Significance
DHPS is one of the most abundant organosulfonates on this planet. The mechanisms for DHPS degradation in the anaerobic biosphere are not well understood. Here, we report the bioinformatics-aided discovery, biochemical, and structural characterizations of two O2-sensitive glycyl radical enzymes that use distinct radical-mediated mechanisms for DHPS degradation in anaerobic bacteria from diverse terrestrial and marine sources as well as human gut. These enzymes play an important role in the biogeochemical sulfur cycle and link dietary sulfonates to microbial production of H2S, which is a causative agent of chronic diseases, such as inflammation and colorectal cancer.
Keywords: glycyl radical enzyme, sulfoglycolysis, dihydroxypropanesulfonate, sulfate- and sulfite-reducing bacteria, gut bacteria
Abstract
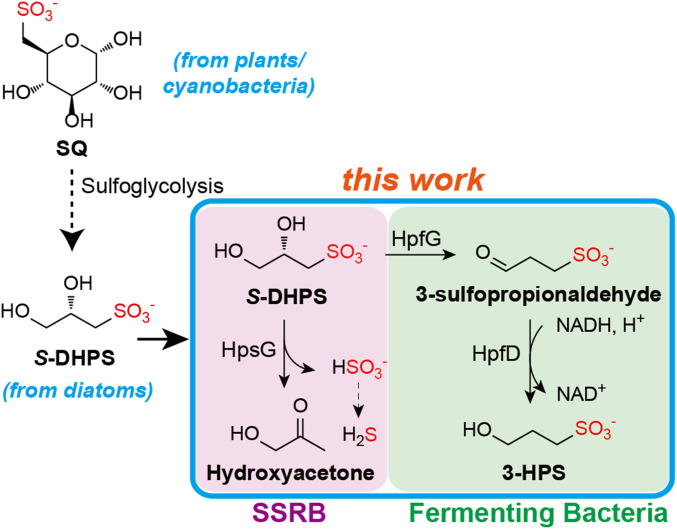
2(S)-dihydroxypropanesulfonate (DHPS) is a microbial degradation product of 6-deoxy-6-sulfo-d-glucopyranose (sulfoquinovose), a component of plant sulfolipid with an estimated annual production of 1010 tons. DHPS is also at millimolar levels in highly abundant marine phytoplankton. Its degradation and sulfur recycling by microbes, thus, play important roles in the biogeochemical sulfur cycle. However, DHPS degradative pathways in the anaerobic biosphere are not well understood. Here, we report the discovery and characterization of two O2-sensitive glycyl radical enzymes that use distinct mechanisms for DHPS degradation. DHPS-sulfolyase (HpsG) in sulfate- and sulfite-reducing bacteria catalyzes C–S cleavage to release sulfite for use as a terminal electron acceptor in respiration, producing H2S. DHPS-dehydratase (HpfG), in fermenting bacteria, catalyzes C–O cleavage to generate 3-sulfopropionaldehyde, subsequently reduced by the NADH-dependent sulfopropionaldehyde reductase (HpfD). Both enzymes are present in bacteria from diverse environments including human gut, suggesting the contribution of enzymatic radical chemistry to sulfur flux in various anaerobic niches.
Organosulfonates are ubiquitous in our environment, our bodies, and the food that we eat. Mechanisms by which they are metabolized by diverse microbes in the human microbiome and in the environment are of great relevance to human health and to the biogeochemical sulfur cycle. Two organosulfonates of special importance are sulfoquinovose and DHPS due to their production in large volumes globally by photoautotrophs (1, 2).
Sulfoquinovose is the polar headgroup of the plant sulfolipid sulfoquinovosyl diacylglycerol (3), a component of photosynthetic thylakoid membranes in all vascular plants, mosses, algae, and most photosynthetic bacteria (1). The annual global production of sulfoquinovose is estimated to be 1010 tons, making it one of the most abundant organic sulfur compounds in nature (1). Bacterial degradation of sulfoquinovose was recently discovered and named sulfoglycolysis due to its resemblance to classical glycolytic pathways. The sulfo-Embden–Meyerhof–Parnas (sulfo-EMP) pathway was characterized in Escherichia coli K-12 and is used during both aerobic growth (4) and anaerobic mixed acid fermentation (5). In this pathway, half of the carbon of sulfoquinovose is converted to dihydroxyacetonephosphate and used for growth, while the other half is excreted as DHPS, which is, subsequently, metabolized by other bacteria (5, 6). A second sulfoglycolytic pathway, the sulfo-Entner–Doudoroff (sulfo-ED) pathway, was characterized in the environmental isolate Pseudomonas putida SQ1 (7) and produces 3-sulfolactate instead of DHPS. It is thought that the sulfo-EMP pathway is favored by fermentative bacteria, while the sulfo-ED pathway is favored by respiratory bacteria (7).
Apart from being a product of sulfoglycolysis, DHPS is also an important organosulfur compound in its own right. It is present in up to millimolar intracellular concentrations in eukaryotic marine phytoplankton, including the highly abundant diatoms (2, 8), which are estimated to contribute ∼20% of the total global primary production (9). These phytoplankton use sulfate, present at ∼30 mM levels in seawater to synthesize a variety of organosulfur compounds including DHPS and sulfoquinovose, Secretion or cell lysis makes these compounds available for degradation by marine heterotrophic bacteria, accounting for a large component of the flux of organic carbon in the surface oceans (8, 10).
Various pathways for the degradation of DHPS have been identified in aerobic environmental bacteria. In Cupriavidus pinatubonensis (11), (S)-DHPS is racemized in two steps through two DHPS-2-dehydrogenases (HpsO and HpsP). A DHPS-1-dehydrogenase (HpsN) then converts (R)-DHPS into (R)-sulfolactate (SL), followed by C–S cleavage by the enantiospecific (R)-sulfolactate-sulfolyase (SuyAB) (SI Appendix, Fig. S1A). The sulfite released is oxidized by the periplasmic sulfite dehydrogenase and excreted as sulfate. Apart from direct C–S cleavage by SuyAB, two other processes are known for the desulfonation of (R)-SL. In the aerobic marine bacterium Roseovarius nubinhibens ISM (12), SL is converted to cysteate followed by C–S cleavage by cysteate sulfolyase and converted to sulfoacetaldehyde followed by C–S cleavage by sulfoacetaldehyde actyltransferase (SI Appendix, Fig. S1A).
In the anaerobic biosphere, which includes environments ranging from ocean sediments to the digestive tracts of marine and terrestrial animals, degradation of DHPS by sulfate- and sulfite-reducing bacteria (SSRB) results in conversion of the sulfonate sulfur to H2S (5). This process is of particular interest in the human gut where fermentation of dietary sulfoquinovose rich in fruits and vegetables produces DHPS, and generation of H2S by SSRB has been linked to diseases, such as inflammation and colorectal cancer (13). Despite its importance, pathways for the degradation of DHPS in anaerobic bacteria are not well understood. The only organism in which it has been studied is Desulfovibrio sp. DF1, an isolate from sewage sludge (5). In this bacterium, (S)-DHPS is imported into the cell through an ABC transporter and oxidized sequentially to sulfolactate by two NAD+-dependent dehydrogenase DhpA and SlaB (SI Appendix, Fig. S1B). C–S cleavage by SuyAB releases sulfite, which is used as a terminal electron acceptor (TEA), producing H2S as an end product.
