Significance
N-terminal protein acetylation is poorly understood in bacteria. Herein, we report the identification of an Nα acetyltransferase (NAT) that modulates the activity of a sirtuin deacylase in a human pathogen. This is significant because the alluded enzyme (named N-acyltransferase A [NatA], formerly YiaC) is a prokaryotic non-Rim–type NAT, and N-terminal acetylation of a bacterial sirtuin has not been reported. Also significant is the fact that NatA affects the metabolism of acetate, a short-chain fatty acid known to play an important role in pathogenesis in the human gut.
Keywords: sirtuin, CobB sirtuin deacylase, posttranslational modification, N-terminal acetylation, bacterial GNAT
Abstract
In eukaryotic cells, the N-terminal amino moiety of many proteins is modified by N-acetyltransferases (NATs). This protein modification can alter the folding of the target protein; can affect binding interactions of the target protein with substrates, allosteric effectors, or other proteins; or can trigger protein degradation. In prokaryotes, only ribosomal proteins are known to be N-terminally acetylated, and the acetyltransferases responsible for this modification belong to the Rim family of proteins. Here, we report that, in Salmonella enterica, the sirtuin deacylase CobB long isoform (CobBL) is N-terminally acetylated by the YiaC protein of this bacterium. Results of in vitro acetylation assays showed that CobBL was acetylated by YiaC; liquid chromatography-tandem mass spectrometry (LC-MS/MS) was used to confirm these results. Results of in vitro and in vivo experiments showed that CobBL deacetylase activity was negatively affected when YiaC acetylated its N terminus. We report 1) modulation of a bacterial sirtuin deacylase activity by acetylation, 2) that the Gcn5-related YiaC protein is the acetyltransferase that modifies CobBL, and 3) that YiaC is an NAT. Based on our data, we propose the name of NatA (N-acyltransferase A) in lieu of YiaC to reflect the function of the enzyme.
Protein acylation is common in prokaryotes and eukaryotes, and it is an effective and rapid means of controlling protein function in response to diverse stimuli (1, 2). What stands out about protein acylation is the diversity of organic acids used by cells to modify proteins (e.g., acetate, propionate, malonate, succinate, etc.) and the large number of acyltransferases that catalyze the modifications (1). Many of the acyltransferases that modify proteins and small molecules belong to the protein superfamily PF00583, and among this family, many proteins contain the so-called GNAT (GCN5-related N-acetyltransferase) domain (IPR000182). GNATs acylate free amino groups of proteins or small molecules (1, 2). For example, there is a subset of well-studied GNATs that modify the ε amino group (Nε) in lysine side chains (3, 4), while other GNATs modify the α amino group (Nα) of the starting residue of proteins (5–8).
To frame the work reported here, we note that many eukaryotic proteins are acetylated on their N termini and that the acetyltransferases responsible for these modifications are referred to as N-acetyltransferases (NATs). In general, NATs catalyze the transfer of the acetyl group from acetyl-Coenzyme A (AcCoA) to a primary amine of a small molecule or the N-terminal amino group of a peptide or protein. In eukaryotes, Nα acetylation has been suggested to alter protein folding, protein–protein interactions, and protein degradation (9–12). In contrast, little is known about the enzymes that catalyze N-terminal protein acetylation in prokaryotes and what the physiological reasons for such modification may be. Examples of N-terminal acetylation of bacterial proteins, where the acetyltransferase is known, are the acetylation of ribosomal proteins S18, S5, and L7/L12 by acetyltransferases RimI, RimJ, and RimL (13–15). These acetyltransferases were thought to have high substrate specificity until recently, when the Mycobacterium tuberculosis RimI was shown to acetylate different peptides in vitro (16). Several other proteins are known to be Nα acetylated in bacteria, such as secretion chaperone SecB of Escherichia coli and the virulence factor ESAT-6 (ExsA) of several Mycobacterium species. In the case of the ESAT-6 (ExsA) protein, acetylation of its N terminus abolished binding interactions with its protein partner CFP10 (ExsB) and attenuated Mycobacterium marinum virulence (17–19). Even though there has been identification of N-terminal acetylation in prokaryotes, the acetyltransferases responsible for the modifications have not been identified. For example, recently published bacterial N-terminal acetylomes of Pseudomonas aruginosa, Acinetobacter baumannii, and M. tuberculosis showed that roughly ∼10% of the proteins were N-terminally acetylated (8, 11, 20), but the enzymes responsible for such modifications were not identified.
