Significance
Sphingolipids (SLs) are complex lipids that constitute hundreds of subspecies. All SLs share a long-chain base (LCB) as a defining structural component. LCBs are formed by serine-palmitoyltransferase (SPT) in the first and rate-limiting step of SL de novo synthesis. SPT consists of three subunits that show tissue-specific expression. In presence of the SPTLC3 subunit, the enzyme forms a spectrum of straight and branched LCBs with distinct biochemical and biophysical properties. This alters the composition of cellular membranes and might influence the dynamics of membrane-related transport and signaling events. SPTLC3 is particularly abundant in skin, and changes in SPT activity are related to dermal pathologies. Genetic variants of SPTLC3 are associated with metabolic conditions such as dyslipidemia and atherosclerosis.
Keywords: serine-palmitoyltransferase, long-chain base, omega-3-methyl-sphingosine
Abstract
Sphingolipids (SLs) are chemically diverse lipids that have important structural and signaling functions within mammalian cells. SLs are commonly defined by the presence of a long-chain base (LCB) that is normally formed by the conjugation of l-serine and palmitoyl-CoA. This pyridoxal 5-phosphate (PLP)-dependent reaction is mediated by the enzyme serine-palmitoyltransferase (SPT). However, SPT can also metabolize other acyl-CoAs, in the range of C14 to C18, forming a variety of LCBs that differ by structure and function. Mammalian SPT consists of three core subunits: SPTLC1, SPTLC2, and SPTLC3. Whereas SPTLC1 and SPTLC2 are ubiquitously expressed, SPTLC3 expression is restricted to certain tissues only. The influence of the individual subunits on enzyme activity is not clear. Using cell models deficient in SPTLC1, SPTLC2, and SPTLC3, we investigated the role of each subunit on enzyme activity and the LCB product spectrum. We showed that SPTLC1 is essential for activity, whereas SPTLC2 and SPTLC3 are partly redundant but differ in their enzymatic properties. SPTLC1 in combination with SPTLC2 specifically formed C18, C19, and C20 LCBs while the combination of SPTLC1 and SPTLC3 yielded a broader product spectrum. We identified anteiso-branched-C18 SO (meC18SO) as the primary product of the SPTLC3 reaction. The meC18SO was synthesized from anteiso-methyl-palmitate, in turn synthesized from a precursor metabolite generated in the isoleucine catabolic pathway. The meC18SO is metabolized to ceramides and complex SLs and is a constituent of human low- and high-density lipoproteins.
Sphingolipids (SLs) share the presence of a long-chain base (LCB) backbone as a common structural element. LCBs are aliphatic amino alcohols and formed in the first and rate-limiting step of SL de novo synthesis (SI Appendix, Fig. S1). This reaction is catalyzed by the enzyme serine-palmitoyltransferase (SPT). SPT is a pyridoxal 5‐phosphate (PLP)-dependent α‐oxoaminotransferase that consists of three core subunits—SPTLC1, SPTLC2, and SPTLC3—that share a mutual homology. SPTLC2 is 68% identical to SPTLC3 (84% similarity), whereas SPTLC1 is more distinct, sharing ∼21% identity (45% similarity) with SPTLC2 and SPTLC3 (1). The PLP-binding motif is present in SPTLC2 and SPTLC3 but not in SPTLC1. While SPTLC1 and SPTLC2 are ubiquitously expressed, SPTLC3 expression is restricted to specific tissues, such as placenta, skin, and some glands (2–6).
LCBs vary structurally within and across species. In mammals, the most abundant LCB is sphingosine (SO; d18:1), which represents ∼60% of the total LCBs in human plasma (7). The remaining LCBs differ with respect to chain length, desaturation, and hydroxylation (reviewed in ref. 8). Plants and fungi, including yeast, mostly form phytosphingosine (phytoSO; t18:0), which is also present at low levels in humans. Insects mainly form short-chain LCBs in the range of C14 to C16 (8).
SPT activity is metabolically controlled by negative feedback regulation. This mechanism is well understood in yeast, where SPT activity is regulated by two phosphoproteins, Orm1 and Orm2 (9, 10). Another protein, Tsc3, is required for maximal SPT activation (11). SPT together with Orm1 and Orm2, Tsc3, and the phosphoinositide phosphatase Sac1 form a multisubunit complex (10). Orthologs of these proteins are also found in mammals, but their roles in regulating SL metabolism are less well understood. Mammalian cells express three ORM orthologs (ORMDL1, 2, and 3) but lack the regulatory phosphorylation sites of yeast Orm proteins (10, 12). The polypeptides ssSPTa and ssSPTb are functional orthologs of Tsc3 that appear to modulate SPT activity and substrate affinity (13–15). While ssSPTa promotes canonical C18 LCB synthesis, ssSPTb is associated with increased synthesis of C20 LCBs. In mice, a single gain-of-function mutation in ssSPTb (H56L) was shown to increase C20 LCB formation in the brain, leading to retinopathy and central neurodegeneration (14). However, the role of the individual SPT subunits with respect to SPT enzyme activity and substrate affinity has not yet been addressed systematically.
In the present study, using SPTLC1-, SPTLC2-, and SPTLC3-deficient cell models, we demonstrated that SPTLC1 is essential for formation of an active enzyme. In contrast, SPTLC2 and SPTLC3 are partly redundant but differ in function and substrate specificity. Furthermore, we identified a novel, methyl-branched LCB as a specific product of human SPTLC3. Methyl-branched LCBs are the major forms in lower invertebrates, such as Caenorhabditis elegans (16), but have not been reported in humans until now.
Results
SPTLC1 Is Indispensable for De Novo SPT Function.
To investigate the role of individual subunits on SPT activity, we generated SPTLC1 and SPTLC2 knockout (KO) HAP1 cell lines using a CRISPR/Cas9 approach. HAP1 cells are haploid and derived from chronic myelogenous leukemia (CML) cells (17). Natively, HAP1 cells express SPTLC1 and SPTLC2 but not SPTLC3 (SI Appendix, Fig. S2A). The CRISPR/Cas9 induced loss of SPTLC1 and SPTLC2 was confirmed by Western blot analysis (Fig. 1A). Surprisingly, in the absence of SPTLC1, we observed a concomitant loss of SPTLC2, whereas deletion of SPTLC2 did not influence SPTLC1 levels (Fig. 1A). This suggested that SPTLC2 is unstable in the absence of SPTLC1, whereas SPTLC1 is stable on its own.
Fig. 1.
