Abstract
Background and Purpose
To determine whether the postcessation weight gain modifies the protective effect of smoking on the development of Parkinson's disease (PD).
Methods
This nationwide cohort study included 3,908,687 Korean males aged ≥40 years who underwent at least 2 health checkups biennially between 2009 and 2015. They were grouped into current smokers; quitters with body mass index (BMI) increase, maintenance, and decrease; and never smokers. The occurrence of incident PD was tracked, and Cox proportional-hazard models were used to adjust for potential confounding factors. We also analyzed the impact of weight change regardless of smoking status in the study population.
Results
There were 6,871 incident PD cases observed during the 13,059,208 person-years of follow-up. The overall risk of PD was significantly lower in quitters than in never smokers [hazard ratio (HR)=0.78, 95% confidence interval (CI)=0.70–0.86]. The risk of PD was still lower in quitters with BMI increase (HR=0.80, 95% CI=0.65–0.98) and in those with BMI maintenance (HR=0.77, 95% CI=0.68–0.87). This tendency was also observed in quitters with BMI decrease (HR=0.76, 95% CI=0.55–1.06), although it was not as robust as in the other two groups. With respect to weight change alone, BMI increase (HR=1.10, 95% CI=1.02–1.18) but not BMI decrease (HR=1.06, 95% CI=0.98–1.14) significantly increased the PD risk compared to BMI maintenance.
Conclusions
Postcessation weight gain in males did not offset the protective impact of smoking on PD development, although overall weight gain predicted an increased risk of PD.
Keywords: Parkinson disease, smoking cessation, weight gain, body mass index, risk factor
INTRODUCTION
Parkinson's disease (PD) is the second-most-common neurodegenerative disorder, and its prevalence is increasing.1 PD affected 2.5 million individuals globally in 1990, increasing to 6.1 million in 20162 and it is predicted to be approximately 9 million by 2030.3 The increasing prevalence of PD is associated with higher morbidity, mortality, and healthcare costs.4
While the etiology of PD remains poorly understood, numerous studies have shown that environmental factors play key roles in its pathogenesis.5 Cigarette smoking is suggested to be one of the main protective factors for PD.6,7,8,9 The risk-lowering effect of smoking on PD has been observed even in ex-smokers. A meta-analysis found that the risk of PD was 58% lower in current smokers and 24% lower in ex-smokers than in never smokers.10
The prevalence of smoking cessation is increasing gradually both in Korea11 and globally.12 Smoking cessation is associated with substantial health benefits for smokers, but subsequent weight gain is of concern in those who attempt to quit smoking.13 Postcessation weight gain usually occurs within the first few months after quitting smoking, and sometimes persists over time.14 Weight gain negatively affects various metabolic conditions of the body,15,16 which might increase the risk of PD because changes induced by weight gain are related to the pathogenesis of PD.4,17 Thus, we hypothesized that weight gain following smoking cessation offsets the benefit of smoking on PD risk. However, it is unknown how postcessation weight gain is expected to modify the risk of PD in ex-smokers.
This study investigated changes in PD risk according to postcessation weight change using nationwide population-based cohort data obtained by the Korean National Health Insurance Service (KNHIS). To determine the overall risk-modification effect more precisely, we also analyzed the overall PD risk according to weight change regardless of smoking status in our study cohort.
METHODS
Study design and population
The KNHIS collects all medical records of the entire population in Korea. Details of the standardized protocol for data acquisition by the KNHIS have been published elsewhere.18 Korean adults aged 40 years or above are required to participate in the National Health Screening Program (NHSP) at least biennially, and all NHSP data are entered into the KNHIS database. The NHSP includes a self-reported health-survey questionnaire, measurements of height, weight, and blood pressure, and blood and urine tests.