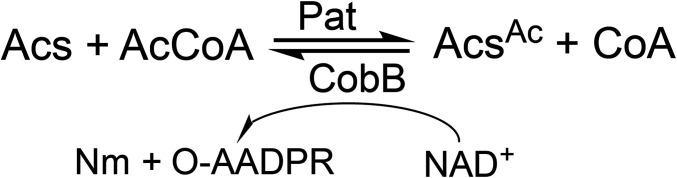
The Salmonella enterica genome contains ∼26 GNATs, but the function of about a third of them has yet to be defined. In addition, the S. enterica genome possesses one nicotinamide adenine dinucleotide (NAD+)-dependent sirtuin deacylase CobB, whose function works in concert with the protein acetyltransferase (Pat) enzyme to reversibly modulate the activity of acetyl-CoA synthetase (Acs) (21, 22). As shown in (Scheme 1, the CobB-dependent deacetylation reaction consumes NAD+ and yields O-acetyl-ADP (adenosine diphosphate ribose) and nicotinamide.
Scheme 1.
Interestingly, this bacterium synthesizes two biologically active isoforms of CobB, referred to as CobBS (CobB short isoform) and CobBL (CobB long isoform), which differ in size by a 37-residue N-terminal extension of the catalytic core (Fig. 1).
Fig. 1.
Biologically active CobB sirtuin deacylase isoforms of S. enterica. N-terminal amino acid sequence of CobBL. Yellow highlighted residues represent hydrophobic amino acids; red residues are arginines, and blue residues are lysines.
Our group showed that both isoforms of CobB deacetylated their bona fide protein substrate (i.e., acetylated acetyl-CoA synthetase [AcsAc]) in vivo and in vitro (23). However, the physiological relevance of the two CobB isoforms in S. enterica is unknown. Here, we report that the CobBL sirtuin deacylase isoform of this bacterium is N-terminally acetylated and that the putative YiaC acetyltransferase acetylates the N terminus of CobBL. We also report in vivo and in vitro evidence that YiaC-dependent N-terminal acetylation of CobBL negatively affects its deacetylase activity.
Results
YiaC Acetylates CobBL but Not CobBS.
A search for protein substrates for the S. enterica putative GNATs led us to discover that the S. enterica YiaC protein acetylated S. enterica CobBL but not CobBS. As shown in Fig. 2, when both isoforms of S. enterica CobB were incubated with [1-14C]-AcCoA as a function of YiaC, radiolabel was transferred to CobBL but not to CobBS (Fig. 2, lanes 4 and 5, respectively). Since CobBS was not acetylated, we surmised that the sites of acetylation were located within the 37-amino acid N-terminal, arginine-rich motif of CobBL (Fig. 1).
Fig. 2.
YiaC acetylates CobBL but not CobBS. YiaC-dependent acetylation of CobB isoforms was assessed after incubation of the proteins with [1-14C]-AcCoA (20 μM) for 1 h at 37 °C. Detailed conditions of the assay are described in Materials and Methods. Controls included incubation of the CobB isoforms with [1-14C]-AcCoA in the absence of YiaC. Proteins were resolved by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis) and visualized by Coomassie Brilliant Blue R stain (Upper) using Precision Plus protein (Bio-Rad) standards as molecular mass markers (lane 1, MM, kilodaltons). Radiolabel signal was visualized by phosphor imaging (Lower). *Radiolabeled acetyl moieties.
YiaC Does Not Acetylate Nε Amino Groups of Lysine Residues.
The N-terminal extension of CobBL contains two lysines (K14, K16), which we investigated as possible acetylation sites. To test this possibility, we changed K14 and K16 to alanine, independently and in combination. The three variants (CobBLK14A, CobBLK16A, and CobBLK14A,K16A) were overproduced, isolated, and incubated with YiaC in the presence of [1-14C]-AcCoA. Surprisingly, YiaC acetylated all three CobBL variants (Fig. 3, lanes 5, 7, and 9), suggesting that, under the assay conditions used, YiaC did not modify the Nε position of either K14 or K16. We note that the intensity of the signal for acetylated CobBLK14A,K16A variant was less than the single-amino acid variants and that the signal intensity was commensurate to the amount of protein loaded on the gel. The yield of CobBLK14A,K16A variant was lower than those of the single-amino acid variants.
Fig. 3.
