Abstract
Background and Purpose
The myelin oligodendrocyte glycoprotein (MOG) antibody is detected at a high rate in childhood acquired demyelinating syndrome (ADS). This study aimed to determine the diagnostic value of the MOG antibody in ADS and the spectrum of MOG-antibody-positive demyelinating diseases in children.
Methods
This study included 128 patients diagnosed with ADS (n=94) or unexplained encephalitis (n=34). The MOG antibody in serum was tested using an in-house live-cell-based immunofluorescence assay.
Results
The MOG antibody was detected in 48 patients (46 ADS patients and 2 encephalitis patients, comprising 23 males and 25 females). Acute disseminated encephalomyelitis (ADEM) (35.4%) was the most-common diagnosis, followed by the unclassified form (17.4%), isolated optic neuritis (ON) (15.2%), neuromyelitis optica spectrum disorder (13.0%), multiple sclerosis (MS) (10.8%), other clinically isolated syndromes [monophasic event except ADEM, isolated ON, or transverse myelitis (TM)] (8.7%), and unexplained encephalitis (4.3%). At the initial presentation, 35 out of the 46 patients with ADS had brain lesions detected in magnetic resonance imaging, and 54% of these 35 patients had encephalopathy. Nine of the 11 patients without brain lesions exhibited only ON. Thirty-nine percent of the patients experienced a multiphasic event during the mean follow-up period of 34.9 months (range 1.4–169.0 months). Encephalopathy at the initial presentation was frequently confirmed in the monophasic group (p=0.011).
Conclusions
MOG antibodies were identified in all pediatric ADS phenotypes except for monophasic TM. Therefore, the MOG antibody test is recommended for all pediatric patients with ADS, especially before a diagnosis of MS and for patients without a clear diagnosis.
Keywords: demyelinating disease, myelin oligodendrocyte glycoprotein, antibodies
INTRODUCTION
Acquired demyelinating syndrome (ADS) is an acute neurological deficit that results from demyelination of the central nervous system. The ADS phenotypes depend on the clinical symptoms, signs, and supporting neuroimaging information.1 ADS comprises a heterogeneous group of diseases with relapse phenotypes, such as multiple sclerosis (MS), neuromyelitis optica spectrum disorder (NMOSD), recurrent optic neuritis (ON), and multiphasic disseminated encephalomyelitis (MDEM). Among recurrent ADSs, recurrent ON, NMOSD, and MDEM have relatively clear diagnostic criteria, whereas MS is essentially diagnosed by exclusion.2 In addition, some recurrent ADSs such as acute disseminated encephalomyelitis (ADEM) followed by ON are difficult to diagnose using the currently available diagnostic criteria.3 Further, the rate of aquaporin-4 (AQP4) antibody positivity is lower in children with NMOSD than in adults with NMOSD.4,5 This situation often makes the accurate diagnosis of relapsing ADS challenging, especially in children due to the presentation of atypical MS or overlapping symptoms.
The myelin oligodendrocyte glycoprotein (MOG) antibody is detected at a relatively high rate in children with ADS, and it has recently attracted attention because a more-specific diagnosis is possible based on the underlying pathogenetic mechanisms in childhood ADS.6,7 The MOG antibody is frequently detected in patients with ADEM, AQP4-antibody-negative NMOSD, isolated ON, and MDEM.8,9,10,11,12,13,14 Although recent studies suggest that MOG antibodies are rarely found in patients with MS,11,12 it is unclear whether they are actually absent in MS patients, especially in those with pediatric ADS. Several studies have shown that the MOG antibody may be present in encephalitis, a syndrome that is quite different from typical ADS.15,16,17 These findings suggest that MOG-antibody-associated diseases have a broad clinical spectrum, and so further studies are needed to clarify the clinical characteristics of these related diseases. Accordingly, this study aimed to determine the clinical spectrum of MOG-antibody-associated demyelinating diseases in children and also the diagnostic value of the MOG antibody in pediatric ADS.
METHODS
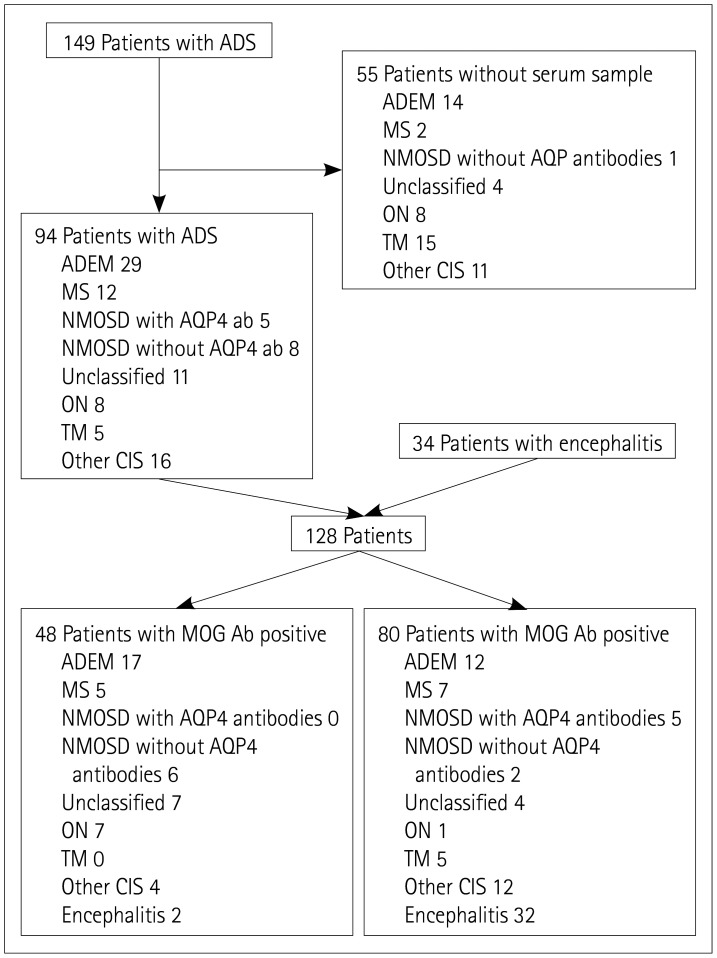
This study was approved by the Institutional Review Board (IRB) of the Seoul National University Hospital (IRB No., 1811-150-989). The study recruited 149 patients diagnosed with ADS at the Seoul National University Children's Hospital from March 2013 to May 2018, of which 94 were included in the study after excluding 55 patients for whom serum samples were not available. In addition, 34 patients with unexplainable encephalitis were also included, and so 128 patients were finally included in this study (Fig. 1). All of the included patients or their parents provided written informed consent.
