Significance
The human brain exploits subtle differences between the inputs to the paired eyes and ears to construct three-dimensional experiences and navigate the environment. Whether and how it does so for olfaction is unclear, although humans also have two separate nasal passages that simultaneously sample from nonoverlapping regions in space. Here, we demonstrate that a moderate internostril difference in odor intensity consistently biases recipients’ perceived direction of self-motion toward the higher-concentration side, despite that they cannot report which nostril smells a stronger odor. The findings indicate that humans have a stereo sense of smell that subconsciously guides navigation.
Keywords: binaral disparity, olfactory navigation, heading perception, optic flow
Abstract
Human navigation relies on inputs to our paired eyes and ears. Although we also have two nasal passages, there has been little empirical indication that internostril differences yield directionality in human olfaction without involving the trigeminal system. By using optic flow that captures the pattern of apparent motion of surface elements in a visual scene, we demonstrate through formal psychophysical testing that a moderate binaral concentration disparity of a nontrigeminal odorant consistently biases recipients’ perceived direction of self-motion toward the higher-concentration side, despite that they cannot verbalize which nostril smells a stronger odor. We further show that the effect depends on the internostril ratio of odor concentrations and not the numeric difference in concentration between the two nostrils. Taken together, our findings provide behavioral evidence that humans smell in stereo and subconsciously utilize stereo olfactory cues in spatial navigation.
The human nose, the most protruding part of the face, bears two nostrils that are separated by the nasal septum and inspire air from nonoverlapping regions (roughly 3.5 cm apart) in space (1). Theoretically, this provides a computational advantage to localize odor sources as compared with sampling at one point in space (2). Empirically, however, results have been mixed and largely negative with regard to human olfactory localization by means of bilateral inputs (3–8). Whereas depriving internostril differences hampers scent-tracking performance in humans (1), it is suspected that the binaral directional information comes from trigeminal rather than olfactory cues. Pure odorants, those that selectively stimulate the olfactory system, have repetitively been demonstrated as unlocalizable (5–8), that is, when such odorants are presented to one of the two nostrils, recipients are at chance in reporting which nostril smells an odor. Meanwhile, the ability to localize an odor during unilateral presentation is routinely viewed both as a sign that the odor elicits trigeminal activities and as an index of one’s nasal trigeminal chemosensitivity (9). Odor-related spatial information in general seems to arise from a combination of trigeminal perception and the comparison of sequential sniffs (10, 11).
By and large, the existent literature does not indicate that the human olfactory system exploits binaral odor-concentration disparity to compute direction, an ability that has been identified in mammals such as rats (12) and moles (13). It is worth noting, though, that the utilization of a spatial cue in action or navigation could be independent of whether that spatial cue reaches awareness or can be verbally reported (14–16). To address whether humans navigate with stereo olfaction, the current study thus opted to directly examine how binaral concentration disparities of a pure odorant act on motion direction perception. We took advantage of a unique type of visual stimuli called optic flow, which critically guides navigation and induces the illusory feeling of self-movement in stationary observers (17). By precisely controlling the expansion pattern of the optic flow, we quantified in a psychophysical procedure the extent to which observers’ heading judgments are influenced by various levels of binaral disparity in the concentration of phenylethyl alcohol, a nontrigeminal rose-like odorant, or vanillin, a nontrigeminal vanilla-like odorant (18).
Results
Binaral Concentration Disparity Modulates Heading Perception.
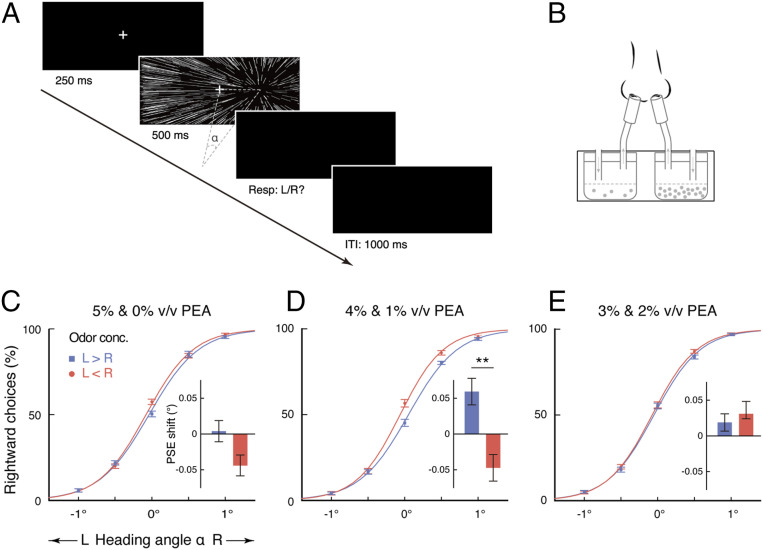
Experiment 1 employed a heading-judgment task (Fig. 1A) using visually presented optic-flow stimuli that simulated self-movement toward a three-dimensional cube of dots (Movie S1). Heading angle α was defined as the angle between straight ahead and the center of expansion of the radial pattern and varied horizontally between 2° leftward (−2°) and 2° rightward (2°) in 7 logarithmic steps, i.e., 0, ±0.5, ±1, and ±2°. Participants were randomly assigned to 3 groups of 24 each and performed the task under continuous dichorhinic exposures to various concentrations of phenylethyl alcohol, i.e., to one concentration in a nostril and a second concentration in the other nostril at the same time (Fig. 1B). In each trial, they viewed an optic-flow field for 500 ms while fixating on a stationary central cross and made a forced-choice judgment on whether he or she seemed to be heading to the left or right of fixation cross. No eye pursuit or rotation was involved. Specifically, there were 4 levels of binaral concentration disparity: high (5% volume/volume [v/v] in propylene glycol in one nostril and 0% in the other nostril), intermediate (4 and 1% v/v), low (3 and 2% v/v), and zero (2.5% v/v in both nostrils). Each participant performed 6 blocks of the task, including 4 blocks under either high, intermediate, or low binaral disparity, where they received the higher concentration of phenylethyl alcohol in the left nostril (left [L] > right [R]) in 2 blocks and in the right nostril (L < R) in the other 2 blocks, and 2 additional blocks under zero binaral disparity (L = R), which served as the reference.