Previously, we (14) and others (15) reported a radical-dependent mechanism for C–S cleavage of 2-hydroxyethylsulfonate (isethionate), which is structurally similar to DHPS, in anaerobic bacteria. The reaction is catalyzed by the O2-sensitive enzyme IseG, a member of the glycyl radical enzyme (GRE) superfamily (16–18). GREs contain an essential O2-sensitive glycyl radical (G•) cofactor, generated by an activating enzyme through chemistry involving S-adenosylmethionine (SAM) and a [4Fe–4S]1+ cluster (19, 20), and catalyze diverse radical-dependent reactions. IseG belongs to a subset of GREs catalyzing 1,2 lyase reactions, including C–O, C–N, and C–S lyases (16). The substrates of these lyases contain a C1–OH group and a variable C2 leaving the group. Since DHPS contains two OH groups, radical-dependent cleavage could, in theory, lead to two possible outcomes: C–S or C–O cleavage (Scheme 1). Here, we describe our bioinformatics-aided discovery, biochemical and structural characterizations of two GREs, a (S)-DHPS sulfolyase (HpsG) and a (S)-DHPS dehydratase (HpfG). HpsG is present in SSRB, while HpfG is present in fermenting bacteria, and their differing distribution and metabolic roles are discussed.
Scheme 1.
Radical-dependent (S)-DHPS degradation pathways involving the sulfolyase HpsG and dehydratase HpfG.
Results
Identification and Biochemical Characterization of the DHPS-Sulfolyase HpsG.
During our previous crystallographic studies of Desulfovibrio vulgaris IseG (DvIseG, Protein Data Bank [PDB] accession no.: 5YMR) (14), we noted that some close homologs of DvIseG contain variations in substrate-interacting residues, suggesting the possibility that they may accept structurally related sulfonate substrates. We focused on the sulfite-reducing human gut bacterium Bilophila wadsworthia, which encodes two homologs of DvIseG (UniProt: E5Y378 and E5Y7I4, 60% identity between the two homologs). Previously, growth of B. wadsworthia RZATAU with isethionate as the sole TEA resulted in the induction of only E5Y378 but not E5Y7I4 (14). The substrate-interacting residues of DvIseG were identical to those of E5Y378 and were highly similar but not identical to those of E5Y7I4 (SI Appendix, Fig. S2), suggesting a structurally similar substrate. We hypothesized that the substrate is (S)-DHPS due to the abundance of this organosulfonate in nature.
To test this hypothesis, we recombinantly produced E5Y7I4 and its adjacent activating enzyme E5Y7I3 from B. wadsworthia 3_1_6 (SI Appendix, Fig. S3). Biochemical characterization confirmed that it was, indeed, a DHPS sulfolyase (Fig. 1), and we renamed the GRE and activating enzyme HpsG and HpsH, respectively. HpsG could be activated by anaerobically reconstituted HpsH (SI Appendix, Fig. S4), forming 0.04 ± 0.02 (out of a theoretical maximum of 1) (19) G• per dimer (Fig. 1A). The low radical yield is an issue with many GREs and requires further analysis. Incubation of activated HpsG with racemic DHPS resulted in the production of hydroxyacetone (Fig. 1 B–D) and sulfite as detected using a colorimetric Fuchsin assay (21) (SI Appendix, Fig. S5A). HpsG exhibited Michaelis–Menten kinetics for DHPS desulfonation (kcat = 26 ± 1 s−1, Km = 13 ± 2 mM, kcat/Km = 1.9 ± 0.2 mM−1 s−1) (SI Appendix, Fig. S5 B and C) with a much lower activity for isethionate desulfonation (kcat = 0.57 ± 0.03 s−1, Km = 34 ± 5 mM, kcat/Km = 0.017 ± 0.003 mM−1 s−1) (Fig. 1B and SI Appendix, Fig. S5 D and E). (The kcat reported is normalized by radical content.)
Fig. 1.
EPR spectra and enzymatic assays of HpsG. (A) X-band EPR spectrum of HpsG (40 μM) reconstituted with HpsH (80 μM), SAM (1 mM), and reductant Ti (III) citrate (100 μM), acquired at a temperature of 90 K and microwave power of 1 mW. (B) Specific activity of HpsG-catalyzed conversion of DHPS to hydroxyacetone and isethionate to acetaldehyde, measured using NADH-coupled spectrophotometric assays with E. coli glycerol dehydrogenase (GldA) and Saccharomyces cerevisiae alcohol dehydrogenase (ADH1), respectively. Error bars represent the SD of three individual experiments. (C) Detection of the products of HpsG-catalyzed reactions by derivatization with 2,4-dinitrophenylhydrazine (DNPH) and liquid chromatography–mass spectrometry (LC–MS) analysis. (D) Negative ionization mass spectra of DNPH-derivatized reaction products, corresponding to peaks 1 and 2 in C. (Theoretical mass of monoanions, DNPH hydroxyacetone = 253.0, and DNPH acetaldehyde = 223.0).
Crystal Structure of HpsG in Complex with (S)-DHPS.
To further examine the origin of the different substrate specificities of HpsG, we determined the crystal structure of HpsG in complex with racemic DHPS at 2.2 Å (Fig. 2 and SI Appendix, Fig. S6 and Table S1). The C121 crystals contain four monomers per asymmetric unit, each exhibiting a canonical β/α barrel fold common to other GREs. The overall structure of HpsG is similar to that of DvIseG with a rmsd of 0.73 Å between 796 Cα atoms (SI Appendix, Fig. S6A). The well-defined electron density for (S)-DHPS in the active site of HpsG enabled unambiguous assignment of the substrate. The C2–OH moiety of the bound (S)-DHPS is located at a position similar to the C1–OH of isethionate in IseG and is positioned for deprotonation by Glu464 (Fig. 2A). The substrate sulfonate group is also coordinated in a similar fashion as in IseG by Arg183, Gln187, and Arg672 of HpsG. Three water molecules contribute to the extensive hydrogen bond network interacting with the substrate (Fig. 2A). However, the orientation of the C3 methylene group of (S)-DHPS is markedly different from that of the corresponding C2 methylene group of isethionate with a 160° rotation between the C2–C3–S plane of (S)-DHPS relative to the C1–C2–S plane of isethionate (SI Appendix, Fig. S6 B and C). The replacement of Phe680 and Thr310 in IseG with the less bulky Ile676 and Ala306 in HpsG accommodates the new orientation of the C3 methylene group and the additional C1 hydroxymethyl group of (S)-DHPS (SI Appendix, Fig. S6 B and C). Binding of DHPS in the active site of HpsG is further established by a water molecule bridging C1–OH of the substrate and the hydroxyl group of Ser305 in HpsG, which replaces a Gly309 in IseG at the same position.
Fig. 2.
HpsG active-site structure. (A) Structure of the HpsG active site in complex with the (S)-DHPS (Chain D). The proposed pathway for hydrogen atom transfer is indicated by red arrows, and hydrogen bonds are indicated by black dotted lines. 2Fo–Fc electron densities for isethionate are shown at 1.0σ. The two rotamers observed for Cys462 are shown. (B) Proposed mechanism of DHPS cleavage by HpsG. The thiyl radical, which is transiently generated by the G• cofactor in all GREs, abstracts a hydrogen (shown in red) from the substrate DHPS, and returns it to form the product hydroxyacetone.
