Abstract
Rationale
Menthol is a widely used tobacco constituent that has shown to enhance nicotine’s reinforcing effects.
Objective
To determine whether injected menthol also alters nicotine’s stimulus effects, we used a drug discrimination task.
Methods
57 adult Sprague-Dawley rats (28M, 29F) received 20 positive and 20 negative days (intermixed) of discrimination training. On positive days, rats received a group-specific menthol and nicotine injection (VEH+0.1NIC, 1M+0.1NIC, 5M+0.1NIC, VEH+0.4NIC, 1M+0.4NIC, 5M+0.4NIC; mg/kg) before eight 15-sec cue light presentations (conditioned stimulus; CS), each followed by 4-sec sucrose access. On negative days, all rats were injected with vehicle and saline before eight non-reinforced CS presentations. Next, rats underwent generalization testing with 30 dose combinations of menthol and nicotine. The change in drug-mediated anticipatory goal tracking during the CS was calculated as a difference score (CS minus pre-CS responding).
Results
All groups readily acquired a drug discrimination. However, difference scores for the 5M+0.1 NIC group were lower for females. Additionally, females had lower scores for 0.05, 0.1, and 0.4 mg/kg nicotine generalization tests. The lowest nicotine dose discriminable from saline was 0.05 mg/kg for females but 0.025 mg/kg for males. Co-administration with 5 or 10 mg/kg menthol weakened discrimination performance between 0.1 and 0.4 mg/kg and between 0.1 and 0.05 mg/kg nicotine for 0.1 mg/kg nicotine training groups.
Conclusions
Female rats that were trained with 0.1 mg/kg nicotine were more sensitive to menthol’s modulatory effects on nicotine’s stimulus effects. This highlights the importance of taking sex and training dose into account when evaluating the interoceptive stimulus effects of nicotine and menthol.
Keywords: nicotine, menthol, drug discrimination, occasion setter, reinforcement learning, sex differences, rats, tobacco
INTRODUCTION
More than 6 million people die globally from tobacco-related diseases each year, making tobacco use the leading cause of preventable death (WHO Report on the Global Tobacco Epidemic, 2011). In the U.S. alone, smoking-related illnesses cost upwards of $300 billion each year (United States Department of Health and Human Services, 2014; Xu, Bishop, Kennedy, Simpson, and Pechacek, 2015). To improve public health and diminish tobacco use, the Family Smoking Prevention and Tobacco Control Act permitted the US Food and Drug Administration (FDA) to regulate the manufacture, distribution, and marketing of tobacco products (Family Smoking Prevention and Tobacco Control Act, 2009). Within the purview of this legislation, the sale of all flavored cigarettes was banned except for menthol.
Menthol is widely used in most commercial cigarettes, even those not explicitly labeled as menthol flavored (Ai et al., 2016). Menthol-preferring smokers make up nearly one-third of current U.S. smoking population (Kuiper et al., 2017). With respect to tobacco products, there is evidence suggesting that menthol contributes more than a cooling, minty flavor (Farco and Grundmann, 2013). Behaviorally, menthol can increase nicotine self-administration in rats (Biswas et al., 2016; Harrison et al., 2017). However, this effect may rely on the route of administration for both menthol and nicotine (Nesil, Narmeen, Bakhti-Suroosh, and Lynch, 2018). For example, oral menthol did not affect oral nicotine consumption (Wickham et al., 2018), but oral menthol increased intravenous nicotine self-administration (Wang, Wang, and Chen, 2014). Interestingly, menthol alters nAChR receptor density, expression, and function (Henderson et al., 2016; Henderson et al., 2017; Alsharari et al., 2015) possibly via negative allosteric modulation (Hans, Wilhelm, and Swandulla, 2012). Further, menthol may also mediate dopamine (DA) neurotransmission by altering neuronal firing frequency (Henderson et al., 2016; Henderson et al., 2017; Zhang, Harrison, Biswas, Tran, and Liu, 2018). In rats, menthol pretreatment potentiated nicotine-mediated dopamine increases in the nucleus accumbens relative to rats that only received nicotine. However, pretreatment with menthol alone had no effect on DA neurotransmission (Zhang, Harrison, Biswas, Tran, and Liu, 2018). Previous studies have examined the effect of menthol on nicotine metabolism. However, it is unclear whether menthol slows nicotine clearance (Alsharari et al., 2015; Benowitz, Herrera, and Jacob, 2004) or increases clearance (Abobo et al., 2012). Combined, these data suggest that menthol can alter the reinforcing effects of nicotine as well as related pharmacokinetic and pharmacodynamic factors. In contrast, much less is known about how menthol may alter the interoceptive discriminative stimulus effects of the nicotine.
Previous work in our lab found that intraperitoneally administered menthol does not serve as an interoceptive stimulus in an appetitive drug discrimination task (Huynh et al., 2019). Specifically, rats were pretreated with menthol–prepared at doses which altered nicotine self-administration–before a 20-min session with a series of sucrose-reinforced cue-light presentations. During separate, intermixed sessions in which rats were pretreated with the menthol vehicle (50% DMSO:H2O), cue-light presentations occurred without sucrose reinforcement. Rats did not discriminate between menthol- and vehicle-treated sessions regardless of the menthol dose (0.0183, 1, or 5 mg/kg) or injection-to-placement interval (i.e., duration between injection and chamber placement [IPI]; 5 or 15 min). That is, menthol did not set the occasion for when light illumination was followed by sucrose presentation. In contrast, drugs such as amphetamine, nicotine, and caffeine acquire control of conditioned responding in this same occasion setting task (cf. Besheer, Palmatier, Metschke, and Bevins, 2004; Murray, Li, Palmatier, and Bevins, 2007; Palmatier, Peterson, Wilkinson, and Bevins, 2004; Palmatier, Wilkinson, Metschke, and Bevins, 2005). Further, menthol did not affect an already acquired nicotine discrimination (Huynh et al., 2019). In the present research, we aimed to assess the potential modulatory effects of intraperitoneally administered menthol (cf. Biswas et al., 2016) on the interoceptive stimulus effects of nicotine using a drug discrimination occasion setting task that was procedurally identical to Huynh et al. (2019) in rats without a history of drug discrimination training. Given that previous research has suggested that menthol can amplify nicotine-mediated behavior and biology, we hypothesized that a menthol-nicotine co-treatment will increase differential control of goal-tracking compared to nicotine-only treatment.
