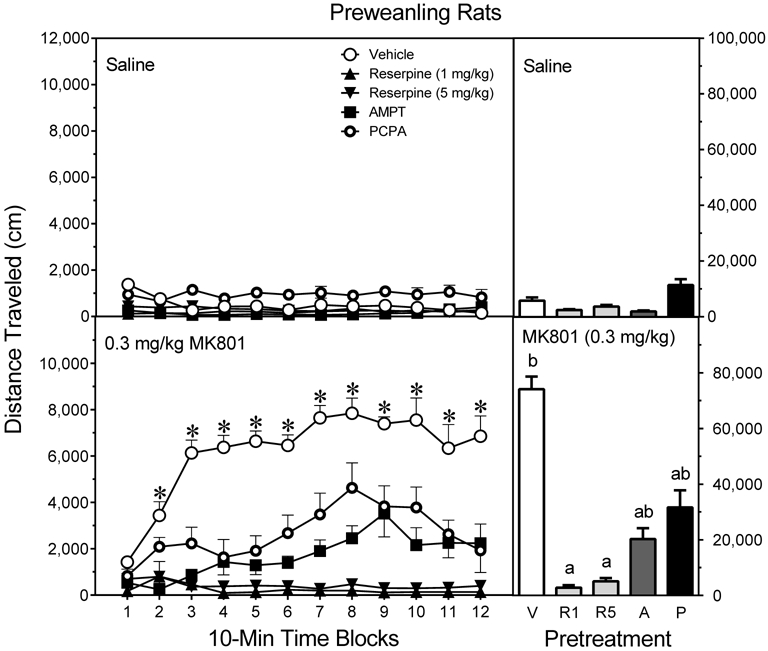
Mean distance traveled scores (±SEM) of male and female preweanling rats (n = 8 per group) tested with saline (upper graph) or 0.3 mg/kg MK801 (lower graph) on PD 21. Rats had been pretreated with vehicle (“V”), reserpine (1 or 5 mg/kg, “R1” or “R5”), AMPT (2 × 200 mg/kg, “A”), or PCPA (3 × 200 mg/kg, “P”). The right panels show total distance traveled collapsed across the testing session. * = Significantly different from all other groups on the same time block. a = Significantly different from the Vehicle-MK801 group. b = Significantly different from saline-treated rats injected with the same pretreatment drug (compare upper and lower graphs). Note: To enhance visual clarity, statistically significant differences between saline- (upper graph) and MK801- (lower graph) treated rats are presented in the bar graphs and text of the Results section, but are not represented by significance symbols on the line graphs.
A finer grain analysis examining time effects showed that vehicle-pretreated rats injected with MK801 (i.e., the Vehicle-MK801 group) exhibited greater distance traveled than saline-treated controls (i.e., the Vehicle-Saline group) on time blocks 2–12[aPretreatment × Drug × Time block interaction, F25,435 =5.06, P<0.001]. On the same time blocks (i.e., time blocks 2–12), reserpine (1 or 5 mg/kg ), AMPT, and PCPA significantly reduced the distance traveled scores of MK801-treated rats (Fig. 3, lower graph, left panel). On time block 3 and time blocks 5–10, 5 mg/kg reserpine caused a greater reduction in MK801-induced locomotor activity than AMPT; whereas, 5 mg/kg reserpine produced a greater reduction than PCPA on time blocks 8 and 9 (Tukey tests). Consistent with these findings, AMPT- and PCPA-pretreated rats injected with MK801 had greater distance traveled scores than the AMPT-Saline group on time blocks 7–9 and the PCPA-Saline group on time block 2 and time blocks 6–10, respectively (Tukey tests). At this preweanling age, no sex differences in drug responsiveness were apparent.
Experiment 2: Effects of combined AMPT and PCPA treatment on the MK801-induced locomotor activity of male and female preweanling rats
Both reserpine (5 mg/kg) and combined treatment with AMPT+PCPA fully attenuated the distance traveled of MK801-treated preweanling rats (Fig. 4) [Pretreatment main effect, F2,21 =53.76, P<0.001]. On all time blocks, vehicle-pretreated rats exhibited greater distance traveled scores than reserpine- or AMPT+PCPA-treated rats [aPretreatment × Time block interaction, F7,57 =8.46, P<0.001].
Fig. 4.
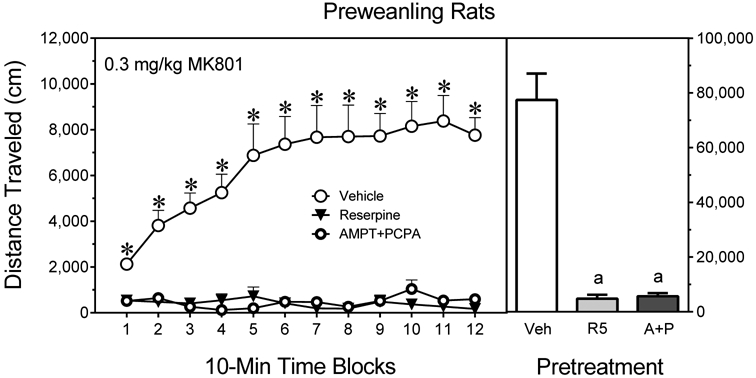
Mean distance traveled scores (±SEM) of male and female preweanling rats (n = 8 per group) tested with 0.3 mg/kg MK801 on PD 21. Rats had been pretreated with vehicle, reserpine (5 mg/kg, “R5”), or AMPT (2 × 200 mg/kg) plus PCPA (3 × 200 mg/kg) (“A+P”). The right panels show total distance traveled collapsed across the testing session. * = Significantly different from all other groups on the same time block. a = Significantly different from the Vehicle-MK801 group (open bar).
Experiment 3: Effects of reserpine, AMPT, or PCPA on the MK801-induced locomotor activity of male and female adolescent rats
Overall, female adolescent rats had greater distance traveled scores than male rats [Sex main effect, F1,140 =4.36, P<0.05], and this effect was moderated by pretreatment condition, test drug, and time block [Sex × Pretreatment × Drug interaction, F4,140 =7.19, P<0.001; aSex × Pretreatment × Drug × Time Block interaction, F21,740 =3.25, P<0.001]. For this reason, the distance traveled scores of male and female adolescent rats were analyzed separately.
Female adolescent rats
MK801 increased distance traveled scores [Drug main effect, F1,70 =144.63, P<0.001], but this effect was restricted to specific pretreatment conditions [Pretreatment main effect, F4,70 =33.30, P<0.001]. Among saline-treated rats (Fig. 5, upper graph, right panel), pretreatment condition did not significantly affect basal locomotor activity. Among MK801-treated rats (Fig. 5, lower graph, right panel), AMPT and 5 mg/kg reserpine caused an almost complete attenuation of MK801-induced locomotion [Pretreatment × Drug interaction, F4,70 =23.23, P<0.001]. In contrast, PCPA caused a partial attenuation of MK801’s locomotor activating effects, because: (1) the PCPA-MK801 group had significantly reduced distance traveled scores when compared to the Vehicle-MK801 group, and (2) the PCPA-MK801 group had greater distance traveled scores than both the PCPA-Vehicle group and the High-Dose Reserpine-MK801 group (Tukey tests).Interestingly, 1 mg/kg reserpine did not affect the MK801-induced locomotion of female adolescent rats.
Fig. 5.