YiaC does not acetylate lysyl residues of CobBL. To query the site of modification, lysine variants of CobBL were purified and incubated with YiaC and [1-14C]-AcCoA. The goal was to determine whether or not YiaC was an Nα acetyltransferase. This experiment was conducted as described for Fig. 2 where CobBL variants (3 μM, except CobBLK14A,K16A was at 2 μM) were incubated with [1-14C]-AcCoA and either with or without YiaC (1 μM). Lanes 4 and 5 contained CobBLK14A, lanes 6 and 7 contained CobBLK16A, and lanes 8 and 9 contained CobBLK14A,K16A. Results of control experiments using CobBL plus AcCoA with or without YiaC are shown in lanes 2 and 3, respectively. Lane 1 represents molecular mass marker (MM) in kilodaltons. SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis. *Radiolabeled acetyl moieties.
YiaC Modifies the N Terminus of CobBL In Vitro.
To support our hypothesis that CobBL is Nα acetylated, liquid chromatography-tandem mass spectrometry (LC-MS/MS) was conducted. Results of peptide fingerprinting analysis of acetylated CobB long isoform (AcCobBL) unequivocally showed that the N terminus of CobBL was acetylated by YiaC (Fig. 4).
Fig. 4.
Mass spectrometry analysis of AcCobBL. CobBL (5 μM) was incubated with AcCoA (1 mM) with and without YiaC (3 μM) at 37 °C for 1 h. Reaction mixture components were resolved by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis). CobBL was excised from the gel and sent to the University of Wisconsin-Madison Biotechnology Center Mass Spectrometry Facility for LC-MS/MS (liquid chromatography-tandem mass spectrometry) analysis. (A) Chromatographic trace for the selected ion of interest (AcGTMQSR). EIC, extracted ion chromatogram. (B) Mass spectrum of AcCobBL. b ions are the series of fragments that extend from the N terminus; y ions are the series of fragments that extend from the C terminus. The x axis of m/z stands for mass (m) over charged number of ions (z). MASCOT software (http://www.matrixscience.com) was the online search engine used to identify peptides on the basis of their masses.
To confirm the LC-MS/MS data, two peptides of the first 50 amino acids of CobBL were synthesized (Peptide 2.0, Virginia): one started with unmodified l-Met, and the second one started with l-MetAc. In vitro acetylation assays were performed with the above-mentioned peptides as substrates for YiaC. When [1-14C]-AcCoA was added to the reaction mixture, YiaC acetylated the CobBL peptides whose N-terminal amino group was not modified (Fig. 5, lanes 2 and 8) but did not acetylate the CobBL peptide whose first residue was l-MetAc (Fig. 5, lane 5). Collectively, these data showed that YiaC was an Nα protein acetyltransferase (NAT).
Fig. 5.
YiaC acetylates the N-terminal methionine of CobBL. SDS-PAGE and phosphor imaging analysis of unacetylated or N-terminally acetylated synthetic peptides of amino acids spanning the first 50 residues of CobBL that were incubated with YiaC without [1-14C]-AcCoA (lanes 3 and 6), with [1-14C]-AcCoA but no YiaC (lanes 4 and 7), or with YiaC and [1-14C]-AcCoA (lanes 2, 5, and 8). Lanes 1 and 9 are the Precision Plus protein standard molecular mass markers (MM) (kilodaltons). SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis. *Radiolabeled acetyl moieties.
N-Terminally AcCobBL Cannot Be Deacetylated by Either Isoform of CobB.
To test whether N-terminally AcCobB could be deacetylated by either isoform of CobB, acetylation assays with YiaC, CobBL, and [1-14C]-AcCoA were conducted. Radiolabeled, acetylated CobB long isoform (*AcCobBL) samples were freed of excess AcCoA and were incubated with either CobBL or CobBS, with or without NAD+. Positive controls were also added to show CobBL and CobBS deacetylated its bona fide substrate, AcsAc. As seen in Fig. 6, CobBL and CobBS deacetylated radiolabeled, acetylated acetyl-CoA synthetase (Acs*Ac; lanes 3 and 5) but did not to deacetylate *AcCobBL (lanes 7 and 9). This showed that N-terminal acetylation of CobBL was not reversed by either CobB isoform.
Fig. 6.
Nα acetylated proteins are not substrates for CobB. [1-14C]-AcCobBL was incubated with either CobBL or CobBS with (lanes 7 and 9) and without NAD+ (lanes 6 and 8). Positive controls of unlabeled CobBL and CobBS were also tested for their ability to deacetylate [1-14C]-AcsAc (lanes 2 to 5). Lane 1 represents molecular mass standards (MM) reported in kilodaltons. Samples were resolved by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), and transfer of [1-14C] label was revealed by phosphor image analysis. *Radiolabeled acetyl moieties.
