Significance
RIG-I–like receptors (RLRs) direct innate immunity as our first line of defense against RNA virus infections. Mitochondria were previously established as the platform for RLR-mediated innate immune signaling, based upon crude mitochondrial fractionation studies. By using high resolution subcellular fractionation separating mitochondria from other organelles, electron microscopy, and analysis of spatiotemporal RLR dynamics, we show that activation and regulation of innate immune signaling do not take place on mitochondria but instead occur at membranes derived from the endoplasmic reticulum. Our data argue against the classical view of the spatial organization of innate immune signaling to reveal insights into RLR signaling dynamics and regulation by LGP2.
Keywords: MAVS, LGP2, RIG-I, IRF3, innate immunity
Abstract
RIG-I, MDA5, and LGP2 comprise the RIG-I–like receptors (RLRs). RIG-I and MDA5 are essential pathogen recognition receptors sensing viral infections while LGP2 has been described as both RLR cofactor and negative regulator. After sensing and binding to viral RNA, including double-stranded RNA (dsRNA), RIG-I and MDA5 undergo cytosol-to-membrane relocalization to bind and signal through the MAVS adaptor protein on intracellular membranes, thus directing downstream activation of IRF3 and innate immunity. Here, we report examination of the dynamic subcellular localization of all three RLRs within the intracellular response to dsRNA and RNA virus infection. Observations from high resolution biochemical fractionation and electron microscopy, coupled with analysis of protein interactions and IRF3 activation, show that, in resting cells, microsome but not mitochondrial fractions harbor the central components to initiate innate immune signaling. LGP2 interacts with MAVS in microsomes, blocking the RIG-I/MAVS interaction. Remarkably, in response to dsRNA treatment or RNA virus infection, LGP2 is rapidly released from MAVS and redistributed to mitochondria, temporally correlating with IRF3 activation. We reveal that IRF3 activation does not take place on mitochondria but instead occurs at endoplasmic reticulum (ER)-derived membranes. Our observations suggest ER-derived membranes as key RLR signaling platforms controlled through inhibitory actions of LGP2 binding to MAVS wherein LGP2 translocation to mitochondria releases MAVS inhibition to facilitate RLR-mediated signaling of innate immunity.
Innate immunity provides our first line of antiviral defense. During acute virus infection, the innate immune response is triggered first within the infected cell when viral macromolecules known as pathogen associated molecular patterns (PAMPs) are recognized as nonself by host cell pathogen recognition receptors (PRRs). Viral nucleic acid represents a major PAMP wherein cytosolic viral DNA, or viral RNA marked with nonself motifs, are recognized by specific PRRs. PAMP recognition and binding by PRRs trigger intracellular signaling that directs innate immune activation and induction of antiviral defenses (1, 2).
The retinoic acid inducible gene-I–like receptor (RLR) family of RNA helicases are cytosolic PRRs. Retinoic acid inducible gene-I (RIG-I) is the charter member of the RLR family that also includes the melanoma differentiation antigen 5 (MDA5) and laboratory of genetics and physiology 2 (LGP2) proteins. RIG-I and MDA5 function as PRRs to recognize most RNA viruses, thus serving to induce innate immunity for protection against infection (2, 3). In particular, RNA viruses represent the largest class of contemporary and emerging human pathogens (4). The RLR proteins are expressed at low basal levels in most nucleated cells to facilitate surveillance for viral PAMPs and are induced to higher level expression in response to interferon (IFN). Whereas RIG-I and MDA5 contain tandem amino-terminal caspase activation and recruitment domains (CARDs) that mediate signaling interaction with the adaptor protein mitochondrial antiviral signaling (MAVS), LGP2 lacks CARDs (2). In response to binding to PAMP RNA produced during viral RNA replication, RIG-I and MDA5 undergo conformation change and cofactor interaction leading to complex formation with MAVS that facilitates activation of downstream transcription factors, including IFN regulatory factor (IRF) 3 and NF-κB (2, 3). This process induces the expression of target genes, including type 1 and 3 IFNs. IFNs are secreted cytokines that signal through their cognate receptor both locally and systemically to direct the expression of IFN-stimulated genes (ISGs) whose products restrict viral replication and spread, and also mediate immune modulatory actions leading to immune polarization and systemic immune activation (2, 5). Governance of this RLR signaling program that underlies immune activation is critical to protect against immune pathology and autoimmunity while suppressing virus infection (6, 7).
Biochemical and in vitro studies have shown that LGP2 mediates suppression of RIG-I signaling (8–11) while it can also serve as a cofactor that facilitates MDA5 signaling (12–15). Moreover, studies of mice lacking LGP2 expression or LGP2 RNA binding function have diametrically shown that LGP2 functions as an essential cofactor of RLR signaling or as a negative regulator of RLR actions in vivo in response to various RNA virus infections (16, 17). While the RLRs are cytosolic proteins, they undergo redistribution among intracellular membranes to mediate innate immune signaling after PAMP binding (18–20). Notably, most RNA viruses replicate their genome within the cytoplasm in collaboration with intracellular membranes, often derived from the endoplasmic reticulum (ER), to build up the replication compartments that harbor viral RNA and proteins (21). Viral PAMP recognition and initiation of innate immune signaling by the RLRs thus relies on their dynamic redistribution in virus-infected cells (18–20, 22). As such, the spatiotemporal dynamics of intracellular redistribution across virus infection likely could explain the variable functions ascribed to LGP2 (8–11, 13, 16, 17, 23–25), as well as RIG-I and MDA5, for differential control of RLR actions.
A classical model of RLR-mediated innate immune signaling by RIG-I and MDA5 implies that, in response to PAMP recognition, the RLRs translocate from the cytosol to bind to the central adaptor MAVS on the outer mitochondrial membrane (OMM) (2). In cell-free models, this interaction triggers prion-like oligomerization of MAVS (26). RIG-I or MDA5 binding to MAVS imparts the formation of a high mass MAVS signalosome, which involves TNF receptor-associated factor (TRAF) proteins and MAVS phosphorylation by TANK-binding kinase 1 (TBK1) or inhibitor of kappa-B kinase (IKK) to allow binding of the transcription factor IRF3 and other signaling proteins with MAVS (26–28). In unstimulated cells, IRF3 exists as nonphosphorylated and hypophosphorylated monomeric protein. Upon binding to MAVS, TBK1- or IKKε-mediated phosphorylation of IRF3 occurs on Serine residues (S386, S396) and enables its dimerization, nuclear translocation, and induction of antiviral target gene expression (29–32). MAVS possesses a membrane anchor motif that places it on the OMM while it has also been shown to localize to peroxisomes and mitochondria-associated membranes (MAMs), the latter of which represent ER membranes tethered to the OMM (22, 33). In addition to the OMM, peroxisomes and MAMs have been shown to play a role as RLR innate immune signaling platforms via MAVS (22, 33). However, the dynamics of LGP2 and its actions in RLR signaling among these and other cellular compartments is not known.
The intracellular landscape of mammalian cells is characterized as an organellar mesh in which organelles are apposed and their membranes connected via protein bridges, with a typical distance of 10 to 30 nm between the membrane interfaces (34, 35). The ER is connected this way to most organelles and is heavily involved in the regulation of interorganelle communication, morphology, and trafficking (34). Biochemically, the ER can be isolated as part of the microsome (MIC) fraction which contains vesicles derived from intracellular membranes (36). Recent work has shown that mitochondria are also in contact with most organelles, including endosomes, lysosomes, peroxisomes, and the ER (35). As noted above, the ER membranes that are connected to the OMM constitute the MAM, which can be visualized by electron microscopy (EM) and advanced microscopy techniques or studied by subcellular fractionation (34, 37, 38). However, conventional confocal microscopy (resolution limit 200 nm) or crude mitochondrial fractions that contain mitochondria and other organelles do not provide the resolution required to distinguish between mitochondria and MAMs or other organelles. Earlier studies reported that the OMM was the central platform of RLR-MAVS signaling, but these observations were based on low resolution biochemical separation and imaging of RLR-MAVS localization, nor did these studies conduct parallel examination of all three RLRs (39, 40). Thus, we sought to assess RLR dynamics, including LGP2 function, using a combination of high resolution subcellular fractionation (41), electron microscopy, and RLR expression with LGP2 mutational analyses to assess the spatiotemporal events taking place during innate immune signaling across organelles.