SPT composition and activity in SPTLC1- and SPTLC2-deficient HAP1 cells. (A) Western blot showing CRISPR/Cas9-mediated loss of SPTLC1 and SPTLC2 expression in the respective HAP1 KO lines. The loss of SPTLC1 led to a concomitant loss of SPTLC2 expression. Calnexin served as a loading control. (B) SPT activity in SPTLC1- and SPTLC2-2 deficient cells compared with HAP1 WT cells. SPT activity was measured by the incorporation of (2,3,3-D3,15N)-l-serine into de novo formed LCBs. One deuterium is lost during the conjugation reaction, which results in a mass shift of +3 Da for the de novo formed LCBs. No LCBs were formed in presence of the SPT inhibitor myriocin. (C) Only SPTLC1 expression rescued activity in SPTLC1 KO cells, while expression of either SPTLC2 or SPTLC3 rescued activity in SPTLC2 KO cells. (D) Western blot of reconstituted HAP1 SPTLC1 KO cells. Expression of SPTLC1 restored the endogenous expression of SPTLC2. (E) Immunoprecipitation of V5-tagged SPT proteins in HAP1 WT and SPTLC2 KO cells. The precipitated proteins were separated by SDS-PAGE and analyzed by Western blotting. (F) SPTLC2 and SPTLC3 form a distinct LCB spectrum when expressed in a SPTLC2 null HAP1 background. Bars represent mean ± SEM; n = 3. **P < 0.01, unpaired t test. nd, not detected.
Next, SPT activity was measured by the time-dependent incorporation of isotope-labeled l-serine (2,3,3-D3,15N) into the de novo formed LCBs. As one of the deuteriums is lost during the conjugation reaction (18), the de novo synthesized LCBs had an additional mass of +3 Da. In HAP1 wild-type (WT) cells, we observed a significant formation of C18SO+3, which was completely abrogated in the absence of either SPTLC1 or SPTLC2 (Fig. 1B). Reconstitution of SPTLC1 and STPLC2 expression in the respective null background restored enzyme activity (Fig. 1C). SPTLC1 expression in the SPTLC1 KO cells rescued endogenous SPTLC2 expression (Fig. 1D). Expression of SPTLC3 in the absence of SPTLC2 resulted in an active SPT enzyme but with significantly lower formation of C18SO+3 compared with STPLC1-SPTLC2 expressing cells (Fig. 1C). These results suggested that SPTLC1–SPTLC3 interaction is independent of SPTLC2.
The physical interaction between the individual SPT subunits was confirmed by coimmunoprecipitation assays (Fig. 1E). Antibody precipitation of V5 epitope-tagged constructs of SPTLC1 (SPTLC1-V5) or SPTLC2 (SPTLC2-V5) showed coprecipitation of the respective nontagged SPTLC1 and SPTLC2 subunits. Interestingly, the pull-down of SPTLC3-V5 in SPTLC2 KO cells coprecipitated SPTLC1, while the pull-down of SPTLC3-V5 in WT cells showed coprecipitation of both SPTLC1 and SPTLC2 (Fig. 1E). This suggests that the mammalian SPT is a holoenzyme formed by the assembly of multiple subunits, which is in line with earlier reports (5).
SPT Forms a Variety of LCBs with Different Aliphatic Chain lengths.
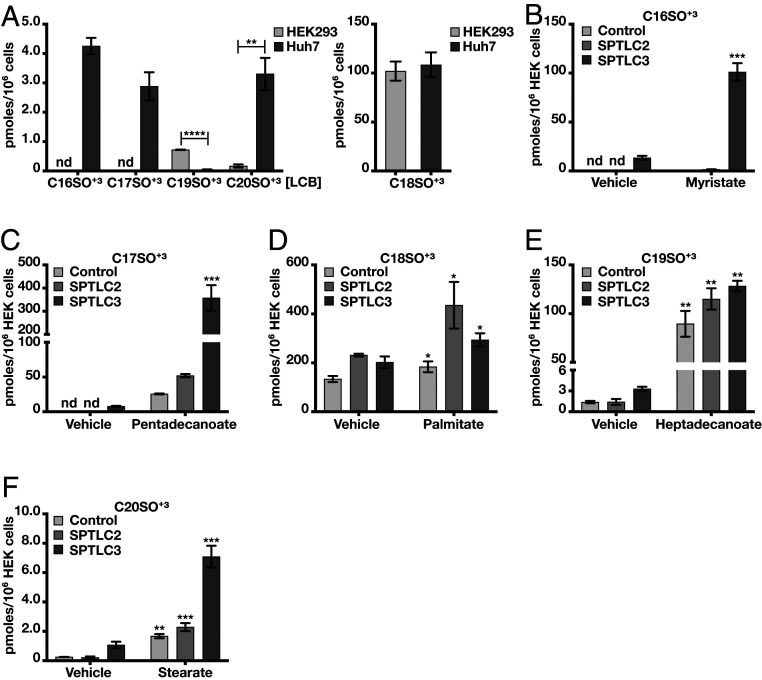
Next, we analyzed the spectrum of LCBs formed in SPTLC2 KO HAP1 cells expressing SPTLC2 or SPTLC3. Although C18SO+3 was the dominant product formed in SPTLC1-SPTLC2–expressing cells, we also observed minor formation of C19SO+3 and C20SO+3 (Fig. 1F). In contrast, SPTLC1-SPTLC3–expressing cells formed a greater variety of LCBs ranging from C16SO+3 to C20SO+3 (Fig. 1F). To exclude that these additional LCBs are artifacts caused by SPTLC3 overexpression, we compared the LCB spectrum between HEK293 and Huh7 cells. Both cell types express SPTLC1 and SPTLC2, however Huh7 cells express SPTLC3 as well (SI Appendix, Fig. S2C). The predominantly formed LCB in either cell type was C18SO+3. C20SO+3 was also formed in both cell types, although the levels were higher in Huh7 cells (Fig. 2A). In contrast, C19SO+3 was primarily formed in HEK293 cells, whereas C16SO+3 and C17SO+3 were present exclusively in Huh7 cells (Fig. 2A).
Fig. 2.
