Abstract
Background:
22q11.2 deletions and duplications are copy number variations (CNVs) that predispose to developmental neuropsychiatric disorders. Both CNVs are associated with autism spectrum disorder (ASD), while the deletion confers disproportionate risk for schizophrenia. Neurobehavioral profiles associated with these reciprocal CNVs in conjunction with brain imaging measures have not been reported.
Method:
We profiled the impact of 22q11.2 CNVs on neurobehavioral measures relevant to ASD and psychosis in 106 22q11.2 deletion carriers, 38 22q11.2 duplication carriers, and 82 demographically-matched controls. To determine whether brain-behavior relationships were altered in CNV carriers, we further tested for interactions between group and regional brain structure on neurobehavioral domains.
Results:
Cognitive deficits were observed in both CNV groups, with the lowest IQs in deletion carriers. ASD and dimensionally-measured ASD traits were elevated in both CNV groups; however, duplication carriers exhibited increased stereotypies compared to deletion carriers. Moreover, discriminant analysis using ASD sub-domains distinguished between CNV cases with 76% accuracy. Both psychotic disorder diagnosis and dimensionally-measured positive and negative symptoms were elevated in deletion carriers. Finally, control participants showed an inverse relationship between processing speed and cortical thickness in heteromodal association areas, which was absent in both CNV groups.
Conclusions:
22q11.2 CNVs differentially modulate intellectual functioning and psychosis-related symptomatology but converge on broad ASD-related symptomatology. However, subtle differences in ASD profiles distinguish CNV groups. Processing speed impairments, coupled with the lack of normative relationship between processing speed and cortical thickness in CNV carriers, implicate aberrant development of the cortical mantle in the pathology underlying impaired processing speed ability.
Keywords: CNV, social behavior, cognition, Autism Spectrum Disorder, Schizophrenia
Introduction
Developmental neuropsychiatric disorders such as schizophrenia (SCZ) and autism spectrum disorders (ASD) have remarkable genetic and phenotypic heterogeneity. These biological complexities have impeded efforts to elucidate disease pathophysiology. One potential strategy to overcome this challenge is to adopt a reverse-genetics approach to characterize brain and behavioral patterns in individuals that carry defined genetic mutations that predispose toward neuropsychiatric illness. Further, variation in specific symptoms and dimensional traits may more precisely represent underlying biological variation than does diagnostic category (1, 2). Thus, dimensional phenotyping of these high-impact mutations offers a window to elucidating neurobiological mechanisms underlying major neuropsychiatric disorders (3, 4).
22q11.2 copy number variants (i.e. deletions or duplications, CNVs) confer some of the greatest known genetic risks for psychiatric disorders (5-7). As such, they represent a particularly powerful model to yield biological insights into how 22q11.2 gene dosage may influence downstream brain and behavioral consequences (8, 9). The 22q11.2 locus is a genetic hotspot that harbors highly-conserved genes critical for brain and cognitive development (10, 11). A 1.5-3 megabase deletion at this locus results in the most commonly-known microdeletion disorder, 22q11.2 Deletion Syndrome (also known as DiGeorge or Velocardiofacial syndrome; OMIM #188400, #192430). It has an estimated prevalence of 1 in ~4,000 live births and has been extensively characterized for associated congenital malformations and neurodevelopmental comorbidities, including Autism Spectrum Disorder (ASD), intellectual disability (ID), and developmental delay (DD) (5, 12-14). Most notably, the deletion is one of the greatest known genetic risk factors for schizophrenia, with up to 20-fold increased risk compared to population-base rates (3, 15-18). Moreover, 22q11.2 deletions are found in approximately 0.3% of schizophrenia cases in the general population (18-20).
In contrast, data on the neurobehavioral phenotype of the 22q11.2 duplication is just starting to emerge, in part due to its more recent discovery as a recurrent CNV (21-23). A recent population-based study found that 22q11.2 duplications occur ~2.5 times as often as the deletion and confer elevated risk for neurodevelopmental and psychiatric conditions (14). However, most available clinical knowledge to date comes from case reports (24-28) and recent studies that assayed multiple CNVs across the genome (29-31). These studies indicate incomplete penetrance and variable expressivity of the duplication, including mild to severe forms of dysmorphia and ID/DD.
Interestingly, multiple large-scale studies have recently shown 22q11.2 duplications to be significantly less common in schizophrenia cases than in the general population, suggesting the first putative protective mutation for schizophrenia (17, 32, 33). While statistical significance varies depending on sample size (18), the overall pattern across such large studies offers consistent evidence that SCZ risk is gene dosage-specific with respect to the 22q11.2 locus. Thus, while preliminary, this intriguing distinction indicates copy-number dependency for risk or putative protective factors for schizophrenia versus copy-number irrelevance for risk of ASD and ID, and suggests that there may be both general and specific effects of gene dosage on disease evolution.
No study has yet directly compared reciprocal 22q11.2 CNVs to each other and to typically developing controls across multiple neurobehavioral traits relevant to schizophrenia, ASD, and neurocognitive functioning, in conjunction with brain imaging measures. This ‘deep-phenotyping’ approach is crucial to establishing clinical context and functional relevance for how variable gene dosage may contribute to shared or distinct effects on brain and behavioral traits. Here, we sought to obtain a finer granularity of dimensional neurobehavioral traits to capture intermediate phenotypes of cognitive and behavioral dysfunction associated with these CNVs. Moreover, while 22q11.2 gene dosage is associated with global opposing effects on brain structure (34), it is unknown whether these opposing effects are associated with similar or distinct neurobehavioral impairments. Integration of brain and high-dimensional cognitive and behavioral assays may help elucidate common or discrete brain biomarkers and biological pathways that lead to cognitive and/or psychiatric dysfunction.
Here, in the largest known cohort of phenotypically well-characterized 22q11.2 reciprocal CNV carriers, we first compared the impact of 22q11.2 deletions and duplications on 15 dimensional traits relevant to intellectual functioning, ASD, and SCZ. Next, given evidence that both 22q11.2 deletions and duplications predispose to ASD, we assessed whether there are differences in specific aspects of the ASD profile between CNV carrier groups. Finally, we asked whether the relationship between brain structure and neurobehavioral traits differed between these genetically-defined groups and controls, which would inform neuroanatomic substrates of neurocognitive and behavioral impairment in 22q11.2 CNVs.
Methods and Materials
PARTICIPANTS
The sample consisted of 226 individuals: 106 with molecularly confirmed 22q11.2 deletions (52 males; 54 females), 38 with confirmed 22q11.2 duplications (22 males; 16 females), and 82 demographically-matched typically developing controls (42 males; 40 females; see Table 1 for participant demographics). Patients were ascertained as part of an ongoing longitudinal study at the University of California at Los Angeles, and were ascertained from local medical or genetics clinics, or from national or local support groups and websites. Demographically comparable, typically-developing participants were recruited from local communities via web-based advertisements and flyers/brochures in local schools, pediatric clinics, and other community sites.
Table 1.
Demographic information between groups at baseline.
| 22q11.2 Deletion Carriers |
Typically- developing Controls |
22q11.2 Duplication Carriers |
|
|---|---|---|---|
| N | 106 | 82 | 38 |
| Age (SD) | 15.94 (10.3) | 14.61 (7.5) | 15.87 (12.4) |
| Age Range | 6 to 61 | 6 to 45 | 5 to 49 |
| N males (%) | 52 (49.1%) | 41 (50.0%) | 22 (57.9%) |
| N White (%)a,b,c | 92 (86.8%) | 52 (63.4%) | 37 (97.4%) |
| N Other (%)a,b | 2 (1.9%) | 18 (16.8%) | 1 (2.7%) |
| N Multi-racial (%)b,c | 12 (11.2%) | 12 (14.6%) | 0 (0%) |
| Highest Parental Education, in years (SD) | 16.1 (2.7) | 16.0 (3.3) | 15.8 (2.5) |
| N ASD (%)a,b | 49 (46.2%) | 0 (0%) | 17 (44.7%) |
| N Psychosis (%)a,c | 13 (12.3%) | 0 (0%) | 0 (0%) |
| N ADHD (%)a,b | 47 (44.4%) | 5 (6.1%) | 15 (39.4%) |
corrected 22qDEL-CON difference
corrected 22qDUP-CON differences
corrected 22qDEL-22qDUP difference.
Exclusion criteria for all study participants included significant neurological or medical conditions (unrelated to 22q11.2 CNV) that might affect brain structure or function, history of head injury with loss of consciousness, insufficient fluency in English, and/or substance or alcohol use disorder within the past 6 months (see Supplement for details). All participants underwent a verbal and written informed consent process. Participants under the age of 18 years provided written assent, while their parent or guardian completed written consent. The UCLA Institutional Review Board approved all study procedures and informed consent documents.