METHODS
Subjects
Sixty Sprague-Dawley rats (30 males, 30 females) were obtained from Envigo at 9-weeks old (Indianapolis, IN, USA). Each rat was individually housed in clear polycarbonate tubs (35.5 × 32 × 18 cm; l × w × h) in a temperature- and humidity-controlled colony room. All rats were handled and allowed to acclimate to their home cage for three days before they were food restricted at 85% of their free-feeding weight. This target weight increased by 2 g each month to account for typical growth. Water was available ad libitum in the home cage. All experimental sessions were performed during the light phase of a 12-h light-dark cycle (6AM-6PM). The protocols for this study were approved by the University of Nebraska-Lincoln Institutional Animal Care and Use Committee.
Apparatus
Ten standard conditioning chambers (ENV-008CT; Med Associates IV, Georgia, VT, USA) were placed inside sound- and light-attenuating cubicles. The chambers (30.5 cm × 24.1 cm × 21 cm; l × w × h) had aluminum sidewalls, metal rod floors, and clear polycarbonate ceiling, front, and back walls. The dipper receptacle was mounted on the right aluminum sidewall occupying a 5.2 × 5.2 × 3.8 cm (l × w × h) recessed space. Here, a dipper arm with an attached 0.1-ml cup was raised to provide access to a solution inside the chamber. An infrared beam located within the dipper receptacle recorded the number of dipper entries. A white cue light (2.54-cm diameter; 28 V, 100 mA) 14.6 cm above the rod floor and 3.5 cm from the nearest polycarbonate wall was mounted on each side of the dipper receptacle. Med Associates interface and software (Med-PC for Windows, version IV) were used to collect data and present programmed events. All Med-PC programs are available upon request.
Drugs
L-(−)-Menthol (Fisher Scientific, Atlanta, Georgia, USA) was dissolved in 50% dimethyl sulfoxide (DMSO; Sigma; Atlanta, Georgia, USA) and distilled water (v/v). Menthol and vehicle (50% DMSO: H2O, v/v) solutions were freshly prepared weekly and injected intraperitoneally (IP) at 1 ml/kg with a 5-min IPI. All menthol and vehicle solutions were heated to 50°C before each session to ensure menthol solubility. All nicotine and menthol training doses were selected based on previous literature (Biswas et al., 2016; Huynh et al., 2019). (−)-Nicotine bitartrate dihydrate (MP Biomedicals; Solon, Ohio, USA) was dissolved in 0.9% saline, titrated with NaOH to a pH of 7.0 ± 0.2, and injected subcutaneously (SC) at 1 ml/kg with a 5-min IPI. All nicotine doses are expressed as the free base form and all menthol doses are expressed as the salt form.
Dipper Training
All rats received three days of dipper training (50 min/day), where 26% liquid sucrose (w/v) was presented for 4 sec using a variable time (VT) schedule of reinforcement (see Figure 1 for a timeline of the experiment). For each session, the reinforcement density decreased at 20 min into the session and again at 40 min. On the first training day, the reinforcement schedule progressed from VT11 sec to VT16 sec, and finally VT26 sec. The sequence for the second day was VT11 sec, VT26 sec, then VT56 sec. The sequence for the third training session was VT56 sec, VT88 sec, then VT116 sec. Robust dipper-entry behavior was evident for all rats after completion of this training.
Fig.1.

Timeline of the experiment including dipper training, discrimination training, and generalization testing. Generalization testing was conducted in 5 day test-cycles: 4 sessions of discrimination training to maintain conditioned behavior followed by 1 test session to generate a dose-response curve for nicotine (saline, 0.0125, 0.025, 0.05, 0.1, 0.4 mg/kg) and menthol (vehicle, 0.5, 1, 5, 10 mg/kg) combinations. Generalization testing continued until all rats completed all 30 nicotine-menthol dose combinations. mg/kg = milligram per kilogram.
General Discrimination Training
Discrimination sessions were structured in 4-day cycles. Each rat received 2 positive and 2 negative sessions in a unique intermixed order with no more than two consecutive positive or negative sessions. Each 20-min session consisted of eight trials, each comprised of 15-sec dual cue light illuminations. On positive sessions, 4-sec access to sucrose was provided in the dipper receptacle immediately upon offset of the 15-sec illumination. For negative sessions, sucrose was withheld after light offset. The time to the first light presentation and inter-trial intervals (ITI; time between light presentations) were varied to reduce predictability of events (Murray et al., 2007). Rats were split into six training groups (n=10) balanced for sex. IP injections were given immediately before the SC injections. On positive sessions, rats received a group-specific injection of one of the following combinations of menthol (M) or vehicle (VEH) with nicotine (NIC): VEH+0.1NIC, VEH+0.4NIC, 1 M + 0.1 NIC, 5 M + 0.1 NIC, 1 M + 0.4 NIC, or 5 M + 0.4 NIC. On negative sessions, all groups received a vehicle + saline injection (see Table 1 for group assignments). For example, rats in the 1M+0.4NIC group received 1 mg/kg menthol (IP) plus 0.4 mg/kg nicotine (SC) 5 min before positive sessions and vehicle (IP) plus saline (SC) before negative sessions. Because previous work from our laboratory found that menthol alone did not serve as an occasion setter, we did not include a menthol-only condition (i.e., menthol + saline) in the present study (Huynh et al., 2019).
Table 1.