SPTLC3 forms a broader LCB spectrum than SPTLC2. (A) Profile of de novo formed LCBs in HEK293 and Huh7 cells. While SPTLC1 and 2 are expressed by both, Huh7 cells also express SPTLC3. C16SO and C17SO formation was seen only in Huh7 cells, while C18SO, C19SO, and C20SO were formed in both lines. (B–F) LCB formation in SPTLC2- and SPTLC3-overexpressing HEK293 cells after FA supplementation (50 µM): myristate (B), pentadecanoate (C), palmitate (D), heptadecanoate (E), and stearate (F). De novo formed LCBs were determined by the incorporation of (2,3,3-D3,15N)-l-serine. For statistical evaluation, absolute levels are compared between FA-treated and untreated cells (vehicle) of the same type. Bars represent mean ± SEM; n = 3. *P < 0.05; **P < 0.01; ***P < 0.001; ****P < 0.0001, unpaired t test. nd, not detected.
To confirm that these LCBs are generated by SPT directly and not formed by downstream modifications, we supplemented SPTLC2 and SPTLC3 overexpressing HEK293 cells with the respective fatty acid (FA) substrates. The addition of myristate (C14:0) stimulated C16SO+3 formation in SPTLC3 expressing cells only (Fig. 2B). In contrast, pentadeconate (C15:0) stimulated C17SO+3 synthesis in both SPTLC2 and SPTLC3 cells, although the levels were significantly higher in SPTLC3 expressing cells (approximately eightfold relative to SPTLC2 cells) (Fig. 2C). The addition of palmitate (C16:0) increased C18SO+3 levels mostly in SPTLC2- overexpressing cells (Fig. 2D), whereas supplementing heptadecanoic acid (C17:0) stimulated C19SO+3 formation in all cells (Fig. 2E). Stearate (C18:0) increased C20SO+3 formation in all cell lines, but the overall levels were the highest in SPTLC3-overexpressing cells, more than threefold higher compared with stearate-treated SPTLC2-expressing cells (Fig. 2F).
ssSPTb Stimulates C20 LCB Formation.
It has been previously reported that the accessory SPT subunits ssSPTa and ssSPTb modulate the product spectrum of SPT (13). HAP1 cells express ORMDL1-3 and ssSPTa but not ssSPTb (SI Appendix, Fig. S2B). Endogenous ssSPTa and b mRNA levels were not significantly influenced by the expression of SPTLC2 or SPTLC3 (SI Appendix, Fig. S2B), excluding that the observed shift in the LCB profile for SPTLC2- and SPTLC3-expressing cells is caused by a mutual change in ssSPTa or ssSPTb expression. Similarly, HEK293 cells also expressed primarily ssSPTa, while ssSPTb expression was low and not influenced by the presence of SPTLC3 (SI Appendix, Fig. S2D). Overexpression of ssSPTa in HEK293 WT cells (SI Appendix, Fig. S2D) had a minor stimulatory effect on C18SO+3 synthesis (Fig. 3A); however, this effect was not observed in SPTLC2- or SPTLC3- overexpressing cells that already showed increased C18SO+3 formation relative to vector-transfected controls (Fig. 3A). Overexpressing ssSPTa or ssSPTb (SI Appendix, Fig. S2D) did not induce synthesis of C16SO+3 or C17SO+3 in SPTLC1-SPTLC2–expressing cells (Fig. 3 B and C). However, by trend, their formation in SPTLC3-expressing cells appeared to be slightly reduced in the presence of ssSPTa and b (Fig. 3 B and C). On the other hand, ssSPTb expression significantly stimulated the formation of C20SO+3 (Fig. 3D). Although this effect was also seen in ssSPTb-transfected HEK293 WT cells, it was even stronger for cells coexpressing SPTLC2 or SPTLC3 (Fig. 3D). Overexpression of ssSPTa had no effect on C20SO+3 formation. Independent of ssSPTb expression, C20SO+3 formation was generally higher in SPTLC3-expressing cells, indicating that the presence of ssSPTb is not essential, but has a stimulatory effect on the formation of C20 LCBs.
Fig. 3.
LCB formation by SPT is modulated through ssSPTa and ssSPTb. (A) ssSPTa induces the formation of C18SO in HEK293 WT but has no effect on synthesis in SPTLC2- and SPTLC3-overexpressing cells. (B and C) The de novo formation of C16SO and C17SO is independent of ssSPTa and ssSPTb expression. (D) ssSPTb stimulates the formation of C20SO in SPTLC2- and SPTLC3-expressing cells. This stimulatory effect is higher in SPTLC3-expressing cells. Bars represent mean ± SEM; n = 3. *P < 0.05; ***P < 0.001, unpaired t test. nd, not detected.
C19 LCBs Are Formed as Two Isomeric Species.
In addition to the LCBs described above, we also observed the formation of an additional metabolite that was present only in SPTLC3-expressing cells (Fig. 4A and SI Appendix, Figs. S3 and S4A). This LCB was isotope-labeled with D3-15N-l-serine, and its formation was blocked by the SPT inhibitor myriocin (SI Appendix, Fig. S4A). This novel LCB was isobaric to C19SO but differed in retention time (RT) by 0.42 min (SI Appendix, Fig. S3). Despite the difference in RT, the fragmentation pattern of this LCB was identical to that of C19SO+3 (SI Appendix, Fig. S5 A–C); therefore, we hypothesized that this metabolite could be a methylated C18 LCB. The hypothesis was tested by supplementing SPTLC3-expressing HEK293 cells with the branched-chain fatty acid (BCFA) iso- or anteiso-methyl-palmitate (iso-mePA; ante-mePA). The addition of ante-mePA, but not of iso-mePA or heptadecanoate (C17:0), stimulated the synthesis of this LCB (Fig. 4B). This identified the novel metabolite as 16-(omega-3-) methyl-branched sphingosine, which was confirmed by comparison to a chemically synthesized 16-methyl-C18SO standard (SI Appendix, Fig. S6). The RT of the synthetic standard was identical to that of the endogenously formed LCB, confirming it as an omega-3-methyl-C18SO (meC18SO) (Fig. 4C). Similar to C16SO and C17SO, the synthesis of meC18SO in SPTLC3-expressing HEK293 cells was only marginally decreased by ssSPTa or ssSPTb expression (SI Appendix, Fig. S4B). However, the 80- to 100-fold increase in meC18SO+3 observed in these cells after supplementation with ante-mePA (SI Appendix, Fig. S4C) indicated that primarily substrate availability limits the formation of this branched LCB.
Fig. 4.