Fig. 1.
Effect of binaral concentration disparity on heading perception based on optic flow. (A) Schematic illustration of an exemplar trial in the heading-judgment task. Heading angle α was defined as the angle between straight ahead and the center of expansion of an optic-flow stimulus and varied from trial to trial in random order. ITI, intertrial interval; Resp: L/R?, response: left or right?. (B) Participants performed the heading-judgment task while smelling one concentration of a nontrigeminal odorant in a nostril and a second concentration in the other nostril. (C–E) Percentages of rightward responses plotted as a function of the physical heading angle α of the optic-flow stimuli, when the 3 groups of participants in Experiment 1 smelled a higher concentration of phenylethyl alcohol (PEA) in the left nostril (L > R) and in the right nostril (L < R), respectively fitted with sigmoidal curves. Insets illustrate the corresponding PSE shifts induced by high (C), intermediate (D), and low (E) binaral disparities relative to zero disparity. The average percentages of rightward responses at α = −2 and 2° were less than 1% and over 99%, respectively, and are not displayed. Error bars indicate SEMs adjusted for individual differences. **P < 0.01. conc., concentration.
To quantify performance, we obtained psychometric curves that depicted the probability of rightward judgments as a function of the heading angle α of the optic-flow stimuli (Fig. 1 C–E). This allowed us to determine the point of subjective equality (PSE), the heading angle yielding leftward and rightward reports with equal probability, and difference limen, essentially the slope of the curve at the PSE point. Across participants, the average PSE under zero binaral concentration disparity was close to 0° (−0.0023°, t71 = −0.13, P = 0.89). Importantly, PSEs differed significantly across dichorhinic manipulations (L > R, L < R, L = R) in the group of participants subjected to intermediate (F2, 46 = 3.68, P = 0.033, partial η2 = 0.14), but not high (F2, 46 = 1.59, P = 0.22) or low (F2, 46 = 0.88, P = 0.42), binaral concentration disparity. Smelling 4% v/v phenylethyl alcohol in the left nostril and 1% v/v in the right nostril, as opposed to the other way around, led to a systematic bias to perceive oneself as moving leftward (mean PSE shift: 0.11° [about a third of the difference limen or heading threshold 0.34°], t23 = 2.87, P = 0.009, Cohen’s d = 0.59), with an 11.5% decrease in rightward judgments at α = 0°, where the visual heading information was most ambiguous (t23 = −3.02, P = 0.006, Cohen’s d = 0.62), and vice versa (Fig. 1D). This was not the case with 5 and 0% v/v (t23 = 1.62, P = 0.12; Fig. 1C) or 3 and 2% v/v phenylethyl alcohol (t23 = −0.93, P = 0.36; Fig. 1E). There was no significant change in heading-judgment sensitivity across dichorhinic manipulations in all groups of participants (Fs2, 46 < 1.87, Ps > 0.16, Bayes factors in favor of H1 over H0 [BFs10] < 0.47), as measured by their difference limens. When explicitly asked which nostril smelled a stronger odor, the participants performed at chance irrespective of the group they belonged to (mean accuracies under high, intermediate, and low disparities vs. chance: 0.52, 0.55, and 0.48, respectively, vs. 0.5; Ps > 0.13), consistent with earlier reports (5–8). The results thus suggested that binaral disparity in odor concentration acts as a subliminal directional cue in human motion perception, and yet, somewhat counterintuitively at a first glance, the strength of the directional cue does not scale with the amount of internostril concentration difference. Before continuation, we sought to replicate this finding in a strictly controlled within-subjects design.
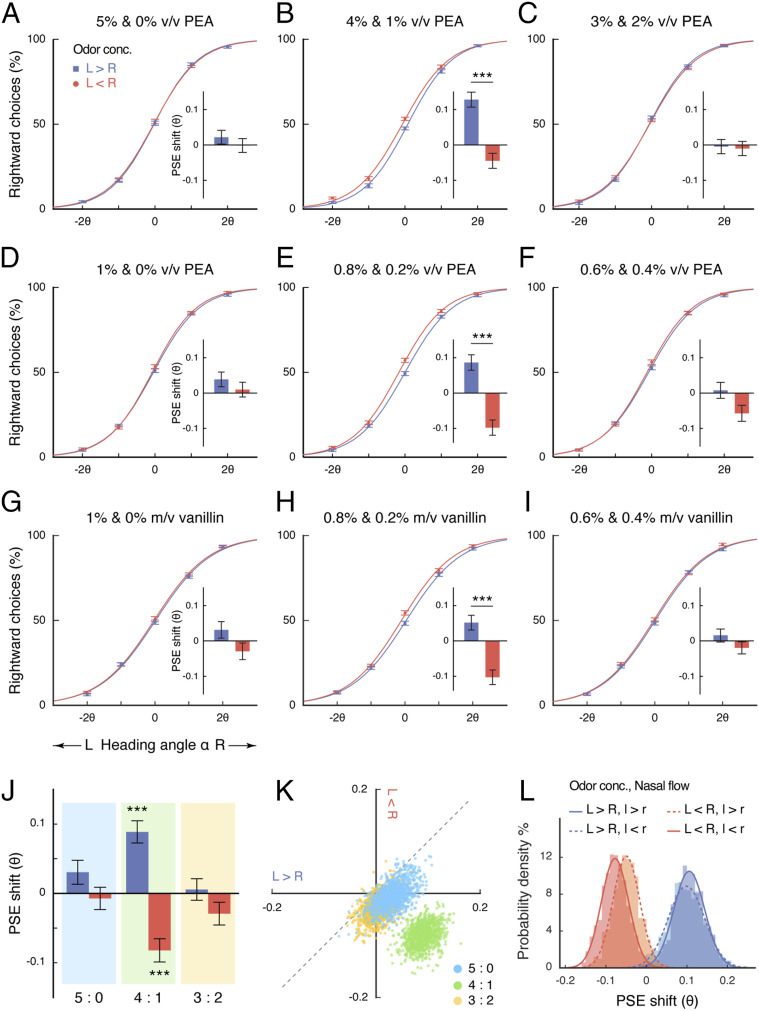
In Experiment 2, 36 new participants each performed 16 blocks of the heading-judgment task over 2 days: 4 blocks each under high (5 and 0% v/v), intermediate (4 and 1% v/v), and low (3 and 2% v/v) levels of binaral concentration disparity (higher concentration in the left nostril in 2 blocks and in the right nostril in the other 2 blocks), and another 4 blocks under zero binaral concentration disparity (2.5% v/v in both nostrils in 2 blocks and 0% in both nostrils in the other 2 blocks). For each participant, heading angle of the optic-flow stimuli varied across 7 steps: 0°, ±θ, ±2θ, and ±4θ, where θ roughly corresponded to his or her difference limen in heading perception and was individually assessed and set prior to the actual experiment in the absence of olfactory stimuli. Again, we observed that PSEs were reliably shifted by which nostril received a higher concentration of phenylethyl alcohol under intermediate (t35 = 4.09, P < 0.001, Cohen’s d = 0.68), but not high (t35 = 0.60, P = 0.55) or low (t35 = 0.14, P = 0.89), binaral disparity, with a significant difference between intermediate and high (t35 = 2.48, P = 0.018, Cohen’s d = 0.41) as well as between intermediate and low (t35 = 2.89, P = 0.007, Cohen’s d = 0.48) disparities (Fig. 2 A–C).
