Abstract
Rationale.
Understanding factors contributing to individual differences in vulnerability to opioid addiction is essential for developing more effective preventions and treatments, yet few reliable behavioral predictors of subsequent opioid self-administration have been identified in rodents. Sensitivity to the acute effects of initial drug exposure predicts later addiction vulnerability in both humans and animals, but the relationship of sensitivity to withdrawal from initial drug exposure and later drug use vulnerability is unclear.
Objective.
The goal of the current study was to evaluate whether the degree of anhedonia experienced during withdrawal from early opioid exposure predicts subsequent vulnerability to opioid self-administration.
Methods.
Rats were first tested for withdrawal sensitivity following acute injections of morphine (i.e., “acute dependence”), measured as elevations in intracranial self-stimulation (ICSS) thresholds (anhedonia-like behavior) during naloxone-precipitated and spontaneous withdrawal. Rats were then tested for addiction-like behavior using various measures of i.v. morphine self-administration (MSA) including acquisition, demand, extinction, and reinstatement induced by morphine, stress, and/or drug-associated cues.
Results.
Greater naloxone-precipitated withdrawal across repeated morphine injections and greater peak spontaneous withdrawal severity following a single morphine injection were associated with lower addiction-like behavior on multiple MSA measures. Withdrawal-induced anhedonia predicted a wider range of MSA measures than did any individual measure of MSA itself.
Conclusions.
Our data establish WIA as one of the first behavioral measures to predict individual differences in opioid SA in rodents. This model promises to be useful for furthering our understanding of behavioral and neurobiological mechanisms underlying vulnerability to opioid addiction.
Keywords: Intracranial self-stimulation, opioid, withdrawal, anhedonia, self-administration
Introduction
Opioid addiction poses a substantial burden on public health (Center for Behavioral Health Statistics and Quality, 2018; Jones et al., 2018). Identifying behavioral and neurobiological factors contributing to the marked individual differences in opioid addiction vulnerability is essential for developing more effective preventions and treatments (Belin et al., 2016; Wang et al., 2019). However, few behavioral measures have been identified in preclinical models that reliably predict individual differences in i.v. opioid self-administration (SA). SA is often considered the “gold standard” for modeling addiction-like behavior in animals because it involves volitional drug consumption as occurs in humans (Yap & Miczek, 2008).
Sensitivity to the initial acute effects of drugs (e.g., euphoria, aversion) is a key predictor of addiction vulnerability in humans (O’Loughlin et al., 2003; DiFranza et al., 2007; Schuckit et al., 2004) and the direction of the relationship depends on the drug effects measured. For example, greater aversive effects of initial drug exposure can be protective against the subsequent development of addiction (DiFranza et al., 2004; Fowler & Kenny, 2014; Sartor et al., 2010). Similarly, several preclinical studies indicate that sensitivity to the initial effects of acute drug injections (e.g., locomotor activity or depression, antinociception) predict voluntary drug intake in an i.v. SA model (Deminiere et al., 1989; Chappell & Weiner, 2008; Nishida et al., 2016).
Acute drug injections also produce negative affective (emotional) states (e.g., anhedonia, or diminished reward sensitivity) during withdrawal. These withdrawal effects are induced even after a single drug exposure in both humans and animals (“acute dependence”; Harris & Gewirtz, 2004; Liu & Schulteis, 2004; Schulteis et al., 2004; Harris & Gewirtz, 2005), and often become more severe with repeated drug exposures (Engelmann et al., 2004; Harris et al., 2004; Kenny et al., 2003). Some authors have proposed that greater sensitivity to the negative affective consequences of withdrawal may be protective against addiction (Carroll et al., 2008; Dess et al., 2005; Holtz et al., 2015; O’Dell, 2009; O’Dell et al., 2006), and that anhedonia may reduce the motivation for reward-seeking (Wise, 2004). Consistent with these predictions, rats selectively bred for high voluntary alcohol consumption showed lower withdrawal-induced anhedonia (WIA) after initial alcohol exposure (Chester et al., 2006). Similarly, we found that saccharin-preferring rats, which exhibit greater SA of opioids and other drugs (Carroll et al., 2002), exhibit lower WIA during withdrawal from acute morphine exposure (Holtz et al., 2015). Nevertheless, the relationship between sensitivity to withdrawal from acute drug exposure and drug SA has not been directly tested within the same animals or in outbred rats, which can differ from selectively bred rats in terms of determinants of addiction vulnerability (Ambrosio et al., 1995; Zhou et al., 2008).
The goal of this study was to evaluate the ability of WIA to predict individual differences in subsequent i.v. morphine SA (MSA) in outbred rats. WIA was measured as increases in intracranial self-stimulation (ICSS) thresholds, which is one of the most commonly used measures of the anhedonic consequences of withdrawal from opioids and other drugs in rats and has considerable predictive validity (Bruijnzeel et al., 2007; Cahill et al., 2009; Igari et al., 2014). ICSS was also used in the studies discussed above demonstrating reduced WIA in rodents selectively bred for greater addiction vulnerability (Chester et al., 2006; Holtz et al., 2015) Several common measures of MSA (e.g., acquisition, demand, reinstatement) were used because they model distinct aspects of addiction and can be differentially associated with other behavioral predictors of drug SA (Belin et al., 2011; Belin et al., 2008). It was hypothesized that greater WIA severity would be associated with lower MSA vulnerability.
Materials and Methods
Animals
Male adult Sprague Dawley rats (Harlan/Envigo, Indianapolis, IN) weighing 276–300 g at arrival were used. All rats were individually housed in a temperature- and humidity-controlled colony room with unlimited access to water under a reversed 12-h light/dark cycle (lights off at 10:00 hr). All behavioral testing occurred during the dark (active) phase. Beginning one week following arrival, food was restricted to 18 g/day to facilitate operant performance, as well as to avoid detrimental health effects of long-term ad libitum feeding and limit catheter migration as recommended by our veterinarian. All procedures were approved by The Institutional Animal Care and Use Committee (IACUC) of the Hennepin Health Research Institute in accordance with the 2011 NIH Guide for the Care and Use of Laboratory Animals and the 2003 National Research Council Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research.
Overview of experimental protocol
Male adult Sprague-Dawley rats were tested under the experimental protocol shown in Figure 1. Rats were first tested for WIA during naloxone-precipitated and spontaneous withdrawal from acute morphine injections (Phase 1). Rats were then tested for opioid addiction vulnerability using several measures of i.v. MSA including acquisition, elasticity of demand, and reinstatement (Phase 2). Finally, WIA was again tested to provide a preliminary characterization of the relationship between MSA and withdrawal sensitivity during a more advanced stage of dependence (“late-stage dependence”, Phase 3).
Figure 1.
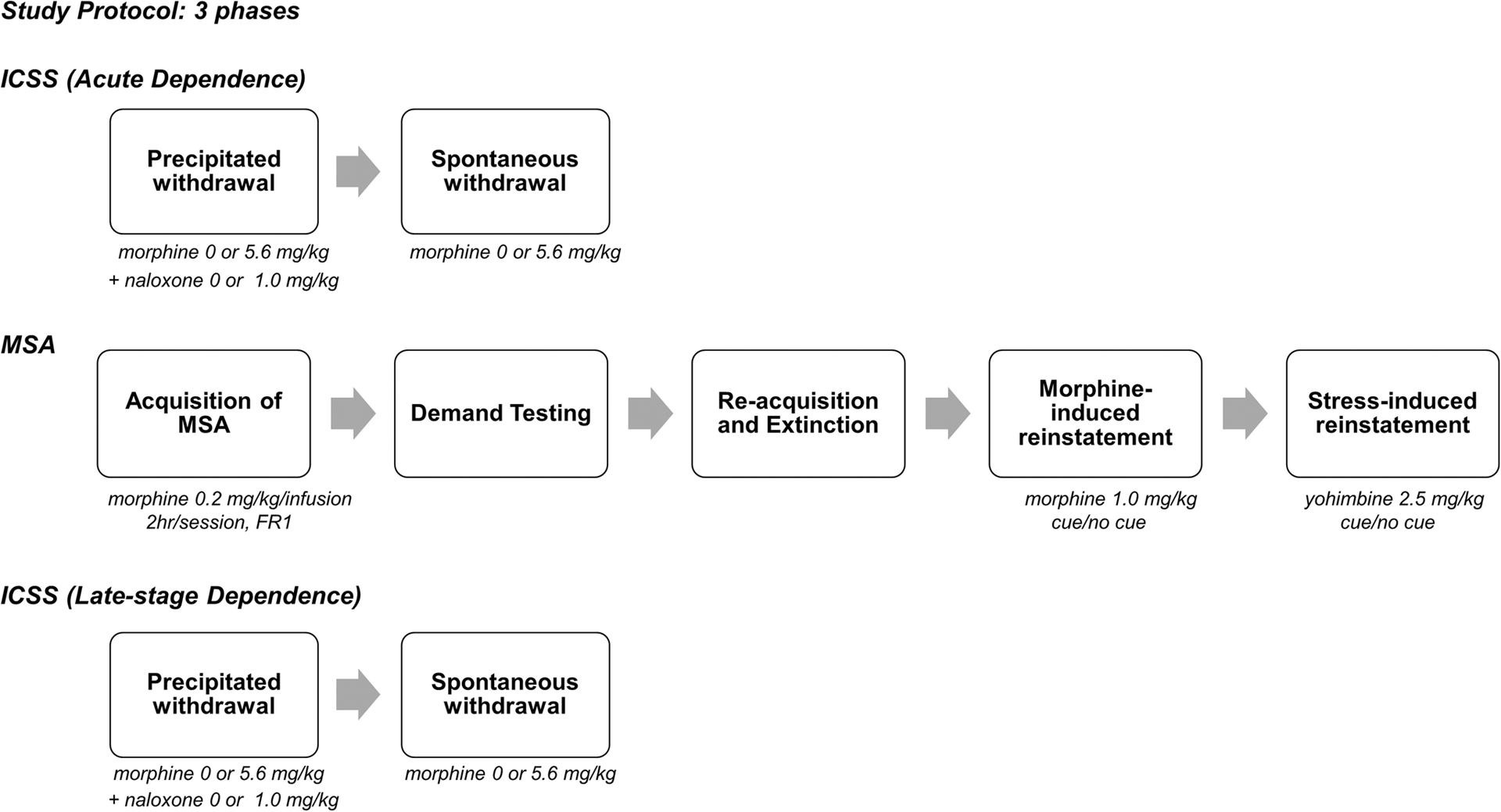
Overview of experimental protocol. During the acute dependence phase (Phase 1), precipitated withdrawal was tested repeatedly over 5 consecutive days. On each day, rats were injected with morphine (0 or 5.6, mg., s.c.), followed 1 hr 50 min later by naloxone (0 or 1.0 mg/kg), and then tested for ICSS 10 min later (length of ICSS session ≈ 45 min). After precipitated withdrawal, rats were injected with morphine (0 or 5.6 mg/kg) and tested for ICSS 2, 6, 26, 30, 50, 64, 74, 98, and 170 hours later (Phase 2). After completion of spontaneous withdrawal testing, all animals were tested using various measures of MSA (e.g., acquisition, demand, reinstatement) in daily 2 hr sessions. Following completion of the MSA protocol, rats were again tested for precipitated and spontaneous withdrawal as described for the acute dependence phase (late-stage dependence, Phase 3).
