Abstract
Fish have somehow colonized isolated water bodies all over the world without human assistance. It has long been speculated that these colonization events are assisted by waterbirds, transporting fish eggs attached to their feet and feathers, yet empirical support for this is lacking. Recently, it was suggested that endozoochory (i.e., internal transport within the gut) might play a more important role, but only highly resistant diapause eggs of killifish have been found to survive passage through waterbird guts. Here, we performed a controlled feeding experiment, where developing eggs of two cosmopolitan, invasive cyprinids (common carp, Prussian carp) were fed to captive mallards. Live embryos of both species were retrieved from fresh feces and survived beyond hatching. Our study identifies an overlooked dispersal mechanism in fish, providing evidence for bird-mediated dispersal ability of soft-membraned eggs undergoing active development. Only 0.2% of ingested eggs survived gut passage, yet, given the abundance, diet, and movements of ducks in nature, our results have major implications for biodiversity conservation and invasion dynamics in freshwater ecosystems.
Keywords: long-distance dispersal, freshwater, fish distribution, invasion, endozoochory
How organisms are able to overcome barriers to dispersal is a central question in biology (1). Dispersal events are key to gene flow, distribution ranges, metapopulation dynamics, speciation, and invasiveness (2). Among these, long-distance dispersal events are generally rare and stochastic (1), but have major influence over species range expansions (3). It is especially intriguing how aquatic organisms move across an inhospitable terrestrial matrix between river catchments (4). Fish are often able to colonize remote lakes or ponds, but suggested mechanisms have gained little empirical support (5, 6). It has been speculated that the most likely dispersal mechanism for fish over dry land is the external transport of eggs on the feathers or feet of waterbirds (5). Nonetheless, there is almost no evidence for this (7), calling for the investigation of alternative mechanisms that allow fish to disperse to isolated waterbodies.
An ability to disperse inside terrestrial vertebrates following ingestion (endozoochory) is one such mechanism. Endozoochory by waterbirds has been demonstrated for a range of aquatic invertebrates, including insect larvae (8), as well as soft plant parts and seeds (9–11). A recent study found that diapaused eggs of annual killifish survive passing the gut of coscoroba swans (6). That study provided the first evidence that endozoochory might play a role in the long-distance dispersal of vertebrates. However, killifish possess adaptations that enable them to survive hostile environments. Killifish zygotes often enter diapause for years during periodic droughts in their ephemeral habitats, while the highly specialized chorionic membrane isolates the embryo and allows it to survive anoxia, hypersalinity, or desiccation (12). The water permeability of diapausing embryos can be orders of magnitude lower than that of other teleost fishes, while the enveloping cell layer acts as a highly efficient barrier to ion exchange (12). It is therefore not so surprising that these resistant eggs were able to survive the acidic, anoxic environment in the waterfowl digestive tract, but the enveloping cell layer of annual killifishes is unique among teleost fish (12). Thus, it remains to be determined whether endozoochory can be a dispersal mechanism in soft-membraned eggs found in the overwhelming majority of freshwater fish.
Here, we experimentally investigate the ability of two soft-chorion fish species to disperse by endozoochory. Our aim was to establish whether fish embryos undergoing active development can survive gut passage through waterfowl. We chose two cyprinid fish species with wide geographic ranges, that are effective invaders, and have nonresistant chorionic membranes, similar to most teleosts.