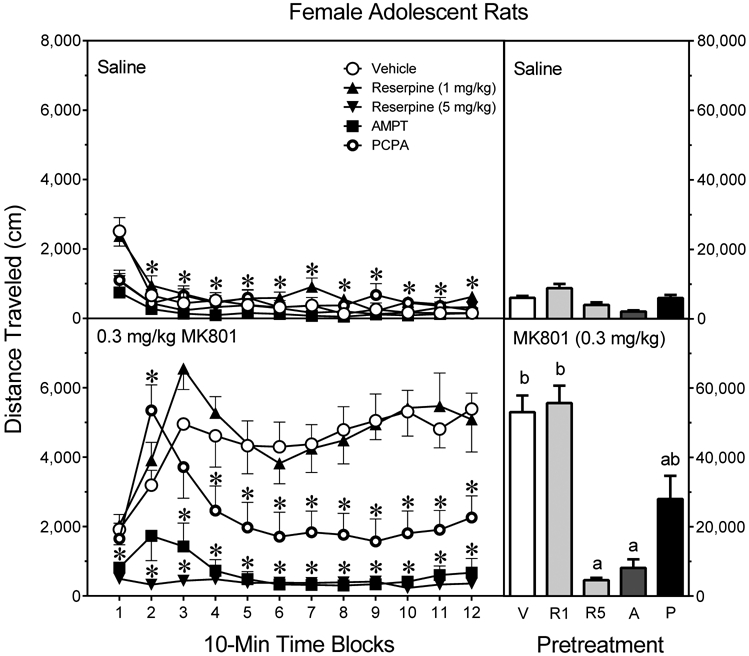
Mean distance traveled scores (±SEM) of female adolescent rats (n = 8 per group) tested with saline (upper graph) or 0.3 mg/kg MK801 (lower graph) on PD 41. Rats had been pretreated with vehicle (“V”), reserpine (1 or 5 mg/kg, “R1” or “R5”), AMPT (2 × 200 mg/kg, “A”), or PCPA (3 × 200 mg/kg, “P”). The right panels show total distance traveled collapsed across the testing session. * = Significantly different from the Vehicle-MK801 group on the same time block. a = Significantly different from the Vehicle-MK801 group. b = Significantly different from saline-treated rats injected with the same pretreatment drug (compare upper and lower graphs). Note: To enhance visual clarity, statistically significant differences between saline- (upper graph) and MK801- (lower graph) treated rats are presented in the bar graphs and text of the Results section, but are not represented by significance symbols on the line graphs.
Analyses involving the repeated measures factor showed that AMPT and 5 mg/kg reserpine reduced the distance traveled scores of MK801-treated female rats on time blocks 3–12 and time blocks 1–12, respectively (Fig. 5, bottom graph, left panel) [aPretreatment × Drug × Time Block interaction, F19,331 =5.21, P<0.001]. Relative to the Vehicle-MK801 group, PCPA decreased the locomotor activity of MK801-treated female rats on time blocks 4–12. This PCPA-induced effect did not represent a complete attenuation of MK801-induced locomotion because the PCPA-MK801 group had greater distance traveled scores than the PCPA-Saline group on time blocks 2–4 and time block 12 (Tukey tests). The Vehicle-MK801 and 1 mg/kg Reserpine-MK801 groups exhibited more locomotor activity than their saline-treated controls (i.e., the Vehicle-Saline and 1 mg/kg Reserpine-Saline groups) on time blocks 2–12 (Tukey tests). Among saline-treated female rats (Fig. 5, upper graph, left panel), AMPT, PCPA, and 5 mg/kg reserpine only reduced distance traveled scores on time block 1 (Tukey tests).
Male adolescent rats
MK801 enhanced the distance traveled scores of male adolescent rats [Drug main effect, F1,70 =108.75, P<0.001]. This MK801-induced effect was not altered by AMPT or PCPA pretreatment (Fig. 6, lower graph, right panel), although 1 mg/kg reserpine caused a small, but significant, potentiation of MK801’s locomotor activating effects [Pretreatment × Drug interaction, F4,70 = 8.72, P<0.001]. All of the aforementioned MK801 groups (i.e., the Vehicle-MK801, AMPT-MK801, PCPA-MK801, and 1 mg/kg Reserpine-MK801 groups) evidenced more locomotor activity than saline-treated rats from the same pretreatment condition (Tukey tests). Even so, only 5 mg/kg reserpine fully attenuated the MK801-induced locomotion of male adolescent rats (Tukey tests). None of the pretreatment regimens differentially affected the distance traveled scores of saline-treated rats (Fig. 6, upper graph, right panel).
Fig. 6.
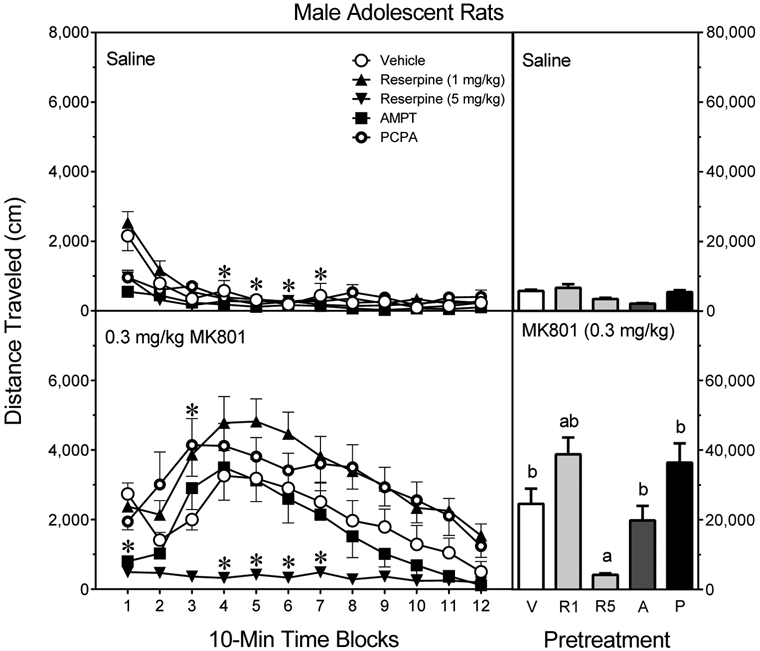
Mean distance traveled scores (±SEM) of male adolescent rats (n = 8 per group) tested with saline (upper graph) or 0.3 mg/kg MK801 (lower graph) on PD 41. Rats had been pretreated with vehicle (“V”), reserpine (1 or 5 mg/kg, “R1” or “R5”), AMPT (2 × 200 mg/kg, “A”), or PCPA (3 × 200 mg/kg, “P”). The right panels show total distance traveled collapsed across the testing session. * = Significantly different from the Vehicle-MK801 group on the same time block. a = Significantly different from the Vehicle-MK801 group. b = Significantly different from saline-treated rats injected with the same pretreatment drug (compare upper and lower graphs). Note: To enhance visual clarity, statistically significant differences between saline- (upper graph) and MK801- (lower graph) treated rats are presented in the bar graphs and text of the Results section, but are not represented by significance symbols on the line graphs.
Among saline-treated rats (Fig. 6, upper graph, left panel), AMPT, PCPA, and 5 mg/kg reserpine significantly reduced basal Locomotor activity on time block 1 [aPretreatment × Drug × Time Block interaction, F21,356 =2.76, P<0.001]. On subsequent time blocks, the various saline-treated groups exhibited minimal amounts of locomotion. Among MK801-treated rats (Fig. 6, lower graph, left panel), 5 mg/kg reserpine fully attenuated locomotor responding across the testing session, while the locomotor activity of the other groups was best represented by an inverted U-shaped function of approximately the same magnitude.MK801-treated rats given vehicle or AMPT responded almost identically, with these two groups exhibiting more locomotor activity than the Vehicle-Saline, AMPT-Saline, and 5 mg/kg Reserpine-MK801 groups on time blocks 4–7 (Tukey tests). Surprisingly, MK801-treated rats given PCPA or 1 mg/kg reserpine were generally more active than MK801 rats pretreated with vehicle, although this effect only reached statistical significance on time block 3. The PCPA-MK801 and 1 mg/kg Reserpine-MK801 groups had more distance traveled than the PCPA-Saline and 1 mg/kg Reserpine-Saline groups on time blocks 2–12, and more locomotor activity than the 5 mg/kg Reserpine-MK801 group on time blocks 1–12 (Tukey tests).