Acute dependence
Rats were prepared and trained on a discrete-trial ICSS procedure (see Supplemental Material) in daily sessions conducted Mon-Fri until ICSS thresholds were stable (<10% variability over 5 days) and habituated to saline injections as described previously (see Harris et al., 2013). On the first test day, rats were injected with morphine sulfate (MOR, 0 or 5.6 mg/kg, s.c., expressed as the salt). One hour and fifty minutes later, rats were injected with the opioid antagonist naloxone (NX, 0 or 1.0 mg/kg, s.c.) and tested for ICSS 10 minutes later. These morphine and naloxone doses and this pretreatment interval produce significant negative affective morphine withdrawal signs, including WIA (Schulteis et al., 2004; Harris et al., 2004; Holtz et al., 2015). Immediately after ICSS testing, somatic withdrawal signs were assessed as a secondary withdrawal measure (see Supplementary Material). There was a total of 4 groups in a 2 (MOR dose) × 2 (NX dose) factorial design. The MOR + NX group (n = 29) was larger than the MOR + SAL, SAL + NX, and SAL + SAL groups (n = 10–11/group) in order to have adequate power for correlation analysis (see below). These procedures were repeated each day for 5 consecutive days. Animals were then tested for ICSS under drug-free conditions for at least one week and until ICSS thresholds were stable (same stability criteria as above).
To test spontaneous withdrawal, the same rats received a single injection of 5.6 mg/kg MOR (MOR+NX and MOR+SAL groups) or 0 mg/kg MOR (SAL+NX and SAL+SAL groups) and ICSS was tested 2, 6, 26, 30, 50, 64, 74, 98, and 170 hours later. The purpose of the 2 hour time point was to detect any reinforcement-facilitating (ICSS threshold-lowering) effects of morphine itself (see Altarifi & Negus, 2011). The selection of subsequent time points was based on the time course of spontaneous withdrawal from acute morphine exposure determined using ICSS and other measures (Harris & Gewirtz, 2004; Rothwell et al., 2010; Liu & Schulteis, 2004). Somatic withdrawal signs were recorded immediately after ICSS testing at the 26 hour time point (based on Allahverdiyev et al., 2015). ICSS testing was suspended after the 170 hour time point.
Locomotor activity
Within 48 hours after the final ICSS test, locomotor activity in a novel environment (i.e., “sensation-seeking” (Blanchard et al., 2009; Pawlak et al., 2008; Piazza et al., 1989)) was tested for 2 hours as a secondary predictor of MSA (Swain et al., 2019, see Supplemental Materials for detailed description of the apparatus).
MSA
Approximately 24–48 hours after completion of the locomotor activity test, animals from all groups were implanted with i.v. catheters using our standard procedures (Swain et al., 2018). Following a 7–10 day recovery period, all rats were allowed to acquire i.v. morphine SA (0.2 mg/kg/inf) during daily 2 hr sessions conducted Mon-Fri using our standard apparatus and procedures (see Supplementary Material). Rats were tested under a fixed ratio (FR) 1 schedule for at least 10 sessions and until acquisition criteria were met (≥5 infusions per session, ≤20% coefficient of variation, and ≥ 2:1 response ratio on the active lever to inactive lever) across 3 sessions. To test elasticity of demand (reinforcing efficacy), the FR requirement was increased every 3–4 sessions as follows: FR 2, 3, 6, 12, 24, and doubled thereafter until infusion rates during the last 2 sessions at a given FR were reduced by 90% compared to baseline (FR 1). Morphine consumption under this protocol is well described by the current exponential demand function (Swain et al., 2018; Hursh & Silberberg, 2008). Data from Mondays were excluded from data analysis to avoid potential spontaneous recovery of responding after the weekend. Therefore, if one of the three sessions at a given FR occurred on a Monday, rats were tested in an additional session at that FR.
After completion of demand testing, rats were allowed to reacquire MSA under an FR1 schedule for at least 5 sessions and until MSA was stable (same stability criteria as above). Extinction conditions were subsequently introduced in which the morphine dose was replaced with saline and the drug-associated cue light was no longer presented upon infusion. Extinction was tested for at least 10 sessions and until animals exhibited a 75% reduction in active lever pressing for 2 consecutive sessions.
To test morphine- and cue-induced reinstatement, rats were injected s.c. on separate days with either saline or morphine (1.0 mg/kg) 10 min prior to the SA session. This morphine dose and pretreatment interval reliably reinstated drug-seeking in our lab (data not shown) and others (Vassoler et al., 2017). Responses on the active lever resulted in a saline infusion and either presentation of the drug-associated cue light (“cue” condition) or no programmed consequences (“no cue” condition). Reinstatement testing was therefore conducted using a 2 (morphine dose) x 2 (cue condition) design, resulting in a total of 4 reinstatement tests. These tests were conducted on Tuesdays and Fridays, provided that active lever pressing returned to extinction levels during the preceding session, and the order of reinstatement conditions was counterbalanced. Following completion of morphine- and cue-induced reinstatement testing, rats were tested under extinction conditions for at least 5 sessions and until extinction criteria were again met. Stress- and cue-induced reinstatement was then tested using the above procedure, except that rats were injected i.p. with either deionized water or the stress-inducing α2-adrenergic antagonist yohimbine (2.5 mg/kg) 30 min prior to each SA test. This dose of yohimbine and pretreatment interval reliably reinstate extinguished SA (Shepard et al., 2004). Similar within-subject designs are commonly used to study multiple forms of reinstatement (Le et al., 1998; Liu & Weiss, 2002).
Late-stage dependence
Following completion of all MSA procedures, rats were again tested for ICSS until thresholds were stable. Rats (N = 26) were subsequently tested for precipitated (MOR + NX: n = 14, n = 4/group for other groups) and spontaneous (MOR: n = 15, SAL: n = 5) morphine withdrawal using the same protocol as described for acute dependence testing above. The small group sizes for this exploratory phase reflect the considerable attrition rate by this stage of the protocol (see below).
Statistical analysis
ICSS.
ICSS thresholds (a measure of brain reinforcement function) and response latencies (a measure of non-specific motoric effects; Markou & Koob, 1992) during naloxone-precipitated and spontaneous withdrawal were measured as percentage of baseline (average of the last 5 days prior to onset of withdrawal testing). To provide a composite measure of naloxone-precipitated withdrawal severity for each animal, percentage scores were standardized into z-scores and then averaged across average and peak ICSS thresholds during all 5 precipitated withdrawal tests and degree of sensitization of WIA (the difference score in ICSS thresholds between test days 1 and 5) with the following formula:
Spontaneous withdrawal severity was measured as peak withdrawal severity during hours 6 – 98 after morphine injection. This measure accounts for the considerable individual differences in the time course of changes in ICSS thresholds during spontaneous withdrawal (see Harris et al., 2011). In the few cases (n = 2 for precipitated withdrawal during 1 or 3 sessions and n = 1 for spontaneous withdrawal during 1 session) where rats failed to respond for any ICSS current intensity, we arbitrarily assigned ICSS threshold and latency values based on those obtained in the animal achieving the highest ICSS threshold in that phase of the experiment (see Harris et al., 2015; Markou & Koob, 1991).
MSA.
MSA acquisition was measured as the mean number of infusions per session during the first 10 days of acquisition. To determine opioid reinforcing efficacy during FR escalation, exponential demand curve analyses were conducted as described in detail elsewhere (Swain et al., 2018; Hursh & Silberberg, 2008). Consistent with previous studies (Diergaarde et al., 2008; Swain et al., 2018; Hursh & Silberberg, 2008), we used α as our primary demand measure. This outcome refers to the rate of change in consumption with increases in unit price (elasticity of demand), with higher α values indicating lower reinforcement efficacy. Zero values in consumption were replaced with 0.01 (1/10th of our lowest non-zero consumption level) to provide better curve fits and more accurate parameter estimates of demand for individual rats (Koffamus et al., 2015; Murphy et al., 2009). α values were log-transformed due to non-normal distribution. Extinction was measured as mean number of infusions per session during the first 10 sessions of extinction. Degree of reinstatement (the reinstatement score) was defined as the difference between active and inactive lever responses during each reinstatement test (Cippitelli et al., 2010; Le et al., 2005; Tran-Nguyen et al., 1998). All statistical analyses and graphing were performed in GraphPad Prism 7 or R 3.4.3, with significance level set at α = 0.05 for all tests. In general, data were analyzed using ANOVA followed by Holm-Sidak’s or Dunnet’s multiple comparison tests (see Results for more details). Relationships between ICSS and MSA measures in the MOR + NX group were assessed using Pearson’s correlation.
Results
Acute dependence
Precipitated withdrawal: ICSS.