Materials and Methods
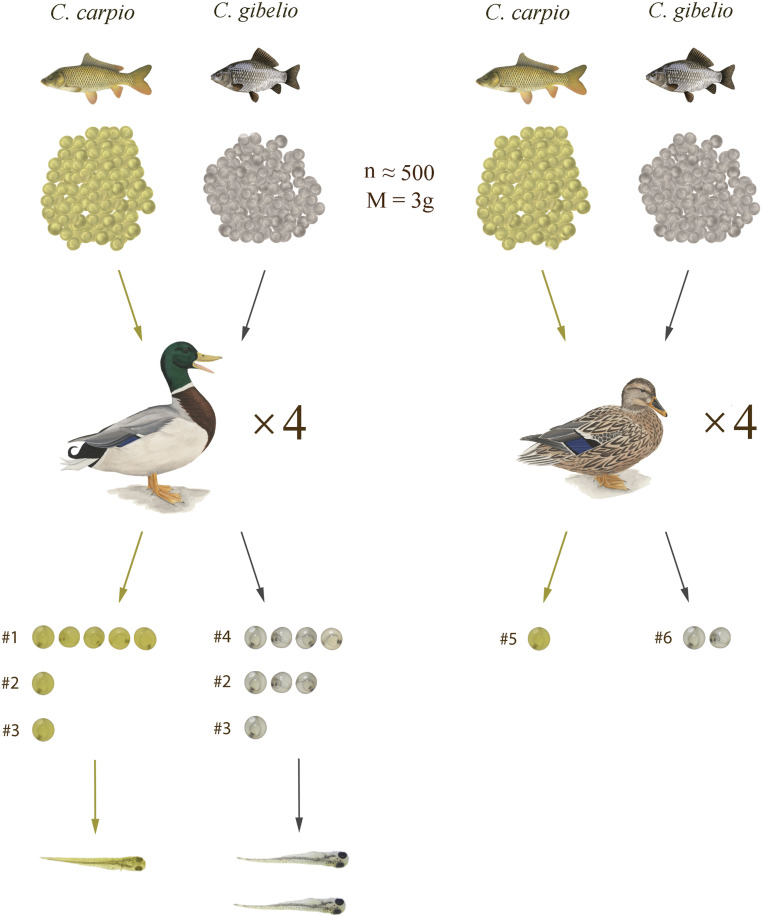
We conducted two feeding experiments with mallards (Anas platyrhynchos), a cosmopolitan dabbling duck with a key role in the dispersal of terrestrial and aquatic plants (11). Twenty-four hours prior to the experiment, ducks were housed in individual cages (60 × 40 × 50 cm) built of wire mesh. Plastic trays were placed under each cage to collect droppings. Water and food (a mixture of wheat, corn, and sunflower seeds) was provided ad libitum throughout. All birds were 1 y old and raised under identical conditions.
Mallards were force-fed with common carp eggs in the first experiment and with Prussian carp eggs in the second. All eggs were obtained from the Research Institute for Fisheries and Aquaculture, Szeged, Hungary, by means of live harvest. Eggs and milk were mixed, with a 100% and 30% fertilization rate for common and Prussian carp, respectively. After artificial insemination, eggs were incubated at 22 °C in Zuger glass for 24 h prior to the experiment. The embryos were at the morula stage at the time of force-feeding. Three batches of 100 eggs were weighed for each species (mean ± SD for common carp = 0.63 ± 0.05 g; Prussian carp = 0.55 ± 0.05 g). In each experiment, 3 g (ca. 500 eggs) were force-fed to each of eight birds (four males, four females).
Feces were collected from the plastic trays at 1, 2, 4, 6, 8, 12, and 24 h after force-feeding, then immediately soaked in filtered river water (from the East Main Channel, Hungary). Samples were sieved (100 µm), and intact eggs (containing embryos) were collected. Eggs were placed in FIAP Proficare©K30 1,5% solution for 15 min to counter fungal infection during incubation. To test their viability, we moved all recovered eggs to aquaria filled with filtered river water and equipped with air pumps. Eggs were placed in plastic tubes with fine mesh bottoms to allow water circulation. Eggs collected from a given mallard at a given hour were placed in the same tube during hatching. We used 50 fertilized eggs of each species from the same experimental batch of eggs as controls, which were handled in the same way as those recovered from mallards. Aquaria were kept at room temperature (ca. 26 °C). The experiment was approved by the scientific council of the Babeş-Bolyai University, Cluj-Napoca (14689/31.08.2018).