Here, we report that all major components required for innate immune signaling, including RLRs, MAVS, TBK1, TRAF, TRIM25, and IRF3, were present in the MIC fraction in absence of stimulation. We found that LGP2 was constitutively bound to MAVS in the MIC fraction but that activation of RLR signaling by treatment of cells with double-stranded RNA (dsRNA) resulted in release of LGP2 from MAVS coincident with IRF3 activation and LGP2 translocation to mitochondria. Functional analysis of wild-type and mutant LGP2 indicates that LGP2 acts as a negative feedback regulator of RLR signaling, possibly by binding to MAVS, blocking an interaction between RIG-I and MAVS, and thereby preventing IRF3 activation. We show that activated IRF3 itself is absent from mitochondria but enriched in MIC and cytosolic fractions, showing that IRF3 activation does not take place on the OMM but instead occurs at microsomal membranes including ER, and MAM, reflecting the location of virus replication and high RNA PAMP concentration.
Results
Subcellular Fractionation Provides Improved Resolution of Protein Localization in Relation to Mitochondria and Their Associated Membranes.
The classical view of innate immune signaling via RLRs and MAVS (illustration depicted in SI Appendix, Fig. S1A), positioning mitochondria at the center of IRF3 activation, is based on analyses by confocal microscopy or crude mitochondrial fractionation (39, 40). These techniques do not provide a sufficient resolution to distinguish between mitochondria and MAMs or other organelles, such as peroxisomes that have more recently been implicated in RLR signaling (22, 33). We first evaluated the distribution of MAVS, mitochondria, and ER by confocal microscopy, analyzing MAVS, TOM20 (mitochondrial outer membrane marker) and PDIA3 (ER marker) in A549 human lung epithelial cells and PH5CH8 immortalized human hepatocytes (Fig. 1A and SI Appendix, Fig. S1B). We found a strong overlap of the protein patterns in both cell lines. This spatial overlap in fluorescent signals was confirmed by analysis of line profiles and zooming into selected areas of the cytoplasm (Fig. 1A and SI Appendix, Fig. S1B, yellow arrow and enlarged views of boxed area). Thus, a classical confocal microscopy approach cannot fully resolve the subcellular structures implicated in RLR signaling. To study the spatiotemporal dynamics of innate immune signaling, we examined the immunocompetent cell line A549 that demonstrates intact RLR-MAVS signaling in response to RNA virus infection as an epithelial cell experimental model (42). Using transmission electron microscopy (TEM), we first examined the distribution of ER and mitochondria in A549 cells. TEM micrographs show the close apposition of ER and OMM, with an approximate distance of 20 nm between these two subcellular compartments (43) (Fig. 1B). A part of the ER appears to be linked to the OMM via membrane connections (Fig. 1 B, Bottom), further defining the MAM as a subdomain of the ER (38). These results confirm the close apposition of mitochondria and ER with MAM–OMM linkage. We therefore employed a high resolution subcellular fractionation scheme to separate mitochondria from their associated membranes and isolate mitochondria (Mito), MAMs, microsomes (MICs), and cytosol (Cyto) from cultured cells (Fig. 1C) (22, 41). A549 cells were subjected to this subcellular fractionation, and the obtained fractions were analyzed for various cellular markers to control for their enrichment (Fig. 1D and SI Appendix, Fig. S1 C and D). Immunoblot analyses demonstrate that the MIC fraction was enriched for ER (Calnexin, PDIA3, and FACL4) and ribosomes (S6 ribo) and additionally contained Golgi (Golgin97), endosomes (EEA1), and lysosomes (Lamp1) while the MAM fraction primarily consisted of ER (Calnexin, PDIA3, and FACL4) but also contained peroxisomes (Catalase), in agreement with an interaction between the OMM and peroxisomes (35). Importantly, the Mito fraction proved to be highly enriched for mitochondrial markers (SOD2; CoxIV [inner mitochondrial membrane]; CytC [intermembrane space]; VDAC [OMM]) while the Cyto fraction was marked with α-tubulin. Catalase, Golgin97, and EEA1, proteins linked with peroxisomes, golgi, and endosomes, were also found in the cytosol. Mitofusin 1 (MFN1) was found to be enriched in the mitochondrial fraction. Both MAM and mitochondrial fractions contained MFN2 in line with its known localization as a MAM and OMM-linked protein (22, 38) while Caveolin-1 was primarily detected in MAM as reported earlier (44). Importantly, MAVS was detected in all three membrane fractions: MIC, MAM, and Mito (Fig. 1D). Thus, the following proteins are used as quality control markers throughout this study: Calnexin, ER marker, enriched in MIC and MAM; MFN2, mitofusin 2, enriched in MAM and Mito; CoxIV, inner mitochondrial membrane, enriched in Mito; and α-tubulin, enriched in cytosol.
Fig. 1.
Subcellular fractionation provides improved resolution of protein localization in relation to mitochondria and their associated membranes. (A) Pattern of MAVS, ER, and mitochondria in PH5CH8 cells. Cells were stained with antibodies directed against MAVS, PDIA3 (ER-marker), and TOM20 (outer mitochondrial membrane marker) and analyzed on a Nikon Eclipse Ti confocal microscope. Merged images are derived from maximum intensity projections of z-stacks. Enlarged views of the boxed areas are shown next to the merge. The graph depicts the intensity profile for the indicated markers along the yellow line shown in the merged image. Depicted are representative images of two independent experiments (n = 2). (Scale bar: 10 μm.) (B) Routine EM imaging of ER and mitochondria in A549 cells at magnifications of 10,000, 25,000, and 50,000. (Scale bars: 200 nm, 100 nm, 50 nm, respectively.) N, nucleus; asterisks, mitochondria; ER, endoplasmic reticulum; arrowheads point to mitochondria-associated ER membrane (MAM). (C) Schematic presentation of subcellular fractionation protocol used to isolate MAM, mitochondria (Mito), microsomes (MIC), and cytosol (Cyto). (D) A549 cells were subjected to subcellular fractionation. The distribution of various compartmental marker proteins was analyzed by Western blot. CoxIV, inner mitochondria; MFN2, MAM + mitochondria; Calnexin/PDIA3, ER; Catalase, peroxisomes; Golgin97, Golgi; EEA1, α-tubulin, cytosol. WCL, whole cell lysate.
dsRNA Imparts Dynamic Localization of LGP2 and Regulated Interaction with MAVS.
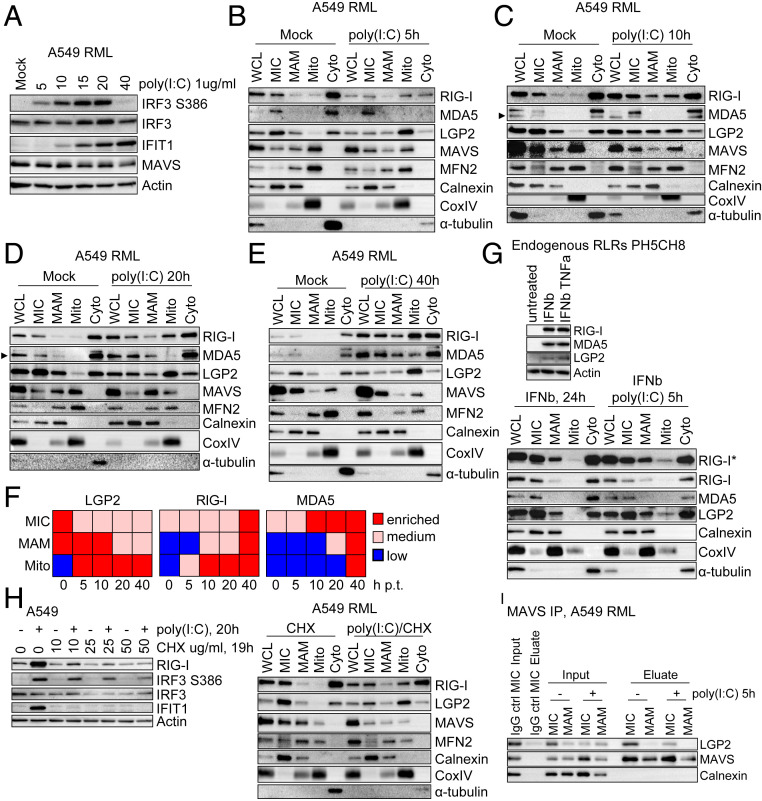
To achieve reliable detection of RLR proteins, we used lentiviral vectors to generate cell lines stably expressing RIG-I, MDA5, and LGP2 (RML) (SI Appendix, Fig. S2B). To stimulate RLR-MAVS mediated IRF3 activation, A549-RML cells were treated with dsRNA via poly inosine:cytosine (polyI:C) transfection or were infected with SeV, a negative sense RNA virus that activates RIG-I (3). When treated with dsRNA or infected with SeV, A549-RML cells responded to undergo innate immune activation marked by IRF3 phosphorylation and expression of the IRF3-target gene, IFIT1 (SI Appendix, Fig. S2 C–F). A membrane flotation assay and immunoblot analyses of the recovered fractions from A549-RML cells revealed that LGP2 was basally abundant in the membrane fraction, even in the absence of stimulation (SI Appendix, Fig. S2 G and H), while RIG-I and MDA5 were present in cytosolic fractions in nontreated cells and accumulated in the membrane fraction following dsRNA treatment. Thus, LGP2 has distinct membrane association dynamics compared to RIG-I and MDA5.