Fig. 2.
Dependence of directional smelling on internostril concentration ratio. (A–I) Psychometric functions and PSE shifts for Experiments 2 (A–C), 3 (D–F), and 4 (G–I). The numerical internostril concentration differences under high (A, D, and G), intermediate (B, E, and H), and low (C, F, and I) binaral disparities varied among the experiments, but the internostril concentration ratios were maintained. The average percentages of rightward responses at α = −4θ and 4θ were less than 1% and over 99%, respectively, and are not displayed. (J) Overall PSE shifts under different internostril concentration ratios across Experiments 2 to 4. (K) Bivariate distributions of 1,000 bootstrapped sample means for the PSE shifts under 5:0 (cyan), 4:1(lime), and 3:2 (orange) binaral disparities. The x and y axes, respectively, represent PSE shifts induced by receiving a higher concentration in the left nostril (L > R) and in the right nostril (L < R), relative to zero disparity (L = R). (L) Histogram distributions (with normal curves) of bootstrapped sample means for the PSE shifts under 4:1 binaral disparity with regard to the left–right relationships in odor concentration (L > R, L < R) and nasal airflow (l > r, l < r). Error bars indicate SEMs adjusted for individual differences. ***P < 0.001. conc., concentration; PEA, phenylethyl alcohol.
Directional Smelling Depends on Internostril Concentration Ratio.
We wondered whether it was the internostril concentration ratio (4%:1% = 4:1) or the numerical internostril concentration difference (4% − 1% = 3% v/v) under intermediate binaral disparity that caused the motion perception biases observed in Experiments 1 and 2. To this end, we systematically lowered the concentrations of phenylethyl alcohol in Experiment 3 to one-fifth of those in Experiments 1 and 2. This greatly reduced the numerical internostril concentration differences under high, intermediate, and low binaral disparities but maintained the internostril concentration ratios. The procedure was otherwise identical to that of Experiment 2. Analyses of the PSEs revealed the same pattern of results (Fig. 2 D–F). Heading judgments were significantly biased toward the higher concentration side under intermediate (t35 = 4.27, P < 0.001, Cohen’s d = 0.71), but not high (t35 = 0.69, P = 0.49) or low (t35 = 1.42, P = 0.16), binaral disparity, with a significant difference between intermediate and high (t35 = 3.00, P = 0.005, Cohen’s d = 0.50) as well as between intermediate and low (t35 = 2.03, P = 0.050, Cohen’s d = 0.34) disparities. Hence, the olfactory directional information was derived from a moderate internostril concentration ratio and was unrelated to the numerical internostril concentration difference of phenylethyl alcohol.
To further verify whether this inference generalizes to other odorants, Experiment 4 adopted a different nontrigeminal compound and a different solvent—vanillin dissolved in distilled water. The olfactory stimuli corresponding to high, intermediate, and low levels of binaral concentration disparity were 1 and 0%, 0.8 and 0.2%, and 0.6 and 0.4% mass/volume (m/v) (i.e., mg/mL) vanillin (4 blocks each), respectively, and those corresponding to zero binaral disparity were 0.5 or 0% m/v (water only) vanillin in both nostrils (2 blocks each). Here, the numerical internostril concentration differences were distinct from those in Experiments 1 to 3 (in different units), and yet the internostril concentration ratios were retained. The procedure was otherwise the same as in Experiments 2 and 3. As shown in Fig. 2 G–I, the participants’ PSEs were again shifted by which nostril received a higher concentration under intermediate (t35 = 3.72, P < 0.001, Cohen’s d = 0.62), but not high (t35 = 1.29, P = 0.21) or low (t35 = 0.94, P = 0.35), binaral disparity, with a significant difference between intermediate and low (t35 = 2.09, P = 0.044, Cohen’s d = 0.35) and a marginally significant difference between intermediate and high (t35 = 1.71, P = 0.095) disparities. The results thus echoed with those obtained with phenylethyl alcohol.