The present analysis included males aged 40 years or older for whom complete data were available on the smoking status at a first health checkup performed between 2009 and 2013, and also a second health checkup with a 2-year interval. We did not consider female individuals in this study due to the very low proportion of females who reported quitting smoking between the first and second health checkups. We excluded subjects whose records indicated 1) a PD diagnosis before the follow-up, 2) the development of incident PD or death during the 1-year lag period, 3) that they were exsmokers at the first health checkup, or 4) that they had missing data for at least one variable. We determined the date of the second health checkup as the baseline and tracked the eligible subjects until the date of death or December 31, 2017.
This study was approved by the Institutional Review Board (IRB) at the Seoul Metropolitan Government-Seoul National University Boramae Medical Center (IRB No. 07-2018-14/062). The requirement to obtain written informed consent was waived because the KNHIS database was constructed after anonymization in accordance with a strict legal confidentiality guideline.
Key variables
We collected data on age, smoking status, alcohol consumption, physical activity, height, weight, blood glucose, total serum cholesterol, and the medical history of diabetes mellitus for all participants at the time of the second health examination. All blood samples were obtained in the fasting state. The body mass index (BMI) was calculated by dividing the weight in kilograms by the height in meters squared. Regular exercise was defined as ≥20 minutes of vigorous-intensity physical activity on ≥3 days per week or ≥30 minutes of moderate-intensity physical activity on ≥5 days per week. For alcohol consumption, the data on the frequency of drinking per week and the amount of alcohol consumed on each occasion were transformed into the amount of alcohol consumed per day, as reported previously.19 Then, the alcohol consumption was categorized into nondrinking, moderate drinking (<30 grams per day), and heavy drinking (≥30 grams per day).
BMI increase was defined as a change of more than +5% in BMI at the second health checkup compared to the first health checkup.20 Similarly, we defined BMI maintenance as a BMI change between −5% and +5%, and BMI decrease as a BMI change of more than −5%. The participants were then categorized into the following five groups: current smokers; quitters with BMI increase, maintenance, and decrease; and never smokers. To investigate the effect of weight change itself regardless of the smoking status on PD risk, the participants were also separately categorized into the three groups of BMI increase, maintenance, and decrease.
The KNHIS database manages claims using the sixth edition of the Korean Classification of Disease, which is a modified version of the tenth edition of the International Classification of Diseases (ICD-10).21 Because the medical expenses of PD patients are legally supported by the benefit expansion policy in Korea, all PD cases should be registered in the government system. In this study, incident PD was defined as being present if both the ICD-10 code for PD (G20) and the registration for the governmental benefit expansion were satisfied during the follow-up period in individuals who did not have PD at baseline.
Statistical analysis
Statistical calculations were performed using SAS (version 9.4, SAS Institute, Cary, NC). We used the Kaplan-Meier method to compare the cumulative incidence of PD between the groups. Multivariable Cox proportional-hazards models were used to assess the adjusted hazard ratios (HRs) and 95% confidence intervals (CIs) for the risk of PD. The considered covariates included age, BMI, alcohol consumption (nondrinking, moderate drinking, or heavy drinking), regular exercise (yes or no), blood glucose, total cholesterol, and medical history of diabetes mellitus (yes or no). The HRs for incident PD according to weight change itself were additionally adjusted for smoking status (never smoker, ex-smoker, or current smoker). All statistical tests were two-tailed, and a p value of <0.05 was considered statistically significant.
RESULTS
From 2009 to 2015, 6,223,562 males aged 40 years or older underwent biennial health checkups. Of these, 10,859 males were diagnosed with PD before the follow-up period and 2,061,379 were ex-smokers at the first health examination. Additionally, 2,411 males were newly diagnosed with PD, 16,102 died during the 1-year lag period, and 224,124 had missing data for any variable. Thus, 3,908,687 males were finally included in this study. We identified 6,871 incident PD cases during 13,059,208 person-years of follow-up.
At the second health checkup, 9% of the participants (n=344,173) reported that they had quit smoking since the first health checkup. Among the quitters, 231,594 (67%) had no significant BMI change, whereas 89,139 (26%) experienced BMI increase and 23,440 (7%) experienced BMI decrease. The general characteristics of the participants are summarized in Table 1.