NEUROBEHAVIORAL PHENOTYPING ASSESSMENT
Cognitive and behavioral traits were chosen based on their relevance for developmental psychiatric disorders, with emphasis on ASD and SCZ (15, 35-37). Age-appropriate, gold-standard psychiatric and cognitive assessments were administered to all participants and included a battery of structured interviews, self-reports, and cognitive tests, spanning several neuropsychological domains. Behavioral questionnaires were also given to parents (see Table 2 for assessment descriptions). Supervised clinical psychology doctoral students administered neurocognitive and psychodiagnostic evaluations to participants to assess for DSM diagnoses (SCID/CDISC). To assess for ASD, the Autism Diagnostic Observation Schedule (ADOS) was administered to participants (38), and the Autism Diagnostic Interview-Revised (ADI-R) was administered to participant’s parent/primary caretaker (39).
Table 2.
Cognitive and behavioral trait descriptions.
| Domain | Test | Trait | Measure |
|---|---|---|---|
| Neurocognition | Wechsler Abbreviated Scale of Intelligence or Wechsler Adult Intelligence Scale, Ed 4 | Full-scale IQ | Standard score |
| Nonverbal IQ | Standard score | ||
| Verbal IQ | Standard score | ||
| Letter Number Sequencing, WISC-IV | Working Memory | Percent correct | |
| California Verbal Learning Test | Verbal Memory | Sum of raw scores | |
| Brief Assessment of Cognition Symbol coding/WISC-IV Coding A or B | Processing Speed | Percent correct | |
| Social Cognition | The Awareness of Social Inference Test A: Part 3 | Lie Detection | Sum of scores |
| Sarcasm Detection | Sum of scores | ||
| Penn Computerized Neurocognitive Battery | Emotion Recognition | Sum of scores | |
| Emotion Differentiation | Sum of scores | ||
| ASD-related | Short Sensory Profile, Adolescent/Adult Sensory Profile | Sensory Insensitivity | Sum of scores |
| Repetitive Behavior Scale | Restrictive and Repetitive Behavior | Sum of scores | |
| Social Responsiveness Scale | Reciprocal Social Behavior | Summary T-score | |
| Psychosis-related | Structured Interview for Prodromal Syndromes | Positive Symptoms | sum of P1-P5 |
| Negative Symptoms | sum of N1-N6 |
All diagnoses were determined by trained clinicians who participated in an ongoing quality assurance program. Training, reliability, and ongoing quality assurance procedures for psychodiagnostic assessments and clinical rating scales are detailed in prior publications (40, 41).
Cognitive functioning:
We assessed cognition using measures of global cognitive function, including: full-scale, verbal IQ, and nonverbal IQ (i.e., Matrix Reasoning (42, 43)), working memory, processing speed (43), and verbal memory (44).
Social cognition measures:
Social cognition impairments are hallmark features of both idiopathic ASD and SCZ (45-47). Thus, four measures were analyzed to capture different aspects of social cognition. To assess theory of mind, we analyzed participants’ ability to ascertain sarcasm or ‘white lies’ from conversational exchanges in video vignettes (48). In addition, we used the Penn-CNB to test ability to recognize different facial expressions of emotion and to differentiate the intensity of facial expressions of emotion (49).
Autism spectrum measures:
Because ASD diagnosis is prevalent in both CNV groups (5, 50), we aimed to assess three cardinal dimensions of ASD-relevant symptomatology, including: sensory sensitivity (51), restrictive/repetitive behavior (52), and social responsiveness (53). Within each of those composite measures, we additionally investigated sub-scale traits. For the Short Sensory Profile, we looked at subdomains of tactile sensitivity, taste/smell sensitivity, movement sensitivity, under-responsivity/seeking sensation, auditory filtering, low energy/weakness and visual/auditory sensitivity. Restrictive/repetitive behavior was further subdivided into categories of stereotyped behavior, self-injurious behavior; compulsive behavior, ritualistic behavior, sameness behavior, and restricted behavior. The Social Responsiveness Scale was subdivided into social awareness, social cognition, social communication, social motivation, and autistic mannerisms.
Psychosis-relevant measures:
The Structured Interview for Prodromal Syndromes (SIPS; (54)) was used to capture dimensional psychosis-relevant traits by quantifying positive symptoms (e.g. unusual thought content/delusional ideas, suspiciousness/persecutory ideas, perceptual abnormalities/hallucinations, grandiosity, and disorganized communication) as well as negative symptoms (social anhedonia, avolition, decreased emotional expression or experience, decreased ideational richness, and occupational impairments).
STRUCTURAL MRI
Measures of brain structure were obtained with high-resolution structural MRI. Scanning was conducted on an identical 3 Tesla Siemens Trio MRI scanner with a 12-channel head coil at the UCLA Brain Mapping Center or at the Center for Cognitive Neuroscience. T1-weighted structural scans were analyzed in an unbiased, whole-brain approach using well-validated analysis and quality control protocols (55), previously applied by our group and others (56-59). Details of the scanning protocol, image pre-processing, and quality control procedures are included in Supplemental Material.
STATISTICAL ANALYSIS
All statistical analyses were performed using R 3.5.2 (60) except for the discriminant analyses which were conducted in Matlab version 2017a (61). First, we tested the extent to which 22q11.2 CNVs influenced neurobehavioral domains relevant to intellectual functioning, ASD, and SCZ by comparing group differences across 15 measures (see Figure 1, Table 2). Toward this end, we performed an omnibus analysis of covariance (ANCOVA) to test the effect of group on each measure, adjusting for age and sex. Given the number of measures analyzed, we took a conservative approach to ensure standardized removal of potential age and sex biases by applying the same covariate adjustments for each univariate model. Post-hoc pairwise comparisons between groups were conducted using Tukey contrasts. Multiple comparisons correction using false discovery rate (FDR) was applied to account for the number of traits and group comparisons (62). FDR-adjusted g-values < 0.05 were considered significant.
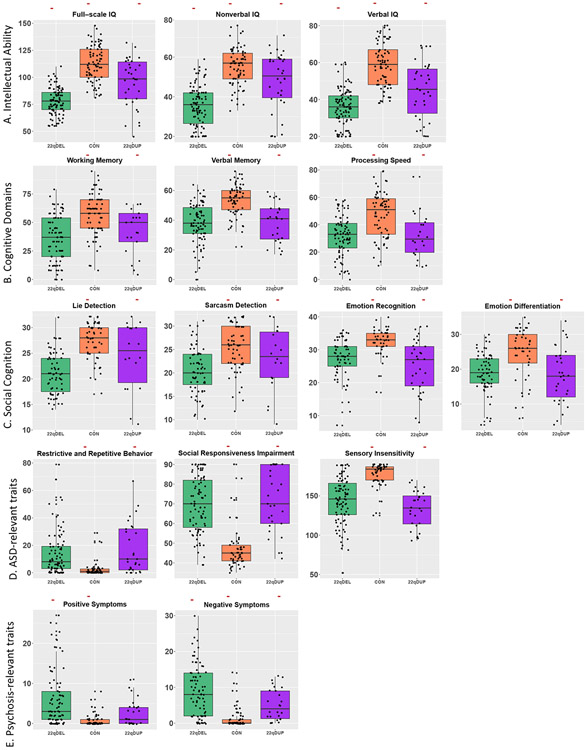
Figure 1. Combined box- and scatterplots of average group scores for 15 traits relevant for intellectual ability, ASD, and SCZ, adjusted for sex and age.
Red, horizontal lines indicate FDR-corrected pairwise differences at the post-hoc level. For all measures, there was a significant omnibus effect of group (q < 0.05). At the post-hoc pairwise level, 22q11.2 deletion carriers exhibited significantly elevated positive symptoms compared to duplication carriers and controls. There were significant pairwise differences between all three groups for Full-scale, Nonverbal, and Verbal IQ as well as for negative symptoms. For the remaining cognitive, social cognitive, and ASD-related measures, both the CNV groups were impaired compared to controls, but did not significantly differ from each other.