SPTLC3 activity induces formation of omega-3-methylated LCB (meC18SO). (A) meC18SO is exclusively formed by SPTLC3-expressing SPTLC2 KO HAP1 cells. (B) Formation of meC18SO in SPTLC3-expressing HEK293 cells is specifically enhanced by supplementation (10 µM FA) with ante-mePA and not by iso-mePA or heptadecanoate. (C) De novo formed meC18SO+3 and the chemically synthesized 16-methyl-C18SO standard have the same retention time. (D) Scheme showing the formation of ante-mePA from isotope-labeled 6[13C] isoleucine and its incorporation in omega-3 branched LCB. Filled circles reflect the position of labeled [13C]. (E) Dose-dependent incorporation of isotope-labeled isoleucine in meC18SO in SPTLC3-expressing HEK293 cells. Besides the formation of 5[13C]-meC18SO, we also observed the formation of 3[13C]-C17SO due to the metabolic conversion of isoleucine into propionyl-CoA. Bars represent mean ± SEM; n = 3. ****P < 0.0001, ANOVA followed by Bonferroni correction. nd, not detected.
BCFAs are synthesized from branched-chain amino acids (BCAAs) such as valine (Val), leucine (Leu), and isoleucine (Ile) (19). Therefore, we supplemented SPTLC3 expressing HEK293 cells with increasing amounts of stable isotope labeled 6[13C]-isoleucine and 6[13C]-leucine. Since ante-mePA is synthesized from 2-methyl-butyryl-CoA generated early in the Ile catabolic pathway (20), 5[13C] from the Ile label are expected to be incorporated into meC18SO (Fig. 4D). We observed a dose-dependent incorporation of label from Ile, but not from Leu, into the de novo formed meC18SO (Fig. 4E and SI Appendix, Fig. S4D). We also observed the de novo formation of 3[13C]C17SO in the 6[13C]-isoleucine–supplemented cells (Fig. 4E). This may be explained by the fact that Ile is terminally catabolized to propionyl-CoA, which then acts as a three-carbon precursor for the synthesis of odd chain fatty acids (reviewed in ref. 19). These results lend further support to the idea that C17SO is formed directly from the odd chain FA pentadecanoate (C15:0).
Branched meC18 LCBs Are Incorporated into Ceramides and Complex SLs.
The de novo formed LCBs are metabolized to ceramides (Cer) and further to complex SLs, such as sphingomyelins (SM) and glycosphingolipids. To determine whether meC18SO is also incorporated into complex SLs, we performed a sphingolipidomic analysis of D3-15N-l-serine–supplemented SPTLC2- and SPTLC3-overexpressing HEK293 cells. The shift in RT between C19SO+3 and meC18SO+3 was retained for the complex forms, which allowed for the chromatographic differentiation among the isobaric SL species. C18SO+3 in Cer and SM was primarily conjugated to C16:0, C22:0, C24:0, and C24:1 FAs (SI Appendix, Fig. S7 A and B). For Cer, the relative species abundance increased with longer N-acyl chains (C24:0 or C24:1; SI Appendix, Fig. S7A); however, for SM, the C16:0 N-acylated species was the most abundant (SI Appendix, Fig. S7B). In contrast, meC18SO+3-based Cer (meCer) and SM (meSM) preferentially contained longer N-acyl chains (C22:0, C24:0, and C24:1), while the levels with a conjugated C16:0 FA were very low (Fig. 5A). Supplementation with ante-mePA significantly increased meC18SO+3-based SL levels in SPTLC3-expressing HEK293 cells, but the N-acylation pattern was conserved (SI Appendix, Fig. S7 C and D). Surprisingly, among C19SO-derived SLs, C22:0 N-acyl Cer(d19:1/22:0) was the most abundant (SI Appendix, Fig. S7 E and F). Comparative analysis (mol%) of detected Cer and SM species revealed conspicuous differences in N-acylation patterns of C18SO, meC18SO, and C19SO (Fig. 5B). In addition, for C18SO- and meC18SO-based SLs, the total de novo produced SM was threefold higher than the total Cer produced from either LCB (Fig. 5C); however, for C19SO- based SLs, total Cer were more abundant than SM containing this LCB (Fig. 5C).
Fig. 5.
meC18SO is efficiently incorporated into ceramides and sphingomyelins. (A) The profile of de novo formed Cer and SM species containing meC18SO. The LCB is preferably N-acylated with longer FA chains. (B) The relative N-acyl distribution for Cer and SM differs for meC18SO-, C19SO-, and C18SO-based SL species. The de novo formed lipid species are compared (mol%) between WT and SPTLC3-expressing HEK293 cells. (C) Total Cer:SM ratio indicating that C19SO is retained in Cer, whereas meC18SO and C18SO are efficiently converted to SM. Bars represent mean ± SEM; n = 3. *P < 0.05; **P < 0.01; ****P < 0.0001, ANOVA followed by Bonferroni correction.
Branched-Chain LCBs in Plasma.
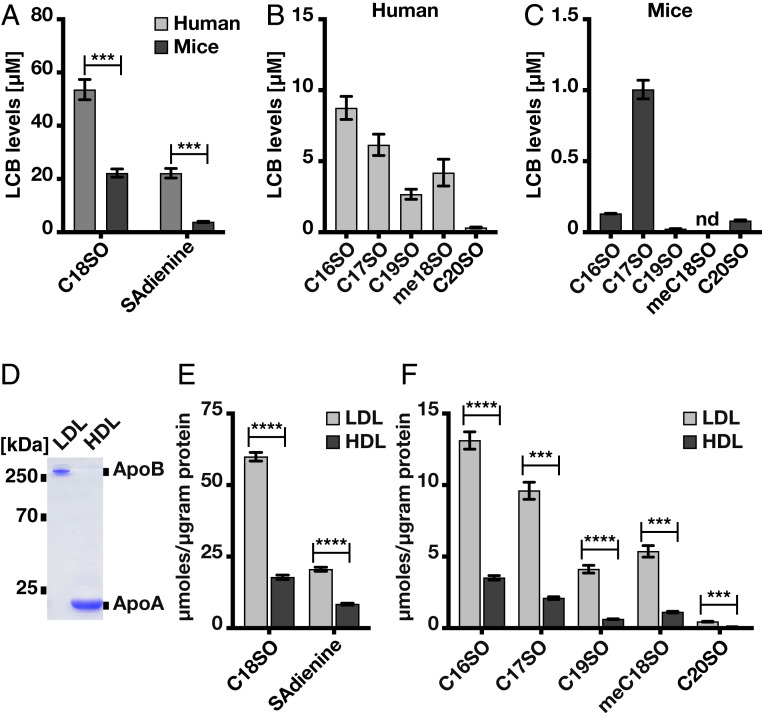
Finally, we wanted to examine the extent to which meC18SO is present in plasma. To do so, we compared the LCB profiles in plasma from mice and humans. Total SL levels were significantly lower in mice than in humans. In both species, C18SO was the most abundant LCB, followed by C18SAdienine, a dienic downstream product of SO (7) (Fig. 6A). Among the list of SPTLC3-specific LCBs, C16SO and C17SO were the most abundant in humans and mice, respectively (Fig. 6 B and C). Although C20SO was present in both, it was the least abundant of the LCBs detected in human plasma (Fig. 6 B and C). Interestingly, meC18SO was detected only in human plasma and was absent in mouse plasma (Fig. 6 B and C).
