Abstract
Background and Purpose
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is the most-common form of autoimmune encephalitis, but its early diagnosis is challenging. This study aimed to identify the risk factors for a poor prognosis in anti-NMDAR encephalitis and construct a prognostic composite score for obtaining earlier predictions of a poor prognosis.
Methods
We retrospectively analyzed the clinical data, laboratory indexes, imaging findings, and electroencephalogram (EEG) data of 60 patients with anti-NMDAR encephalitis. The modified Rankin Scale (mRS) scores of patients were collected when they were discharged from the hospital. The mRS scores were used to divide the patients into two groups, with mRS scores of 3–6 defined as a poor prognosis. Logistic regression analysis was used to analyze independent risk factors related to a poor prognosis.
Results
This study found that 23 (38.3%) and 37 (61.7%) patients had good and poor prognoses, respectively. Logistic regression analysis showed that age, disturbance of consciousness at admission, and ≥50% slow waves on the EEG were significantly associated with patient outcomes. An age, consciousness, and slow waves (ACS) composite score was constructed to predict the prognosis of patients with anti-NMDAR encephalitis at an early stage based on regression coefficients.
Conclusions
Age, disturbance of consciousness at admission, and ≥50% slow waves on the EEG were independent risk factors for a poor prognosis. The ACS prognostic composite score could play a role in facilitating early predictions of the prognosis of anti-NMDAR encephalitis.
Keywords: Anti-NMDAR encephalitis, electroencephalogram, prognostic composite score
INTRODUCTION
Anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis is the most-common form of autoimmune encephalitis. The popularization of autoimmune encephalitis antibody detection has resulted in its reported worldwide incidence increasing.1 Its mechanism is well known, although the pathogenesis remains unclear.2 The populations in certain geographic areas appear to have a genetic or racial susceptibility.3,4 This type of encephalitis may be complicated with tumors, and some cases may be secondary to virus infection in the central nervous system (CNS).5 The anti-NMDAR antibody binds to the NR1 subunits in NMDARs on the cell surface, which results in patients rapidly developing severe CNS symptoms such as mental behavior abnormalities, speech dysfunction, seizures, movement disorders, disturbance of consciousness, and autonomic dysfunction.6 Approximately 75% of patients with anti-NMDAR encephalitis need intensive care in a neurology intensive care unit (NICU).7 Admission to the NICU is associated with longer hospital stays and higher hospitalization expenses, which imposes high mental and economic stresses on the patients' families and reduces patient compliance, thus affecting further treatment and the prognosis. Although studies have shown that 81% of patients have a good prognosis after 2 years of immunotherapy and rehabilitation exercise,7 most of them will have neurological deficits that affect their quality of life for a long time, and 5–11% of the patients will die.8 Therefore, identifying early risk factors that affect the prognosis of anti-NMDAR encephalitis would facilitate the formulation of supportive care and individualized treatment regimens for patients with different clinical outcomes, and reduce the incidence rates of sequelae and death.
Many previous studies have found risk factors based on clinical data and laboratory indexes that may affect the prognosis of anti-NMDAR encephalitis.8,9,10,11 Other studies have focused on the predictive value of electroencephalogram (EEG) data for the prognosis of anti-NMDAR encephalitis.12,13 Each type of study has advantages and disadvantages, but a single indicator has low sensitivity and specificity for predicting prognoses. Moreover, no previous study has combined clinical data, laboratory indexes, imaging examinations, and neuroelectrophysiology data in a multimodal analysis of the prognosis of this disease, or to construct a prognostic composite score.
This study retrospectively enrolled 60 patients with anti-NMDAR encephalitis to analyze their clinical, EEG, laboratory, and imaging data. The clinical features of the disease and the risk factors affecting prognoses were analyzed. A prognostic composite score for anti-NMDAR encephalitis was established according to the identified risk factors to provide a theoretical basis for individualized treatment in clinical practice.
METHODS
Study subjects
The 60 study patients were diagnosed with anti-NMDAR encephalitis in The First Affiliated Hospital of Chongqing Medical University from January 2014 to February 2019. Fifty-eight of these patients had a definitive diagnosis of anti-NMDAR encephalitis based on the following14: 1) at least one of the six main symptoms of behavioral abnormalities or cognitive impairment; speech dysfunction (pressured speech, verbal reduction, or mutism); seizures; movement disorder, dyskinesias, or rigidity/abnormal postures; disturbance of consciousness; or autonomic dysfunction or central hypoventilation; 2) positive for cerebrospinal fluid (CSF) anti-NMDAR (GluN1 subunit) IgG antibodies; and 3) reasonable exclusion of other disorders. The other two patients were negative for CSF and serum autoimmune encephalitis antibodies, but were included in this study because their clinical manifestations and imaging, CSF, and EEG data all met the diagnostic criteria for probable anti-NMDAR encephalitis,14 and also their symptoms improved significantly after immunotherapy. The exclusion criteria included the following: 1) presence of intracranial infection or other types of autoimmune encephalitis, 2) refusal from family members for the patient to participate in the study, and 3) incomplete data. This study was approved by the Medical Ethics Committee of The First Affiliated Hospital of Chongqing Medical University (chiCTR1800014324). All subjects included in the study provided informed consent by signing an informed-consent form.
Data collection
This study utilized a retrospective investigative method, with all data collected by two experienced doctors. The collected data included the following:
1. Demographics, including age and sex.
2. Clinical features, including initial symptoms, clinical manifestations, consciousness level at admission, presence of tumors, admission to the NICU, use of mechanical ventilation, interval between onset and treatment, immunotherapy, and remaining symptoms at discharge.
3. Laboratory indexes and imaging examination results, including serum albumin levels, immune indexes (antinuclear, antibody, antineutrophil cytoplasmic antibodies, thyroid-related antibodies, and humoral immunity index), opening pressure in lumbar puncture, white blood cell count, protein in CSF, serum and CSF anti-NMDAR antibody titers [classified into strongly positive (+++), positive (++), weakly positive (+), and negative (−)], and brain magnetic resonance imaging (MRI).
