Abstract
Objective
Malignant pleural mesothelioma (MPM) is a lethal malignancy with poor survival and high local recurrence rates despite multimodal therapy with cytoreduction and chemoradiation. We evaluated the anti-tumor efficacy of a paclitaxel-loaded pH-responsive expansile nanoparticle (PTX-eNP) in two clinically relevant murine xenograft models of MPM.
Methods
Luciferase-transfected MSTO-211H human mesothelioma cells were injected into the thoracic cavity of immunodeficient Nu/J mice. Tumor burden was monitored by bioluminescent imaging. Animals were randomized into two models of disease treatment (1) chemotherapy with PTX-eNPs alone delivered locally for “early” limited disease or (2) cytoreductive surgery plus local PTX-eNP chemotherapy for advanced disease. Within each disease model, anti-tumor efficacy of PTX-eNP was compared against standard formulation paclitaxel (PTX-C/E) and drug-empty nanoparticles (unloaded-eNP). Impact on survival was calculated. Fluorescently labeled PTX-eNPs and immunohistochemistry evaluated in vivo drug localization to tumor.
Results
Intrathoracic injection of MSTO-211H resulted in large tumor deposits distributed within the pleural space of the murine thoracic cavity. Local multi-dose treatment with PTX-eNPs alone in limited stage disease more than doubled survival compared to unloaded-eNP (p≤0.0001) and PTX-C/E (p=0.0004). In the model of advanced disease, local multi-dose treatment with PTX-eNPs following cytoreductive surgery also prolonged survival by 126% and 69.4% compared to unloaded-eNP (p=0.0018) and PTX-C/E (p=0.03457), respectively. Immunohistology demonstrated PTX-eNP accumulation within tumor cells in vitro and in vivo.
Conclusion
Local delivery of paclitaxel via expansile nanoparticles confers prolonged survival in a murine model of MPM as single modality treatment for limited disease and in combination with cytoreductive surgery for advanced disease.
INTRODUCTION
Malignant mesothelioma is a highly aggressive malignancy of the serosal membranes. Malignant pleural mesothelioma (MPM) constitutes 80% of mesothelioma cases, and incidence rates are increasing worldwide.1 MPM is often diagnosed at an advanced stage, carrying a poor prognosis with survival of less than one year from time of diagnosis.2 Treatment involves a multimodality approach that includes surgery and adjuvant therapy.1,3 Unfortunately, anatomic limitations and the diffuse nature of the disease means that it is essentially impossible to achieve complete R0 (i.e., microscopically negative) margins.4 Instead, the goal of surgery is macroscopic complete resection. Surgical debulking can revert disease to a microscopic stage with adjuvant chemotherapy, with or without radiation, used for additional disease control.1,3,5 However, despite this aggressive approach, locoregional recurrence remains a significant cause of morbidity and mortality in MPM with recurrence rates of 12–65%.6
Efficacy of systemically administered chemotherapy to treat residual or locally recurrent tumor is limited due to short local residence time, low intratumoral penetration, rapid systemic clearance and severe dose-limiting off-target effects. Therefore, locoregional delivery of chemotherapy is being explored to increase intratumoral drug levels while reducing systemic toxicity. Intracavitary chemotherapy, for example, has been shown to deliver significantly higher doses of drug locally for MPM.7–9 While taxane-based intracavitary chemotherapy has seen positive results in multiple large clinical trials for optimally debulked ovarian cancer10–11, this approach has been investigational for MPM.
An emerging alternative approach for potentially achieving higher intratumoral drug concentrations utilizes polymer-based drug release platforms such as fibrin glues, hydrogels, and polymeric nanoparticles.12–13 Previously, our group demonstrated markedly improved anti-tumor efficacy of paclitaxel-loaded pH-responsive expansile nanoparticles (PTX-eNPs) in a murine xenograft model of malignant peritoneal mesothelioma. Intraperitoneal delivery of PTX-eNPs decreased tumor burden and significantly prolonged survival compared to an equivalent dose of the standard clinical formulation of paclitaxel (i.e., Taxol).14–15
However, intrathoracic mesothelioma represents the overwhelming majority of mesothelioma cases and carries a significantly worse prognosis than peritoneal mesothelioma16, and there have been few investigations of these polymer-based drug release platforms in the thoracic cavity. This study therefore investigates the effectiveness of PTX-eNPs against mesothelioma in this setting using two novel murine treatment models of intrathoracic mesothelioma. Clinically, patients diagnosed with MPM early (i.e., with minimal disease) logically fare better than patients presenting with bulky late-stage disease, highlighting that treatment options and prognosis are directly related to the extent of disease at presentation.2 Therefore, we established two clinically relevant murine xenograft models of intrathoracic human mesothelioma and evaluated the efficacy of PTX-eNPs in both a non-surgical treatment regimen designed to mimic treatment of early-stage, low-volume disease (“early”), as well as a cytoreductive surgical approach to mimic the clinical treatment of locally advanced intrathoracic disease (“late”). We hypothesize that PTX-eNPs will be well tolerated within the pleural cavity, facilitate intratumoral delivery of paclitaxel to sites of mesothelioma in the chest and result in decreased tumor burden, delayed disease progression and, ultimately, improved survival in both early and late disease models.
METHODS
Cell lines
A human malignant pleural mesothelioma cell line, MSTO-211H (ATCC, Manassas, VA), and the luciferase transfected line, MSTO-211H-luc (J. Rheinwald, Harvard Medical School, Boston, MA), were maintained at 37 °C and 5% CO2 in complete RPMI 1640 supplemented with 10% (v/v) fetal bovine serum, streptomycin (100 mg/mL), and penicillin (100 units/mL).
