Significance
The use of BRAF V600E-selective inhibitor dabrafenib and MEK inhibitor trametinib is a standard first-line treatment for human cancers harboring BRAF V600E. However, drug resistance/tumor recurrence is common with this treatment and the outcomes are unpredictable. We demonstrate here that dabrafenib and trametinib robustly induce cell apoptosis and hence abolishment of growth/regrowth of cancers harboring both BRAF V600E and TERT promoter mutations with little effect in cells/tumors harboring only BRAF V600E. Thus, TERT promoter mutation, by governing the apoptotic and hence therapeutic sensitivities of BRAF-mutant cancer cells to BRAF/MEK inhibitors, may potentially be useful in helping patient selection for the inhibitor treatment and predicting therapeutic outcomes.
Keywords: BRAF V600E, TERT promoter mutation, BRAF inhibitor, MEK inhibitor, apoptosis
Abstract
Combination use of BRAF V600E inhibitor dabrafenib and MEK inhibitor trametinib has become a standard treatment for human cancers harboring BRAF V600E. Its anticancer efficacies vary, however, with dramatic efficacy in some patients and drug resistance/tumor recurrence in others, which is poorly understood. Using thyroid cancer, melanoma, and colon cancer cell models, we showed that dabrafenib and trametinib induced robust apoptosis of cancer cells harboring both BRAF V600E and TERT promoter mutations but had little proapoptotic effect in cells harboring only BRAF V600E. Correspondingly, the inhibitors nearly completely abolished the growth of in vivo tumors harboring both mutations but had little effect on tumors harboring only BRAF V600E. Upon drug withdrawal, tumors harboring both mutations remained hardly measurable but tumors harboring only BRAF V600E regrew rapidly. BRAF V600E/MAP kinase pathway is known to robustly activate mutant promoter of TERT, a strong apoptosis suppressor. Thus, for survival, cancer cells harboring both mutations may have evolved to rely on BRAF V600E-promoted and high-TERT expression-mediated suppression of apoptosis. As such, inhibition of BRAF/MEK can trigger strong apoptosis-induced cell death and hence tumor abolishment. This does not happen in cells harboring only BRAF V600E as they have not developed reliance on TERT-mediated suppression of apoptosis due to the lack of mutant promoter-driven high-TERT expression. TERT promoter mutation governs BRAF-mutant cancer cells’ apoptotic and hence therapeutic responses to BRAF/MEK inhibitors. Thus, the genetic duet of BRAF V600E and TERT promoter mutation represents an Achilles Heel for effective therapeutic targeting and response prediction in cancer.
BRAF and MEK inhibitors have been applied to the treatment of human cancers harboring the BRAF V600E mutation for many years. However, the anticancer efficacies of these inhibitors vary widely with two extremes—with excellent responses in some cancers but drug resistance/tumor recurrence in others. As an example, the therapeutic responses of BRAF-mutant cancers to these inhibitors ranged from a response rate of 48% in melanoma to 5% in colorectal cancer (1, 2). Although several molecular mechanisms for such drug resistance have been proposed (3, 4), little clinical progress has occurred in tackling it. Moreover, there has been no study to investigate the role of cell apoptosis in the drug effect of this anticancer treatment, which is critical to cell death and would therefore determine the therapeutic effects.
This BRAF V600E-targeted treatment is widely used because BRAF V600E is common, seen in about 60% of melanomas, 45% of thyroid cancers, and 10% of colorectal cancers, and represents one of the best-defined therapeutic targets in human cancers (5, 6). Combination use of BRAF/MEK inhibitors, represented by the BRAF V600E-selective inhibitor dabrafenib and the MEK inhibitor trametinib, improves the treatment compared with their individual use and is now a standard Food and Drug Administration (FDA)-approved therapy for BRAF-mutant cancers (7, 8). Yet, the efficacies of even this combination treatment still has two extremes; they may induce dramatic tumor shrinkage or even complete tumor resolution in some cases while drug resistance/tumor recurrence occur in others (9, 10). The reason remains unclear and, in particular, there is no known underlying genetic background. There is also currently no way clinically to predict the therapeutic responses of BRAF-mutant cancers to BRAF/MEK inhibitors.