Table 1. Baseline characteristics of the study participants.
| Variable | Never smokers | Smoking cessation | |||
|---|---|---|---|---|---|
| Quitters with BMI increase | Quitters with BMI maintenance | Quitters with BMI decrease | Current smokers | ||
| Number of cases | 1,504,789 | 89,139 | 231,594 | 23,440 | 2,059,725 |
| Age, years | 56.0±11.5 | 51.6±9.7 | 52.4±9.8 | 54.3±11.1 | 51.3±9.4 |
| BMI, kg/m2 | 24.3±2.9 | 25.1±3.0 | 24.6±2.9 | 23.1±3.0 | 24.1±3.1 |
| Change in BMI, kg/m2 | 0 [-0.65 to 0.68] | 1.77 [1.38 to 2.29] | 0.28 [-0.33 to 0.69] | -1.75 [-2.34 to -1.37] | 0 [-0.65 to 0.68] |
| Fasting blood glucose, mg/dL | 102.0±24.5 | 102.9±23.1 | 104.3±27.5 | 107.8±39.5 | 103.2±27.9 |
| Serum total cholesterol, mg/dL | 193.1±36.0 | 204.5±38.6 | 199.1±37.8 | 189.2±38.9 | 198.0±37.1 |
| History of diabetes mellitus | 204,924 (13.6) | 10,611 (11.9) | 35,576 (15.4) | 4,985 (21.3) | 290,842 (14.1) |
| Regular exercise* | 884,948 (58.8) | 54,053 (60.6) | 150,260 (64.9) | 14,492 (61.8) | 1,162,153 (56.4) |
| Alcohol consumption† | |||||
| Nondrinking | 769,612 (51.1) | 26,195 (29.4) | 64,476 (27.8) | 9,242 (39.4) | 512,185 (24.9) |
| Moderate drinking | 655,685 (43.6) | 50,901 (57.1) | 137,619 (59.4) | 11,884 (50.7) | 1,225,750 (59.5) |
| Heavy drinking | 79,492 (5.3) | 12,043 (13.5) | 29,499 (12.7) | 2,314 (9.9) | 321,790 (15.6) |
| Cigarettes smoked per day | |||||
| <10 | - | 6,618 (7.4) | 30,054 (13.0) | 3,215 (13.7) | 211,261 (10.3) |
| 0–19 | - | 29,973 (33.6) | 91,883 (39.7) | 8,889 (37.9) | 814,597 (39.6) |
| 20–29 | - | 41,853 (47.0) | 91,715 (39.6) | 9,488 (40.5) | 895,767 (43.5) |
| ≥30 | - | 10,695 (12.0) | 17,942 (7.8) | 1,848 (7.9) | 138,100 (6.7) |
| Smoking duration | |||||
| <10 years | - | 4,456 (5.0) | 17,618 (7.6) | 1,891 (8.1) | 87,391 (4.2) |
| 10–19 years | - | 14,043 (15.8) | 42,849 (18.5) | 4,466 (19.1) | 262,476 (12.7) |
| 20–29 years | - | 39,231 (44.0) | 92,311 (39.9) | 8,443 (36.0) | 922,807 (44.8) |
| ≥30 years | - | 31,409 (35.2) | 78,816 (34.0) | 8,640 (36.9) | 787,051 (38.2) |
| Pack-years of smoking | |||||
| <10 | - | 13,963 (15.7) | 55,085 (23.8) | 5,768 (24.6) | 331,736 (16.1) |
| 10–19 | - | 23,749 (26.6) | 66,360 (28.7) | 6,304 (26.9) | 616,685 (29.9) |
| 20–29 | - | 23,360 (26.2) | 51,070 (22.1) | 4,991 (21.3) | 557,834 (27.1) |
| ≥30 | - | 28,067 (31.5) | 59,079 (25.5) | 6,377 (27.2) | 553,470 (26.9) |
Data are n (%), median [interquartile range], or mean±standard-deviation values.