Fig. 6.
LCB profile in human and mouse plasma and in isolated human lipoprotein fractions. (A–C) The most abundant LCB in mouse and human plasma was C18SO, followed by SAdienine. meC18SO was detected in human plasma. (D) SDS gel of the purified LDL and HDL fractions (Coomassie blue-stained). (E and F) LCB analysis from purified LDL and HDL fractions. Bars represent mean ± SEM; n = 4. ***P < 0.001; ****P < 0.0001, multiple t test using Bonferroni–Dunn correction. nd, not detected.
SLs are mostly carried by low- and high-density lipoproteins (LDL and HDL, respectively) (21); therefore, we determined the LCB profiles in purified human LDL and HDL fractions (Fig. 6D). LDL contained >70% of C18SO and SAdienine (Fig. 6E). Moreover, C19 and C20SO, as well as the SPTLC3-specific LCBs C16, C17, and meC18SO, were relatively more abundant in LDL (Fig. 6F) but were also present in HDL.
Discussion
SPT is an essential enzyme in mammals, and its deficiency is embryonically lethal. Here we investigated the function of each of the three SPT core subunits. Expression of SPTLC2 and SPTLC3 in the SPTLC1 null background did not result in an active enzyme, whereas both generated a functional SPT enzyme in presence of SPTLC1 (Fig. 1C). Interestingly, the deletion of SPTLC1 caused a concomitant loss of SPTLC2, whereas SPTLC1 levels were essentially unchanged when SPTLC2 was deleted (Fig. 1A). This confirms earlier reports showing that SPTLC2 is degraded in the absence of SPTLC1 even though mRNA levels are not altered (22). Immunoprecipitation showed that both SPTLC2 and SPTLC3 interact with SPTLC1 independently. SPTLC3 coprecipitated with SPTLC1 and SPTLC2 in WT HAP1 cells but also precipitated with SPTLC1 in the absence of SPTLC2 (Fig. 1E). Similarly, antibody precipitation of ectopically expressed SPTLC2-V5 coeluted with untagged (endogenous) SPTLC2 (Fig. 1E). This points to a higher-order structure for the SPT complex and suggests that the individual SPTLC2 and SPTLC3 subunits can replace each other within this structure. In this respect, SPTLC1 might be important to anchor the enzyme to the endoplasmic reticulum through its N-terminal membrane-binding domain. In yeast, the N-terminal domain of SPTLC1 mediates binding to Orm proteins, which are integral membrane proteins as well (23, 24). As SPTLC3 expression is specific to certain tissues, the composition of the SPT complex might be altered in response to the relative tissue expression levels of SPTLC1, 2, and 3. Such concentration-dependent substitutions within complexes have also been described for some sequence-neutral DNA-binding proteins (25).
In addition to C18 LCBs, SPTLC1-2–expressing cells also synthesize C19 and C20 LCBs (Fig. 1F). However, in the presence of SPTLC3, cells formed a broader spectrum of odd and even LCBs, ranging from C16 to C20 (Figs. 1F and 2 A–F). The overexpression of ssSPTa mildly stimulated C18 LCB synthesis in WT cells but had no effect in SPTLC2- or SPTLC3-overexpressing cells, which already show increased SPT activity compared with WT cells (Fig. 3A). However, ssSPTb overexpression had a significant effect on the synthesis of C20SO. Compared with WT cells, basal C20SO synthesis was increased in SPTLC2-overexpressing and even more so in SPTLC3-overexpressing HEK293 cells, while coexpression of ssSPTb had an additional stimulatory effect on C20SO synthesis (Fig. 3D). The stimulation of C20SO synthesis by ssSPTb expression was more pronounced than stearate supplementation (Figs. 2F and 3D). This indicates that C20SO formation is regulated beyond the level of substrate availability. As stearate is one of the most abundant FAs, and accumulation of C20SO (or of SL derived from it) is neurotoxic (14), such regulation might be necessary to maintain physiological levels of this important LCB.
In addition to the LCBs formed from straight-chain saturated fatty acids, we identified a previously undescribed methyl-branched LCB exclusively formed by SPTLC3 (Fig. 4A and SI Appendix, S4 A and B). Through MS fragmentation, metabolic labeling, and comparison with a chemically synthesized standard, the LCB was identified as meC18SO (SI Appendix, Fig. S5 and Fig. 4 B–E). We showed that this branched LCB is metabolized to complex SLs, although we observed significant differences in the N-acyl profiles for C18SO-, C19SO-, and meC18SO-containing Cer and SM (Fig. 5 A and B and SI Appendix, Fig. S7 A–F). All lipids were formed within the same cell type and thus on exactly the same background of ceramide synthase (CerS) isoforms. This suggests that not only the spectrum of expressed CerS enzymes, but also the type of LCB might influence the N-acyl spectrum of Cer and complex SLs.
Interestingly, the LCBs formed by either SPTLC2 or SPTLC3 do not follow a clear continuum. SPTLC2 showed the highest activity with palmitate (C16:0), followed by heptadecanoate (C17:0), and only minor activity was seen with stearate (C18:0) (Fig. 2 D–F). In contrast, SPTLC3 had the highest activity with ante-mePA, followed by pentadecanoate (C17:0) and myristate (C14:0) (SI Appendix, Fig. S4C and Fig. 2 C and B). This differs from earlier in vitro data indicating a direct association of SPT activity with the length of the FA substrate (relative to palmitate) (3). Therefore, cellular SPT activity seems to depend on additional factors, such as the microenvironment, lost when structural integrity of the cell is destroyed.