A dual conformation of the thiyl radical residue Cys462 is observed in HpsG, suggesting high conformational flexibility of this residue. The minimal distance between the thiyl radial and the Cα of the glycyl radical residue Gly799 is 4.27 Å (chain D), and the minimal distance between the C2 of DHPS and the thiyl radical is 4.09 Å (chain D), which would allow C–H abstraction to occur (Fig. 2B and SI Appendix, Fig. S6C). In the structure of DvIseG, the orientation of isethionate suggested that its pro-R hydrogen is abstracted. The C2–H of (S)-DHPS has the same orientation, implicating a similar stereochemistry of abstraction (SI Appendix, Fig. S6C). In contrast, the hydrogen of the opposite stereochemistry is thought to be abstracted in all other mechanistically related GREs: CutC, propanediol dehydratase, glycerol dehydratase, and ribonucleotide reductase (16).
Investigation of DHPS Transporters in SSRB.
To identify HpsG in other organisms, we examined the UniRef50 cluster UniRef50_A0A348AJ86 (where each member shares ≥50% sequence identity and ≥80% overlap with the seed sequence of the cluster) (22), which contains IseG and HpsG sequences. A sequence similarity network (SSN) was constructed for these proteins, revealing an organization into two major clusters (Fig. 3A). The first contained 103 proteins, the majority of which have active-site residues identical to those of IseG from D. vulgaris or D. bizertensis (SI Appendix, Fig. S2). The second cluster contained 14 proteins with active-site residues identical to those of B. wadsworthia HpsG (Fig. 3A and SI Appendix, Table S2) and are, thus, assigned as HpsG.
Fig. 3.
SSN and genome neighborhoods of HpsG homologs. (A) SSN for IseG homologs belonging to UniRef50_A0A348AJ86, displayed at the E-value cutoff of 10−360. Each node represents a unique protein sequence, and the most highly related proteins are grouped together in clusters, putatively sharing the same function. Blue, active-site residues identical to D. vulgaris or Desulfovibrio bizertensis IseG; red, active-site residues identical to B. wadsworthia HpsG. (B) Genome neighborhoods of selected homologs in the HpsG cluster. (C) Proposed pathway for DHPS import and HpsG-dependent degradation in B. wadsworthia.
To find other genes associated with DHPS degradation, the genome neighborhoods of the HpsG sequences were examined using the enzyme function initiative–genome neighborhood tool (Fig. 3B) (23). Two of them (Desulfovibrio litoralis German Collection of Microorganisms [DSM] 11393, uncultured Desulfovibrio sp Tax ID: 167968) were associated with transporters in the MFS family (Pfam PF07690). These were closely related to the Cupriavidus pinatubonensis (S)-DHPS transporter HpsU (60% sequence identity) (11), suggesting that they are DHPS transporters (Fig. 3C).
Several of the HpsG homologs, including B. wadsworthia HpsG, were also associated with a tripartite ATP-independent periplasmic (TRAP) transporter, a family of transporters that catalyzes the import of carboxylic and sulfonic acids (24) and includes the previously characterized isethionate transporter IseKLM from Desulfovibrio piger (14). TRAP transporters are reliant on a soluble periplasmic substrate-binding protein (SBP), which is amenable to biochemical characterization by isothermal titration calorimetry (ITC) (14, 25). The recombinant SBP (E5Y7I1) of the HpsG-associated TRAP transporter in B. wadsworthia (SI Appendix, Fig. S7A) showed enthalpy-driven binding of DHPS with a Kd of 6.7 μM (SI Appendix, Fig. S7B) but no binding of isethionate or 3-hydroxypropane-1-sulfonate (3-HPS) (SI Appendix, Fig. S7 C and D), confirming that it is a DHPS TRAP transporter (HpsKLM) (Fig. 3C).
HpsG Is Involved in DHPS Dissimilation in B. wadsworthia.
We further studied the ability B. wadsworthia RZATAU to use DHPS as a TEA. When pyruvate is supplied as a carbon and electron source, growth was supported with DHPS as the sole TEA (Fig. 4A), accompanied by the production of H2S as detected using a methylene blue assay (Fig. 4A). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS/PAGE) analysis revealed a prominent protein band with molecular weight of ∼95 kDa (Fig. 4C), present in DHPS—but not thiosulfate-grown cells. Mass spectrometric analysis identified this protein as HpsG (Dataset S3), suggesting its involvement in DHPS dissimilation in this bacterium.
Fig. 4.
DHPS-dependent growth of B. wadsworthia and H2S formation. (A) Various sulfur containing TEAs support the growth of B. wadsworthia. (B) Formation of methylene blue by reacting the headspace gas with N,N-dimethyl-pphenylenediamine dihydrochloride and the absorbance of the respective reaction mixtures at 670 nm. The assays were performed in triplicate and are presented with SDs. (C) B. wadsworthia grown on pyruvate plus thiosulfate (lane 2), taurine (lane 3), or pyruvate plus DHPS (lane 4). The arrows indicate a ∼95-kDa band identified as HpsG.
Identification and Biochemical Characterization of the DHPS Dehydratase HpfG.
Apart from desulfonation, GREs could, in theory, also catalyze radical-mediated DHPS dehydration, converting it to 3-sulfopropionaldehyde (Scheme 1). Because of the global abundance of (S)-DHPS, we hypothesized the existence of a GRE (S)-DHPS dehydratase, which would provide an alternative pathway for DHPS degradation. The catalytic mechanism of GRE diol dehydratases has been extensively studied both experimentally (26) and computationally (27). The reaction involves five conserved active-site residues: a Cys thiyl radical involved in substrate radical generation, Glu involved in substrate C1–OH deprotonation, and a triad of two His and one Asp involved in coordination and protonation of the substrate C2–OH leaving group.
To search for a GRE (S)-DHPS dehydratase, 674 candidate diol dehydratase sequences containing the five conserved active-site residues were extracted from the 22,000 sequences in the GRE superfamily (InterPro IPR004184). A SSN was constructed for these proteins, revealing an organization into two major clusters (Fig. 5A). The first contained previously characterized Clostridium butyricum glycerol dehydratase (GDH) and Roseburia inulinivorans propanediol dehydratase. The second cluster contained 74 GREs of unknown function, corresponding to the UniRef cluster UniRef50_E3H691 (SI Appendix, Table S3). Examination of their genome neighborhoods revealed genes that could be involved in DHPS degradation (Fig. 5B). Like other diol dehydratases, this GRE is associated with a metal-dependent alcohol dehydrogenase (ADH), which catalyzes the reduction of the aldehyde product. In addition, this GRE is associated with a very characteristic transporter belonging to the TauE family (PF01925), which includes bacterial sulfite, isethionate, and sulfoacetate exporters, suggesting a link to sulfonate metabolism. We, therefore, hypothesized a pathway in which (S)-DHPS is converted to 3-sulfopropionaldehyde by the GRE (HpfG), reduced to sulfopropanol by the ADH (HpfD), and excreted through the exporter (HpfE) (Fig. 5C).
Fig. 5.
SSN and genome neighborhoods of HpfG homologs. (A) SSN for putative diol dehydratases in the GRE superfamily, containing conserved residues thought to be required for catalysis, displayed at the E-value cutoff of 10−260. Each node represents a unique protein sequence, and the most highly related proteins are grouped together in clusters, putatively sharing the same function. (B) Genome neighborhoods of selected homologs in the HpfG cluster. (C) Proposed pathways for DHPS import and HpfG-dependent degradation.