In Vitro and In Vivo Evidence That Nα Acetylation of CobBL Alters Its Deacetylase Activity.
In vitro evidence.
The N-terminal acetylation of CobBL by YiaC (Fig. 2) raised questions regarding the effect of the modification on the enzymatic activity of CobBL. To answer this question, we performed in vitro protein deacetylation assays with Acs*Ac and AcCobBL to enhance our ability to detect deacetylation events. The protocols for the generation of Acs*Ac and *AcCobBL are described in Materials and Methods. After we had Acs*Ac and *AcCobBL, the following experiment was performed. CobBL or *AcCobBL was added to a reaction mixture that contained Acs*Ac and NAD+, and samples (25 μL each) were incubated for 1 h. As seen in Fig. 7A, unacetylated CobBL deacetylated Acs*Ac as indicated by the disappearance of the signal in the phosphor image corresponding to Acs*Ac (molecular mass ∼72 kDa) (Fig. 7A, lane 5). In contrast, *AcCobBL did not deacetylate Acs*Ac as efficiently as CobBL (Fig. 7A, lane 3 vs. lane 5). Quantitative densitometry of the signals of AcsAc radiolabel disappearance showed an average of ∼50% reduction in CobBL deacetylase activity when acetylated. The results shown in Fig. 7 are representative of a set of six separate experiments. These data showed that the enzymatic activity of *AcCobBL was negatively affected by the modification.
Fig. 7.
Acetylation of the N terminus of CobBL negatively affects its deacetylase activity in vitro and in vivo. (A) To assess the enzymatic activity of AcCobBL in vitro, Acs protein radiolabeled with [1-14C]-AcCoA (AcsAc*) was incubated with either CobBL (lane 5) or *AcCobBL (lane 3) and NAD+ to visualize the removal of the radiolabeled acetyl group from Acs. CobBL was acetylated with YiaC using [1-14C]-AcCoA as substrate. *AcCobBL helped visualize the mobility of AcCobBL on the phosphor image. Negative controls included reactions listed above except no NAD+ was added (lanes 2 and 4). Lane 1 shows molecular mass marker (MM) in kilodaltons. SDS-PAGE, sodium dodecyl sulfate polyacrylamide gel electrophoresis. (B) All strains were grown on NCE (no-carbon essential) minimal medium supplemented with acetate (10 mM) as the sole source of carbon and energy. The strains used were ΔcobB/pCV1/pCV3 (black triangles; negative control), ΔcobB/pcobB+ (CobBS)/pCV1 (open squares; complementation), ΔcobB/pcobB+ (CobBL)/pCV1 (black squares; complementation), cobB+/pCV1/pCV3 (gray squares; wild-type control), cobB+/pyiaC+/pCV3 (open circles), ΔcobB/pcobB+ (CobBS)/pyiaC+ (open triangles), and ΔcobB/pcobB+ (CobBL)/pyiaC+ (gray circles). Cloning vectors pCV1 and pCV3 contain the same arabinose-inducible promoter (24, 25). Maps of cloning vectors pCV1 and pCV3 can be found in ref. 25. The concentration of arabinose used was 100 μM. Each strain was grown in biological and technical triplicate, and analyses were conducted three independent times. Error bars represent SD of technical triplicates. *Radiolabeled acetyl moieties.
In vivo evidence.
To verify that acetylation of the N terminus of CobBL affected its enzymatic activity, we performed in vivo experiments to assess the activity of CobBL as a function of YiaC during growth on minimal medium containing a low concentration of acetate (10 mM). It is known that, under such conditions, CobB function is required to maintain Acs deacetylated, hence active (3, 22, 26). For this purpose, we moved two plasmids into an S. enterica ΔcobB strain. One plasmid encoded either CobBL(M37A,M38A) or CobBS(M1A); the second plasmid encoded YiaC or the vector control. Genes cobB and yiaC were under the control of the l-(+)-arabinose–inducible promoter ParaBAD (24). To get only CobBL and no CobBS protein, the plasmid encoding CobBL contained the natural starting methionine (M1) for CobBL but had two mutations that change the starting methionine and neighboring methionine for CobBS (i.e., M37 and M38) to alanines. The cobB allele encoding CobBM37A,M38A effectively blocks the synthesis of CobBS (23). Conversely, for the cell to synthesize only CobBS, the CobBS-encoding plasmid has a cobB allele with the first methionine changed to encode alanine so that CobBL protein cannot be made from M1, resulting in the exclusive synthesis of CobBS starting at position M38 (23). The growth behaviors of strains ΔcobB and cobB+ harboring empty cloning vectors were used as controls. An additional control used a cobB+ strain harboring a plasmid carrying yiaC+ (open circles in Fig. 7B). All strains were grown on minimal medium with acetate (10 mM) as the sole carbon and energy source.