Baseline ICSS thresholds did not differ between groups (Table S1). A two-way ANOVA on ICSS thresholds during precipitated withdrawal revealed a significant main effect of group (F (3, 54) = 36.62, p < 0.0001) and a significant interaction between group and session (F (12, 216) = 3.181, p = 0.0003). Dunnett’s multiple comparisons indicated that ICSS thresholds were significantly elevated in the MOR+NX group compared to SAL+SAL controls during all 5 sessions (all q ≥ 3.162, all p ≤ 0.005). In contrast, thresholds in the MOR+SAL and SAL+NX groups did not differ from the SAL + SAL group during any session (Figure 2A). A repeated-measures ANOVA showed a significant effect of session in the MOR+NX rats (F (2.863, 77.30) = 18.40, p < 0.001), with Dunnett’s multiple comparisons indicating significantly higher ICSS thresholds during sessions 2–5 compared to session 1 (all q ≥ 4.68, all p ≤ 0.001). In contrast, there was no effect of session in any of the control groups (all p > 0.05).
Figure 2.
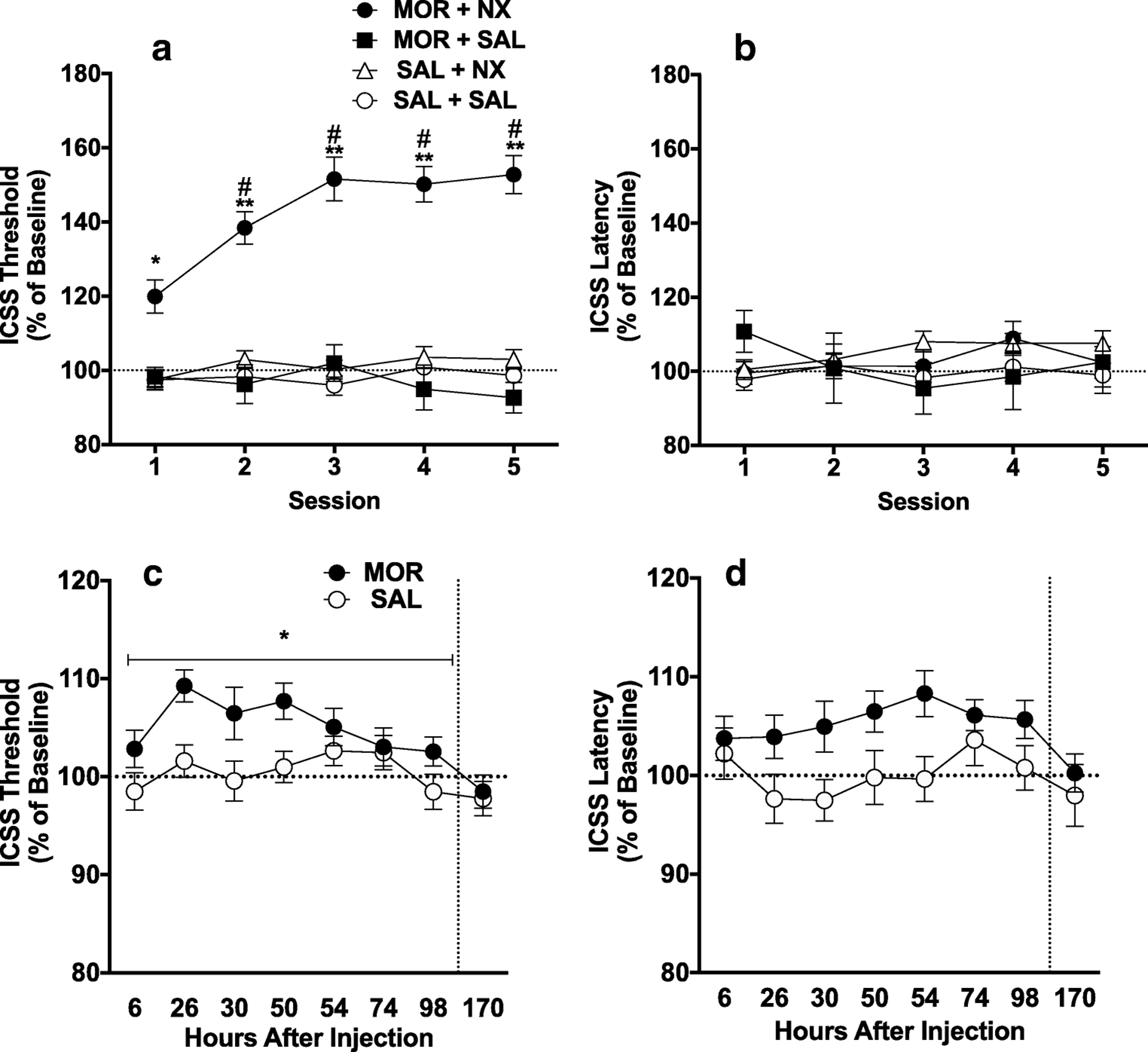
Mean (± SEM) ICSS thresholds (A) and response latencies (B) (expressed as percent of baseline) during naloxone-precipitated withdrawal (acute dependence). Mean (± SEM) ICSS thresholds (C) and latencies (D) as percent of baseline during spontaneous withdrawal (acute dependence). *,** Different from SAL+SAL group at that session or SAL condition during hours 6–98 (main effect), p < 0.05, 0.01. # Different from Session 1 in that group, p < 0.05.
No significant differences were observed in baseline ICSS response latencies between groups (Table S1). Latencies also did not differ between groups during precipitated withdrawal testing (Figure 2B), indicating the absence of non-specific (e.g., motoric) effects.
Spontaneous withdrawal: ICSS.
Baseline ICSS thresholds did not differ between groups (Table S1). ICSS threshold data during hours 2 (i.e., acute effect of MOR itself) and hours 6 – 98 (i.e., withdrawal period) of spontaneous withdrawal did not differ between the two groups receiving MOR (MOR + NX and MOR + SAL groups) or between the two groups receiving SAL (SAL + SAL and SAL + NX groups). Therefore, data from these groups were combined into single MOR (n = 37) and SAL (n = 20) groups for further analysis. Welch’s corrected t-test showed no significant difference in ICSS thresholds between MOR and SAL rats 2 hours after injection (MOR: 107.7 ±5.35%; SAL: 100.4 ± 2.50%), indicating that MOR itself did not affect ICSS. Two-way ANOVA on ICSS thresholds during spontaneous withdrawal 6–98 hours after morphine injection revealed a significant main effect of time (F (7, 385) = 4.831, p < 0.0001) and group (morphine vs. saline) (F (1, 55) = 4.012, p = 0.05), but no significant interaction (Figure 2C). After correcting for multiple comparisons, ICSS did not significantly differ between groups at any individual time-point post injection. However, peak ICSS threshold values between hours 6 and 98 (regardless of the time point at which they occurred) differed significantly between the morphine (117.1 ± 2.15%) and saline (110.4 ± 1.63%) groups (Welch-corrected t(54.9) = 2.50, p = 0.02).
No significant differences were observed in ICSS response latencies between groups during baseline sessions (Table S1), 2 hours after injection (MOR: 103.2 ± 3.01%; SAL: 96.43 ± 1.82%), or during spontaneous withdrawal (Figure 2D).
Precipitated withdrawal: Somatic signs.
One-way ANOVA on total somatic signs during the 5th session of naloxone-precipitated withdrawal indicated a significant effect of group (F (3, 52) = 11.51, p < 0.0001). Dunnett’s multiple comparison test revealed significantly higher scores in the MOR + NX group compared to the SAL + SAL group (q(52) =2.62, adjusted p = 0.03). In contrast, neither the MOR + SAL or SAL + NX group differed significantly from the SAL + SAL group (Figure 3A).
Figure 3:
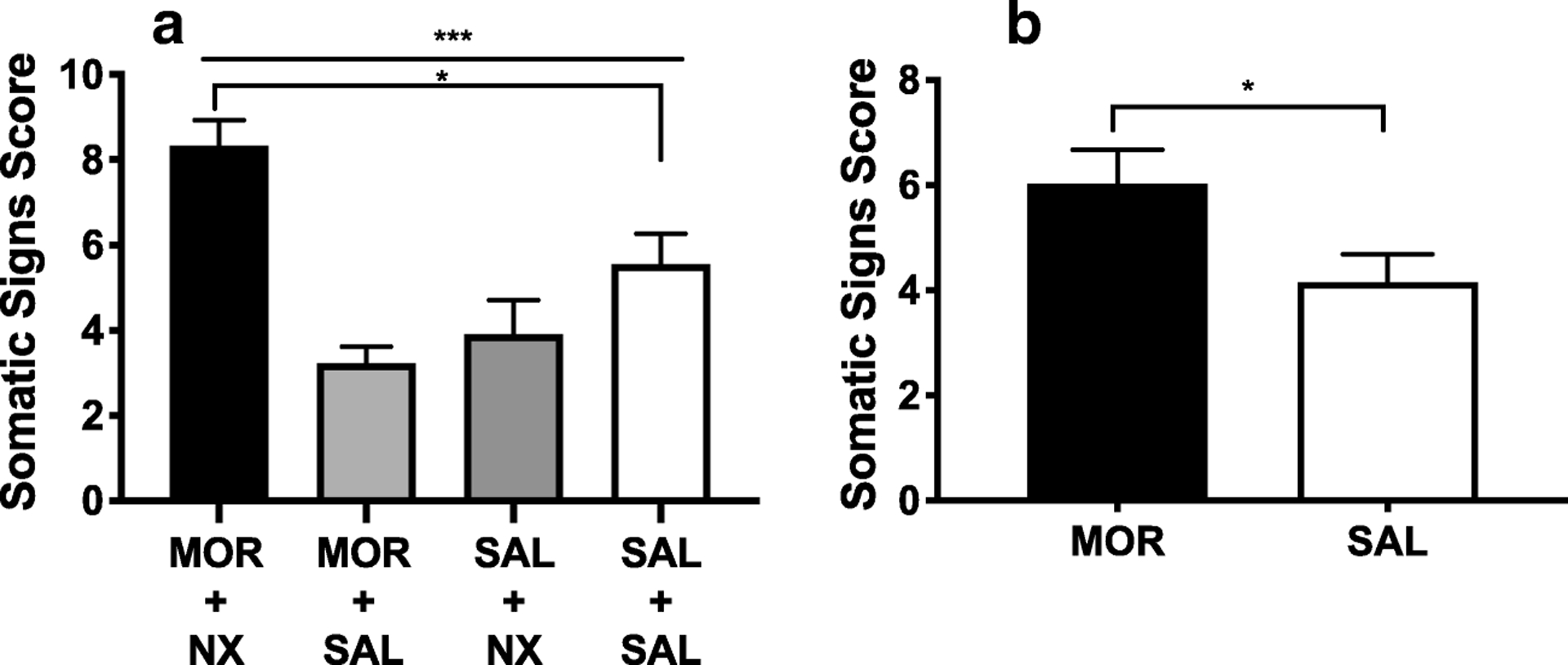
(A) Mean (± SEM) somatic signs in groups on the 5th day of acute dependence precipitated withdrawal testing. (B) Mean (± SEM) somatic signs in the MOR and SAL condition 26 hours after injection during acute dependence spontaneous withdrawal testing. *** Significant effect of group, p < 0.001. * Different from SAL + SAL group or SAL condition, p < 0.05.