The genetic duet of BRAF V600E and TERT promoter mutations has been identified in several human cancers, including thyroid cancer and melanoma, which synergistically promotes aggressive clinicopathological outcomes, including disease recurrence and patient mortality (11–14). The mechanism underpinning this synergistic oncogenicity of the two mutations involves their strong cooperation in up-regulating the mutant TERT through a BRAF V600E/MAP kinase pathway → FOS → GABP → mutant TERT axis (15). A key element in this process is the specific binding and activation of the mutant TERT promoter by GABP, which is promoted by the BRAF V600E/MAP kinase pathway signaling. In this way, strong oncogenic cooperation and synergism between the two mutations occur. Given this unique molecular mechanism, we tested here our hypothesis that the TERT promoter mutation may be a key determining factor for the therapeutic efficacy of BRAF and MEK inhibitors in BRAF-mutant cancers. As apoptosis leading to cell death is a critical determinant for effective drug treatment of cancers, here we particularly investigated the apoptotic and suppressive responses of BRAF-mutant cancer cells and tumors to dabrafenib and trametinib with respect to the genetic status of TERT.
Results
Effects of Dabrafenib and Trametinib on Apoptosis of Cancer Cells Harboring Only BRAF V600E Mutation or Both BRAF V600E and TERT Promoter Mutations.
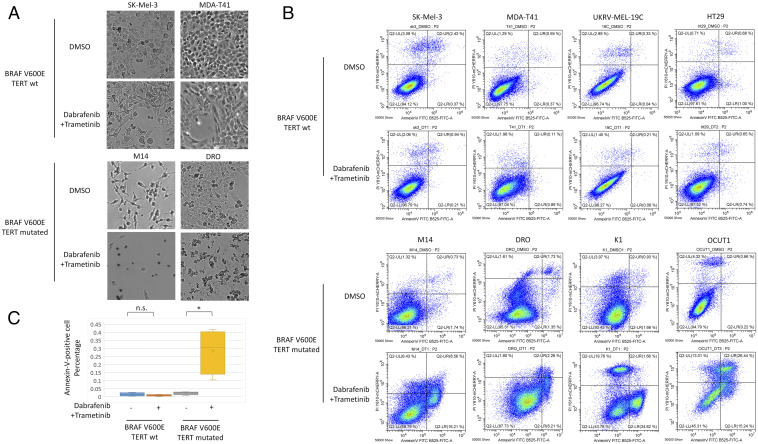
We found that cancer cells harboring BRAF V600E and wild-type TERT (termed “BRAF-only cells” hereafter) survived well under the combination treatment of dabrafenib and trametinib while the same treatment induced severe death, morphologically, of cells harboring both BRAF V600E and TERT promoter mutations (termed “genetic-duet cells” hereafter) (Fig. 1A). Correspondingly, the genetic-duet cells displayed robust apoptosis with the treatment, with Annexin V-positive cells rising from ∼3% in the control group to 10–40% in the drug treatment group (Fig. 1 B and C), while no apoptosis occurred in BRAF-only cells (Fig. 1 A–C), under the test conditions.
Fig. 1.
Effects of the combination treatment with dabrafenib and trametinib on apoptosis of cancer cells harboring only BRAF V600E mutation (BRAF-only) or both BRAF V600E and TERT promoter mutations (genetic-duet). (A) Optical microscopic examination (400×) of morphological death changes after drug or control DMSO treatment of BRAF-only cells (SK-Mel-3 and MDA-T41) and genetic-duet cells (M14 and DRO). (B) Flow cytometry analysis of apoptosis, in terms of percentage of Annexin V and PI positivities, in BRAF-only cells (SK-Mel-3, MDA-T41, UKRV-Mel-19c, and HT29) and genetic-duet cells (M14, DRO, K1, and OCUT1) after dabrafenib + trametinib or DMSO treatment. (C) Bar graph summary of the average percentages of Annexin V-positivity in genetic-duet cells and BRAF-only cells after treatments in each cell group corresponding to Fig. 1B. *P < 0.05; n.s.: P > 0.05.
Effects of Dabrafenib and Trametinib on Apoptotic Proteins in Cancer Cells Harboring Only BRAF V600E Mutation or Both BRAF V600E and TERT Promoter Mutations.