Each row represents one of the six discrimination training groups. Each group differs with regard to the menthol and nicotine dose injected before a positive discrimination training session as listed in the table. On negative discrimination training sessions, all rats received a vehicle + saline injection (not listed above). The right column, n, represents the total number of rats and the total number of females (F) and males (M) per training group. All drug doses are in units of milligram per kilogram rat (mg/kg). IP = intraperitoneal injection; SC = subcutaneous injection.
| Menthol Dose (mg/kg, IP) | Nicotine Dose (mg/kg, SC) | n |
|---|---|---|
| Vehicle | 0.1 | 8 (4F, 4M) |
| 1 | 0.1 | 10 (5F, 5M) |
| 5 | 0.1 | 10 (5F, 5M) |
| Vehicle | 0.4 | 10 (5F, 5M) |
| 1 | 0.4 | 10 (5F, 5M) |
| 5 | 0.4 | 9 (5F, 4M) |
Generalization Tests and Maintenance Sessions
Following 40 sessions of discrimination training (20 positive and 20 negative days, intermixed), rats received a series of 4-min tests to generate a dose-response curve for 30 menthol-nicotine combinations. Testing was conducted in 5-day cycles: four maintenance sessions (2 positive and 2 negative discrimination sessions, intermixed) followed by one test day. Rats that met testing criteria (defined later) received a menthol injection (vehicle, 0.5, 1, 5, or 10 mg/kg menthol) immediately followed by a nicotine injection (saline, 0.0125, 0.025, 0.05, 0.1, or 0.4 mg/kg nicotine) 5 min before chamber placement. Each rat received a randomized testing order and completed each test combination. Test sessions consisted of one non-reinforced light presentation (15 sec). The initial blackout period before the single cue-light test was varied to reduce predictability (120-195 sec).
Before each generalization test, a rat was required to meet two a priori criteria to ensure that the discrimination was maintained. First, the contrast between trial 1 difference scores from the last positive and negative session must have been ≥ 1. Second, the contrast between mean trial difference scores from the last two positive sessions (averaged) and last two negative sessions (averaged) must have been ≥ 3. These testing criteria were selected based on previous work from our laboratory (cf. Palmatier et al., 2005). Due to poor discrimination performance (i.e., rats did not meet testing criteria for over three consecutive months), two rats were removed from the study. One additional rat was excluded from analysis due to death from natural causes (N=57; refer to Table 1 for final group sizes).
Dependent Measure
The primary dependent measure was the difference between dipper entries made during the 15-sec cue light illumination minus entries made during the 15 sec immediately before the light illumination (Bevins et al., 2006; Palmatier et al., 2004; Palmatier and Bevins, 2008). Difference scores were calculated for trial 1 only (cf. Huynh et al., 2019). Positive trial 1 difference scores would indicate that dipper entries primarily occurred during the 15 sec of light illumination rather than during the 15 sec of darkness. Further, higher trial 1 scores on positive days versus negative days would suggest that responding to the light illumination was controlled by the interoceptive stimulus effects of the drug that was administered on the positive day.
Data Analysis
All analyses were performed separately for each nicotine dose during discrimination training. That is, the three 0.1 mg/kg nicotine training groups [VEH+0.1NIC (n=8), 1M+0.1NIC (n=10), 5M+0.1NIC (n=10)] were analyzed together and the three 0.4 mg/kg nicotine training groups [VEH+0.4NIC (n=10), 1M+0.4NIC (n=10), 5M+0.4NIC (n=9)] were analyzed together. A four-way mixed factors analysis of variance (ANOVA) compared the mean trial 1 difference scores across Program (positive or negative session) × Group × Sex × Session for the initial training phase (20 positive and 20 negative sessions). Significant interactions were followed by post-hoc analyses with Tukey corrections, which corrects for family-wise error rate in normally distributed data (Tukey, 1949). Statistical significance was set at p<0.05. All statistical analyses were completed using the ‘afex’ package (Singmann, Bolker, Westfall, and Aust, 2019) and ‘emmeans’ package (Lenth, 2019) for the R statistical system (Version 3.6.1; R Core Team, 2019). Graphs were created with the ‘ggplot2’ package for R (Wickham, 2016).
RESULTS
Discrimination Training
0.1 mg/kg Nicotine Groups:
In the four-way mixed factors ANOVA for 0.1 mg/kg nicotine groups, there was a main effect of Program [F(1,22)=180.86, p <0.0001], Session [F(19,418)=15.61, p<0.0001], a Program × Session interaction [F(19,418)=22.44,p<0.0001], and a Group × Sex × Program × Session interaction [F(38,418)=1.76,p=0.004]. In post-hoc tests examining the Program × Session interaction, mean positive day scores were greater than mean negative day scores across sessions 7-20 (ps<0.0001), indicating that the discrimination was readily acquired and maintained (see Figure 2 for discrimination data by group). Given the complexity of the four-way interaction, specific contrasts were selected a priori to examine effects related to the experimental hypothesis. First, we compared difference scores between the sexes for each 0.1 mg/kg nicotine training group. On positive sessions, females responded higher than males in the VEH+0.1NIC group (sessions 8, 13, 20; ps≤ 0.039), lower than males in the 1M+0.1NIC group (sessions 3, 11, 14, 17; ps≤0.041), and lower than males in the 5M+0.1NIC group (sessions 11, 13, 14, 15, 18, and 20; ps≤0.032; see Figure 3); females scored higher than males in the 5M+0.1NIC group on session 2 only (p=0.036). Sex effects were minimal on negative sessions such that only 1 or 2 sessions were significantly different per group (data not shown).
Fig. 2.