4. EEG data, including the posterior dominant rhythm (PDR), poorly sustained PDR, extreme delta brush (EDB), epileptiform discharges, status epilepticus, proportion of slow waves, and slow-wave amplitudes.
5. Outcome indexes, including remaining symptoms at discharge, length of hospital stay, hospitalization expenses, and time interval between disturbance of consciousness and awakening.
All of the patients were in the initial stage of onset (within 14 days after onset).15
A 32-channel EEG monitoring system (Neuron-Spectrum-5, Neurosoft Company, Ivanovo, Russia) was used for continuous EEG monitoring via 16 recording electrodes placed on the scalp in compliance with the international 10–20 system. The patients received 1 to 10 EEG examinations. The selected EEG data were the first EEG records during the first 14 days after onset (the initial stage). All EEG recordings were performed during the same daytime period (2–5 p.m.) and without sedation. Monitoring lasted for at least 2 hours so that each record contained a complete sleep cycle. The use of antiepileptic drugs and sedative drugs was recorded simultaneously. The EEG was assessed visually by at least two certified neurophysiologists, and any inconsistent conclusions were excluded.
Assessment of prognosis
All of the patients were followed up for prognosis after being discharged from the hospital. During the follow-up, two experienced doctors used modified Rankin Scale (mRS) scores to evaluate the recovery of neurological function. The mRS score on discharge was used to divide the patients into good-prognosis and poor-prognosis groups (mRS scores of 0–2 and 3–6, respectively).6
Definition of variables
This study used the Glasgow Coma Scale (GCS) to score the level of consciousness of patients when they were admitted to the hospital, with a GCS score of <15 defined as disturbance of consciousness.16 Slow waves were defined as 4–8 Hz theta waves or 0.5–4 Hz delta waves,17 and slow waves occurring during sleep were not included in the count of the proportion of slow waves. Limotai et al.12 divided PDR into poorly sustained PDR and well-sustained PDR according to the number of slow waves included with the alpha signals in the posterior head region. A poorly sustained PDR was defined as slow-wave activity mixed with a large proportion (≥50% of the recording time) of small-amplitude (<20 µV) to large-amplitude (>50 µV) signals in PDR, noticeable in the T5-O1 and T6-O2 electrodes. Posterior slow waves in adolescents were regarded as physiological waves. Well-sustained PDR was defined when the PDR was completely lacking slow waves or minimally (<50% of the recording time) intermixed with slow waves. Schmitt et al.18 defined EDB as a large amount of beta activity at 20–30 Hz superimposed on the peaks of rhythmic delta activity at 1–3 Hz.
Statistical analyses
Statistical analyses were performed with SAS (version 9.2, SAS Institute, Cary, NC, USA) statistical software. Measurement data are quantified as rates. Chi-square tests and Fisher's exact tests were used for intergroup comparisons. Measurement data conforming to a normal distribution are expressed as mean and standard-deviation values, and t-tests of two independent samples were used for intergroup comparisons. Measurement data with skewed distributions are described as median and interquartile-range values, and the Mann-Whitney U-test was used for intergroup comparisons.
Univariate analysis was applied to identify predictive factors that potentially affect the prognosis. The variables with p<0.05 in the univariate analysis were included in the multifactor logistic regression analysis of the composite score, and binary classification logistic regression was used to analyze the risk factors for patient prognoses. A prognostic composite score was established for anti-NMDAR encephalitis based on the regression coefficient, with 1 point assigned if its absolute value was ≤1, and 2 points assigned for absolute values >1.
The receiver operating characteristic (ROC) curve was used to evaluate the effectiveness of the scale. An area under the ROC curve (AUC) of larger than 0.8 was considered to indicate good diagnostic accuracy. The ROC curve was also used to determine the optimal value in terms of sensitivity and specificity for distinguishing different prognoses. A penalized maximum likelihood estimate was used to separate data in the logistic regression. A probability value of p<0.05 was considered indicative of statistical significance.
RESULTS
Demographic data and univariate analysis
Among the 60 patients with anti-NMDAR encephalitis enrolled in this study, 23 (38.3%) had a good prognosis and 37 (61.7%) had a poor prognosis when they were discharged from the hospital. One patient died of multiple-organ failure during hospitalization. Tables 1 and 2 compare the basic information and clinical characteristics between the two groups. The ages of the included individuals varied from 11 to 60 years, with a median age of 25 years (interquartile range=20–35 years). There was a significant age difference between the two groups (p=0.041). Most (60.0%) of the patients were female, and none of the characteristics differed significantly between the sexes (p=0.665). All of the patients received routine tumor screening, such as abdominal Doppler ultrasonography, chest computed tomography, and measurements of tumor markers. Six (10.0%) female patients were clinically diagnosed as ovarian teratoma, and three (5.0%) patients received tumorectomy at 4 months, 64 days, and 68 days after the onset of disease. All 60 patients received first-line immunotherapy, 19 received methylprednisolone pulse therapy alone (methylprednisolone at 500–1,000 mg/day for five consecutive days, reduced to oral prednisone at 1 mg/kg/day and then slowly decreased until stopped), 6 received intravenous immunoglobulin (IVIg, 0.4 g/kg/day for 5 consecutive days), and 35 received combined corticosteroids and IVIg. No patient received any second-line immunotherapy including rituximab or cyclophosphamide. Azathioprine was applied to three (5.0%) patients.