Results
Eight intact common carp eggs (ca. 0.2% of those ingested) and 10 Prussian carp eggs (ca. 0.25%) were recovered from feces (Fig. 1). All eggs were passed in the first hour following force-feeding, except for one common carp egg egested between 4 h and 6 h after feeding. All passed common carp eggs and four Prussian carp eggs had viable embryos, as indicated by the movement of embryos within the translucent eggs. The remaining six eggs of Prussian carp appeared to have been fertilized, but the embryo died during gut passage. Six mallards out of the eight egested at least one egg (Fig. 1). Male birds passed more eggs than females (M:F = 15:3 eggs).
Fig. 1.
Numbers of eggs fed, of intact eggs recovered, and of fry that hatched following gut passage; #number refers to duck ID in the experiment.
During incubation, seven common carp and two Prussian carp embryos died due to fungal infection. The common carp egg passed after 4 h to 6 h subsequently hatched 68 h after ingestion. Two Prussian carp eggs (passed within 1 h after feeding) hatched 49 h after ingestion. All control common carp eggs died due to fungal infection. Of control Prussian carp eggs, 14 hatched, and the rest died due to fungal infection.
Discussion
Our experiment provides evidence that soft-chorion fish embryos, undergoing active embryonic development, can survive passage through the digestive tract of vertebrates, enabling endozoochory. Such survival was not a freak event, and occurred in 75% of the experimental ducks and in both fish species studied, which have eggs and embryos typical of teleost fish. Our study thus provides a potential explanation in a long-standing debate on how fish colonize remote, isolated water bodies, including crater lakes, desert lakes, and temporary wetlands within agricultural fields (13).
Although only 0.2% of eggs survived gut passage through mallards, endozoochory of fish is likely to be frequent in nature, given the frequent feeding by birds on fish roe, which are rich in proteins and lipids (14). Diet studies record fish eggs in the digestive tracts of dabbling and diving ducks (15, 16), gulls, and shorebirds (14). As many as 217 fish eggs have been recorded in a single mallard (17), and fish eggs, when available, often take up 100% of the stomach content of birds, reaching a maximum of 63,501 eggs in a glaucous gull (14). Five common bird species were estimated to consume 857 t (ca. 31% of all) of Pacific herring (Clupea pallasi) eggs within 27 d at one spawning site (14). Therefore, the large number of eggs available in the water during spawning, combined with the high abundance and diversity of waterbirds that may consume eggs, provide considerable potential for frequent dispersal events via endozoochory. Both fish studied here are highly fecund. A single common carp lays up to 1.5 million eggs at a spawning event, compared to 400,000 for Prussian carp. Mallard numbers are in the tens of millions (18), and they are likely to use any Eurasian or American wetland with carp. The invasive success of these fish species might thus be related to their high fecundity, potential for endozoochorous dispersal, and broad ecological niche, as well as to the ability of the Prussian carp to reproduce asexually (19).
During the experiment, we recovered 18 eggs from mallards, of which 12 contained viable embryos. During the hatching process, only three of these eggs hatched successfully, the rest being lost to a fungal infection that caused similarly high embryo mortality in the control groups. Thus, the cause of low hatchability was likely to be suboptimal culture conditions, and not a consequence of gut passage. Our experimental setup may have facilitated fungal infection, due to weak antifungal treatment, small water volume, a shared tank for eggs placed for hatching, and insufficient water circulation around the eggs. These technical issues regularly increase the susceptibility of eggs to fungal infection in artificial fish breeding (20). In contrast, eggs are unlikely to experience fungal infection in the wild, especially due to larger water volume and optimal spacing of the eggs (21).