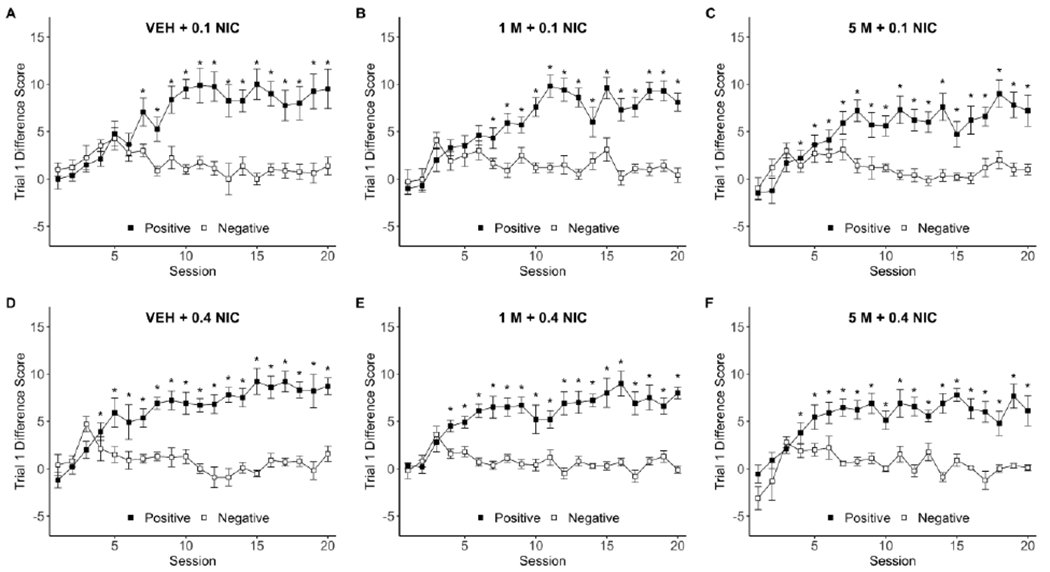
A-F Each panel displays trial 1 difference scores (±SEM) for the first forty discrimination training sessions (20 positive, 20 negative sessions) for one of the six training groups [VEH+0.1NIC (n=8), 1M+0.1NIC (n=10), 5M+0.1NIC (n=10), VEH+0.4NIC (n=10), 1M+0.4NIC (n=10), 5M+0.4NIC (n=9)]. Each of the training groups is labeled at the top of the graphs with the menthol (IP) and nicotine (SC) dose combination administered on positive sessions (filled squares). All groups received a vehicle (IP) and a saline (SC) pre-injection during the negative sessions (open squares). All drug doses are measured in milligrams per kilogram rat. M = menthol; NIC = nicotine; * indicates significant discrimination between the positive and negative day, p<0.01.
Fig. 3.
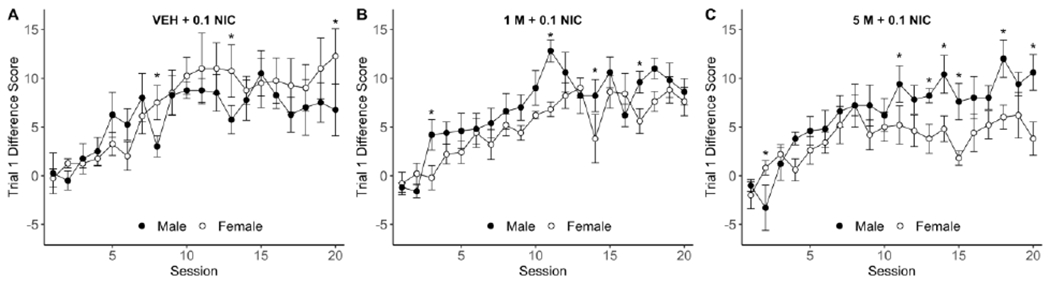
A-C Panels compare male (filled circle) and female (open circle) trial 1 discrimination performance (±SEM) during positive discrimination training sessions for 0.1 mg/kg nicotine training groups only [VEH+0.1NIC (n=8), 1M+0.1NIC (n=10), 5M+0.1NIC (n=10)]. All drug doses are in units of milligram per kilogram rat. M = menthol; NIC = nicotine; * indicates significant sex difference, p<0.05.
Next, we compared the 0.1 mg/kg nicotine training groups for each sex. For females, the VEH+0.1NIC group scored higher than the 5M+0.1NIC group during positive sessions 10 to 13, 15, 16, and 20 (ps<0.03). The VEH+0.1NIC group scored higher than the 1M+0.1NIC group during session 14 only (p=0.045) and the 1M+0.1NIC group scored higher than the 5M+0.1NIC group during session 15 only (p=0.0015). For males, the VEH+0.1NIC group scored lower than the 5M+0.1NIC group in session 18 only (p=0.042). Group-related effects were minimal across negative sessions for both sexes (≤2 significant sessions per sex; data not shown).
0.4 mg/kg Nicotine Groups:
In the four-way ANOVA, there were no main effects or interactions with Sex or Group (Fs≤1.11, ps≥0.3). Thus, a subsequent two-way repeated-measures ANOVA with Program × Session was performed. There was a main effect of Program [F(1,28)=368.14,p<0.0001], Session [F(19,532)=13.24,p<0.0001], and a Program × Session interaction [F(19,532)=15.68,p<0.0001]. In follow-up tests, difference scores for positive days were greater than scores for negative days in sessions 4-20 (ps≤0.0024), indicating that the discrimination was quickly acquired and maintained after session 4 (Figure 2).
Generalization Testing
To assess difference scores for the 30 generalization tests (5 menthol and 6 nicotine test doses), a four-way mixed factors ANOVA with Group × Sex × Menthol × Nicotine was conducted separately for 0.1 and 0.4 mg/kg nicotine training groups.
0.1 mg/kg Nicotine Groups:
There was a main effect of Nicotine [F(5,110)=60.38,p<0.0001], a Group × Menthol interaction [F(8,88)=2.15, p=0.04], Sex × Nicotine interaction [F(5,110)=2.68, p=0.03], and Menthol × Nicotine interaction [F(20,440)=1.97, p=0.008]. In post-hoc tests that assessed the Menthol × Group interaction, difference scores for the vehicle were higher than scores for 0.5 mg/kg menthol in the 1M+0.1NIC group (p=0.046). For both sexes, scores for 0.1 mg/kg nicotine were higher than scores for saline, 0.0125, 0.025, and 0.4 mg/kg nicotine (ps≤0.001) but were similar to 0.05 mg/kg nicotine (p=0.16). Average scores for saline tests were lower than average scores for 0.05, 0.1, and 0.4 mg/kg nicotine tests (ps≤0.009) in both males and females. However, scores for saline were also lower than that of 0.025 mg/kg nicotine in males (p=0.027) but not in females (p=0.49). That is, the lowest nicotine dose discriminable from saline was 0.05 mg/kg for females and 0.025 mg/kg for males. Additionally, males scored higher with 0.05, 0.1, and 0.4 mg/kg nicotine than females (ps≤0.036; see Figure 4).
Fig. 4.