Spontaneous withdrawal: Somatic signs.
During spontaneous withdrawal, somatic sign scores did not differ between the two groups receiving MOR (MOR + NX and MOR + SAL groups) or between the two groups receiving SAL (SAL + SAL and SAL + NX groups). Data from these groups were therefore combined into a single MOR (n = 37) or SAL (n = 20) condition. T-tests showed that scores were significantly higher in the MOR condition compared to the SAL condition (Welch-corrected t(51.31) = 2.25, p = 0.03) (Figure 3B). Eye blinking, facial fasciculations and swallowing movements were the most commonly observed somatic signs during both precipitated and spontaneous withdrawal (Table S4, S5).
MSA in the Mor + NX Group
Acquisition (n = 29).
Two-way ANOVA revealed a significant main effect of lever (active vs inactive) (F (1, 28) = 58.38, p < 0.0001) and session (F (9, 252) = 2.896, p = 0.003) on responses during the first 10 days of acquisition (Figure 4A). Sidak’s multiple comparison test showed significantly higher responses on the active lever during all acquisition sessions (all t ≥ 4.88, all p < 0.0001).
Figure 4.
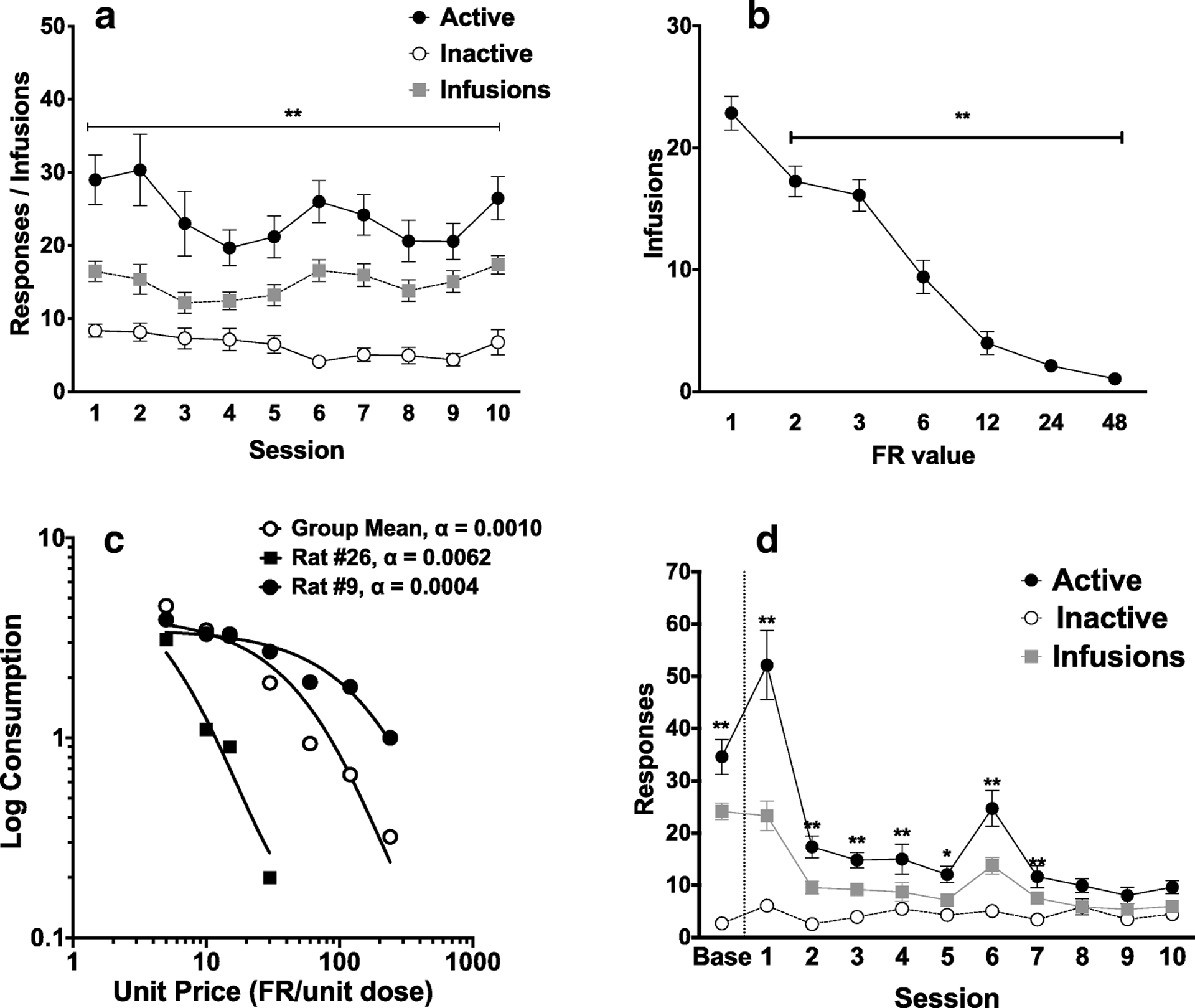
MSA in MOR + NX rats. (A) Mean (± SEM) active and inactive lever presses and infusions during the first 10 sessions of acquisition. ** Different between active and inactive lever presses, p < 0.01. (B) Mean (± SEM) infusions at each FR during demand testing. ** Different compared to infusions at FR1, p < 0.01. (C) Exponential demand curve describing morphine consumption as a function of unit price for rats as a group, and for individual rats with relatively high (rat #26) and low (rat #9) elasticity of demand (α). (D) Mean (± SEM) infusions during baseline and during the first 10 extinction sessions, ** Different compared to pre-extinction (baseline), p < 0.01. The increase in infusion rates during session 6 reflects spontaneous recovery following the weekend break in extinction testing.
Demand (n = 25 due to attrition, see Supplementary Material).
Increases in FR requirement resulted in a progressive reduction in morphine consumption (Figure 4B). A one-way repeated measures ANOVA revealed a significant effect of FR on number of morphine infusions (F (3.59, 82.63) = 79.43, p < 0.0001). A post-hoc Dunnett’s multiple comparisons test showed that infusions at all subsequent FRs were significantly lower than at FR 1 (all q ≥ 4.23, all p ≤ 0.002). Morphine consumption during demand testing was well-described by an exponential demand function, with R2 values typically ≥ 0.80 for individual animals (Table S2) and R2 = 0.94 for rats as a group, with considerable individual variability in elasticity of demand (see Figure 4C).
Extinction (n = 24).
Repeated-measures ANOVA revealed a significant main effect of session (F (10, 230) = 27.14, p < 0.001 ), main effect of lever (F (1, 23) = 67.44, p < 0.001; Figure 4D), and an interaction between session and lever (F (10, 229) = 26.98, p < 0.001) during extinction. A post-hoc Holm-Sidak’s multiple comparisons test showed that active lever presses were significantly higher than inactive lever presses during sessions 1–7 (all p < 0.05), but not during sessions 8–10.
Morphine-Induced Reinstatement (n = 23).
One-way repeated measures ANOVA of reinstatement scores (responses on the active lever - inactive lever) during morphine-induced reinstatement showed an overall effect of treatment (F(1.12, 24.73) = 18.18, p < 0.001) (Figure 5A). Holm-Sidak’s multiple comparisons test revealed that the MOR + NO CUE, VEH + CUE and MOR + CUE conditions all resulted in significantly higher responding compared to the VEH + NO CUE condition (all t ≥ 4.82, all p < 0.001). MOR+CUE resulted in significantly higher responding than VEH + CUE (t = 3.83, p = 0.002) and MOR + NO CUE (t = 3.80, p = 0.002).
Figure 5.