Further research is needed to assess what species traits of fish (e.g., chorion structure, hatching time, fecundity, spawning time, and substrate) or their avian vectors (e.g., diet, movement) influence the potential for fish endozoochory. Benthic spawners may be more likely to be dispersed by benthic feeding birds such as diving ducks, and fish spawning on macrophytes like carp may be more likely to be dispersed by dabbling ducks and herbivorous waterfowl. Mass and multiple spawners may also differ in endozoochory potential. Mass spawners produce large masses of eggs synchronously, where birds might gorge on large quantities of eggs within a short period of time, increasing survival of gut passage (22). Multiple spawners produce eggs asynchronously, ensuring availability of eggs over a longer time frame, exposing these to a larger pool of bird species, and increasing chances of overlap with their migratory periods. Furthermore, it remains to be determined how biological (e.g., pathogens), physical (e.g., temperature), and chemical (e.g., acidity) conditions within the digestive tract influence hatchability of fish eggs or their postembyronic development in nature.
Despite the relatively short retention times of fish eggs within the digestive tract of mallards, these birds are still likely to play a significant role in long-distance dispersal of carp. In our experiment, most fish eggs were passed within 1 h of ingestion, suggested a maximum dispersal range of 60 km based on flight speed of mallards (23), although one egg hatched after retention in the gut for between 4 h and 6 h, indicating potential dispersal of up to 360 km. Like insect (24) and zooplankton eggs, fish eggs will be dispersed over shorter distances during migration events than plant seeds, but modeling of banding data supports dispersal over 100 km, and suggests that dispersal distances by mallards are greater in North America than in Europe (25). High-resolution movement data indicate that the great majority of fish eggs carried in mallards will be dispersed between feeding and roosting sites within 10 km of each other (26). How often such dispersal events lead to the successful establishment of new populations of invasive freshwater fish is a critical question for future research.
Data Availability.
All data are provided in Dataset S1.
Supplementary Material
Acknowledgments
We thank Gabriella Bodnár for her assistance during the experiment. The experiment was financed by the Hungarian National Research, Development and Innovation Office (NKFIH OTKA Grants FK-127939 and KH129520). B.A.L., Á.L.-K., and O.V. were supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences and by the New National Excellence Programme of the Hungarian Ministry of Innovation and Technology. A.J.G. was supported by the Spanish Ministerio de Economía, Industria y Competitividad Project CGL2016-76067-P (Agencia Estatal de Investigación/Fondo Europeo de Desarrollo Regional, European Union).
Footnotes
The authors declare no competing interest.
Data deposition: All data are given in the manuscript and Dataset S1.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2004805117/-/DCSupplemental.
References
- 1.Nathan R., Long-distance dispersal of plants. Science 313, 786–788 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Hui C., Richardson D. M., Invasion Dynamics, (Oxford University Press, 2017). [Google Scholar]
- 3.Hastings A.et al., The spatial spread of invasions: New developments in theory and evidence. Ecol. Lett. 8, 91–101 (2004). [Google Scholar]
- 4.Docherty C., Ruppert J., Rudolfsen T., Hamann A., Poesch M., Assessing the spread and potential impact of Prussian carp Carassius gibelio (Bloch, 1782) to freshwater fishes in western North America. BioInvasions Rec. 6, 291–296 (2017). [Google Scholar]
- 5.Hirsch P. E., N’Guyen A., Muller R., Adrian-Kalchhauser I., Burkhardt-Holm P., Colonizing islands of water on dry land—On the passive dispersal of fish eggs by birds. Fish Fish. 19, 502–510 (2018). [Google Scholar]