To evaluate the dynamics of RLRs during innate immune signaling, we subjected A549-RML cells to dsRNA treatment, followed by subcellular fractionation. IRF3 activation and the distribution of RLRs in MIC, MAM, Mito, and Cyto fractions were determined through 40 h of dsRNA treatment (Fig. 2 A–E). In the absence of treatment, all RLRs were absent from Mito but present in MIC and Cyto while LGP2 was also basally present in MAM. Strikingly, LGP2 accumulated in Mito at all time points following dsRNA treatment while RIG-I accumulated in MAM and Mito over the time course (Fig. 2 B–F). Compared to RIG-I, the amount of MDA5 increased in MAM and Mito at later time points while distribution of MAVS, TRIM25, and the specific organelle markers did not change (SI Appendix, Fig. S3A). A dsRNA-induced enrichment of LGP2 in Mito was also revealed in PH5CH8-RML cells, confirming that dsRNA triggers the redistribution of LGP2 from MAM to Mito (SI Appendix, Fig. S3B). To further assess the spatial distribution of endogenous RLRs, parent PH5CH8 cells were pretreated for 24 h with IFN-β to induce RLR expression before dsRNA treatment to thereby increase endogenous RLR abundance. Endogenous LGP2 was found in the Mito fraction after dsRNA treatment but not after IFN-β treatment alone, demonstrating that the LGP2 translocation events observed in the RML cell lines are triggered by dsRNA and also account for endogenous receptors (Fig. 2G). While α-tubulin levels were qualitatively variable after dsRNA treatment of cells (Fig. 2 B–E and G), immunofluorescent staining of cells shows that Actin (Phalloidin Alexa Fluor 488) and α-tubulin patterns remained unaltered in A549-RML cells after dsRNA treatment (SI Appendix, Fig. S3C).
Fig. 2.
Spatiotemporal dynamics of RLRs upon dsRNA stimulation. (A) Kinetics of innate immune signaling after poly(I:C) transfection of A549-RML cells. Cells were lysed at the indicated time points and analyzed by Western blot. Depicted is one representative blot out of two independent experiments (n = 2). (B–E) Subcellular fractionation of A549-RML cells after 5 h (B), 10 h (C), 20 h (D), and 40 h (E) of poly(I:C) transfection. Fractions were analyzed by Western blot using antibodies directed against RLRs, MAVS, or marker proteins (MFN2, MAM + mitochondria; Calnexin, ER; CoxIV, inner mitochondria; α-tubulin, Cytosol). A total of eight independent fractionations comprise this set of experiments; per each time point, two fractionations were performed. (F) Graphical summary of observations made in B–E. (G) Analysis of endogenous RLRs. (Upper) PH5CH8 cells were treated with 500 IU/mL IFN-β or IFN-β and 10 ng/mL TNF-α to detect expression of endogenous RLRs. (Lower) PH5CH8 cells were treated with IFN-β (500 IU/mL, 24 h) and either mock transfected or transfected with 1 μg/mL poly(I:C) to study dynamics of endogenous RLRs. Note that IFN treatment seems to affect separation of MAM and mitochondria. Depicted is one representative out of two independent fractionations. (H, Left) A549 cells were transfected with poly(I:C) followed by treatment with the indicated concentrations of Cycloheximide (CHX) 1 h posttransfection (p.t.). Then, 20 h after poly(I:C) stimulation, cell lysates were harvested and analyzed by Western blot to monitor induction of RIG-I protein expression. (H, Right) A549-RML cells were mock transfected or transfected with 1 μg/mL poly(I:C) and treated with 10 mg/mL CHX at 1 h p.t. Then, 16 h after poly(I:C) stimulation, cells were subjected to subcellular fractionation. Fractions were analyzed by Western blot. (I) Immunoprecipitation of endogenous MAVS from MIC and MAM subcellular fractions after mock treatment or 5 h of stimulation with poly(I:C). An equal amount of IgG control antibody was incubated with the MIC fraction (mock). For Western blot analysis, 1/20 of the Input and total eluate were loaded onto an SDS gel. Eluates were analyzed for coimmunoprecipitation of LGP2 or Calnexin (loading control). One representative dataset out of two independent experiments is shown. Arrowheads point to specific protein band. Cyto, cytosol; MAM, mitochondria-associated membranes; MIC, microsomes; Mito, mitochondria; WCL, whole cell lysate.
To determine whether the enrichment of RLRs in different fractions resulted from translocation of already existing protein or from transport of newly expressed protein, we subjected A549 cells to dsRNA treatment, followed by cycloheximide (CHX) to block translation elongation and prevent induction of RLR expression due to innate immune signaling (Fig. 2H). Addition of 10 mg/mL CHX to A549 cell cultures was sufficient to block IRF3 target gene and ISG expression (RIG-I, IFIT1) otherwise induced by dsRNA (Fig. 2 H, Left). Subcellular fractionation of A549-RML cells treated with dsRNA followed by CHX treatment, but not CHX treatment alone, showed enrichment of LGP2 and RIG-I in the mitochondrial fraction. These results indicate that dsRNA induces the translocation of preexisting RLRs rather than de novo RLRs produced in response to dsRNA signaling (Fig. 2 H, Right). Based on qualitative comparison of LGP2 protein in different fractions obtained from mock and dsRNA treated cells, we found that, in response to dsRNA, LGP2 became enriched in Mito while decreasing in MIC and Cyto. These data indicate that LGP2 translocates from the ER and cytosol to mitochondria upon stimulation.
To determine if LGP2 translocation and membrane association are linked with MAVS interaction, we evaluated LGP2/MAVS binding in subcellular fractions via coimmunoprecipitation/immunoblot analyses. We established MAVS immunoprecipitation (IP) from MIC and MAM fractions and probed for LGP2 within the recovered products. These analyses indeed revealed an interaction of LGP2 and MAVS primarily in MIC fractions. In addition, MIC fractions from dsRNA-treated cells contained less LGP2 (Input), in line with the previously observed LGP2 translocation resulting in a lower amount of LGP2 in complex with MAVS (Fig. 2I). Calnexin served as loading control as well as control for the nonspecific pulldown of membrane-associated proteins. Taken together, these observations indicate that, in the absence of stimulation, LGP2 is associated with membranes of the ER or other organelles present in MIC via direct or indirect interaction with MAVS while dsRNA treatment triggers LGP2 release from MAVS and translocation to mitochondria. Fractionation analysis of nontargeting A549 RML cells (gNT RML) in comparison to MAVS knockout RML cells (gMAVS RML) suggested that MAVS was not required for LGP2 membrane association or relocalization, indicating that LGP2 interacts with other factors independent of MAVS to regulate its membrane association (SI Appendix, Fig. S3D).
Ultrastructural Analysis Defines dsRNA-Induced Accumulation of LGP2 on Mitochondria.
To define the intracellular localization dynamics of LGP2 on an ultrastructural level, we assessed the membrane-to-mitochondria translocation of LGP2 by TEM using immunogold labeling. For this purpose, we generated A549 cells stably expressing hemagglutinin (HA)-tagged LGP2 (Fig. 3A). Based on the homogeneous expression level of LGP2 in the cell population as determined by immunofluorescent microscopy (SI Appendix, Fig. S4A), we chose A549 cells expressing C-terminally tagged LGP2 for further analyses. After confirming the dsRNA-induced translocation of LGP2-HA in A549 cells expressing RIG-I, MDA5, and LGP2-HA (A549-LGP2-HA-RM cells) (Fig. 3B), we subjected these cells to immunogold labeling using a monoclonal anti-HA antibody and analyzed the samples by TEM. As seen in Fig. 3C, yellow arrowheads point to nonmitochondrial gold particles while the red arrowhead indicates gold particles associated with mitochondria (Fig. 3 C, Right and SI Appendix, Fig. S4B). Image analyses demonstrated no significant difference between the total number of mitochondria per image or the total number of gold particles per image between mock and dsRNA-treated cells (Fig. 3 D, Top Graph). In contrast, we found a significant increase in the number of gold particles on mitochondria per image in dsRNA-treated cells compared to mock-treated cells (Fig. 3 D, Bottom Graph), confirming that dsRNA induces translocation of LGP2 to mitochondria.
Fig. 3.