Nanoparticle synthesis
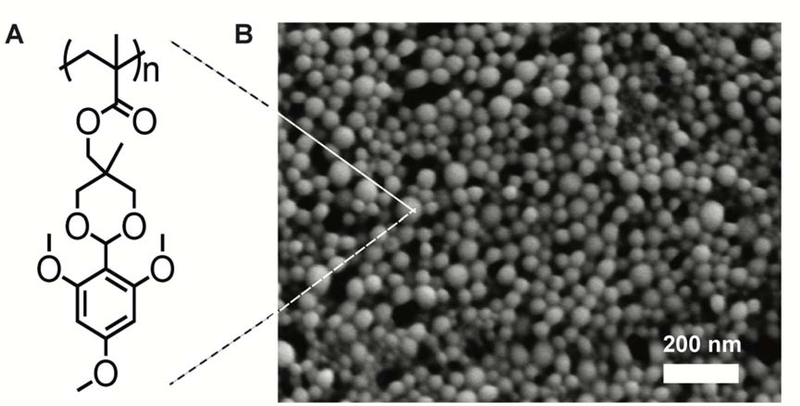
Paclitaxel-loaded and unloaded expansile nanoparticles (PTX-eNPs and unloaded-eNPs, respectively) were synthesized using a mini-emulsion polymerization technique17 with quantification of paclitaxel loading performed according to previously published protocols.18 These nanoparticles measured 30- to 50-nm in size under scanning electron microscopy. Fluorescently-labeled nanoparticles (Rho-eNPs) incorporated rhodamine B (Polysciences Inc., Warrington, PA) into the polymer backbone during polymerization. Oregon Green® 488-conjugated paclitaxel (OG-PTX; Invitrogen) was encapsulated within Rho-eNPs (OG-PTX-Rho-eNPs) in the same manner as non-fluorescently labeled paclitaxel.18
Intrathoracic human mesothelioma xenograft models of early and late tumor burdens
Animal care and procedures were conducted with the approval of the Animal Care and Use Committee of the Dana-Farber Cancer Institute in strict compliance with federal and institutional guidelines. Under isoflurane anesthesia and in the right lateral recumbent position, female Nu/J mice (Jackson Laboratory, Bar Harbor, ME) were injected at the fifth intercostal space with 106 MSTO-211H-luc cells using a 27-gauge blunt tip needle fitted with a plastic sleeve to limit insertion depth to <5-mm for intrapleural injection. Animals were randomly assigned to the “early” stage disease model cohort where chemotherapy was initiated on day 3 after intrathoracic tumor injection or to the “late” stage disease model where tumor progressed for 8 days, at which time cytoreductive surgery followed by adjuvant chemotherapy treatment was instituted.
Intrathoracic drug administration
Tumor-bearing animals were randomly assigned to receive intrathoracic treatment with unloaded-eNPs or 4-mg/kg paclitaxel either suspended in a solution of 1:1 Cremophor EL and absolute ethanol (PTX-C/E) or encapsulated within eNP (PTX-eNPs). Four mg/kg was the highest single dose that could be given in the 100-uL maximum volume allowable within the constraints of the murine chest cavity and injected in the same manner as described above with tumor cell injection. Animals were monitored daily for clinical signs of toxicity (as assessed by appearance and activity) and sacrificed upon evidence of clinically morbid disease progression.
Bioluminescent imaging
Bioluminescent imaging (BLI) was performed under isoflurane anesthesia. Following intraperitoneal injection of 2.25-mg firefly luciferin, images were taken with 10-second exposure time with a Xenogen IVIS-50 bioluminescence camera (Caliper Life Sciences, Hopkinton, MA).
Cytoreductive surgery and pneumonectomy
Animals randomized to the “late” stage disease cohort were anesthetized using ketamine (120 mg/kg, IP) and xylazine (10 mg/kg, IP) and intubated with a 20-gauge IV catheter (BD Angiocath, Becton Dickinson) connected to a MiniVent 845 animal ventilator (Harvard Apparatus). Via a 10-mm thoracotomy incision, all visible intrapleural tumor was removed followed by left hilar ligation with 5–0 silk suture and left pneumonectomy. Ventilation tidal volume was reduced by 30%. Animals were then randomized to an adjuvant drug treatment group (unloaded-eNP, PTX-C/E, or PTX-eNP) administered in the thoracic cavity, and the incision was closed. Subsequent doses were given by intrathoracic injection, and clinical signs of morbidity and survival were monitored as described above.
Nanoparticle uptake studies
Twenty-thousand MSTO-211H-luc cells were seeded in 35-mm glass bottom dishes (MatTek Corporation, Ashland, MA) with complete RPMI-1640 before incubation with OG-PTX-Rho-eNPs (50 μg/ml polymer concentration, 24 hours, 37 °C). Dishes were washed with phenol red-free Hank’s Buffered Saline Solution (HBSS) to remove adherent particles before fixation with 2% formaldehyde, staining with 0.2 μg/mL Hoechst 33342 (Life Technologies, Carlsbad, CA) at room temperature, and mounting with Prolong Gold Anti-Fade (Invitrogen). Confocal microscopic images were obtained with Zeiss LSM510 inverted confocal laser scanning microscope with Plan-Apochromat 10x/0.45 for frozen tissues or C-Apochromat 40×1.2W corrective lens for cultured cells (Carl Zeiss Microscopy, Thornwood, NY).
In vivo localization of eNPs to mesothelioma
Fourteen days after intrathoracic tumor inoculation, animals were given 100-μL intrathoracic injection of OG-PTX-Rho-eNPs and sacrificed four days later. The chest cavity was photographed under ambient and ultraviolet (254 nm) light from a Wood’s lamp. Following gross imaging, tumor was embedded in OCT (Tissue Tek), snap frozen in 2-methylbutane cooled by liquid nitrogen, stored at −80°C and sectioned at 5-μm thickness. After rehydration with HBSS, tissue slides were counterstained with 0.2 μg/mL Hoechst 33342.
Statistics
All computations were performed by Prism 5.0 software. Median survival between treatment groups was compared by Kaplan-Meier method. All significance tests and quoted p-values are two-sided with p<0.05 considered significant.