*Regular exercise means ≥20 minutes of vigorous-intensity physical activity ≥3 days per week or ≥30 minutes of moderate-intensity physical activity ≥5 days per week, †Daily alcohol consumption was categorized as follows: heavy drinker, ≥30 g/day; moderate drinker, <30 g/day; and nondrinker.
BMI: body mass index.
Kaplan-Meier estimates showed that the incidence of PD did not differ significantly between the quitters with BMI increase and those with BMI maintenance (p=0.1290) (Fig. 1), while it was higher in quitters with BMI decrease than in those with BMI maintenance (p<0.0001).
Fig. 1. Kaplan-Meier estimates for the probability of incident PD up to 7 years according to postcessation weight change. BMI: body mass index, PD: Parkinson's disease.
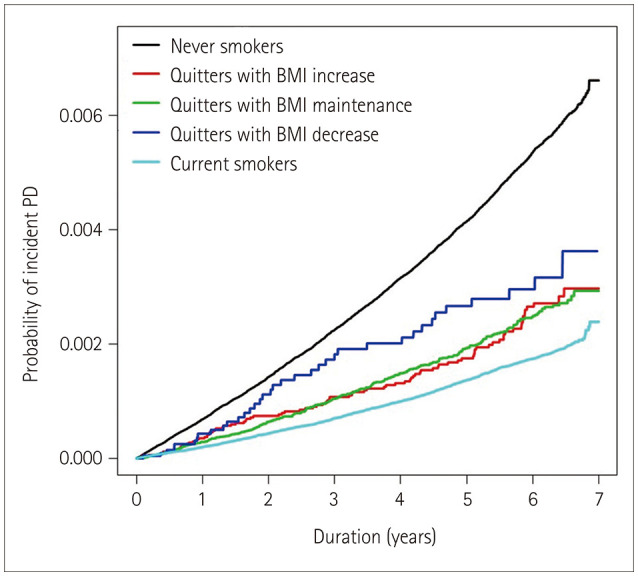
The results of the Cox regression analyses are presented in Table 2. The overall risk of PD was significantly lower in quitters than in never smokers (adjusted HR=0.78, 95% CI= 0.70–0.86, p<0001). The decreased risk for PD associated with smoking persisted among quitters with BMI increase (adjusted HR=0.80, 95% CI=0.65–0.98, p=0.0315) compared with the never smokers. This impact was similar in quitters with BMI maintenance (adjusted HR=0.77, 95% CI=0.68–0.87, p<0001). There was also a trend toward a risk-lowering effect of smoking in quitters with BMI decrease (adjusted HR=0.76, 95% CI=0.55–1.06, p=0.1026), but it was less robust with a wide 95% CI.
Table 2. Incidence rates, HRs, and associated 95% CIs for Parkinson's disease according to postcessation weight change.
| Never smokers | Smoking cessation | ||||
|---|---|---|---|---|---|
| Quitters with BMI increase | Quitters with BMI maintenance | Quitters with BMI decrease | Current smokers | ||
| Total number | 1,504,789 | 89,139 | 231,594 | 23,440 | 2,059,725 |
| Events | 4,587 | 94 | 276 | 37 | 1,877 |
| Person-years | 5,324,944 | 234,593 | 702,275 | 71,848 | 6,725,548 |
| Incidence rate* | 0.86 | 0.40 | 0.39 | 0.51 | 0.28 |
| Crude HR (95% CI) | 1 | 0.47 (0.38–0.58) | 0.46 (0.41–0.52) | 0.61 (0.44–0.84) | 0.33 (0.31–0.34) |
| p | NA | <0.0001 | <0.0001 | 0.0025 | <0.0001 |
| Adjusted HR† (95% CI) | 1 | 0.80 (0.65–0.98) | 0.77 (0.68–0.87) | 0.76 (0.55–1.06) | 0.61 (0.57–0.64) |
| p | NA | 0.0315 | <0.0001 | 0.1026 | <0.0001 |
*Incidence rates are expressed per 1,000 person-years, †Adjusted for age, BMI, alcohol consumption, physical activity, fasting blood glucose, total cholesterol, and the presence of diabetes mellitus.