Ultrastructural analyses showing LGP2 translocation to mitochondria following dsRNA treatment. (A) Immunoblot analysis of A549 cells stably expressing HA-LGP2 or LGP2-HA (N- or C-terminal HA-tag, respectively) using antibodies directed against LGP2 or HA. (B) A549 cells stably expressing LGP2-HA (C-terminal HA-tag) together with RIG-I and MDA5 (A549 LGP2-HA RM) were subjected to subcellular fractionation 16 h after poly(I:C) transfection. One representative experiment is shown from two independent fractionations. (C) Immunogold-labeling of A549 LGP2-HA RM cells 16 h after poly(I:C) or mock transfection using a monoclonal antibody directed against HA. Arrowheads point to immunogold in the cytosol (yellow) or on mitochondria (red). The images in the third column are a magnified view of the boxed areas in column 2 and the images in the second column are a magnified view of the boxed areas in column 1. M, Mitochondria. (Scale bars in second column: 200 nm.) (D, Top) Number of mitochondria or gold particles per image of mock or poly(I:C) (pIC) treated A549 LGP2-HA RM cells. At least 45 images per condition were analyzed. (D, Bottom) Number of gold particles on mitochondria per image. At least 45 images per condition were analyzed manually using the ImageJ software (version 1.52; NIH). Each data point represents one image. To test for statistical significance, a one-way ANOVA was performed, followed by Sidak’s multiple comparison test using GraphPad Prism. ns, not significant; *P < 0.05. Enzo, Enzo Life Sciences, Inc.; CST, Cell Signaling Technology.
LGP2 Translocation Induction by dsRNA Correlates with IRF3 Activation.
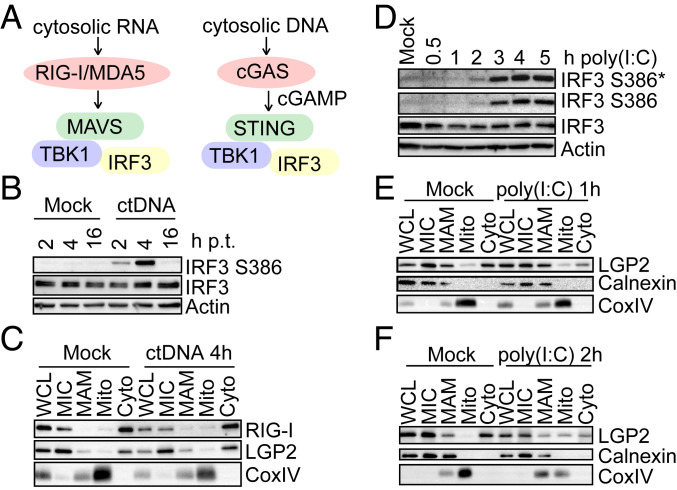
To determine if RLR dynamics are specific for activation of the RLR-MAVS pathway or instead represent a general response to innate immune activation, we assessed the response to calf thymus DNA (ctDNA), which activates IRF3 via the cGAS-STING pathway (Fig. 4A) (1). ctDNA treatment of A549-RML cells induced rapid and robust IRF3 activation (Fig. 4B) but did not alter the subcellular localization of LGP2 or RIG-I (Fig. 4C). Thus, RLR spatial dynamics are specifically responsive to dsRNA. Since the LGP2/MAVS interaction was reduced upon dsRNA stimulation, we reasoned that LGP2 might block or inhibit downstream IRF3 activation in resting cells by interaction with MAVS, possibly preventing MAVS interaction with RIG-I or another accessory signaling component required to impart IRF3 activation. We therefore measured IRF3 activation at early time points after dsRNA treatment of A549-RML cells, identifying 2 h posttreatment as the earliest time point of detectable IRF3 phosphorylation (Fig. 4D). This time point corresponded with mitochondrial translocation of LGP2 which was only detectable at 2 h after treatment (Fig. 4 E and F), thus linking dynamic relocalization of LGP2 with the onset of IRF3 activation in response to dsRNA.
Fig. 4.
Temporal correlation of LGP2 translocation and IRF3 activation kinetics. (A) Pathways leading to IRF3 activation upon stimulation with cytosolic RNA [poly(I:C)] or cytosolic ctDNA. (B) IRF3 activation in A549-RML cells upon ctDNA transfection (1 μg/mL). Cells were harvested at the indicated time points and analyzed by Western blot. (C) Subcellular fractionation of A549-RML cells after 4 h of ctDNA transfection (1 μg/mL). Fractions were analyzed by Western blot for presence of LGP2 and RIG-I (n = 2). (D) Early kinetics of poly(I:C)-induced IRF3 activation. A549-RML cells were lysed at the indicated time points after poly(I:C) transfection, and IRF3 phosphorylation at Serine residues S386 and S396 was analyzed by Western blot. One representative out of two independent experiments is depicted. (E and F) Kinetics of LGP2 to mitochondria translocation analyzed by subcellular fractionation of A549-RML cells after 1 h (E) or 2 h (F) of poly(I:C) transfection. The four independent fractionations performed for this set of experiments comprise two fractionations per time point. *, An asterisk indicates longer exposure.
LGP2 Is a Negative Regulator of RIG-I Signaling.
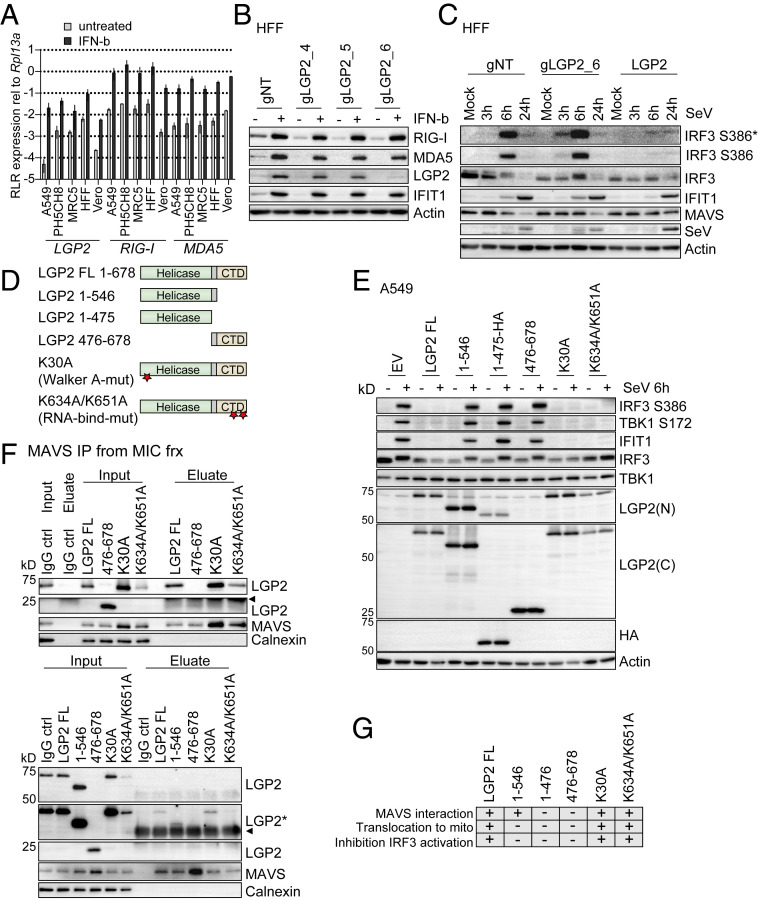
To assess the impact of LGP2 on dsRNA-induced IRF3 activation, we examined the stoichiometric relationship of LGP2 expression and IRF3 activation in dsRNA-treated cells. We first measured the expression of the RLRs across a set of human cell lines and hTert immortalized human foreskin fibroblasts (HFFs) that were mock-treated or treated with IFN-β. We found that baseline RIG-I and MDA5 expression were relatively consistent between the five cell lines under resting conditions (Fig. 5A). In contrast, baseline LGP2 expression was remarkably variable, with lowest basal level of LGP2 expressed in A549 cells and highest present in HFFs (123.55-fold difference, SD ± 49.26 between these two cell types). In addition, transcript expression of all RLRs was strongly induced by IFN-β across all cell lines. We next assessed the response to dsRNA of LGP2 HFF knockout (KO) cells generated by CRISPR/Cas9 gene targeting (HFF-gLGP2_6 cells). Immunoblot analysis of IFN-β–treated HFF-gLGP2_6 cells revealed a specific reduction of LGP2 protein level, but neither RIG-I nor MDA5 expression were affected (Fig. 5B). Comparison of the response to acute SeV infection of control cells harboring nontargeting guide RNA (HFF-gNT) and HFF-gLGP2_6 cells demonstrated that loss of LGP2 expression confers enhanced kinetics of IRF3 activation, as marked by enhanced virus-induced IRF3 S386 phosphorylation in the HFF-gLGP2_6 cells (Fig. 5C). Moreover, we found that mouse embryonic fibroblasts (MEFS) from LGP2−/− mice had significantly enhanced Mx1 mRNA expression kinetics in response to SeV infection compared to wild-type MEFs (SI Appendix, Fig. S5A). Conversely, HFFs with ectopic LGP2 expression exhibited a suppressed response to SeV infection, exhibiting reduced IRF3 activation, with slower activation kinetics and markedly reduced IFIT1 concomitant with increased viral protein abundance (Fig. 5C). Together these data affirm LGP2 as a negative regulator of RIG-I-MAVS innate immune signaling and suggest that the major effect of LGP2 regulatory actions on RLR signaling is exerted after IFN-mediated induction of LGP2 expression (8–11). We note that SeV is known to primarily stimulate innate immune activation via RIG-I, such that these analyses do not assess the impact of LGP2 on MDA5-driven IRF3 activation (13–16).