To test this hypothesis, we recombinantly produced HpfG (A0A318FL05) and its activating enzyme HpfH (A0A318FEA4) from Klebsiella oxytoca (SI Appendix, Fig. S8) and HpfD (A0A374P995) from Hungatella hathewayi (SI Appendix, Fig. S9A). HpfD catalyzed NAD+-dependent oxidation of 3-HPS (SI Appendix, Fig. S9B) and is presumed to also catalyze the NADH-dependent sulfopropionaldehyde reduction because of the thermodynamic reversibility of this reaction. HpfG could be activated by anaerobically reconstituted HpfH (SI Appendix, Fig. S1A), forming 0.03 ± 0.01 (out of a theoretical maximum of 1) (19) G• per dimer (Fig. 6A). The low radical yield is an issue with many GREs, and requires further analysis. Incubation of activated HpfG with racemic DHPS resulted in the production of 3-sulfopropionaldehyde (Fig. 6 B–D). HpfG exhibited Michaelis–Menten kinetics for DHPS dehydration (kcat = 130 ± 4 s−1, Km = 5.0 ± 0.6 mM, and kcat/Km = 26 ± 3 mM−1 s−1) (SI Appendix, Fig. S10). (The kcat reported is normalized by radical content.)
Fig. 6.
EPR spectra and enzymatic assays. (A) X- band EPR spectrum of HpfG (40 μM) reconstituted with HpfH (80 μM), SAM (1 mM), and reductant Ti (III) citrate (100 μM), acquired at a temperature of 90 K and microwave power of 1 mW. (B) Specific activity of HpfG-catalyzed conversion of DHPS to 3-sulfopropionaldehyde, measured using a NADH-coupled spectrophotometric assay with sulfopropionaldehyde reductase (HpfD). Error bars represent the SD of three individual experiments. (C) Detection of HpfG-catalyzed 3-sulfopropionaldehyde formation by derivatization with DNPH and LC–MS analysis. (D) Negative ionization mass spectra of the DNPH-derivatized reaction product, corresponding to peak 1 c. (Theoretical mass of the monoanion of DNPH sulfopropionaldehyde = 317.0).
Homology Model and Molecular Docking of HpfG.
A homology model of HpfG was constructed using a C. butyricum glycerol dehydratase structure (PDB 1R9D) as a template (37% sequence identity between the two proteins), followed by docking of the DHPS substrate. The position and the pose of (S)-DHPS in the model is similar to that of glycerol in GDH (SI Appendix, Fig. S11). The positions of the conserved Cys464, Glu466, and the triad Asp478, His191, and His308 (SI Appendix, Fig. S11) are consistent with the conserved mechanism for these diol dehydratases. The substrate sulfonate group interacts with Arg363, Val677, Tyr481, and Asp478, which are conserved in the members of the HpfG cluster. However, given the low sequence identity between HpfG and the used template, further structural studies are required and are currently underway.
Discussion
DHPS is produced in large volumes globally, and the ability of its two hydroxyl groups to serve as reactive handles for enzymatic chemistry provides a wide range of possible pathways for DHPS degradation in metabolically diverse microbes. The two O2-sensitive GREs, HpsG and HpfG, described in this study, provide radical-dependent pathways for DHPS degradation in anaerobic bacteria and highlight the diversity of O2-sensitive chemistry occurring in anoxic environments.
The HpsG sequences that we have identified are present in gram-negative SSRB (SI Appendix, Table S2). Like in B. wadsworthia, the HpsG gene clusters appear to be involved in the uptake of (S)-DHPS, followed by desulfonation, producing sulfite for use as a TEA (Fig. 3C). The HpfG sequences are present in gram-negative Gammaproteobacteria, and gram-positive Clostridia and Lactobacilliales bacteria (SI Appendix, Table S3). Several of the HpfG sequences in Gammaproteobacteria are associated with homologs of E. coli sulfoglycolysis enzymes, including sulfoquinovose isomerase (YihS), sulfofructose kinase (YihV), sulfofructose-1-phosphate aldolase (YihT), and sulfolactaldehyde reductase (YihU) (Fig. 5B), suggesting the metabolism of (S)-DHPS produced internally through sulfoglycolysis (Fig. 5C). HpfG sequences in other bacteria are associated with a protein annotated as a sodium:sulfate symporter (PF00939), which we hypothesize is a (S)-DHPS importer, suggesting the uptake and metabolism of (S)-DHPS from the environment (Fig. 5C).
Many of the HpsGs and HpfGs are from gut bacteria, including strains from the human gut microbiome (SI Appendix, Tables S2 and S3). The production of H2S by human gut SSRB has been linked to diseases, and HpsG and HpfG may provide competing pathways for DHPS degradation in metabolically distinct bacteria. HpsG and HpfG are also present in environmental bacteria, including marine bacteria, and identification of their sequences allows further investigation of their roles in the recycling of abundant marine sulfonates (2, 8). HpsG is present in Desulfovibrio desulfuricans ND132, isolated from mesohaline Chesapeake Bay sediments and Desulfovibrio sp. HK-II, isolated from sediments of a partial salt lake (SI Appendix, Table S2). HpfG is present in many marine bacteria, including gram-negative Vibrio and Photobacterium species isolated from seawater, sediments, and marine animals and gram-positive Epulopiscum species found in fish gut (SI Appendix, Table S3), suggesting a role in marine anaerobic niches.
In conclusion, our study has expanded the repertoire of enzymes and transporters involved in the biodegradation of DHPS, a highly abundant organosulfonate on this planet. Existing sequence databases reveal the occurrence of these proteins in microbes inhabiting diverse anaerobic environments, and their identification facilitates further bioinformatics studies of the biological recycling of this compound.
Materials and Methods
Materials and General Methods.
Lysogeny Broth (LB) was prepared with yeast extract and tryptone purchased from Oxoid, UK. For LC–MS, high-purity methanol and acetonitrile from Concord Technology and formic acid from Merck were used. The ultrapure deionized water used in all experiments was prepared using a Millipore Direct-Q. Synthetic oligonucleotide primers were purchased from Tsingke, Inc. (Beijing, China). “ÄKTA pure” or “ÄKTA prime plus” fast protein liquid chromatography machines were used to perform all protein purification chromatographic steps. Proteins were quantified by measuring their absorption at 280 nm, using a BioPhotometer D30 (Eppendorf, Hamburg, Germany). A Lab2000 glovebox (Etelux, Beijing, China) was used to conduct all anaerobic experiments in an atmosphere of N2 and with the O2 maintained at less than 5 ppm.
Gene Synthesis, Molecular Cloning, and Plasmid Construction.
DNA fragments encoding E. coli codon-optimized sequences of B. wadsworthia HpsG (BwHpsG, E5Y7I4), HpsH (BwHpsH, E5Y7I3), HpsK (BwHpsK, E5Y7I1), K. oxytoca HpfG (KoHpfG, A0A318FL05), HpfH (KoHpfH, A0A318FEA4), and H. hathewayi HpfD (HhHpfD, A0A374P995) were synthesized and inserted into plasmids by General Biosystems, Inc. (Anhui, China). Plasmids used in this study are pET-28a(+) and modified pET28 vectors including HMT (containing, in tandem, a His6-tag, maltose binding protein [MBP], and a tobacco etch virus (TEV) protease cleavage site, followed by the construct of interest), and HT (containing a His6-tag and a TEV protease cleavage site, followed by the construct of interest). The BwHpsG, KoHpsG, and HhHpfD fragments were inserted into HT at the SspI site. For the production of soluble BwHpsH, BwHpsK, and KoHpfH, the respective fragments were inserted into HMT at the SspI site. The N-terminal 22-a.a. (amino acid) signal peptide of BwHpsK was omitted. For the production of high-purity BwHpsG with a N-terminal 20-a.a. truncation for crystallography, the BwHpsG fragment was amplified by PCR using primers 1F and 1R (SI Appendix, Table S4) and inserted into the HMT at the SspI site to form HMT-BwHpsG (-20 a.a.). A surface-entropy reduction mutation, changing a.a. 129–132 from TDMQ to AAAA, was introduced by QuikChange site-directed mutagenesis using primers 2F and 2R to form HMT-BwHpsG (-20 a.a. 129TDMQ132-AAAA).