Fig. 1. Diagram of the selection of patients. Ab: antibodies, ADEM: acute disseminated encephalomyelitis, ADS: acquired demyelinating syndrome, AQP: aquaporin, MOG: myelin oligodendrocyte glycoprotein, MS: multiple sclerosis, NMOSD: neuromyelitis optica spectrum disorder, ON: optic neuritis, Other CIS: clinically isolated syndrome except for isolated ON or isolated TM, TM: transverse myelitis.
Clinical information including age at onset, sex, length of the follow-up period, symptoms and signs at presentation, brain and spine magnetic resonance imaging (MRI) findings, recurrence status, and treatment outcomes were retrospectively collected from their electronic medical records. We used this information to divide the patients into the following seven groups: MS, NMOSD, unclassified form, ADEM, isolated ON, isolated transverse myelitis (TM), and other clinically isolated syndromes (CISs). The 2013 criteria of the International Pediatric Multiple Sclerosis Study Group (IPMSSG) were used for diagnosing MS, ADEM, and other CIS, while the 2015 revision was applied for diagnosing NMOSD.18
Serum was collected from patients within 1 month of the onset and stored in a liquid-nitrogen tank after centrifugation. MOG antibodies were detected qualitatively using a live-cell-based indirect immunofluorescence assay.19 Briefly, MOG-human embryonic kidney 293 cells were seeded on eight-well chambered slides (SPL Life Sciences, Pocheon, Korea) and incubated in 5% CO2 at 37℃ overnight. The cells were then blocked with a blocking buffer [1×phosphate-buffered saline (PBS) containing 5% bovine serum albumin] at room temperature for 1 h, diluted serum (1:20) was added to each well, and the slides were incubated at room temperature for 2 h. The cells were then fixed using 2% paraformaldehyde at room temperature for 45 min. After washing, the cells were stained with Alexa-594 (Jackson ImmunoResearch, West Grove, PA, USA; diluted 1:2,000 with 1×PBS) conjugated antihuman immunoglobulin G (IgG) for MOG-IgG for 1 h at room temperature in the dark. The cells were then washed three times before being mounted using VECTASHIELD antifade reagent with DAPI (Vector Laboratories, Burlingame, CA, USA). Each experiment was performed in duplicate, and green or red fluorescence in cell membranes was visually examined by the investigators.
Continuous variables were analyzed using independent-samples t-tests. The chi-square test or Fisher's exact test was used to analyze categorical variables. SPSS software (version 22.0, IBM Corp., Armonk, NY, USA) was used for statistical analyses, and p values less than 0.05 were considered statistically significant.
RESULTS
Demographics and characteristics of patients
The 94 ADS patients (40 males and 54 females) were aged 101.1±48.6 months (mean±SD) and followed up for 35.1±39.0 months. The 34 patients with unexplained encephalitis were aged 122.3±63.1 months followed up for 20.4±29.4 months. MRI revealed that the sites of involvement included the brain, spine, and anterior optic pathway (including the optic nerves and chiasm).
The 28 patients in the ADS group who were diagnosed with ADEM exhibited encephalopathy as their initial symptoms, had polyfocal lesions, and had not experienced relapse beyond 3 months after onset. One patient was diagnosed with multiphasic ADEM due to an encephalopathy relapse at 3 months after the onset. Twelve patients diagnosed with MS satisfied the IPMSSG criteria. Thirteen patients with NMOSD satisfied the 2015 revision criteria for NMOSD, of which five tested positive for AQP4 antibodies. Eleven patients were diagnosed with the unclassified form and experienced one or more relapses after the first attack, but they were not diagnosed with either NMOSD or MS. One of the eight patients diagnosed with isolated ON experienced recurrence. Five patients diagnosed with isolated TM were monophasic. Other CIS (16 patients) included patients who experienced a monophasic event, except those diagnosed with ADEM, isolated ON, or isolated TM. However, patients diagnosed with NMOSD or MS despite experiencing monophasic events were excluded from the other-CIS group.