Fig. 5.
LGP2 is a negative feedback regulator of RIG-I signaling. (A) SYBR green qPCR analysis of RLR expression levels in various cell lines. The indicated cell lines were left untreated or treated with 500 IU/mL IFN-β for 8 h. cDNA was subjected to qPCR analysis using primer pairs specific for LGP2, RIG-I, or MDA5. Data were normalized to Rpl13a housekeeping gene expression and are derived from two (MRC5, Vero) or three (A549, PH5CH8, HFF hTert) independent experiments measured in triplicate. Presented are mean and SD on a log10 scale. (B) Western blot analysis of CRISPR/Cas9-mediated LGP2 knockout in HFF hTert cells. HFF hTert cells were transduced with different guide RNAs (nontargeting [gNT] or targeting LGP2 [gLGP2_4, gLGP2_5, gLGP2_6]) and treated with 500 IU/mL IFN-β for 24 h to monitor LGP2, RIG-I, and MDA5 protein expression. (C) Western blot analysis of innate immune signaling in HFF hTert cells with LGP2 KO (gLGP2_6), LGP2 expression (LGP2), or nontargeting control cells (gNT). Cells were mock infected or infected with SeV (40 hemagglutinating unit [HAU]/mL) and harvested at the indicated time points. (D) Illustration of LGP2 deletion and point mutants. Stars indicate position of point mutations. (E) A549 cells were transduced with lentiviral particles carrying empty vector (EV) or different LGP2 constructs. Cells were selected for stable expression with Blasticidin. Cells were infected with 40 HAU/mL SeV for 6 h and subjected to Western blot analysis. LGP2(C), antibody directed against the LGP2 C-terminal region; LGP2(N), antibody directed against the LGP2 N-terminal region. Depicted is one representative out of two independent experiments (n = 2). (F) MAVS protein was immunoprecipitated from MIC fractions of subcellular fractionation experiments performed with A549 cells expressing the indicated LGP2 mutants (SI Appendix, Fig. S5). Inputs and eluates were analyzed using antibodies directed against LGP2, MAVS, and Calnexin. A polyclonal IgG antibody incubated with the MIC fraction served as control for unspecific binding. The presented data are each derived from one representative out of two independent experiments (n = 2). Arrowheads indicate signals derived from IgG heavy chain or light chain. (G) Graphic summary of the observations made with wild-type LGP2 and LGP2 mutants. *, An asterisk indicates longer exposure. −, indicates no interaction with MAVS, no translocation to mito, regular IRF3 activation; +, indicates interaction with MAVS, translocation to mito, inhibition of IRF3 activation induced by SeV infection.
LGP2-Mediated Feedback Inhibition of RLR Signaling Requires Binding to MAVS.
LGP2 has RNA-binding activity and is an ATPase (2). To determine the requirement for each activity in regulation of RLR signaling and mitochondrial translocation, we generated A549 cells stably expressing full-length LGP2 (LGP2 FL) or LGP2 deletion mutants containing only the helicase domain (1-546 or HA-tagged 1-475) or the C-terminal regulatory domain (476-678), or full-length constructs harboring a Walker A = ATP-binding point mutant (K30A), or a double point mutation that abrogates RNA binding (K634A/K651A) (Fig. 5D) (10, 16, 45–47). In comparison to empty vector (EV) control cells, expression of LGP2 FL, LGP2-K30A, or LGP2-K634A/K651A in A549 cells efficiently blocked the acute activation of IRF3 and IFIT1 induction 6 h after SeV infection while expression of the helicase constructs (1-546 or HA-tagged 1-475) or the regulatory domain (476-678) alone did not impact SeV-induced innate immune activation (Fig. 5E). We note that, 24 h after SeV infection, IFIT1 was induced in all cell lines, but it was unclear if this was due to incomplete block of signaling by ectopic LGP2 or continued accumulation of SeV PAMPs to overcome LGP2 inhibition of signaling (SI Appendix, Fig. S5B). The negative regulation of SeV-induced innate immune activation by wild-type LGP2, and the ATP-binding and RNA-binding LGP2 mutants was also confirmed in HFFs expressing each construct (SI Appendix, Fig. S5C).
We next examined the subcellular distribution of LGP2 constructs in A549 cells to determine the association of LGP2 regulation of innate immune signaling with specific translocation dynamics of each. We examined the enrichment of LGP2 protein in Mito fractions after dsRNA treatment of A549 cells (SI Appendix, Fig. S5 D–J). We found that wild-type LGP2 (LGP2 FL), HA-tagged LGP2 (LGP2 FL_HA), LGP2-K30A, and LGP2-K634A/K651A each accumulated in the Mito fraction after dsRNA treatment of cells (summarized in Fig. 5G, data shown in SI Appendix, Fig. S5 D, G, H, and J). Conversely, the helicase mutants 1-546 or HA-tagged 1-475 (1-475_HA) and the regulatory domain 476-678 did not change in their distribution, remaining chiefly in the Cyto and MIC fractions (Fig. 5G and SI Appendix, Fig. S5 E, F, and I). We further assessed the interaction of the LGP2 mutant proteins with MAVS in MIC fractions from mock-treated samples (Fig. 5 F and G and SI Appendix, Fig. S5K). In line with the observed translocation dynamics and their inhibitory capacity, LGP2 and both point mutants (ATP-binding mutant, RNA-binding mutant) interacted with MAVS in MIC fractions while 1-475_HA and the regulatory domain (476-678) did not (Fig. 5 F and G and SI Appendix, Fig. S5K). Interestingly, we found evidence for an interaction of the helicase construct 1-546 with MAVS in MIC fractions, implicating the region of amino acids 476 to 546 as a potential LGP2-MAVS interaction site. We note however, that possible misfolding of the 1-475 or 476-678 constructs might impact their ability to bind MAVS. Altogether, these data support the notion that LGP2 acts as a negative regulator of innate immune signaling by binding to MAVS at the ER, MAM, or other membranes that are part of the microsome fraction, thus preventing IRF3 activation.
IRF3 Activation Does Not Take Place on Mitochondria.