RESULTS
Characterization of mesothelioma xenograft models as a function of tumor burden
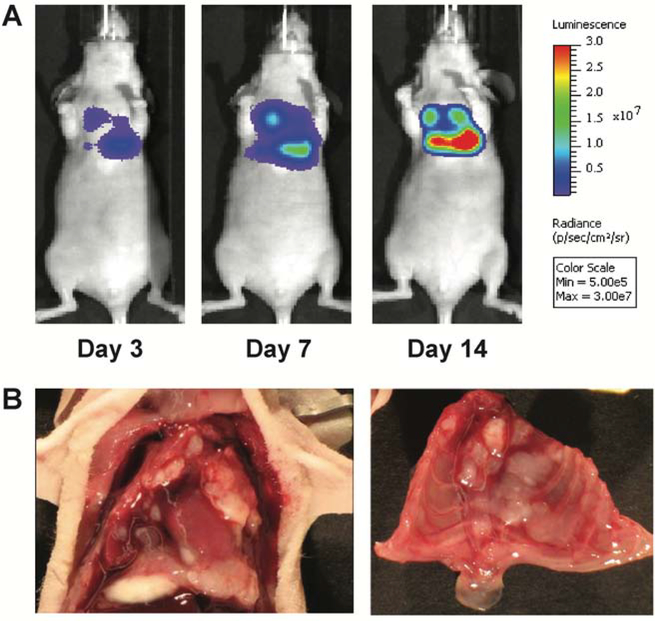
The human mesothelioma cell line MSTO-211H exhibits aggressive biphasic disease in previous murine peritoneal xenograft models, and we desired to recapitulate similar disease characteristics in the chest to evaluate the treatment efficacy in this clinically challenging subset for which surgery is not favored.19 We utilized the luciferase-transfected reporter cell line, MSTO-211H-luc, administered as a single injection of 106 cells into the intrapleural space of immunodeficient Nu/J mice to establish disease. The luciferase reporter permitted in vivo qualitative monitoring of tumor establishment, disease burden, and treatment response by serial BLI. Pleural mesothelioma was established in the chest as early as day 3. An increase in signal intensity was noted over 14 days, correlating with rapid tumor growth within the thorax (Figure 1A). Representative necropsy at day 20 revealed multiple bilateral large tumor deposits diffusely distributed within the pleural lining of the chest cavity (Figure 1B). Compared to our peritoneal mesothelioma model14, 20, this pleural model exhibited a more aggressive and accelerated disease course despite using 5-fold fewer tumor cells, thus modeling the greater clinical aggression of MPM seen clinically.
Figure 1. Murine orthotopic xenograft model of malignant pleural mesothelioma.
(A) After intrathoracic (IT) injection of 106 MSTO-211H-luciferase cells, animals were serially imaged on days 3, 7, and 14. Representative images show that tumor is clearly established in the chest at day 3 with progression to significant tumor burden over time. (B) Representative necropsy findings show multiple pleural tumor deposits diffusely distributed throughout the bilateral chest cavities.
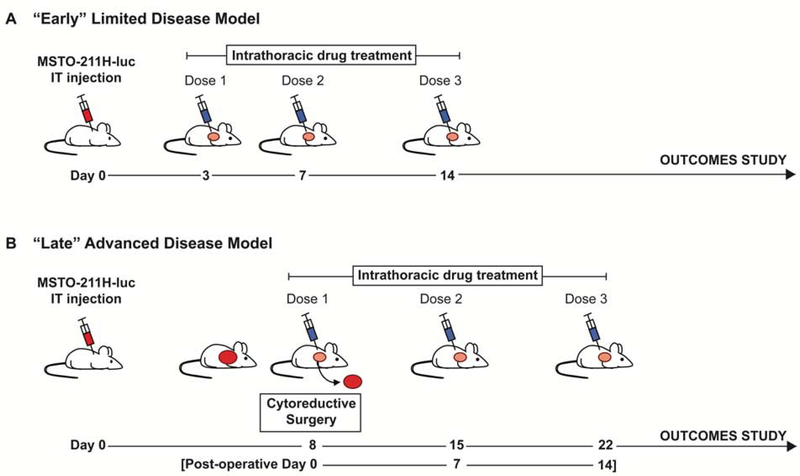
Using these orthotopic xenografts, we investigated the efficacy of PTX-eNP (Figure 2) in the setting of two different disease stages and their corresponding treatment models. The “early”, non-surgical model (Figure 3A) reflected a clinical scenario in which disease is diagnosed via effusion with positive cytology and detectable low volume disease on initial imaging. BLI demonstrated that pleural disease was established as early as 3 days. Therefore, drug treatment was initiated on day 3 in this early limited disease model. The “late” advanced stage surgical model (Figure 3B) mimicked the more common clinical scenario in which disease is diagnosed at a more locally advanced stage. In this model, disease progressed for a longer duration after MSTO-211H-luc injection, therefore developing greater tumor burden that was surgically debulked on day 8 followed by multi-dose adjuvant chemotherapy.
Figure 2. Expansile nanoparticle (eNP) structure.
(A) Chemical structure of the expansile nanoparticle polymer into which hydrophobic paclitaxel drug is incorporated. (B) Scanning electron micrograph shows the spherical shape and size variation of eNPs, which range between 30- and 50-nm.
Figure 3. Treatment models for “early” limited and “late” advanced stage murine malignant pleural mesothelioma.
Pleural mesothelioma is established by intrathoracic (IT) injection of MSTO-211H-luciferase (MSTO-211H-luc) cells, and animals are randomized to two different disease stage models and their corresponding treatment. (A) Experimental design for multi-dose drug treatment for “early” minimal disease. After intrathoracic injection of MSTO-211H-luciferase cells, the first intrathoracic drug dose is administered on day 3 when disease is known to be established. Subsequent drug doses are given on days 7 and 14. Animals are then monitored for survival. (B) Experimental design for the “late” disease model which includes surgical cytoreduction followed by multi-dose adjuvant drug therapy. Disease is allowed to progress until day 8, at which time cytoreductive surgery (resection of visible tumor deposits and pneumonectomy) is performed immediately followed by the first dose of intrathoracic adjuvant drug therapy. The remaining two doses are administered at weekly intervals on post-operative day 7 and 14. Animals are then monitored for survival.