Data presented in Fig. 7B show the growth behavior of strains of interest in the presence of inducer [l-(+)-arabinose, 100 μM]. The following observations were made. 1) As expected, the ΔcobB strain failed to grow on 10 mM acetate (black triangles in Fig. 7B) because Acs remained acetylated, hence inactive. 2) The phenotype of the ΔcobB strain was corrected by ectopic expression of cobB alleles that directed the synthesis of functional CobBS (open squares in Fig. 7B) or CobBL (black squares in Fig. 7B). 3) Synthesis of YiaC did not affect the growth of the cobB+ strain (open circles vs. gray squares in Fig. 7B). 4) Synthesis of CobBS and YiaC by a strain with a genomic deletion of cobB resulted in a growth behavior that was very similar to that of the cobB+ strain that synthesized YiaC (open triangles vs. gray squares and open circles in Fig. 7B). 5) Synthesis of CobBL and YiaC by the ΔcobB strain prematurely arrested growth (gray circles in Fig. 7B). These results were consistent with our in vitro data, which showed that N-terminal acetylation of CobBL by YiaC had a negative effect on the deacetylase activity of CobBL (Fig. 7A). Again, under the growth conditions used, a reduction in deacetylase activity would prevent the deacetylation (hence reactivation) of Acs, blocking the conversion of acetate into AcCoA with the concomitant negative effect on cell growth.
As predicted by the above results, the specific activity of Acs was different between the ΔcobB strain that ectopically synthesized CobBL and YiaC and the ΔcobB strain that synthesized CobBL but did not ectopically synthesized YiaC (Fig. 8A). In the above-mentioned strains, we measured a statistically significant reduction of Acs activity when YiaC was overproduced. These results were consistent with the data showing that YiaC-dependent acetylation of the CobBL N terminus reduced its activity, maintaining Acs acetylated (hence inactive) and thus, arresting growth due to reduced levels of AcCoA.
Fig. 8.
Acs activity is decreased when YiaC is overproduced in S. enterica. (A) Strains of S. enterica with cobB deleted and with only pCobBLM37A,M38A in trans with or without pYiaC were grown to midlogarithmic phase with 10 mM acetate as the sole carbon and energy source and were lysed and tested for Acs-specific activity from 4 μg of lysate. Specific activity (micromoles AMP [adenosine monophosphate] minute−1 milligram−1) was calculated using a continuous spectrophotometric assay described in Materials and Methods. The activity of Acs decreased in lysates of the strain with pYiaC overexpressed. The experiment was performed in biological triplicate with nine technical replicates each. *P = 0.03. (B) Acs protein concentration is the same in both strains tested for Acs activity based on quantitative anti-Acs western blot analysis of lysates used in the experiment in A. Error bars represent unpaired t test with equal SD; not significant (ns) P value = 0.5118.
To verify that the concentration of Acs was the same in both strains, quantitative western blots using rabbit polyclonal anti-Acs antibodies were performed to determine the amount of Acs protein present in lysates used to assay for Acs activity. Indeed, lysates of strains that either overexpressed yiaC+ or carried the empty vector contained the same amount of Acs (Fig. 8B and SI Appendix, Fig. S2).
Acetylation of CobBL Does Not Cause CobBL Degradation In Vivo.
In eukaryotes, some N-terminally acetylated proteins are targeted for degradation (27). Other examples suggest that N-terminal acetylation stabilizes acetylated proteins and prevents degradation (28, 29). To assess whether acetylation of CobBL altered CobBL protein levels in vivo, we used rabbit polyclonal anti-CobB antibodies to quantify levels of CobB in cell-free extracts of three strains: yiaC+/vector, yiaC::kan+/vector, and yiaC::kan+/pYiaC. Strains were grown in minimal medium supplemented with acetate (10 mM) and with l-(+)-arabinose (500 μM) or in rich medium (Lysogeny Broth, LB) containing l-(+)-arabinose (1 mM). In both cases, growth was monitored at 600 nm. Data presented in Fig. 9 show that during growth in rich medium or in minimal medium with a low concentration of acetate, the concentration of CobBL did not vary in any of the strains. Pure protein controls of CobBL and CobBS were added as positive controls, and an anti-DnaK blot was included to ensure that all samples were loaded equally. Statistical analysis of pixel density of the anti-CobB western blots shows no difference in CobBL concentration in any of the strains tested, as well as no difference in CobBS or the internal control protein DnaK (Fig. 9C and SI Appendix, Fig. S5).