Experiment 4: Effects of combined AMPT and PCPA treatment on the MK801-induced locomotor activity of male and female adolescent rats
As in Experiment 3, female adolescent rats exhibited more locomotor activity than male rats [Sex main effect, F1,42 =15.88, P<0.001; aSex × Pretreatment × Time Block interaction, F7,153 =6.53, P<0.001], thus the distance traveled scores of male and female rats were presented and analyzed separately.
Female adolescent rats
Overall, female adolescent rats given reserpine or AMPT+PCPA had significantly smaller distance traveled scores than vehicle-treated female rats (Fig. 7, upper graph, right panel) [Pretreatment main effect, F2,21 =43.49, P<0.001]. When collapsed across time blocks, reserpine and AMPT+PCPA did not differentially affect the MK801-induced locomotion of adolescent female rats (Tukey tests, P=0.20).
Fig. 7.
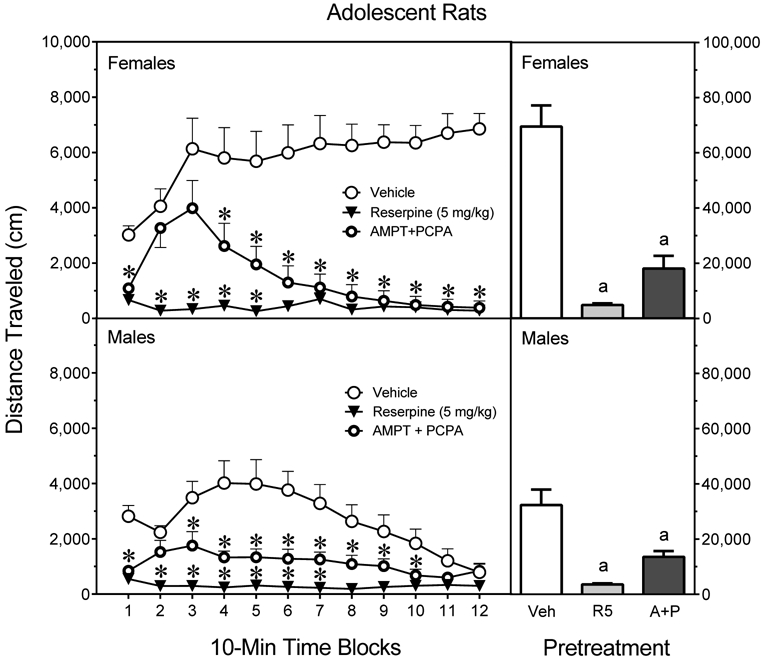
Mean distance traveled scores (±SEM) of male and female adolescent rats (n = 8 per group) tested with 0.3 mg/kg MK801 on PD 41. Rats had been pretreated with vehicle (“Veh”), reserpine (5 mg/kg, “R5”), or AMPT (2 × 200 mg/kg) plus PCPA (3 × 200 mg/kg) (“A+P”). The right panels show total distance traveled collapsed across the testing session. * = Significantly different from the same-sex vehicle group on the same time block. a = Significantly different from the same-sex vehicle group (open bars).
Reserpine significantly reduced the MK801-induced distance traveled scores of female rats on time blocks 1–12; whereas, AMPT+PCPA only decreased MK801-induced locomotion on time blocks 1 and 4–12 (Fig. 7, upper graph, left panel) [aPretreatment × Time Block interaction, F7,71 =6.42, P<0.001]. On time blocks 2 and 3, MK801-treated rats given AMPT+PCPA exhibited significantly greater distance traveled scores than reserpinized rats (Tukey tests).
Male adolescent rats
Among MK801-treated male adolescent rats (Fig. 7, lower graph, right panel), both reserpine and AMPT+PCPA significantly reduced distance traveled scores [Pretreatment main effect, F2,21 =17.48, P<0.001]. Reserpine caused significant reductions on time blocks 1–10, while AMPT+PCPA decreased MK801-induced locomotor activity on time blocks 1 and 3–8 [Pretreatment × Time Block interaction, F8,80 =4.90, P<0.001].
Separate analyses assessing sex-dependent differences in the MK801-induced locomotor activity of preweanling and adolescent rats
When data were collapsed across Experiments 1 and 2, it was evident that the MK801 induced locomotor activity of preweanling rats did not vary according to sex (Fig. 8, upper graph). The absence of sex differences was apparent in preweanling rats that were pretreated with vehicle as well as reserpine (1 or 5 mg/kg), AMPT, PCPA, or AMPT+PCPA.
Fig. 8.
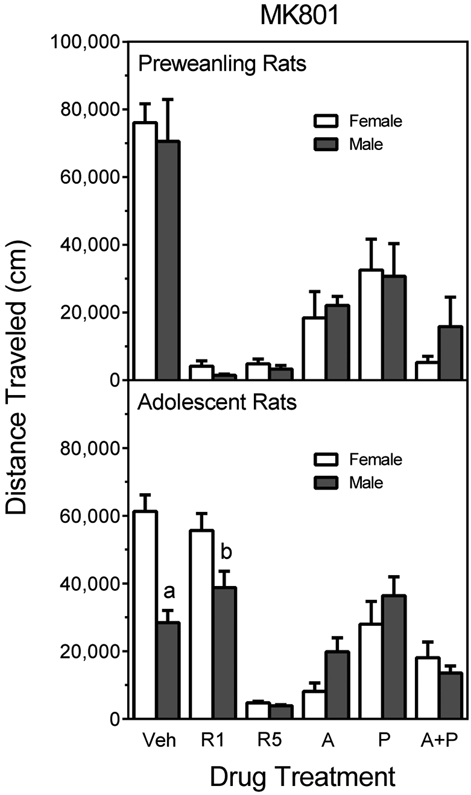
Mean distance traveled scores (±SEM) of male and female preweanling and adolescent rats tested with 0.3 mg/kg MK801 on PD 21 or PD 41 (these are the same rats shown in Fig. 1-5). Rats had been pretreated with vehicle (“Veh”), reserpine (1 or 5 mg/kg, “R1” or “R5”), AMPT (2 × 200 mg/kg, “A”), PCPA (3 × 200 mg/kg, “P”), or AMPT+PCPA (“A+P”). a = Significantly different from adolescent female rats pretreated with vehicle (adjacent open bar). b = Significantly different from adolescent female rats pretreated with 1 mg/kg reserpine (adjacent open bar).
In contrast, female adolescent rats from Experiments 3 and 4 had significantly greater distance traveled scores than male adolescent rats (Fig. 8, lower graph) [Pretreatment main effect, F1,116 =6.15, P<0.05]. This sex effect varied according to pretreatment condition [Pretreatment × Sex interaction, F5,116 =10.40, P<0.001], as Tukey tests (P<0.05) indicated that only vehicle-pretreated rats showed a sex difference after MK801 administration. Female adolescent rats given reserpine (1 mg/kg) and MK801 appeared to exhibit more locomotor activity than similarly-treated male rats, but this effect was only statistically significant when an independent t test was used [t14 =2.41, P<0.05] and not when family-wise error rate was controlled for (Tukey test, P>0.05).
Effects of reserpine and PCPA on the body weights of male and female preweanling and adolescent rats
Although male rats weighed more than female rats (Table 1) [Sex main effect, F1,176 = 132.68, P<0.001], this sex effect was only evident in adolescent rats [Age × Sex interaction, F1,176 = 92.79, P<0.001]. Reserpine (5 mg/kg) and PCPA (3 × 200 mg/kg) significantly reduced body weights at both ages and in both sexes [Pretreatment main effect, F2,176 = 44.70, P<0.001; Pretreatment × Age interaction, F2,176 = 7.38, P<0.001]. Rats treated with AMPT and 1 mg/kg reserpine were not included in the analyses, because these drugs were administered only 4 h and/or 2 h before testing.
Table 1.
