Significance
Inbreeding reduces fitness leading to selection for incest avoidance in many organisms. Passive processes, such as sex-biased dispersal, may reduce inbreeding risk, but when dispersal is limited, inbreeding may still be minimized by animals actively recognizing and discriminating kin from nonkin when choosing mates. We investigated inbreeding costs, risk, and avoidance in a cooperative bird species in which opposite-sex adults disperse locally to breed and frequently associate. We identified a reduction in fitness in inbred individuals and have shown that despite a substantial inbreeding risk, breeders alleviate this by discriminating against close kin as partners. We show that the increased vocal similarity among relatives offers a probable recognition mechanism for this observed level of kin discrimination during mate choice.
Keywords: inbreeding, kin discrimination, cooperative breeder, mate choice
Abstract
Inbreeding is often avoided in natural populations by passive processes such as sex-biased dispersal. But, in many social animals, opposite-sexed adult relatives are spatially clustered, generating a risk of incest and hence selection for active inbreeding avoidance. Here we show that, in long-tailed tits (Aegithalos caudatus), a cooperative breeder that risks inbreeding by living alongside opposite-sex relatives, inbreeding carries fitness costs and is avoided by active kin discrimination during mate choice. First, we identified a positive association between heterozygosity and fitness, indicating that inbreeding is costly. We then compared relatedness within breeding pairs to that expected under multiple mate-choice models, finding that pair relatedness is consistent with avoidance of first-order kin as partners. Finally, we show that the similarity of vocal cues offers a plausible mechanism for discrimination against first-order kin during mate choice. Long-tailed tits are known to discriminate between the calls of close kin and nonkin, and they favor first-order kin in cooperative contexts, so we conclude that long-tailed tits use the same kin discrimination rule to avoid inbreeding as they do to direct help toward kin.
Inbreeding is generally maladaptive because it increases homozygosity and hence the unmasking of deleterious recessive alleles, which, when expressed, result in a reduction in fitness among inbred individuals termed "inbreeding depression” (1, 2). Inbreeding may be tolerated (3, 4), however, if avoidance is costly, or if the costs of inbreeding are outweighed by the inclusive fitness benefits accrued from breeding with or interacting socially with relatives (5, 6). Thus, the selection pressures on alternative inbreeding strategies depend on the fitness consequences of inbreeding, typically inferred by the strength of inbreeding depression and the costs of inbreeding avoidance. Inbreeding depression is often difficult to quantify in natural populations (7), but it has been shown to select for various avoidance mechanisms (8–10). Passive processes that disrupt opposite-sex kin associations, such as sex-biased dispersal, are widespread (11, 12), but when dispersal is constrained (13) or when there is countervailing selection for kin association (14), individuals may frequently encounter kin as potential mates. This is the case in most cooperative breeders, where delayed natal dispersal creates structured populations within which opposite-sex kin associate beyond reproductive maturity (15). In such situations, inbreeding may be minimized by extragroup matings (16–20) or by abstention from breeding (21–23). The latter often results in a strong reproductive skew, with reproduction monopolized by a minority of dominant individuals within groups, aided by subordinate helpers (24–26).
Most cooperative species live in discrete groups that occupy exclusive territories, but in some others, helping (providing care to others’ offspring) follows local natal dispersal that results in continued association among relatives across extended social networks known as “kin neighborhoods” (27). Kin neighborhoods are characterized by a diffuse kin structure where mean relatedness among socially interacting individuals is low. This degree of social organization also exists in colonial breeders, such as sociable weavers (Philetairus socius), in which males and females may recruit as breeders within their natal colony (28). Such social structures select for strong kin discrimination in helping behavior because of the risk of directing care toward nonkin (29), and if adult associations include opposite-sex relatives, then strong inbreeding depression would also be expected to select for a mechanism for active incest avoidance.
However, the extent to which variation in relatedness across social systems influences inbreeding risk and the strength of kin discrimination exercised during mate choice remain relatively understudied. Fitness costs of inbreeding (30) or of being inbred have been identified in several cooperative breeders (19, 20, 31), and active incest avoidance has been demonstrated in western bluebirds (Sialia mexicana) (32) and inferred in red-winged fairy-wrens (Malurus elegans) (33) and gray-crowned babblers (Pomatostomus temporalis) (34). However, the discrimination rules used to avoid inbreeding and the recognition mechanisms that effectively minimize its costs have not been determined.
Here, we present a comprehensive study of inbreeding depression, inbreeding risk, and inbreeding avoidance in long-tailed tits (Aegithalos caudatus). Long-tailed tits breed in kin neighborhoods and exhibit redirected helping, whereby failed breeders acquire indirect fitness by helping to provision nondescendant kin (35). Although dispersal is female-biased, natal dispersal distances of both sexes are short (36), creating a fine-scale genetic structure within breeding populations (37). This kin structure facilitates kin-selected helping, but also results in both kin and nonkin being available as partners when monogamous pairs form each spring (38). Using a long-term genetic and life-history dataset (39), we assess the evidence for inbreeding depression and a risk of incest and test putative rules for inbreeding avoidance to determine the likely kin-recognition mechanism (40, 41).