All encephalitis patients showed encephalopathy and fever, but MRI did not reveal any clear lesions in their brains other than meningeal enhancement. The findings of their cerebrospinal fluid (CSF) examinations (bacterial cultures) were negative, and polymerase chain reaction did not detect herpes simplex virus type 1, herpes simplex virus type 2, human herpesvirus 6, or enterovirus.
Seropositivity of ADS and encephalitis patients
Among all 128 patients, 48 (37.5%) showed MOG antibody positivity: 46 of the 94 ADS patients and 2 of the 34 encephalitis patients. The most-common diagnosis in the MOG antibody-positive patients was ADEM (35.4%), followed by the unclassified form (17.4%), isolated ON (15.2%), NMOSD (13.0%; all patients were negative for AQP4 antibodies), MS (10.8%), other CIS (8.7%), and encephalitis (4.3%). None of the patients who had monophasic TM during the follow-up showed positivity for MOG antibodies.
The proportion of patients with MOG antibody positivity was evaluated according to the clinical classification of ADS. Isolated-ON patients exhibited the highest rate of MOG positivity (7 of 8 patients, 77.8%), followed by 7 (63.6%) of the 11 patients with the unclassified form, 17 (58.6%) of the 29 patients with ADEM, 6 (46.1%) of the 13 patients with NMOSD, 5 (41.6%) of the 12 MS patients, and 4 (25.0%) of the 16 patients with other CIS.
MOG-antibody-positive ADS versus MOG-anti body-negative ADS
MOG-antibody-positive patients tended to be younger at the onset (p=0.081) and had a higher probability of encephalopathy (p=0.291) than did MOG-antibody-negative patients. MOG-antibody-positive patients had a higher incidence of optic nerve involvement during the follow-up period than did MOG-antibody-negative patients (46% versus 21%, p=0.015) (Table 1).
Table 1. MOG-antibody-positive versus MOG-antibody-negative patients.
| Positive (n=46) | Negative (n=48) | p | |
|---|---|---|---|
| Age at onset, months | 92.6±47.5 | 110.1±48.9 | 0.081 |
| Sex, males:females | 21:25 | 18:30 | 0.530 |
| Follow-up period, months | 34.8±36.4 | 36.4±41.9 | 0.882 |
| Recurrence | 18 (39) | 17 (35) | 0.710 |
| Encephalopathy | 21 (46) | 16 (33) | 0.291 |
| Involvement site | |||
| Brain demyelination | 39 (85) | 40 (83) | 1.000 |
| Optic nerve | 21 (46) | 10 (21) | 0.015 |
| Spinal cord | 15 (33) | 18 (38) | 0.670 |
Data are mean±SD, n, or n (%) values.
MOG: myelin oligodendrocyte glycoprotein.
Initial presentation of MOG-antibody-positive ADS patients
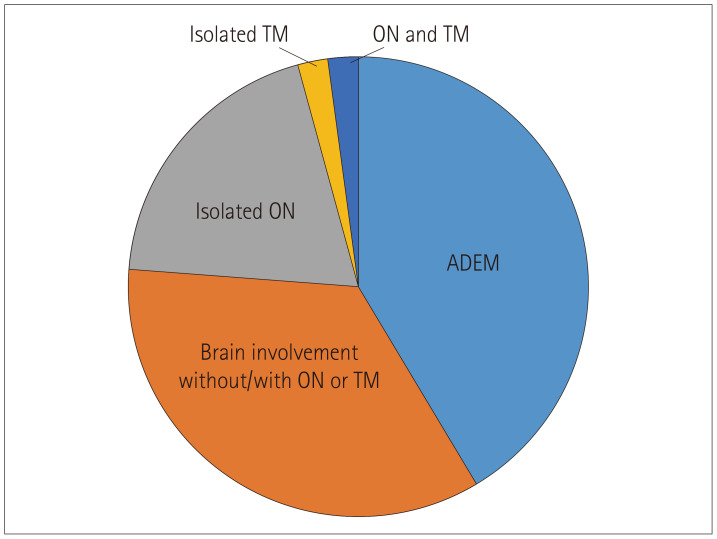
Thirty-five (76.1%) of the 46 MOG-antibody-positive patients exhibited brain demyelination at the first presentation, of whom 19 (54.3%) had encephalopathy, while 9 (81.8%) of the 11 patients without brain demyelination exhibited only ON. We therefore divided the patients into the following three categories based on these characteristics: brain demyelination with encephalopathy (n=19, 41.3%), brain demyelination without encephalopathy (n=16, 34.7%), and only ON (n=9, 19.6%) (Fig. 2).
Fig. 2. The initial presentation of myelin oligodendrocyte glycoprotein positive acquired demyelinating syndrome patients. ADEM: acute disseminated encephalomyelitis, ON: optic neuritis, TM: transverse myelitis.