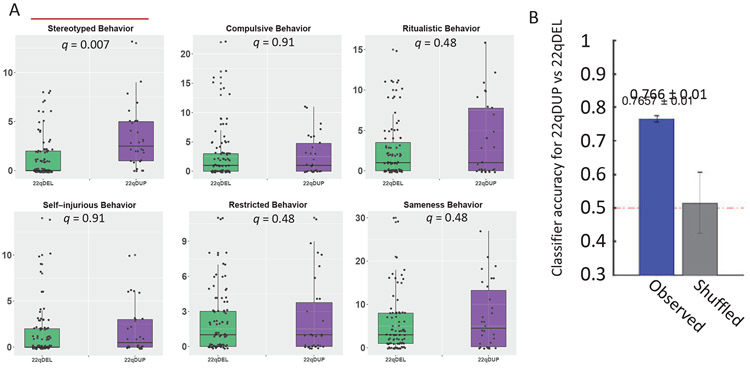
Second, within CNV carriers, we asked whether ASD profiles between deletion and duplication carriers differed across subscale measures using a mass univariate as well as multivariate approach. The univariate model directly assessed group differences for each subscale measure by modeling the effect of group, while correcting for age and sex. FDR correction was performed for the number of subscale measures within each composite trait. The multivariate model used Fisher’s linear discriminant analysis to distinguish between CNV carriers, based on all 18 ASD subscale measures. Model performance was assessed using leave one out cross-validation. To account for differences in sample size between the groups and equalize class representation, data from the duplication cohort was uniformly oversampled. “Control” models were constructed by randomly shuffling class identities within the training set prior to model construction and cross-validation. Mean and standard deviations of model accuracy represent statistics that were computed over 100 simulations of model construction and testing over all subjects. The p-value was generated based on the fraction of the shuffled simulations in which performance exceeded the observed value (see Figure 2).
Figure 2. Modeling subdomains of ASD traits reveals distinct profiles between 22q11.2 deletion and duplication carriers.
A. The univariate model showed that duplication carriers exhibited significantly more stereotyped behaviors compared to deletion carriers (indicated by red, horizontal line) after adjusting for age and sex, at a corrected q < 0.05. B. The multivariate model using discriminant analysis of all 18 subscales correctly classified deletion versus duplication carriers 76.6% of the time, suggesting distinct characteristics of ASD-relevant symptomatology in deletion versus duplication carriers.
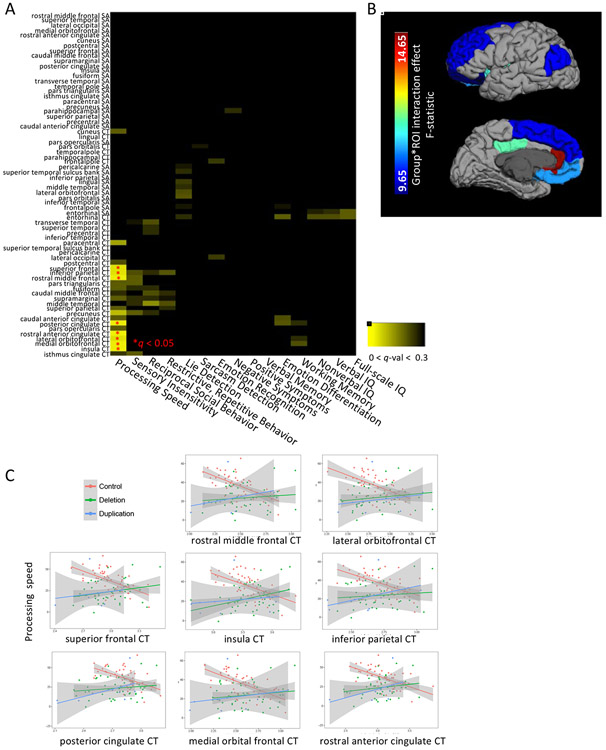
Finally, we asked whether the relationship between regional brain structure and each of the cognitive/behavioral traits differed as a function of group. To reduce the number of comparisons, measures of cortical thickness (CT) and surface area (SA) were averaged across homologous regions-of-interest (ROIs) between each hemisphere, generating 34 total ROIs, for which 2 brain metrics (CT or SA) were assessed. First, as before, each neurobehavioral trait score was adjusted for effects of age and sex. Then, the main effect of group, brain metric, and group-by-brain metric interaction term were included as predictors in separate linear models for each residualized neurobehavioral score. Because we were interested in relationships between brain and behavior that differed between groups, we focused on the interaction effect (see Figure 3, Figure S4). Finally, for traits in which there was a significant, corrected interaction effect, post-hoc comparisons were performed to test pairwise differences. Also, for those traits and ROIs which showed a significant group-by-brain interaction effect, within-group linear models were conducted to directly characterize the relationships between brain structure and trait (see Table 4). FDR correction at q < 0.05 was used to account for the number of ROIs, neurobehavioral traits, and brain metrics in the omnibus test, while correction of the post-hoc tests accounted for the number of pairwise comparisons.
Figure 3: Regional cortical thickness (CT) of frontal, inferior parietal, and medial regions differentially explains processing speed depending on group.
After regressing out age and sex from cognitive measures, a linear model was applied to estimate the interaction effect of brain and group for each of the 15 traits using measures of CT or SA. False discovery rate correction was applied for 34 ROIs,15 traits, and each brain metric (SA or CT). Panel A depicts a heatmap of the q-values for the significance of the interaction term associated with each test, from q = 0 to q = 0.30. Panel B depicts the ROIs for which there was a corrected interaction effect, plotting the associated F-statistic. Panel C displays the within-group correlation between processing speed and CT for each of the 8 regions that showed a significant interaction effect. The control group showed a consistent negative correlation between CT and processing speed that was not found in either CNV group.
Table 4.
Differential effects of regional cortical thickness by group on processing speed.
| Brain region (CT) | Group*Brain Term q-val |
Control slope |
Control q-val |
Control R2 |
22qDEL slope |
22qDEL q-val |
22qDEL R2 |
22qDUP slope |
22qDUP q-val |
22qDUP R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Inferior parietal gyrusa,b | 0.04 | −45.4 | 4.70E-04 | 0.31 | 53.7 | 0.85 | 0.01 | 76.4 | 0.76 | 0.16 |
| Insulaa,b | 0.02 | −35.2 | 2.80E-03 | 0.25 | 56.7 | 0.13 | 0.12 | 43.1 | 0.93 | 0.03 |
| Lateral orbitofrontal gyrusa,b | 0.04 | −33.7 | 3.90E-04 | 0.32 | 42.1 | 0.76 | 0.03 | 44 | 0.91 | 0.04 |
| Medial orbitofrontal gyrusa,b | 0.02 | −36.1 | 1.30E-04 | 0.38 | 44.3 | 0.76 | 0.02 | 47.5 | 0.87 | 0.05 |
| Posterior cingulate gyrusa,b | 0.02 | −40 | 1.30E-03 | 0.27 | 48.7 | 0.8 | 0.02 | 71.6 | 0.67 | 0.28 |
| Rostral anterior cingulate gyrusa,b | 0.02 | −28.1 | 2.80E-03 | 0.35 | 37.4 | 0.7 | 0.04 | 50.9 | 0.7 | 0.24 |
| Rostral middle frontal gyrusa | 0.02 | −42.2 | 1.30E-04 | 0.39 | 49.2 | 0.84 | 0.01 | 62.3 | 0.85 | 0.08 |
| Superior frontal gyrusa | 0.03 | −39.7 | 8.00E-04 | 0.29 | 54.8 | 0.51 | 0.06 | 53.3 | 0.93 | 0.03 |
The "Group-by-brain Term q-value" column includes the q-value of the interaction term for the model: processing speed ~ group + brain + group * brain. The subsequent columns include the slope, FDR-corrected q-value, and R2 terms for the within-group model for each group: processing speed ~ brain. Post-hoc, pairwise Tukey tests showed that the relationships between processing speed and CT were different between controls and each of the CNV groups, except for the rostral middle frontal gyrus and superior frontal gyrus for which the duplication group did not differ significantly from the control group.
corrected 22qDEL-CON difference
corrected 22qDUP-CON differences
corrected 22qDEL-22qDUP difference.
Associations between ASD-related impairments and cognitive abilities:
We further explored whether ASD-related impairments correlated with cognitive function in deletion and duplication carriers. As these results were beyond the main scope of this paper, they are included in Supplementary Material (Figure S2).
Social-emotional behavior and real-world function:
Secondary analyses of group differences in measures of social-emotional behavior and real-world function not specific to ASD or SCZ are included in Supplemental Material, as these were also beyond the main scope of this paper (Figure S3, Tables S1 and S2).
Results
22q11.2 CNVs produce convergent phenotypes relevant for intellectual disability and ASD but divergent psychosis-relevant phenotypes
At q < 0.05, 22q11.2 deletion carriers exhibited the lowest Full-Scale IQ, control participants displayed the highest IQ, while duplication carriers were intermediate (del<dup<con; see Figure 1, Table 3). The same pattern remained when Full-Scale IQ was broken down into its constituent parts, verbal and non-verbal IQ. Regarding specific cognitive domains, deletion and duplication carriers exhibited significant impairments in working memory, verbal memory, and processing speed compared to controls, but did not significantly differ from each other.