Mean (SEM) body weights (g) of vehicle-, reserpine-, and PCPA-pretreated male and female preweanling and adolescent rats on the test day
| Pretreatment | Age |
|||
|---|---|---|---|---|
| Preweanling | Adolescent | |||
| Male | Female | Male | Female | |
| Vehicle | 63.03 (1.3) | 59.79 (1.4) | 207.76 (2.8) a | 165.07 (1.8) |
| Reserpine (5 mg/kg) | 53.55 (1.5) b | 49.70 (2.5) b | 176.69 (3.9) ab | 144.67 (2.3) b |
| PCPA (3 × 200 mg/kg) | 51.90 (2.4) b | 48.75 (1.7) b | 184.19 (4.0) ab | 144.12 (3.4) b |
Significantly different from adolescent female rats.
Significantly different from vehicle-treated rats of the same sex and age.
Discussion
It is well established that administering MK801 to adult rodents causes substantially more locomotor activity in females than males (Blanchard et al. 1992; Criswell et al. 1993; Frantz and Van Hartesveldt 1999b; Giordano and Mejia-Vigevano 2001). In adult male rats, this MK801-induced locomotion is attenuated by moderate and high doses (5 and 10 mg/kg) of reserpine (Maj et al. 1991; Starr and Starr 1994; Narayanan et al. 1996), while low doses of reserpine (0.5–2 mg/kg) are sufficient to block the locomotor activity of MK801-treated adult male mice (Kuribara et al. 1992). In the present study, 0.3 mg/kg MK801 robustly increased the locomotor activity of adolescent female rats and preweanling rats (no sex differences were apparent at this age). Although MK801 also enhanced the locomotor activity of adolescent male rats, the increase was less than half (37%) of that evidenced by adolescent females. Thus, only in adolescent and adult rats, but not preweanling rats, do females show more MK801-induced locomotor activity than males (see also Duke et al. 1997; Frantz and Van Hartesveldt 1999a, 1999b).
The effects of reserpine also differed according to both sex and age. In terms of sex differences, 1 mg/kg reserpine potentiated the MK801-induced locomotor activity of male adolescent rats, while not significantly altering the behavior of female adolescent rats. The reason for this seemingly odd effect is uncertain, but reserpine was previously reported to potentiate the locomotor activity of d-amphetamine-treated adolescent and adult male rats and mice (Smith 1963; Stolk and Rech 1967; Crawford et al. 2020). In terms of age differences, both doses of reserpine (1 and 5 mg/kg) completely attenuated the MK801-induced locomotion of preweanling rats; while only the higher dose of reserpine (5 mg/kg) significantly reduced the MK801-induced locomotor activity of male and female adolescent rats. This effect is almost certainly due to age differences in monoamine depletion, because 1 mg/kg reserpine causes large reductions in the dorsal striatal DA and 5-HT content (94.2% and 74.9% declines, respectively) of preweanling rats; whereas, the same dose of reserpine produces only small reductions in the DA and 5-HT content (13.6% and 14.3% declines, respectively) of adolescent rats (Crawford et al. 2020). Thus, it appears that modest depletions of DA and/or 5-HT are insufficient to attenuate MK801’s locomotor activating effects. An important caveat to this conclusion is that the effects of reserpine, as well as AMPT and PCPA, on monoamine content were only assessed in the dorsal striatum of preweanling and adolescent rats, and not in other brain regions that might be critical for NMDA/monoamine interactions (Crawford et al. 2020; McDougall et al. 2020). Even so, available evidence using striatal tissue suggests that the amount of monoamine depletion is a critical factor affecting the occurrence of MK801-induced locomotor activity.
DA and 5-HT synthesis inhibitors caused age- and sex-dependent behavioral differences in MK801-treated rats. For example, AMPT and PCPA produced substantial declines (72.7–84.6% and 47.2–57.3%, respectively) in the MK801-induced locomotor activity of preweanling rats and female adolescent rats, while neither drug significantly reduced the MK801-induced locomotor activity of male adolescent rats. The latter effect is also observed in adult mice, as Kuribara and colleagues (1992), as well as Lapin and Rogawski (1995), reported that AMPT did not decrease the MK801-induced locomotor activity of adult male mice (but see Maj et al. 1991). Likewise, Martin et al. (1998) conducted a series of six experiments in which PCPA did not significantly reduce MK801’s locomotor activating effects in adult male rats. Only when a meta-analysis of these data was conducted did Martin et al. (1998) note that PCPA caused a small, but statistically significant reduction (17%) in the MK801-induced locomotor activity of adult rats. What appears certain, is that the age- and sex-dependent behavioral effects of AMPT and PCPA are not due to differences in the effectiveness of synthesis inhibition, because the ability of AMPT and PCPA to reduce dorsal striatal DA and 5-HT levels are similar in male and female preweanling and adolescent rats (McDougall et al. 2020).
In both age groups and with both sexes, 5 mg/kg reserpine caused a greater reduction in MK801-induced locomotor activity than PCPA alone; likewise, 5 mg/kg reserpine produced a more pronounced decline in MK801-induced locomotion than AMPT in all but female adolescent rats. These behavioral differences may be a consequence of reserpine depleting both DA and 5-HT, while the synthesis inhibitors affect only a single neurotransmitter (Crawford et al. 2020; McDougall et al. 2020). This explanation received only partial support from the present behavioral results, as combined treatment with AMPT and PCPA, like reserpine, fully attenuated the MK801-induced locomotor activity of preweanling rats; however, 5 mg/kg reserpine reduced the MK801-induced locomotion of adolescent rats to a greater extent than AMPT+PCPA. An alternative explanation involves the mechanism of action of each compound, as reserpine depletes DA and 5-HT located in vesicular storage pools, whereas AMPT and PCPA block the synthesis of new DA and 5-HT (Callaway et al. 1989; Cummings and Walker 1996; Watanabe et al. 2005). This mechanistic difference can have behavioral implications. For example, the d-amphetamine-induced locomotor activity of adult rats is more sensitive to AMPT than reserpine (Finn et al. 1990), presumably because d-amphetamine primarily releases newly synthesized DA from cytosolic pools rather than from storage vesicles (Kuczenski 1983; Parker and Cubeddu 1986). In terms of our adolescent rats, reserpine (5 mg/kg) caused a greater reduction in MK801-induced locomotor activity than AMPT+PCPA, thus it is possible that MK801’s locomotor activating effects are more sensitive to manipulations that deplete monoamines from vesicular stores.
Regardless of interpretation, results using PCPA and/or AMPT suggest that both DA and 5-HT systems are involved in mediating the MK801-induced locomotor activity of preweanling rats and female adolescent rats. Using adult male rats and mice, Carlsson, Martin, Svensson and colleagues came to a similar conclusion, as they reported abundant evidence that DA systems were involved in mediating MK801-induced locomotion (Svensson et al. 1992, 1994; Martin et al. 1994, 1997, 1998). These same authors viewed the role of 5-HT neurotransmission as being more complex, since they concluded that multiple 5-HT receptor subtypes were involved in mediating MK801-induced locomotion, with individual subtypes exerting either excitatory or inhibitory effects (Martin et al. 1998). In adult male rodents, Martin et al. (1998) found that excitatory influences were “almost completely counterbalanced” by inhibitory influences, which is consistent with the pattern of effects exhibited by our male adolescent rats (i.e., PCPA neither reduced nor potentiated MK801-induced locomotor activity). In contrast, PCPA reduced the MK801- and ketamine-induced locomotor activity of preweanling rats and female adolescent rats (McDougall et al. 2020), suggesting that in a normosensitive state the excitatory influences of 5-HT receptor subtypes outweigh inhibitory influences. If Martin et al.’s (1998) model is accurate, our results may indicate that the balance of excitatory and inhibitory 5-HT receptor systems mediating MK801-induced locomotor activity differ between male and female rats, and across ontogeny. Specifically, the locomotor activation produced by NMDA receptor open channel blockers is more dependent on 5-HT neurotransmission during the preweanling period and with female adolescent rats, than with male adolescent and adult rats (see also McDougall et al. 2020).