Production of BwHpsGH and KoHpfGH for Enzymatic Assays.
BwHpsGH and KoHpfGH were heterologously expressed in E. coli BL21 (DE3) cells harboring the plasmids HT-BwHpsG, HMT-BwHpsH and HT-KoHpfG, HMT-KoHpfH, respectively. For BwHpsH, the cells were cotransformed with the plasmid pTf16 (TaKaRa) for coexpression of the tig chaperone. Expression was carried out in LB supplemented with the appropriate antibiotics (50 μg/mL kanamycin for BwHpsG, KoHpfG, and MBP-KoHpfH, 50 μg/mL kanamycin and 25 μg/mL chloramphenicol for MBP-BwHpsH). Starter cultures (5 mL) were inoculated from single colonies and grown overnight at 37 °C followed by dilution into 1 L of medium. To induce chaperone expression for the MPB-BwHpsH culture, 2.5 mg/mL l-arabinose was added. The cultures were grown in a shaking incubator at 37 °C and 220 rpm, up to an optical density at 600 nm (OD600) of 0.6–0.8. The temperature was then decreased to 18 °C, followed by addition of isopropyl β-d-1-thiogalactopyranoside (IPTG) to a final concentration of 0.4 mM, and continued shaking for 16 h. The cultures were centrifuged (8,000 × g for 10 min at 4 °C), and the cell pellets (∼1 g wet weight) were suspended in 5 mL of lysis buffer (50 mM Tris/HCl, pH 8.0, 200 mM KCl, 1 mM phenylmethanesulfonyl fluoride (PMSF), 0.2 mg/mL lysozyme, 0.03% Triton X-100, and 0.02 mg/mL of DNase I), and frozen in a −80 °C freezer.
For protein purification, the cells were thawed and incubated at room temperature (RT, 25 °C) for 50 min, during which cell lysis occurs. Some 5 mM β-mercaptoethanol (BME) was added, and nucleic acid was removed by precipitation with 1% streptomycin sulfate. For BwHpsG and KoHpfG, the protein solution was loaded on to a 5 mL TALON Co2+ column (Clontech Laboratories, Inc.), preequilibrated with buffer A [20 mM Tris/HCl, pH 7.5, 5 mM BME, and 0.2 M KCl]. The column was washed with 10 column volumes (CV) of buffer A, and protein was eluted with 5 CV of buffer A containing 150 mM imidazole. The eluted protein was precipitated with solid (NH4)2SO4 to 70% saturation and isolated by centrifugation (20,000 × g for 10 min at 4 °C). The pellet was dissolved in 5 mL of buffer B [20 mM Tris/HCl, pH 7.5, 100 mM KCl, and 5 mM BME] and desalted using a G25 column (GE, thermostat jacket tube XK16/20, packed 15 cm × 2 cm, 30 mL), preequilibrated with buffer B.
For BwHpsH and KoHpfH, the protein solution was loaded on to a column packed with 20 mL amylose resin (New England Biolabs), and protein was eluted with buffer A containing 10 mM maltose. The purified BwHpsG (ε280 = 122,000 M−1 cm−1), MBP-BwHpsH (ε280 = 88,200 M−1 cm−1), KoHpfG (ε280 = 118,000 M−1 cm−1), and MBP-KoHpfH (ε280 = 101,000 M−1 cm−1) were examined by SDS/PAGE on a commercial gel (SurePAGE, Bis-Tris, 8–16%).
Production of N-Terminal 20 a.a. Truncated BwHpsG for Protein Crystallization.
Expression of a MBP-BwHpsG mutant for crystallography was carried out in E. coli BL21 (DE3) cells harboring the plasmid pET28-HMT-BwHpsG (-20 a.a. 129TDMQ132-AAAA). The transformant was grown in LB medium containing kanamycin (50 μg/mL) at 37 °C in flasks in a shaker incubator at 220 rpm and induced for the expression of MBP-BwHpsG with 0.3 mM IPTG for 16 h at 18 °C. Typically, cells from 6 L culture were harvested by centrifugation (8,000 × g for 10 min) and were suspended in 5 mL (∼1 g wet weight) of buffer C [50 mM Tris/HCl, pH 8.0, 200 mM KCl, 1 mM PMSF, 0.03% Triton X-100]. Then, the cells were lysed with sonication (Scientz Biotechnology, Ningbo, China). The lysate was then centrifuged (20,000 × g 30 min at 4 °C) to remove unbroken cells and cell debris. The protein solution was then applied to a 10 mL TALON column. The protein was eluted with buffer A containing 150 mM imidazole. The eluate was then diluted twofold with buffer A and loaded on a column packed with 40 mL amylose resins. The column was then washed with 5 CV buffer A and eluted with 5 CV buffer A containing 10 mM maltose. The eluate premixed with recombinant His6-tagged TEV protease (15:1) was dialyzed overnight against 2 L buffer A. The dialyzed sample was loaded on a 10 mL TALON column to retain TEV and MBP proteins. The flow through was collected and dialyzed against 2 L buffer D [20 mM Tris⋅HCl, pH 7.5, 5 mM BME] for 3 h before it was applied to a 5 mL Q Sepharose high performance column. The column was eluted with 10 CV linear salt gradient from 100 to 500 mM KCl in buffer D. A prominent peak containing BwHpsG (-20 a.a.) was collected and concentrated to a final volume of 5 mL (4 mg/mL) using a centrifuge concentrator (30K MWCO; Millipore). This protein solution was then injected into a Superdex200 gel filtration column (300 mL) and eluted with buffer E [20 mM Tris⋅HCl, pH 7.5, 0.2 M KCl, 1 mM dithiothreitol (DTT)]. The eluate from gel filtration column was reconcentrated and buffer exchanged with the storage buffer [10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid/KOH, pH 7.4, 50 mM KCl, 1 mM Tris-(2-carboxyethyl)-phosphine hydrochloride]. The final concentration is 10 mg/mL The purified protein was examined by SDS/PAGE gel.
[Fe-S] Cluster Reconstitution for MBP-HpsH and MBP-HpfH.
Reconstitution of the [Fe–S] clusters of MBP-HpsH and MBP-HpfH, quantitation of Fe and S contents, and measurement of their absorption spectra, were carried out according to previously described procedures (14). The purity of MBP-BwHpsH and MBP-KoHpfH was estimated to be 69% and 73% based on densitometry analysis of the Coomassie-stained SDS/PAGE gel using the software ImageJ and used to estimate the Fe and S contents per monomer.