Table 3.
ANCOVA results including all 15 measures, covarying for age and sex.
| Trait | F-statistic | 22qDEL-Control Estimate (SE) |
22qDUP-Control Estimate (SE) |
22qDUP-22qDEL Estimate (SE) |
|---|---|---|---|---|
| Nonverbal Iqa,b,c | 75.68 | −19.93 (−1.64) | −7.25 (−2.22) | 12.68 (−2.14) |
| Full-scale IQa,b,c | 105.82 | −35.52 (−2.45) | −17.38 (−3.29) | 18.14 (−3.19) |
| Verbal IQa,b,c | 87.05 | −23.29 (−1.77) | −13.86 (−2.38) | 9.43 (−2.3) |
| Working Memorya,b | 31.73 | −21.86 (−2.8) | −18.99 (−4.41) | 2.87 (−4.3) |
| Verbal Memorya,b | 44.27 | −16.1 (−1.78) | −15.28 (−2.56) | 0.82 (−2.48) |
| Processing Speeda,b | 38.66 | −16.49 (−2.02) | −18.74 (−3.12) | −2.24 (−3.04) |
| Lie Detectiona,b | 42.05 | −6.71 (−0.73) | −3.94 (−1.12) | 2.77 (−1.08) |
| Sarcasm Detectiona,b | 16 | −4.95 (−0.88) | −3.58 (−1.34) | 1.37 (−1.3) |
| Emotion Recognitiona,b | 17.35 | −5.08 (−1.07) | −6.89 (−1.27) | −1.81 (−1.12) |
| Emotion Differentiationa,b | 13.65 | −5.64 (−1.19) | −6.22 (−1.45) | −0.57 (−1.34) |
| Sensory Insensitivitya,b | 44.12 | −36.19 (−4.45) | −44.58 (−5.77) | −8.39 (−5.58) |
| Restrictive and Repetitive Behaviora,b | 14.6 | 11.9 (−2.59) | 14.8 (−3.3) | 2.92 (−3.2) |
| Social Responsiveness Impairmenta,b | 48.91 | 21.7 (−2.45) | 24.48 (−3.14) | 2.79 (−3.03) |
| Positive Symptomsa,c | 19.53 | 5.19 (−0.84) | 2.02 (−1.15) | −3.18 (−1.11) |
| Negative Symptomsa,b,c | 33.79 | 7.77 (−0.95) | 3.69 (−1.3) | −4.08 (−1.26) |
corrected 22qDEL-CON difference
corrected 22qDUP-CON differences
corrected 22qDEL-22qDUP difference.
Both CNV groups had significantly higher rates of ASD diagnosis compared to controls (see Table 1). Dimensionally, as expected compared to controls, both 22q11.2 CNV groups showed significantly poorer social cognition (e.g. emotion differentiation, emotion recognition, lie detection, sarcasm detection), as well as elevated scores on reciprocal social behavior, sensory sensitivity, and restrictive, repetitive behavior, which were not significantly different from each other. In contrast, deletion carriers diverged from duplication carriers in the psychosis domain, with higher rates of psychotic disorder diagnosis (12.1%; Table 1), as well as significantly elevated positive and negative symptom scores (Table 3).
Deletion and duplication carriers did not differ from each other in rates of ADHD diagnosis, but both groups had significantly elevated rates of ADHD relative to controls (see Table 1). Further, compared to controls, both CNV groups had significant impairments in dimensional measures of executive and daily life functioning (i.e. somatic complaints, thought problems, withdrawn/depressed, attention problems, anxious/depressed, aggression, role functioning, social functioning, and global functioning). CNV groups did not significantly differ from each other except in terms of role functioning, for which deletion carriers were more impaired (Figure S3, Tables S1 and S2).
22q11.2 CNVs involve subtle differences in ASD profile, despite similar rates of ASD diagnosis
Assessments on the subdomains of the 3 composite ASD measures of social responsiveness, sensory sensitivity, and restrictive/repetitive behavior revealed differences in ASD-relevant symptom profiles between the CNV groups (See Figure 2). Specifically, the univariate model revealed significantly increased stereotyped behaviors in duplication relative to deletion carriers (q < 0.05). No corrected differences between the deletion and duplication groups were found for any of the other individual subscale measures at q < 0.05 (see Figure S1). However, the multivariate model that incorporated all 18 subscale features correctly classified deletion versus duplication carriers 76.6% of the time (p < 10−3.), suggesting distinctiveness in the overall picture of ASD symptomatology between deletion and duplication carriers. Further, verbal IQ was correlated with social cognition traits in both CNV groups, with larger effects in duplication carriers (q<0.05; Figure S2).
Controls showed an inverse relationship between cortical thickness in heteromodal association areas and processing speed that was absent in CNV carriers
Lastly, CT in frontal, medial, and inferior parietal regions differentially explained processing speed, depending on group (see Figure 3). Table 4 includes ROIs that showed a significant, FDR-corrected group-by-brain interaction effect on processing speed. Specifically, processing speed was inversely correlated with CT in controls in frontal, inferior parietal, and medial regions (anterior cingulate and insula), but not in either CNV group. No significant group-dependent relationships between surface area and any cognitive/behavioral trait survived correction. See Figure S4 for uncorrected p-values across all ROIs for both CT and SA.
Discussion
This study revealed several novel findings regarding the impact of 22q11.2 CNVs on neurobehavioral function, in that they: (i) produce comparable impairments in traits relevant for ASD and intellectual functioning but differential impairments in psychosis-related traits; (ii) comprise distinct differences in ASD profile at the subdomain level; and (iii) both lack the normative relationships between processing speed and cortical thickness in higher-order brain regions.
Notably, these reciprocal CNVs conferred broad ‘hits’ across multiple neuropsychiatric domains, with shared as well as unique effects on dimensional traits of ASD and psychosis. Specifically, we found convergent effects of both CNVs on intellectual abilities, specific cognitive domains, and social cognition, with greater impairment in intellectual abilities in the deletion group, consistent with elevated rates of ID in 22q11.2 deletion versus duplication (6, 13, 14). Rates of ASD were similar in deletion and duplication carriers (45.8% and 44.7%, respectively), whereas 12.1% of deletion carriers (and no duplication carriers) had a psychotic disorder diagnosis.
Both CNV groups also exhibited elevations in dimensional, ASD-related traits compared to controls, consistent with the significantly higher rates of ASD diagnosis across both CNV groups (5, 14, 50). These findings suggest that both over and under-expression of genes within the 22q11.2 locus may result in downstream pathogenic effects on cognition and social behavior. Considering only our 22q11.2 deletion participants who have mostly passed the risk period for schizophrenia onset (≥ 25 years old; (63)), the rate of schizophrenia increases to 25%, which is more comparable to rates reported in other studies that typically include adults. Further, deletion carriers exhibited greater positive and negative symptoms compared to the other two groups. This specific association of both categorically- and dimensionally-measured SCZ-related symptoms with the deletion suggests that under-expression of 22q11.2 genes may represent a specific biological mechanism predisposing toward psychotic symptomatology.
Although mean global IQ in deletion carriers was significantly lower than duplication cases, the CNV groups showed similar impairments in other cognitive and behavioral domains. Dimensionally, these include working memory, verbal memory, processing speed, social cognition (e.g. lie/sarcasm detection and emotion differentiation/recognition), and composite ASD-related traits (e.g. restrictive/repetitive behavior, social responsiveness, and sensory sensitivity) compared to controls. These findings are compatible with results from the population-based registry study on the cumulative incidence of psychiatric diagnoses in 22q11.2 CNV carriers in the Danish population, which found greater ID in 22q11.2 deletion carriers but increased rates of developmental neuropsychiatric disorders in both deletion and duplication carriers (13, 14). The comorbidity of ID and psychiatric disorders, namely for ASD and SCZ, has been extensively discussed in the literature, particularly with regard to CNVs (17, 30, 31, 64). However, the mechanisms by which reciprocal deletions and duplications at the same locus can have divergent effects on intellectual dysfunction and psychosis-related traits (17, 18, 32, 33) but converge on similar impairments for specific cognitive domains and social cognition remain unknown. Notably, ASD diagnosis does not appear to increase risk of subsequent development of schizophrenia in 22q11.2 deletion carriers, suggesting pleiotropic effects of 22q11.2 gene dosage, at least with regard to these neurobehavioral outcomes (65, 66).