PTX-eNPs decrease tumor burden and improve survival in limited stage mesothelioma
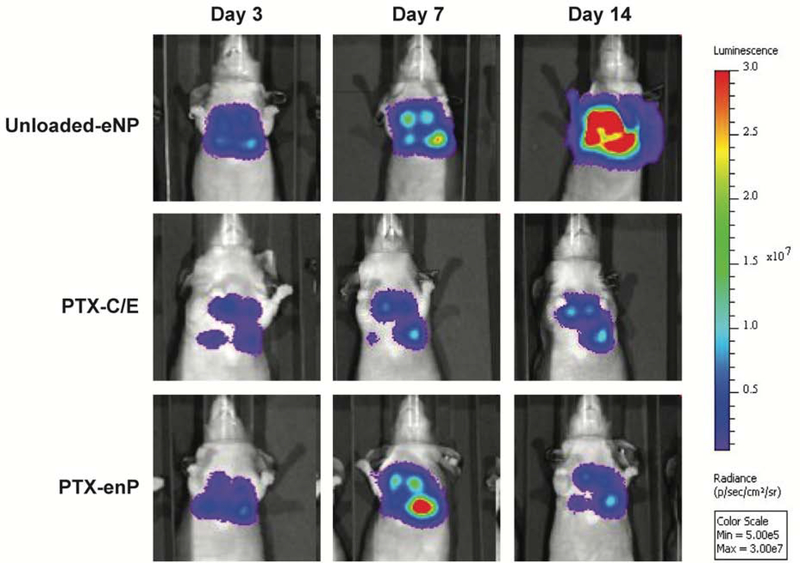
Tumor-bearing Nu/J mice randomized to the “early” limited stage cohort received intrapleural administration of 4-mg/kg/dose paclitaxel given as either standard PTX-C/E (n=6, i.e. the clinically-used formulation of Taxol) or encapsulated in PTX-eNPs (n=12) on days 3, 7, and 14 following tumor inoculation (Figure 3A). Untreated controls received intrathoracic injections of unloaded-eNPs (n=11) at the same time points. Compared to unloaded-eNPs, animals treated with either PTX-C/E or PTX-eNP demonstrated an overall lower tumor burden as evidenced by both smaller bioluminescent signal area and by lower signal intensity. Although PTX-C/E appeared initially to be more effective than PTX-eNP at day 7, tumor burden was reduced in the PTX-eNP group by day 14 resulting in comparable tumor burden to the PTX-C/E group as demonstrated by similar bioluminescent intensities (Figure 4).
Figure 4. Multi-dose intrathoracic treatment with PTX-eNPs decreases MSTO-211 tumor burden.
Panels display representative serial bioluminescent images of pleural MSTO-211H-luciferase implants in animals treated with unloaded-eNPs (no drug expansile nanoparticle control), PTX-C/E (standard formulation paclitaxel) and PTX-eNPs (paclitaxel-loaded expansile nanoparticles) for three doses. PTX-C/E and PTX-eNP were given at dose equivalents of 4mg/kg/dose paclitaxel. At day 7, PTX-C/E-treated animals demonstrate the lowest qualitative tumor burden as indicated by smaller area of bioluminescence and lower overall signal intensity. At day 14, tumor burden in PTX-C/E- and PTX-eNP-treated animals is significantly decreased compared to no drug control (unloaded-eNP). Tumor burden is comparable between PTX-C/E and PTX-eNP treatment groups at day 14.
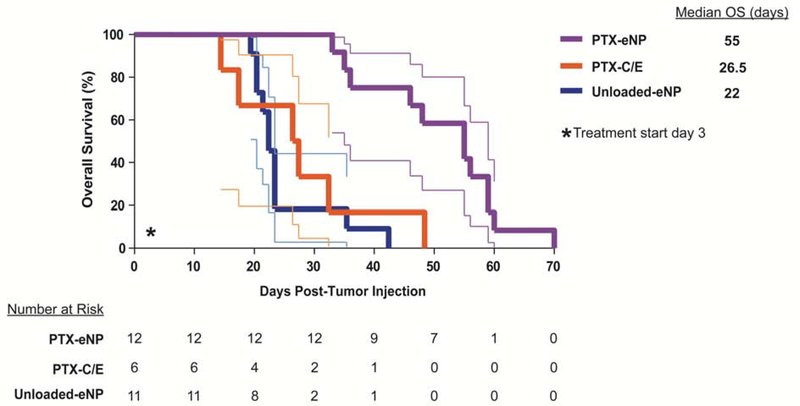
Notably, this initial improvement increased with time, likely due to the cumulative intratumoral release of paclitaxel from PTX-eNPs over time as compared to the immediate effects of locally injected PTX-C/E.11 Specifically, the median overall survival (OS) for PTX-eNP-treated animals was 55 days, more than double the 26.5 days seen in PTX-C/E-treated animals despite both groups receiving an equivalent dose of paclitaxel (p=0.0004). Interestingly, for the PTX-C/E treatment, the initially favorable anti-tumor response at day 7 did not translate into a significant survival advantage over untreated mice that received unloaded-eNPs whose median OS was 22 days (p≤0.0001) (Figure 5).
Figure 5. Local multi-dose treatment with PTX-eNPs prolongs survival in a model of limited stage pleural mesothelioma.
On day 3 following disease establishment via intrathoracic injection of MSTO-211H-luc cells, animals were randomized to intrathoracic injections of the following treatments for three doses: PTX-eNPs (n=12; paclitaxel-loaded expansile nanoparticles) at 4-mg/kg/dose, PTX-C/E (n=6; standard formulation paclitaxel) at 4-mg/kg/dose, or unloaded-eNPs (n=11; no drug expansile nanoparticle control). Survival more than doubled in mice treated with PTX-eNPs with a median overall survival (OS) of 55 days, as compared to 26.5 and 22 days following treatment with equivalent paclitaxel dosing of PTX-C/E (p=0.0004) or unloaded-eNP (p≤0.0001), respectively. Thus, PTX-eNP increased median OS by over 100% for PTX-C/E and 150% for unloaded-eNP.
The benefits of cytoreductive surgery are enhanced by PTX-eNPs in a multimodality treatment model for advanced disease
While we have shown that PTX-eNPs significantly increased survival in the early stage disease model, most patients with MPM present with locally advanced disease for which chemotherapy alone is ineffective. Treatment regimens consisting of cytoreductive surgery to remove bulky tumors and subsequent adjuvant chemotherapy have met with limited success due to a high rate of locally recurrent disease. We hypothesized that the improved efficacy of PTX-eNPs observed against low volume disease may also translate to improved efficacy after cytoreduction. Therefore, to model “late” stage disease, tumor was allowed to grow to day 8 at which time gross tumor deposits were resected. To account for possible cytoreductive variability, all animals underwent a pneumonectomy and were post-surgically randomized to a multi-dose adjuvant chemotherapy regimen, receiving either unloaded-eNP, PTX-C/E (4-mg/kg/dose) or PTX-eNPs (4-mg/kg/dose) via intrathoracic injection on post-operative day 0, 7, and 14 (i.e. post-tumor inoculation days 8, 15 and 22; Figure 3B).