A-B Displays the averaged male (filled square) and female (open square) mean difference scores (±SEM) for 0.1 (n=28) and 0.4 (n=29) nicotine training groups across six nicotine test doses (saline, 0.0125, 0.025, 0.05, 0.1, 0.4 mg/kg). All drug doses are measured in milligrams per kilogram rat. NIC = nicotine; * indicates significant sex difference (p<0.05); # indicates lowest nicotine dose discriminable from saline for males; @ indicates lowest nicotine dose discriminable from saline for females.
When co-administered with 0.1 mg/kg nicotine, there was a significant effect of the menthol dose (see Figure 5). Specifically, difference scores were lower for 5 mg/kg menthol than for the menthol vehicle or 1 mg/kg menthol (ps≤0.022) but not for 0.5 mg/kg menthol (p=0.11). Additionally, scores were lower for 10 mg/kg menthol than for 1 mg/kg menthol (p=0.002) but not for VEH (p=0.11), 0.5 (p=0.38), or 5 mg/kg menthol (p=0.97).
Fig. 5.

A-E Displays all thirty menthol-nicotine generalization tests averaged for all 0.1 mg/kg nicotine-trained rats (n=28Each panel compares the mean difference scores (±SEM) for each of the six nicotine test doses for each menthol test dose (labeled above each graph). All drug doses are measured in milligram per kilogram rat. Sal = saline; NS = not significant (p>0.05).
Difference scores for saline were significantly lower than 0.05, 0.1, and 0.4 mg/kg nicotine regardless of menthol dose (ps≤0.008). For example, VEH+SAL test scores were lower than scores for the VEH+0.05NIC, VEH+0.1NIC, and VEH+0.4NIC tests (see Figure 5 for nicotine dose comparisons across menthol dose). However, saline scores were not different from scores for 0.0125 or 0.025 mg/kg nicotine tests regardless of menthol dose (ps≥0.13). These data suggest that menthol did not affect the nicotine discrimination threshold.
Scores for tests using 0.1 mg/kg nicotine (i.e., the nicotine training dose) were higher than for saline, 0.0125, and 0.025 mg/kg nicotine tests (ps<0.0001) regardless of menthol dose. Scores for 0.1 mg/kg nicotine were also greater than scores for 0.4 mg/kg nicotine, but only in combination with VEH, 0.05, or 1 mg/kg menthol (ps≤0.012) and not with 5 or 10 mg/kg menthol (ps≥0.36). Further, scores for 0.1 mg/kg nicotine tests were greater than scores for 0.05 mg/kg nicotine tests only with VEH and 1 mg/kg menthol (ps≤0.047). This data pattern suggests that higher menthol doses (5 and 10 mg/kg) may modulate the stimulus effects of the 0.1 mg/kg nicotine training dose.
0.4 mg/kg Nicotine Groups:
In the four-way mixed factor ANOVA with Group, Sex, Menthol Dose, and Nicotine Dose conducted, there was a main effect of Nicotine Dose [F(5,155)=no.37, p<0.0001]. No other main effects or interactions were found (see Figure 4). Mean difference scores were higher for 0.4 mg/kg nicotine tests than for any other nicotine doses (ps<0.0001), indicating peak scores at the 0.4 mg/kg nicotine dose. Furthermore, scores for the three highest nicotine doses (0.05, 0.1, and 0.4 mg/kg) were higher than saline (ps<0.01). This outcome suggests that the nicotine discrimination threshold for rats that were trained with 0.4 mg/kg nicotine was between 0.05 and 0.025 mg/kg.
DISCUSSION
In the present study, we found that the stimulus effects of nicotine and menthol varied between male and female rats. Interestingly, the differential sex effects were evident only for rats that received discrimination training with 0.1 mg/kg nicotine (VEH+0.1NIC, 1M+0.1NIC, 5M+0.1NIC groups) but not with 0.4 mg/kg nicotine (VEH+0.4NIC, 1M+0.4NIC, 5M+0.4NIC groups). During discrimination training, menthol differentially modulated nicotine-controlled conditioned responding in female rats. Specifically, females responded higher than males in the vehicle control group (VEH+0.1NIC) whereas females scored lower than males in both menthol training groups (1M+0.1NIC and 5M+0.1NIC). Although the 5M+0.1NIC group (Figure 3C) were the most consistent across the final ten training sessions, the effect of sex varied for all 0.1 mg/kg nicotine training groups (Figure 3A–C). During generalization testing, significant effects of sex were found again only for 0.1 mg/kg nicotine training groups. Regardless of menthol dose, conditioned responding was lower for females at higher nicotine doses (0.05, 0.1, and 0.4 mg/kg) compared to males (Figure 4). The nicotine discrimination threshold also varied by sex such that the lowest nicotine dose discriminable from saline was 0.05 mg/kg for females and 0.025 mg/kg for males.
Few preclinical studies have examined sex as a factor in the nicotine drug discrimination literature (cf. Bevins and Charntikov, 2015). Recently, Charntikov et al. (2017) demonstrated that male and female rats that were trained with 0.4 mg/kg nicotine did not differ during the acquisition or maintenance of a nicotine-saline discrimination nor during the nicotine generalization tests. This pattern was consistent with our findings from the 0.4 mg/kg nicotine training groups. As previously discussed, however, there were differential sex effects for rats in the 0.1 mg/kg nicotine training groups. The differential behavioral effects produced in rats trained with 0.1 versus 0.4 mg/kg nicotine may reflect the unique interoceptive stimulus effects that each dose produces. In Donny et al. (1998), rats that acquired nicotine self-administration at 0.01 mg/kg/infusion had more variable nicotine infusion rates whereas rats that received 0.06 mg/kg/infusion exhibited more stable responding. This suggests that the reinforcing effects of high-dose nicotine was more resistant to subject variability. As such it is possible that interoceptive conditioning with a robust, high dose of nicotine may also obscure any subject variability that is otherwise present in lower nicotine doses. This highlights the importance of training dose selection when evaluating the interoceptive stimulus effects of nicotine (Stolerman et al., 1984). Future work is needed, however, as sex and nicotine’s interoceptive stimulus effects have not been extensively studied (Bevins & Charntikov, 2015; Charntikov et al., 2017).