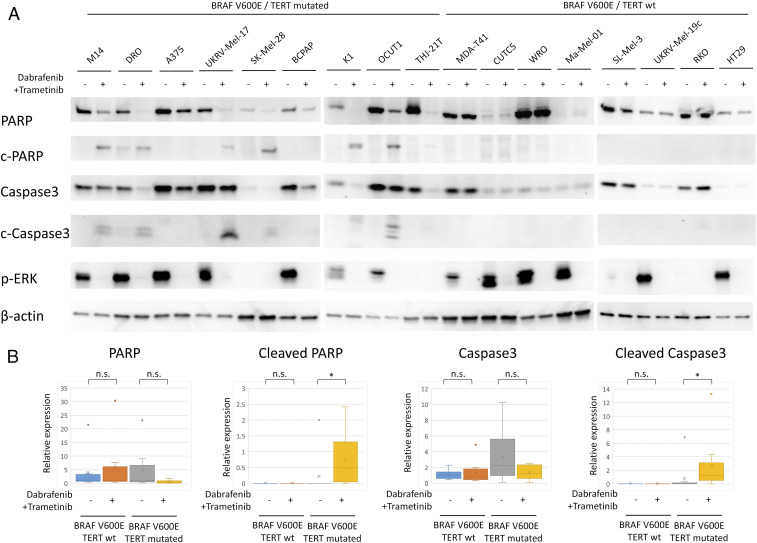
To definitively confirm true apoptosis, we examined and compared apoptosis proteins in genetic-duet cells and BRAF-only cells (Fig. 2 A and B). Total proteins of PARP and caspase-3 were significantly decreased and the cleaved proteins were correspondingly increased with the drug treatment in genetic-duet cells, confirming apoptotic signaling activation. The same treatment had no or little effect on the apoptotic protein patterns in BRAF-only cells. TERT expression was also suppressed by the treatment in genetic-duet cells but not BRAF-only cells. ERK phosphorylation was completely inhibited by dabrafenib and trametinib in both genetic-duet and BRAF-only cells, confirming the specific target effects of the inhibitors.
Fig. 2.
Effects of the combination treatment with dabrafenib and trametinib on apoptotic proteins of 19 different cancer cell lines harboring only BRAF V600E mutation (BRAF-only) or both BRAF V600E and TERT promoter mutations (genetic-duet). (A) Western blotting analysis of proteins in the apoptotic signaling, showing changes in total proteins of PARP and Caspase-3 and their corresponding cleaved proteins (C-PARP and C-Caspase-3) in genetic-duet cells and BRAF-only cells after treatments. The protein quantities were matched for correspondingly paired DMSO and dabrafenib + trametinib treatment groups in each cell as shown by the β-actin levels. (B) Bar graph illustration of the relative level of apoptotic proteins in Fig. 2A, representing the average of band intensities of the indicated protein normalized by corresponding β-actin level of the cells in each treatment groups. The color dots represent outliers of the relative expression. *P < 0.05; n.s.: P > 0.05.
Effects of Dabrafenib and Trametinib on the Growth/Regrowth of Xenograft Tumors Harboring Only BRAF V600E Mutation or Both BRAF V600E and TERT Promoter Mutations.
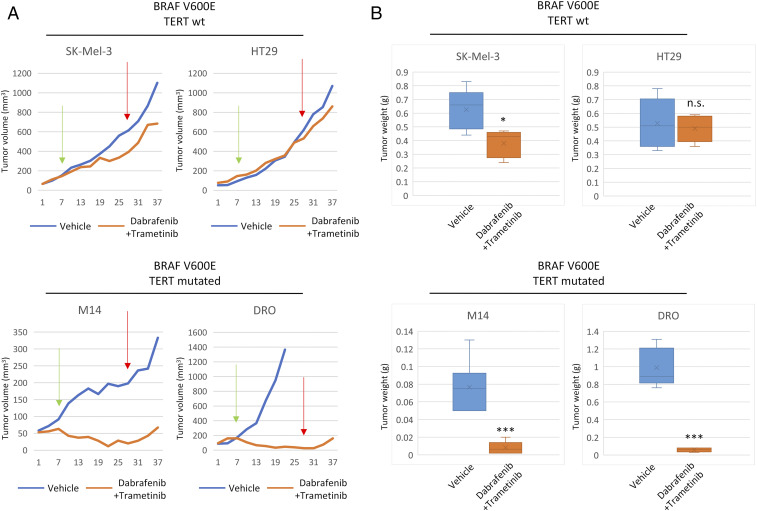
We next examined the therapeutic effects of dabrafenib and trametinib in vivo using BRAF-mutant xenograft tumors with respect to the genetic status of the BRAF and TERT genes. As shown in Fig. 3A, genetic-duet tumor growth was virtually completely abolished with the combination drug treatment, while BRAF-only tumors showed only partial growth slowdown with the drug treatment. After 21 d of continuous drug treatment, genetic-duet tumors became virtually unmeasurable and did not regrow in the subsequent 10 d after drug treatment was withheld; the final tumor weights in the drug treatment group were sharply lower than those in the control group (Figs. 3B and 4). In contrast, BRAF-only tumors kept regrowing rapidly after drug withdrawal and, in some cases, approximated the control group; in the end, tumors in the drug-treated group had similar weights as tumors in the control group. As a dramatic case, genetic-duet DRO tumors with control vehicle treatment grew rapidly to the tumor burden limit set by our institutionally approved animal care protocol and the animals had to be killed at 15 d after treatment, while DRO tumors with drug treatment remained nearly completely abolished even after drug withdrawal.