Fig. 9.
YiaC does not alter CobB protein levels in vivo. The concentration of CobB isoform protein in S. enterica strains where yiaC was deleted or complemented by pYiaC was compared with the parent strain (yiaC+/vector) by western blotting. (A) Western blots of cells grown on LB medium with 1 mM l-(+)-arabinose using anti-CobB or anti-DnaK antibodies. Cells were harvested at three different optical densities (OD600 nanometers, nm) of 0.2, 0.5, and 0.7. (B) Western blots of CobB protein levels or DnaK protein levels from cells grown with 10 mM acetate minimal medium supplemented with 500 μM l-(+)-arabinose. Anti-DnaK western blotting was used as positive controls to show that all lanes were loaded equally and to use as a standard for densitometry calculations. (C) The density of pixels for the CobBL-only sample of the anti-CobB western blot from B showing the concentration of CobBL in all strains are the same. Calculations were conducted using one-way ANOVA, and differences of CobBL protein concentrations are not significant with a P value of 0.9747.
Discussion
In S. enterica, the YiaC protein is an Nα acetyltransferase that controls the activity of the long isoform of the CobB sirtuin deacylase. Data reported here support several conclusions regarding the function of the S. enterica YiaC protein. First, YiaC has Nα acetyltransferase activity. Our data also show that YiaC modifies the long isoform of the CobB sirtuin deacylase (CobBL) of this bacterium but not the short isoform of CobB (Fig. 2). These results placed the sites of acetylation within the 37-amino acid, N-terminal extension of CobBL (Fig. 1). Second, YiaC does not modify Nε amino groups of lysine side chains of the CobBL protein (Figs. 3 and 4). Whether YiaC can modify lysyl residues of other proteins remains to be determined. Third, YiaC appears to have somewhat broad specificity for its target since it acetylated the Nα amino group of the glycyl residue that remained fused to the protein after protease treatment to remove the MBP (maltose binding protein)-H6 tag (Figs. 2 and 4). YiaC also acetylated the Nα amino group of the N-terminal methionine of the C-terminally H6-tagged CobBL, and the N-terminal methionine of a synthetic peptide comprised the first 50 amino acids of CobBL (Fig. 5 and SI Appendix, Fig. S4).
We note that repeated attempts to isolate N-terminally AcCobBL from cells were unsuccessful, despite the fact that several different proteases were used in combination prior to mass spectrometry analysis. We posit that the amino acid composition of the N terminus of CobBL makes this analysis difficult because it is so rich in arginines. However, when we removed the N-terminal tag from MBP-H6-CobBL, the resulting protein had two additional residues on its N terminus, namely Gly-Thr (GT-CobBL). YiaC acetylated GT-CobBL in vitro (Fig. 4 and SI Appendix, Fig. S4) but did not acetylate a protein that had an N-terminal hexahistidine tag (H6-CobBL protein) (SI Appendix, Fig. S4).
Collectively, our results show that YiaC is an Nα acetyltransferase (NAT) and that the CobBL isoform is a substrate of it. To the best of our knowledge, YiaC is a bacterial NAT that does not belong to the Rim family of proteins. Based on our data, we propose to change the name of YiaC to NatA, to reflect the fact that it is an Nα acetyltransferase.
Can YiaC Acetylate the ε Amino Group of Lysine Side Chains?
Recently, Christensen et al. (30) showed that overexpression of yiaC in an E. coli pta patZ acs cobB deletion strain displayed increased protein acetylation measured by western blot analysis using α-AcK antibodies. These results are interesting because these investigators conducted AcK enrichment and mass spectrometry to identify putative YiaC protein lysine targets. CobB was not identified from the aforementioned proteome because the cobB gene was deleted in the strains used, and N-terminal acetylation of proteins was not reported. As mentioned above, at present we cannot rule out the possibility that YiaC acetylates lysyl residues as suggested by Christensen et al. (30). Additional work is needed to determine whether YiaC can perform Nα and Nε protein acetylation. The identified activity of YiaC as an N-terminal acetyltransferase raises questions about the role of N-terminal acetylation in prokaryotic cell physiology.