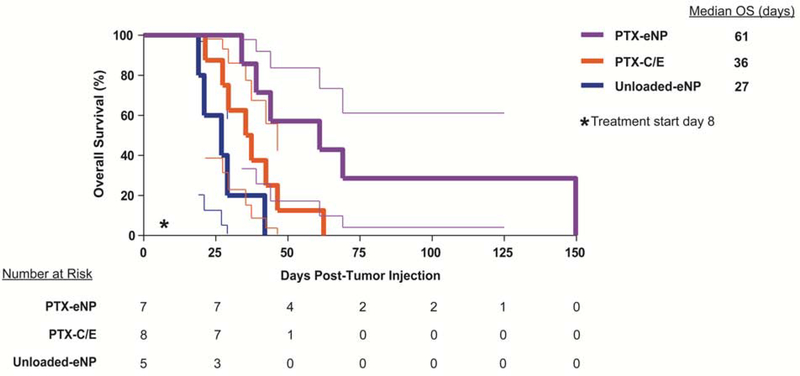
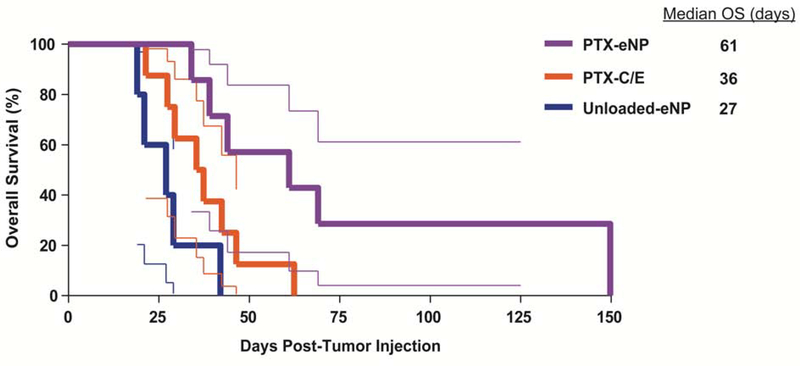
Despite the initiation of treatment in the setting of more advanced disease, survival in the setting of cytoreductive surgery was prolonged in all groups as compared to non-surgical treatment in the “early” limited disease model. Median OS in animals treated with unloaded-eNPs following cytoreductive surgery was 27 days as compared to 22 days in animals with early stage disease treated without surgery. Similarly, median OS (36 versus 26.5 days) was greater in animals treated for more advanced disease with the combination of cytoreductive surgery and PTX-C/E. The greatest survival benefit, however, was seen in animals treated with PTX-eNPs after cytoreductive surgery with survival beyond 140 days and a median OS of 61 days, resulting in a 126% greater median OS than unloaded-eNPs (p=0.0018) and 69.5% greater than PTX-C/E (p=0.0357) groups (Figure 6).
Figure 6. Local multi-dose treatment with PTX-eNPs prolongs survival following cytoreductive surgery as compared to PTX-C/E.
On day 8 after establishment of pleural mesothelioma tumors via intrathoracic injection of MSTO-211H-luciferase cells, animals underwent cytoreductive surgery then post-surgically randomized to adjuvant treatment with 4mg/kg/dose PTX-eNPs (n=7, paclitaxel-loaded expansile nanoparticles), an equivalent paclitaxel dose of PTX-C/E (n=8; standard formulation paclitaxel), or unloaded-eNPs (n=5; no drug expansile nanoparticle control). The first dose was given at the time of surgery, and subsequent treatments were given on days 15 and 22 (post-operative days 7 and 14). Animals treated with PTX-eNPs exhibited prolonged survival with a median overall survival (OS) of 61 days as compared to median OS of only 36 and 27 days for animals treated with PTX-C/E (p=0.0357) or unloaded-eNPs (p=0.0018), respectively. This represents an increase in median OS of 69.4% over standard PTX-C/E and 126% over no drug treatment (unloaded-eNP).
PTX-eNPs demonstrate prolonged accumulation within mesothelioma cells in vitro and in vivo
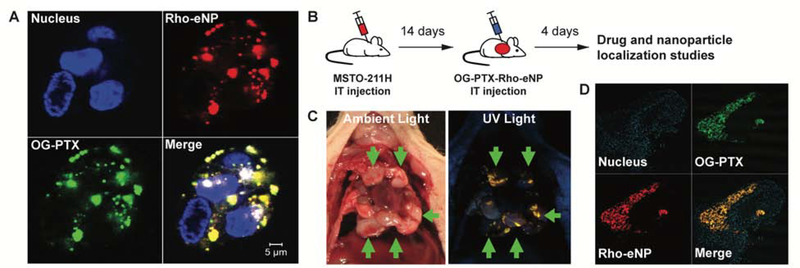
We hypothesized that the improved survival observed in animals treated with PTX-eNP in both the limited disease (non-surgical) and advanced disease (cytoreductive surgery) models was related to the unique drug delivery properties of the PTX-eNP formulation - namely, prolonged release and tumor-localized drug delivery. To evaluate our hypothesis, rhodamine-labeled eNPs (Rho-eNP) containing paclitaxel conjugated to the Oregon-Green fluorophore (OG-PTX) were synthesized (OG-PTX-Rho-eNPs) to allow visualization of both polymer (eNP) and drug (PTX) components of the nanoparticle. After 24-hour co-incubation of OG-PTX-Rho-eNPs with MSTO-211H cells in vitro, confocal microscopy showed Rho-eNPs (red) and OG-PTX (green) to be present within the cytoplasm of MSTO-211H tumor cells, confirming intracellular uptake. Merged fluorescence demonstrated that polymer and drug were co-localized (yellow) confirming that paclitaxel entered the cell while encapsulated within the eNP (Figure 7A) allowing paclitaxel drug release in the cytoplasm resulting in tumor cytotoxicity.
Figure 7. PTX-eNPs accumulate within cells in vitro and concentrate at sites of tumor in vivo.
(A) Paclitaxel and the polymer components of paclitaxel expansile nanoparticles (PTX-eNPs) were labeled with the respective fluorophores Oregon Green (OG-PTX) and rhodamine (Rho-eNP) and co-incubated with MSTO-11H cells. Representative confocal images show that OG-PTX-Rho-eNPs accumulate within MSTO-211H cells after 24 hours of in vitro co-incubation. (B) Experimental design to assess in vivo localization of OG-PTX-Rho-eNPs to tumor. Fourteen days after establishment of MSTO-211H xenografts, animals received an intrathoracic (IT) injection of OG-PTX-Rho-eNPs. Animals were euthanized 4 days later for drug and nanoparticle co-localization studies. (C) High-resolution photographs were taken of the opened chest containing multiple tumor deposits under ambient light and long-wave ultraviolet (UV) light. Rho-eNPs, which appear yellow-orange under UV light, are concentrated at the sites of tumor within the thoracic cavity (arrows). (D) Frozen histology sections of tumor tissue under fluorescence microscopy reveal accumulation and co-localization of Rho-eNPs and OG-PTX within tumor demonstrating co-localization of both particle and paclitaxel drug delivery to tumor.