Among all tested FA substrates, SPTLC3 showed the highest activity with the BCFA ante-mePA (Fig. 2 B–F and SI Appendix, Fig. S4C). BCFAs are formed through the catabolism of BCAAs, and we found that de novo formed meC18SO incorporated isotope-labeled Ile but not Leu (Fig. 4E and SI Appendix, Fig. S4D). Suppression of BCAA catabolism drives the development and progression of hepatic cancer, and their hepatic accumulation correlates with tumor multiplicity and size in mice (26). A high-fat diet induces hepatic expression of SPTLC3, but not of SPTLC1 or SPTLC2 in mice, which is correlated with the development of hepatocarcinoma (27, 28). High plasma BCAA levels are also found in other metabolic conditions and considered to be predictive markers for insulin resistance and type 2 diabetes (T2DM) (29, 30). Given the presence of meC18SO in human plasma (Fig. 6B), it would be interesting to test whether plasma meC18SO levels are altered in patients with hepatic cancer or T2DM.
The noncanonical SPTLC3-derived LCBs are relatively low in plasma (Fig. 6 A and B). We cannot exclude the possibility that diet and microbiota contribute to the LCB profile in plasma as well. However, the strong stimulatory effect of FA supplementation on LCBs formed by SPTLC3 (in particular for meC18SO) indicates that primarily substrate availability limits the formation of these LCBs in SPTLC3-expressing cells. Several genome-wide association studies have implicated variants in the SPTLC3 genomic locus in altered plasma lipids (31–33) and also in LDL cholesterol levels (34). Polymorphisms at an intergenic locus in SPTLC3 influence plasma Cer levels and the risk for cardiovascular disease (35). We also detected SPTLC3-derived SLs in LDL and HDL fractions isolated from human plasma (Fig. 6F), even though the physiological relevance of this association remains unclear. Furthermore, the greatest variety of LCBs have been reported from skin (36), which is also one of the tissues with the highest SPTLC3 expression (1). Here SPTLC3 and FA metabolism might have coevolved to maintain skin-specific functions, such as the water permeability barrier. In addition, Ile is efficiently metabolized to anteiso-fatty acids in skin (37), indicating a possible role for meC18SO-derived SLs in this tissue.
In conclusion, we have shown that SPT forms a variety of different LCB structures, particularly when the SPTLC3 subunit is present. This, in combination with the variability of the N-acyl chain and the diversity of conjugated head group structures, increases the already vast number of potential SL species in mammals. To obtain a comprehensive picture of SL metabolism, the inclusion of noncanonical SL will be important for future lipidomic and metabolic studies. This will help unveil the physiological and pathophysiological relevance of SPTLC3 and of noncanonical SL species in mammals.
Materials and Methods
Cell Lines, Cell Culture, and Transfections.
HAP1 cells were cultured in Iscove’s modified Dulbecco’s medium (Thermo Fisher Scientific) supplemented with 10% FBS (Thermo Fisher Scientific; FSA15-043), 4 mM l-glutamine, and 1% penicillin/streptomycin. HEK293 and Huh7 cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich) with 10% FCS. Cells were grown at 37 °C in a 5% CO2 atmosphere. HAP1 SPTLC1/SPTLC2 KO cells were generated by a commercial service (Horizon Discovery). The introduced frame-shift mutation resulting in a premature stop was confirmed by sequencing.
Standard molecular biology techniques were used for generation of all plasmid constructs used in the study. Transient plasmid transfections were performed with Lipofectamine 3000 (Thermo Fisher Scientific) for HAP1 WT and SPTLC1 and 2 deletion cells or Viromer yellow (Lipocalyx) for HEK293 cells, according to the supplier’s protocol. Transgenic HEK293 cell lines were selected for growth in DMEM containing 400 µg/mL Geneticin (Thermo Fisher Scientific).
Western Blot Analysis.
Total protein was extracted from frozen cell pellets using HET lysis buffer (50 mM Hepes pH 8.0, 1 mM EDTA, 0.2% Triton-X-100). Lysates containing 50 µg of protein were separated by sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and, after blotting onto a PVDF membrane, detected with either tag-specific or protein-specific (SPTLC1, SPTLC2) polyclonal antibodies.
Immunoprecipitation Assays.
Cells were harvested by scraping in ice-cold extraction buffer (Hepes-NaOH 50 mM, pH 7.4) containing potassium acetate (150 mM), magnesium acetate (2 mM), calcium chloride (1 mM), and glycerol (15%) without detergent. After centrifugation, cell pellets were resuspended in extraction buffer containing Triton-X-100 (0.5%) and incubated on ice for 45 min, followed by centrifugation at 13,000 rpm at 4 °C. Buffer-equilibrated Sepharose beads (40 µL) conjugated to mouse anti-V5 antibody were added, followed by incubation for 1.5 h at 4 °C with agitation. Beads were collected by centrifugation and washed three times with extraction buffer (1 mL). Finally, the bound protein was specifically eluted by competition with V5-peptide (Sigma-Aldrich, 2 µg/mL) for 15 min at room temperature, and the beads removed by centrifugation (1,800 rpm for 5 min). After addition of 5× SDS loading buffer to the supernatant, proteins were separated by SDS-PAGE, and coprecipitating proteins were detected by Western blot analysis with anti-V5 and protein-specific antibodies.
Isotope Labeling Assay.
The l-serine labeling assay and SPT activity measurements were performed as described previously (38). Cells were grown to 70% confluence in DMEM growth media. For labeling, the media was exchanged to l-serine–free DMEM (Genaxxon Bioscience) containing 10% FBS, 1% penicillin/streptomycin, and isotope-labeled D3-15N-l-serine (1 mM) (Cambridge Isotope Laboratories). Cells were grown for another 16 h in the labeling media. Inhibitors (when used) were added together with the labeling media. Fatty acid supplementation was performed by adding the respective FA (10 or 50, µM as indicated) in labeling media for the duration of the assay. For lipid analysis, cells were harvested on ice and frozen after counting (Z2 Coulter Counter; Beckman Coulter).
Lipid Analysis.
Sphingoid base extraction from frozen cell pellets or plasma (100 µL) was performed as described previously (38). Hydrolyzed lipids were resuspended in 200 µL of reconstitution buffer (70% methanol, 10 mM ammonium acetate, pH 8.5). LCBs were separated via a reverse-phase C18 column (Uptisphere, 120 Å, 5 μm, 125 × 2 mm; Interchim) connected to a QTRAP 6500+ LC-MS/MS System (Sciex). For chromatography, a binary solvent system consisting of solvent A (50% methanol, 10 mM ammonium formate, 0.2% formic acid) and solvent B (100% methanol) at a constant flow rate of 0.6 mL/min was used. The column was equilibrated with 30% solvent B, and a linear gradient to 50% B was run over 9 min. The concentration of solvent B was increased to 100% over 0.5 min. After 2 min at 100% solvent B, the column was equilibrated using 30% solvent B for 1.5 min. Sample ionization was achieved via electrospray ionization in positive ion mode.