BMI: body mass index, CI: confidence interval, HR: hazard ratio.
With respect to the overall impact of the 2-year weight change in this study population, 3,023,791 (77%) had no significant BMI change, 393,552 (10%) had BMI increase, and 491,344 (13%) had BMI decrease. BMI increase regardless of smoking status was expected to increase the risk of PD relative to BMI maintenance (adjusted HR=1.10, 95% CI= 1.02–1.18, p=0.0097). By contrast, BMI decrease did not significantly change the risk of PD development (adjusted HR= 1.06, 95% CI=0.98–1.14, p=0.1550) (Table 3).
Table 3. Incidence rates, HRs, and associated 95% CIs for Parkinson's disease according to weight change.
| Total number | Events | Person-years | Incidence rate* | Crude HR (95% CI) | p | Adjusted HR† (95% CI) | p | |
|---|---|---|---|---|---|---|---|---|
| BMI increase | 393,552 | 995 | 1,355,717 | 0.73 | 1.49 (1.39–1.59) | <0.0001 | 1.10 (1.02–1.18) | 0.0097 |
| BMI maintenance | 3,023,791 | 5,066 | 10,203,312 | 0.50 | 1 | NA | 1 | NA |
| BMI decrease | 491,344 | 810 | 1,500,179 | 0.54 | 1.09 (1.01–1.18) | 0.0199 | 1.06 (0.98–1.14) | 0.1550 |
*Incidence rates are expressed per 1,000 person-years, †Adjusted for age, BMI, alcohol consumption, physical activity, fasting blood glucose, total cholesterol, the presence of diabetes mellitus, and smoking status.
BMI: body mass index, CI: confidence interval, HR: hazard ratio.
DISCUSSION
To the best of our knowledge, this study is the first to perform a big-data analysis with the aim of determining the impact of postcessation weight gain on PD risk. Our data showed that approximately 26% of quitters gained weight, which was more than twofold higher than the proportion for all participants. This finding supports the notion that smoking cessation is associated with weight gain. Even though weight gain itself was shown to increase the risk of PD in the overall population, individuals with postcessation weight gain were expected to show a persistent protective effect from smoking on the development of PD.
The negative metabolic effects of excess adiposity include the development of insulin resistance, oxidative stress, and enhanced inflammation,22 and these changes are regarded as pathomechanisms underlying PD.23,24 Weight gain also promotes such adverse metabolic conditions,15,16 which may explain the significant relationship between weight gain and PD risk found in the current study. However, there was only a minimal difference in the risk-lowering effect of smoking on PD between quitters with weight gain and those with weight maintenance. These findings indicate that smoking may be a much more powerful factor than weight gain for PD development. Alternatively, heavy smokers are prone to develop postcessation weight gain, which is the leading cause of resuming smoking.13 Thus, these smoking behaviors might have affected the results of our analyses. However, the potential protective effect from smoking was also observed in the other quitter groups, indicating that postcessation weight change is not expected to significantly alter the PD risk in individuals with a smoking history. On the other hand, we cannot exclude the possibility that our follow-up duration was too short to fully assess the effect of postcessation weight gain on PD risk.
We observed a wide 95% CI for the estimated HR in quitters with weight loss. This result is probably attributable to their relatively small number, which limits the ability to drawn definitive conclusions. However, weight loss could occur early in the disease course or even in the prodromal stage of PD,25 suggesting that individuals with undiagnosed PD or PD in the prodromal stage are included in quitters with weight loss. This finding might be partly responsible for the weakening of the protective effect of smoking in quitters with weight loss. Further epidemiological studies are needed to clarify this issue.