Fig. 3.
Effects of the combination treatment with dabrafenib and trametinib on the growth of xenograft tumors harboring only BRAF V600E mutation (BRAF-only) or both BRAF V600E and TERT promoter mutations (genetic-duet). (A) Time courses of the growth of BRAF-only tumors (SK-Mel-3 and HT29) and genetic-duet tumors (M14 and DRO) treated with DMSO (vehicle) or combined dabrafenib and trametinib as described in Materials and Methods. Green arrows indicate day 7 (day 14 for M14 cells; labeled as “Day 7” for unified formality of the x axis of the figure) as the start of the treatment after cell inoculation and red arrows indicate day 28 (actually day 35 for M14 cells) as the termination of the treatment. (B) Bar graph illustration of the average final tumor weights, corresponding to Fig. 2A. *P < 0.05, ***P < 0.001, n.s.: P > 0.05.
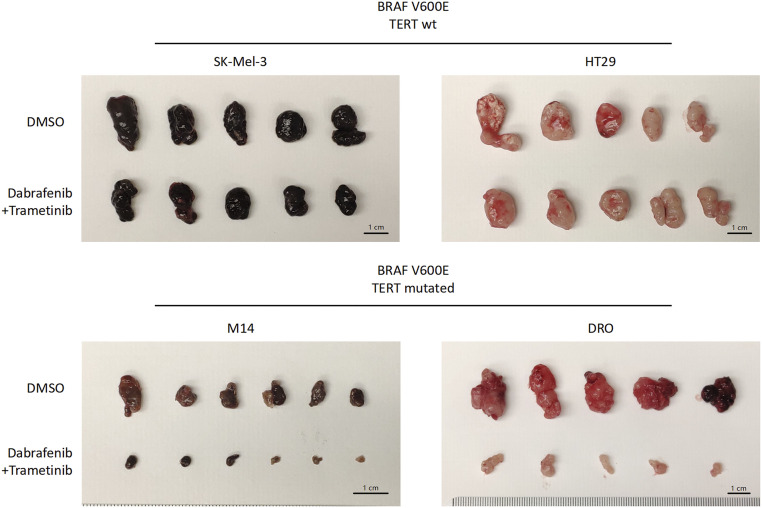
Fig. 4.
Photograph of the xenograft tumors surgically removed. Animals in this figure correspond to those in Fig. 3. Specifically, animals with xenograft tumors grown from cancer cells SK-Mel-3 and HT29 harboring only BRAF V600E or cells M14 and DRO harboring both BRAF V600E and TERT promoter mutations were treated with DMSO (vehicle) or combined dabrafenib and trametinib as described in Fig. 3. At the end of the experiment shown in Fig. 3, animals were killed and tumors were surgically removed and photographed. Each group had 5–6 animals.
Discussion
It remains unclear why the clinical outcomes of anticancer treatment with BRAF and MEK inhibitors, exemplified by the FDA-approved and widely used BRAF inhibitor dabrafenib and MEK inhibitor trametinib, vary so widely in BRAF-mutant human cancers (1, 2, 9, 10). This preclinical study investigated the TERT promoter mutation for its possible role in this clinical challenge. In particular, given the critical importance of apoptosis-induced cancer cell death in achieving effective anticancer treatment, we focused on the role of cell apoptosis in the therapeutic effects of these inhibitors with respect to the TERT promoter mutation status.