We subsequently combined the data from Experiments 2 to 4 to better characterize heading performances under different internostril concentration ratios (Fig. 2J). Relative to zero binaral disparity, we found that PSEs were significantly shifted under 4:1 disparity both when the higher concentration appeared in the left nostril (t107 = 4.25, P < 0.001, Cohen’s d = 0.41) and in the right nostril (t107 = −3.70, P < 0.001, Cohen’s d = 0.36). The strengths of the effect were comparable between the two nostrils (t107 = 0.19, P = 0.85). No significant PSE shift was detected under 5:0 (Ps = 0.22 and 0.78) or 3:2 (Ps = 0.78 and 0.23) disparities. The central tendencies of the PSE shifts are highlighted in Fig. 2K, generated by using a standard bootstrapping procedure (19), where the x and y axes, respectively, represent PSE shifts induced by receiving a higher concentration in the left nostril (x axis) and in the right nostril (y axis), relative to receiving equal concentrations in both nostrils (zero disparity). They form two distinct clusters: the bootstrapped sample means for 4:1 binaral disparity (lime dots) fall in the fourth quadrant, whereas those for 5:0 (cyan dots) and 3:2 (orange dots) disparities lie close to the origin. Heading-judgment sensitivities, as indexed by difference limens, remained unchanged across all combinations of olfactory conditions (F6, 642 = 0.46, P = 0.84, BF10 = 0.001). Like in Experiment 1, the participants in Experiments 2 to 4 were at chance in reporting which nostril smelled a stronger odor regardless of the internostril concentration ratio (mean accuracies under 5:0, 4:1, and 3:2 disparities vs. chance: 0.48, 0.51, and 0.49, respectively, vs. 0.5; Ps > 0.12).
A potential confound in the calculation of internostril concentration ratio, however, was nasal flow. Due to the periodical alternations of partial congestion and decongestion of the nasal cavities (i.e., nasal cycle), bilateral nasal airflows are typically unequal and the nostril that takes in more air switches every few hours (20, 21). To assess the impact of such airflow imbalances on the extraction of olfactory directional information, we inspected the partitioning of airflow between the two nostrils in the blocks with 4:1 binaral disparity across Experiments 2 to 4. Out of the 432 blocks (108 participants × 4 blocks each), we were able to determine which nostril had a higher airflow for 398 blocks (92%; nasal-flow records for the remaining 8% were incomplete) and grouped them into 4 categories according to odor concentration (L > R or L < R) and nasal airflow (l > r or l < r). Analysis of the PSE shifts (relative to zero binaral disparity) in these blocks showed no significant interaction between the left–right relationships in odor concentration and nasal airflow (F1, 394 = 0.13, P = 0.72). There was a significant main effect of which nostril received a higher odor concentration and no significant effect of which nostril had a higher airflow (Fs1, 394 = 21.12 and 0.23; P < 0.001 and P = 0.63). Fig. 2L displays the distributions of the bootstrapped sample means for the 4 categories of blocks, where the x and y axes respectively represent PSE shift and probability density: those for the L > R blocks mainly fall on the positive side of the x axis (showing a leftward bias in heading perception), whereas those for the L < R blocks mainly fall on the negative side (showing a rightward bias), irrespective of which nostril had a higher airflow (l > r or l < r).
Taken together, the results of Experiments 1 to 4 convergently demonstrated that a moderate (4:1), but not high (5:0) or low (3:2), internostril concentration ratio provides a reliable albeit subliminal directional cue that contributes to self-motion perception, in a manner largely independent of nasal cycle. As only 3 internostril concentration ratios were tested, the results did not fully characterize how olfactory directional information varies with internostril concentration ratio. Nonetheless, the observed effect is noteworthy given the subtlety of the olfactory manipulations and humans’ superior ability to recover heading from optic-flow cues (22).
Binaral Intensity Disparity as a Potential Computational Basis for Olfactory Direction.
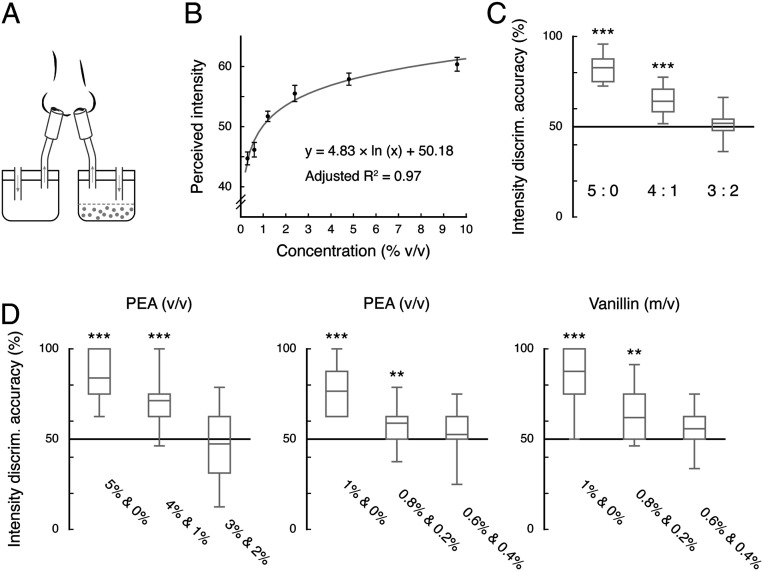
How does the olfactory system, without direct access to the physical concentrations of odorants, compute internostril concentration ratio? We verified in an independent sample of 12 participants in Experiment 5 that the relationship between the subjective intensity of unirhinally presented phenylethyl alcohol (Fig. 3A) and its physical concentration (ranged between 0.3 and 9.6% v/v) was approximately logarithmic (adjusted R2 = 0.97, P = 0.0002; Fig. 3B), in accordance with Fechner’s law (23). Hence, internostril concentration ratio is roughly equivalent to binaral disparity in perceived odor intensity. Given that the perceptual feature of odor intensity arises from neuronal responses in the olfactory bulb (24) and is unaffected by nasal cycle (25), it is plausible that binaral intensity disparity serves as a computational basis for directionality in the olfactory system.
Fig. 3.