Recurrence and outcomes
Eighteen (39.1%) of the 46 MOG-antibody-positive patients experienced recurrence. The median follow-up duration of patients who tested positive for the MOG antibody was 36.6 months (range 1.4–169.0 months), and the median time from onset to the first relapse was 4.2 months (range 1.5–31.3 months). Fourteen (77.8%) and 16 (88.9%) of these 18 patients experienced the first relapse within 12 months and 2 years, respectively. Only 2 of the 46 patients received maintenance therapy after the first attack, including immunosuppressant and disease-modifying medication: one was prescribed steroids and the other was prescribed azathioprine. Relapse occurred in 16 and 2 of the 44 patients who did and did not receive maintenance therapy, respectively. Fifteen of the 18 patients who experienced a relapse experienced fewer than 4 attacks (2 attacks in 9 patients and 3 attacks in 6 patients). Interferon-beta was administered to three patients after the second attack (two with MS and one with the unclassified form). One of the MS patients did not experience recurrence after interferon-beta use, while the other experienced one additional attack but no relapse after dosage escalation. The third patient received azathioprine after the third attack and then subsequently experienced two additional attacks (Table 2). We found no significant difference with sex, age at onset, or the site of involvement at the initial presentation (monophasic versus multiphasic), whereas encephalopathy was more common in the monophasic group (p=0.011) (Table 3).
Table 2. Summary of myelin oligodendrocyte glycoprotein-antibody-positive patients.
| Patient number | Onset age, years | Sex | Clinical diagnosis | Number of demye-lination events | Disease course | Maintenance therapy regimen (number of events)* | Outcome (EDSS score) |
|---|---|---|---|---|---|---|---|
| 1 | 14 | Male | Recurrent ON | 6 | Unilateral ON (left)×5 → Unilateral ON (right) | Mycophenolic acid (5) | 0 |
| 2 | 13 | Female | Unclassified | 5 | Brain demyelination×5 | Interferon (2) | 0 |
| Azathioprine (4) | |||||||
| 3 | 5.6 | Male | MS | 4 | Brain demyelination×4 | Azathioprine, glucocorticoid (3) | 0 |
| Mycophenolic acid (4) | |||||||
| 4 | 2.8 | Male | MS | 3 | Brain demyelination×2 → brain | Interferon (2) | 0 |
| 5 | 11.2 | Female | MS | 3 | Brain demyelination×3 | Glucocorticoid (3) | 0 |
| 6 | 12.3 | Female | Unclassified | 3 | Brain demyelination×3 | Azathioprine (2) | 2 |
| 7 | 7.3 | Female | MS | 3 | Brain demyelination×3 | Glucocorticoid (2) | 0 |
| 8 | 16.6 | Female | Unclassified | 2 | Brain demyelination → brain demyelination+myelitis (long) | Azathioprine (1) | 0 |
| 9 | 3.3 | Female | NMOSD | 3 | Bilateral ON → brain demyelination+myelitis (long) → brain demyelination | Azathioprine (3) | 0 |
| 10 | 10.1 | Female | Unclassified | 3 | Bilateral ON → brain demyelination×2 | None | 0 |
| 11 | 9.4 | Male | MS | 2 | Brain demyelination(e) → brain demyelination | Interferon (2) | 0 |
| 12 | 5.3 | Female | Unclassified | 2 | Brain demyelination(e) → ON | None | 0 |
| 13 | 6.7 | Female | Unclassified | 2 | Bilateral ON+brain demyelination → brain demyelination(e) | Glucocorticoid (2) | 0 |
| 14 | 11.6 | Female | ADEM | 1 | Brain demyelination(e) | None | 0 |
| 15 | 9.3 | Male | Unclassified | 2 | Bilateral ON+brain demyelination → bilateral ON | None | 0 |
| 16 | 7.9 | Male | NMOSD | 2 | Brain demyelination+myelitis (long) → unilateral ON (right) | Azathioprine, glucocorticoid (2) | 0 |
| 17 | 6.5 | Female | NMOSD | 2 | Brain demyelination+bilateral ON → myelitis (long)+bilateral ON+brain demyelination | Glucocorticoid (1) | 0 |
| Mycophenolic acid (2) | |||||||
| 18 | 10 | Female | NMOSD | 2 | Myelitis (long) → unilateral ON (right)+brain demyelination | Azathioprine, glucocorticoid (2) | 0 |
| 19 | 5.4 | Female | NMOSD | 2 | Brain demyelination+myelitis (long) → brain demyelination+unilateral ON (right) | None | 0 |
| 20 | 4.7 | Male | ADEM | 1 | Brain demyelination(e) | None | 0 |
| 21 | 4.2 | Male | ADEM | 1 | Brain demyelination(e)+myelitis (short) | None | 0 |
| 22 | 6.1 | Male | ADEM | 1 | Brain demyelination(e)+myelitis (long) | None | 0 |
| 23 | 6.1 | Male | ADEM | 1 | ADEM | None | 0 |
| 24 | 5.7 | Male | ADEM | 1 | Brain demyelination(e) | None | 0 |
| 25 | 11.6 | Male | ADEM | 1 | Brain demyelination(e) | None | 0 |
| 26 | 2.4 | Male | ADEM | 1 | Brain demyelination(e) | None | 4 |
| 27 | 3.7 | Male | ADEM | 1 | Brain demyelination(e) | None | 0 |
| 28 | 4.9 | Male | ADEM | 1 | Brain demyelination(e)+myelitis (short)+bilateral ON | None | 0 |
| 29 | 1.3 | Male | ADEM | 1 | Brain demyelination(e)+myelitis (long) | None | 0 |
| 30 | 1.9 | Female | ADEM | 1 | Brain demyelination(e) | None | 0 |
| 31 | 6.6 | Female | ADEM | 1 | Brain demyelination(e) | None | 0 |
| 32 | 5.3 | Female | ADEM | 1 | Brain demyelination(e)+myelitis (long) | None | 1 |
| 33 | 4.3 | Female | ADEM | 1 | Brain demyelination(e) | None | 0 |
| 34 | 5.7 | Female | ADEM | 1 | Brain demyelination(e)+myelitis (long) | None | 0 |
