Abstract
The Casparian strip (CS) is a tight junction-like structure formed by lignin impregnation on the walls of endodermal cells in plant roots. The CS membrane domain (CSDM), demarked by the CASP proteins, is important for orienting lignification enzymes. Here, we report that an endodermis-expressed multicopper oxidase, LACCASE3 (LAC3) in Arabidopsis, locates to the interface between lignin domains and the cell wall during early CS development prior to CASP1 localizing to CSDM and eventually flanks the mature CS. Pharmacological perturbation of LAC3 causes dispersed localization of CASP1 and compensatory ectopic lignification. These results support the existence of a LAC3-based CS wall domain which coordinates with CSDM to provide bidirectional positional information that guides precise CS lignification.
Keywords: Casparian strip, laccase, lignin, positional information, copper
Tight junctions blocking ion flux, pathogen entry, and protein movement through the paracellular pathway are a conserved feature of multicellular organisms (1). In higher plants, the Casparian strip (CS) is a crucial apoplastic diffusion barrier built by the endodermis (2). Unlike junctions of animal cells that are formed by simultaneously pulling together and sealing off adjacent cytoplasmic membranes with large protein complexes, the existence of a primary wall surrounding plant cells has rendered two fundamental features in CS formation. First, CS sealing is controlled by precise spatial information, resulting in a ring-like structure aligning the anticlinal and transversal cell walls (3). Second, in plants such as Arabidopsis, CS is primarily sealed with lignin, a phenylpropanoid polymer that deposits in the primary cell wall to span the predefined extracellular space between neighboring cells (4). Part of the spatial information is encoded by CASPs, which demark the CS membrane domain (CSDM), a specialized membrane region excluding most proteins and lipid dyes (5, 6). CSDM is thought to form a scaffold for recruiting and spatially restricting lignification enzymes such as PER64 (7), which is under surveillance by a peptide signaling pathway that comprises the ligands CIF1/CIF2, the leucine-rich repeat receptor kinase SGN3, and the polarly localized kinase SGN1 (8–11). Because lignin deposition takes place in the primary wall, we speculate there may exist a CS wall domain (CSDW) which coordinates with CSDM and the CIF-SGN3 module to provide bidirectional positional information for accurate CS lignification.
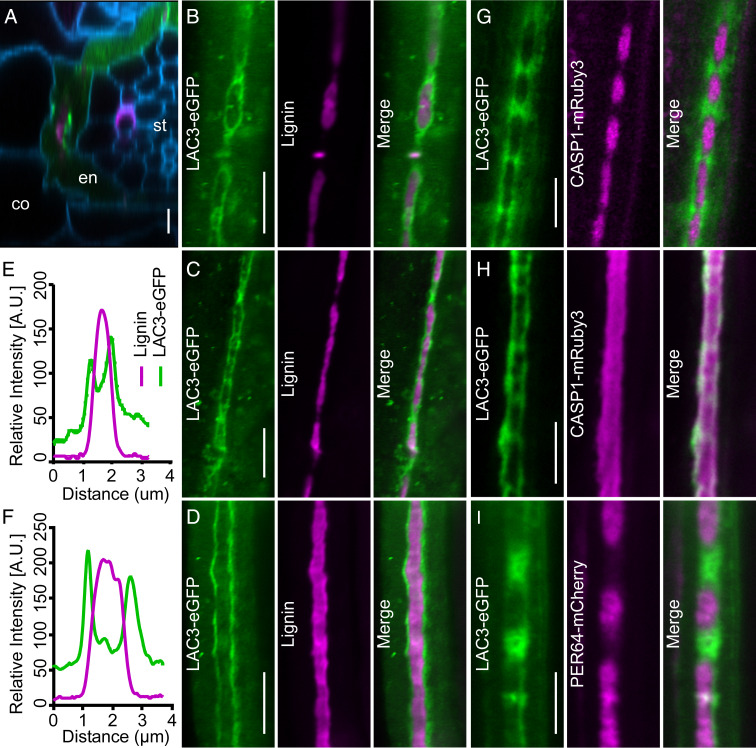
Laccases are secretory multicopper oxidases involved in lignin polymerization (12). Gene expression profiling of root subzones (13) reveals that transcript of LAC3, among the 17 laccase-encoding genes in Arabidopsis, preferentially accumulates in endodermal cells. Using the proLAC3::LAC3-eGFP transgenic line that expresses LAC3-eGFP driven by the native promoter, we observed that LAC3 is expressed in endodermis and remains secretory between 4.5 ± 1.5 and 17.5 ± 2.5 cells after onset of cell elongation (Fig. 1A and Movies S1 and S2), which temporally spans CS development (5–7). At the subcellular level, CS lignification initiates in a string-of-pearls pattern, after which the discrete lignin domains fuse to form a completely sealed ring-like structure (6). LAC3 initially surrounds nascently formed lignin “pearls” and accumulates between two adjacent “pearls” where new lignin would deposit (Fig. 1B). As the lignin domains expand, LAC3 signals are pushed outward and gradually localize to the borders of fused CS regions (Fig. 1 C and E). Eventually, LAC3 locates to the borders flanking the continuous lignin band (Fig. 1 D and F and Movie S3). Thus, LAC3 participates in a dynamic CSDW at the interface between the primary cell wall and deposited lignin during CS formation.