Binaral intensity disparity as a rough equivalent for internostril concentration ratio. (A and B) Intensity ratings of unirhinally presented phenylethyl alcohol increased logarithmically with its concentration. Error bars indicate SEM adjusted for individual differences. (C and D) Intensity discrimination (discrim.) accuracies for the olfactory stimuli used in Experiments 1 to 4 that formed 5:0, 4:1, and 3:2 disparities. The two concentrations in a pair were presented unirhinally, one after another to different nostrils. The overall accuracies are shown in C; the respective results for the stimuli used in Experiments 1 and 2, 3, and 4 are shown in D. In each box and whisker plot, the central line denotes the mean, and the bottom and top edges of the box indicate the 25th and 75th percentiles. The ends of the whiskers represent 90% CI. **P < 0.01; ***P < 0.001 (corrected). PEA, phenylethyl alcohol.
Following this reasoning, two concentrations that robustly yield directionality when presented dichorhinically should likely be differentiable in perceived intensity when presented separately, one at a time. In Experiment 6, 24 participants were tested for the intensity discrimination of the olfactory stimuli used in Experiments 1 to 4. They were presented with the odorants that formed high (5:0), intermediate (4:1), and low (3:2) concentration disparities in pairs. In each trial, the two concentrations in a pair were presented unirhinally, one after another to different nostrils, and the participants reported which smelled stronger. Overall, their accuracies were significantly above chance for the concentrations that formed high and intermediate (mean accuracies vs. chance: 0.83 and 0.64, respectively, vs. 0.5; Ps < 0.001, Bonferroni corrected), but not low (mean accuracy: 0.52; P = 0.86, corrected), disparities (Fig. 3C). Importantly, the concentrations that biased heading judgments in Experiments 1 to 4 (forming an intermediate 4:1 binaral disparity) were consistently perceived as different in intensity when smelled individually (mean accuracies: 0.71, 0.59, and 0.62 for those in Experiments 1 and 2, 3, and 4, respectively; Ps < 0.007, corrected; Fig. 3D). These data hence added support to the deduction that the olfactory system exploits binaral intensity disparity to compute direction, although the nostril-of-origin information is not accessible to awareness. It is conceivable that a low binaral concentration disparity, which may still carry directional information, is insufficient to bias self-motion perception based on optic flow. On the other hand, further measurements indicated that an extreme binaral concentration disparity like 5:0 is not ecologically possible (SI Appendix, Supplemental Experiment and Fig. S1), which could be why it failed to generate a directional cue.
Discussion
Spatial navigation requires dynamic representations of the relations between the body and the environment and is an important function of olfaction (26) that has been less appreciated in humans. Here, we present psychophysical evidence that a moderate binaral intensity disparity produces a subliminal directional cue that reliably modulates the perception of self-motion independent of trigeminal activity or nasal cycle. Put differently, humans smell in stereo and utilize olfactory stereo cues in the determination of heading direction, despite that they are not verbally aware of such cues. Our finding reconciles earlier observations that internostril differences contribute to human scent tracking (1) and that pure odorants cannot be verbally localized (5–8) and is also in line with the notion that olfaction is a “muted” sense (27). Moreover, it underscores the multisensory nature of heading perception (28, 29) and provides guidance for the design and development of olfactory virtual-reality systems for humans (30).
Whereas the present work focuses on the effect of internostril intensity difference on human heading perception from optic flow, in natural settings, olfactory spatial information can also be extracted from comparison of sequential sniffs (1, 10) and possibly internostril timing difference. The latter has been demonstrated in rats and sharks (12, 31). Considering that the perceived heading direction is a weighted sum of the estimates based on each sensory cue alone, with weights proportional to the relative reliabilities of the cues (32), we expect these olfactory cues to play a more pronounced role in navigation in circumstances where visual and other sensory cues are more vague or unavailable, for instance, tracking an odor or finding one’s way in the dark.
Together with other studies in the field, the current study points to interesting parallels between olfaction and vision, which may bring insights into the neural computational mechanisms underlying stereo olfaction. In vision, small differences between the retinal images of the two eyes (binocular disparity) give rise to stereoscopic depth perception (33). When the two retinal images are rendered distinctively different, observers experience binocular rivalry, in which one eye’s image dominates for several seconds and is then replaced by that of the other eye (34). Similarly, a moderate, but not high, binaral disparity in odor intensity generates directionality (present study), and when two distinctly different odorants are presented to the two nostrils, recipients experience alternating odor percepts, a phenomenon termed binaral rivalry (35). Extensive research has shown that binocular rivalry originates from inhibitory interactions among monocular neurons and binocular pattern-selective neurons (34), whereas a stereoscopic visual percept arises from binocular neurons tuned for disparity in the primary visual cortex as well as in both dorsal and ventral pathways (33). In the olfactory system, the first site of convergence between binaral inputs is the anterior olfactory nucleus (36, 37), which has been found to play a critical role in internostril odorant comparison and source localization in mice (38). We postulate that the tuning profiles of binaral neurons in the anterior olfactory nucleus and/or other downstream medial temporal regions (39) could set the limit for disparities that are converted to directional information. Moreover, the entorhinal cortex receives not only olfactory inputs but also cortical afferents from a wide range of areas including the medial superior temporal cortex (40) that mediates the extraction of heading information from visual optic flow as well as vestibular signals (41, 42). As such, it is ideally situated to integrate multisensory directional cues and support navigation (43, 44). Exactly how the computation and utilization of olfactory stereo information are implemented in the brain awaits future experimentation to clarify, which will also help test the olfactory spatial hypothesis that olfaction evolved for the primary purpose of navigating in a chemical world (26).
Materials and Methods
Participants.
A total of 216 healthy nonsmokers took part in the study, 72 in Experiment 1 (38 females; mean age ± SD: 22.6 ± 2.0 y), 36 in Experiment 2 (17 females, 22.2 ± 2.6 y), 36 in Experiment 3 (19 females, 22.3 ± 2.6 y), 36 in Experiment 4 (20 females, 22.8 ± 2.5 y), 12 in Experiment 5 (8 females, 22.2 ± 3.0 y), and 24 in Experiment 6 (12 females, 22.2 ± 2.8 y). All participants reported to have normal or corrected-to-normal vision, a normal sense of smell, and no respiratory allergy or upper respiratory infection at the time of testing. None had significant nasal septal deviation as assessed by nasal spirometry (GM Instruments) (nasal partitioning ratios ranged between −0.6 and 0.5) (45). They were blind to the experimental purposes. Written informed consent and consent to publish were obtained from all participants in accordance with ethical standards of the Declaration of Helsinki. The study was approved by the Institutional Review Board at the Institute of Psychology, Chinese Academy of Sciences.