Sequence alignments suggested that HpsH and HpfH, like many other previously characterized GRE activating enzymes, contain one [4Fe–4S] cluster in a radical SAM domain, and two [4Fe–4S] clusters in a ferredoxin domain (SI Appendix, Fig. S12) (28), giving a theoretical maximum of 12 Fe and 12 S per monomer. Anaerobic reconstitution of the [4Fe–4S] clusters resulted in 7.9 ± 0.3 Fe and 8.4 ± 0.1 S per HpsH monomer and 5.4 ± 0.2 Fe and 5.3 ± 0.4 S per HpfH monomer, suggesting that our protocol gives rise to incomplete reconstitution.
The ultraviolet–visible (UV–Vis) absorption spectra exhibited features characteristic of [4Fe–4S]2+ clusters, which disappeared upon reduction (SI Appendix, Fig. S4). Correcting for the 69% purity of MBP-BwHpsH and 73% purity of MBP-KoHpfH, the extinction coefficients of the reconstituted MBP-BwHpsH and MBP-KoHpfH [4Fe–4S] clusters were estimated to be 27 and 23 mM−1 cm−1. Assuming an approximate ε410nm of 15 mM−1 cm−1 per cluster (29), we estimate that for MBP-BwHpsH and MBP-KoHpfH, ∼1.8 [4Fe–4S] clusters and 1.5 [4Fe–4S] clusters per monomer, respectively, were reconstituted consistent with the measured Fe and S contents.
Electron Paramagnetic Resonance Detection of Glycyl Radical Formation.
Continuous wave X-band electron paramagnetic resonance (EPR) spectroscopy was used to characterize the glycyl radical. A 200 μL reaction mixture containing 20 mM Tris, pH 7.5, 0.1 M KCl, 40 μM BwHpsG/KoHpfG, 80 μM reconstituted MBP-BwHpsH/MBP-KoHpfH, 1 mM SAM, 100 μM Ti(III) citrate was incubated at RT for 60 min/15 min in the glovebox. Then, 10% glycerol for HpsG, or 10% d-sorbitol for HpfG was added into the reactions. Samples were loaded into EPR tubes with 4-mm outer diameter and 8-in. length (Wilmad Lab-Glass, 734-LPV-7), sealed with a rubber stopper, removed from the glovebox, and frozen in liquid nitrogen prior to EPR analysis. The experimental spectra for the glycyl radical were modeled with Bruker Xepr spin fit to obtain g values, hyperfine coupling constants, and linewidths. Double integration of the simulated spectra was used to measure spin concentration (14). For both HpsG and HpfG enzymes, EPR quantitation was performed with triplicate biological samples. The perpendicular mode X-band EPR spectra were recorded using a Bruker E500 EPR spectrometer. Data acquisition was performed with Xepr software (Bruker). The EPR spectra represent an average of 30 scans and were recorded under the following conditions: temperature, 90 K; center field, 3,370 G; range, 200 G; microwave power, 1 mW; microwave frequency, 9.43 GHz; modulation amplitude, 2 mT; modulation frequency, 100 kHz; time constant, 20.48 ms; conversion time, 40 ms; scan time, 81.92 s; and receiver gain, 23 dB.
LC–MS/MS Assay for Hydroxyacetone and 3-Sulfopropionaldehyde Detection.
BwHpsG and KoHpfG activation was carried out as described for the EPR experiments except that glycerol/sorbitol was omitted. For BwHpsG, a 100 μL reaction mixture containing 10 μM activated BwHpsG, 0.1 mM Ti(III) citrate and 50 mM DHPS (AA blocks) was incubated at 30 °C for 30 min in the glovebox. Two negative controls omitting SAM or DHPS were also performed. Isethionate (Adamas) added instead of DHPS was also performed. For KoHpfG, a 100 μL reaction mixture containing 10 μM activated KoHpfG, 0.1 mM Ti(III) citrate, and 20 mM DHPS was incubated at 30 °C for 30 min in the glovebox. Three negative controls omitting KoHpfG, SAM, or DHPS were also performed. Derivatization with DNPH and LC–MS analysis were carried out according to previously described procedures (14). A commercial hydroxyacetone standard (LabNetwork, Wuxi, China) was also prepared.
Fuchsin Assay for Sulfite Detection.
Sulfite was detected using a colorimetric Fuchsin assay according to previously described procedures (14). BwHpsG activation was carried out as described for the EPR experiments except that glycerol was omitted. A 100 µL reaction mixture containing 10 μM activated BwHpsG, 20 mM DHPS or isethionate was incubated at 30 °C for 1 h in the glovebox. A negative control omitting substrate was also performed. While the reaction incubated, stock solution A (0.8 M H2SO4, 0.08% Fuchsin, and 1.6% formaldehyde, mixed 7:2:1) was freshly prepared. A 50 µL portion per reaction sample was mixed with 950 µL of solution A, incubated for 10 min, and the UV–Vis absorption spectra were collected.
Spectrophotometric Activity Assay for H. hathewayi HpfD.
A 200 μL reaction mixture containing 20 mM Tris⋅HCl buffer, pH 9.5, 100 mM KCl, 0.6 ng HhHpfD, 2 mM NAD+, and 100 mM 3-HPS (Aladdin, Shanghai, China) was incubated at RT in a 96-well plate, and the absorbance at 340 nm was monitored using a plate reader (Tecan M200, Männedorf, Switzerland).
Enzymatic Assay for Hydroxyacetone Formation by HpsG.
A 200 µL reaction mixture containing 20 mM Tris⋅HCl, pH 7.5, 0.1 M KCl, 1 µM activated BwHpsG, 10 µM E. coli GldA, 0.4 mM NADH, and 50 mM DHPS was incubated at RT for 20 min in the glovebox. The mixture was removed from the glovebox, and its absorbance at 340 nm was measured using a plate reader (Tecan M200). Control assays omitting either SAM or BwHpsG were also performed. To investigate the substrate specificity of BwHpsG, assays substituting DHPS and GldA with 50 mM isethionate and excess S. cerevisiae ADH1 were also performed as previously described for IseG (14).
Coupled Spectrophotometric Activity Assays for HpsG.
A 100 µL reaction mixture containing 20 mM Tris⋅HCl, pH 7.5, 0.1 M KCl, and typically, 1 µM activated BwHpsG, 10 µM GldA, 0.2 mM NADH, and 100 mM DHPS was incubated at RT in a 1 cm Eppendorf cuvette in the glovebox. The absorbance at 340 nm was monitored at 4 s intervals using the cuvette mode of a Thermo Scientific Nanodrop OneC in the glovebox. To obtain the Michaelis–Menten kinetic parameters, DHPS concentration was varied while keeping a fixed enzyme concentration of 1 μM BwHpsG. Assays substituting DHPS and GldA with isethionate and ADH1 were also prepared, and the absorbance 340 nm was monitored at 15 s intervals. These assays contained, typically, a saturating substrate concentration of 500 mM isethionateand 5 μM BwHpsG. The kinetic data presented were obtained from a HpsG sample with 0.06 G• per protein dimer. GraphPad Prism6 was used for data analysis.
Enzymatic Assay for 3-Sulfopropionaldehyde Formation by HpfG.
A 100 µL reaction mixture containing 20 mM Tris⋅HCl, pH 7.5, 0.1 M KCl, 0.5 μM activated KoHpfG, 10 µM sulfopropionaldehyde reductase (HhHpfD), 0.2 mM NADH, and 100 mM DHPS was incubated at RT for 2 min in the glovebox. Additionally, then its absorbance at 340 nm was measured using the cuvette mode of a Nanophotometer NP80 Mobile in the glovebox. Control assays omitting either DHPS or KoHpfG were also performed.