In comparing the results of the present study using MK801 to previous ontogenetic studies using ketamine (Crawford et al. 2020; McDougall et al. 2020), it appears that at each age and with each sex, the individual pretreatment agents (reserpine, AMPT, or PCPA) affected MK801-and ketamine-induced locomotor activity similarly. The only exceptions involved low-dose reserpine treatment, as 1 mg/kg reserpine attenuated the locomotor activity of ketamine-, but not MK801-treated male and female adolescent rats. In preweanling rats the opposite was the case, as 1 mg/kg reserpine attenuated the effects of MK801, but not ketamine. Therefore, it appears that MK801-treated adolescent rats are less sensitive to the effects of monoamine depletion than ketamine-treated adolescents, whereas preweanling rats show the opposite pattern. Despite these exceptions involving 1 mg/kg reserpine, the lack of a major dissociation between MK801 and ketamine is informative, because it suggests that the two NMDA receptor open channel blockers affect locomotor activity through common mechanisms.
Independent of monoamine depletion and synthesis inhibition, it is interesting that female rats and preweanling rats, when compared to adolescent and adult male rats, are more sensitive to the locomotor activating effects of NMDA receptor open channel blockers (e.g., MK801, PCP, and ketamine). One possibility is that ontogenetic and sex-dependent differences in NMDA, DA, and/or 5-HT systems underlie these behavioral effects. Not only do NMDA receptor binding site densities differ according to age and sex (Cyr et al. 2001; Insel et al. 1990), but DA and 5-HT systems show pronounced changes depending on age and sex. For example, DA release and uptake is greater in female rats than male rats (Walker et al. 2000, 2006), and the same is true when adolescent rats are compared to adults (Walker and Kuhn 2008). It is also the case that D1 and D2 receptor sites are transiently over-produced in adolescence, with the magnitude of the effect being much larger in males than females (Andersen et al. 1997; Andersen and Teicher 2000). In terms of serotonergic systems, 5-HT content and turnover is greater in females than males (Carlsson et al. 1985; Carlsson and Carlsson 1988; Staiti et al. 2011), while various 5-HT receptor subtypes vary in number depending on both age and sex (Zilles et al. 1985; Mendelson and McEwen 1991; Morilak and Ciaranello, 1993; Morilak et al. 1994; Sumner and Fink 1997; Schiller et al. 2006). Drug pharmacokinetics is another important factor that is at least partially responsible for the ontogenetic and sex-dependent differences in responsiveness to NMDA receptor open channel blockers. Specifically, total drug availability, as well as peak plasma and brain concentrations of MK801, PCP, and ketamine are significantly greater in adult female rats than male rats (Nabeshima et al. 1984; Andiné et al. 1999; Shelnutt et al. 1999; Saland and Kabbaj 2018; McDougall et al. 2019). Likewise, peak brain concentrations of ketamine are significantly elevated in preweanling rats when compared to male adolescent or adult rats (McDougall et al. 2019). In sum, pharmacokinetic factors, along with age and sex-dependent differences in NMDA, DA, and 5-HT elements, may be responsible for the behavioral differences exhibited by MK801-treated male and female preweanling and adolescent rats.
In terms of limitations, an often unmentioned issue is that reserpine and PCPA cause weight loss, or a reduction in normal weight gain, in rats of all ages. In the present study, reserpine (5 mg/kg) and PCPA (3 × 200 mg/kg) significantly reduced the body weights of male (15.0% and 17.7%, respectively) and female (16.9% and 18.5%, respectively) preweanling rats, as well as male (15.0% and 11.4%, respectively) and female (12.4% and 12.7%, respectively) adolescent rats. Whether these body weight changes systematically affected locomotor activity is doubtful, because 5 mg/kg reserpine and PCPA did not reduce the basal locomotor activity of saline-treated rats. That being said, a floor effect could have masked differences between the drug and vehicle groups, thus leaving open the possibility that weight loss might have contributed to differences between the groups. A separate issue is whether the monoamine depleting agent and synthesis inhibitors caused a general suppression of locomotor activity irrespective of the neural mechanism mediating MK801’s actions. This concern is mitigated by the finding that at least one male or female age group treated with AMPT, PCPA, or reserpine (1 mg/kg) exhibited as much MK801-induced locomotor activity as the Vehicle-MK801 group. In terms of high-dose reserpine treatment, we previously reported that 5 mg/kg reserpine did not attenuate the d-amphetamine-induced locomotor activity of male and female preweanling and adolescent rats (Crawford et al. 2020). The latter set of findings suggest that young rats are physically capable of exhibiting substantial locomotor activity when pretreated with 5 mg/kg reserpine. Nonetheless, these pretreatment agents do affect locomotion, so care must be taken when interpreting their behavioral effects, especially when given in conjunction with locomotor activating drugs.
In summary, MK801’s locomotor activating effects differ according to both age and sex, with preweanling rats and adolescent female rats exhibiting more MK801-induced locomotor activity than adolescent male rats. Preweanling rats and adolescent female rats were more sensitive to the effects of DA and 5-HT synthesis inhibitors, as AMPT or PCPA caused only small reductions in the MK801-induced locomotor activity of adolescent male rats. In contrast, manipulations that produced substantial and simultaneous reductions in both DA and 5-HT levels caused profound reductions in MK801-induced locomotor activity regardless of age or sex. The present results are consistent with other studies showing that NMDA receptor open channel blockers stimulate locomotor activity through mechanisms that involve DA and 5-HT neurotransmission, and that these systems differ according to sex and across ontogeny.
Acknowledgments
Funding sources:
This research was supported by NIGMS training grant GM083883.