Table 1. Basic information on the study subjects.
| Variable | Total | Prognosis | Statistical value | p | |
|---|---|---|---|---|---|
| Good (n=23) | Poor (n=37) | ||||
| Age, years | 25 [20–35] | 22 [16–28] | 27 [21–36] | -2.048* | 0.041 |
| Sex | |||||
| Male | 24 (40.0) | 10 (43.5) | 14 (37.8) | 0.188† | 0.665 |
| Female | 36 (60.0) | 13 (56.5) | 23 (62.2) | ||
| Initial symptoms | - | 0.352‡ | |||
| Mental behavioral disorders/cognitive dysfunction | 34 (56.7) | 11 (47.8) | 23 (62.2) | ||
| Speech disorders | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Seizures | 19 (31.6) | 9 (39.1) | 10 (27.0) | ||
| Movement disorders/involuntary movement | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Disturbance of consciousness | 1 (1.7) | 0 (0.0) | 1 (2.7) | ||
| Autonomic dysfunction | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Complicated with tumors | 6 (10.0) | 0 (0.0) | 6 (16.2) | - | 0.073‡ |
| Disturbance of consciousness | 31 (51.7) | 6 (26.1) | 25 (67.6) | 9.773† | 0.002 |
| Immunotherapy | 58 (96.7) | 23 (100.0) | 35 (94.6) | - | 0.519‡ |
| Admission to neurology intensive care unit | 46 (76.7) | 14 (60.9) | 32 (86.5) | 5.203† | 0.023 |
| Mechanical ventilation | 16 (26.7) | 2 (8.7) | 14 (37.8) | 6.160† | 0.013 |
Data are median [interquartile range] or n (%) values.
*Z value in the Mann-Whitney U-test, †χ2, ‡p value in Fisher's exact test.
Table 2. Results from the univariate analysis of clinical, laboratory, imaging, and EEG data of the two groups.
| Variable | Total | Prognosis | Statistical value | p | |
|---|---|---|---|---|---|
| Good (n=23) | Poor (n=37) | ||||
| Age, years | 25 [20–35] | 22 [16–28] | 27 [21–36] | -2.048† | 0.041 |
| Clinical manifestation | |||||
| Mental behavioral disorders/cognitive dysfunction | 56 (93.3) | 19 (82.6) | 37 (100.0) | - | 0.018§ |
| Speech disorders | 25 (41.7) | 9 (39.1) | 16 (43.2) | 0.099‡ | 0.753 |
| Seizures | 40 (66.7) | 15 (65.2) | 25 (67.6) | 0.035‡ | 0.851 |
| Movement disorders/involuntary movement | 31 (51.7) | 9 (39.1) | 22 (59.5) | 2.347‡ | 0.126 |
| Disturbance of consciousness | 31 (51.7) | 6 (26.1) | 25 (67.6) | 9.773‡ | 0.002 |
| Autonomic dysfunction | 32 (53.3) | 8 (34.8) | 24 (64.9) | 5.157‡ | 0.023 |
| Admission to neurology intensive care unit | 46 (76.7) | 14 (60.9) | 32 (86.5) | 5.203‡ | 0.023 |
| Mechanical ventilation | 16 (26.7) | 2 (8.7) | 14 (37.8) | 6.160‡ | 0.013 |
| Interval between onset and immunotherapy, days | 12 [9–26] | 15 [7–23] | 0.024† | 0.981 | |
| Immunotherapy | - | 0.278§ | |||
| Corticosteroids | 19 (31.7) | 10 (43.5) | 9 (23.3) | ||
| IVIg | 6 (10.0) | 2 (8.7) | 4 (10.8) | ||
| Corticosteroids+IVIg | 35 (58.3) | 11 (47.8) | 24 (64.9) | ||
| CSF examination | |||||
| Opening pressure in lumbar puncture, mmH2O | 165.35±65.67 | 166.66±47.92 | -0.089* | 0.929 | |
| Increased white blood cell count | 21 (35.0) | 8 (34.8) | 13 (35.1) | 0.001‡ | 0.978 |
| Increased protein in CSF | 39 (65.0) | 17 (73.9) | 22 (59.5) | 1.302‡ | 0.254 |
| CSF anti-NMDAR antibody titer | -1.086† | 0.278 | |||
| Negative | 2 (3.3) | 0 (0.0) | 2 (5.4) | ||
| Weakly positive | 16 (26.7) | 9 (39.1) | 7 (18.9) | ||
| Positive | 19 (31.7) | 7 (30.4) | 12 (32.4) | ||
| Strongly positive | 23 (38.3) | 7 (30.4) | 16 (43.2) | ||
| Serum anti-NMDAR antibody titer | -0.512† | 0.609 | |||
| Negative | 21 (35.0) | 9 (39.1) | 12 (32.4) | ||
| Weakly positive | 15 (25.0) | 6 (26.1) | 9 (24.3) | ||
| Positive | 20 (33.3) | 6 (26.1) | 14 (37.8) | ||
| Strongly positive | 4 (6.7) | 2 (8.7) | 2 (5.4) | ||
| Biochemical indexes | |||||
| Abnormal immune indexes | 16 (26.7) | 5 (21.7) | 11 (29.7) | 0.463‡ | 0.496 |
| Serum albumin before treatment | 0.093‡ | 0.761 | |||
| ≥40 g/L | 43 (71.7) | 17 (73.9) | 26 (70.3) | ||
| <40 g/L | 17 (28.3) | 6 (26.1) | 11 (29.7) | ||
| Abnormal tumor markers | 8 (13.3) | 3 (13.0) | 5 (13.5) | - | >0.999§ |
| MRI abnormality | 18 (30.0) | 5 (21.7) | 13 (35.1) | 1.212‡ | 0.271 |
| EEG | |||||
| Disappearance of PDR | 32 (53.3) | 6 (10.0) | 26 (43.4) | 11.125‡ | 0.001 |
| Poorly sustained PDR | 23 (38.3) | 12 (52.2) | 11 (29.7) | 3.022‡ | 0.082 |
| Rhythmic delta activity | 20 (33.3) | 3 (13.0) | 17 (45.9) | 6.910‡ | 0.009 |
| Epileptic discharge | 16 (26.7) | 5 (21.7) | 11 (29.7) | 0.463‡ | 0.496 |
| Extreme delta brush | 12 (20.0) | 1 (4.3) | 11 (29.7) | - | 0.020§ |
| Slow waves | |||||
| ≥50% slow waves | 44 (73.3) | 11 (47.8) | 33 (89.2) | 12.409‡ | <0.001 |
| Slow-wave amplitude | -2.072† | 0.038 | |||
| Small | 8 (13.3) | 5 (21.7) | 3 (8.1) | ||
| Moderate | 40 (66.7) | 16 (69.6) | 24 (64.7) | ||
| Large | 12 (20.0) | 2 (8.7) | 10 (27.0) | ||
Data are median [interquartile range], n (%), or mean±standard-deviation values. -, no value available.