Similarly, we assessed eNP localization and paclitaxel delivery to tumors in vivo via intrapleural administration of OG-PTX-Rho-eNP 14 days after establishment of MSTO-211H xenografts (Figure 7B). Four days after OG-PTX-Rho-eNP injection, imaging with long-wave ultraviolet light demonstrated that OG-PTX-Rho-eNPs concentrated to sites of tumor (Figure 7C). Intratumoral penetration of OG-PTX-Rho-eNPs and eNP-mediated delivery of OG-PTX directly to the tumor was also confirmed on tissue histology (Figure 7D).
DISCUSSION
Malignant pleural mesothelioma is an aggressive cancer that is very difficult to treat. Due to the presence of large bulky tumors, local invasion, and anatomic limitations, residual microscopic disease is nearly always present despite aggressive cytoreductive surgery. The addition of adjuvant chemotherapy, with or without radiation, aims to improve local control, but locoregional recurrence remains the primary cause of death.3,6
Conventional systemic (i.e., intravenous) administration of paclitaxel results in broad drug distribution with relatively low drug accumulation within the tumor itself, with >75% of drug excreted within 48 hours.21 Consequently, off-target toxicity limits the maximum dose that can be administered. Furthermore, relatively high surgical morbidity and mortality prevents many patients from completing trimodal therapy. We have previously shown that expansile nanoparticles result in prolonged drug delivery with nearly a 100-fold increase in local drug concentrations within tumor compared to levels achieved following systemic administration.15 This intratumoral delivery of high-dose chemotherapy with prolonged release kinetics has the potential to minimize off-target effects, allow early initiation of therapy, decrease the incidence of local recurrence, and improve survival.
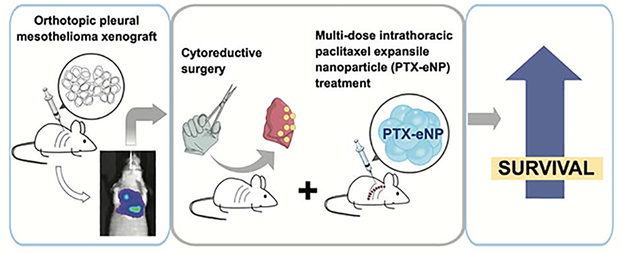
Our present study demonstrates that nanoparticle-based delivery of chemotherapy in the thoracic cavity is feasible, safe, and superior to standard chemotherapy in a murine model of both limited and advanced stage MPM. Our key findings include: (1) PTX-eNPs effectively decrease tumor burden in vivo; (2) local, multi-dose intrathoracic treatment with PTX-eNPs in limited stage disease prolongs survival; (3) cytoreductive surgery with adjuvant multi-dose intrathoracic treatment with PTX-eNPs significantly prolongs survival for locally advanced disease (Figure 8); (4) PTX-eNP localizes to and exhibits sustained presence in tumor tissue in vivo; and, (5) intrathoracic administration of multiple doses of PTX-eNPs is well-tolerated.
Figure 8. Local multi-dose treatment with paclitaxel-loaded expansile nanoparticles prolongs survival after cytoreductive surgery in an orthotopic xenograft model of locally advanced pleural mesothelioma.
Pleural mesothelioma is established by intrathoracic injection of the human aggressive and biphasic mesothelioma cell line MSTO-11H. Locally advanced disease in the chest is visualized by bioluminescent imaging (via a luciferase reporter transfected in the cell line). Cytoreductive surgery with pneumectomy is performed to remove visible disease followed by three doses of paclitaxel-loaded expansile nanoparticles (PTX-eNP) administered into the thoracic cavity. This treatment resulted in significantly increased survival.
Several groups have described murine orthotopic xenograft models of early pleural mesothelioma.22–24 We used the human mesothelioma MSTO-11 cell line with a luciferase reporter to allow for non-invasive and continuous assessment of disease progression and treatment response, which contrasts with studies that assessed these parameters at the time of sacrifice. Intrathoracic injection of these MSTO-11H-luc cells produced reliable establishment of homogeneous disease and progression, allowing for successful cytoreductive surgery to be performed. Our observation that MSTO-211H is significantly more aggressive in the thorax than in the abdomen is consistent with the clinically observed aggression of pleural versus peritoneal mesothelioma, thus further validating this model. BLI at multiple time points as well as survival data shows that PTX-eNPs are effective against this aggressive and biphasic type of mesothelioma. This is important given that biphasic mesothelioma is a extremely difficult subset of mesothelioma to treat as surgery is generally not recommended for these patients. To reflect a more challenging clinical scenario, the late, advanced stage model allowed assessment of PTX-eNPs as adjuvant therapy following cytoreduction. This novel approach involving a model of surgical debulking shows a clear difference in disease response between non-drug treated controls, conventional PTX-C/E, and PTX-eNP as adjuvant therapy.
We have previously shown that eNPs can accumulate within malignant MSTO-211H cells in vitro in as quickly as two hours, exhibiting both faster and greater intracellular uptake than non-malignant cells.15 In the current study, we provide evidence of preferential in vivo accumulation of PTX-eNPs to tumor deposits within the chest and the sustained presence of both drug and eNP components intracellularly over at least 4 days. The localization of particles to tumor results from the eNP characteristic termed Materials-Based Targeting.13 The exact mechanism by which adjuvant intrapleural treatment with PTX-eNP is superior to an equivalent dose of intrapleural PTX-C/E remains under investigation, but prior studies demonstrate that rapid PTX-eNP uptake by malignant mesothelial cells occurs via endocytosis15, 25, with subsequent endosomal pH-triggered swelling of the eNP initiating slow and controlled release of paclitaxel intracellularly.17 This swelling prevents expulsion of the now larger nanoparticle which accumulates within autophagosomes and inhibits lysosome-mediated degradation. 15 Paclitaxel remains trapped within the tumor cell regardless of cell cycle and can also induce apoptosis25, thereby increasing susceptibility compared to an equivalent dose of paclitaxel delivered systemically which is rapidly cleared. Therefore, the net effect is enhancement of paclitaxel-induced cytotoxicity by virtue of greater intratumoral drug concentration and sustained local paclitaxel exposure.