Coupled Spectrophotometric Activity Assays for HpfG.
A 100 µL reaction mixture containing 20 mM Tris⋅HCl, pH 7.5, 0.1 M KCl, and, typically, 0.5 μM activated KoHpfG, 10 µM HpfD, 0.2 mM NADH, and 100 mM DHPS was incubated at RT in a 1 cm Eppendorf cuvette in the glovebox. The absorbance at 340 nm was monitored at 5 s intervals using the cuvette mode of a Nanophotometer NP80 Mobile in the glovebox. To obtain the Michaelis–Menten kinetic parameters, DHPS concentration was varied while keeping a fixed enzyme concentration of 500 nM KoHpfG. The kinetic data presented were obtained from a HpfG sample with 0.04 G• per protein dimer. GraphPad Prism6 was used for data analysis.
X-ray Crystal Structure of BwHpsG.
Initial screening of BwHpsG crystals was performed using an automated liquid handling robotic system (Gryphon, Art Robbins) in 96-well format by the sitting-drop vapor diffusion method. The screens were set up at 295 K using various sparse matrix crystal screening kits from Hampton Research and Molecular Dimensions. Several crystallization conditions gave thin plate-shape crystals. After further optimization using the hanging-drop vapor-diffusion method in 24-well plates, we obtained crystals large enough for single crystal X-ray diffraction studies. The best condition yielding large platelike crystals was 0.2 M sodium acetate and 0.1 M Bis–Tris propane, pH 6.5, 16% (wt/vol) PEG3350 plus 500 mM DHPS.
Crystals were flash cooled in liquid nitrogen using reservoir solution containing 25% glycerol as a cryoprotectant. Diffraction data were collected on a local Rigaku X-ray diffractor (XtaLAB P200 MM007HF, Tokyo, Japan) to a resolution of 2.20 Å. The data set was indexed, integrated, and scaled using HKL3000 suite (30). Molecular replacement was performed by PHENIX (31) using the crystal structure of IseG (PDB 5YMR). The structure was manually built according to the modified experimental electron density using Coot (32) and further refined by PHENIX (31) in iterative cycles before it was deposited in the RCSB Protein Data Bank (accession no. 6LON). The SI Appendix, Table S1 contains the statistics for data collection and final refinement. All structural figures were generated with UCSF Chimera (33).
Homology Modeling and Docking.
A homology model of HpfG was created by Prime module of Schrödinger software (Schrödinger LLC, New York, NY) using C. butyricum glycerol dehydratase structure (PDB 1R9D) as a template. The sequence identity between the HpfG and the template protein is ∼37%. The ligand (S)-DHPS was sketched in Chem-DrawUltra 13.0 and, subsequently, prepared using the LigPrep module of Schrödinger software. (S)-DHPS was docked by Glide module of Schrödinger using an extra precision mode to a reference position in the binding site of HpfG similar as glycerol in the template structure (PDB 1R9D).
Growth of B. wadsworthia with Different TEAs.
B. wadsworthia strain RZATAU (DSM 11045) was purchased from DSMZ. Cells were first inoculated into ABB medium supplemented with 5 mM taurine and cultivated anaerobically at 30 °C for 3–7 d. Then, 100 μL portions of the starter culture were transferred into three anaerobic bottles containing 5 mL modified DSM 503 medium, omitting taurine, and supplemented with 60 mM Na-formate and 200 µg/ L 1,4-naphthochinone. Different TEAs (20 mM) were added: 1) Na2S2O3 (with 20 mM sodium pyruvate was added as a carbon and electron source), 2) taurine, and 3) DHPS (with 20 mM sodium pyruvate was added as a carbon and electron source). After 3–7 d incubation at 30 °C, all three cultures became turbid and contained a black precipitate. H2S in the headspace gas was detected using a methylene blue assay as previously described (14). The samples were diluted prior to absorbance measurement to ensure that they fall within the linear region of the spectrometer.
Protein Identification by SDS/PAGE and Mass Spectrometry.
Cells were harvested by centrifugation, lysed by boiling in Laemmli loading buffer, and analyzed on a 10% SDS/PAGE gel. Prominent protein bands induced by growth on sulfonate substrates were manually excised. After in-gel digestion and extraction, the peptide mixtures were loaded onto Orbitrap Fusion LUMOS MS. The MS/MS spectra from each LC–MS/MS run were searched against the B. wadsworthia protein database GCF_000185705.2 (Bilo_wads_3_1_6_V2) from UniProt (release date of March 19, 2014; 68,406 entries) using an in-house Proteome Discoverer (Version PD1.4, Thermo-Fisher Scientific). Protein identifications were performed based on Sequest HT (34). Source data underlying Fig. 4C are provided as in Dataset S1.
ITC Assays for BwHpsK.
ITC experiments were carried out on a 50 μM solution of BwHpsK in buffer containing 20 mM Tris⋅HCl, pH 7.5 and 200 mM KCl with 1 mM DTT using a PEAQ-ITC instrument (Malvern). The samples were titrated with 19 injections of 2 μL of 1 mM DHPS, isethionate, or 3-HPS at 25 °C and a stirring speed of 750 rpm. Deionized water was used for the reference cell. Background titrations were obtained using protein-free buffer, and subtracted from the raw titrations. The data were fitted to a single-site binding model using the MicroCal PEAQ-ITC software to estimate the stoichiometry, binding affinity, and changes in enthalpy (ΔH) and entropy (ΔS). A Kd of 6.7 μM was measured for DHPS with a N value of 2.2. No interaction was observed for isethionate and 3-HPS.
Data Availability.
Desulfovibrio vulgaris IseG deposited in the Protein Data Bank, www.wwpdb.org (accession no. 5YMR), two homologs of DvIseG in UniProt, https://www.uniprot.org/ (accession nos. E5Y378 and E5Y7I4), and coordinates and structure factors of HpsG in complex with (S)-DHPS have been deposited in the RCSB Protein Data Bank, https://www.rcsb.org (accession no. 6LON). All other data required to evaluate the paper’s conclusions are present in the paper and/or the SI Appendix.
Supplementary Material
Acknowledgments
We thank the instrument analytical center of School of Pharmaceutical Science and Technology at Tianjin University for providing the LC–MS analysis, Zhi Li, and Drs. Xinghua Jin, Yan Gao, and Xiangyang Zhang for helpful discussion. This work was supported by the National Key R&D Program of China (Grant 2019YFA0905700), the National Natural Science Foundation of China (Grant 31870049) (Y. Zhang), National Key R&D Program of China (Grant 2017YFD0201400, Grant 2017YFD0201403), National Natural Science Foundation of China (Grant NSFC31972287), the State Key Laboratory of Ecological Pest Control for Fujian and Taiwan Crops (Grant SKL2019001, Grant SKL2019003) (Z.Y.), and the Agency for Science, Research and Technology of Singapore Visiting Investigator Program Grant 1535j00137 (H.Z.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Desulfovibrio vulgaris IseG has been deposited in the Protein Data Bank, www.wwpdb.org (accession no. 5YMR); two homologs of DvIseG have been deposited in UniProt, https://www.uniprot.org/ (accession nos. E5Y378 and E5Y7I4); and the crystallography, atomic coordinates, and structure factors have been deposited in the RCSB Protein Data Bank, https://www.rcsb.org (accession no. 6LON).
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2003434117/-/DCSupplemental.