Animal models of 22q11.2 CNVs involving over or under-expression of specific genes within the homologous 22q11.2 locus offer crucial insight into potential mechanisms that underlie brain and behavioral dysfunction in human CNV carriers. Such experimental paradigms can elucidate the ways in which reciprocal gene dosage contributes to impairment in the same cognitive domain at single-gene resolution. For example, transgenic mice constitutively overexpressing a 190 kb human chromosomal 22q11.2 segment, including TXNRD2, COMT, and ARVCF, showed impairments in prolonged maintenance of working memory during a delay task (67). In another murine model, region-specific overexpression of COMT and Tbx1 in the hippocampal dentate gyrus resulted in reduced developmental maturation of working memory capacity, as well as reduced proliferation and migration of adult neural stem/progenitor cells (68). Working memory impairments using a T-maze paradigm (69) have also been observed in mice heterozygous for Tbx1. Because the authors found Tbx1 expression in postnatally generated neurons and in cells that differentiate into glial cells, they posit altered neurogenesis or reactive glial proliferation as potential mechanisms that may underlie working memory impairment. Moreover, mouse studies of Dgcr8 deficiency have also implicated potential mechanisms of working memory impairment in the form of reduced adult hippocampal neurogenesis (70), as well as perturbed short-term synaptic plasticity in prefrontal cortex (71). Whether similar mechanisms explain convergent effects on other cognitive domains such as verbal memory and processing speed is unknown, as a remaining challenge will be overcoming the animal to human translation to test these and other human-specific traits.
One important implication of these findings is that lack of genetic material is more broadly deleterious than excess of the same genetic material, suggesting that certain 22q11.2 genes are sensitive to haploinsufficiency. This is consistent with the prediction that deletion carriers would be more severely affected than duplication carriers (72), given that the deletion is more likely to arise de novo (28), whereas the duplication is more frequently inherited (25). In the context of other reciprocal CNVs, 16p11.2 deletion has been associated with a 2 standard deviation decrease in IQ relative to controls, while the duplication was associated with only a 1 standard deviation decrease (73-75). Also similar to our findings, there were no differences in the prevalence of ASD between 16p11.2 CNV groups, suggesting multiple pathways to ASD that include over and under-expression of genes implicated in neurodevelopment across multiple genomic locations. However, divergent from our findings, 16p11.2 duplication carriers showed increased frequency of psychotic symptoms and severe psychiatric disorders relative to 16p11.2 deletion carriers, further demonstrating increased specificity for copy number and genomic location in mediating psychosis risk, compared to ASD (76). More direct and targeted genomic assays are warranted to disentangle how deletions or duplications may lead to transcriptomic dysregulation to cause widespread, convergent as opposed to specific, divergent effects on downstream brain and behavioral impairments.
Despite global similarities in social cognitive and social behavioral impairments across 22q11.2 CNVs, at a more granular level, 22q11.2 duplication carriers exhibited more stereotyped behaviors than 22q11.2 deletion carriers. The only other in-depth investigation of ASD-related phenotypes in 22q11.2 CNV carriers to date also found elevated stereotypies in duplication carriers, albeit in a smaller sample (50). Murine models have implicated the nigrostriatal dopamine pathway in the mediation of stereotypies, where administration of indirect and direct dopamine agonists or selective dopamine uptake inhibitors have been shown to induce stereotypic behaviors (77, 78). These findings suggest neuroanatomical relevance and potential biochemical mechanisms for increased 22q11.2 gene dosage leading to greater stereotyped behaviors. However, ASD is notoriously heterogeneous and not diagnosable by a single behavioral scale. Thus, using a multivariate discriminant analyses, we showed that a collection of ASD-associated traits was informative in classifying deletion versus duplication carriers with 76.6% accuracy. This significantly above-chance performance indicates that the overall manifestation of ASD-relevant traits is complex and differs between reciprocal 22q11.2 CNVs in a way that was not captured by traditional univariate methods. Thus, while rates of ASD diagnosis and composite dimensional measures may not statistically differ between groups, subtle and meaningful differences in clinical profile exist. Other studies have also used multivariate approaches and found ASD-related behavioral signatures of ASD cases who carry 22q11.2 deletions compared to ASD cases with different genetically-defined syndromes (e.g., Down's syndrome, Prader-Willi, tuberous sclerosis complex (79, 80)). Collectively, these findings emphasize the utility of a “genetics-first” approach for disentangling the heterogeneity and revealing biological underpinnings of ASD. In sum, our findings suggest a need for more comprehensive clinical screening of 22q11.2 duplication carriers, as well as informed guidance in genetic counseling, optimization of health care, and clinical follow-up.
We also found that thickness of heteromodal association regions explained processing speed ability in typical development, but not in either CNV group. More specifically, cortical thinning in these regions within the control group was associated with better processing speed, suggesting that developmental thinning within the cortical mantle underlies the neural circuitry that supports processing speed ability. Processing speed refers to the efficiency of information transfer and manipulation (81) and is a complex, multidimensional ability, which improves over adolescent development and constrains other higher-order cognitive processes in health and disease (82-85). Not surprisingly, impairments in processing speed have also been implicated in various complex neuropsychiatric disorders. In high-functioning ASD, processing speed task performance has been correlated with communication abilities, indicating its importance to functional outcomes in ASD (86, 87). In patients with schizophrenia, processing speed ability is strongly associated with illness risk (88-90), illness severity (91) and functional disability (92). The specific association with cortical thickness as opposed to surface area suggests that developmentally-mediated, normative cortical thinning (for circuit refinement via white matter expansion from increasing myelination and/or pruning of inefficient synaptic connections (93-95)) may underlie processing speed ability. As such, distributed neural network operations supported by myelinated axonal fibers are likely relevant to processing speed (96, 97). White matter alterations have been consistently observed in schizophrenia (98), ASD (99), and 22q11.2 Deletion syndrome via human post-mortem and in-vivo imaging studies (100). As specific genes within the 22q11.2 region are implicated in axonal development and myelination (101, 102), abnormal dosage of these genes may contribute to the underlying pathophysiology of processing speed deficits that cross many developmental psychiatric disorders (103, 104). Thus, these findings highlighting the link between 22q11.2 gene dosage and cortical thickness provide opportunities to test novel mechanistic hypotheses regarding the interaction between under- or over-expression of 22q11.2 genes and processing speed on cortical development in cell cultures and animal models.
Several limitations to this study should be considered. As with any study involving a clinically-ascertained cohort, the possibility of ascertainment bias may influence representativeness of our cohort. As such, differences we observed between CNV carriers and controls may be attenuated in an epidemiologic cohort, particularly for 22q11.2 duplication carriers who are more likely under-diagnosed than deletion carriers due to the lack of associated congenital and medical issues that prompt genetic testing (105). Importantly, our rates of childhood psychiatric disorders are largely comparable to those observed in epidemiologically-based studies of 22q11.2 CNVs (13, 14). Further, while some duplication carriers in our study were diagnosed based on developmental or medical concerns, our duplication cohort also includes relatives who were not clinically ascertained and are otherwise clinically unremarkable. In addition, CNV size/breakpoints may impact phenotypic severity (56); however, given variability in CNV breakpoints, the current study was under-powered to investigate these effects. As sample sizes increase, future multisite investigations should integrate information on CNV breakpoints to better characterize effects of specific genes within the locus. Finally, sample size limits our capability to model non-linear effects of age, particularly in the duplication cohort. However, our findings of both convergent and divergent 22q11.2 CNV effects motivate longitudinal assays of brain and behavioral development within these CNV groups to better delineate changes in developmental trajectories that result from these genetic perturbations.
This deep phenotyping approach offers a unique opportunity to characterize the functional consequences of high-impact genetic mutations such as 22q11.2 CNVs. Future directions include integrating genomic information to assess whether transcriptomic signatures of these genetic conditions explain differences in downstream brain and behavioral traits, as well as longitudinal studies to assess developmental trajectories. Finally, our findings demonstrate the utility of using 22q11.2 CNVs as a model for bridging the gap between genes, brain, and behavior. Crucially, these findings allow generation of hypothesis-driven, translational experiments using in vivo or in vitro models to validate meaningful biological insights into molecular pathways and identification of relevant cell types and circuits.