| 35 | 3.3 | Female | ADEM | 1 | Brain demyelination(e) | None | 2 |
| 36 | 10.8 | Male | Other CIS | 1 | Brain demyelination+myelitis (long) | None | 0 |
| 37 | 17.4 | Male | Other CIS | 1 | Brain demyelination | None | 0 |
| 38 | 14.9 | Male | ON | 1 | Unilateral ON (left) | None | 0 |
| 39 | 5.4 | Male | ON | 1 | Unilateral ON (left) | None | 0 |
| 40 | 13.8 | Female | Other CIS | 1 | Brain demyelination+bilateral ON | None | 0 |
| 41 | 6.4 | Female | ON | 1 | Bilateral ON | None | 0 |
| 42 | 9.0 | Female | Other CIS | 1 | Brain demyelination | None | 0 |
| 43 | 6.1 | Female | ON | 1 | Bilateral ON | None | 0 |
| 44 | 10.5 | Female | ON | 1 | Bilateral ON | None | 0 |
| 45 | 9.0 | Female | NMOSD | 1 | Unilateral ON+myelitis (long) → brain demyelination (subclinical) | None | 0 |
| 46 | 5.3 | Male | ON | 1 | Unilateral ON | None | 0 |
| 47 | 1.7 | Male | Encephalitis | 0 | Encephalitis | 0 | |
| 48 | 11.8 | Male | Encephalitis | 0 | Encephalitis → epilepsy | 0 |
*The timing of starting after the (nth) attack.
ADEM: acute disseminated encephalomyelitis, EDSS: Expanded Disability Status Scale, MS: multiple sclerosis, NMOSD: neuromyelitis optica spectrum disorder, ON: optic neuritis, Other CIS: clinically isolated syndrome other than isolated ON or isolated transverse myelitis, (e): with encephalopathy, (long): at least three vertebral segments, (short): fewer than three vertebral segments.
Table 3. Comparison of groups with and without recurrence in myelin oligodendrocyte glycoprotein-antibody-positive patients with acquired demyelinating syndrome.
| Recurrence (n=18) | No recurrence (n=28) | p | |
|---|---|---|---|
| Age at onset, months | 104.6±45.1 | 84.9±48.1 | 0.167 |
| Sex, males:females | 6:12 | 15:13 | 0.179 |
| Encephalopathy | 4 (22) | 17 (61) | 0.011 |
| Involvement site | |||
| Brain demyelination | 17 (94) | 22 (79) | 0.144 |
| Optic nerve | 6 (33) | 10 (36) | 0.091 |
| Spinal cord | 6 (33) | 9 (32) | 0.933 |
Data are mean±SD, n, or n (%) values.
Patients with encephalitis with MOG antibody positivity
Patient 47 exhibited prolonged seizures and fever during the initial presentation (which are suggestive of encephalitis), but normal CSF and brain MRI findings. Patient 48 exhibited fever, headache, and vomiting at the initial presentation, and the CSF examination showed pleocytosis while brain MRI revealed only leptomeningeal enhancement. Spine MRI was not performed in either of the two patients. Patient 47 received antibiotics and intravenous immunoglobulin, and patient 48 received antibiotics, acyclovir, and steroids. Both patients had an Expanded Disability Status Scale score of 0 points, but patient 48 experienced epilepsy after the initial event and showed no clear demyelinating lesions.
DISCUSSION
The proportion of pediatric patients with MOG antibody positivity in ADS has reportedly ranged from 15% to 40%.9,10,11,12,20 This wide range is thought to be due to differences in the proportions of phenotypes in the study cohorts.9,10,11,12,20 Specific phenotypes such as ADEM, AQP4-antibody-negative NMOSD, and recurrent ON have high rates of MOG antibody positivity,9,10,11,12,13,21 and so including large numbers of these phenotypes may increase the MOG-antibody-positive rate in the overall ADS sample. We avoided this bias by selecting studies that included all ADS phenotypes.9,12,20 The present study detected MOG antibody positivity in 48.9% of the children with ADS, in contrast to previous studies finding rates of MOG antibody positivity ranging between 18% and 42%. The proportion of patients diagnosed with ADEM in our study was 30%, which is similar to ADEM patients accounting for 20–42% of the cohorts in the previous studies. In addition, the proportion of ON patients in our study was 8.5%, which is lower than that in previous studies.9,12 These comparisons confirm that the high percentage of MOG antibodies in the present study was not due to the inclusion of specific phenotypes such as ADEM and isolated ON.
There are three possible reasons for the high rate of MOG antibody positivity in this study. First, since our hospital is a tertiary hospital, the study included many patients with a high recurrence and severe symptoms (referral bias). The second possible reason is different racial backgrounds, considering that a study in California found that the proportion of ADS phenotypes might vary between races.22 In addition, a Japanese study found that 52% of the 17 participants were positive for the MOG antibody,23 which is a similar proportion to that in our study. Unfortunately, to the best of our knowledge, no large-scale study has investigated all types of ADS patients in East Asia. Therefore, further prospective studies are needed to confirm whether the proportion of ADS patients who are positive for the MOG antibody is higher in East Asia than in other regions. Finally, unlike other studies, the present study found high MOG-antibody-positive rates in other CIS, the unclassified form, and MS.