*p value in a t-test, †Z value in the Mann-Whitney U-test, ‡χ2, §p value in Fisher's exact test.
CSF: cerebrospinal fluid, EEG: electroencephalogram, NMDAR: N-methyl-D-aspartate receptor, IVIg: intravenous immunoglobulin, PDR: posterior dominant rhythm.
Serum and CSF were collected from all patients and tested for autoimmune encephalitis antibodies, which revealed that 58 (96.7%) patients were positive for anti-NMDAR antibodies in their CSF, while only 39 (65.0%) patients were positive in their serum. At least 1 cranial MRI examination and lumbar puncture were performed in all 60 patients, and at least 1 EEG examination was performed in the initial stage of the disease.
The following evaluated parameters were correlated with a poor prognosis at discharge: the clinical factors of mental behavioral disorders/cognitive dysfunction (p=0.018), disturbance of consciousness (p=0.002), and autonomic dysfunction (p=0.023); admission to the NICU (p=0.023); and mechanical ventilation (p=0.013). The following evaluated parameters were not significantly correlated with the prognosis: various initial symptoms (p=0.352), speech disorders at admission (p=0.753), seizures (p=0.851), movement disorders/involuntary movements (p=0.126), complicated with tumors (p=0.073), the interval between onset to immunotherapy (p=0.981), and different treatment methods (p=0.278). The following laboratory indexes were not significantly correlated with the prognosis: opening pressure in lumbar puncture (p=0.929), white blood cell count (p=0.978), protein in the CSF (p=0.254), anti-NMDAR antibody titers in the serum (p=0.609) and CSF (p=0.278), immune indexes (p=0.496), serum albumin before treatment (p=0.761), and tumor markers (p>0.999).
Forty (66.7%) of the 60 patients underwent cranial MRI enhanced scanning, which revealed cranial MRI abnormalities in 18 (30.0%) patients that mainly involving the bilateral temporal lobe, occipital lobe, parietal lobe, hippocampus, and insula. Different degrees of localized linear enhancement of the meninges and enhancement of the pia mater in the bilateral cerebral hemispheres were seen in the corresponding regions. However, the observed imaging abnormalities did not differ significantly between the groups with different prognoses (p=0.271).
Forty-eight (80.0%) of the 60 patients had abnormal EEG findings, 3 (5.0%) patients received diazepam therapy, and 24 (60.0%) patients received various antiepileptic drugs. Focal and diffuse moderate-to-large-amplitude slow waves were the most-common EEG manifestations. Forty-four (73.3%) patients had ≥50% slow waves on the EEG. PDR disappeared in 32 (53.3%) patients, and PDR was poorly sustained in 23 of the remaining 28 patients with PDR. The EEGs of 16 (26.7%) patients showed epileptiform discharges, and partial seizure was a common clinical manifestation, with nine (15.0%) patients showing status epilepticus. A poor prognosis was significantly correlated with EEG characteristics of PDR disappearance (p=0.001), rhythmic delta activity (p=0.009), EDB (p=0.02), ≥50% slow waves on the EEG (p<0.001), and slow-wave amplitudes (p=0.038), but not with poorly sustained PDR (p=0.082) or epileptiform discharges (p=0.496).
Multivariate analysis
The variables with p<0.05 in the univariate analysis (i.e., age, mental behavioral disorders/cognitive dysfunction at admission, disturbance of consciousness, autonomic dysfunction, admission to the NICU, mechanical ventilation, disappearance of PDR, rhythmic delta activity, EDB, ≥50% slow waves, and slow-wave amplitude) were included in the multivariate analysis of the factors influencing the prognosis. The results showed that age [odds ratio (OR)=1.146, 95% confidence interval (CI)=1.042–1.262], disturbance of consciousness (OR=6.19, 95% CI=1.434–26.723), and ≥50% slow waves on the EEG (OR=38.985, 95% CI=3.592–423.073) were significant risk factors in patients with anti-NMDAR encephalitis (Table 3).
Table 3. Results from the multivariate logistic regression analysis of a poor prognosis in patients.
| Variable | β | SE | Wald χ2 | p | OR | 95% CI |
|---|---|---|---|---|---|---|
| Age | 0.137 | 0.049 | 7.829 | 0.005 | 1.146 | 1.042–1.262 |
| Disturbance of consciousness | 1.823 | 0.746 | 5.968 | 0.015 | 6.190 | 1.434–26.723 |
| ≥50% slow waves | 3.663 | 1.217 | 9.067 | 0.003 | 38.985 | 3.592–423.073 |
CI: confidence interval, OR: odds ratios, SE: standard error.