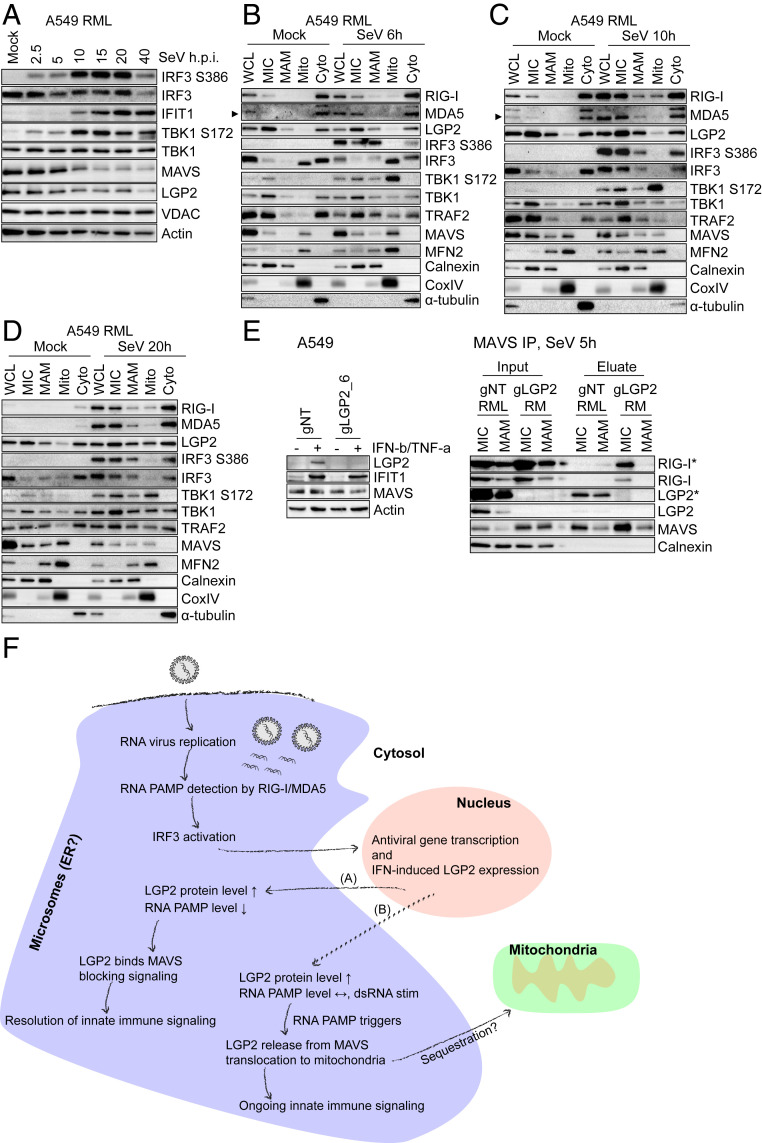
The presence of LGP2 at membranes contained in the microsome fraction, where it interacts with MAVS in resting cells, suggests that IRF3 activation is regulated through LGP2 at microsomal sites of innate immune signaling, including ER, and likely MAM, and peroxisomes. To better understand where IRF3 activation takes place, we assessed SeV-induced innate immune signaling kinetics and component activation in A549-RML cells (Fig. 6A). IRF3 and TBK1 activation was detectable early at 2.5 and 5 h postinfection (h.p.i.), and phosphorylation/activation of each was strongly increased through 20 h. A reduction in MAVS protein level, marking MAVS activation (48), was observed from 10 h after infection and associated with strong IRF3 activation. At 40 h after infection, innate immune signaling was reduced/down-regulated as determined by lower abundance of phospho-IRF3 (Fig. 6A).
Fig. 6.
IRF3 activation does not take place on mitochondria but occurs at the ER-derived membrane network. (A) Innate immune signaling kinetics in A549-RML cells upon SeV infection. A549-RML cells were infected with 40 HAU/mL SeV and harvested for Western blot analysis at the indicated time points. Shown is one representative Western blot derived from two independent experiments (n = 2). (B–D) Subcellular fractionation of A549-RML cells after 6 h (B), 10 h (C), and 20 h (D) of SeV infection. Six independent fractionations were conducted for this experiment, comprising two fractionations per time point. (E, Left) CRISPR/Cas9-mediated LGP2 knockout in A549 cells. A549 cells were transduced with gNT or gLGP2_6 and treated with 500 IU/mL IFN-β + 10 ng/mL TNF-α 48 h prior to Western blot analysis to monitor LGP2 protein expression. (E, Right) MAVS immunoprecipitation from MIC and MAM fractions after subcellular fractionation of A549 gNT RML and A549 gLGP2 RM cells infected with 40 HAU/mL SeV for 5 h. Depicted is one representative out of two independent experiments (n = 2). Asterisks (*) indicate longer exposure. (F) Hypothetical model of RLR-mediated innate immune signaling. Upon RNA virus infection and RNA replication in viral replication compartments that are often generated at the ER, viral RNA PAMPs are detected by RIG-I/MDA5. This leads to interaction with MAVS at ER-derived membranes and IRF3 activation resulting in antiviral gene transcription, inhibition of virus replication, and IFN-induced up-regulation of LGP2 protein expression. In the case of viral PAMP reduction (A), LGP2 binds to MAVS, blocking an interaction of MAVS and RIG-I supporting the resolution of innate immune signaling. In the case of viral persistence or resistance (B), high viral RNA levels trigger LGP2 release from MAVS and its translocation to mitochondria, possibly to sequester LGP2 and allow ongoing innate immune signaling. Arrowheads point to a specific protein band.
We also examined the subcellular localization of activated IRF3 following early (6 h), mid (10 h, and late (20 h) time points of SeV infection (Fig. 6 B–D). At all time points phosphorylated/activated IRF3 (IRF3 S386P) was absent from Mito but present in MIC, MAM, and Cyto fractions (Fig. 6 B–D). This distribution was also confirmed for IRF3 S396P (SI Appendix, Fig. S6A). Interestingly, by using the anti-IRF3 D83B9 antibody (4302; Cell Signaling), which has a higher sensitivity for the detection of baseline variants of total IRF3, nonphosphorylated IRF3, and hypophosphorylated IRF3 (30), we revealed a differential distribution of these two IRF3 phospho-species. While hypophosphorylated IRF3 (upper protein band) was mainly present in MIC and Cyto, nonphosphorylated IRF3 (lower protein band) was enriched in Mito (SI Appendix, Fig. S6A; see IRF3 (CST #4302)). Curiously, nonphosphorylated IRF3 was previously suggested to represent a dormant IRF3 pool while hypophosphorylated IRF3 was suggested to be primed for activation (30). In PH5CH8-RML cells that were mock infected or infected with Mengovirus, an RNA virus that signals innate immune activation through MDA5 (2), we observed a similar distribution of activated and total IRF3 (SI Appendix, Fig. S6B). We note that, in these experiments, although activated/phosphorylated TBK1 (TBK1 S172) was present in the Mito fraction, no activated IRF3 was present in the Mito fraction but instead was detected in MAM, microsomes, and cytosol, indicating that IRF3 activation does not take place on mitochondria (Fig. 6 B–D).
Similar to the dsRNA-induced spatiotemporal dynamics of RIG-I and MDA5, SeV infection resulted in acute enrichment of both RLRs in MAM and Mito fractions. LGP2 translocation to mitochondria occurred relatively late at 20 h post-SeV infection in comparison to dsRNA signaling (Fig. 6 B–D; compare to Fig. 2 B–E). Similar translocation dynamics were also observed for TRAF2 and TBK1. In contrast to Mito, all analyzed factors that are known to be central components of the MAVS signalosome (RLRs, MAVS, TRAF2, TBK1, and IRF3) were present in MIC across the time course.
We next assessed if LGP2 regulates the MAVS–RIG-I interaction in response to SeV infection. Therefore, we produced A549-gLGP2_6 cells expressing ectopic RIG-I and MDA5 (gLGP2-RM cells) and A549-gNT cells expressing RML (A549-gNT-RML cells) and probed for RIG-I protein after MAVS immunoprecipitation from MIC and MAM fractions after SeV infection. Indeed, the virus-induced RIG-I–MAVS interaction was highly increased in the absence of LGP2 (Fig. 6E and SI Appendix, Fig. S6C). Thus LGP2 regulates the RIG-I–MAVS interaction.
Discussion
Our observations support a model where the low level of LGP2 present in resting cells basally occupies interaction with MAVS on microsomal membranes, such as ER, and including peroxisomes, and MAM. Upon RNA virus infection, viral replication takes place in close proximity to membranes that are mostly derived from the ER (21) so that viral PAMPs, including dsRNA and other viral RNAs, accumulate at these membrane sites. Once activated by PAMP binding, RIG-I (and possibly MDA5) mediate a stable interaction with MAVS at microsomal membranes that could displace LGP2 for sequestration at mitochondria via ER-to-mitochondria translocation, thus initiating innate immune signaling and the activation of IRF3 at microsomal membrane sites independent of the OMM. This process facilitates innate immune activation marked by the expression of IRF3-target genes, IFN production, and ISG expression that includes increased RLR expression. Upon IFN-induced suppression of viral PAMP production and increase of LGP2 protein expression, a negative feedback loop is initiated in which LGP2 binds to microsomal MAVS and disrupts or prevents the RIG-I–MAVS interaction, resulting in resolution of innate immune signaling. These actions of LGP2 are summarized in Fig. 6F and are supported by the following observations. 1) Only full-length LGP2 constructs that bind to MAVS inhibit IRF3 activation, and the RIG-I–MAVS interaction is stronger in the absence of LGP2, suggesting that the LGP2–MAVS interaction hinders RIG-I–MAVS binding and thereby inhibits IRF3 activation. 2) The LGP2 1-546 construct binds to MAVS but does not block IRF3 activation, pointing to a possible steric hindrance of RIG-I–MAVS interaction by full-length LGP2. 3) We confirmed the LGP2–MAVS interaction, concluding that LGP2 indeed binds to MAVS and that this interaction facilitates disruption of RIG-I signaling, thus implicating MAVS in LGP2 regulation of RIG-I signaling. Further, we propose that, in case of viral persistence and high PAMP level, as mimicked by dsRNA treatment, LGP2 is released from MAVS (Fig. 6F, pathway B) and is translocated to mitochondria, possibly to sequester LGP2, allowing ongoing innate immune signaling.