In addition to its classical telomerase function, TERT is well known to also be a strong suppressor of cell apoptosis (16, 17). It has been recently established that the BRAF V600E-activated MAP kinase pathway signaling robustly and specifically activates the mutant TERT promoter and up-regulates TERT expression (15). The present study now demonstrates that inhibition of the BRAF V600E-activated MAP kinase pathway by dabrafenib and trametinib induces robust apoptosis and nearly completely abolishes tumors of genetic-duet cancer cells but not BRAF-only cells. These results, taken together, suggest that genetic-duet cells have evolved to develop a strong survival dependence on BRAF V600E-promoted suppression of apoptosis through specific activation of the mutant TERT promoter and hence up-regulation of the TERT gene, which is absent in BRAF-only cells. Consequently, abrupt inhibition of the MAP kinase pathway can induce cell apoptosis and tumor disappearance in genetic-duet cancer cells but not BRAF-only cells. As a result, after drug withdrawal virtually no tumor regrowth occurred with genetic-duet cancer cells but tumor regrew rapidly with BRAF-only cells. Thus, on one hand, the duet of BRAF V600E and TERT promoter mutations is a genetic background that, as previously demonstrated, is a robust driver for aggressiveness of human cancer; on the other hand, this genetic duet is an Achilles Heel that, as demonstrated in the present study, represents a most vulnerable therapeutic target in cancer.
Our results on these differential proapoptotic effects of BRAF/MEK inhibitors in BRAF-mutant cancer cells, determined by the genetic status of TERT, are different from previous studies showing their inhibitory effects on the proliferation of virtually all BRAF-mutant cancer cells, including, most likely, BRAF-only cells (TERT promoter mutation was not known in those years) (18, 19). It is conceivable that inhibition of cell proliferation by BRAF and MEK inhibitors can occur in all BRAF-mutant cells since the proproliferative role of the BRAF V600E-promoted MEK kinase is universal. However, even cell proliferation can be inhibited without apoptosis, cytostatic cells can resume proliferation, and tumor can resume growth after drug withdrawal. This explains the partial inhibition of the BRAF-only tumors by dabrafenib and trametinbib and their regrowth after drug withdrawal observed in the present study. Consistently, previous studies have demonstrated that cell-cycle arrest induced by another BRAF V600E inhibitor, vemurafenib, led to selection and expansion of subclones of thyroid cancer cells with intrinsic drug resistance and apoptosis refractoriness (20). In striking contrast, due to robust apoptosis and hence death of cells induced by the BRAF and MEK inhibitors, genetic-duet tumors nearly completely disappeared with drug treatment and virtually no tumor regrowth occurred after drug withdrawal.
In the current clinical practice, only BRAF V600E is used to help select patients for cancer treatment with BRAF and MEK inhibitors. It is likely that cancers clinically seen responding well to BRAF and MEK inhibitors harbor the genetic duet of BRAF V600E and TERT promoter mutations while those responding poorly or having disease relapse after drug withdrawal harbor only BRAF V600E. This concept is consistent with the fact that both BRAF V600E and TERT promoter mutation and their genetic duet are highly prevalent in melanoma and its response rate to BRAF/MEK inhibitors is high too while the opposite is all true with colon cancer (1, 2, 21). Similarly, undifferentiated thyroid cancer, which has a high prevalence of the genetic duet of BRAF V600E and TERT promoter mutations (11), has robust responses to the combination treatment of dabrafenib and trametinib (22), while differentiated thyroid cancer, which has a low rate of the genetic duet, has modest responses (23). These clinical data, together with the preclinical results in the present study, strongly support our concept that it is the apoptotic responsiveness governed by the TERT promoter mutation status that determines the therapeutic effects of BRAF/MEK inhibitors in BRAF-mutant cancers.
Our study thus reveals a genetic background explanation for the variations at two extremes of the therapeutic responses of BRAF-mutant cancers to dabrafenib and trametinib seen clinically. The study provides strong clinical translational implications that the genetic status of TERT promoter may assist the selection of patients with BRAF-mutant cancers for effective treatment with dabrafenib and trametinib and help predict treatment outcomes. The study also demonstrates that the genetic duet of BRAF V600E and TERT promoter mutations represents an Achilles Heel in cancer for effective therapeutic targeting.
Materials and Methods
Inhibitors.
The BRAF V600E-selective inhibitor dabrafenib (S2807) and the MEK inhibitor trametinib (S2673) were purchased from Selleck Chemicals, dissolved in Dimethyl sulfoxide (DMSO) with a stock concentration of 10 mM, and stored at −20 °C. Combination of dabrafenib and trametinib, as currently clinically applied, was used to treat cancer cells as drug treatment for 48 h at concentrations of 0.5 and 0.1 μM, respectively. DMSO was used as the vehicle control.