- 6.Silva G. G.et al., Killifish eggs can disperse via gut passage through waterfowl. Ecology 100, e02774 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Riehl R., Können einheimische Fische anhand ihrer Eier durch Wasservögel verbreitet werden? Zeitschrift für Fischkd. 1, 79–83 (1991). [Google Scholar]
- 8.Green A. J., Sánchez M. I., Passive internal dispersal of insect larvae by migratory birds. Biol. Lett. 2, 55–57 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilkinson D. M., Lovas-Kiss A., Callaghan D. A., Green A. J., Endozoochory of large bryophyte fragments by waterbirds. Cryptogam. Bryol. 38, 223–228 (2017). [Google Scholar]
- 10.Silva G. G.et al., Whole angiosperms Wolffia columbiana disperse by gut passage through wildfowl in South America. Biol. Lett. 14, 20180703 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lovas-kiss Á.et al., Endozoochory of aquatic ferns and angiosperms by mallards in Central Europe. J. Ecol. 106, 1714–1723 (2018). [Google Scholar]
- 12.Machado B. E., Podrabsky J. E., Salinity tolerance in diapausing embryos of the annual killifish Austrofundulus limnaeus is supported by exceptionally low water and ion permeability. J. Comp. Physiol. B 177, 809–820 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Williams D. D., The Biology of Temporary Waters, (Oxford University Press, 2005). [Google Scholar]
- 14.Bishop M. A., Green S. P., Predation on Pacific herring (Clupea pallasi) spawn by birds in Prince William Sound, Alaska. Fish. Oceanogr. 10, 149–158 (2001). [Google Scholar]
- 15.Vermeer K., Diet of the harlequin duck in the Strait of Georgia, British Columbia. The Murrelet 64, 54 (1983). [Google Scholar]
- 16.Stempniewicz L. E. C. H., Feeding ecology of the long-tailed duck Clangula hyemalis wintering in the Gulf of Gdansk (southern Baltic Sea). Ornis Svec. 5, 133–142 (1995). [Google Scholar]
- 17.Street M., The food of mallard ducklings in a wet gravel quarry, and its relation to duckling survival. Wildfowl 28, 13 (1977). [Google Scholar]
- 18.Wetlands International , Waterbird population estimates. http://wpe.wetlands.org/search?form%5Bspecies%5D=anas+platyrhynchos&form%5Bpopulation%5D=&form%5Bpublication%5D=5. Accessed 20 April 2020.
- 19.Tóth B., Várkonyi E., Hidas A., Meleg E., Váradi L., Genetic analysis of offspring from intra- and interspecific crosses of Carassius auratus gibelio by chromosome and RAPD analysis. J. Fish Biol. 66, 784–797 (2005). [Google Scholar]
- 20.Bruno D., van West P., Beakes G., “Saprolegnia and other oomycetes” in Fish Diseases and Disorders, Viral, Bacterial and Fungal Infections, Woo P., Bruno D., Eds. (CAB International, ed. 2, 2013), pp. 669–720. [Google Scholar]
- 21.van den Berg A. H., McLaggan D., Diéguez-Uribeondo J., van West P., The impact of the water moulds Saprolegnia diclina and Saprolegnia parasitica on natural ecosystems and the aquaculture industry. Fungal Biol. Rev. 27, 33–42 (2013). [Google Scholar]
- 22.Green A. J., Soons M., Brochet A. L., Kleyheeg E., “Dispersal of plants by waterbirds” in Why Birds Matter: Avian Ecological Function and Ecosystem Services, Şekercioğlu Ç. H., Wenny D., Whelan C. J., Floyd C., Eds. (University of Chicago Press, 2016), pp. 147–195. [Google Scholar]
- 23.Welhun C. V. J., Flight speeds of migrating birds: A test of maximum range speed predictions from three aerodynamic equations. Behav. Ecol. 5, 1–8 (1994). [Google Scholar]
- 24.Laux J. J., Kölsch G., Potential for passive internal dispersal: Eggs of an aquatic leaf beetle survive passage through the digestive system of mallards. Ecol. Entomol. 39, 391–394 (2014). [Google Scholar]
- 25.Viana D. S., Santamaría L., Michot T. C., Figuerola J., Migratory strategies of waterbirds shape the continental-scale dispersal of aquatic organisms. Ecography 36, 430–438 (2013). [Google Scholar]
- 26.Kleyheeg E., Treep J., de Jager M., Nolet B. A., Soons M. B., Seed dispersal distributions resulting from landscape-dependent daily movement behaviour of a key vector species, Anas platyrhynchos. J. Ecol. 105, 1279–1289 (2017). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data are provided in Dataset S1.