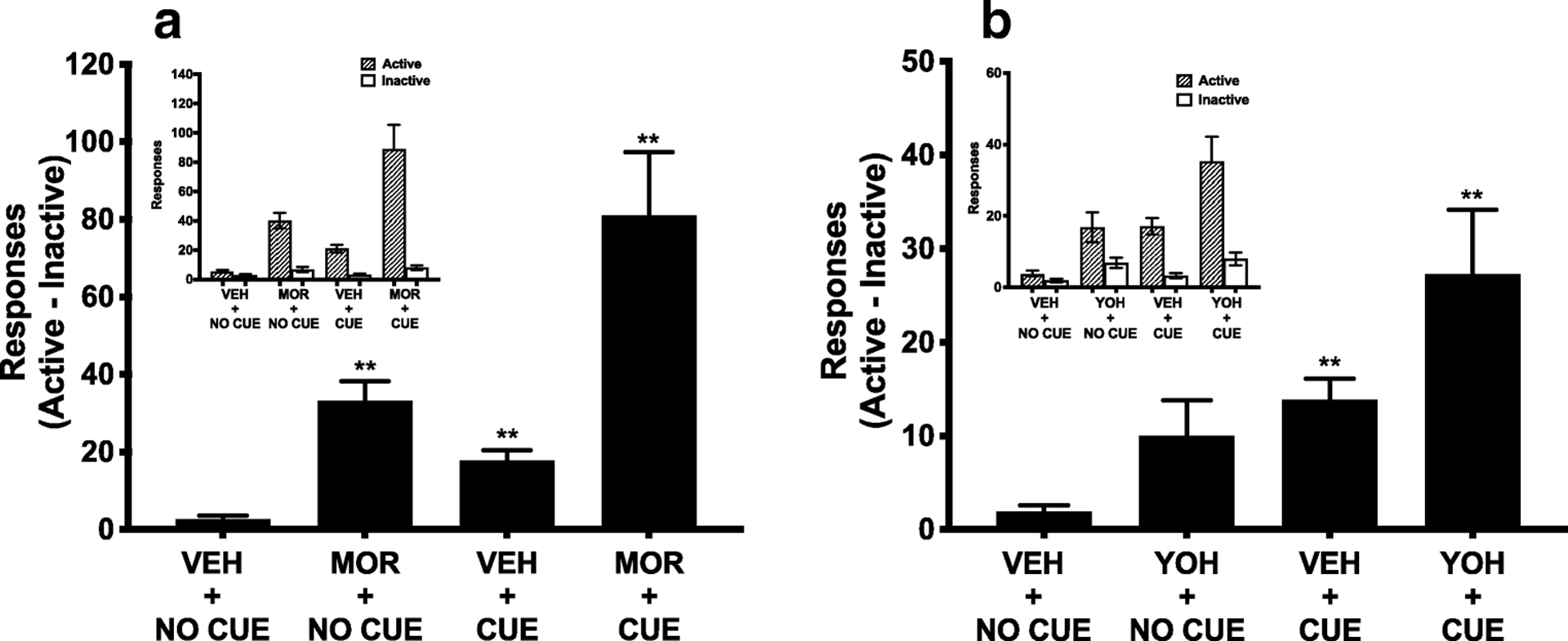
Mean (± SEM) reinstatement scores (differences between active and inactive lever responses) during morphine- and cue-induced reinstatement (E) and yohimbine-and cue-induced reinstatement (F). Active and inactive lever presses during each reinstatement test are shown in the insets. ** Different compared to VEH + NO CUE, p < 0.01.
Stress-Induced Reinstatement (n = 22).
There was an overall effect of treatment on reinstatement scores during stress-induced reinstatement (F(1.68, 35.35) = 8.92, p = 0.001) (Figure 5B). Holm-Sidak’s multiple comparisons test revealed that VEH + CUE (t = 5.98, p < 0.001) and YOH + CUE treatment (t = 3.75, p = 0.006) resulted in higher responding than the VEH + NO CUE control condition, whereas the YOH + NO CUE condition did not. Responding during YOH + CUE reinstatement was significantly higher than during the YOH + NO CUE condition (t = 3.17, p = 0.02), but did not differ from the VEH + CUE condition.
Correlations in the MOR + NX group
Composite z-scores for ICSS thresholds during precipitated withdrawal were significantly correlated with all individual measures: peak ICSS threshold (r = 0.92, p < 0.001), average ICSS threshold (r = 0.86, p < 0.001) and degree of sensitization of WIA (r = 0.54, p = 0.003). This validates our use of z-scores to measure cumulative precipitated withdrawal severity (see Belin et al., 2009; Belin & Deroche-Gamonet, 2012). Pearson’s r revealed that greater composite WIA severity during precipitated withdrawal correlated with lower infusions during acquisition of MSA and higher elasticity of demand (i.e., lower reinforcing efficacy) (Table 1, Figure 6). Greater peak ICSS threshold elevation during spontaneous withdrawal was associated with lower acquisition and reinstatement induced by morphine alone or morphine + cue (Table 1, Figure 7), and marginally correlated with elasticity of demand (p = 0.06, Figure 7). Most MSA measure correlated significantly with at least one other MSA measure (Table 1). However, no individual MSA measure correlated with as wide a range of other MSA measures as did WIA (Table 1).
Table 1.
Correlation between ICSS withdrawal measures and MSA measures (Pearson’s R) in the Mor + NX group. 1. Standardized composite score of average WIA, peak WIA, and degree of sensitization of WIA during naloxone-precipitated withdrawal. 2. Peak ICSS threshold during spontaneous withdrawal. 3. Average infusions during first 10 days of acquisition. 4. Log-transformed elasticity of demand computed from exponential demand model. Higher log α = lower reinforcement efficacy. 5. Average infusions during first 10 days of extinction; 6.–10. Reinstatement score (active - inactive lever pressing) during reinstatement induced by morphine with cue (MOR+CUE), morphine with no cue (MOR+ NO CUE), yohimbine with cue (YOH+CUE), yohimbine with no cue (YOH+ NO CUE) and cue alone (averaged across VEH + CUE condition during morphine- and yohimbine-induced reinstatement testing).*,**,*** p ≤ 0.05, 0.01, 0.001.
| Variables | 1. Precip WIA | 2. Spont. WIA | 3. Acquisition | 4. Log α | 5. Extinction | 6. MOR+ CUE | 7. MOR+ NO CUE | 8. YOH+ CUE | 9. YOH + NO CUE |
|---|---|---|---|---|---|---|---|---|---|
| 1. Precip. WIA | – | ||||||||
| 2. Spont. WIA | .41* | – | |||||||
| 3. Acquisition | −.39* | −.55** | – | ||||||
| 4. Log α | .43* | .40 | −.42* | – | |||||
| 5. Extinction | −.01 | .−.26 | .35 | −.39 | – | ||||
| 6. MOR+CUE | −.07 | −.53** | .15 | −.13 | .20 | – | |||
| 7. MOR + NOCUE | −.21 | −.63** | .33 | −.21 | .35 | −81*** | – | ||
| 8. YOH + CUE | −.32 | −.17 | .18 | −.57** | .35 | −.13 | −.05 | – | |
| 9. YOH + NOCUE | .11 | .04 | −.12 | −.26 | −.20 | −.16 | −.26 | .61** | – |
| 10. VEH+ CUE | −.17 | −.06 | .16 | −.66*** | .38* | −.10 | −.11 | .77*** | .59** |
Figure 6:
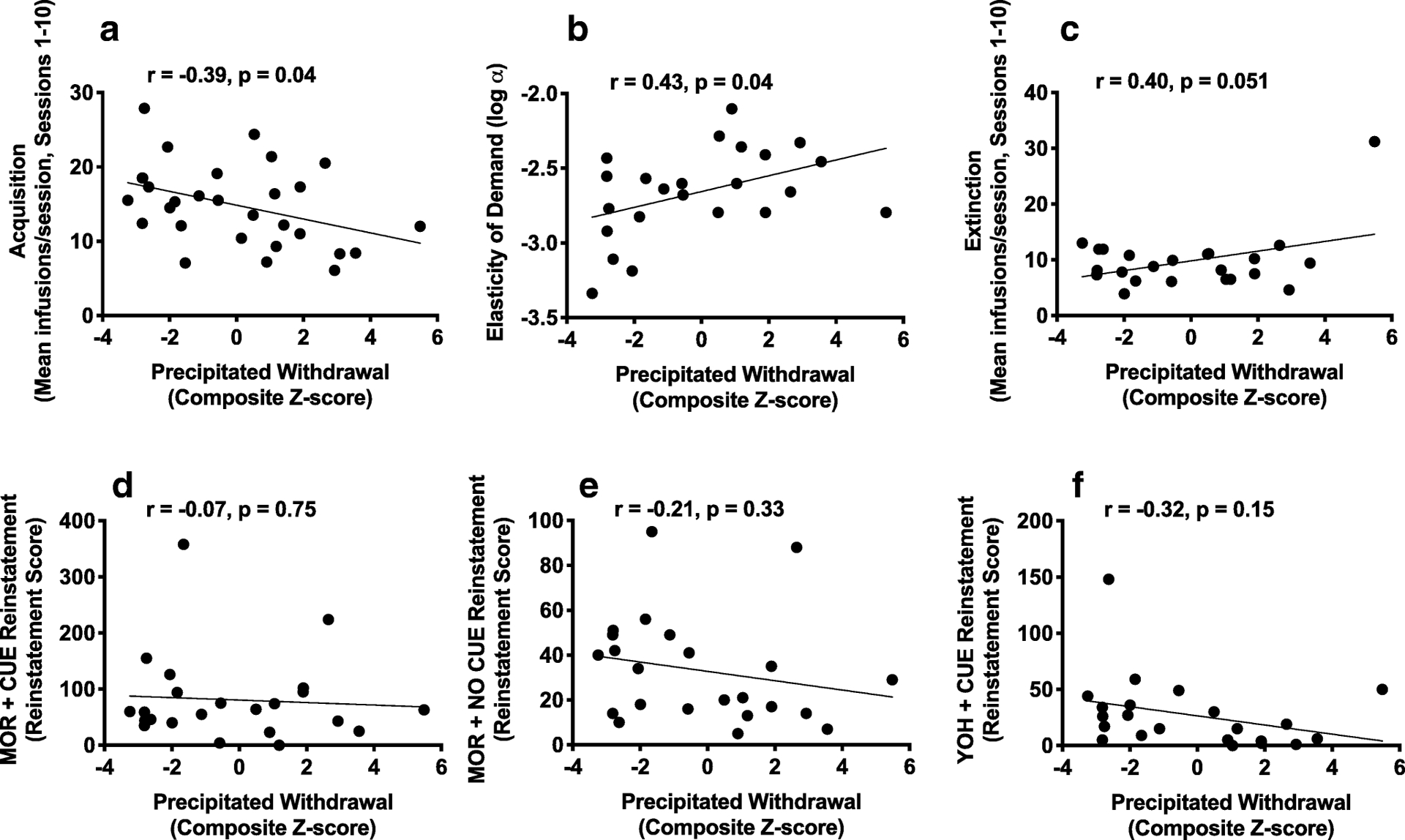
Scatterplots with regression line depicting the relationship between naloxone-precipitated withdrawal measured using ICSS (composite z-score) and infusions during first 10 sessions of acquisition (A), log α (B), infusions during first 10 sessions of extinction (C), and reinstatement score during MOR + CUE (D), MOR + NO CUE (E) and YOH + CUE (F) reinstatement. Higher log α (elasticity of demand) = lower reinforcement efficacy.