Cell Lines.
The following human cancer cell lines were used in the present study as gifts originally from the indicated providers, to whom we acknowledge here with great gratitude:
Thyroid cancer cell lines were from the following sources: WRO from Motoyasu Saji, Ohio State University Wexner Medical Center, Columbus, OH; CUTC5 from Rebecca E. Schweppe, University of Colorado Cancer Center, Aurora, CO; OCUT1 from Naoyoshi Onoda, Osaka City University Graduate School of Medicine, Osaka, Japan; BCPAP from Massimo Santoro, University of Federico II, Naples, Italy; THJ-21T from John A. Copland III, Mayo Clinic Comprehensive Cancer Center, Jacksonville, FL; K1 from David Wynford-Thomas, University of Wales College of Medicine, Cardiff, UK; and MDA-T41 from American Type Culture Collection (ATCC).
Melanoma cell lines were from the following sources: Ma-Mel-1, UKRV-Mel-17, and UKRV-Mel-19c from Dirk Schadendorf, University Hospital Essen, Essen, Germany; DRO from Guy J.F. Juillard, University of California, Los Angeles School of Medicine, Los Angeles, CA; and A375, M14, SK-MEL-3, and SK-MEL-28 from ATCC.
Colon cancer cell lines RKO and HT-29 were purchased from ATCC.
K1 cell line was reported to be contaminated with the GLAG-66, which is also a human papillary thyroid cancer-derived cell line, in the International Cell Line Authentication Committee database (24). The DRO cell line, which had been early thought to have originated from anaplastic thyroid cancer, was later confirmed to be an A-375 melanoma cell-line–derived cell subclone (24). Our genetic analysis of the K1 and DRO used in the present study confirmed typical heterozygous BRAF V600E and TERT promoter mutations. The WRO cell line was reported to carry BRAF V600E in some previous publications (25, 26) but not in others (15, 27), suggesting that there are two lines of WRO with different genetic alterations. The WRO cell line used in this study was from Motoyasu Saji, Ohio State University Wexner Medical Center, Columbus, OH, and was confirmed to harbor heterozygous BRAF V600E mutation and wild-type TERT promoter. The thyroid cancer originality of OCUT1 cell was not certain. Our genetic analysis, however, showed that it harbored heterozygous BRAF V600E and TERT promoter mutations. Thus, K1, DRO, WRO, and OCUT1 cells used here met the purpose of the present study to investigate the role of BRAF V600E and TERT promoter mutations in determining the apoptotic and therapeutic responses of BRAF-mutant human cancers to BRAF and MEK inhibitors. Other cell lines were authenticated by short tandem repeat analyses and tested for mycoplasma.
Cell Culture.
OCUT1, CUTC5, THJ-21T, BCPAP, K1, MDA-T41, M14, DRO, Ma-Mel-1, UKRV-Mel-17, and UKRV-Mel-19c were grown at 37 °C and 5% CO2 in RPMI-1640 medium containing 10% fetal bovine serum (FBS) (F2442; Sigma-Aldrich, St Louis, MO). A375 and WRO were grown in Dulbecco’s Modified Eagle Medium supplemented with 10% FBS. SK-MEL-28 and RKO cells were grown in EMEM medium with 10% FBS. SK-MEL-3 cell was grown in McCoy’s 5A medium with 15% FBS and HT29 cell was grown in the same medium but with 10% FBS.
Mutational Analysis.
All of the cell lines were genetically examined and confirmed for the status of BRAF V600E and TERT promoter mutations chr5:1,295,228C > T and chr5:1,295,250 C > T (TERT C228T and TERT C250T) by sequencing of genomic DNA. The TERT promoter region was amplified by PCR using primers 5′-AGTGGATTCGCGGGCACAGA-3′ (forward) and 5′-CAGCGCTGCCTGAAACTC-3′ (reverse); the BRAF V600E mutation hot spot region was amplified using primers 5′-TCATAATGCTTGCTCTGATAGGA-3′ (forward) and 5′-GGCCAAAAATTTAATCAGTGGA-3′ (reverse). The PCR products were subjected to Sanger sequencing for the detection of BRAF V600E and TERT promoter mutations.
Flow Cytometry.