Supplementary Material
KEY RESOURCES TABLE
| Resource Type | Specific Reagent or Resource | Source or Reference | Identifiers | Additional Information |
|---|---|---|---|---|
| Add additional rows as needed for each resource type | Include species and sex when applicable. | Include name of manufacturer, company, repository, individual, or research lab. Include PMID or DOI for references; use “this paper” if new. | Include catalog numbers, stock numbers, database IDs or accession numbers, and/or RRIDs. RRIDs are highly encouraged; search for RRIDs at https://scicrunch.org/resources. | Include any additional information or notes if necessary. |
| Deposited Data; Public Database | 106 22q11.2 human deletion carriers (52 male), 38 22q11.2 duplication carriers (22 male), 82 typically-developing control participants (41 male) | Bearden lab; this paper | data reported in this paper are being deposited in NIMH Data Archive (https://nda.nih.gov/) | |
| Software; Algorithm | R 3.5.2; Matlab version 2017a | Core Team R (2013): R: A language and environment for statistical computing. R Foundation for statistical computing, Vienna.; MathWorks I (2017): MATLAB and Statistics and Machine Learning Toolbox, Release 2017a. The MathWorks Inc. Natick, Massachusetts. | ||
| Protocol | Enigma Quality Assesment protocol for neuroimaging analyse | http://enigma.ini.usc.edu/protocols/imaging-protocols/ | ENIGMA Cortical Quality Control Protocol 2.0 (April 2017) |
Acknowledgements:
This work was supported by grants from the National Institute of Mental Health: RO1 MH085953 (CEB), U01MH101719 (CEB), Neurobehavioral Genetics Predoctoral Training Grant (5T32MH073526), and the Simons Foundation (SFARI Explorer Award to CEB).
Footnotes
Financial Disclosures: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Casey BJ, Craddock N, Cuthbert BN, Hyman SE, Lee FS, Ressler KJ (2013): DSM-5 and RDoC: progress in psychiatry research? Nat Rev Neurosci. 14:810–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cuthbert BN (2014): The RDoC framework: facilitating transition from ICD/DSM to dimensional approaches that integrate neuroscience and psychopathology. World Psychiatry. 13:28–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Malhotra D, Sebat J (2012): CNVs: harbingers of a rare variant revolution in psychiatric genetics. Cell. 148:1223–1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Simons Vip C (2012): Simons Variation in Individuals Project (Simons VIP): a genetics-first approach to studying autism spectrum and related neurodevelopmental disorders. Neuron. 73:1063–1067. [DOI] [PubMed] [Google Scholar]
- 5.Schneider M, Debbane M, Bassett AS, Chow EW, Fung WL, van den Bree M, et al. (2014): Psychiatric disorders from childhood to adulthood in 22q11.2 deletion syndrome: results from the International Consortium on Brain and Behavior in 22q11.2 Deletion Syndrome. Am J Psychiatry. 171:627–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Niarchou M, Zammit S, van Goozen SH, Thapar A, Tierling HM, Owen MJ, et al. (2014): Psychopathology and cognition in children with 22q11.2 deletion syndrome. Br J Psychiatry. 204:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monks S, Niarchou M, Davies AR, Walters JT, Williams N, Owen MJ, et al. (2014): Further evidence for high rates of schizophrenia in 22q11.2 deletion syndrome. Schizophr Res. 153:231–236. [DOI] [PubMed] [Google Scholar]
- 8.Hiroi N, Takahashi T, Hishimoto A, Izumi T, Boku S, Hiramoto T (2013): Copy number variation at 22q11.2: from rare variants to common mechanisms of developmental neuropsychiatric disorders. Mol Psychiatry. 18:1153–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiroi N, Yamauchi T (2019): Modeling and Predicting Developmental Trajectories of Neuropsychiatric Dimensions Associated With Copy Number Variations. Int J Neuropsychopharmacol. 22:488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meechan DW, Maynard TM, Tucker ES, Fernandez A, Karpinski BA, Rothblat LA, et al. (2015): Modeling a model: Mouse genetics, 22q11.2 Deletion Syndrome, and disorders of cortical circuit development. Prog Neurobiol. 130:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guna A, Butcher NJ, Bassett AS (2015): Comparative mapping of the 22q11.2 deletion region and the potential of simple model organisms. J Neurodev Disord. 7:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonald-McGinn DM, Sullivan KE, Marino B, Philip N, Swillen A, Vorstman JAS, et al. (2015): 22q11.2 deletion syndrome. Nat Rev Dis Primers. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoeffding LK, Trabjerg BB, Olsen L, Mazin W, Sparso T, Vangkilde A, et al. (2017): Risk of Psychiatric Disorders Among Individuals With the 22q11.2 Deletion or Duplication: A Danish Nationwide, Register-Based Study. JAMA Psychiatry. 74:282–290. [DOI] [PubMed] [Google Scholar]
- 14.Olsen L, Sparso T, Weinsheimer SM, Dos Santos MBQ, Mazin W, Rosengren A, et al. (2018): Prevalence of rearrangements in the 22q11.2 region and population-based risk of neuropsychiatric and developmental disorders in a Danish population: a case-cohort study. Lancet Psychiatry. 5:573–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Green T, Gothelf D, Glaser B, Debbane M, Frisch A, Kotler M, et al. (2009): Psychiatric disorders and intellectual functioning throughout development in velocardiofacial (22q11.2 deletion) syndrome. J Am Acad Child Adolesc Psychiatry. 48:1060–1068. [DOI] [PubMed] [Google Scholar]
- 16.Chow EW, Watson M, Young DA, Bassett AS (2006): Neurocognitive profile in 22q11 deletion syndrome and schizophrenia. Schizophr Res. 87:270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rees E, Kendall K, Pardinas AF, Legge SE, Pocklington A, Escott-Price V, et al. (2016): Analysis of Intellectual Disability Copy Number Variants for Association With Schizophrenia. JAMA Psychiatry. 73:963–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marshall CR, Howrigan DP, Merico D, Thiruvahindrapuram B, Wu W, Greer DS, et al. (2017): Contribution of copy number variants to schizophrenia from a genome-wide study of 41,321 subjects. Nat Genet. 49:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kirov G, Rees E, Walters JT, Escott-Price V, Georgieva L, Richards AL, et al. (2014): The penetrance of copy number variations for schizophrenia and developmental delay. Biol Psychiatry. 75:378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rees E, Walters JT, Georgieva L, Isles AR, Chambert KD, Richards AL, et al. (2014): Analysis of copy number variations at 15 schizophrenia-associated loci. Br J Psychiatry. 204:108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Firth HV (1993): 22q11.2 Duplication In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, et al. , editors. GeneReviews((R)). Seattle (WA). [Google Scholar]
- 22.Ensenauer RE, Adeyinka A, Flynn HC, Michels VV, Lindor NM, Dawson DB, et al. (2003): Microduplication 22q11.2, an emerging syndrome: clinical, cytogenetic, and molecular analysis of thirteen patients. Am J Hum Genet. 73:1027–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Portnoi MF (2009): Microduplication 22q11.2: a new chromosomal syndrome. Eur J Med Genet. 52:88–93. [DOI] [PubMed] [Google Scholar]
- 24.Wentzel C, Fernstrom M, Ohrner Y, Anneren G, Thuresson AC (2008): Clinical variability of the 22q11.2 duplication syndrome. Eur J Med Genet. 51:501–510. [DOI] [PubMed] [Google Scholar]
- 25.Ou Z, Berg JS, Yonath H, Enciso VB, Miller DT, Picker J, et al. (2008): Microduplications of 22q11.2 are frequently inherited and are associated with variable phenotypes. Genet Med. 10:267–277. [DOI] [PubMed] [Google Scholar]
- 26.Yu S, Cox K, Friend K, Smith S, Buchheim R, Bain S, et al. (2008): Familial 22q11.2 duplication: a three-generation family with a 3-Mb duplication and a familial 1.5-Mb duplication. Clin Genet. 73:160–164. [DOI] [PubMed] [Google Scholar]
- 27.Woodward KJ, Stampalia J, Vanyai H, Rijhumal H, Potts K, Taylor F, et al. (2019): Atypical nested 22q11.2 duplications between LCR22B and LCR22D are associated with neurodevelopmental phenotypes including autism spectrum disorder with incomplete penetrance. Molecular Genetics & Genomic Medicine. 7:e00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bassett AS, Marshall CR, Lionel AC, Chow EW, Scherer SW (2008): Copy number variations and risk for schizophrenia in 22q11.2 deletion syndrome. Hum Mol Genet. 17:4045–4053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chawner S, Owen MJ, Holmans P, Raymond FL, Skuse D, Hall J, et al. (2019): Genotype-phenotype associations in children with copy number variants associated with high neuropsychiatric risk in the UK (IMAGINE-ID): a case-control cohort study. Lancet Psychiatry. 6:493–505. [DOI] [PubMed] [Google Scholar]