Establishment of the age, consciousness, and slow waves prognostic composite score and ROC-curve analysis
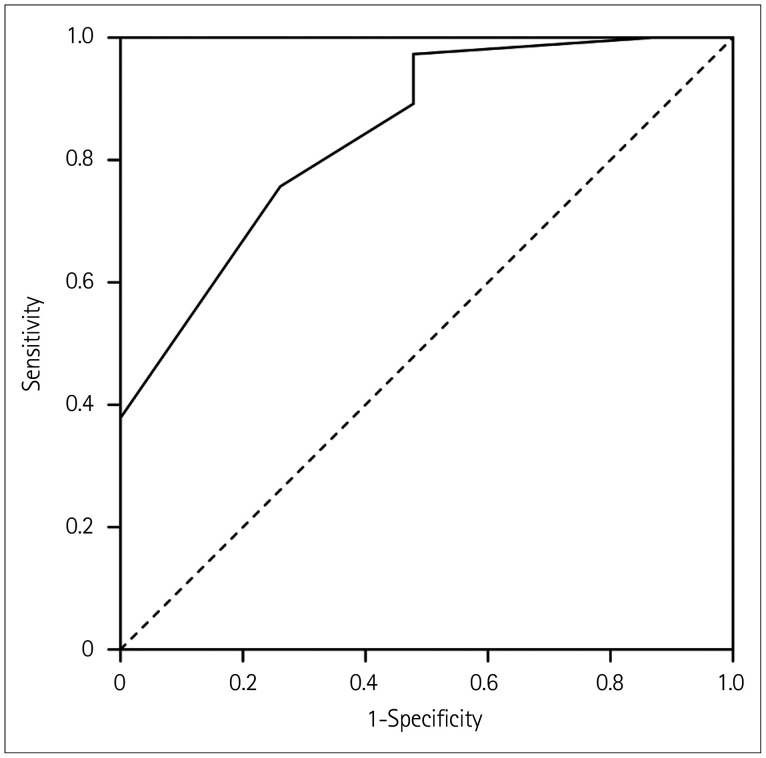
Based on the analysis results and coefficients included in the logistic regression of the composite score, three factors were chosen to construct a composite score called the age, consciousness, and slow waves (ACS) score for predicting prognoses in patients with anti-NMDAR encephalitis. Table 4 lists the assignments of the ACS table. Fig. 1 shows that the AUC was 0.853 (p<0.001, 95% CI 0.756–0.950) when the cutoff value of the ACS score was 3. Applying this cutoff value produced overall sensitivity and specificity values of 83.78% and 73.91%, respectively (92.86% and 80.00%, respectively, in males, and 78.26% and 69.23% in females) (Table 5).
Table 4. Assignment of age, consciousness, and slow waves scores based on the regression coefficients.
| Variable | Score |
|---|---|
| Age, years | |
| <25 | 0 |
| ≥25 | 1 |
| Disturbance of consciousness at admission | |
| No | 0 |
| Yes | 1 |
| Proportion of slow waves on the electroencephalogram | |
| <50% | 0 |
| ≥50% | 2 |
Fig. 1. ROC curve. The area under the ROC curve value for the ACS score was 0.853, with 95% CI=0.756–0.950 (p<0.001). The ACS cutoff value was 3. ACS: age, consciousness, and slow waves, ROC: receiver operating characteristic.
Table 5. Outcomes according to age, consciousness, and slow waves scores.
| Good prognosis | Poor prognosis | Accuracy (%) | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | |
|---|---|---|---|---|---|---|---|
| Total | 23 | 37 | 80.0 | 83.78 | 73.91 | 83.78 | 73.91 |
| Score <3 | 17 | 6 | |||||
| Score ≥3 | 6 | 31 | |||||
| Males | 10 | 14 | 87.5 | 92.86 | 80.00 | 86.67 | 88.89 |
| Score <3 | 8 | 1 | |||||
| Score ≥3 | 2 | 13 | |||||
| Females | 13 | 23 | 75.0 | 78.26 | 69.23 | 81.82 | 64.29 |
| Score <3 | 9 | 5 | |||||
| Score ≥3 | 4 | 18 |
Analysis of indexes of patient outcomes
The main symptoms presented by the patients in this study after hospital discharge (63.3%) were mental behavioral disorders/cognitive dysfunction. The patients with less-severe symptoms presented with personality changes, emotional instability, anxiety, and nervousness, while those with severe symptoms experienced impulsive aggressive behaviors, depression, and attempted suicide. Patients with a poor prognosis had greater mental behavioral disorders/cognitive dysfunction after hospital discharge (p=0.012). The other outcome indexes that differed significantly between the two groups were movement disorders/involuntary movement after discharge (p=0.019), length of hospital stay (p=0.026), hospitalization expenses (p=0.002), and time interval between disturbance of consciousness and awakening (p=0.003). However, no significant difference was observed in speech disorders at discharge (p=0.107), seizures (p=0.519), disturbance of consciousness (p=0.519), or autonomic dysfunction (p=0.279) (Table 6).
Table 6. Analysis of outcomes differences between the two groups.
| Variable | Total | Prognosis | Statistical value | p | |
|---|---|---|---|---|---|
| Good (n=23) | Poor (n=37) | ||||
| Symptoms after hospital discharge | |||||
| Mental behavioral disorders/cognitive dysfunction | 38 (63.3) | 10 (43.5) | 28 (75.7) | 6.332† | 0.012 |
| Speech disorders | 12 (20.0) | 2 (8.7) | 10 (27.0) | - | 0.107‡ |
| Seizures | 2 (3.3) | 0 (0.0) | 2 (5.4) | - | 0.519‡ |
| Dyskinesia/involuntary movement | 8 (13.3) | 0 (0.0) | 8 (21.6) | - | 0.019‡ |
| Disturbance of consciousness | 2 (3.3) | 0 (0.0) | 2 (5.4) | - | 0.519‡ |
| Autonomic dysfunction | 3 (5.0) | 0 (0.0) | 3 (8.1) | - | 0.279‡ |
| Hospital stay, days | 24 [19–31] | 32 [21–53] | -2.221* | 0.026 | |
| Hospitalization expenses, US$ (×10,000) | 0.45 [0.31–0.93] | 1.61 [0.83–4.40] | -3.086 | 0.002 | |
| Time interval between disturbance of consciousness and awakening, days | 0 [0–4] | 5 [0–16] | -2.973* | 0.003 | |
Data are median [interquartile range] or n (%) values. -, no value available.
*Z value in the Mann-Whitney U-test, †χ2, ‡p value in Fisher's exact test.