Cells were collected and stained for Annexin and PI using the TACS Annexin V-FITC Apoptosis Detection Kit (R&D systems, Minneapolis, MN). Data were collected using a CytoFLEX LX (Beckman Coulter) and analyzed using CytExpert 2.0 (Beckman Coulter). Apoptotic cells were gated as Annexin V+.
Western Blotting.
Western blotting analyses were performed as previously described (15). Briefly, cells were lysed in the RIPA buffer (sc-24948; Santa Cruz Biotechnology, Santa Cruz, CA) supplemented with protease inhibitor mixture (P8340; Sigma-Aldrich) and phosphatase inhibitor mixture (P0044; Sigma-Aldrich). Cell lysates were denatured by boiling the sample at 95 °C for 5 min and resolved by sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS/PAGE). Proteins were transferred to Amersham Hybond-P polyvinylidene difluoride membrane (10600023; GE Healthcare Life Sciences, Germany) and blocked with 5% nonfat milk in Tris-buffered saline buffer with 0.1% Tween-20 (TBST) at room temperature for 1 h. The membranes were then sliced according to the molecular weights and incubated with primary antibodies at 4 °C overnight, washed with TBST, and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies at room temperature for 2 h. Signals were detected by SuperSignal West Femto Maximum Sensitivity Substrate (34095; Thermo Fisher Scientific). The primary antibodies, including anti-PARP1 (F-2), anticleaved PARP-1 (194C1439), anticaspase-3 (E-8), and anti–β-actin (C-4), were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The anticleaved caspase-3 (Asp175) and anti–phospho-ERK1/2 (Thr202/Tyr204) antibodies were purchased from Cell Signaling Technology (Beverly, MA). HRP-linked secondary antibodies, including anti-mouse IgG (7076S) and anti-rabbit IgG (7074S), were purchased from Cell Signaling Technology.
Xenograft Tumor Growth Assay.
All animal studies were approved and performed according to the guidelines of the Institutional Animal Care and Use Committee of Johns Hopkins University. Four-week-old female nude mice (Hsd:Athymic Nude-Foxn1nu mice) were purchased from Harlan Laboratories (Frederick, MD). M14, DRO, SK-Mel-3, and HT29 (1 × 107) cells were injected subcutaneously in the back of nude mice (10 mice per group). At 1 wk of inoculation of cells (2 wk for M14 cells) when the tumors approached about 5 mm, each group of animals was divided further into two subgroups (5–6 mice per subgroup) and treated daily with vehicle [0.5% hydroxypropyl methylcellulose (Sigma-Aldrich, St. Louis, MO) and 0.2% Tween 80 (Sigma-Aldrich)] or a combination of 30 mg/kg dabrafenib and 0.6 mg/kg trametinib dissolved in the above vehicle medium by oral gavage. Tumor size was measured every 3 d on the skin surface of the animal using a caliper and tumor volume was calculated using the formula (width2 × length) × 0.5. After 3 wk of continuous treatment, all treatments were stopped and measurement of tumor size continued. At the end of 10 d of subsequent observation, all mice were killed and tumors were surgically removed, photographed, and weighted.
Statistics.
Two-tailed Student’s t test was used to determine the significance of difference between two groups in the assays of flow cytometry, Western blotting, and tumor formation in nude mice. For flow cytometry, three independent experiments were carried out, and each was done in triplicate. All of the Western blotting analyses of proteins were repeated at least twice in independent experiments with similar results. Gray values of protein bands were obtained using Image Lab 6.0.1 (Bio-Rad Laboratories, Inc., CA). Relative protein expression was defined as the gray value of target protein normalized by that of β-actin. All P values were two-sided and a P < 0.05 was considered significant. Analyses were performed using SPSS 19.0 (IBM Corp., NY) and GraphPad Prism 6 (GraphPad Software, Inc., San Diego, CA).
Data Availability.
All data are included and freely available in this article.