Concluding Remarks.
We have shown that N-terminal acetylation of CobBL occurs both in vitro and in vivo, as well as that YiaC-dependent acetylation of CobBL negatively impacts its deacetylase activity, which then negatively affects growth on acetate. How the addition of an acetyl group to the N terminus of CobBL impacts its activity is a question of interest, and ongoing studies in our laboratory are focused on answering this question. YiaC is an NAT that does not belong to the Rim protein family of Nα amino group acetyltransferases. Also, we report acylation of a prokaryotic sirtuin deacylase. As pointed above, based on the evidence reported here, we propose to change the name of YiaC to NatA (for Nα acetyltransferase) to reflect the biochemical activity of the enzyme.
Materials and Methods
Detailed protocols used in this study are presented in SI Appendix. SI Appendix contains protocols for the purification of CobBS, CobBL, and YiaC proteins; size exclusion chromatography; in vitro acetylation and deacetylation assays; a protocol for the in vitro determination of Acs activity; quantitative western blot analysis; lists of strains, plasmids, and primers; methods for the construction of strains and plasmids; culture media, growth conditions, and growth behavior analysis; and mass spectrometry analysis of the acetylation state of proteins of interest. SI Appendix also contains tables which contain information about strains, plasmids, and primers, and YiaC and CobB homologs in other enteric bacteria, respectively (SI Appendix, Tables S1–S4). In addition, SI Appendix contains figures (SI Appendix, Figs. S1–S5) that present results of control experiments, data regarding the oligomeric state of YiaC in solution, and quantification of western blots of CobB isoforms or DnaK (control) used in this study.
Data Availability.
All data of this work are reported in the paper.
Supplementary Material
Acknowledgments
We thank Grzegorz Sabat from the Biotechnology Center of The University of Wisconsin–Madison for the performance of the LC-MS/MS analysis. Rachel Burckhardt first observed CobB acetylation. This work was supported by NIH Grant R35 GM130399 (to J.C.E.-S.).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005296117/-/DCSupplemental.
References
- 1.Hentchel K. L., Escalante-Semerena J. C., Acylation of biomolecules in prokaryotes: A widespread strategy for the control of biological function and metabolic stress. Microbiol. Mol. Biol. Rev. 79, 321–346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.VanDrisse C. M., Escalante-Semerena J. C., Protein acetylation in bacteria. Annu. Rev. Microbiol. 73, 111–132 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starai V. J., Escalante-Semerena J. C., Identification of the protein acetyltransferase (Pat) enzyme that acetylates acetyl-CoA synthetase in Salmonella enterica. J. Mol. Biol. 340, 1005–1012 (2004). [DOI] [PubMed] [Google Scholar]
- 4.Gardner J. G., Escalante-Semerena J. C., Biochemical and mutational analyses of AcuA, the acetyltransferase enzyme that controls the activity of the acetyl coenzyme a synthetase (AcsA) in Bacillus subtilis. J. Bacteriol. 190, 5132–5136 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takakura H., Tsunasawa S., Miyagi M., Warner J. R., NH2-terminal acetylation of ribosomal proteins of Saccharomyces cerevisiae. J. Biol. Chem. 267, 5442–5445 (1992). [PubMed] [Google Scholar]
- 6.Yoshikawa A., Isono S., Sheback A., Isono K., Cloning and nucleotide sequencing of the genes rimI and rimJ which encode enzymes acetylating ribosomal proteins S18 and S5 of Escherichia coli K12. Mol. Gen. Genet. 209, 481–488 (1987). [DOI] [PubMed] [Google Scholar]
- 7.Walsh C. J., “Protein N-acetylation” in Posttranslational Modification of Proteins: Expanding Nature’s Inventory, (Roberts and Company Publishers, Greenwood Village, CO, 2006), pp. 151–170. [Google Scholar]
- 8.Kentache T., Jouenne T., Dé E., Hardouin J., Proteomic characterization of Nα- and Nε-acetylation in Acinetobacter baumannii. J. Proteomics 144, 148–158 (2016). [DOI] [PubMed] [Google Scholar]