Alternatively, dried lipid extracts were dissolved in 75 μL of derivatization mix (methanol/ethanol/H2O, 85:55:15 [vol/vol/vol]), and 5 μL of οrtho-phthalaldehyde (OPA) working solution (990 μL of boric acid, 10 µL of OPA [50 mg/mL in EtOH] and 1.5 μL of β-mercaptoethanol) was added. Samples were separated via the reverse-phase C18 column (Uptisphere 120 Å, 5 μm, 125 × 2 mm) connected to a Q-Exactive MS analyzer (Thermo Fisher Scientific). Solvent A (50% methanol, 2.5 mM ammonium acetate) and solvent B (100% methanol) at a constant flow rate of 0.3 mL per min were used. The column was equilibrated in 50% solvent B, and lipids were eluted with a linear gradient to 100% solvent B (25 min), followed by 100% solvent B (5 min) and then reequilibration at 50% solvent B (5 min). Atmospheric pressure chemical ionization was in positive ion mode (39, 40).
For lipidomics profiling, frozen cell pellets were resuspended in 50 μL of PBS and extracted with 1 mL of methanol/methyl-tert-butyl ether/chloroform (MMC) (4:3:3 [vol/vol/vol]) containing D7SA (d18:0), D7SO (d18:1), dhCer (d18:0/12:0), ceramide (d18:1/12:0), glucosylceramide (d18:1/8:0), SM (d18:1/18:1 [D9]), and D7-S1P. After brief vortexing, the samples were mixed continuously (1,400 rpm for 30 min) in a Thermomixer (Eppendorf) at 37 °C. The single-phase supernatant was collected, dried under N2, and dissolved in 100 μL of methanol/isopropanol (1/1). Untargeted lipid analysis was performed on a high-resolution Q-Exactive MS analyzer (Thermo Scientific) as described previously (41).
Chemical Synthesis.
The synthesis of meC18SO was carried out in eight consecutive steps (SI Appendix, Fig. S6) from a previously described intermediate (42) and in analogy to the synthetic schemes for deoxy-sphingosines (43). Full synthetic details will be soon reported elsewhere.
Isolation of LDL and HDL.
LDL (1.006 < d <1.063 g/mL) and HDL (1.063 < d < 1.21 g/mL) were isolated from fresh human plasma as described previously (44, 45). Isolated fractions were quantified for protein amounts and aliquots separated by SDS-PAGE (10%) for quality assurance. The fractions were stored at −20 °C. Lipoprotein particle fractions amounting to 200 µg of protein were aliquoted and processed for lipid extraction and LCB analysis.
Statistical Analysis.
Data are expressed as mean ± SEM. Statistical evaluation was performed using Student’s t test for unpaired data, a multiple t test using Bonferroni–Dunn correction, or one-way ANOVA with Bonferroni correction. P values < 0.05 were considered statistically significant. Statistical analyses were performed with Prism 8.0 (GraphPad Software).
Data Availability Statement.
All data discussed in this study are included in the main text and SI Appendix.
Supplementary Material
Acknowledgments
We thank Jeannette Fries and Irina Alecu for critically reviewing the manuscript. Financial support for this work was provided by the Swiss National Foundation (Projects 31003A_153390 and 31003A_179371), the Novartis Foundation (Project 18B081), and the Fundação para a Ciência e Tecnologia (Project PTDC/BBB-BQB/3710/2014).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission. H.R. is a guest editor invited by the Editorial Board.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2002391117/-/DCSupplemental.
References
- 1.Hornemann T., Richard S., Rütti M. F., Wei Y., von Eckardstein A., Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase. J. Biol. Chem. 281, 37275–37281 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Weiss B., Stoffel W., Human and murine serine-palmitoyl-CoA transferase: Cloning, expression and characterization of the key enzyme in sphingolipid synthesis. Eur. J. Biochem. 249, 239–247 (1997). [DOI] [PubMed] [Google Scholar]
- 3.Merrill A. H. Jr., Nixon D. W., Williams R. D., Activities of serine palmitoyltransferase (3-ketosphinganine synthase) in microsomes from different rat tissues. J. Lipid Res. 26, 617–622 (1985). [PubMed] [Google Scholar]
- 4.Hornemann T.et al., The SPTLC3 subunit of serine palmitoyltransferase generates short-chain sphingoid bases. J. Biol. Chem. 284, 26322–26330 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hornemann T., Wei Y., von Eckardstein A., Is the mammalian serine palmitoyltransferase a high-molecular-mass complex? Biochem. J. 405, 157–164 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Russo S. B., Tidhar R., Futerman A. H., Cowart L. A., Myristate-derived d16:0 sphingolipids constitute a cardiac sphingolipid pool with distinct synthetic routes and functional properties. J. Biol. Chem. 288, 13397–13409 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Karsai G.et al., FADS3 is a delta14Z sphingoid base desaturase that contributes to gender differences to the human plasma sphingolipidome. J. Biol. Chem. 295, 1889–1897 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pruett S. T.et al., Biodiversity of sphingoid bases (“sphingosines”) and related amino alcohols. J. Lipid Res. 49, 1621–1639 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han S., Lone M. A., Schneiter R., Chang A., Orm1 and Orm2 are conserved endoplasmic reticulum membrane proteins regulating lipid homeostasis and protein quality control. Proc. Natl. Acad. Sci. U.S.A. 107, 5851–5856 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breslow D. K.et al., Orm family proteins mediate sphingolipid homeostasis. Nature 463, 1048–1053 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gable K., Slife H., Bacikova D., Monaghan E., Dunn T. M., Tsc3p is an 80-amino acid protein associated with serine palmitoyltransferase and required for optimal enzyme activity. J. Biol. Chem. 275, 7597–7603 (2000). [DOI] [PubMed] [Google Scholar]
- 12.Roelants F. M., Breslow D. K., Muir A., Weissman J. S., Thorner J., Protein kinase Ypk1 phosphorylates regulatory proteins Orm1 and Orm2 to control sphingolipid homeostasis in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. U.S.A. 108, 19222–19227 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Han G.et al., Identification of small subunits of mammalian serine palmitoyltransferase that confer distinct acyl-CoA substrate specificities. Proc. Natl. Acad. Sci. U.S.A. 106, 8186–8191 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao L.et al., Elevation of 20-carbon long chain bases due to a mutation in serine palmitoyltransferase small subunit b results in neurodegeneration. Proc. Natl. Acad. Sci. U.S.A. 112, 12962–12967 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimberlin A. N.et al., Arabidopsis 56-amino acid serine palmitoyltransferase-interacting proteins stimulate sphingolipid synthesis, are essential, and affect mycotoxin sensitivity. Plant Cell 25, 4627–4639 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannich J. T., Mellal D., Feng S., Zumbuehl A., Riezman H., Structure and conserved function of iso-branched sphingoid bases from the nematode Caenorhabditis elegans. Chem. Sci. 8, 3676–3686 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Essletzbichler P.et al., Megabase-scale deletion using CRISPR/Cas9 to generate a fully haploid human cell line. Genome Res. 24, 2059–2065 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ikushiro H., Fujii S., Shiraiwa Y., Hayashi H., Acceleration of the substrate Calpha deprotonation by an analogue of the second substrate palmitoyl-CoA in serine palmitoyltransferase. J. Biol. Chem. 283, 7542–7553 (2008). [DOI] [PubMed] [Google Scholar]