Figure 7:
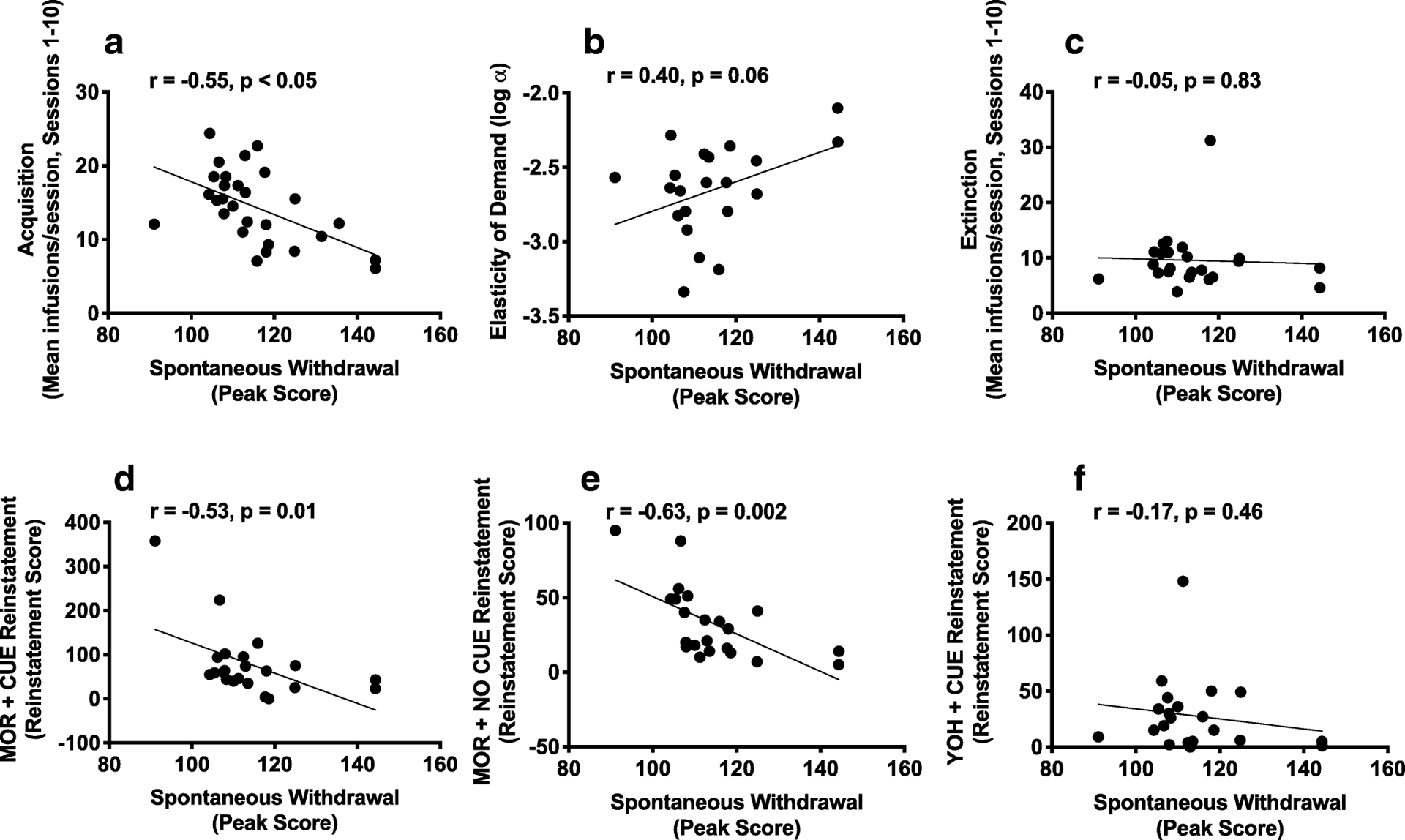
Scatterplots with regression line depicting the relationship between peak spontaneous withdrawal during ICSS testing and infusions during first 10 sessions of acquisition (A), log α (B), infusions during first 10 sessions of extinction (C), and reinstatement score during MOR + CUE (D), MOR + NO CUE (E) and YOH + CUE (F) reinstatement in the Mor + NX group. Higher log α (elasticity of demand) = lower reinforcement efficacy.
Additional Analyses
Secondary correlations in the MOR + NX group.
There were no significant correlations between any of the secondary predictors (i.e., somatic signs during acute dependence testing, locomotor activity) and any measure of MSA (all p-values > 0.05, data not shown).
Locomotor activity and MSA in control groups.
Rats in the control groups did not differ from the MOR + NX group in terms of locomotor activity or on any primary MSA measure (see Supplementary Materials, Figure S1A, S2).
Late-stage dependence.
ICSS thresholds were significantly elevated during precipitated withdrawal during late-stage dependence (Figure S3), while somatic signs were significantly elevated during spontaneous withdrawal (Figure S3). However, these effects were not correlated with most MSA measures (see Supplementary Materials).
Discussion
Greater WIA during antagonist-precipitated and spontaneous withdrawal in an acute dependence model was associated with lower vulnerability on multiple measures of subsequent i.v. MSA (e.g., elasticity of demand, reinstatement). In fact, WIA predicted a wider range of MSA measures than did any individual measure of MSA. These findings are consistent with the principle that initial drug sensitivity is an important predictor of subsequent drug use (Deminiere et al., 1989; Chappell & Weiner, 2008; Nishida et al., 2016), and also support the notion that drug withdrawal sensitivity may be protective against drug addiction (Carroll et al., 2008; Holtz et al., 2015; Radke et al., 2015). In particular, our findings with outbred rats complement findings of lower WIA in rats bred for high saccharin consumption (Holtz et al., 2015), a line that exhibits greater SA of opioids and other drugs (Carroll et al., 2002). Together, these data identify WIA as a potential target for understanding behavioral and neurobiological mechanisms underlying the emergence of opioid addiction.
Several features of WIA during acute dependence distinguish it from other behavioral measures of vulnerability to addiction-like behaviors. First WIA is unique in that it reliably predicts individual differences in opioid SA in outbred rats, whereas numerous established behavioral markers of individual differences in stimulant and alcohol SA (e.g., sensation-seeking as measured by open-field locomotor activity, impulsivity) do not (Swain et al., 2018; Dileen et al., 2012; McNamara et al., 2010). Indeed, open-field activity was not correlated with any measure of MSA in this study (see Supplementary Material), consistent with our previous findings using a more limited set of MSA measures (Swain et al., 2018). WIA also differs from other behavioral predictors of SA of other drugs in that it predicted a variety of measures of SA (Belin et al., 2016). An additional unique feature of WIA is that it is an outcome of early opioid exposure, as opposed to a preexisting disposition. Therefore, WIA may represent a neuroadaptive mechanism underlying addiction vulnerability, as opposed to only a behavioral indicator. As such, WIA promises to provide unique information on addiction vulnerability to complement findings obtained using existing behavioral markers of addiction vulnerability.
In contrast to WIA, somatic signs during acute dependence did not predict any primary MSA measure. These data complement previous findings indicating that affective/emotional and somatic withdrawal signs are mediated by distinct neurobiological mechanisms (Koob & Le Moal, 1997; Nestler & Carlezon, 2006), and supports the notion that the former have greater relevance to addiction vulnerability (Schulteis et al., 1994; Baker et al., 2004; Koob & Le Moal, 2005).
The current findings contrast with some studies reporting a positive relationship between withdrawal sensitivity and addiction vulnerability (Ahmed et al., 2002; Kenny et al., 2006; Funk et al., 2006). Numerous methodological differences between studies could account for this discrepancy (e.g., drug class studied, etc). In addition, the current acute dependence model isolates the earliest stages of dependence, while prior studies involved subjects in which dependence had already been established. As such, withdrawal sensitivity may shift from being a protective factor to a vulnerability factor for addiction as dependence develops (Kiluk et al., 2019). The fact that WIA during late stage dependence was associated with greater resistance to extinction (see Supplemental Materials) may be consistent with this possibility. The lack of correlation between WIA during acute dependence and late-stage dependence (Supplementary Material) also suggests that each of these stages may provide unique information. Use of larger group sizes in order to provide adequate statistical power is needed to further address this issue, which was not a primary goal of this study.
Comparison of locomotor activity and MSA in the MOR + NX and control groups suggests that a history of morphine exposure and/or withdrawal had limited or no effects on these measures. The MSA data contrast with findings that repeated, experimenter-administered acute drug injections (and presumably spontaneous withdrawal episodes) can enhance subsequent drug SA (Piazza et al., 1989; Mendrek et al., 1998; Shoaib et al., 1997). Methodological differences across studies (e.g., types of drugs, duration of interval between the final acute injection and onset of SA) may account for the similar MSA across groups in this study. Importantly, results from this secondary comparison do not impact interpretation of the correlations between WIA and measures of MSA in the Mor + NX group, which was our primary outcome.
Behavioral economics has been useful for understanding individual differences in addiction vulnerability in both humans and animals (Chase et al., 2013; Worley et al., 2015; Diergaarde et al., 2008; Grebenstein et al., 2013; Hursh & Silberberg, 2008; LeSage et al., 2016), but has not been applied extensively to MSA. Consistent with our previous study (Swain et al., 2018), an exponential demand function generally provided a good fit for morphine consumption under an FR escalation procedure. There were also considerable individual differences in α (reinforcing efficacy) that were correlated with severity of WIA during precipitated withdrawal, and a similar trend was observed for spontaneous withdrawal. Together, these data further support the utility and sensitivity of behavioral economics to study individual differences in opioid addiction vulnerability.