- 9.Nguyen K. T.et al., N-terminal acetylation and the N-end rule pathway control degradation of the lipid droplet protein PLIN2. J. Biol. Chem. 294, 379–388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen K. T., Mun S. H., Lee C. S., Hwang C. S., Control of protein degradation by N-terminal acetylation and the N-end rule pathway. Exp. Mol. Med. 50, 1–8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thompson C. R., Champion M. M., Champion P. A., Quantitative N-terminal footprinting of pathogenic mycobacteria reveals differential protein acetylation. J. Proteome Res. 17, 3246–3258 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ree R., Varland S., Arnesen T., Spotlight on protein N-terminal acetylation. Exp. Mol. Med. 50, 1–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yaguchi M.et al., Cooperative control of translational fidelity by ribosomal proteins in Escherichia coli. II. Localization of amino acid replacements in proteins S5 and S12 altered in double mutants resistant to neamine. Mol. Gen. Genet. 142, 35–43 (1975). [DOI] [PubMed] [Google Scholar]
- 14.Wittmann-Liebold B., Greuer B., The primary structure of protein S5 from the small subunit of the Escherichia coli ribosome. FEBS Lett. 95, 91–98 (1978). [DOI] [PubMed] [Google Scholar]
- 15.Terhorst C., Wittmann-Liebold B., Möller W., 50-S ribosomal proteins. Peptide studies on two acidic proteins, A 1 and A 2, isolated from 50-S ribosomes of Escherichia coli. Eur. J. Biochem. 25, 13–19 (1972). [DOI] [PubMed] [Google Scholar]
- 16.Pathak D., Bhat A. H., Sapehia V., Rai J., Rao A., Biochemical evidence for relaxed substrate specificity of Nα-acetyltransferase (Rv3420c/rimI) of Mycobacterium tuberculosis. Sci. Rep. 6, 28892 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith V. F., Schwartz B. L., Randall L. L., Smith R. D., Electrospray mass spectrometric investigation of the chaperone SecB. Protein Sci. 5, 488–494 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okkels L. M.et al., CFP10 discriminates between nonacetylated and acetylated ESAT-6 of Mycobacterium tuberculosis by differential interaction. Proteomics 4, 2954–2960 (2004). [DOI] [PubMed] [Google Scholar]
- 19.Mediero A., Perez-Aso M., Cronstein B. N., Activation of EPAC1/2 is essential for osteoclast formation by modulating NFκB nuclear translocation and actin cytoskeleton rearrangements. FASEB J. 28, 4901–4913 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ouidir T., Jarnier F., Cosette P., Jouenne T., Hardouin J., Characterization of N-terminal protein modifications in Pseudomonas aeruginosa PA14. J. Proteomics 114, 214–225 (2015). [DOI] [PubMed] [Google Scholar]
- 21.Starai V. J., Celic I., Cole R. N., Boeke J. D., Escalante-Semerena J. C., Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science 298, 2390–2392 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Starai V. J., Takahashi H., Boeke J. D., Escalante-Semerena J. C., Short-chain fatty acid activation by acyl-coenzyme A synthetases requires SIR2 protein function in Salmonella enterica and Saccharomyces cerevisiae. Genetics 163, 545–555 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker A. C., Escalante-Semerena J. C., Biologically active isoforms of CobB sirtuin deacetylase in Salmonella enterica and Erwinia amylovora. J. Bacteriol. 192, 6200–6208 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzman L. M., Belin D., Carson M. J., Beckwith J., Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177, 4121–4130 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.VanDrisse C. M., Escalante-Semerena J. C., New high-cloning-efficiency vectors for complementation studies and recombinant protein overproduction in Escherichia coli and Salmonella enterica. Plasmid 86, 1–6 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chan C. H., Garrity J., Crosby H. A., Escalante-Semerena J. C., In Salmonella enterica, the sirtuin-dependent protein acylation/deacylation system (SDPADS) maintains energy homeostasis during growth on low concentrations of acetate. Mol. Microbiol. 80, 168–183 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hwang C. S., Shemorry A., Varshavsky A., N-terminal acetylation of cellular proteins creates specific degradation signals. Science 327, 973–977 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jörnvall H., Acetylation of protein N-terminal amino groups structural observations on alpha-amino acetylated proteins. J. Theor. Biol. 55, 1–12 (1975). [DOI] [PubMed] [Google Scholar]
- 29.Persson B., Flinta C., von Heijne G., Jörnvall H., Structures of N-terminally acetylated proteins. Eur. J. Biochem. 152, 523–527 (1985). [DOI] [PubMed] [Google Scholar]
- 30.Christensen D. G.et al., Identification of novel protein lysine acetyltransferases in Escherichia coli. MBio 9, e01905–e01918 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data of this work are reported in the paper.