Footnotes
Conflict of interest:
All authors declare no conflict of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- Adams B, Moghaddam B (1998) Corticolimbic dopamine neurotransmission is temporally dissociated from the cognitive and locomotor effects of phencyclidine. J Neurosci 18:5545–5554. 10.1523/JNEUROSCI.18-14-05545.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH (2000) Sex differences in dopamine receptors and their relevance to ADHD. Neurosci Biobehav Rev 24:137–141. 10.1016/s0149-7634(99)00044-5 [DOI] [PubMed] [Google Scholar]
- Andersen SL, Rutstein M, Benzo JM, Hostetter JC, Teicher MH (1997) Sex differences in dopamine receptor overproduction and elimination. Neuroreport 8:1495–1498. 10.1097/00001756-199704140-00034 [DOI] [PubMed] [Google Scholar]
- Andiné P, Widermark N, Axelsson R, Nyberg G, Olofsson U, Martensson E, Sandberg M (1999) Characterization of MK-801-induced behavior as a putative rat model of psychosis. J Pharmacol Exp Ther 290:1393–1408. [PubMed] [Google Scholar]
- Blanchard DC, Blanchard RJ, De Padua Carobrez A, Veniegas R, Rodgers RJ, Shepherd JK (1992) MK-801 produces a reduction in anxiety-related antipredator defensiveness in male and female rats and a gender-dependent increase in locomotor behavior. Psychopharmacology 108:352–362. 10.1007/bf02245123 [DOI] [PubMed] [Google Scholar]
- Bolshakov KV, Gmiro VE, Tikhonov DB, Magazanik LG (2003) Determinants of trapping block of N-methyl-d-aspartate receptor channels. J Neurochem 87:56–65. https://doi.Org/10.1046/j.1471-4159.2003.01956.x [DOI] [PubMed] [Google Scholar]
- Callaway CW, Kuczenski R, Segal DS (1989) Reserpine enhances amphetamine stereotypies without increasing amphetamine-induced changes in striatal dialysate dopamine. Brain Res 505:83–90. 10.1016/0006-8993(89)90118-2 [DOI] [PubMed] [Google Scholar]
- Campbell BA, Lytle LD, Fibiger HC (1969) Ontogeny of adrenergic arousal and cholinergic inhibitory mechanisms in the rat. Science 166:635–637. https://doi.Org/10.1126/science.166.3905.635 [DOI] [PubMed] [Google Scholar]
- Can A, Zanos P, Moaddel R, Kang HJ, Dossou KS, Wainer IW, Cheer JF, Frost DO, Huang XP, Gould TD (2016) Effects of ketamine and ketamine metabolites on evoked striatal dopamine release, dopamine receptors, and monoamine transporters. J Pharmacol Exp Ther 359:159–170. 10.1124/jpet.116.235838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson M, Carlsson A (1988) A regional study of sex differences in rat brain serotonin. Prog Neuropsychopharmacol Biol Psychiatry 12:53–61. 10.1016/0278-5846(88)90061-9 [DOI] [PubMed] [Google Scholar]
- Carlsson M, Svensson K, Eriksson E, Carlsson A (1985) Rat brain serotonin: biochemical and functional evidence for a sex difference. J Neural Transm 63:297–313. 10.1007/bf01252033 [DOI] [PubMed] [Google Scholar]
- Castellani S, Adams PM (1981) Acute and chronic phencyclidine effects on locomotor activity, stereotypy and ataxia in rats. Eur J Pharmacol 73:143–154. 10.1016/0014-2999(81)90086-8 [DOI] [PubMed] [Google Scholar]
- Crawford CA, Moran AE, Baum TA, Apodaca MG, Montejano NR, Park GI, Gomez V, McDougall SA (2020) Effects of monoamine depletion on the ketamine-induced locomotor activity of preweanling, adolescent, and adult rats: sex and age differences. Behav Brain Res 379:112267 10.1016/j.bbr.2019.112267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criswell HE, Johnson KB, Mueller RA, Breese GR (1993) Evidence for involvement of brain dopamine and other mechanisms in the behavioral action of the N-methyl-D-aspartic acid antagonist MK-801 in control and 6-hydroxydopamine-lesioned rats. J Pharmacol Exp Ther 265:1001–1010. [PubMed] [Google Scholar]
- Cummings TJ, Walker PD (1996) Serotonin depletion exacerbates changes in striatal gene expression following quinolinic acid injection. Brain Res 743:240–248. 10.1016/s0006-8993(96)01053-0 [DOI] [PubMed] [Google Scholar]
- Cyr M, Ghribi O, Thibault C, Morissette M, Landry M, Di Paolo T (2001) Ovarian steroids and selective estrogen receptor modulators activity on rat brain NMDA and AMPA receptors. Brain Res Rev 37:153–161. 10.1016/s0165-0173(01)00115-1 [DOI] [PubMed] [Google Scholar]
- Duke MA, O'Neal J, McDougall SA (1997) Ontogeny of dopamine agonist-induced sensitization: role of NMDA receptors. Psychopharmacology 129:153–160. 10.1007/s002130050175 [DOI] [PubMed] [Google Scholar]
- Feinstein I, Kritzer MF (2013) Acute N-methyl-d-aspartate receptor hypofunction induced by MK801 evokes sex-specific changes in behaviors observed in open-field testing in adult male and proestrus female rats. Neuroscience 228:200–214. https://doi.Org/10.1016/j.neuroscience.2012.10.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes A, Wojcik T, Baireddy P, Pieschl R, Newton A, Tian Y, Hong Y, Bristow L, Li YW (2015) Inhibition of in vivo [3HMK-801 binding by NMDA receptor open channel blockers and GluN2B antagonists in rats and mice. Eur J Pharmacol 766:1–8. https://doi.Org/10.1016/j.ejphar.2015.08.044 [DOI] [PubMed] [Google Scholar]
- Finn IB, Iuvone PM, Holtzman SG (1990) Depletion of catecholamines in the brain of rats differentially affects stimulation of locomotor activity by caffeine, d-amphetamine, and methylphenidate. Neuropharmacology 29:625–631. 10.1016/0028-3908(90)90023-k [DOI] [PubMed] [Google Scholar]
- Ford LM, Norman AB, Sanberg PR (1989) The topography of MK-801-induced locomotor patterns in rats. Physiol Behav 46:755–758. 10.1016/0031-9384(89)90363-6 [DOI] [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C (1999a) The locomotor effects of MK801 in the nucleus accumbens of developing and adult rats. Eur J Pharmacol 368:125–135 [DOI] [PubMed] [Google Scholar]
- Frantz K, Van Hartesveldt C (1999b) Locomotion elicited by MK801 in developing and adult rats: temporal, environmental, and gender effects. Eur J Pharmacol 369:145–157. 10.1016/s0091-3057(99)00162-8 [DOI] [PubMed] [Google Scholar]
- French ED, Ceci A (1990) Non-competitive N-methyl-d-aspartate antagonists are potent activators of ventral tegmental A10 dopamine neurons Neurosci Lett 119:159–162. 10.1016/0304-3940(90)90823-r [DOI] [PubMed] [Google Scholar]
- Gartside SE, Cole AJ, Williams AP, McQuade R, Judge SJ (2007) AMPA and NMDA receptor regulation of firing activity in 5-HT neurons of the dorsal and median raphe nuclei. Eur J Neurosci 25:3001–3008. https://doi.Org/10.1111/j.1460-9568.2007.05577.x [DOI] [PubMed] [Google Scholar]
- Giordano M, Mejía-Viggiano MC (2001) Gender differences in spontaneous and MK-801-induced activity after striatal lesions. Brain Res Bull 56:553–561. 10.1016/s0361-9230(01)00627-x [DOI] [PubMed] [Google Scholar]
- Glasgow NG, Wilcox MR, Johnson JW (2018) Effects of Mg2+ on recovery of NMDA receptors from inhibition by memantine and ketamine reveal properties of a second site. Neuropharmacology 137:344–358. 10.1016/j.neuropharm.2018.05.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock PJ, Stamford JA (1999) Stereospecific effects of ketamine on dopamine efflux and uptake in the rat nucleus accumbens. Br J Anaesth 82:603–608. https://doi.Org/10.1093/bja/82.4.603 [DOI] [PubMed] [Google Scholar]
- Hiramatsu M, Cho AK, Nabeshima T (1989) Comparison of the behavioral and biochemical effects of the NMDA receptor antagonists, MK-801 and phencyclidine. Eur J Pharmacol 166:359–366. 10.1016/0014-2999(89)90346-4 [DOI] [PubMed] [Google Scholar]