We designated patients with no definite diagnosis as the unclassified form. In pediatric ADS patients with relapses, it is often challenging to make an accurate diagnosis since some patients do not clearly fulfill the diagnostic criteria. In our study, 36 of the 94 participants experienced a relapse, among which 11 patients had no clear diagnosis. We found that a large proportion (n=7, 63.6%) of these 11 patients were positive for the MOG antibody. This finding is highly significant since it will facilitate the diagnosis of children who cannot be accurately diagnosed based on clinical features and neuroimaging findings alone.
Whether patients diagnosed with MS are positive for the MOG antibody remains controversial. Some studies have indicated that the MOG antibody is rarely found in patients with MS.11,20,21 In contrast, one study found that 33% of MOG-antibody-positive adult patients fulfilled the McDonald criteria,24 and some pediatric studies have found MOG antibody positivity in patients diagnosed with MS.9,23 This controversy is probably due to diagnosing MS being more difficult in children than in adults for the following reasons: 1) caution is required when applying the McDonald criteria to children younger than 12 years because the likelihood of MS is lower in their population , 2) there are many atypical initial presentations of pediatric MS, such as ON, encephalopathy, and brainstem involvement,25,26 3) pediatric patients with MS have ill-defined lesions in brain MRI and are less likely to have a CSF oligoclonal band,5 and 4) it is difficult to achieve agreement between symptoms and neuroimaging data. Therefore, many pediatric ADS patients have been classified according to the 2013 IPMSSG criteria rather than the McDonald criteria. In this study, 5 (41.6%) of the 12 patients diagnosed with MS were found to be positive for the MOG antibody: 3 experienced repeated brain demyelinating events without encephalopathy, ON, or TM; 1 experienced nonencephalopathic events following ADEM, and 1 experienced ADEM after nonencephalopathic events. These patients fulfilled the criteria for dissemination in both time and space, and are therefore likely to be diagnosed with MS according to the 2013 IPMSSG criteria.3
The findings of the present study indicate that the spectrum of MOG-antibody-positive demyelinating diseases is quite wide and that some patients may even satisfy the criteria for MS. However, although these patients meet the criteria for MS, they are unlikely to have MS. Because MOG-antibody-associated disease and MS are different diseases with different underlying pathophysiologies and prognoses, there is a clear need for them to be discriminated.26,27 In addition, it is very important to differentiate MS from other ADSs since MS has different therapeutic options (immunomodulating agents). We therefore recommend checking for the presence of MOG antibodies before diagnosing MS in order to determine if MOG-related diseases are present.
We divided the included patients into three groups according to their initial presentations. ADEM (brain demyelination with encephalopathy) episodes were the most-common symptom (41%), followed by other CIS [brain demyelination without encephalopathy (35%)] and isolated ON (20%). Jurynczyk et al.27 found that MOG-antibody-positive ADS patients younger than 20 years presented with isolated ON and ADEM at onset more frequently than with TM, and Ketelslegers et al.12 reported that ADEM and ON were the most-common initial presentations of MOG-antibody-positive ADS in children. These findings are consistent with our results, but a relatively large proportion (35%) of patients presented with brain demyelination without encephalopathy in the current study. Patients with nonencephalopathic brain demyelination show various phenotypes according to the involvement of the spinal cord and optic nerve, which makes it difficult to clearly define the characteristics of these patients. Our findings suggest that the initial presentation in children with the MOG antibody is far wider and more varied than demonstrated by previous studies. We therefore recommend testing for MOG antibodies in patients diagnosed with ADEM or ON, including those diagnosed with other CIS.
We attempted to determine differences at the initial presentation between the monophasic and multiphasic groups. Previous studies found that patients with MOG antibodies had a bimodal distribution according to their age, encephalopathy, and sex. In the younger group, patients were predominantly male and had ADEM features and a lower risk of relapse, whereas the older patients were predominantly female, were more likely to have ON, and had a higher risk of relapse.10,20 The present study found no statistically significant differences in age, sex, or optic nerve involvement between the monophasic and multiphasic groups. Encephalopathy was the only significant group difference found without recurrence during the initial presentation.
This study detected the MOG antibody in two patients who had no distinct parenchymal lesions in brain MRI and only exhibited symptoms of encephalitis. Consistently, there is accumulating evidence that the MOG antibody can cause encephalitis-like symptoms such as seizures and encephalopathy with cortical involvement.15,16,17,28 However, since the patients in the present study did not have any lesions, further studies are needed to determine whether the MOG antibody causes encephalitis symptoms in patients without lesions. Previous studies found that 1.5% (range 0–6%) of control participants were positive for MOG antibody, and so we cannot exclude the possibility that there is no association between the MOG antibody and encephalitis symptoms in patients.21 In addition, our patient 48, who had leptomeningeal enhancement in the initial brain MRI, underwent another brain MRI scan 2 months later, which again revealed leptomeningeal enhancement and also suspected high signal intensity in T2-weighted and fluid-attenuated inversion recovery imaging in the right posterior temporal lobe. This finding suggests that the ability to detect the lesion depends on when brain MRI is conducted.