Visual Stimuli.
The visual optic-flow stimuli (Movie S1) in Experiments 1 to 4 were made up of 1,800 moving white dots on a black background that simulated the self-motion of the observer toward a three-dimensional cube of points at 5 m/s. The coherence level was held at 75%, i.e., 25% of the dots (noise dots) appeared at random on each frame, rather than following the motion trajectory. They were displayed on a 34-inch curved monitor and subtended 70.2° horizontally and 33.2° vertically at 57-cm viewing distance. The center of expansion indicates the heading (Fig. 1A) and varied horizontally between 4θ leftward (−4θ) and 4θ rightward (4θ) in 7 logarithmic steps, i.e., 0°, ±θ, ±2θ, and ±4θ, where θ was set as 0.5° in Experiment 1 and roughly corresponded to the observer’s difference limen in heading perception in Experiments 2 to 4. Specifically, in Experiments 2 to 4, θ was individually adjusted and set prior to the actual experiment in the absence of olfactory stimuli and ranged between 0.25 and 0.7° across participants.
Olfactory Stimuli.
The olfactory stimuli consisted of various concentrations of phenylethyl alcohol dissolved in propylene glycol and vanillin dissolved in distilled water, as follows: Experiments 1 and 2: 0 (solvent only), 1, 2, 2.5, 3, 4, and 5% v/v phenylethyl alcohol in propylene glycol; Experiment 3: 0, 0.2, 0.4, 0.5, 0.6, 0.8, and 1% v/v phenylethyl alcohol in propylene glycol; Experiment 4: 0, 0.2, 0.4, 0.5, 0.6, 0.8, and 1% m/v (i.e., mg/mL) vanillin in water; Experiment 5: 0.3, 0.6, 1.2, 2.4, 4.8, and 9.6% v/v phenylethyl alcohol in propylene glycol; Experiment 6: all of those in Experiments 1 to 4 other than 2.5 and 0.5% v/v phenylethyl alcohol and 0.5% m/v vanillin. They were presented in identical 40-mL polypropylene jars; each jar contained 10 mL of clear liquid and was fitted with a Tygon R-3603 tube that connected to a Teflon nosepiece. In Experiments 1 to 4, the olfactory stimuli were presented dichorhinically (Fig. 1B) to form 4 levels of binaral concentration disparity: high (Experiments 1 to 2: 5% v/v in one nostril and 0% in the other; Experiment 3: 1 and 0% v/v; Experiment 4: 1 and 0% m/v), intermediate (Experiments 1 to 2: 4 and 1% v/v; Experiment 3: 0.8 and 0.2% v/v; Experiment 4: 0.8 and 0.2% m/v), low (Experiments 1 and 2: 3 and 2% v/v; Experiment 3: 0.6 and 0.4% v/v; Experiment 4: 0.6 and 0.4% m/v), and zero (Experiment 1: 2.5% v/v in both nostrils; Experiment 2: 0 or 2.5% v/v in both nostrils; Experiment 3: 0 or 0.5% v/v in both nostrils; Experiment 4: 0 or 0.5% m/v in both nostrils). Specifically, the jars (not including the tubes and nosepieces) were placed in pairs inside identical carton boxes (one pair per box) and covered from view to ensure that participants were unaware of which nostril smelled from which jar.
Procedure.
Each trial of the heading-judgment task (Fig. 1A) began with a stationary fixation cross (0.5° × 0.5°) for 250 ms, followed by an optic-flow field superimposed on the fixation cross for 500 ms (30 frames). The observer then made a forced choice judgment of whether he or she seemed to be heading to the left or right of fixation. The next trial began 1,000 ms after a response was made. In Experiment 1 there were 70 trials per block, with 10 trials per heading angle in random order and a 30 s break after 35 trials. Participants were randomly assigned to 3 groups of 24 each and performed 4 blocks of the task under either high, intermediate, or low binaral concentration disparity (between-subjects factor), where they received the higher concentration in the left nostril (L > R) in 2 blocks and in the right nostril (L < R) in the other 2 blocks, and 2 additional blocks under zero binaral disparity (L = R), where they received 2.5% v/v phenylethyl alcohol in both nostrils. The order of left–right relationship (within-subjects factor; L > R, L < R, L = R) was counter-balanced across participants in each group.
Experiments 2 to 4 adopted a fully within-subjects design. To efficiently and accurately determine the PSE point, which was estimated to be around 0° on the basis of the results of Experiment 1, we employed a hybrid psychometric procedure based on maximum-likelihood estimation (46) and the method of constant stimuli. There were 75 trials per block, with 15, 15, 10, and 5 trials per heading angle for 0°, ±θ, ±2θ, and ±4θ, respectively, and a 30-s break after every 25 trials. Participants completed a total of 16 blocks over 2 days (8 blocks per day). On each day, they performed 2 blocks under each of high, intermediate, and low levels of binaral concentration disparity, where they received the higher concentration in the left nostril in one block and in the right nostril in the other block, and 2 blocks under zero binaral disparity, where they received 2.5% v/v phenylethyl alcohol (Experiment 2), 0.5% v/v phenylethyl alcohol (Experiment 3), or 0.5% m/v vanillin (Experiment 4) in both nostrils in one block and 0% (solvent only) in both nostrils in the other block. Blocks of the same level of concentration disparity were performed consecutively. The order of the olfactory stimuli (concentration disparity and left–right relationship) was otherwise randomized within participants.