- 30.Stefansson H, Meyer-Lindenberg A, Steinberg S, Magnusdottir B, Morgen K, Arnarsdottir S, et al. (2014): CNVs conferring risk of autism or schizophrenia affect cognition in controls. Nature. 505:361–366. [DOI] [PubMed] [Google Scholar]
- 31.Kendall KM, Bracher-Smith M, Fitzpatrick H, Lynham A, Rees E, Escott-Price V, et al. (2019): Cognitive performance and functional outcomes of carriers of pathogenic copy number variants: analysis of the UK Biobank. Br J Psychiatry.1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rees E, Kirov G, Sanders A, Walters JT, Chambert KD, Shi J, et al. (2014): Evidence that duplications of 22q11.2 protect against schizophrenia. Mol Psychiatry. 19:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Z, Chen J, Xu Y, Yi Q, Ji W, Wang P, et al. (2016): Genome-wide Analysis of the Role of Copy Number Variation in Schizophrenia Risk in Chinese. Biol Psychiatry. 80:331–337. [DOI] [PubMed] [Google Scholar]
- 34.Lin A, Ching CRK, Vajdi A, Sun D, Jonas RK, Jalbrzikowski M, et al. (2017): Mapping 22q11.2 Gene Dosage Effects on Brain Morphometry. J Neurosci. 37:6183–6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vorstman JAS, Breetvelt EJ, Duijff SN, Eliez S, Schneider M, Jalbrzikowski M, et al. (2015): Cognitive Decline Preceding the Onset of Psychosis in Patients With 22q11.2 Deletion Syndrome. Jama Psychiatry. 72:377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lord C, Cook EH, Leventhal BL, Amaral DG (2013): Autism spectrum disorders. Autism: The Science of Mental Health. 28:217. [Google Scholar]
- 37.Morgan VA, Leonard H, Bourke J, Jablensky A (2008): Intellectual disability co-occurring with schizophrenia and other psychiatric illness: population-based study. Br J Psychiatry. 193:364–372. [DOI] [PubMed] [Google Scholar]
- 38.Lord C, Risi S, Lambrecht L, Cook EH Jr., Leventhal BL, DiLavore PC, et al. (2000): The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 30:205–223. [PubMed] [Google Scholar]
- 39.Rutter M, LeCouteur A, Lord C (2003): Autism diagnostic interview revised (ADI-R) manual (WPS Edition) WPS: Los Angeles, CA. [Google Scholar]
- 40.Jalbrzikowski M, Ahmed KH, Patel A, Jonas R, Kushan L, Chow C, et al. (2017): Categorical versus dimensional approaches to autism-associated intermediate phenotypes in 22q11.2 microdeletion syndrome. Biol Psychiatry Cogn Neurosci Neuroimaging. 2:53–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jalbrzikowski M, Jonas R, Senturk D, Patel A, Chow C, Green MF, et al. (2013): Structural abnormalities in cortical volume, thickness, and surface area in 22q11.2 microdeletion syndrome: Relationship with psychotic symptoms. Neuroimage Clin. 3:405–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wechsler D (1999): Manual for the Wechsler Abbreviated Intelligence Scale (WASI) The Psychological Corporation; San Antonio, Tx. [Google Scholar]
- 43.Wechsler D (2008): Wechsler adult intelligence scale–Fourth Edition (WAIS–IV). San Antonio, TX: NCS Pearson; 22:498. [Google Scholar]
- 44.Delis DC, Kramer JH, Kaplan E, Thompkins BAO (1987): CVLT: California verbal learning test-adult version: manual. Psychological corporation. [Google Scholar]
- 45.Pinkham AE, Penn DL, Perkins DO, Lieberman J (2003): Implications for the neural basis of social cognition for the study of schizophrenia. Am J Psychiatry. 160:815–824. [DOI] [PubMed] [Google Scholar]
- 46.Couture S, Penn D, Losh M, Adolphs R, Hurley R, Piven J (2010): Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychological medicine. 40:569–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pilowsky T, Yirmiya N, Arbelle S, Mozes T (2000): Theory of mind abilities of children with schizophrenia, children with autism, and normally developing children. Schizophr Res. 42:145–155. [DOI] [PubMed] [Google Scholar]
- 48.McDonald S, Bornhofen C, Shum D, Long E, Saunders C, Neulinger K (2006): Reliability and validity of The Awareness of Social Inference Test (TASIT): a clinical test of social perception. Disabil Rehabil. 28:1529–1542. [DOI] [PubMed] [Google Scholar]
- 49.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, et al. (2010): A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 187:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wenger TL, Miller JS, DePolo LM, de Marchena AB, Clements CC, Emanuel BS, et al. (2016): 22q11.2 duplication syndrome: elevated rate of autism spectrum disorder and need for medical screening. Mol Autism. 7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dunn W (1999): Short sensory profile. San Antonio, TX: Psychological Corporation. [Google Scholar]
- 52.Lam KS, Aman MG (2007): The Repetitive Behavior Scale-Revised: independent validation in individuals with autism spectrum disorders. Journal of autism and developmental disorders. 37:855–866. [DOI] [PubMed] [Google Scholar]
- 53.Constantino J, Gruber C (2007): Social responsiveness scale (SRS): Western Psychological Services; Los Angeles. CA. [Google Scholar]
- 54.McGlashan TH, Miller TJ, Woods SW, Hoffman RE, Davidson L (2001): Instrument for the assessment of prodromal symptoms and states. Early intervention in psychotic disorders.135–149. [Google Scholar]
- 55.Thompson PM, Stein JL, Medland SE, Hibar DP, Vasquez AA, Renteria ME, et al. (2014): The ENIGMA Consortium: large-scale collaborative analyses of neuroimaging and genetic data. Brain imaging and behavior. 8:153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun D, Ching CRK, Lin A, Forsyth JK, Kushan L, Vajdi A, et al. (2018): Large-scale mapping of cortical alterations in 22q11.2 deletion syndrome: Convergence with idiopathic psychosis and effects of deletion size. Mol Psychiatry. [DOI] [PMC free article] [PubMed]
- 57.Hibar DP, Westlye LT, Doan NT, Jahanshad N, Cheung JW, Ching CRK, et al. (2018): Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol Psychiatry. 23:932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schmaal L, Veltman DJ, van Erp TG, Samann PG, Frodl T, Jahanshad N, et al. (2016): Subcortical brain alterations in major depressive disorder: findings from the ENIGMA Major Depressive Disorder working group. Mol Psychiatry. 21:806–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Erp TG, Hibar DP, Rasmussen JM, Glahn DC, Pearlson GD, Andreassen OA, et al. (2016): Subcortical brain volume abnormalities in 2028 individuals with schizophrenia and 2540 healthy controls via the ENIGMA consortium. Mol Psychiatry. 21:585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Core Team R (2013): R: A language and environment for statistical computing R Foundation for statistical computing, Vienna. [Google Scholar]
- 61.MathWorks I (2017): MATLAB and Statistics and Machine Learning Toolbox, Release 2017a. The MathWorks Inc; Natick, Massachusetts. [Google Scholar]
- 62.Benjamini Y, Hochberg Y (1995): Controlling the False Discovery Rate - a Practical and Powerful Approach to Multiple Testing. J Roy Stat Soc B Met. 57:289–300. [Google Scholar]
- 63.Gur RE, Bassett AS, McDonald-McGinn DM, Bearden CE, Chow E, Emanuel BS, et al. (2017): A neurogenetic model for the study of schizophrenia spectrum disorders: the International 22q11.2 Deletion Syndrome Brain Behavior Consortium. Mol Psychiatry. 22:1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Thygesen JH, Wolfe K, McQuillin A, Vinas-Jornet M, Baena N, Brison N, et al. (2018): Neurodevelopmental risk copy number variants in adults with intellectual disabilities and comorbid psychiatric disorders. Br J Psychiatry. 212:287–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fiksinski AM, Breetvelt EJ, Duijff SN, Bassett AS, Kahn RS, Vorstman JAS (2017): Autism Spectrum and psychosis risk in the 22q11.2 deletion syndrome. Findings from a prospective longitudinal study. Schizophr Res. 188:59–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vorstman JA, Breetvelt EJ, Thode KI, Chow EW, Bassett AS (2013): Expression of autism spectrum and schizophrenia in patients with a 22q11.2 deletion. Schizophr Res. 143:55–59. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki G, Harper KM, Hiramoto T, Funke B, Lee M, Kang G, et al. (2009): Over-expression of a human chromosome 22q11.2 segment including TXNRD2, COMT and ARVCF developmentally affects incentive learning and working memory in mice. Hum Mol Genet. 18:3914–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boku S, Izumi T, Abe S, Takahashi T, Nishi A, Nomaru H, et al. (2018): Copy number elevation of 22q11. 2 genes arrests the developmental maturation of working memory capacity and adult hippocampal neurogenesis. Molecular psychiatry. 23:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hiramoto T, Kang G, Suzuki G, Satoh Y, Kucherlapati R, Watanabe Y, et al. (2011): Tbx1: identification of a 22q11. 2 gene as a risk factor for autism spectrum disorder in a mouse model. Human molecular genetics. 