DISCUSSION
The incidence of anti-NMDAR encephalitis is increasing, but its pathogenesis remains unclear.2 Considering that anti-NMDAR encephalitis is a serious but treatable disease, the ability to perform early, timely, and accurate assessments of the prognosis is conducive to individualized adjustment of treatment plans, and over the long term this will improve adherence among patients and their family members. We retrospectively analyzed the clinical data, laboratory parameters, imaging findings, and EEG data of 60 patients with anti-NMDAR encephalitis, and found that age, disturbance of consciousness at admission, and ≥50% slow waves on the EEG were independent risk factors for a poor prognosis. To predict the prognoses of these patients, we constructed an ACS prognostic composite score based on the regression coefficient, which had an AUC value of 0.853 (p<0.001, 95% CI=0.756–0.950), a sensitivity of 83.78%, and a specificity of 73.91%. The ACS score can be used by doctors to assess affected patients (especially males) in the early stage of disease.
Anti-NMDAR encephalitis occurs more frequently in young patients. Previous studies have found the median age of patients to be between 21 and 28 years,7,8,15,19 while this was 25 years in the present study. Furthermore, we found that older patients were more likely to have a poor prognosis, which is consistent with most studies.19,20 In addition to irreversible age-related factors, atypical or relatively mild symptoms experienced by elderly patients lead to delayed diagnosis and treatment, which contributes to their poor recovery of neurological function. Also, initial immunotherapy may be beneficial to patients.20
As the most commonly used scale for assessing the level of consciousness, the GCS is now widely used to evaluate prognoses in patients with severe neurological diseases. Previous studies have shown that disturbance of consciousness at admission (GCS score ≤8 points) can be used as a predictor of death and is an independent risk factor for a poor prognosis.8,10,21 In the present study, we found that the prognosis of patients with disturbance of consciousness at admission was even worse, which is consistent with previous studies. Disturbance of consciousness that occurs during the course of anti-NMDAR encephalitis can be caused by status epilepticus, elevated intracranial pressure, or inflammation itself.22 Prolonged bed rest and tracheal intubation increase the risk of multiple complications such as pneumonia, urinary tract infection, sepsis, and deep vein thrombosis that can lead to a poor prognosis. Therefore, doctors should closely monitor the conscious state of patients with anti-NMDAR encephalitis when they are admitted to the hospital, including continually assessing and removing any potential causes of disturbance of consciousness as soon as possible.
EEG abnormalities are one of the criteria used to diagnose anti-NMDAR encephalitis. Diffuse slow waves are the mostcommon EEG presentation in anti-NMDAR encephalitis.14,23 One possible mechanism is that NMDARs specifically bind to anti-NMDAR antibodies, resulting in shortened cell depolarization,24 which causes slow waves. Blockade of the NMDAR will disturb the thalamic cortical rhythm, resulting in low-frequency delta oscillations.12 Simultaneous with these manifestations, subcortical lesions lead to the loss of afferent impulses in the cortex and are important causes of diffuse slow waves. In this study, 48 (80.0%) patients had EEG abnormalities, including 44 (73.3%) with EEGs that showed focal or diffuse slow waves. Our multivariate analysis performed showed that ≥50% slow waves on the EEG was an independent risk factor for a poor prognosis. Therefore, because EEG changes are closely related to the severity of brain damage, EEG changes—especially in the proportion of slow waves—can be used as a clinical prognostic indicator.
The ACS score includes the patient's age, disturbance of consciousness at admission, and ≥50% slow waves on the EEG. When the cutoff value of the ACS score was 3, the predicted sensitivity and specificity were 83.78% and 73.91%, respectively. The information provided by the ACS score can help the doctor to assess the possible clinical outcomes in individual patients at an early stage and provide them with reasonable advice. This might help alleviate the anxiety of patients and their family members and improve compliance with a customized therapeutic plan. The sensitivity and specificity of the ACS score were higher when applied to males (92.86% and 80.00%, respectively) than females (78.26% and 69.23%), which indicates that the ACS score is more accurate when applied to evaluate a prognosis in male patients.
Previous studies have indicated that a lack of normal background activity on an EEG is associated with severe neurological dysfunction and a poor prognosis.25 Limotai et al.12 found that poor PDR has significant value in diagnosing anti-NMDAR encephalitis; however, PDR did not differ significantly between the two groups in the present study. It was particularly interesting that all of the patients with PDR in our poor prognosis group had poorly sustained PDR. This phenomenon needs to be examined further in a larger sample. There remains some disagreement about the predictive value of EDB on EEG. Most authors believe that patients with EDB have more-severe symptoms, longer hospital stays, and worse prognosis,18 whereas others believe that there is no significant correlation between EDB and prognoses.24 EDB was observed on the EEGs of 12 (20.0%) patients in the present study, and the proportion of patients with a poor prognosis was significantly higher in these patients than in those with a good prognosis (p=0.02). Therefore, further evidence is needed on the role of EDB in predicting prognoses. However, the neuron-specific enolase and S-100B proteins have been widely used as biochemical indicators in evaluating the prognosis of CNS injury, but they are rarely used in anti-NMDAR encephalitis. Only one relevant study has shown that the neuron-specific enolase or S100B concentration in the CSF obtained within 72 hours after onset can be utilized to assess a patient's prognosis.10 More researches are needed in the future. Jang et al.26 found that patients with low albumin levels before treatment exhibited worse responses to immunotherapy as well as poor short-term and long-term prognoses. However, there was no significant difference in pretreatment albumin levels between the two groups in this study, which was probably due to the differences in the sizes of the target populations. Furthermore, Titulaer et al.7 showed that early treatment was one of the predictors of good outcome, whereas in our study the interval between onset to immunotherapy (p=0.981) was not correlated with the prognosis. This difference may be related to the different durations for evaluating the prognosis as well as the limitations of the small sample. A long-term investigation of a larger cohort needs to be conducted in a future study.