Peripherally administered menthol also modulated the stimulus effects of nicotine for rats that trained with 0.1 mg/kg nicotine. Co-administration with menthol did not affect the nicotine discrimination threshold during generalization testing (i.e., discrimination between nicotine and saline). However, co-administration with 5 or 10 mg/kg menthol suppressed discrimination between 0.1 and 0.4 mg/kg nicotine and between 0.1 and 0.05 mg/kg (Figure 5). Additionally, 0.5 mg/kg menthol weakened the discrimination between 0.1 and 0.05 mg/kg nicotine. Collectively, these data suggest that peripherally administered menthol, particularly 5 and 10 mg/kg, may weaken the interoceptive stimulus effects of 0.1 mg/kg nicotine in rats that trained with this nicotine dose.
Previous work from Huynh et al. (2019) found that peripherally administered menthol did not acquire control of conditioned responding (i.e., there was no discrimination between menthol and the vehicle). Furthermore, menthol did not affect the nicotine-saline discrimination performance in rats with an established nicotine-saline discrimination training history (Huynh et al., 2019). In the present study, we demonstrated that rats without previous discrimination training also acquired and maintained a nicotine discrimination regardless of menthol co-administration (VEH, 1, or 5 mg/kg menthol; Figure 2). Given that the vehicle groups in the present study (VEH+0.1NIC and VEH+0.4NIC) readily acquired a discrimination, the lack of menthol-vehicle discrimination in Huynh et al. (2019) was not likely affected by any non-specific effect of the menthol vehicle (50% DMSO + H2O). Although menthol alone did not control conditioned responding in previous work, the current findings suggest that it may function to modulate the interoceptive stimulus effects of nicotine.
The present study adds the modulation of nicotine discrimination to the list of effects that menthol has on nicotine. Previously, menthol has been shown to potentiate nicotine-induced locomotor sensitization (Thompson et al., 2017), reduce oral nicotine aversion (Fan et al., 2016), and prevent (Henderson et al., 2016; chronic menthol exposure delivered by osmotic mini pump) or enhance (Henderson et al., 2017; acute menthol exposure delivered by IP injection) nicotine place conditioning. There is also evidence suggesting that menthol affects nicotine reinforcement. In rodents, menthol enhanced nicotine-mediated increases in DA release (Zhang et al., 2018; Wickham et al., 2018; Henderson et al., 2017). However, administration of menthol alone (i.e., without nicotine) decreased DA release relative to baseline (Henderson et al., 2016). Menthol can alter intravenous (IV) nicotine self-administration during extinction and reinstatement (Biswas et al., 2016; Harrison et al., 2017; Wang et al., 2014). However, this effect appeared to be contingent upon the dose of menthol and nicotine. For example, Biswas et al. (2016) found that 5 mg/kg menthol (IP) increased IV nicotine self-administration at low nicotine doses (0.0075 and 0.015 mg/kg/infusion), decreased self-administration for 0.03 mg/kg/infusion nicotine, and had no effect for 0.06 mg/kg/infusion nicotine. Additionally, 0.01% oral menthol increased 0.03 mg/kg/infusion IV nicotine self-administration (Wang et al., 2014) while 0.005% oral menthol had no effect (Wickham et al., 2018).
Among current U.S. smokers, approximately 27-32% use mentholated cigarettes (Kasza et al., 2014; Kuiper et al., 2017). Although the data are mixed (Cubbin, Soobader, and Leclere, 2010; Foulds, Hooper, Pletcher, and Okuyemi, 2010), previous research has shown that menthol-preferring cigarette smokers may exhibit lower smoking cessation rates (Smith, Fiore, and Baker, 2014), poorer nicotine discrimination (Perkins et al., 2017; DeVito, Valentine, Herman, Jensen, and Sofuoglu, 2016), and are more likely to be female (Giovino et al., 2013; Smith, Akpara, Haq, Miniawi, and Thompson, 2017). This is particularly concerning given that there is both clinical and preclinical evidence suggesting that females may be more sensitive to the cues associated with nicotine than to nicotine’s direct reinforcing effects (McClemon, Kozink, and Rose, 2008; Perkins, Karelitz, and Kunkle, 2018; Sofuoglu and Mooney, 2009; Jensen, DeVito, Valentine, Gueorguieva, and Sofuoglu, 2016; Donny et al., 2000; Chaudhri et al., 2005).
To date, no other studies to our knowledge have assessed the discriminative stimulus effects of menthol and nicotine in both male and female rats. Contrary to our initial hypothesis, the present study found that co-administration of menthol and nicotine diminished differential control of conditioned responding compared to co-administration with the menthol vehicle. However, these effects were contingent upon the nicotine training dose (0.1 vs. 0.4 mg/kg), biological sex, menthol dose, and nicotine dose. More research is needed to determine whether these differential sex effects persist at lower nicotine training doses. Given that there are significant sex differences with regard to the reinforcement and discriminative stimulus effects of menthol and nicotine, future studies should include both sexes to more thoroughly assess these effects. Further, replications of the current study should be performed to determine whether these sex effects persist in larger sample sizes for each sex and additional work should investigate whether specific biological factors such as gonadal hormones play a role in the discriminative effects of menthol and nicotine.
Acknowledgments
This study was supported by a grant from the National Institutes of Health: R01-DA034389 and R01-DA046109. The funding sources had no other role than financial support. There were no potential conflicts of interest reported by the authors.
We thank Ashlyn Saeger and Allissa Flynn for help with conducting daily experimental sessions.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
CONFLICT OF INTEREST
On behalf of all authors, the corresponding author states that there is no conflict of interest.