Several potential limitations should be considered when interpreting the current data. First, although the first and second health checkups provide information on smoking cessation, the exact date when each person stopped smoking between the checkups was not available in the KNHIS database. In addition, any changes in smoking habits after the second health checkup were not accounted for. However, this study focused on providing data on how clinicians can predict PD risk from a single event of quitting smoking and postcessation weight change, so our results should be interpreted with such a clinical meaning. Second, the KNHIS database depends on clinicians assigning diagnostic codes for PD. A PD diagnosis in clinical practice is not generally followed by a pathological confirmation, and so misdiagnoses of PD might be present. Furthermore, since the diagnosis of parkinsonism is also classified as the G20 code, it is possible that atypical parkinsonian disorders such as the parkinsonian subtype of multiple-system atrophy or progressive supranuclear palsy with predominant parkinsonism were included in this code. However, considering that such cases are relatively rare compared to PD, this bias is less likely to be significant in our big data set. Third, there is probably a delay between the onset and diagnosis of PD. However, our sensitivity analysis using a 2-year lag period produced similar results (Supplementary Table 1 in the online-only Data Supplement), making bias with temporal associations unlikely. Finally, this study only analyzed from male individuals, and so further investigation is warranted for female individuals.
In conclusion, this national cohort big-data analysis has demonstrated that the protective effect of smoking on PD development tends to persist in males regardless of their postcessation weight gain or maintenance. However, our findings should be interpreted with caution due to the shortness of the follow-up. Further research with a longer follow-up could help to confirm the impact of postcessation weight change on the risk of PD.
Acknowledgements
We thank the KNHIS for providing access to their data (No. NHIS-2018-1-442).
Footnotes
- Conceptualization: Ryul Kim, Kyungdo Han, Jee-Young Lee.
- Data curation: Kyungdo Han.
- Formal analysis: Kyungdo Han.
- Investigation: Ryul Kim, Kyungdo Han, Jee-Young Lee.
- Methodology: Ryul Kim, Kyungdo Han, Jee-Young Lee.
- Supervision: Jee-Young Lee.
- Writing—original draft: Ryul Kim.
- Writing—review & editing: Dallah Yoo, Yu Jin Jung, Kyungdo Han, Jee-Young Lee.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Supplementary Material
The online-only Data Supplement is available with this article at https://doi.org/10.3988/jcn.2020.16.3.455.
Incidence rates, HRs, and associated 95% CIs for Parkinson's disease when using a 2-year lag period
References
- 1.Kalia LV, Lang AE. Parkinson's disease. Lancet. 2015;386:896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 Parkinson's Disease Collaborators. Global, regional, and national burden of Parkinson's disease, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17:939–953. doi: 10.1016/S1474-4422(18)30295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, et al. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- 4.Nam GE, Kim SM, Han K, Kim NH, Chung HS, Kim JW, et al. Metabolic syndrome and risk of Parkinson disease: a nationwide cohort study. PLoS Med. 2018;15:e1002640. doi: 10.1371/journal.pmed.1002640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. 2016;15:1257–1272. doi: 10.1016/S1474-4422(16)30230-7. [DOI] [PubMed] [Google Scholar]
- 6.Hernan MA, Zhang SM, Rueda-deCastro AM, Colditz GA, Speizer FE, Ascherio A. Cigarette smoking and the incidence of Parkinson's disease in two prospective studies. Ann Neurol. 2001;50:780–786. doi: 10.1002/ana.10028. [DOI] [PubMed] [Google Scholar]