In this study, mental behavioral disorders/cognitive dysfunction were the most-common symptoms in the initial stages (55.0%), during the disease course (93.3%), and at discharge (63.3%). Therefore, the mental and cognitive symptoms observed in anti-NMADAR encephalitis should be of particular concern to doctors. Compared with patients with primary mental illness, patients with anti-NMDAR encephalitis have more-prominent confusion and behavioral disorders, such as agitation, emotional instability, and stress disorder.27,28 Studies have found that the possible mechanisms underlying psychotic symptoms, including the notion that anti-NMDAR antibodies can cause NMDAR internalization, which in turn leads to the progressive loss of surface receptors, result in reduced inhibition of the prefrontal cortex by γ-aminobutyric acid, cortical dysfunction, excessive release of acetylcholine, and overactivation of the glutamic acid pathway, thereby inducing various psychiatric symptoms.29 Because the process is reversible, psychotic symptoms have been shown to improve in parallel with antibody clearance, although mental behavioral disorders and cognitive dysfunction can often coexist for a long time.30,31,32 The remaining psychiatric symptoms include personality changes, anxiety, and irritability, which mostly manifest to a mild extent, but some patients show long-term depression and others exhibit emotional changes30 that can even lead to suicide.8,33 Therefore, after immunotherapy, it is necessary to perform strict neuropsychological monitoring of such patients and to distinguish them from recurrent patients.
In conclusion, in this study we analyzed the risk factors for a poor prognosis in anti-NMDAR encephalitis. Age, disturbance of consciousness at admission, and ≥50% slow waves on the EEG were independent risk factors for a poor prognosis. On this basis, we constructed the ACS prognostic composite score for assessing the prognosis of individual patients with anti-NMDAR encephalitis at an early stage. We found that an ACS score of ≥3 points indicates a higher likelihood of a poor prognosis, especially in male patients. Due to the limitations of the small sample and single-center observations in this study, more predictors need to be identified, explored, and validated in large-sample, multicenter, randomized controlled trials in the future.
Acknowledgements
This study was supported by the National Science Foundation of Chongqing Science and Technology Commission (cstc, 2016jcyjA1406).
Footnotes
- Conceptualization: Yejia Mo, Li Wang, Feng Li, Gang Yu.
- Data curation: Yetao Luo.
- Formal analysis: Li Wang, Feng Li, Gang Yu.
- Funding acquisition: Gang Yu.
- Investigation: Yejia Mo, Libo Zhu, Meng Ni.
- Methodology: Yejia Mo, Li Wang.
- Project administration: Gang Yu.
- Resources: Feng Li, Gang Yu.
- Software: Feng Li, Yetao Luo.
- Writing—original draft: Yejia Mo, Li Wang.
- Writing—review & editing: Feng Li, Gang Yu, Libo Zhu, Meng Ni.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
References
- 1.Dubey D, Pittock SJ, Kelly CR, McKeon A, Lopez-Chiriboga AS, Lennon VA, et al. Autoimmune encephalitis epidemiology and a comparison to infectious encephalitis. Ann Neurol. 2018;83:166–177. doi: 10.1002/ana.25131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dalmau J, Armangué T, Planagumà J, Radosevic M, Mannara F, Leypoldt F, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models. Lancet Neurol. 2019;18:1045–1057. doi: 10.1016/S1474-4422(19)30244-3. [DOI] [PubMed] [Google Scholar]
- 3.Ho AC, Chan SH, Chan E, Wong SS, Fung ST, Cherk SW, et al. Anti-N-methyl-d-aspartate receptor encephalitis in children: incidence and experience in Hong Kong. Brain Dev. 2018;40:473–479. doi: 10.1016/j.braindev.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 4.Jones HF, Mohammad SS, Reed PW, Dunn PP, Steele RH, Dale RC, et al. Anti-N-methyl-d-aspartate receptor encephalitis in MXMLLink_XYZori and Pacific Island children in New Zealand. Dev Med Child Neurol. 2017;59:719–724. doi: 10.1111/dmcn.13420. [DOI] [PubMed] [Google Scholar]
- 5.Armangue T, Spatola M, Vlagea A, Mattozzi S, Cárceles-Cordon M, Martinez-Heras E, et al. Frequency, symptoms, risk factors, and outcomes of autoimmune encephalitis after herpes simplex encephalitis: a prospective observational study and retrospective analysis. Lancet Neurol. 2018;17:760–772. doi: 10.1016/S1474-4422(18)30244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10:63–74. doi: 10.1016/S1474-4422(10)70253-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Titulaer MJ, McCracken L, Gabilondo I, Armangué T, Glaser C, Iizuka T, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12:157–165. doi: 10.1016/S1474-4422(12)70310-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chi X, Wang W, Huang C, Wu M, Zhang L, Li J, et al. Risk factors for mortality in patients with anti-NMDA receptor encephalitis. Acta Neurol Scand. 2017;136:298–304. doi: 10.1111/ane.12723. [DOI] [PubMed] [Google Scholar]
- 9.Balu R, McCracken L, Lancaster E, Graus F, Dalmau J, Titulaer MJ. A score that predicts 1-year functional status in patients with anti-NMDA receptor encephalitis. Neurology. 2019;92:e244–e252. doi: 10.1212/WNL.0000000000006783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu B, Xie Z, Liu G, Gu Y, Pan S, Wang H. Elevated neuron-specific enolase and S100 calcium-binding protein B concentrations in cerebrospinal fluid of patients with anti-N-methyl-d-aspartate receptor encephalitis. Clin Chim Acta. 2018;480:79–83. doi: 10.1016/j.cca.2018.01.016. [DOI] [PubMed] [Google Scholar]
- 11.Wang W, Li JM, Hu FY, Wang R, Hong Z, He L, et al. Anti-NMDA receptor encephalitis: clinical characteristics, predictors of outcome and the knowledge gap in southwest China. Eur J Neurol. 2016;23:621–629. doi: 10.1111/ene.12911. [DOI] [PubMed] [Google Scholar]