- Hoffman DC (1992) Typical and atypical neuroleptics antagonize MK-801-induced locomotion and stereotypy in rats. J Neural Transm 89:1–10. 10.1007/bf01245347 [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B (1992) Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol 14:221–228. 10.1016/0892-0362(92)90020-B [DOI] [PubMed] [Google Scholar]
- Honack D, Loscher W (1993) Sex differences in NMDA receptor mediated responses in rats. Brain Res 620:167–170. 10.1016/0006-8993(93)90287-w [DOI] [PubMed] [Google Scholar]
- Huettner JE, Bean BP (1988) Block of N-methyl-d-aspartate-activated current by the anticonvulsant MK-801: Selective binding to open channels. Proc Natl Acad Sci USA 85:1307–1311. 10.1073/pnas.85.4.1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh H, Feldt LS (1976) Estimation of the Box correction for degrees of freedom from sample data in randomized block and split-plot designs. J Educ Stat 1:69–82. https://doi.Org/10.2307/1164736 [Google Scholar]
- Insel TR, Miller LP, Gelhard RE (1990) The ontogeny of excitatory amino acid receptors in rat forebrain—I. N-methyl-d-aspartate and quisqualate receptors. Neuroscience 35:31–43. 10.1016/0306-4522(90)90117-m [DOI] [PubMed] [Google Scholar]
- Jackisch R, Link T, Neufang B, Koch R (1992) Studies on the mechanism of action of the antiparkinsonian drugs memantine and amantadine: no evidence for direct dopaminomimetic or antimuscarinic properties. Arch Int Pharmacodyn Ther 320:21–42. [PubMed] [Google Scholar]
- Jacobs PS, Taylor BM, Bardgett ME (2000) Maturation of locomotor and Fos responses to the NMDA antagonists, PCP and MK-801. Dev Brain Res 122:91–95. 10.1016/s0165-3806(00)00059-6 [DOI] [PubMed] [Google Scholar]
- Kiss JP, Tóth E, Lajtha A, Vizi ES (1994) NMDA receptors are not involved in the MK-801-induced increase of striatal dopamine release in rat: a microdialysis study. Brain Res 641:145–148. 10.1016/0006-8993(94)91828-7 [DOI] [PubMed] [Google Scholar]
- Kuribara H, Uchihashi Y (1993) SCH 23390 equivalently, but YM-09151-2 differentially reduces the stimulant effects of methamphetamine, MK-801 and ketamine: assessment by discrete shuttle avoidance in mice. Jpn J Pharmacol 62:111–114. 10.1254/jjp.62.111 [DOI] [PubMed] [Google Scholar]
- Kuribara H, Asami T, Ida I, Tadokoro S (1992) Characteristics of the ambulation-increasing effect of the noncompetitive NMDA antagonist MK-801 in mice: assessment by the coadministration with central-acting drugs. Jpn J Pharmacol 58:11–18. 10.1254/jjp.58.11 [DOI] [PubMed] [Google Scholar]
- Kuczenski R (1983) Biochemical actions of amphetamine and other stimulants In Creese I (ed), Stimulants: Neurochemical, behavioral and clinical perspectives. Raven Press, New York, pp 31–61. [Google Scholar]
- Lapin IP, Rogawski MA (1995) Effects of D1 and D2 dopamine receptor antagonists and catecholamine depleting agents on the locomotor stimulation induced by dizocilpine in mice. Behav Brain Res 70:145–151. 10.1016/0166-4328(95)80004-2 [DOI] [PubMed] [Google Scholar]
- Liljequist S (1991) Genetic differences in the effects of competitive and non-competitive NMDA receptor antagonists on locomotor activity in mice. Psychopharmacology 104:17–21. 10.1007/bf02244548 [DOI] [PubMed] [Google Scholar]
- Loscher W, Honack D (1992) The behavioural effects of MK-801 in rats: involvement of dopaminergic, serotonergic and noradrenergic systems. Eur J Pharmacol 215:199–208 10.1016/0014-2999(92)90029-4 [DOI] [PubMed] [Google Scholar]
- MacDonald JF, Bartlett MC, Mody I, Pahapill P, Reynolds JN, Salter MW, Schneiderman JH, Pennefather PS (1991) Actions of ketamine, phencyclidine and MK-801 on NMDA receptor currents in cultured mouse hippocampal neurones. J Physiol 432:483–508. 10.1113/jphysiol.1991.sp018396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maj J, Rogoz Z, Skuza G (1991) Locomotor hyperactivity induced by MK-801 in rats. Pol J Pharmacol Pharm 43:449–458. [PubMed] [Google Scholar]
- Martin P, Svensson A, Carlsson A, Carlsson ML (1994) On the roles of dopamine D-1 vs. D-2 receptors for the hyperactivity response elicited by MK-801. J Neural Transm Gen Sect 95:113–121. 10.1007/bf01276430 [DOI] [PubMed] [Google Scholar]
- Martin P, Waters N, Waters S, Carlsson A, Carlsson ML (1997) MK-801-induced hyperlocomotion: differential effects of M100907, SDZ PSD 958 and raclopride. Eur J Pharmacol 335:107–116. 10.1016/s0014-2999(97)01188-6 [DOI] [PubMed] [Google Scholar]
- Martin P, Waters N, Schmidt CJ, Carlsson A, Carlsson ML (1998) Rodent data and general hypothesis: antipsychotic action exerted through 5-HT2A receptor antagonism is dependent on increased serotonergic tone. J Neural Transm 105:365–396. 10.1007/s007020050064 [DOI] [PubMed] [Google Scholar]
- McDougall SA Moran AE, Baum TA, Apodaca MG, Real V (2017) Effects of ketamine on the unconditioned and conditioned locomotor activity of preadolescent and adolescent rats: impact of age, sex, and drug dose. Psychopharmacology 234:2683–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Park GI, Ramirez GI, Gomez V, Adame BC, Crawford CA (2019) Sex-dependent changes in ketamine-induced locomotor activity and ketamine pharmacokinetics in preweanling, adolescent, and adult rats. Eur Neuropsychopharmacol 29:740–755. 10.1016/j.euroneuro.2019.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall SA, Rios JW, Apodaca MG, Park GI, Montejano NR, Taylor JA, Moran AE, Robinson JAM, Baum TA, Teran A, Crawford CA (2020) Effects of dopamine and serotonin synthesis inhibitors on the ketamine-, d-amphetamine-, and cocaine-induced locomotor activity of preweanling and adolescent rats: sex differences. Behav Brain Res 189:172857 https://doi.Org/10.1016/j.bbr.2019.112302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mele A, Fontana D, Pert A (1997) Alterations in striatal dopamine overflow during rotational behavior induced by amphetamine, phencyclidine, and MK-801. Synapse 26:218–224 [DOI] [PubMed] [Google Scholar]
- Mele A, Thomas DN, Pert A (1998) Different neural mechanisms underlie dizocilpine maleate- and dopamine agonist-induced locomotor activity. Neuroscience 82:43–58. 10.1016/s0306-4522(97)00277-7 [DOI] [PubMed] [Google Scholar]
- Mendelson SD, McEwen BS (1991) Autoradiographic analyses of the effects of restraint-induced stress on 5-HT1A, 5-HT1C and 5-HT2 receptors in the dorsal hippocampus of male and female rats. Neuroendocrinology 54:454–461. 10.1159/000125951 [DOI] [PubMed] [Google Scholar]
- Morilak DA, Ciaranello RD (1993) Ontogeny of 5-hydroxytryptamine2 receptor immunoreactivity in the developing rat brain. Neuroscience 55:869–880. 10.1016/0306-4522(93)90447-n [DOI] [PubMed] [Google Scholar]
- Morilak DA, Somogyi P, Lujan-Miras R, Ciaranello RD (1994) Neurons expressing 5-HT2 receptors in the rat brain: neurochemical identification of cell types by immunocytochemistry. Neuropsychopharmacology 11:157–166. 10.1038/sj.npp.1380102 [DOI] [PubMed] [Google Scholar]
- Nabeshima T, Yamaguchi K, Yamada K, Hiramatsu M, Kuwabara Y, Furukawa H, Kameyama T (1984) Sex-dependent differences in the pharmacological actions and pharmacokinetics of phencyclidine in rats. Eur J Pharmacol 97:217–227. 10.1016/0014-2999(84)90453-9 [DOI] [PubMed] [Google Scholar]