It is notable that bioluminescence on day 7 in PTX-eNP-treated animals is increased and similar to that of untreated controls while PTX-C/E-treated animals exhibit stable bioluminescence at this early time point. This observation may reflect the immediate cytotoxic action of paclitaxel delivered in its free form (PTX-C/E) while minimal anti-tumor response is observed on day 7 with PTX-eNP due to the known absence of drug burst release kinetics from these eNPs. Instead, the anti-tumor effect of PTX-eNPs becomes more apparent on day 14 with an observable decrease in bioluminescence, supporting the hypothesis that subsequent continued release of paclitaxel from PTX-eNPs allows for prolonged effectiveness over time. Importantly, the increased survival seen with PTX-eNPs over all other therapies suggests that prolonged eNP-mediated release of paclitaxel is more effective and that high cumulative doses are well tolerated without prohibitive clinical toxicity or morbidity.
We acknowledge that paclitaxel monotherapy is not first line for pleural mesothelioma due to historically poor clinical response. We have hypothesized that this “clinical resistance” may in part be due to the inadequate dosing or ineffective delivery kinetics associated with systemic paclitaxel administration.10 Although platinum-based chemotherapies are more commonly used, the rationale for prolonged local cavitary delivery of paclitaxel after cytoreduction for mesothelioma explored in this study parallels the clinical work reported by Sugarbaker et al. In these studies, the addition of intraperitoneal paclitaxel in the early post-cytoreduction period and long-term intraperitoneal paclitaxel for patients with peritoneal mesothelioma resulted in a 31% increase in 5-year survival compared to patients treated with cytoreductive surgery and heated perioperative chemotherapy with doxorubicin/cisplatin alone.26 We hypothesized that the prolonged local delivery of high-dose paclitaxel by eNP may prove effective against more aggressive pleural mesothelioma, particularly in the setting of cytoreduction, while avoiding adjuvant radiation therapy and obviating the need for an indwelling catheter and long-term cavitary lavage in a patient population already challenged by significant morbidity. Based on the success of PTX-eNPs in the current study, we are working on developing additional nanoparticle constructs to allow encapsulation of drugs such as pemetrexed, gemcitabine, doxorubicin, and cisplatin/carboplatin. Thus, the novelty of our study is not so much in drug selection but in the method of achieving prolonged intratumoral delivery of drug via eNP that translates to an improvement in survival. The success of PTX-eNPs also supports the notion that prolonged high-dose paclitaxel delivered directly to tumor can enhance cell death in “poor responding” tumors, thus opening the door for possible repurposing of drugs for tumors previously deemed “clinically resistant”.
In summary, the current study establishes two clinically relevant murine xenograft models of intrathoracic mesothelioma that reflect early treatment of low volume mesothelioma versus adjuvant therapy following surgical cytoreduction for advanced disease. Local administration of PTX-eNPs confers a marked improvement in survival compared to an equivalent dose of locally administered PTX-C/E. This improved in vivo efficacy is seen in the setting of a slow and sustained mechanism of drug release coupled with high-dose local concentrations focused within the tumor cells. Our findings further validate nanoparticle drug delivery as a feasible and effective strategy for the treatment of microscopic tumor and for prevention of tumor recurrence after surgical resection.
CENTRAL MESSAGE
Delivery of paclitaxel via nanoparticles confers prolonged survival in pleural mesothelioma as single modality therapy for limited disease and in combination with cytoreduction for advanced disease.
CENTRAL FIGURE LEGEND
Treatment with paclitaxel nanoparticles (PTX-eNPs) after cytoreduction prolongs survival.
PERSPECTIVE STATEMENT.
Locoregional chemotherapy is an important adjunct in the treatment of pleural mesothelioma after cytoreductive surgery. Nanoparticle-based delivery of high dose chemotherapy in the thoracic cavity is feasible and safe and can offer superior survival in the treatment of pleural mesothelioma.
Acknowledgements
The authors express their appreciation to the staff of the Animal Resources Facility at Dana-Faber Cancer Institute for their excellent care of the research animals and assistance with bioluminescent imaging.
Sources of Funding: This research was supported by the National Institutes of Health/National Cancer Institute R0I CA227433 and R01 CA232056, the National Institutes of Health Small Business Innovation Research Program R43 CA189215, R43 CA213538-01A1, and R43 CA2247391 as well as the Michael A. Bell Family Distinguished Chair in Healthcare Innovation (YLC) and the Thoracic Surgery Foundation Resident Research Award (NQC).
Conflict of Interest Statement: YLC has a sponsored research agreement with Canon USA and equipment loan from Stryker Novadaq Industries (both outside of the submitted work). There are two patents issued: “Films and Particles for Delayed and Locoregional Delivery of Agents” (US7671095B2) and “Compliant Composites for Application of Drug Eluting Coatings to Tissue Surfaces” (US8795707B2). MWG has ownership in AcuityBio and Ionic Pharmaceuticals and has the above two patents pending (US7671095B2 and US8795707B2). AHC reports ownership in Ionic Pharmaceuticals. The remaining authors have no disclosures.