A potential limitation of this study is that all rats underwent the experimental phases in a fixed order. It could therefore be argued that the relationships we observed might not hold if a different order of phases was used. It is important to note that not all aspects of the protocol were fixed, as order of reinstatement conditions (drug + cue, cue alone, etc) within each reinstatement phase (morphine/cue, yohimbine/cue) were counterbalanced across rats. In addition, the current order of phases was to some extent unavoidable due to the goals of the study (e.g., assessment of acute dependence prior to MSA) or to the required procedure for assessing certain MSA measures (e.g., testing extinction of MSA prior to reinstatement). Also, it was prudent to test yohimbine-induced reinstatement as the final MSA measure because of the well-established long-term effects of yohimbine or other stressors on behavior, including drug sensitivity (Ball et al., 2015; Barsy et al., 2011; Pizzimenti et al., 2017). Nonetheless, we cannot rule out the possibility that the relationships observed here were specific to this order of experimental phases.
In conclusion, this study establishes WIA as one of the first behavioral measures to reliably predict individual differences in future opioid SA. Evaluating the generality of the current findings to other subject variables (e.g., genetic background using inbred rat strains), opioids (e.g., heroin, fentanyl), withdrawal measures (e.g., other measures of anhedonia such as sucrose preference, or other negative affective withdrawal signs such as elevated startle responding), and orders of experimental phases, will be an important direction for future research. Given the numerous sex differences in addiction vulnerability reported in both humans and animals (Back et al., 2011; Becker & Koob, 2016; Becker et al., 2017), evaluating effects of sex in this model is also of interest. Furthermore, measures of opioid SA that model other aspects of addiction (e.g., escalation of SA under extended access conditions, a model of compulsive drug use,) are also an important direction for future research. Finally, characterizing the neurobiological mechanisms underlying WIA will allow addiction-related effects (i.e., those uniquely related to severity of WIA) to be differentiated from other, corollary effects of opioids. Hence, further use of this model promises to provide novel insights into neurobiological mechanisms underlying vulnerability and/or resilience to opioid addiction.
Supplementary Material
Acknowledgements
The authors thank Mary Krueger, Joseph Tombers, Haley Rudnick, and Nettie Enshayan for their excellent technical assistance.
Funding
Supported by NIH / NIDA grant R21 DA037728 (Gewirtz/Harris, Co-PIs), the Hennepin Healthcare Research Institute (formerly Minneapolis Medical Research Foundation) Translational Addiction Research Program (Harris PI), a Hennepin Healthcare Research Institute Career Development Award for PhD Investigators (Harris PI), and NIDA training grant T32 DA007097 (Swain, Y; Molitor T, PI).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflict of Interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
References
- Ahmed SH, Kenny PJ, Koob GF, Markou A (2002) Neurobiological evidence for hedonic allostasis associated with escalating cocaine use. Nat Neurosci 5: 625–6. [DOI] [PubMed] [Google Scholar]
- Allahverdiyev O, Turkmen AZ, Nurten A, Sehirli I, Enginar N (2015) Spontaneous withdrawal in intermittent morphine administration in rats and mice: effect of clonidine coadministration and sex-related differences. Turk J Med Sci 45: 1380–9. [PubMed] [Google Scholar]
- Altarifi AA, Negus SS (2011) Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol 22: 663–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosio E, Goldberg SR, Elmer GI (1995) Behavior genetic investigation of the relationship between spontaneous locomotor activity and the acquisition of morphine self-administration behavior. Behav Pharmacol 6: 229–237. [PubMed] [Google Scholar]
- Back SE, Lawson KM, Singleton LM, Brady KT (2011) Characteristics and correlates of men and women with prescription opioid dependence. Addict Behav 36: 829–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC (2004) Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol Rev 111: 33–51. [DOI] [PubMed] [Google Scholar]
- Ball KT, Jarsocrak H, Hyacinthe J, Lambert J, Lockowitz J, Schrock J (2015) Yohimbine reinstates extinguished 3, 4-methylenedioxymethamphetamine (MDMA; ecstasy) seeking in rats with prior exposure to chronic yohimbine. Behav brain research 294:1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsy B, Mikics É, Barsvári B, Haller J (2011) The long-term impact of footshock stress on addiction-related behaviors in rats. Neuropharmacology 60(2–3):267–73. [DOI] [PubMed] [Google Scholar]
- Becker JB, Koob GF (2016) Sex Differences in Animal Models: Focus on Addiction. Pharmacol Rev 68: 242–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker JB, McClellan ML, Reed BG (2017) Sex differences, gender and addiction. J Neurosci Res 95: 136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Balado E, Piazza PV, Deroche-Gamonet V (2009) Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry 65: 863–8. [DOI] [PubMed] [Google Scholar]
- Belin D, Belin-Rauscent A, Everitt BJ, Dalley JW (2016) In search of predictive endophenotypes in addiction: insights from preclinical research. Genes Brain Behav 15: 74–88. [DOI] [PubMed] [Google Scholar]
- Belin D, Berson N, Balado E, Piazza PV, Deroche-Gamonet V (2011) High-novelty-preference rats are predisposed to compulsive cocaine self-administration. Neuropsychopharmacology 36: 569–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Deroche-Gamonet V (2012) Responses to novelty and vulnerability to cocaine addiction: contribution of a multi-symptomatic animal model. Cold Spring Harb Perspect Med 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ (2008) High impulsivity predicts the switch to compulsive cocaine-taking. Science 320: 1352–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanchard MM, Mendelsohn D, Stamp JA (2009) The HR/LR model: Further evidence as an animal model of sensation seeking. Neurosci Biobehav Rev 33: 1145–54. [DOI] [PubMed] [Google Scholar]
- Bruijnzeel AW, Marcinkiewcz C, Isaac S, Booth MM, Dennis DM, Gold MS (2007) The effects of buprenorphine on fentanyl withdrawal in rats. Psychopharmacology (Berl) 191: 931–41. [DOI] [PubMed] [Google Scholar]
- Cahill K, Stead L, Lancaster T (2009) A preliminary benefit-risk assessment of varenicline in smoking cessation. Drug Saf 32: 119–35. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Anker JJ, Perry JL, Dess NK (2008) Selective breeding for differential saccharin intake as an animal model of drug abuse. Behav Pharmacol 19: 435–60. [DOI] [PubMed] [Google Scholar]
- Carroll ME, Morgan AD, Lynch WJ, Campbell UC, Dess NK (2002) Intravenous cocaine and heroin self-administration in rats selectively bred for differential saccharin intake: phenotype and sex differences. Psychopharmacology (Berl) 161: 304–13. [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality (2018) National survey on drug use and health: detailed tables Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Chappell AM, Weiner JL (2008) Relationship between ethanol’s acute locomotor effects and ethanol self-administration in male Long-Evans rats. Alcohol Clin Exp Res 32: 2088–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase HW, Mackillop J, Hogarth L (2013) Isolating behavioural economic indices of demand in relation to nicotine dependence. Psychopharmacology (Berl) 226: 371–80. [DOI] [PubMed] [Google Scholar]
- Chester JA, Rausch EJ, June HL, Froehlich JC (2006) Decreased reward during acute alcohol withdrawal in rats selectively bred for low alcohol drinking. Alcohol 38: 165–72. [DOI] [PubMed] [Google Scholar]
- Cippitelli A, Karlsson C, Shaw JL, Thorsell A, Gehlert DR, Heilig M (2010) Suppression of alcohol self-administration and reinstatement of alcohol seeking by melanin-concentrating hormone receptor 1 (MCH1-R) antagonism in Wistar rats. Psychopharmacology 211(4):367–75. [DOI] [PubMed] [Google Scholar]
- Deminiere JM, Piazza PV, Le Moal M, Simon H (1989) Experimental approach to individual vulnerability to psychostimulant addiction. Neurosci Biobehav Rev 13: 141–7. [DOI] [PubMed] [Google Scholar]
- Dess NK, O’Neill P, Chapman CD (2005) Ethanol withdrawal and proclivity are inversely related in rats selectively bred for differential saccharin intake. Alcohol 37: 9–22. [DOI] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, DeVries TJ (2008) Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry 63(3): 301–308. [DOI] [PubMed] [Google Scholar]
- DiFranza JR, Savageau JA, Fletcher K, Ockene JK, Rigotti NA, McNeill AD, Coleman M, Wood C (2004) Recollections and repercussions of the first inhaled cigarette. Addict Behav 29: 261–72. [DOI] [PubMed] [Google Scholar]
- Dilleen R, Pelloux Y, Mar AC, Molander A, Robbins TW, Everitt BJ, Dalley JW, Belin D (2012) High anxiety is a predisposing endophenotype for loss of control over cocaine, but not heroin, self-administration in rats. Psychopharmacology (Berl) 222: 89–97. [DOI] [PubMed] [Google Scholar]
- Engelmann JM, Radke AK, Gewirtz JC (2009) Potentiated startle as a measure of the negative affective consequences of repeated exposure to nicotine in rats. Psychopharmacology (Berl) 207: 13–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowler CD, Kenny PJ (2014) Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacology 76 Pt B: 533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk CK, O’Dell LE, Crawford EF, Koob GF (2006) Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J Neurosci 26: 11324–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebenstein P, Burroughs D, Zhang Y, LeSage MG (2013) Sex differences in nicotine self-administration in rats during progressive unit dose reduction: implications for nicotine regulation policy. Pharmacol Biochem Behav 114–115: 70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC (2004) Elevated startle during withdrawal from acute morphine: a model of opiate withdrawal and anxiety. Psychopharmacology (Berl) 171: 140–7. [DOI] [PubMed] [Google Scholar]