Fig. 1.
Surface view of LAC3 localization during CS development. (A) Radial section of a proLAC3::LAC3-eGFP root showing LAC3-eGFP (green) in relation to lignin (basic fuchsin, magenta) and the cell wall (calcofluor white, blue). co, cortex; en, endodermis; st, stele. (B–D) Localization of LAC3-eGFP (green) at the string-of-pearls (B), transition (C), and mature (D) stage of CS development in relation to lignin. (E and F) Quantification of relative LAC3-eGFP and basic fuchsin fluorescence intensity in C and D. (G and H) Localization of LAC3-eGFP (green) and CASP1-mRuby3 (magenta) at the string-of-pearls (G) and mature (H) stage in the dual reporter line. (I) Localization of LAC3-eGFP (green) and PER64-mCherry (magenta) at the string-of-pearls stage in the dual reporter line. (Scale bars, 5 μm.)
In the proLAC3::LAC3-eGFP proCASP1::CASP1-mRuby3 dual reporter line, LAC3 accumulates in between two adjacent CASP1 domains (Fig. 1G and Movie S4). These original LAC3-positive but CASP1-negative sites are later marked by CASP1, as the CASP1 domains further fuse and push LAC3 toward the borders (Movie S4). After the CASP1 domains are continuously fused, LAC3 colocalizes with CASP1 on the CS borders (Fig. 1H). In the proLAC3::LAC3-eGFP proPER64::PER64-mCherry reporter line, LAC3 and PER64 display an interspersed spatial relationship in early endodermis similar to LAC3 and CASP1 (Fig. 1I). These observations indicate that LAC3-based CSDW and CASP1-defined CSDM are spatially separated yet coordinated in CS development.