REFERENCES
- Abobo CV, Ma J, Liang D (2012) Effect of menthol on nicotine pharmacokinetics in rats after cigarette smoke inhalation. Nicotine and Tobacco Research, 14(7):801–808. 10.1093/ntr/ntr287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ai J, Taylor KM, Lisko JG, Tran H, Watson CH, Holman MR (2016) Menthol Content in U.S. Marketed Cigarettes. Nicotine & Tobacco Research, 18(7):1575–1580. 10.1093/ntr/ntv162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alsharari SD, King JR, Nordman JC, Muldoon PP, Jackson A, Zhu AZ et al. (2015) Effects of Menthol on Nicotine Pharmacokinetic, Pharmacology and Dependence in Mice. PloS one, 10(9):e0137070 10.1371/journal.pone.0137070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benowitz NL, Herrera B, Jacob P (2004) Mentholated Cigarette Smoking Inhibits Nicotine Metabolism. Journal of Pharmacology and Experimental Therapeutics, 310(3):1208–1215. 10.1124/jpet.104.066902. [DOI] [PubMed] [Google Scholar]
- Besheer J, Palmatier MI, Metschke DM, Bevins RA (2004) Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology, 172(1):108–117. 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- Bevins RA, Charntikov S (2015) We know very little about the subjective effects of drugs in females. ACS: Chemical Neuroscience, 6:359–361. 10.1021/acschemneuro.5b00018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins RA, Wilkinson JL, Palmatier MI, Siebert HL, Wiltgen SM (2006) Characterization of nicotine’s ability to serve as a negative feature in a Pavlovian appetitive conditioning task in rats. Psychopharmacology, 184(3–4):470–481. 10.1007/s00213-005-0079-3 [DOI] [PubMed] [Google Scholar]
- Biswas L, Gong Y, Avusula R, Lee J, Zhang M, Rousselle T et al. (2016) Enhancing effect of menthol on nicotine self-administration in rats. Psychopharmacology, 233(18):3417–3427. 10.1007/s00213-016-4391-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charntikov S, Falco AM, Fink K, Dwoskin LP, Bevins RA (2017) The effect of sazetidine-A and other nicotinic ligands on nicotine controlled goal-tracking in female and male rats. Neuropharmacology, 113(Pt A):354–366. 10.1016/j.neuropharm.2016.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib MA, Craven LA, Allen SS, Sved AF, Perkins KP (2005) Psychopharmacology 180(2):258–266. 10.1007/s00213-005-2152-3 [DOI] [PubMed] [Google Scholar]
- Cubbin C, Soobader MJ, Leclere FB (2010) The intersection of gender and race/ethnicity in smoking behaviors among menthol and non-menthol smokers in the United States. Addiction, 105(SUPPL.1):32–38. 10.1111/j.1360-0443.2010.03191.x [DOI] [PubMed] [Google Scholar]
- DeVito EE, Valentine GW, Herman AI, Jensen KP, Sofuoglu M (2016) Effect of Menthol-preferring Status on Response to Intravenous Nicotine. Tob Regul Sci 2:317–328. 10.18001/TRS.2.4.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donny E, Caggiula A, Mielke M et al. (1998) Acquisition of nicotine self-administration in rats: the effects of dose, feeding schedule, and drug contingency. Psychopharmacology 136, 83–90. 10.1007/s002130050542 [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Rowell PP, Gharib MA, Maldovan V, Booth S et al. (2000) Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology, 151(4):392–405. 10.1007/s002130000497 [DOI] [PubMed] [Google Scholar]
- Family Smoking Prevention and Tobacco Control Act (2009) H.R. 1256. 111th Cong., 1st Sess., 155, E859–859. [Google Scholar]
- Fan L, Balakrishna S, Jabba SV, Bonner PE, Taylor SR, Picciotto MR, Jordt SE (2016) Menthol decreases oral nicotine aversion in C57BL/6 mice through a TRPM8-dependent mechanism. Tobacco control, 25:ii50–ii54. 10.1136/tobaccocontrol-2016-053209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farco JA., Grundmann O (2013) Menthol - Pharmacology of an Important Naturally Medicinal “Cool.” Mini Reviews in Medicinal Chemistry, 13(1):124–131. 10.2174/138955713804484686 [DOI] [PubMed] [Google Scholar]
- Foulds J, Hooper MW, Pletcher MJ, Okuyemi KS (2010) Do smokers of menthol cigarettes find it harder to quit smoking? Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco, 12(Suppl 2):S102–S109. 10.1093/ntr/ntq166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovino GA, Villanti AC, Mowery PD, Sevilimedu V, Niaura RS, Vallone DM, Abrams DB (2013) Differential trends in cigarette smoking in the USA: Is menthol slowing progress? Tobacco Control, 24:28–37. 10.1136/tobaccocontrol-2013-051159 [DOI] [PubMed] [Google Scholar]
- Hans M, Wilhelm M, Swandulla D (2012) Menthol suppresses nicotinic acetylcholine receptor functioning in sensory neurons via allosteric modulation. Chem. Senses, 37:463–469. 10.1093/chemse/bjr128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison E, Biswas L, Avusula R, Zhang M, Gong Y, Liu X (2017) Effects of menthol and its interaction with nicotine-conditioned cue on nicotine-seeking behavior in rats. Psychopharmacology, 234:3443–3453. 10.1007/s00213-017-4736-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, McKinney S, Lester HA (2017) Menthol Enhances Nicotine Reward-Related Behavior by Potentiating Nicotine-Induced Changes in nAChR Function, nAChR Upregulation, and DA Neuron Excitability. Neuropsychopharmacology, 42(12):2285–2291. https://doi.org/10.1038Znpp.2017.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson BJ, Wall TR, Henley BM, Kim CH, Nichols WA, Moaddel R et al. (2016) Menthol Alone Upregulates Midbrain nAChRs, Alters nAChR Subtype Stoichiometry, Alters Dopamine Neuron Firing Frequency, and Prevents Nicotine Reward. Journal of Neuroscience, 36(10): 2957–2974. 10.1523/JNEUROSCI.4194-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huynh YW, Raimondi A, Schuster C, Finkner A, Selleck C, Bevins RA (2019) Investigating the interoceptive stimulus effects of injected menthol in rats. Experimental and Clinical Psychopharmacology. 10.1037/pha0000295 [DOI] [PubMed] [Google Scholar]