- 19.Adeva-Andany M. M., López-Maside L., Donapetry-García C., Fernández-Fernández C., Sixto-Leal C., Enzymes involved in branched-chain amino acid metabolism in humans. Amino Acids 49, 1005–1028 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Wallace M.et al., Enzyme promiscuity drives branched-chain fatty acid synthesis in adipose tissues. Nat. Chem. Biol. 14, 1021–1031 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iqbal J., Walsh M. T., Hammad S. M., Hussain M. M., Sphingolipids and lipoproteins in health and metabolic disorders. Trends Endocrinol. Metab. 28, 506–518 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hanada K., Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism. Biochim. Biophys. Acta 1632, 16–30 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Han G.et al., The ORMs interact with transmembrane domain 1 of Lcb1 and regulate serine palmitoyltransferase oligomerization, activity, and localization. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 245–259 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hjelmqvist L.et al., ORMDL proteins are a conserved new family of endoplasmic reticulum membrane proteins. Genome Biol. 3, RESEARCH0027 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham J. S., Johnson R. C., Marko J. F., Concentration-dependent exchange accelerates turnover of proteins bound to double-stranded DNA. Nucleic Acids Res. 39, 2249–2259 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ericksen R. E.et al., Loss of BCAA catabolism during carcinogenesis enhances mTORC1 activity and promotes tumor development and progression. Cell Metab. 29, 1151–1165 e6 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cinar R.et al., Hepatic cannabinoid-1 receptors mediate diet-induced insulin resistance by increasing de novo synthesis of long-chain ceramides. Hepatology 59, 143–153 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshimine Y.et al., Hepatic expression of the Sptlc3 subunit of serine palmitoyltransferase is associated with the development of hepatocellular carcinoma in a mouse model of nonalcoholic steatohepatitis. Oncol. Rep. 33, 1657–1666 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Gancheva S., Jelenik T., Álvarez-Hernández E., Roden M., Interorgan metabolic crosstalk in human insulin resistance. Physiol. Rev. 98, 1371–1415 (2018). [DOI] [PubMed] [Google Scholar]
- 30.Lynch C. J., Adams S. H., Branched-chain amino acids in metabolic signalling and insulin resistance. Nat. Rev. Endocrinol. 10, 723–736 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hicks A. A.et al., Genetic determinants of circulating sphingolipid concentrations in European populations. PLoS Genet. 5, e1000672 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Illig T.et al., A genome-wide perspective of genetic variation in human metabolism. Nat. Genet. 42, 137–141 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Demirkan A.et al.; DIAGRAM Consortium; CARDIoGRAM Consortium; CHARGE Consortium; EUROSPAN consortium , Genome-wide association study identifies novel loci associated with circulating phospho- and sphingolipid concentrations. PLoS Genet. 8, e1002490 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Willer C. J.et al.; Global Lipids Genetics Consortium , Discovery and refinement of loci associated with lipid levels. Nat. Genet. 45, 1274–1283 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tabassum R.et al.; FinnGen Project , Genetic architecture of human plasma lipidome and its link to cardiovascular disease. Nat. Commun. 10, 4329 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai M. K.et al., Biological effects of naturally occurring sphingolipids, uncommon variants, and their analogs. Neuromolecular Med. 18, 396–414 (2016). [DOI] [PubMed] [Google Scholar]
- 37.Oku H., Yagi N., Nagata J., Chinen I., Precursor role of branched-chain amino acids in the biosynthesis of iso and anteiso fatty acids in rat skin. Biochim. Biophys. Acta 1214, 279–287 (1994). [PubMed] [Google Scholar]
- 38.Zhakupova A.et al., ORMDL3 expression levels have no influence on the activity of serine palmitoyltransferase. FASEB J. 30, 4289–4300 (2016). [DOI] [PubMed] [Google Scholar]
- 39.Othman A.et al., Plasma deoxysphingolipids: A novel class of biomarkers for the metabolic syndrome? Diabetologia 55, 421–431 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Bode H.et al., HSAN1 mutations in serine palmitoyltransferase reveal a close structure-function-phenotype relationship. Hum. Mol. Genet. 25, 853–865 (2016). [DOI] [PubMed] [Google Scholar]
- 41.Karsai G.et al., DEGS1-associated aberrant sphingolipid metabolism impairs nervous system function in humans. J. Clin. Invest. 129, 1229–1239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saied E. M., Banhart S., Bürkle S. E., Heuer D., Arenz C., A series of ceramide analogs modified at the 1-position with potent activity against the intracellular growth of Chlamydia trachomatis. Future Med. Chem. 7, 1971–1980 (2015). [DOI] [PubMed] [Google Scholar]
- 43.Saied E. M., Le T. L., Hornemann T., Arenz C., Synthesis and characterization of some atypical sphingoid bases. Bioorg. Med. Chem. 26, 4047–4057 (2018). [DOI] [PubMed] [Google Scholar]
- 44.Camus M. C., Chapman M. J., Forgez P., Laplaud P. M., Distribution and characterization of the serum lipoproteins and apoproteins in the mouse, Mus musculus. J. Lipid Res. 24, 1210–1228 (1983). [PubMed] [Google Scholar]
- 45.Rohrer L.et al., Binding, internalization and transport of apolipoprotein A-I by vascular endothelial cells. Biochim. Biophys. Acta 1761, 186–194 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data discussed in this study are included in the main text and SI Appendix.