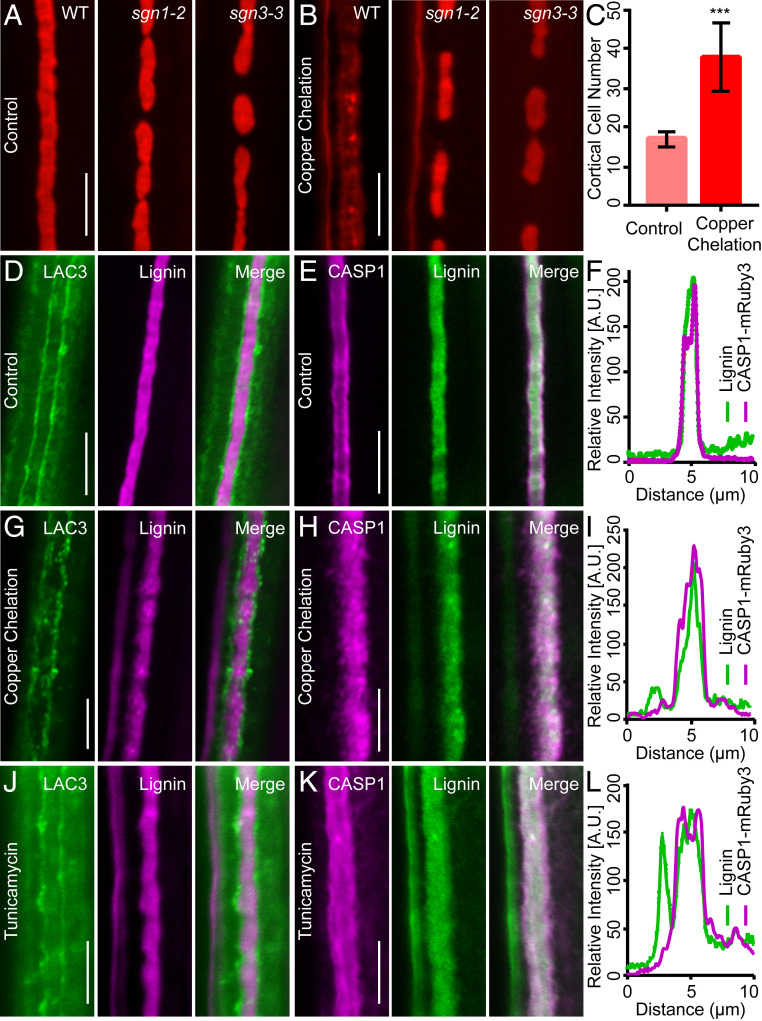
Laccases are genetically redundant glycosylated cuproenzymes (14). We utilized the copper chelator bathocuproinedisulfonic acid and the N-glycosylation inhibitor tunicamycin to assess the potential effects of compromising LAC3 and related laccases. Upon copper chelation, the continuous CS becomes dispersed, compensatory lignification is activated at the cortex-facing side (Fig. 2 A and B), and the function of CS as a diffusion barrier is breached (Fig. 2C). Ectopic lignification induced by copper chelation is specifically blocked in sgn3 (Fig. 2 A and B), suggesting that copper is required for the establishment of a functional CS and prevention of SGN3-mediated compensatory lignification. Upon copper chelation, LAC3 signals continuously aligning the CS borders become discrete and form locally concentrated patches while CASP1 localization, which demarks the CSDM, loses clear borders and becomes more diffused, similar to the dispersed lignin deposition (Fig. 2 D–I). These effects were recapitulated by tunicamycin treatment (Fig. 2 J–L). Although neither bathocuproinedisulfonic acid nor tunicamycin is specific for laccases, commonality of the two treatments on LAC3 and CASP1 localization suggests that LAC3-based CSDW focalizes CSDM to provide positional information guiding CS lignification.
Fig. 2.
Putative pharmacological perturbation of LAC3 disperses lignin and CASP1. (A and B) Mature CS stained with basic fuchsin in the indicated genotypes under control condition (A) and copper chelation (B). (C) Quantification of propidium iodide permeability. Data represent mean and SD (n = 5). ***P < 0.001 by Student’s t test. (D–F) Localization of LAC3-eGFP (green) in relation to CS lignin (basic fuchsin, magenta) (D), CASP1-mRuby3 (magenta) in relation to CS lignin (auramine O, green) (E), and comparison of CASP1-mRuby3 and lignin distribution (F) under the control condition. (G–I) Effects of copper chelation on LAC3-eGFP, CASP1-mRuby3, and CS lignin localization. (J–L) Effects of tunicamycin treatment. (Scale bars, 5 μm.)
Materials and Methods
Plant Materials.
The sgn1-2 and sgn3-3 mutants were respectively Salk_055095 and Salk_043282. Primers CCAAGCTTGCATGCCTGCAGCTTCTTGTGTGCAGGAGCT and GTACCCGGGGATCCTCTAGAGCATCTTGGAAGATCCAATG were used to clone a 4,090-bp genomic region into pCambia1300-native-eGFP for generating proLAC3::LAC3-eGFP. Primers CCAAGCTTGCATGCCTGCAGTGAGAATTGGCGATTAAAGA and GTACCCGGGGATCCTCTAGAATGCCTCTTGAGGGCGATGG were used to clone a 2,954-bp genomic region into pCambia1300-native-mRuby3 for generating proCASP1::CASP1-mRuby3. The proPER64::PER64-mCherry line was as previously described (7). The dual reporter lines were generated by crossing.