In all four experiments, the participants were told beforehand that the purpose of the study was to investigate whether a contextual odor would affect the accuracy of heading perception from optic flow and that they should focus on the optic-flow displays and ignore the contextual odor. No reference was made to dichorhinic stimulation. They were instructed to hold the carton box containing the olfactory stimuli with their nondominant hand, position the nosepieces in the two nostrils (to form seals at the nostrils), and continuously inhale through the nose and exhale through the mouth while they were performing the heading-judgment task. Each block lasted 5 to 6 min. There was a 2-min break in between the blocks to eliminate olfactory adaptation. After the completion of the heading-judgment task, each participant also performed a lateralization task (9), where they were blindfolded, dichorhinically presented with two concentrations of phenylethyl alcohol (Experiments 1 to 3) or of vanillin (Experiments 4) that formed either high, intermediate, or low binaral disparity, and asked to verbally report which nostril smelled a higher concentration or a stronger odor. Those in Experiment 1 performed 10 trials of the level of disparity they were assigned to, whereas those in Experiments 2 to 4 performed 30 trials, with 10 trials per level of binaral disparity, in random order. There was a break of at least 30 s in between two trials. In addition, for the majority of the participants in Experiments 2 to 4, the partitioning of airflow between the two nasal passages was assessed with a rhinospirometer (GM Instruments, UK) immediately before and after the heading-judgment task on each day of testing, which allowed us to extrapolate which nostril had a higher airflow in each block of the task.
In Experiment 5, participants rated on a 100-unit visual analog scale the perceived intensities of 6 different concentrations of phenylethyl alcohol (ranged from 0.3 to 9.6% v/v), presented to one of the two nostrils (the other nostril was presented with an empty jar; Fig. 3A). Each completed a total of 72 trials, with 6 trials per nostril per concentration in random order. In Experiment 6, participants were tested for the intensity discrimination of the odorants that formed high, intermediate, and low concentration disparities in Experiments 1 to 4. In each trial, the two concentrations in a pair were presented one after another to different nostrils (a procedure that mimics dichorhinic presentation), and the participants, blindfolded, reported which one smelled stronger. There were 9 concentration pairs and 8 trials per pair, in random order, resulting in a total of 72 trials. In both Experiments 5 and 6, there was a break of at least 30 s in between two trials to reduce olfactory fatigue.
Analyses.
For each participant under each olfactory condition, we calculated the proportions of rightward judgments and fitted them with a Boltzmann sigmoid function , where corresponds to the physical heading angle (−4θ, −2θ, −θ, 0°, θ, 2θ, and 4θ), corresponds to the PSE, at which the participant made leftward and rightward judgments with equal probability, and half the interquartile range of the fitted function corresponds to difference limen, an index of heading discrimination sensitivity. PSE and difference limen served as the primary dependent variables and were subsequently compared across conditions in a series of repeated-measures ANOVAs and paired sample t tests. We were mainly interested in whether smelling a higher concentration of phenylethyl alcohol or vanillin in the left, as opposed to the right, nostril would bias heading perception toward the left side and vice versa. Note that in Experiments 2 to 4, participants showed no difference in PSE (Ps = 0.91, 0.67, and 0.75, respectively) or difference limen (Ps = 0.88, 0.32, and 0.63, respectively) between receiving equal nonzero concentrations of phenylethyl alcohol (2.5% v/v in Experiment 2, 0.5% v/v in Experiment 3) or vanillin (0.5% m/v in Experiment 4) in both nostrils and receiving only the solvent (0%) in both nostrils. We therefore combined their heading judgments under these two settings and used the PSEs and difference limens of the combined data to characterize performances under zero binaral disparity. All statistical tests were two-tailed.
Data Availability.
Data are included in Dataset S1.
Supplementary Material
Acknowledgments
We thank Yan Zhu and Shenbing Kuang for assistance and Leslie M. Kay for comments. This work was supported by the Key Research Program of Frontier Sciences (QYZDB-SSW-SMC055) and the Strategic Priority Research Program (XDBS01010200) of the Chinese Academy of Sciences, the National Natural Science Foundation of China (31830037), and Beijing Municipal Science and Technology Commission.
Footnotes
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004642117/-/DCSupplemental.
References
- 1.Porter J., et al., Mechanisms of scent-tracking in humans. Nat. Neurosci. 10, 27–29 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Boie S. D., et al., Information-theoretic analysis of realistic odor plumes: What cues are useful for determining location? PLOS Comput. Biol. 14, e1006275 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vonbekesy G., Olfactory analogue to directional hearing. J. Appl. Physiol. 19, 369–373 (1964). [DOI] [PubMed] [Google Scholar]
- 4.Porter J., Anand T., Johnson B., Khan R. M., Sobel N., Brain mechanisms for extracting spatial information from smell. Neuron 47, 581–592 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Kleemann A. M., et al., Trigeminal perception is necessary to localize odors. Physiol. Behav. 97, 401–405 (2009). [DOI] [PubMed] [Google Scholar]
- 6.Croy I., et al., Human olfactory lateralization requires trigeminal activation. Neuroimage 98, 289–295 (2014). [DOI] [PubMed] [Google Scholar]
- 7.Frasnelli J., Charbonneau G., Collignon O., Lepore F., Odor localization and sniffing. Chem. Senses 34, 139–144 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Kobal G., Van Toller S., Hummel T., Is there directional smelling? Experientia 45, 130–132 (1989). [DOI] [PubMed] [Google Scholar]
- 9.Wysocki C. J., Cowart B. J., Radil T., Nasal trigeminal chemosensitivity across the adult life span. Percept. Psychophys. 65, 115–122 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Welge-Lussen A., Looser G. L., Westermann B., Hummel T., Olfactory source localization in the open field using one or both nostrils. Rhinology 52, 41–47 (2014). [DOI] [PubMed] [Google Scholar]