Previous studies of RLR-MAVS signaling were based on confocal microscopy or analysis of crude mitochondrial fractions, neither of which provide the resolution to distinguish between mitochondria and associated organelles. In contrast, here, we employ a higher resolution biochemical analysis of subcellular fractions spanning a broad range of cellular compartments and allowing us to examine highly enriched mitochondrial fractions devoid of other organellar membranes (18, 39, 40). This approach, in combination with TEM analyses and immunoprecipitation, revealed that, in resting cells, LGP2 is bound to MAVS, possibly involving a region spanning amino acid residues 476 to 546. An interaction of LGP2 and MAVS was already reported by Komuro et al. (8), who demonstrated coimmunoprecipitation of the two proteins in whole cell lysates of MAVS and LGP2 cotransfected cells, thus identifying the C-terminal region and transmembrane domain of MAVS as LGP2 interaction domains. Interestingly, the authors found that LGP2 blocked an interaction of MAVS with IKKε. Our data suggest that, during SeV infection, LGP2 similarly blocks the interaction of MAVS and RIG-I, most likely by steric hindrance through full-length LGP2 since an LGP2 deletion mutant (1-546) bound to MAVS but did not block IRF3 activation. This observation is in contrast to studies by Quicke et al. and Parisien et al., who both reported an inhibitory effect of the 1-546 LGP2 helicase region construct (45, 46). Underlying reasons for this discrepancy might be the different experimental setups: While we generated A549 cells stably expressing the 1-546 construct and studied the impact on endogenous IRF3 activation and IFIT1 protein expression by Western blot analysis, the other groups employed transient transfection of LGP2 1-546 into HEK293 cells and measured IFN-β promoter-driven luciferase expression from an ectopic plasmid construct. When comparing the effects of LGP2 KO and ectopic LGP2 expression on SeV-induced IRF3 activation in our cell models, we found that increased expression of LGP2 in our ectopic expression cell models had a much stronger effect on innate immune signaling. Thus, we propose that LGP2 exerts its main regulatory function following its increased expression by IFN. In this respect, LGP2 functions as a negative feedback regulator contributing to resolution of RIG-I (and possibly MDA5) signaling after virus infection. The impact of baseline LGP2 on the initiation of RLR-MAVS signaling is likely to differ between different cell lines and in vivo cell types, considering the variability of basal LGP2 expression level demonstrated in our study.
We found that LGP2 translocation from microsomal MAVS to the OMM occurs coincident with high burden of viral PAMP and IRF3 activation. Therefore, it is plausible that LGP2 release from MAVS and translocation to mitochondria facilitates ongoing innate immune signaling. We note that LGP2 has been shown to serve as a positive cofactor of MDA5 signaling to facilitate the dsRNA interaction of MDA5 that builds signaling-competent MDA5 filaments (13). Although we did not examine the specific MDA5 signaling regulation by LGP2 in the present study, our model allows for this cofactor action of LGP2 as LGP2 could execute distinct functions at different subcellular compartments. We found that RNA binding or ATPase activity of LGP2 are not required for MAVS interaction, mitochondrial translocation, or the negative regulation of SeV-induced IRF3 activation. Therefore, direct binding of RNA by LGP2 is not the inducer of LGP2-to-mitochondria translocation. However, LGP2 release from MAVS might be induced by competitive MAVS binding between RIG-I and LGP2 or be regulated by other RNA-binding proteins. MAVS itself was not required for LGP2 membrane localization or dynamics, suggesting a role for other factors in directing LGP2 membrane association and redistribution. In the context of RLR mitochondrial translocation, the 14-3-3 chaperone proteins have been shown to play a role in directing RIG-I and MDA5 to membranes for innate immune signaling (18, 19). Thus, 14-3-3 proteins might similarly facilitate LGP2 translocation to the OMM in response to RLR signaling. It is well established that, at baseline, LGP2 acts as a positive regulator of MDA5 signaling (13–17, 25) although, at increasing concentrations, LGP2 seems to exert MDA5 negative regulation (15, 25). In our model, LGP2 binding to MAVS blocks the RIG-I–MAVS interaction while viral PAMP RNA induces LGP2 translocation to the OMM after its levels accumulate following IFN signaling. This process may sequester LGP2 to allow innate immune signaling, or, as noted above, it may also allow MDA5 filament formation. We also note that LGP2 has not only been implicated in regulation of RLR signaling regulation but has been assigned other functions, including regulation of DNA-virus and bacteria induced immune responses (49), promoting CD8 T cell expansion and survival (23), and mediating resistance of tumor cells to ionizing radiation (50). It is possible that the intracellular dynamics of LGP2 localization could impact these processes where its distribution among different organellar membranes would serve to regulate these cellular functions.
In summary, we performed a high resolution analysis on the spatiotemporal distribution of RLRs, including LGP2, over a broad range of fractions and spanning various subcellular compartments. We present data that argue against the dogma of mitochondria representing the central platform of innate immune signaling but instead suggest that innate immune signaling is initiated at the ER including MAM, a subdomain of ER membranes. Our observations provide increased details for understanding of the subcellular localization of innate immune signaling components and where innate immune signaling is initiated and governed. Such studies are important to inform strategies in the growing field of RLR-based therapeutics (51–55) so that targeting RLRs at relevant subcellular compartments can be obtained. Our study demonstrates that LGP2 interaction with MAVS as well as dynamic relocalization of LGP2 between microsomal membranes and mitochondria are important features of RLR signaling control.
Materials and Methods
Subcellular Fractionation.
Subcellular fractionation by Percoll density gradient centrifugation was performed as described by Bozidis et al. (41). In brief, cells were centrifuged and resuspended in a sucrose homogenization medium. After breaking up the cells with a Dounce homogenizer, nuclei and unbroken cells were pelleted twice at 600 × g, and the supernatant was separated into crude mitochondria (MAM+mitochondria) and microsomes+cytosol by spinning three times at 10,400 × g for 10 min. Crude mitochondria were resuspended in buffer Mannitol A, homogenized by douncing, and layered onto a 30% Percoll solution. After spinning for 1 h at 95,000 × g, fractions of MAM and mitochondria were harvested and washed with buffer Mannitol B. MAM and MIC were pelleted at 100,000 × g for 1 h. Cytosol was concentrated using an Amicon Ultra-4 filter unit (Millipore Sigma). All membranous fractions were resuspended in lysis buffer (50 mM Tris⋅HCl, 150 mM NaCl, 1% Triton X-100, with freshly added protease and phosphatase inhibitors) (Sigma-Aldrich). The protein concentration of each fraction was measured by Pierce BCA assay (Thermo Fisher Scientific). For Western blot analysis, 2 μg of each fraction were loaded onto a polyacrylamide gel containing 10% sodium dodecyl sulfate (SDS). Detection of bound horseradish peroxidase-coupled secondary antibodies was performed using an ECL prime reagent (GE Healthcare) on a ChemiDoc Imager (Bio-Rad). Polyvinylidene difluoride membranes were stripped for reprobing with a harsh stripping buffer containing 2% SDS and 0.8% β-mercaptoethanol. Antibodies are listed in SI Appendix, Table S2.
Statistical Analysis.
Statistical analysis was performed in GraphPad Prism 7.03 (GraphPad, La Jolla, CA) and is described in figure legends.
Data Availability.
All data, protocols, and reagents are available in the main text or SI Appendix.
Supplementary Material
Acknowledgments
This work was supported by NIH Grants AI04002, AI145296, AI118916, and AI127463. We thank B. Schneider at the electron microscopy core facility of the Fred Hutchinson Cancer Research Center; V. Lohmann, M. Binder, and R. Bartenschlager for plasmids; and A. Sekine and K. Voss for mouse embryonic fibroblasts.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921861117/-/DCSupplemental.