References
- 1.Goddard-Borger E. D., Williams S. J., Sulfoquinovose in the biosphere: Occurrence, metabolism and functions. Biochem. J. 474, 827–849 (2017). [DOI] [PubMed] [Google Scholar]
- 2.Moran M. A., Durham B. P., Sulfur metabolites in the pelagic ocean. Nat. Rev. Microbiol. 17, 665–678 (2019). [DOI] [PubMed] [Google Scholar]
- 3.Benson A. A., Daniel H., Wiser R., A sulfolipid in plants. Proc. Natl. Acad. Sci. U.S.A. 45, 1582–1587 (1959). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denger K.et al., Sulphoglycolysis in Escherichia coli K-12 closes a gap in the biogeochemical sulphur cycle. Nature 507, 114–117 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Burrichter A.et al., Anaerobic degradation of the plant sugar sulfoquinovose concomitant with H2S production: Escherichia coli K-12 and Desulfovibrio sp. strain DF1 as co-culture model. Front. Microbiol. 9, 2792 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denger K., Huhn T., Hollemeyer K., Schleheck D., Cook A. M., Sulfoquinovose degraded by pure cultures of bacteria with release of C3-organosulfonates: Complete degradation in two-member communities. FEMS Microbiol. Lett. 328, 39–45 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Felux A.-K., Spiteller D., Klebensberger J., Schleheck D., Entner-Doudoroff pathway for sulfoquinovose degradation in Pseudomonas putida SQ1. Proc. Natl. Acad. Sci. U.S.A. 112, E4298–E4305 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Durham B. P.et al., Sulfonate-based networks between eukaryotic phytoplankton and heterotrophic bacteria in the surface ocean. Nat. Microbiol. 4, 1706–1715 (2019). [DOI] [PubMed] [Google Scholar]
- 9.Malviya S.et al., Insights into global diatom distribution and diversity in the world’s ocean. Proc. Natl. Acad. Sci. U.S.A. 113, E1516–E1525 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celik E.et al., Metabolism of 2,3-dihydroxypropane-1-sulfonate by marine bacteria. Org. Biomol. Chem. 15, 2919–2922 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Mayer J.et al., 2,3-Dihydroxypropane-1-sulfonate degraded by Cupriavidus pinatubonensis JMP134: Purification of dihydroxypropanesulfonate 3-dehydrogenase. Microbiology 156, 1556–1564 (2010). [DOI] [PubMed] [Google Scholar]
- 12.Denger K.et al., Bifurcated degradative pathway of 3-sulfolactate in Roseovarius nubinhibens ISM via sulfoacetaldehyde acetyltransferase and (S)-cysteate sulfolyase. J. Bacteriol. 191, 5648–5656 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbonero F., Benefiel A. C., Alizadeh-Ghamsari A. H., Gaskins H. R., Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 3, 448 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xing M.et al., Radical-mediated C-S bond cleavage in C2 sulfonate degradation by anaerobic bacteria. Nat. Commun. 10, 1609 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peck S. C.et al., A glycyl radical enzyme enables hydrogen sulfide production by the human intestinal bacterium Bilophila wadsworthia. Proc. Natl. Acad. Sci. U.S.A. 116, 3171–3176 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Backman L. R. F., Funk M. A., Dawson C. D., Drennan C. L., New tricks for the glycyl radical enzyme family. Crit. Rev. Biochem. Mol. Biol. 52, 674–695 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beller H. R.et al., Discovery of enzymes for toluene synthesis from anoxic microbial communities. Nat. Chem. Biol. 14, 451–457 (2018). [DOI] [PubMed] [Google Scholar]
- 18.Liu D.et al., Indoleacetate decarboxylase is a glycyl radical enzyme catalysing the formation of malodorant skatole. Nat. Commun. 9, 4224 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shisler K. A., Broderick J. B., Glycyl radical activating enzymes: Structure, mechanism, and substrate interactions. Arch. Biochem. Biophys. 546, 64–71 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vey J. L.et al., Structural basis for glycyl radical formation by pyruvate formate-lyase activating enzyme. Proc. Natl. Acad. Sci. U.S.A. 105, 16137–16141 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Denger K., Ruff J., Rein U., Cook A. M., Sulphoacetaldehyde sulpho-lyase (EC 4.4.1.12) from Desulfonispora thiosulfatigenes: Purification, properties and primary sequence. Biochem. J. 357, 581–586 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Suzek B. E., Wang Y., Huang H., McGarvey P. B., Wu C. H.; UniProt Consortium , UniRef clusters: A comprehensive and scalable alternative for improving sequence similarity searches. Bioinformatics 31, 926–932 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao S.et al., Prediction and characterization of enzymatic activities guided by sequence similarity and genome neighborhood networks. eLife 3, e03275 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mulligan C., Fischer M., Thomas G. H., Tripartite ATP-independent periplasmic (TRAP) transporters in bacteria and archaea. FEMS Microbiol. Rev. 35, 68–86 (2011). [DOI] [PubMed] [Google Scholar]
- 25.Vetting M. W.et al., Experimental strategies for functional annotation and metabolism discovery: Targeted screening of solute binding proteins and unbiased panning of metabolomes. Biochemistry 54, 909–931 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levin B. J., Balskus E. P., Characterization of 1,2-propanediol dehydratases reveals distinct mechanisms for B12-dependent and glycyl radical enzymes. Biochemistry 57, 3222–3226 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Kovačević B.et al., Computational tale of two enzymes: Glycerol dehydration with or without B12. J. Am. Chem. Soc. 140, 8487–8496 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Selvaraj B., Buckel W., Golding B. T., Ullmann G. M., Martins B. M., Structure and function of 4-hydroxyphenylacetate decarboxylase and its cognate activating enzyme. J. Mol. Microbiol. Biotechnol. 26, 76–91 (2016). [DOI] [PubMed] [Google Scholar]
- 29.Shen G.et al., SufR coordinates two [4Fe-4S]2+, 1+ clusters and functions as a transcriptional repressor of the sufBCDS operon and an autoregulator of sufR in cyanobacteria. J. Biol. Chem. 282, 31909–31919 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Minor W., Cymborowski M., Otwinowski Z., Chruszcz M., HKL-3000: The integration of data reduction and structure solution–from diffraction images to an initial model in minutes. Acta Crystallogr. D Biol. Crystallogr. 62, 859–866 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Adams P. D.et al., PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 66, 213–221 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Emsley P., Cowtan K., Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 60, 2126–2132 (2004). [DOI] [PubMed] [Google Scholar]
- 33.Pettersen E. F.et al., UCSF Chimera–A visualization system for exploratory research and analysis. J. Comput. Chem. 25, 1605–1612 (2004). [DOI] [PubMed] [Google Scholar]
- 34.Eng J. K., McCormack A. L., Yates J. R., An approach to correlate tandem mass spectral data of peptides with amino acid sequences in a protein database. J. Am. Soc. Mass Spectrom. 5, 976–989 (1994). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Desulfovibrio vulgaris IseG deposited in the Protein Data Bank, www.wwpdb.org (accession no. 5YMR), two homologs of DvIseG in UniProt, https://www.uniprot.org/ (accession nos. E5Y378 and E5Y7I4), and coordinates and structure factors of HpsG in complex with (S)-DHPS have been deposited in the RCSB Protein Data Bank, https://www.rcsb.org (accession no. 6LON). All other data required to evaluate the paper’s conclusions are present in the paper and/or the SI Appendix.