- Narayanan S, Willins D, Dalia A, Wallace L, Uretsky N (1996) Role of dopaminergic mechanisms in the stimulatory effects of MK-801 injected into the ventral tegmental area and the nucleus accumbens. Pharmacol Biochem Behav 54:565–573. 10.1016/0091-3057(95)02220-1 [DOI] [PubMed] [Google Scholar]
- National Research Council (2010) Guide for the Care and Use of Laboratory Animals. 8th ed. National Academies Press, Washington. [Google Scholar]
- Niddam R, Arbilla S, Scatton B, Dennis T, Langer SZ (1985) Amphetamine induced release of endogenous dopamine in vitro is not reduced following pretreatment with reserpine. Naunyn-Schmiedebergs Arch Pharmacol 329:123–127. 10.1007/bf00501200 [DOI] [PubMed] [Google Scholar]
- Ouagazzal A, Nieoullon A, Amalric M (1993) Effects of dopamine D1 and D2 receptor blockade on MK-801-induced hyperlocomotion in rats. Psychopharmacology 111:427–434. 10.1007/bf02253532 [DOI] [PubMed] [Google Scholar]
- Overton P, Clark D (1992) Iontophoretically administered drugs acting at the N-methyl-D-aspartate receptor modulate burst firing in A9 dopamine neurons in the rat. Synapse 10:131–140. 10.1002/syn.890100208 [DOI] [PubMed] [Google Scholar]
- Parker EM, Cubeddu LX (1986) Effects of d-amphetamine and dopamine synthesis inhibitors on dopamine and acetylcholine neurotransmission in the striatum. II. Release in the presence of vesicular transmitter stores. J Pharmacol Exp Ther 237:193–203. [PubMed] [Google Scholar]
- Pesić V, Popić J, Milanović D, Loncarević-Vasiljković N, Rakić L, Kanazir S, Ruzdijić S (2010) The effect of MK-801 on motor activity and c-Fos protein expression in the brain of adolescent Wistar rats. Brain Res 1321:96–104. https://doi.Org/10.1016/j.brainres.2010.01.048 [DOI] [PubMed] [Google Scholar]
- Saland SK, Kabbaj M (2018) Sex differences in the pharmacokinetics of low-dose ketamine in plasma and brain of male and female rats. J Pharmacol Exp Ther 367:393–404. 10.1124/jpet.118.251652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller L, Jähkel M, Oehler J (2006) The influence of sex and social isolation housing on pre- and postsynaptic 5-HT1A receptors. Brain Res 1103:76–87. https://doi.Org/10.1016/j.brainres.2006.05.051 [DOI] [PubMed] [Google Scholar]
- Shalaby IA, Spear LP (1980) Psychopharmacological effects of low and high doses of apomorphine during ontogeny. Eur J Pharmacol 67:451–459. 10.1016/0014-2999(80)90186-7 [DOI] [PubMed] [Google Scholar]
- Shelnutt SR, Gunnell M, Owens SM (1999) Sexual dimorphism in phencyclidine in vitro metabolism and pharmacokinetics in rats. J Pharmacol Exp Ther 290:1292–1298. [PubMed] [Google Scholar]
- Smith CB (1963) Enhancement by reserpine and α-methyl dopa of the effects of d-amphetamine upon the locomotor activity of mice. J Pharmacol Exp Ther 142:343–350. [PubMed] [Google Scholar]
- Staiti AM, Morgane PJ, Galler JR, Grivetti JY, Bass DC, Mokler DJ (2011) A microdialysis study of the medial prefrontal cortex of adolescent and adult rats. Neuropharmacology 61:544–549. https://doi.Org/10.1016/j.neuropharm.2011.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr MS, Starr BS (1994) Comparison of the effects of NMDA and AMPA antagonists on the locomotor activity induced by selective D1 and D2 dopamine agonists in reserpine-treated mice. Psychopharmacology 114:469–476. 10.1007/bf02249338 [DOI] [PubMed] [Google Scholar]
- Stolk JM, Rech RH (1967) Enhanced stimulant effects of 1-amphetamine on the spontaneous locomotor activity of rats treated with reserpine. J Pharmacol Exp Ther 158:140–149. [PubMed] [Google Scholar]
- Sumner BE, Fink G (1997) The density of 5-hydoxytryptamine2A receptors in forebrain is increased at pro-oestrus in intact female rats. Neurosci Lett 234:7–10. 10.1016/s0304-3940(97)00651-4 [DOI] [PubMed] [Google Scholar]
- Svensson A, Carlsson A, Carlsson ML (1992) Differential locomotor interactions between dopamine D1/D2 receptor agonists and the NMDA antagonist dizocilpine in monoamine-depleted mice. J Neural Transm 90:199–217. 10.1007/bf01250961 [DOI] [PubMed] [Google Scholar]
- Svensson A, Carlsson ML, Carlsson A (1994) Glutamatergic neurons projecting to the nucleus accumbens can affect motor functions in opposite directions depending on the dopaminergic tone. Prog Neuropsychopharmacol Biol Psychiatry 18:1203–1218. 10.1016/0278-5846(94)90121-x [DOI] [PubMed] [Google Scholar]
- Thomas KL, Rose S, Jenner P, Marsden CD (1992) Acute reserpine treatment induces down regulation of D-1 dopamine receptor associated adenylyl cyclase activity in rat striatum. Biochem Pharmacol 44:83–91. 10.1016/0006-2952(92)90041-g [DOI] [PubMed] [Google Scholar]
- Uchihashi Y, Kuribara H, Tadokoro S (1992) Assessment of the ambulation-increasing effect of ketamine by coadministration with central-acting drugs in mice. Jpn J Pharmacol 60:25–31. 10.1254/jjp.60.25 [DOI] [PubMed] [Google Scholar]
- Usun Y, Eybrard S, Meyer F, Louilot A (2013) Ketamine increases striatal dopamine release and hyperlocomotion in adult rats after postnatal functional blockade of the prefrontal cortex. Behav Brain Res 256:229–237. https://doi.Org/10.1016/j.bbr.2013.08.017 [DOI] [PubMed] [Google Scholar]
- Walker QD, Kuhn CM (2008) Cocaine increases stimulated dopamine release more in periadolescent than adult rats. Neurotoxicol Teratol 30:412–418. 10.1016/j.ntt.2008.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker QD, Rooney MB, Wightman RM, Kuhn CM (2000) Dopamine release and uptake are greater in female than male rat striatum as measured by fast cyclic voltammetry. Neuroscience 95:1061–1070. 10.1016/s0306-4522(99)00500-x [DOI] [PubMed] [Google Scholar]
- Walker QD, Ray R, Kuhn CM (2006) Sex differences in neurochemical effects of dopaminergic drugs in rat striatum. Neuropsychopharmacology 31:1193–1202. 10.1038/sj.npp.1300915 [DOI] [PubMed] [Google Scholar]
- Watanabe S, Fusa K, Takada K, Aono Y, Saigusa T, Koshikawa N, Cools AR (2005) Effects of alpha-methyl-p-tyrosine on extracellular dopamine levels in the nucleus accumbens and the dorsal striatum of freely moving rats. J Oral Sci 47:185–190. 10.2334/josnusd.47.185 [DOI] [PubMed] [Google Scholar]
- Wilson C, Kercher M, Quinn B, Murphy A, Fiegel C, McLaurin A (2007) Effects of age and sex on ketamine-induced hyperactivity in rats. Physiol Behav 91:202–207. https://doi.Org/10.1016/j.physbeh.2007.02.010 [DOI] [PubMed] [Google Scholar]
- Witkin JM, Monn JA, Schoepp DD, Li X, Overshiner C, Mitchell SN, Carter G, Johnson B, Rasmussen K, Rorick-Kehn LM (2016) The rapidly acting antidepressant ketamine and the mGlu2/3 receptor antagonist LY341495 rapidly engage dopaminergic mood circuits. J Pharmacol Exp Ther 358:71–82. 10.1124/jpet.116.233627 [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Nakayama T, Yamaguchi J, Matsuzawa M, Mishina M, Ikeda K, Yamamoto H (2016) Role of the NMDA receptor GluN2D subunit in the expression of ketamine-induced behavioral sensitization and region-specific activation of neuronal nitric oxide synthase. Neurosci Lett 610:48–53. https://doi.Org/10.1016/j.neulet.2015.10.049 [DOI] [PubMed] [Google Scholar]
- Zilles K, Schleicher A, Glaser T, Traber J, Rath M (1985) The ontogenetic development of serotonin (5-HT1) receptors in various cortical regions of the rat brain. Anat Embryol 172:255–264. 10.1007/bf00318973 [DOI] [PubMed] [Google Scholar]
- Zorrilla EP (1997) Multiparous species present problems (and possibilities) to developmentalists. Dev Psychobiol 30:141–150. [DOI] [PubMed] [Google Scholar]