Pharmacological Treatments.
A copper dropout 1/2 Murashige and Skoog (MS) medium was supplemented with 1 µM CuSO4 as control and 50 µM disodium salt of bathocuproinedisulfonic acid (Sigma) for copper chelation. Tunicamycin (Pharmabiology) was added to the 1/2 MS medium at a final concentration of 0.05 μg/mL.
CS Assays.
Basic fuchsin, auramine O, and calcofluor white staining assays were performed on 5-d-old seedlings using the previously described ClearSee protocol (15). Propidium iodide penetration assay was performed as described (5). Confocal images were acquired by an LSM 800 microscope equipped with Airyscan (Zeiss). The original images were processed using Zeiss ZEN software and ImageJ.
Data Availability.
All of the data in this study are included in the paper or Movies S1–S4.
Supplementary Material
Acknowledgments
We thank Drs. N. Geldner, A. L. Samuels, and Q. Zhao for providing genetic materials. This work was supported by grants from the National Key Research and Development Program of China (2017YFA0503800) and the National Natural Science Foundation of China (31621001).
Footnotes
The authors declare no competing interest.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2005429117/-/DCSupplemental.
References
- 1.Anderson J. M., Van Itallie C. M., Physiology and function of the tight junction. Cold Spring Harb. Perspect. Biol. 1, a002584 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nagahashi G., Thomson W. W., Leonard R. T., The Casparian strip as a barrier to the movement of lanthanum in corn roots. Science 183, 670–671 (1974). [DOI] [PubMed] [Google Scholar]
- 3.Geldner N., The endodermis. Annu. Rev. Plant Biol. 64, 531–558 (2013). [DOI] [PubMed] [Google Scholar]
- 4.Naseer S.et al., Casparian strip diffusion barrier in Arabidopsis is made of a lignin polymer without suberin. Proc. Natl. Acad. Sci. U.S.A. 109, 10101–10106 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alassimone J., Naseer S., Geldner N., A developmental framework for endodermal differentiation and polarity. Proc. Natl. Acad. Sci. U.S.A. 107, 5214–5219 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roppolo D.et al., A novel protein family mediates Casparian strip formation in the endodermis. Nature 473, 380–383 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Lee Y., Rubio M. C., Alassimone J., Geldner N., A mechanism for localized lignin deposition in the endodermis. Cell 153, 402–412 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Nakayama T.et al., A peptide hormone required for Casparian strip diffusion barrier formation in Arabidopsis roots. Science 355, 284–286 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Doblas V. G.et al., Root diffusion barrier control by a vasculature-derived peptide binding to the SGN3 receptor. Science 355, 280–284 (2017). [DOI] [PubMed] [Google Scholar]
- 10.Pfister A.et al., A receptor-like kinase mutant with absent endodermal diffusion barrier displays selective nutrient homeostasis defects. eLife 3, e03115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alassimone J.et al., Polarly localized kinase SGN1 is required for Casparian strip integrity and positioning. Nat. Plants 2, 16113 (2016). [DOI] [PubMed] [Google Scholar]
- 12.Yi Chou E.et al., Distribution, mobility, and anchoring of lignin-related oxidative enzymes in Arabidopsis secondary cell walls. J. Exp. Bot. 69, 1849–1859 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Birnbaum K.et al., A gene expression map of the Arabidopsis root. Science 302, 1956–1960 (2003). [DOI] [PubMed] [Google Scholar]
- 14.Turlapati P. V., Kim K. W., Davin L. B., Lewis N. G., The laccase multigene family in Arabidopsis thaliana: Towards addressing the mystery of their gene function(s). Planta 233, 439–470 (2011). [DOI] [PubMed] [Google Scholar]
- 15.Ursache R., Andersen T. G., Marhavý P., Geldner N., A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J. 93, 399–412 (2018). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All of the data in this study are included in the paper or Movies S1–S4.