- Jensen KP, DeVito EE, Valentine G, Gueorguieva R, Sofuoglu M (2016) Intravenous Nicotine Self-Administration in Smokers: Dose-Response Function and Sex Differences. Neuropsychopharmacology, 41(8):2034–2040. 10.1038/npp.2015.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasza KA, Hyland AJ, Bansal-Travers M, Vogl LM, Chen J, Evans SE et al. (2014) Switching between menthol and nonmenthol cigarettes: findings from the U.S. Cohort of the International Tobacco Control Four Country Survey. Nicotine & tobacco research: official journal of the Society for Research on Nicotine and Tobacco, 16(9):1255–1265. 10.1093/ntr/ntuo98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper NM, Gammon D, Loomis B, Falvey K, Wang TW, King BA, Rogers T (2017) Trends in Sales of Flavored and Menthol Tobacco Products in the United States During 2011-2015. Nicotine & Tobacco Research, 20(6):698–706. 10.1093/ntr/ntx123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenth R (2019) Emmeans: Estimated Marginal Means, Aka Least-Squares Means. Retrieved from https://CRAN.R-project.org/package=emmeans
- McClernon FJ, Kozink RV, Rose JE (2008) Individual Differences in Nicotine Dependence, Withdrawal Symptoms, and Sex Predict Transient fMRI-BOLD Responses to Smoking Cues. Neuropsychopharmacology, 33:2148–2157. 10.1038/sj.npp.1301618 [DOI] [PubMed] [Google Scholar]
- Murray JE, Li C, Palmatier MI, Bevins RA (2007) The interoceptive Pavlovian stimulus effects of caffeine. Pharmacology, Biochemistry, and Behavior, 86(4):838–846. 10.1016/j.pbb.2007.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesil T, Narmeen S, Bakhti-Suroosh A, Lynch WJ (2018) Effect of menthol on nicotine intake and relapse vulnerability in a rat model of concurrent intravenous menthol/nicotine self-administration. Psychopharmacology, 236(4): 1219–1232. 10.1007/s00213-018-5128-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Bevins RA (2008) Occasion-setting by drug states: Functional equivalence following similar training history. Behavioural Brain Research, 195(2):260–270. 10.1016/j.bbr.2008.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA (2004) Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behavioural Pharmacology, 15(3): 183–194. 10.1097/01.fbp.0000132915.11693.8e [DOI] [PubMed] [Google Scholar]
- Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA (2005) Stimulus Properties of Nicotine, Amphetamine, and Chlordiazepoxide as Positive Features in a Pavlovian Appetitive Discrimination Task in Rats. Neuropsychopharmacology, 30(4):731–741. 10.1038/sj.npp.1300629 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Kunkle N, Karelitz JL (2017) Threshold dose for behavioral discrimination of cigarette nicotine content in menthol vs. non-menthol smokers. Psychopharmacology, 234(8): 1255–1265. 10.1007/S00213-017-4563-3 [DOI] [PubMed] [Google Scholar]
- Perkins KA, Karelitz JL, Kunkle N (2018) Sex Differences in Subjective Responses To Moderate Versus Very Low Nicotine Content Cigarettes. Nicotine & Tobacco Research, 20(10):1258–1264. 10.1093/ntr/ntx205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2019) R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; Retrieved from https://www.R-project.org/ [Google Scholar]
- Singmann H, Bolker B, Westfall J, Aust F (2019) Afex: Analysis of Factorial Experiments. Retrieved from https://CRAN.R-project.org/package=afex
- Smith PH, Akpara E, Haq R, El-Miniawi M, Thompson AB (2017) Gender and Menthol Cigarette Use in the United States: A Systematic Review of the Recent Literature (2011 - May 2017). Current addiction reports, 4(4):431–438. 10.1007/s40429-017-0175-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Fiore MC, Baker TB (2014) Smoking Cessation in Smokers Who Smoke Menthol and Non-Menthol Cigarettes. Addiction, 109(2):2107–2117. 10.1111/add.12661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Mooney M (2009) Subjective responses to intravenous nicotine: greater sensitivity in women than in men. Experimental and Clinical Psychopharmacology, 17(2):63–69. 10.1037/a0015297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolerman IP, Garcha HS, Pratt JA et al. (1984) Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology, 84(3):413–413. 10.1007/BF00555223 [DOI] [PubMed] [Google Scholar]
- Thompson MF, Poirier GL, Dávila-García MI, Huang W, Tam K, Robidoux M et al. (2017) Menthol enhances nicotine-induced locomotor sensitization and in vivo functional connectivity in adolescence. Journal of Psychopharmacology, 32(3):332–343. 10.1177/0269881117719265 [DOI] [PubMed] [Google Scholar]
- Tukey J (1949). Comparing Individual Means in the Analysis of Variance. Biometrics, 5(2), 99–114. doi: 10.2307/3001913 [DOI] [PubMed] [Google Scholar]
- World Health Organization (2011) WHO report on the global tobacco epidemic, 2011: Warning about the dangers of tobacco. Retrieved from http://apps.who.int/iris/bitstream/handle/10665/44616/9789240687813_eng.pdf;jsessionid=296AA6EC8A23C7AoF83EE98209115B48?sequence=1
- U.S. Department of Health and Human Services (2014) The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General. Atlanta: Retrieved from https://www.surgeongeneral.gov/library/reports/50-years-of-progress/full-report.pdf [Google Scholar]
- Wang T, Wang B, Chen H (2014) Menthol facilitates the intravenous self-administration of nicotine in rats. Front. Behav. Neurosci 8:1–12. 10.3389/fnbeh.2014.00437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Retrieved from https://cran.r-project.org/web/packages/ggplot2/index.html
- Wickham RJ, Nunes EJ, Hughley S, Silva P, Walton SN, Park J, Addy NA (2018) Evaluating oral flavorant effects on nicotine self-administration behavior and phasic dopamine signaling. Neuropharmacology, 128:33–42. 10.1016/j.neur0pharm.2017.09.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Bishop EE, Kennedy SM, Simpson SA, Pechacek TF (2015) Annual Healthcare Spending Attributable to Cigarette Smoking: An Update. American Journal of Preventive Medicine, 48(3):326–333. 10.1016/j.amepre.2014.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Harrison E, Biswas L, Tran T, Liu X (2018) Menthol facilitates dopamine-releasing effect of nicotine in rat nucleus accumbens. Pharmacology, Biochemistry and Behavior, 175(1):47–52. 10.1016/j.pbb.2018.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]