- Harris AC, Gewirtz JC (2005) Acute opioid dependence: characterizing the early adaptations underlying drug withdrawal. Psychopharmacology (Berl) 178: 353–66. [DOI] [PubMed] [Google Scholar]
- Harris AC, Hanes SL, Gewirtz JC (2004) Potentiated startle and hyperalgesia during withdrawal from acute morphine: effects of multiple opiate exposures. Psychopharmacology (Berl) 176: 266–73. [DOI] [PubMed] [Google Scholar]
- Harris AC, Manbeck KE, Schmidt CE, Shelley D (2013) Mecamylamine elicits withdrawal-like signs in rats following a single dose of nicotine. Psychopharmacology (Berl) 225: 291–302. [DOI] [PubMed] [Google Scholar]
- Harris AC, Pentel PR, Burroughs D, Staley MD, Lesage MG (2011) A lack of association between severity of nicotine withdrawal and individual differences in compensatory nicotine self-administration in rats. Psychopharmacology (Berl) 217: 153–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris AC, Tally L, Muelken P, Banal A, Schmidt CE, Cao Q, LeSage MG (2015) Effects of nicotine and minor tobacco alkaloids on intracranial-self-stimulation in rats. Drug Alcohol Depend 153: 330–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtz NA, Radke AK, Zlebnik NE, Harris AC, Carroll ME (2015) Intracranial self-stimulation reward thresholds during morphine withdrawal in rats bred for high (HiS) and low (LoS) saccharin intake. Brain Res 1602: 119–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hursh SR, Silberberg A (2008) Economic demand and essential value. Psychol Rev 115: 186–98. [DOI] [PubMed] [Google Scholar]
- Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW (2014) Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology 39: 455–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CM, Einstein EB, Compton WM (2018) Changes in Synthetic Opioid Involvement in Drug Overdose Deaths in the United States, 2010–2016. JAMA 319: 1819–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Chen SA, Kitamura O, Markou A, Koob GF (2006) Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J Neurosci 26: 5894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Koob GF, Markou A (2003) Conditioned facilitation of brain reward function after repeated cocaine administration. Behav Neurosci 117: 1103–7. [DOI] [PubMed] [Google Scholar]
- Kiluk BD, Yip SW, DeVito EE, Carroll KM, Sofuoglu M (2019) Anhedonia as a Key Clinical Feature in the Maintenance and Treatment of Opioid Use Disorder. Clinical Psychological Science: 2167702619855659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffarnus MN, Franck CT, Stein JS, Bickel WK (2015) A modified exponential behavioral economic demand model to better describe consumption data. Exp Clin Psychopharmacol 23: 504–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (2005) Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci 8: 1442–4. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M (1997) Drug abuse: hedonic homeostatic dysregulation. Science 278: 52–8. [DOI] [PubMed] [Google Scholar]
- Le AD, Harding S, Juzytsch W, Funk D, Shaham Y (2005) Role of alpha-2 adrenoceptors in stress-induced reinstatement of alcohol seeking and alcohol self-administration in rats. Psychopharmacology 179(2):366–73. [DOI] [PubMed] [Google Scholar]
- Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y (1998) Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacol 135(2): 169–74. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Staley M, Muelken P, Smethells JR, Stepanov I, Vogel RI, Pentel PR, Harris AC (2016) Abuse liability assessment of an e-cigarette refill liquid using intracranial self-stimulation and self-administration models in rats. Drug Alcohol Depend 168: 76–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Schulteis G (2004) Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol Biochem Behav 79: 101–8. [DOI] [PubMed] [Google Scholar]
- Liu X, Weiss F (2002) Additive effect of stress and drug cues on reinstatement of ethanol seeking: exacerbation by history of dependence and role of concurrent activation of corticotropin-releasing factor and opioid mechanisms. J of Neurosci 22(18): 7856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markou A, Koob GF (1991) Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology 4: 17–26. [PubMed] [Google Scholar]
- Markou A, Koob GF (1992) Construct validity of a self-stimulation threshold paradigm: effects of reward and performance manipulations. Physiol Behav 51: 111–9. [DOI] [PubMed] [Google Scholar]
- McNamara R, Dalley JW, Robbins TW, Everitt BJ, Belin D (2010) Trait-like impulsivity does not predict escalation of heroin self-administration in the rat. Psychopharmacol (Berl) 212: 453–64. [DOI] [PubMed] [Google Scholar]
- Mendrek A, Blaha CD, Phillips AG (1998) Pre-exposure of rats to amphetamine sensitizes self-administration of this drug under a progressive ratio schedule. Psychopharmacology (Berl) 135: 416–22. [DOI] [PubMed] [Google Scholar]
- Murphy JG, MacKillop J, Skidmore JR, Pederson AA (2009) Reliability and validity of a demand curve measure of alcohol reinforcement. Exp Clin Psychopharmacol 17: 396–404. [DOI] [PubMed] [Google Scholar]
- Nestler EJ, Carlezon WA Jr., (2006) The mesolimbic dopamine reward circuit in depression. Biol Psychiatry 59: 1151–9. [DOI] [PubMed] [Google Scholar]
- Nishida KS, Park TY, Lee BH, Ursano RJ, Choi KH (2016) Individual differences in initial morphine sensitivity as a predictor for the development of opiate addiction in rats. Behav Brain Res 313: 315–323. [DOI] [PubMed] [Google Scholar]
- O’Dell LE (2009) A psychobiological framework of the substrates that mediate nicotine use during adolescence. Neuropharmacology 56 Suppl 1: 263–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Dell LE, Bruijnzeel AW, Smith RT, Parsons LH, Merves ML, Goldberger BA, Richardson HN, Koob GF, Markou A (2006) Diminished nicotine withdrawal in adolescent rats: implications for vulnerability to addiction. Psychopharmacology (Berl) 186: 612–9. [DOI] [PubMed] [Google Scholar]
- O’Loughlin J, DiFranza J, Tyndale RF, Meshefedjian G, McMillan-Davey E, Clarke PB, Hanley J, Paradis G (2003) Nicotine-dependence symptoms are associated with smoking frequency in adolescents. Am J Prev Med 25: 219–25. [DOI] [PubMed] [Google Scholar]
- Pawlak CR, Ho YJ, Schwarting RK (2008) Animal models of human psychopathology based on individual differences in novelty-seeking and anxiety. Neurosci Biobehav Rev 32: 1544–68. [DOI] [PubMed] [Google Scholar]
- Piazza PV, Deminiere JM, Le Moal M, Simon H (1989) Factors that predict individual vulnerability to amphetamine self-administration. Science 245: 1511–3. [DOI] [PubMed] [Google Scholar]
- Pizzimenti CL, Navis TM, Lattal KM (2017) Persistent effects of acute stress on fear and drug-seeking in a novel model of the comorbidity between post-traumatic stress disorder and addiction. Learning & Memory 24(9):422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radke AK, Zlebnik NE, Carroll ME (2015) Cocaine withdrawal in rats selectively bred for low (LoS) versus high (HiS) saccharin intake. Pharmacol Biochem Behav 129: 51–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PE, Gewirtz JC, Thomas MJ (2010) Episodic withdrawal promotes psychomotor sensitization to morphine. Neuropsychopharmacology 35: 2579–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor CE, Lessov-Schlaggar CN, Scherrer JF, Bucholz KK, Madden PA, Pergadia ML, Grant JD, Jacob T, Xian H (2010) Initial response to cigarettes predicts rate of progression to regular smoking: findings from an offspring-of-twins design. Addict Behav 35: 771–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuckit MA, Smith TL, Kalmijn J (2004) The search for genes contributing to the low level of response to alcohol: patterns of findings across studies. Alcohol Clin Exp Res 28: 1449–58. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Gold LH, Stinus L, Koob GF (1994) Relative sensitivity to naloxone of multiple indices of opiate withdrawal: a quantitative dose-response analysis. J Pharmacol Exp Ther 271: 1391–8. [PubMed] [Google Scholar]
- Schulteis G, Morse AC, Liu J (2004) Conditioning processes contribute to severity of naloxone-precipitated withdrawal from acute opioid dependence. Psychopharmacology (Berl) 175: 463–72. [DOI] [PubMed] [Google Scholar]
- Shepard JD, Bossert JM, Liu SY, Shaham Y (2004) The anxiogenic drug yohimbine reinstates methamphetamine seeking in a rat model of drug relapse. Biol Psychiatry 55: 1082–9. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR (1997) Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 129: 35–43. [DOI] [PubMed] [Google Scholar]
- Swain Y, Muelken P, LeSage MG, Gewirtz JC, Harris AC (2018) Locomotor activity does not predict individual differences in morphine self-administration in rats. Pharmacol Biochem Behav 166: 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran-Nguyen LT, Fuchs RA, Coffey GP, Baker DA, O’Dell LE, Neisewander JL (1998) Timedependent changes in cocaine-seeking behavior and extracellular dopamine levels in the amygdala during cocaine withdrawal. Neuropsychopharmacol 19(1):48–59. [DOI] [PubMed] [Google Scholar]
- Vassoler FM, Oliver DJ, Wyse C, Blau A, Shtutman M, Turner JR, Byrnes EM (2017) Transgenerational attenuation of opioid self-administration as a consequence of adolescent morphine exposure. Neuropharmacolo 113: 271–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang SC, Chen YC, Lee CH, Cheng CM (2019) Opioid Addiction, Genetic Susceptibility, and Medical Treatments: A Review. Int J Mol Sci 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (2004) Dopamine, learning and motivation. Nat Rev Neurosci 5: 483–94. [DOI] [PubMed] [Google Scholar]
- Worley MJ, Shoptaw SJ, Bickel WK, Ling W (2015) Using behavioral economics to predict opioid use during prescription opioid dependence treatment. Drug Alcohol Depend 148: 62–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap JJ, Miczek KA (2008) Stress and Rodent Models of Drug Addiction: Role of VTA-Accumbens-PFC-Amygdala Circuit. Drug Discov Today Dis Models 5: 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Cui CL, Schlussman SD, Choi JC, Ho A, Han JS, Kreek MJ (2008) Effects of cocaine place conditioning, chronic escalating-dose “binge” pattern cocaine administration and acute withdrawal on orexin/hypocretin and preprodynorphin gene expressions in lateral hypothalamus of Fischer and Sprague-Dawley rats. Neuroscience 153: 1225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