This study was conducted at a single tertiary hospital and so might have included higher proportions of severely affected patients or patients with relapse. Thus, our cohort cannot represent all children with ADS in Korea, and the referral bias might have contributed to the high MOG-antibody-positive rate in our study. In addition, we could not determine how the prognosis of patients was associated with temporal changes in the MOG antibody because the study did not have a longitudinal design. Moreover, while our study identified differences at the initial presentation between the monophasic and multiphasic groups, it did not examine the effectiveness of treatment. This study did not have a controlled design, the number of patients undergoing maintenance therapy was small, the starting time of maintenance therapy varied, and the regimens administered to patients were inconsistent. Therefore, it is impossible to verify the treatment effect, and so further prospective studies are needed to determine the factors associated with relapses.
This study indicates that the rate of MOG antibody positivity is relatively high in Korean pediatric patients with ADS, and that the spectrum of MOG-antibody-positive demyelinating diseases is wider than previously thought. These findings demonstrate the importance of examining the MOG antibody status regardless of the phenotype in all children with ADS, especially before MS is diagnosed and in patients without a clear diagnosis.
Acknowledgements
This study was supported by the research institute of medical science at the St.Vincent's hospital (SVHR-2018-05).
Footnotes
- Conceptualization: Ki Joong Kim, Jong-Hee Chae, Byung Chan Lim, Il Han Yoo.
- Data curation: WooJoong Kim, Youngkyu Shim, Il Han Yoo.
- Formal analysis: Il Han Yoo.
- Funding acquisition: Il Han Yoo.
- Investigation: Hunmin Kim.
- Methodology: Yeseul Kim, Jae-Won Hyun, Su-Hyun Kim, Kyungho Choi, Ho Jin Kim.
- Project administration: Ki Joong Kim, Jong-Hee Chae.
- Resources: Ki Joong Kim, Jong-Hee Chae, Byung Chan Lim.
- Software: Jieun Choi.
- Supervision: Ki Joong Kim.
- Validation: Hee Hwang.
- Visualization: Sun Ah Choi, Soo Yeon Kim.
- Writing—original draft: Jong-Hee Chae, Ho Jin Kim, Il Han Yoo.
- Writing—review & editing: Jong-Hee Chae, Ho Jin Kim, Il Han Yoo.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Hintzen RQ, Dale RC, Neuteboom RF, Mar S, Banwell B. Pediatric acquired CNS demyelinating syndromes: features associated with multiple sclerosis. Neurology. 2016;87(9 Suppl 2):S67–S73. doi: 10.1212/WNL.0000000000002881. [DOI] [PubMed] [Google Scholar]
- 2.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, Coetzee T, Comi G, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17:162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 3.Krupp LB, Tardieu M, Amato MP, Banwell B, Chitnis T, Dale RC, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19:1261–1267. doi: 10.1177/1352458513484547. [DOI] [PubMed] [Google Scholar]
- 4.Huppke P, Blüthner M, Bauer O, Stark W, Reinhardt K, Huppke B, et al. Neuromyelitis optica and NMO-IgG in European pediatric patients. Neurology. 2010;75:1740–1744. doi: 10.1212/WNL.0b013e3181fc2823. [DOI] [PubMed] [Google Scholar]
- 5.Chabas D, Ness J, Belman A, Yeh EA, Kuntz N, Gorman MP, et al. Younger children with MS have a distinct CSF inflammatory profile at disease onset. Neurology. 2010;74:399–405. doi: 10.1212/WNL.0b013e3181ce5db0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Di Pauli F, Reindl M, Berger T. New clinical implications of anti-myelin oligodendrocyte glycoprotein antibodies in children with CNS demyelinating diseases. Mult Scler Relat Disord. 2018;22:35–37. doi: 10.1016/j.msard.2018.02.023. [DOI] [PubMed] [Google Scholar]
- 7.Wells E, Hacohen Y, Waldman A, Tillema JM, Soldatos A, Ances B, et al. Neuroimmune disorders of the central nervous system in children in the molecular era. Nat Rev Neurol. 2018;14:433–445. doi: 10.1038/s41582-018-0024-9. [DOI] [PubMed] [Google Scholar]
- 8.Cobo-Calvo Á, Ruiz A, D'Indy H, Poulat AL, Carneiro M, Philippe N, et al. MOG antibody-related disorders: common features and uncommon presentations. J Neurol. 2017;264:1945–1955. doi: 10.1007/s00415-017-8583-z. [DOI] [PubMed] [Google Scholar]
- 9.Dale RC, Tantsis EM, Merheb V, Kumaran RY, Sinmaz N, Pathmanandavel K, et al. Antibodies to MOG have a demyelination phenotype and affect oligodendrocyte cytoskeleton. Neurol Neuroimmunol Neuroinflamm. 2014;1:e12. doi: 10.1212/NXI.0000000000000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez-Carbonell C, Vargas-Lowy D, Musallam A, Healy B, McLaughlin K, Wucherpfennig KW, et al. Clinical and MRI phenotype of children with MOG antibodies. Mult Scler. 2016;22:174–184. doi: 10.1177/1352458515587751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hacohen Y, Absoud M, Deiva K, Hemingway C, Nytrova P, Woodhall M, et al. Myelin oligodendrocyte glycoprotein antibodies are associated with a non-MS course in children. Neurol Neuroimmunol Neuroinflamm. 2015;2:e81. doi: 10.1212/NXI.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ketelslegers IA, Van Pelt DE, Bryde S, Neuteboom RF, Catsman-Berrevoets CE, Hamann D, et al. Anti-MOG antibodies plead against MS diagnosis in an acquired demyelinating syndromes cohort. Mult Scler. 2015;21:1513–1520. doi: 10.1177/1352458514566666. [DOI] [PubMed] [Google Scholar]