- 12.Limotai C, Denlertchaikul C, Saraya AW, Jirasakuldej S. Predictive values and specificity of electroencephalographic findings in autoimmune encephalitis diagnosis. Epilepsy Behav. 2018;84:29–36. doi: 10.1016/j.yebeh.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 13.Yildirim M, Konuskan B, Yalnizoglu D, Topaloglu H, Erol I, Anlar B. Electroencephalographic findings in anti-N-methyl-d-aspartate receptor encephalitis in children: a series of 12 patients. Epilepsy Behav. 2018;78:118–123. doi: 10.1016/j.yebeh.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 14.Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15:391–404. doi: 10.1016/S1474-4422(15)00401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y, Liu G, Jiang M, Chen W, He Y, Su Y. Clinical characteristics and prognosis of severe anti-N-methyl-D-aspartate receptor encephalitis patients. Neurocrit Care. 2018;29:264–272. doi: 10.1007/s12028-018-0536-6. [DOI] [PubMed] [Google Scholar]
- 16.Chen C, Ye M, Chen BL, Chen GF, Gao ZQ, Zhou JS, et al. Thrombolysis on ischemic stroke patients with decreased level of consciousness within 4.5 h. CNS Neurosci Ther. 2013;19:48–52. doi: 10.1111/cns.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hirsch LJ, LaRoche SM, Gaspard N, Gerard E, Svoronos A, Herman ST, et al. American Clinical Neurophysiology Society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30:1–27. doi: 10.1097/WNP.0b013e3182784729. [DOI] [PubMed] [Google Scholar]
- 18.Schmitt SE, Pargeon K, Frechette ES, Hirsch LJ, Dalmau J, Friedman D. Extreme delta brush: a unique EEG pattern in adults with anti-NMDA receptor encephalitis. Neurology. 2012;79:1094–1100. doi: 10.1212/WNL.0b013e3182698cd8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Montmollin E, Demeret S, Brulé N, Conrad M, Dailler F, Lerolle N, et al. Anti-N-methyl-d-aspartate receptor encephalitis in adult patients requiring intensive care. Am J Respir Crit Care Med. 2017;195:491–499. doi: 10.1164/rccm.201603-0507OC. [DOI] [PubMed] [Google Scholar]
- 20.Titulaer MJ, McCracken L, Gabilondo I, Iizuka T, Kawachi I, Bataller L, et al. Late-onset anti-NMDA receptor encephalitis. Neurology. 2013;81:1058–1063. doi: 10.1212/WNL.0b013e3182a4a49c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aungsumart S, Ha A, Apiwattanakul M. Abnormal level of consciousness predicts outcomes of patients with anti-NMDA encephalitis. J Clin Neurosci. 2019;62:184–187. doi: 10.1016/j.jocn.2018.11.033. [DOI] [PubMed] [Google Scholar]
- 22.Broadley J, Seneviratne U, Beech P, Buzzard K, Butzkueven H, O'Brien T, et al. Prognosticating autoimmune encephalitis: a systematic review. J Autoimmun. 2019;96:24–34. doi: 10.1016/j.jaut.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 23.Gillinder L, Warren N, Hartel G, Dionisio S, O'Gorman C. EEG findings in NMDA encephalitis-a systematic review. Seizure. 2019;65:20–24. doi: 10.1016/j.seizure.2018.12.015. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Liu G, Jiang MD, Li LP, Su YY. Analysis of electroencephalogram characteristics of anti-NMDA receptor encephalitis patients in China. Clin Neurophysiol. 2017;128:1227–1233. doi: 10.1016/j.clinph.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 25.Gitiaux C, Simonnet H, Eisermann M, Leunen D, Dulac O, Nabbout R, et al. Early electro-clinical features may contribute to diagnosis of the anti-NMDA receptor encephalitis in children. Clin Neurophysiol. 2013;124:2354–2361. doi: 10.1016/j.clinph.2013.05.023. [DOI] [PubMed] [Google Scholar]
- 26.Jang Y, Lee ST, Kim TJ, Jun JS, Moon J, Jung KH, et al. High albumin level is a predictor of favorable response to immunotherapy in autoimmune encephalitis. Sci Rep. 2018;8:1012. doi: 10.1038/s41598-018-19490-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warren N, Siskind D, O'Gorman C. Refining the psychiatric syndrome of anti-N-methyl-d-aspartate receptor encephalitis. Acta Psychiatr Scand. 2018;138:401–408. doi: 10.1111/acps.12941. [DOI] [PubMed] [Google Scholar]
- 28.Gibson LL, Pollak TA, Blackman G, Thornton M, Moran N, David AS. The psychiatric phenotype of anti-NMDA receptor encephalitis. J Neuropsychiatry Clin Neurosci. 2019;31:70–79. doi: 10.1176/appi.neuropsych.17120343. [DOI] [PubMed] [Google Scholar]
- 29.Kuppuswamy PS, Takala CR, Sola CL. Management of psychiatric symptoms in anti-NMDAR encephalitis: a case series, literature review and future directions. Gen Hosp Psychiatry. 2014;36:388–391. doi: 10.1016/j.genhosppsych.2014.02.010. [DOI] [PubMed] [Google Scholar]
- 30.Mariotto S, Tamburin S, Salviati A, Ferrari S, Zoccarato M, Giometto B, et al. Anti-N-methyl-d-aspartate receptor encephalitis causing a prolonged depressive disorder evolving to inflammatory brain disease. Case Rep Neurol. 2014;6:38–43. doi: 10.1159/000358820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKeon GL, Robinson GA, Ryan AE, Blum S, Gillis D, Finke C, et al. Cognitive outcomes following anti-N-methyl-D-aspartate receptor encephalitis: a systematic review. J Clin Exp Neuropsychol. 2018;40:234–252. doi: 10.1080/13803395.2017.1329408. [DOI] [PubMed] [Google Scholar]
- 32.de Bruijn MAAM, Aarsen FK, van Oosterhout MP, van der Knoop MM, Catsman-Berrevoets CE, Schreurs MWJ, et al. Long-term neuropsychological outcome following pediatric anti-NMDAR encephalitis. Neurology. 2018;90:e1997–e2005. doi: 10.1212/WNL.0000000000005605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Sander JW, Zhang L, Jiang XY, Wang W, Shuang K, et al. Suicidality is a common and serious feature of anti-N-methyl-D-aspartate receptor encephalitis. J Neurol. 2017;264:2378–2386. doi: 10.1007/s00415-017-8626-5. [DOI] [PubMed] [Google Scholar]