GLOSSARY OF ABBREVIATIONS
- MPM
malignant pleural mesothelioma
- PTX
paclitaxel
- eNP
expansile nanoparticle
- PTX-eNP
paclitaxel-loaded expansile nanoparticles
- PTX-C/E
paclitaxel cremaphor-ethanol (Taxol)
- BLI
bioluminescent imaging
- OS
overall survival
- IT
intrathoracic
- IP
intraperitoneal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Rossini M, Rizzo P, Bononi I, Clementz A, Ferrari R, Martini F et al. New perspectives on diagnosis and therapy of malignant pleural mesothelioma. Frontiers in Oncology 2018; 8: 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carbone M, Ly BH, Dodson RF, Pagano I, Morris PT, Dogan UA et. Malignant mesothelioma: facts, myths, and hypotheses. J Cell Physiology. 2012; 227: 44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Opitz I Management of malignant pleural mesothelioma - The European experience. J Thorac Disease 2014; 6 (S2): S238–S252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rice D Surgery for malignant pleural mesothelioma. Annals of Diagnostic Pathology 2009; 13: 65–72. [DOI] [PubMed] [Google Scholar]
- 5.McCambridge AJ, Napolitano A, Mansfield AS, Fennell DA, Sekido Y, Nowak AK et al. Progress in the management of pleural mesothelioma in 2017. Journal of Thoracic Oncology 2018; 13: 606–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mahvi DA, Liu R, Grinstaff MW, Colson YL, Raut CP. Local cancer recurrence: the realities, challenges, and opportunities for new therapies. CA Cancer Journal for Clinicians 2018; 68: 488–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rusch VW, Niedzwiecki D, Tao Y, Menendez-Botet C, Dnistrian A, Kelsen D et al. Intrapleural cisplatin and mitomycin for malignant mesothelioma following pleurectomy: pharmacokinetic studies. J Clin Oncol 1992; 10 (6): 1001–6. [DOI] [PubMed] [Google Scholar]
- 8.Lerza R, Esposito M, Vannozzi M, Bottino GB, Bogliolo G, Pannacciulli I. High doses of intrapleural cisplatin in a case of malignant pleural mesothelioma. Clinical observations and pharmacokinetic analyses. Cancer 1994; 73: 79–84. [DOI] [PubMed] [Google Scholar]
- 9.Mujoomdar AA, Sugarbaker DJ. Hyperthermic chemoperfusion for the treatment of malignant pleural mesothelioma. Semin Thorac Cardiovasc Surg 2008; 20(4): 298–304. [DOI] [PubMed] [Google Scholar]
- 10.Monk BJ, Chan JK. Is intraperitoneal chemotherapy still an acceptable option in primary adjuvant chemotherapy for advanced ovarian cancer? Annals of Oncology 2017. 28 (S8): viii40–viii45 [DOI] [PubMed] [Google Scholar]
- 11.Tewari D, Java JJ, Salani R, Armstrong DK, Markman M, Herzog T et al. Long-term survival advantage and prognostic factors associated with intraperitoneal chemotherapy treatment in advanced ovarian cancer: a gynecologic oncology group study. J Clin Oncology 2015; 33(13): 1460–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolinksy JB, Colson YL, Grinstaff MW. Local drug delivery strategies for cancer treatment: gels, nanoparticles, polymeric films, rods, and wafers. J Controlled Release. 2012; 159: 14–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colby AH, Oberlies NH, Pearce CJ, Herrera VL, Colson YL, Grinstaff MW. Nanoparticle drug-delivery systems for peritoneal cancers: a case study of the design, characterization and development of the expansile nanoparticle. Wiley Interdiscip Rev Nanomed Nanobiotechol. 2017; 9(3): 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Colson YL, Liu R, Southard EB, Schulz MD, Wade JE, Griset AP et al. The performance of expansile nanoparticles in a murine model of peritoneal carcinomatosis. Biomaterials 2011; 32: 832–840. [DOI] [PubMed] [Google Scholar]
- 15.Liu R, Colby AH, Gilmore D, Schulz M, Zeng J, Padera RF et al. Nanoparticle tumor localization, disruption of autophagosomal trafficking, and prolonged drug delivery improve survival in peritoneal mesothelioma. Biomaterials 2016; 102: 175–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Siesling S, van der Zwan JM, Izarzugaza I, Jaal J, Treasure T, Foschi R et al. Rare thoracic cancers, including peritoneum mesothelioma. European Journal of Cancer 2012; 48: 949–960. [DOI] [PubMed] [Google Scholar]
- 17.Griset AP, Walpole J, Liu R, Gaffey A, Colson YL, Grinstaff MW. Expansile nanoparticles: synthesis, characterization, and in vivo efficacy of an acid-responsive polymeric drug delivery system. J Am Chem Soc 2009; 131: 2469–71 [DOI] [PubMed] [Google Scholar]
- 18.Zubris KA, Liu R, Colby A, Schulz MD, Colson YL, Grinstaff MW. In vitro activity of paclitaxel-loaded polymeric expansile nanoparticles in breast cancer cells. Biomacromolecules. 2013. June 10; 14(6): 2074–2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyerhoff RR, Yang CJ, Speicher PJ, Gulack BC, Hartwig MG, D’Amico TA et al. Impact of mesothelioma histologic subtype on outcomes in the Surveillance, Epidemiology, and End Results database. J Surg Res 2015; 196(1): 23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schulz MD, Zubris KA, Wade JE, Padera RF, Xu X, Grinstaff MW et al. Paclitaxel-loaded expansile nanoparticles in a multimodal treatment model of malignant mesothelioma. Ann Thorac Surg 2011; 92: 2007–14. [DOI] [PubMed] [Google Scholar]
- 21.Sparreboom A, Scripture CD, Trieu V, Williams PJ, De T, Yang A, et al. Comparative preclinical and clinical pharmacokinetics of a cremophor-free, nanoparticle albumin-bound paclitaxel (ABI-007) and paclitaxel formulated in cremophor (taxol). Clin Cancer Res 2005; 11(11): 4136–4143. [DOI] [PubMed] [Google Scholar]
- 22.Laszlo V, Valko Z, Kovacs I, Ozsvar J, Hoda MA, Klikovits T et al. Nintedanib is active in malignant pleural mesothelioma cell models and inhibits angiogenesis and tumor growth in vivo. Clinical Cancer Research 2018; OF1–OF12. [DOI] [PubMed] [Google Scholar]
- 23.Colin DJ, Cottet-Dumoulin D, Faivre A, Germain S, Triponez F, Serre-Beinier V. Experimental model of human malignant mesothelioma in athymic mice. Int J of Molec Sciences 2018; 19: 1881–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Opitz I, Lardinois D, Arni S, Hillinger S, Vogt P, Odermatt B et al. Local recurrence model of malignant pleural mesothelioma for investigation of intrapleural treatment. European Journal of Cardiothoracic Surgery 2007; 31: 772–778. [DOI] [PubMed] [Google Scholar]
- 25.Lei H, Hofferberth SC, Liu R, Colby A, Tevis KM, Catalano P et al. Paclitaxel-loaded expansile nanoparticles enhance chemotherapeutic drug delivery in mesothelioma 3-dimensional multicellular spheroids. J Thorac Cardiovasc Surg 2015; 149(5): 1417–24. [DOI] [PubMed] [Google Scholar]
- 26.Sugarbaker PH, Chang D. Long-term regional chemotherapy for patients with epithelial malignant peritoneal mesothelioma results in improved survival. EJSO 2017; 43: 1228–1235. [DOI] [PubMed] [Google Scholar]