- 13.Rostásy K, Mader S, Hennes EM, Schanda K, Gredler V, Guenther A, et al. Persisting myelin oligodendrocyte glycoprotein antibodies in aquaporin-4 antibody negative pediatric neuromyelitis optica. Mult Scler. 2013;19:1052–1059. doi: 10.1177/1352458512470310. [DOI] [PubMed] [Google Scholar]
- 14.Baumann M, Hennes EM, Schanda K, Karenfort M, Kornek B, Seidl R, et al. Children with multiphasic disseminated encephalomyelitis and antibodies to the myelin oligodendrocyte glycoprotein (MOG): extending the spectrum of MOG antibody positive diseases. Mult Scler. 2016;22:1821–1829. doi: 10.1177/1352458516631038. [DOI] [PubMed] [Google Scholar]
- 15.Hamid SHM, Whittam D, Saviour M, Alorainy A, Mutch K, Linaker S, et al. Seizures and encephalitis in myelin oligodendrocyte glycoprotein IgG disease vs aquaporin 4 IgG disease. JAMA Neurol. 2018;75:65–71. doi: 10.1001/jamaneurol.2017.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ogawa R, Nakashima I, Takahashi T, Kaneko K, Akaishi T, Takai Y, et al. MOG antibody-positive, benign, unilateral, cerebral cortical encephalitis with epilepsy. Neurol Neuroimmunol Neuroinflamm. 2017;4:e322. doi: 10.1212/NXI.0000000000000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang JJ, Jaunmuktane Z, Mummery C, Brandner S, Leary S, Trip SA. Inflammatory demyelination without astrocyte loss in MOG antibody-positive NMOSD. Neurology. 2016;87:229–231. doi: 10.1212/WNL.0000000000002844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wingerchuk DM, Banwell B, Bennett JL, Cabre P, Carroll W, Chitnis T, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85:177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Hyun JW, Woodhall MR, Oh YM, Lee JE, Jung JY, et al. Refining cell-based assay to detect MOG-IgG in patients with central nervous system inflammatory diseases. Mult Scler Relat Disord. 2020;40:101939. doi: 10.1016/j.msard.2020.101939. [DOI] [PubMed] [Google Scholar]
- 20.Hennes EM, Baumann M, Schanda K, Anlar B, Bajer-Kornek B, Blaschek A, et al. Prognostic relevance of MOG antibodies in children with an acquired demyelinating syndrome. Neurology. 2017;89:900–908. doi: 10.1212/WNL.0000000000004312. [DOI] [PubMed] [Google Scholar]
- 21.Peschl P, Bradl M, Höftberger R, Berger T, Reindl M. Myelin oligodendrocyte glycoprotein: deciphering a target in inflammatory demyelinating diseases. Front Immunol. 2017;8:529. doi: 10.3389/fimmu.2017.00529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Langer-Gould A, Zhang JL, Chung J, Yeung Y, Waubant E, Yao J. Incidence of acquired CNS demyelinating syndromes in a multiethnic cohort of children. Neurology. 2011;77:1143–1148. doi: 10.1212/WNL.0b013e31822facdd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hino-Fukuyo N, Haginoya K, Nakashima I, Sato DK, Takahashi T, Misu T, et al. Clinical features and long-term outcome of a group of Japanese children with inflammatory central nervous system disorders and seropositivity to myelin-oligodendrocyte glycoprotein antibodies. Brain Dev. 2015;37:849–852. doi: 10.1016/j.braindev.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 24.Jarius S, Ruprecht K, Kleiter I, Borisow N, Asgari N, Pitarokoili K, et al. MOG-IgG in NMO and related disorders: a multicenter study of 50 patients. Part 2: epidemiology, clinical presentation, radiological and laboratory features, treatment responses, and long-term outcome. J Neuroinflammation. 2016;13:280. doi: 10.1186/s12974-016-0718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Renoux C, Vukusic S, Mikaeloff Y, Edan G, Clanet M, Dubois B, et al. Natural history of multiple sclerosis with childhood onset. N Engl J Med. 2007;356:2603–2613. doi: 10.1056/NEJMoa067597. [DOI] [PubMed] [Google Scholar]
- 26.Waldman A, Ness J, Pohl D, Simone IL, Anlar B, Amato MP, et al. Pediatric multiple sclerosis: clinical features and outcome. Neurology. 2016;87(9 Suppl 2):S74–S81. doi: 10.1212/WNL.0000000000003028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jurynczyk M, Messina S, Woodhall MR, Raza N, Everett R, Roca-Fernandez A, et al. Clinical presentation and prognosis in MOG-antibody disease: a UK study. Brain. 2017;140:3128–3138. doi: 10.1093/brain/awx276. [DOI] [PubMed] [Google Scholar]
- 28.Fujimori J, Takai Y, Nakashima I, Sato DK, Takahashi T, Kaneko K, et al. Bilateral frontal cortex encephalitis and paraparesis in a patient with anti-MOG antibodies. J Neurol Neurosurg Psychiatry. 2017;88:534–536. doi: 10.1136/jnnp-2016-315094. [DOI] [PubMed] [Google Scholar]