- 11.Sorokowska A., et al., Odor lateralization and spatial localization: Null effects of blindness. Atten. Percept. Psychophys. 81, 2078–2087 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rajan R., Clement J. P., Bhalla U. S., Rats smell in stereo. Science 311, 666–670 (2006). [DOI] [PubMed] [Google Scholar]
- 13.Catania K. C., Stereo and serial sniffing guide navigation to an odour source in a mammal. Nat. Commun. 4, 1441 (2013). [DOI] [PubMed] [Google Scholar]
- 14.Goodale M. A., Westwood D. A., An evolving view of duplex vision: Separate but interacting cortical pathways for perception and action. Curr. Opin. Neurobiol. 14, 203–211 (2004). [DOI] [PubMed] [Google Scholar]
- 15.Garde M. M., Cowey A., “Deaf hearing”: Unacknowledged detection of auditory stimuli in a patient with cerebral deafness. Cortex 36, 71–80 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Paillard J., Michel F., Stelmach G., Localization without content. A tactile analogue of ‘blind sight’. Arch. Neurol. 40, 548–551 (1983). [DOI] [PubMed] [Google Scholar]
- 17.Lappe M., Bremmer F., van den Berg A. V., Perception of self-motion from visual flow. Trends Cogn. Sci. 3, 329–336 (1999). [DOI] [PubMed] [Google Scholar]
- 18.Doty R. L., et al., Intranasal trigeminal stimulation from odorous volatiles: Psychometric responses from anosmic and normal humans. Physiol. Behav. 20, 175–185 (1978). [DOI] [PubMed] [Google Scholar]
- 19.Davison A. C., Hinkley D. V., Bootstrap Methods and Their Application (Cambridge University Press, Cambridge, UK, 1997). [Google Scholar]
- 20.Hasegawa M., Kern E. B., The human nasal cycle. Mayo Clin. Proc. 52, 28–34 (1977). [PubMed] [Google Scholar]
- 21.Kahana-Zweig R., et al., Measuring and characterizing the human nasal cycle. PLoS One 11, e0162918 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowell J. A., Banks M. S., Perceiving heading with different retinal regions and types of optic flow. Percept. Psychophys. 53, 325–337 (1993). [DOI] [PubMed] [Google Scholar]
- 23.Copelli M., Roque A. C., Oliveira R. F., Kinouchi O., Physics of psychophysics: Stevens and Weber-Fechner laws are transfer functions of excitable media. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 65, 060901 (2002). [DOI] [PubMed] [Google Scholar]
- 24.Sirotin Y. B., Shusterman R., Rinberg D., Neural coding of perceived odor intensity. eNeuro 2, ENEURO.0083-15.2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Doty R. L., Frye R., Influence of nasal obstruction on smell function. Otolaryngol. Clin. North Am. 22, 397–411 (1989). [PubMed] [Google Scholar]
- 26.Jacobs L. F., From chemotaxis to the cognitive map: The function of olfaction. Proc. Natl. Acad. Sci. U.S.A. 109 (suppl. 1), 10693–10700 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olofsson J. K., Gottfried J. A., The muted sense: Neurocognitive limitations of olfactory language. Trends Cogn. Sci. 19, 314–321 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fetsch C. R., Turner A. H., DeAngelis G. C., Angelaki D. E., Dynamic reweighting of visual and vestibular cues during self-motion perception. J. Neurosci. 29, 15601–15612 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calabro F. J., Soto-Faraco S., Vaina L. M., Acoustic facilitation of object movement detection during self-motion. Proc. Biol. Sci. 278, 2840–2847 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radvansky B. A., Dombeck D. A., An olfactory virtual reality system for mice. Nat. Commun. 9, 839 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gardiner J. M., Atema J., The function of bilateral odor arrival time differences in olfactory orientation of sharks. Curr. Biol. 20, 1187–1191 (2010). [DOI] [PubMed] [Google Scholar]
- 32.Angelaki D. E., Gu Y., DeAngelis G. C., Multisensory integration: Psychophysics, neurophysiology, and computation. Curr. Opin. Neurobiol. 19, 452–458 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Parker A. J., Binocular depth perception and the cerebral cortex. Nat. Rev. Neurosci. 8, 379–391 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Tong F., Meng M., Blake R., Neural bases of binocular rivalry. Trends Cogn. Sci. 10, 502–511 (2006). [DOI] [PubMed] [Google Scholar]
- 35.Zhou W., Chen D., Binaral rivalry between the nostrils and in the cortex. Curr. Biol. 19, 1561–1565 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yan Z., et al., Precise circuitry links bilaterally symmetric olfactory maps. Neuron 58, 613–624 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Grobman M., et al., A mirror-symmetric excitatory link coordinates odor maps across olfactory bulbs and enables odor perceptual unity. Neuron 99, P800.E6– P813.E6 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Esquivelzeta Rabell J., Mutlu K., Noutel J., Martin Del Olmo P., Haesler S., Spontaneous rapid odor source localization behavior requires interhemispheric communication. Curr. Biol. 27, P1542.E4–P1548.E4 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Wilson D. A., Binaral interactions in the rat piriform cortex. J. Neurophysiol. 78, 160–169 (1997). [DOI] [PubMed] [Google Scholar]
- 40.Insausti R., Amaral D. G., Cowan W. M., The entorhinal cortex of the monkey: II. Cortical afferents. J. Comp. Neurol. 264, 356–395 (1987). [DOI] [PubMed] [Google Scholar]
- 41.Duffy C. J., Wurtz R. H., Sensitivity of MST neurons to optic flow stimuli. I. A continuum of response selectivity to large-field stimuli. J. Neurophysiol. 65, 1329–1345 (1991). [DOI] [PubMed] [Google Scholar]
- 42.Gu Y., DeAngelis G. C., Angelaki D. E., A functional link between area MSTd and heading perception based on vestibular signals. Nat. Neurosci. 10, 1038–1047 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Spiers H. J., Barry C., Neural systems supporting navigation. Curr. Opin. Behav. Sci. 1, 47–55 (2015). [Google Scholar]
- 44.Ravassard P., et al., Multisensory control of hippocampal spatiotemporal selectivity. Science 340, 1342–1346 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roblin D. G., Eccles R., Normal range for nasal partitioning of airflow determined by nasal spirometry in 100 healthy subjects. Am. J. Rhinol. 17, 179–183 (2003). [PubMed] [Google Scholar]
- 46.Pentland A., Maximum likelihood estimation: The best PEST. Percept. Psychophys. 28, 377–379 (1980). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are included in Dataset S1.