Acknowledgments
This study was supported partly by US NIH Grants R01CA215142 and R01CA189224 to M.X.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
References
- 1.Chapman P. B.et al.; BRIM-3 Study Group , Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopetz S.et al., Phase II pilot study of vemurafenib in patients with metastatic BRAF-mutated colorectal cancer. J. Clin. Oncol. 33, 4032–4038 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Prahallad A.et al., Unresponsiveness of colon cancer to BRAF(V600E) inhibition through feedback activation of EGFR. Nature 483, 100–103 (2012). [DOI] [PubMed] [Google Scholar]
- 4.Sun C.et al., Reversible and adaptive resistance to BRAF(V600E) inhibition in melanoma. Nature 508, 118–122 (2014). [DOI] [PubMed] [Google Scholar]
- 5.Karoulia Z., Gavathiotis E., Poulikakos P. I., New perspectives for targeting RAF kinase in human cancer. Nat. Rev. Cancer 17, 676–691 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xing M., Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer 13, 184–199 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhillon S., Dabrafenib plus trametinib: A review in advanced melanoma with a BRAF (V600) mutation. Target. Oncol. 11, 417–428 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Long G. V.et al., Dabrafenib plus trametinib versus dabrafenib monotherapy in patients with metastatic BRAF V600E/K-mutant melanoma: Long-term survival and safety analysis of a phase 3 study. Ann. Oncol. 28, 1631–1639 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corcoran R. B.et al., Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J. Clin. Oncol. 33, 4023–4031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hess K. R., Beneficial effect of adjuvant dabrafenib plus trametinib on recurrence-free survival in patients with resected BRAFV600-mutant stage III melanoma seems to be short-lived. J. Clin. Oncol. 37, 1354–1355 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X.et al., Highly prevalent TERT promoter mutations in aggressive thyroid cancers. Endocr. Relat. Cancer 20, 603–610 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xing M.et al., BRAF V600E and TERT promoter mutations cooperatively identify the most aggressive papillary thyroid cancer with highest recurrence. J. Clin. Oncol. 32, 2718–2726 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macerola E.et al., Coexistence of TERT promoter and BRAF mutations in cutaneous melanoma is associated with more clinicopathological features of aggressiveness. Virchows Arch. 467, 177–184 (2015). [DOI] [PubMed] [Google Scholar]
- 14.Liu R.et al., Mortality risk stratification by combining BRAF V600E and TERT promoter mutations in papillary thyroid cancer: Genetic duet of BRAF and TERT promoter mutations in thyroid cancer mortality. JAMA Oncol. 3, 202–208 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Liu R., Zhang T., Zhu G., Xing M., Regulation of mutant TERT by BRAF V600E/MAP kinase pathway through FOS/GABP in human cancer. Nat. Commun. 9, 579 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson M. P., Klapper W., Emerging roles for telomerase in neuronal development and apoptosis. J. Neurosci. Res. 63, 1–9 (2001). [DOI] [PubMed] [Google Scholar]
- 17.Chakraborty S., Ghosh U., Bhattacharyya N. P., Bhattacharya R. K., Roy M., Inhibition of telomerase activity and induction of apoptosis by curcumin in K-562 cells. Mutat. Res. 596, 81–90 (2006). [DOI] [PubMed] [Google Scholar]
- 18.Solit D. B.et al., BRAF mutation predicts sensitivity to MEK inhibition. Nature 439, 358–362 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xing J., Liu R., Xing M., Trink B., The BRAFT1799A mutation confers sensitivity of thyroid cancer cells to the BRAFV600E inhibitor PLX4032 (RG7204). Biochem. Biophys. Res. Commun. 404, 958–962 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antonello Z. A.et al., Vemurafenib-resistance via de novo RBM genes mutations and chromosome 5 aberrations is overcome by combined therapy with palbociclib in thyroid carcinoma with BRAFV600E. Oncotarget 8, 84743–84760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Killela P. J.et al., TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc. Natl. Acad. Sci. U.S.A. 110, 6021–6026 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subbiah V.et al., Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. J. Clin. Oncol. 36, 7–13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cabanillas M. E., Ryder M., Jimenez C., Targeted therapy for advanced thyroid cancer: Kinase inhibitors and beyond. Endocr. Rev. 40, 1573–1604 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Capes-Davis A.et al., Check your cultures! A list of cross-contaminated or misidentified cell lines. Int. J. Cancer 127, 1–8 (2010). [DOI] [PubMed] [Google Scholar]
- 25.Saiselet M.et al., Thyroid cancer cell lines: An overview. Front. Endocrinol. (Lausanne) 3, 133 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reeb A. N., Ziegler A., Lin R. Y., Characterization of human follicular thyroid cancer cell lines in preclinical mouse models. Endocr. Connect. 5, 47–54 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Namba H.et al., Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J. Clin. Endocrinol. Metab. 88, 4393–4397 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are included and freely available in this article.