20:4775–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ouchi Y, Banno Y, Shimizu Y, Ando S, Hasegawa H, Adachi K, et al. (2013): Reduced adult hippocampal neurogenesis and working memory deficits in the Dgcr8-deficient mouse model of 22q11.2 deletion-associated schizophrenia can be rescued by IGF2. J Neurosci. 33:9408–9419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fenelon K, Xu B, Lai CS, Mukai J, Markx S, Stark KL, et al. (2013): The pattern of cortical dysfunction in a mouse model of a schizophrenia-related microdeletion. J Neurosci. 33:14825–14839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mannik K, Magi R, Mace A, Cole B, Guyatt AL, Shihab HA, et al. (2015): Copy number variations and cognitive phenotypes in unselected populations. JAMA. 313:2044–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.D'Angelo D, Lebon S, Chen Q, Martin-Brevet S, Snyder LG, Hippolyte L, et al. (2016): Defining the Effect of the 16p11.2 Duplication on Cognition, Behavior, and Medical Comorbidities. JAMA Psychiatry. 73:20–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hanson E, Bernier R, Porche K, Jackson FI, Goin-Kochel RP, Snyder LG, et al. (2015): The cognitive and behavioral phenotype of the 16p11.2 deletion in a clinically ascertained population. Biol Psychiatry. 77:785–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hippolyte L, Maillard AM, Rodriguez-Herreros B, Pain A, Martin-Brevet S, Ferrari C, et al. (2016): The Number of Genomic Copies at the 16p11.2 Locus Modulates Language, Verbal Memory, and Inhibition. Biol Psychiatry. 80:129–139. [DOI] [PubMed] [Google Scholar]
- 76.Niarchou M, Chawner S, Doherty JL, Maillard AM, Jacquemont S, Chung WK, et al. (2019): Psychiatric disorders in children with 16p11.2 deletion and duplication. Transl Psychiatry. 9:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanimura Y, Vaziri S, Lewis MH (2010): Indirect basal ganglia pathway mediation of repetitive behavior: attenuation by adenosine receptor agonists. Behav Brain Res. 210:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lewis M, Kim SJ (2009): The pathophysiology of restricted repetitive behavior. J Neurodev Disord. 1:114–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bruining H, de Sonneville L, Swaab H, de Jonge M, Kas M, van Engeland H, et al. (2010): Dissecting the clinical heterogeneity of autism spectrum disorders through defined genotypes. PLoS One. 5:e10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bruining H, Eijkemans MJ, Kas MJ, Curran SR, Vorstman JA, Bolton PF (2014): Behavioral signatures related to genetic disorders in autism. Mol Autism. 5:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kail R, Salthouse TA (1994): Processing speed as a mental capacity. Acta psychologica. 86:199–225. [DOI] [PubMed] [Google Scholar]
- 82.Fry AF, Hale S (2000): Relationships among processing speed, working memory, and fluid intelligence in children. Biological psychology. 54:1–34. [DOI] [PubMed] [Google Scholar]
- 83.Rodríguez-Sánchez JM, Crespo-Facorro B, Gonzalez-Blanch C, Perez-Iglesias R, Vázquez-Barquero JL (2007): Cognitive dysfunction in first-episode psychosis: the processing speed hypothesis. The British Journal of Psychiatry. 191:s107–s110. [DOI] [PubMed] [Google Scholar]
- 84.Span MM, Ridderinkhof KR, van der Molen MW (2004): Age-related changes in the efficiency of cognitive processing across the life span. Acta Psychologica. 117:155–183. [DOI] [PubMed] [Google Scholar]
- 85.Ojeda N, Peña J, Schretlen D, Sanchez P, Aretouli E, Elizagarate E, et al. (2012): Hierarchical structure of the cognitive processes in schizophrenia: the fundamental role of processing speed. Schizophrenia research. 135:72–78. [DOI] [PubMed] [Google Scholar]
- 86.Haigh SM, Walsh JA, Mazefsky CA, Minshew NJ, Eack SM (2018): Processing speed is impaired in adults with autism spectrum disorder, and relates to social communication abilities. Journal of autism and developmental disorders. 48:2653–2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Oliveras-Rentas RE, Kenworthy L, Roberson RB, Martin A, Wallace GL (2012): WISC-IV profile in high-functioning autism spectrum disorders: impaired processing speed is associated with increased autism communication symptoms and decreased adaptive communication abilities. Journal of autism and developmental disorders. 42:655–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Niendam TA, Bearden CE, Rosso IM, Sanchez LE, Hadley T, Nuechterlein KH, et al. (2003): A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. American Journal of Psychiatry. 160:2060–2062. [DOI] [PubMed] [Google Scholar]
- 89.Glahn DC, Almasy L, Blangero J, Burk GM, Estrada J, Peralta JM, et al. (2007): Adjudicating neurocognitive endophenotypes for schizophrenia. American Journal of Medical Genetics Part B: Neuropsychiatric Genetics. 144:242–249. [DOI] [PubMed] [Google Scholar]
- 90.Reichenberg A, Caspi A, Harrington H, Houts R, Keefe RS, Murray RM, et al. (2009): Static and dynamic cognitive deficits in childhood preceding adult schizophrenia: a 30-year study. American Journal of Psychiatry. 167:160–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Dickinson D, Ramsey ME, Gold JM (2007): Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Archives of general psychiatry. 64:532–542. [DOI] [PubMed] [Google Scholar]
- 92.Brekke JS, Hoe M, Long J, Green MF (2007): How neurocognition and social cognition influence functional change during community-based psychosocial rehabilitation for individuals with schizophrenia. Schizophrenia bulletin. 33:1247–1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Glasser MF, Van Essen DC (2011): Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J Neurosci. 31:11597–11616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tamnes CK, Ostby Y, Fjell AM, Westlye LT, Due-Tonnessen P, Walhovd KB (2010): Brain maturation in adolescence and young adulthood: regional age-related changes in cortical thickness and white matter volume and microstructure. Cereb Cortex. 20:534–548. [DOI] [PubMed] [Google Scholar]
- 95.Paus T (2005): Mapping brain maturation and cognitive development during adolescence. Trends in cognitive sciences. 9:60–68. [DOI] [PubMed] [Google Scholar]
- 96.Madden DJ, Whiting WL, Huettel SA, White LE, MacFall JR, Provenzale JM (2004): Diffusion tensor imaging of adult age differences in cerebral white matter: relation to response time. Neuroimage. 21:1174–1181. [DOI] [PubMed] [Google Scholar]
- 97.Bells S, Lefebvre J, Prescott SA, Dockstader C, Bouffet E, Skocic J, et al. (2017): Changes in White Matter Microstructure Impact Cognition by Disrupting the Ability of Neural Assemblies to Synchronize. J Neurosci. 37:8227–8238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kelly S, Jahanshad N, Zalesky A, Kochunov P, Agartz I, Alloza C, et al. (2018): Widespread white matter microstructural differences in schizophrenia across 4322 individuals: results from the ENIGMA Schizophrenia DTI Working Group. Mol Psychiatry. 23:1261–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keller TA, Kana RK, Just MA (2007): A developmental study of the structural integrity of white matter in autism. Neuroreport. 18:23–27. [DOI] [PubMed] [Google Scholar]
- 100.Villalon-Reina JE, Martinez K, Qu X, Ching CRK, Nir TM, Kothapalli D, et al. (2019): Altered white matter microstructure in 22q11.2 deletion syndrome: a multisite diffusion tensor imaging study. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mukai J, Tamura M, Fenelon K, Rosen AM, Spellman TJ, Kang R, et al. (2015): Molecular substrates of altered axonal growth and brain connectivity in a mouse model of schizophrenia. Neuron. 86:680–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perlstein MD, Chohan MR, Coman IL, Antshel KM, Fremont WP, Gnirke MH, et al. (2014): White matter abnormalities in 22q11.2 deletion syndrome: preliminary associations with the Nogo-66 receptor gene and symptoms of psychosis. Schizophr Res. 152:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kochunov P, Coyle TR, Rowland LM, Jahanshad N, Thompson PM, Kelly S, et al. (2017): Association of White Matter With Core Cognitive Deficits in Patients With Schizophrenia. JAMA Psychiatry. 74:958–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kochunov P, Rowland LM, Fieremans E, Veraart J, Jahanshad N, Eskandar G, et al. (2016): Diffusion-weighted imaging uncovers likely sources of processing-speed deficits in schizophrenia. Proc Natl Acad Sci U S A. 113:13504–13509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Grati FR, Molina Gomes D, Ferreira JC, Dupont C, Alesi V, Gouas L, et al. (2015): Prevalence of recurrent pathogenic microdeletions and microduplications in over 9500 pregnancies. Prenat Diagn. 35:801–809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.