References
- 1.Chen Q., Sun L., Chen Z. J., Regulation and function of the cGAS-STING pathway of cytosolic DNA sensing. Nat. Immunol. 17, 1142–1149 (2016). [DOI] [PubMed] [Google Scholar]
- 2.Loo Y. M., Gale M. Jr., Immune signaling by RIG-I-like receptors. Immunity 34, 680–692 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kell A. M., Gale M. Jr., RIG-I in RNA virus recognition. Virology 479–480, 110–121 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Woolhouse M. E., Gowtage-Sequeria S., Host range and emerging and reemerging pathogens. Emerg. Infect. Dis. 11, 1842–1847 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Belgnaoui S. M., Paz S., Hiscott J., Orchestrating the interferon antiviral response through the mitochondrial antiviral signaling (MAVS) adapter. Curr. Opin. Immunol. 23, 564–572 (2011). [DOI] [PubMed] [Google Scholar]
- 6.Gorman J. A.et al., The A946T variant of the RNA sensor IFIH1 mediates an interferon program that limits viral infection but increases the risk for autoimmunity. Nat. Immunol. 18, 744–752 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Funabiki M.et al., Autoimmune disorders associated with gain of function of the intracellular sensor MDA5. Immunity 40, 199–212 (2014). [DOI] [PubMed] [Google Scholar]
- 8.Komuro A., Horvath C. M., RNA- and virus-independent inhibition of antiviral signaling by RNA helicase LGP2. J. Virol. 80, 12332–12342 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rothenfusser S.et al., The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 175, 5260–5268 (2005). [DOI] [PubMed] [Google Scholar]
- 10.Saito T.et al., Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl. Acad. Sci. U.S.A. 104, 582–587 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yoneyama M.et al., Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175, 2851–2858 (2005). [DOI] [PubMed] [Google Scholar]
- 12.Deddouche S.et al., Identification of an LGP2-associated MDA5 agonist in picornavirus-infected cells. eLife 3, e01535 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bruns A. M., Leser G. P., Lamb R. A., Horvath C. M., The innate immune sensor LGP2 activates antiviral signaling by regulating MDA5-RNA interaction and filament assembly. Mol. Cell 55, 771–781 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Childs K. S., Randall R. E., Goodbourn S., LGP2 plays a critical role in sensitizing mda-5 to activation by double-stranded RNA. PLoS One 8, e64202 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bruns A. M.et al., ATP hydrolysis enhances RNA recognition and antiviral signal transduction by the innate immune sensor, laboratory of genetics and physiology 2 (LGP2). J. Biol. Chem. 288, 938–946 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Satoh T.et al., LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl. Acad. Sci. U.S.A. 107, 1512–1517 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Venkataraman T.et al., Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J. Immunol. 178, 6444–6455 (2007). [DOI] [PubMed] [Google Scholar]
- 18.Liu H. M.et al., The mitochondrial targeting chaperone 14-3-3ε regulates a RIG-I translocon that mediates membrane association and innate antiviral immunity. Cell Host Microbe 11, 528–537 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin J. P., Fan Y. K., Liu H. M., The 14-3-3η chaperone protein promotes antiviral innate immunity via facilitating MDA5 oligomerization and intracellular redistribution. PLoS Pathog. 15, e1007582 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riedl W.et al., Zika Virus NS3 mimics a cellular 14-3-3-binding motif to antagonize RIG-I- and MDA5-mediated innate immunity. Cell Host Microbe 26, 493–503.e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero-Brey I., Bartenschlager R., Membranous replication factories induced by plus-strand RNA viruses. Viruses 6, 2826–2857 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Horner S. M., Liu H. M., Park H. S., Briley J., Gale M. Jr., Mitochondrial-associated endoplasmic reticulum membranes (MAM) form innate immune synapses and are targeted by hepatitis C virus. Proc. Natl. Acad. Sci. U.S.A. 108, 14590–14595 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suthar M. S.et al., The RIG-I-like receptor LGP2 controls CD8(+) T cell survival and fitness. Immunity 37, 235–248 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sanchez David R. Y.et al., LGP2 binds to PACT to regulate RIG-I- and MDA5-mediated antiviral responses. Sci. Signal. 12, eaar3993 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Pippig D. A.et al., The regulatory domain of the RIG-I family ATPase LGP2 senses double-stranded RNA. Nucleic Acids Res. 37, 2014–2025 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hou F.et al., MAVS forms functional prion-like aggregates to activate and propagate antiviral innate immune response. Cell 146, 448–461 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu S.et al., Phosphorylation of innate immune adaptor proteins MAVS, STING, and TRIF induces IRF3 activation. Science 347, aaa2630 (2015). [DOI] [PubMed] [Google Scholar]
- 28.Fang R.et al., MAVS activates TBK1 and IKKε through TRAFs in NEMO dependent and independent manner. PLoS Pathog. 13, e1006720 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Servant M. J.et al., Identification of the minimal phosphoacceptor site required for in vivo activation of interferon regulatory factor 3 in response to virus and double-stranded RNA. J. Biol. Chem. 278, 9441–9447 (2003). [DOI] [PubMed] [Google Scholar]
- 30.Servant M. J.et al., Identification of distinct signaling pathways leading to the phosphorylation of interferon regulatory factor 3. J. Biol. Chem. 276, 355–363 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Bergstroem B.et al., Identification of a novel in vivo virus-targeted phosphorylation site in interferon regulatory factor-3 (IRF3). J. Biol. Chem. 285, 24904–24914 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grandvaux N.et al., Transcriptional profiling of interferon regulatory factor 3 target genes: Direct involvement in the regulation of interferon-stimulated genes. J. Virol. 76, 5532–5539 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dixit E.et al., Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141, 668–681 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu H., Carvalho P., Voeltz G. K., Here, there, and everywhere: The importance of ER membrane contact sites. Science 361, eaan5835 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lackner L. L., The expanding and unexpected functions of mitochondria contact sites. Trends Cell Biol. 29, 580–590 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siekevitz P., Protoplasm: Endoplasmic reticulum and microsomes and their properties. Annu. Rev. Physiol. 25, 15–40 (1963). [DOI] [PubMed] [Google Scholar]
- 37.Scorrano L.et al., Coming together to define membrane contact sites. Nat. Commun. 10, 1287 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vance J. E., MAM (mitochondria-associated membranes) in mammalian cells: Lipids and beyond. Biochim. Biophys. Acta 1841, 595–609 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Loo Y. M.et al., Viral and therapeutic control of IFN-beta promoter stimulator 1 during hepatitis C virus infection. Proc. Natl. Acad. Sci. U.S.A. 103, 6001–6006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seth R. B., Sun L., Ea C. K., Chen Z. J., Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-kappaB and IRF 3. Cell 122, 669–682 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Bozidis P., Williamson C. D., Colberg-Poley A. M., Isolation of endoplasmic reticulum, mitochondria, and mitochondria-associated membrane fractions from transfected cells and from human cytomegalovirus-infected primary fibroblasts. Curr. Protoc. Cell Biology 37, 3.27.1-3.27.23 (2007). [DOI] [PubMed] [Google Scholar]
- 42.Esser-Nobis K.et al., Comparative analysis of African and Asian lineage-derived zika virus strains reveals differences in activation of and sensitivity to antiviral innate immunity. J. Virol. 93, e00640-19 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Giacomello M., Pellegrini L., The coming of age of the mitochondria-ER contact: A matter of thickness. Cell Death Differ. 23, 1417–1427 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sala-Vila A.et al., Interplay between hepatic mitochondria-associated membranes, lipid metabolism and caveolin-1 in mice. Sci. Rep. 6, 27351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Quicke K. M., Kim K. Y., Horvath C. M., Suthar M. S., RNA helicase LGP2 negatively regulates RIG-I signaling by preventing TRIM25-mediated caspase activation and recruitment domain ubiquitination. J. Interferon Cytokine Res. 39, 669–683 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Parisien J. P.et al., RNA sensor LGP2 inhibits TRAF ubiquitin ligase to negatively regulate innate immune signaling. EMBO Rep. 19, e45176 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li X.et al., The RIG-I-like receptor LGP2 recognizes the termini of double-stranded RNA. J. Biol. Chem. 284, 13881–13891 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Heaton S. M., Borg N. A., Dixit V. M., Ubiquitin in the activation and attenuation of innate antiviral immunity. J. Exp. Med. 213, 1–13 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pollpeter D., Komuro A., Barber G. N., Horvath C. M., Impaired cellular responses to cytosolic DNA or infection with Listeria monocytogenes and vaccinia virus in the absence of the murine LGP2 protein. PLoS One 6, e18842 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Widau R. C.et al., RIG-I-like receptor LGP2 protects tumor cells from ionizing radiation. Proc. Natl. Acad. Sci. U.S.A. 111, E484–E491 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Probst P.et al., A small-molecule IRF3 agonist functions as an influenza vaccine adjuvant by modulating the antiviral immune response. Vaccine 35, 1964–1971 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pattabhi S.et al., Targeting innate immunity for antiviral therapy through small molecule agonists of the RLR pathway. J. Virol. 90, 2372–2387 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ruzicka M.et al., RIG-I-based immunotherapy enhances survival in preclinical AML models and sensitizes AML cells to checkpoint blockade. Leukemia 34, 1017–1026 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jiang X.et al., Intratumoral delivery of RIG-I agonist SLR14 induces robust antitumor responses. J. Exp. Med. 216, 2854–2868 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Heidegger S.et al., RIG-I activation is critical for responsiveness to checkpoint blockade. Sci. Immunol. 4, eaau8943 (2019). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, protocols, and reagents are available in the main text or SI Appendix.