- 7.Thacker EL, O'Reilly EJ, Weisskopf MG, Chen H, Schwarzschild MA, McCullough ML, et al. Temporal relationship between cigarette smoking and risk of Parkinson disease. Neurology. 2007;68:764–768. doi: 10.1212/01.wnl.0000256374.50227.4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen H, Huang X, Guo X, Mailman RB, Park Y, Kamel F, et al. Smoking duration, intensity, and risk of Parkinson disease. Neurology. 2010;74:878–884. doi: 10.1212/WNL.0b013e3181d55f38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim R, Yoo D, Jung YJ, Han K, Lee JY. Sex differences in smoking, alcohol consumption, and risk of Parkinson's disease: a nationwide cohort study. Parkinsonism Relat Disord. 2020;71:60–65. doi: 10.1016/j.parkreldis.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Li W, Liu G, Shen X, Tang Y. Association between cigarette smoking and Parkinson's disease: a meta-analysis. Arch Gerontol Geriatr. 2015;61:510–516. doi: 10.1016/j.archger.2015.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Korea Centers for Disease Control and Prevention; Ministry of Health and Welfare. 2014 National Health Statistics. Sejong: Ministry of Health and Welfare; 2015. [Google Scholar]
- 12.Bilano V, Gilmour S, Moffiet T, d'Espaignet ET, Stevens GA, Commar A, et al. Global trends and projections for tobacco use, 1990-2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet. 2015;385:966–976. doi: 10.1016/S0140-6736(15)60264-1. [DOI] [PubMed] [Google Scholar]
- 13.Harris KK, Zopey M, Friedman TC. Metabolic effects of smoking cessation. Nat Rev Endocrinol. 2016;12:299–308. doi: 10.1038/nrendo.2016.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Filozof C, Fernández Pinilla MC, Fernandez-Cruz A. Smoking cessation and weight gain. Obes Rev. 2004;5:95–103. doi: 10.1111/j.1467-789X.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- 15.Nam GE, Park YG, Han K, Kim MK, Koh ES, Kim ES, et al. BMI, weight change, and dementia risk in patients with new-onset type 2 diabetes: a nationwide cohort study. Diabetes Care. 2019;42:1217–1224. doi: 10.2337/dc18-1667. [DOI] [PubMed] [Google Scholar]
- 16.Park S, Jeon SM, Jung SY, Hwang J, Kwon JW. Effect of late-life weight change on dementia incidence: a 10-year cohort study using claim data in Korea. BMJ Open. 2019;9:e021739. doi: 10.1136/bmjopen-2018-021739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santiago JA, Potashkin JA. Shared dysregulated pathways lead to Parkinson's disease and diabetes. Trends Mol Med. 2013;19:176–186. doi: 10.1016/j.molmed.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Seong SC, Kim YY, Khang YH, Park JH, Kang HJ, Lee H, et al. Data resource profile: the national health information database of the National Health Insurance Service in South Korea. Int J Epidemiol. 2017;46:799–800. doi: 10.1093/ije/dyw253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi YJ, Lee DH, Han KD, Kim HS, Yoon H, Shin CM, et al. The relationship between drinking alcohol and esophageal, gastric or colorectal cancer: a nationwide population-based cohort study of South Korea. PLoS One. 2017;12:e0185778. doi: 10.1371/journal.pone.0185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dehghani P, Habib B, Windle SB, Roy N, Old W, Grondin FR, et al. Smokers and postcessation weight gain after acute coronary syndrome. J Am Heart Assoc. 2017;6:e004785. doi: 10.1161/JAHA.116.004785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee SH, Lee SJ, Kim YJ. Region-based analysis of prevalence and incidence of Parkinson’s disease: analysis of the national sample cohort in South Korea. J Clin Neurol. 2018;14:478–486. doi: 10.3988/jcn.2018.14.4.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greenberg AS, Obin MS. Obesity and the role of adipose tissue in inflammation and metabolism. Am J Clin Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 23.Aviles-Olmos I, Limousin P, Lees A, Foltynie T. Parkinson's disease, insulin resistance and novel agents of neuroprotection. Brain. 2013;136:374–384. doi: 10.1093/brain/aws009. [DOI] [PubMed] [Google Scholar]
- 24.Hassanzadeh K, Rahimmi A. Oxidative stress and neuroinflammation in the story of Parkinson's disease: could targeting these pathways write a good ending? J Cell Physiol. 2018;234:23–32. doi: 10.1002/jcp.26865. [DOI] [PubMed] [Google Scholar]
- 25.Searles Nielsen S, Warden MN, Camacho-Soto A, Willis AW, Wright BA, Racette BA. A predictive model to identify Parkinson disease from administrative claims data. Neurology. 2017;89:1448–1456. doi: 10.1212/WNL.0000000000004536. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Incidence rates, HRs, and associated 95% CIs for Parkinson's disease when using a 2-year lag period