Results
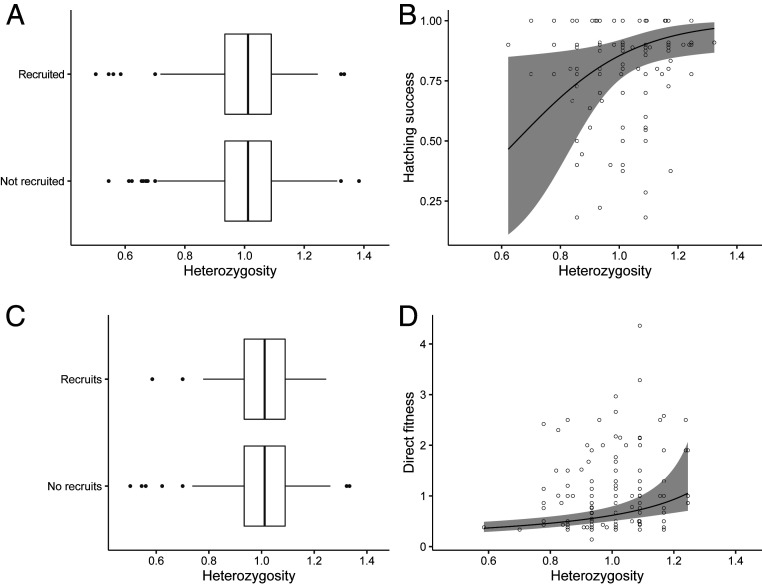
Reduced heterozygosity in inbred individuals is a major source of inbreeding depression, and associations between heterozygosity at microsatellite markers and variation in fitness are widely used as an indirect measure of inbreeding depression when pedigree-derived inbreeding coefficients are unreliable (42). We tested for an association between standardized heterozygosity (H) at 17 microsatellite markers and fitness using 4 fitness-associated life-history traits: whether an individual recruited to the breeding population; the proportion of eggs that hatched in a female’s first clutch; the probability that a breeder produced recruits; and the direct fitness of breeders that produced recruits. Here, direct fitness is a measure of an individual’s lifetime reproductive success that corrects for the contribution of helpers (Materials and Methods). This is important because the presence of helpers has a very substantial effect on fledgling recruitment (35), and this social effect must be removed to reveal the fitness that most closely reflects an individual’s intrinsic “quality.” Heterozygosity was positively associated with the hatching success of females’ clutches (Fig. 1B) and the direct fitness of breeders that produced recruits (Fig. 1D), but there was no association between H and an individual’s probability of recruitment (Fig. 1A) nor the probability that a breeder produced recruits (Fig. 1C). In our analyses, both hatching success and direct fitness are adult traits, and this reduction in fitness of inbred adults indicates that inbreeding has long-term, negative-fitness consequences.
Fig. 1.
The relationship between H at microsatellite loci and fitness components. (A) Probability of recruitment was not associated with H (GLMM: n = 1,924, z = 0.40, P = 0.69). (B) Females’ hatching success was positively associated with H (GLMM: n = 142, z = 2.32, P = 0.02). (C) Probability of producing recruits was not associated with H (GLMM: n = 744, z = −1.77, P = 0.07). (D) The direct fitness of breeders that produced recruits was positively associated with H (GLMM: n = 151, t = −4.65, P < 0.001). Full model outputs are reported in SI Appendix, Tables S3–S6. Lines represent model predictions ± 95% CI constructed using fixed effects; boxplots represent median ± 1.5 × inter-quartile range (IQR).
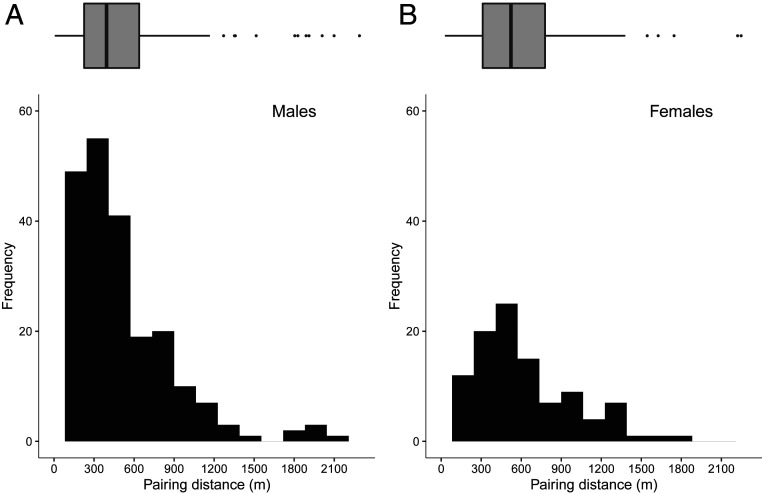
Long-tailed tits exhibit a significantly enhanced level of relatedness between adult males and females within 600 m (37), a range within which pairing typically occurs (Fig. 2). However, based on the pedigree, only 1 of 609 pairs (0.2%) were first-order relatives, and another 2 pairings (0.3%) were between second-order kin (SI Appendix, Table S1). Genetic relatedness estimates (rQG) (43) revealed a similar frequency of close inbreeding (2/609, 0.3%), but substantially more cases of moderate inbreeding (94/609, 15.4%; SI Appendix, Table S2). These results suggest active avoidance of close kin when pairing, rather than retrospective extrapair mating to avoid inbreeding with a related partner. Indeed, the relatively low levels of promiscuity in long-tailed tits (44, 45) make extrapair mating an unlikely mechanism of inbreeding avoidance. Instead, we examined whether inbreeding was actively avoided when choosing a social mate.
Fig. 2.
Frequency distribution and median (+IQR) pairing ranges of (A) male (median = 393 m, n = 230) and (B) female (median = 523 m, n = 109) breeders, calculated as the distance between an individual’s natal nest and their first breeding attempt. Boxplots represent median ± 1.5 × IQR.
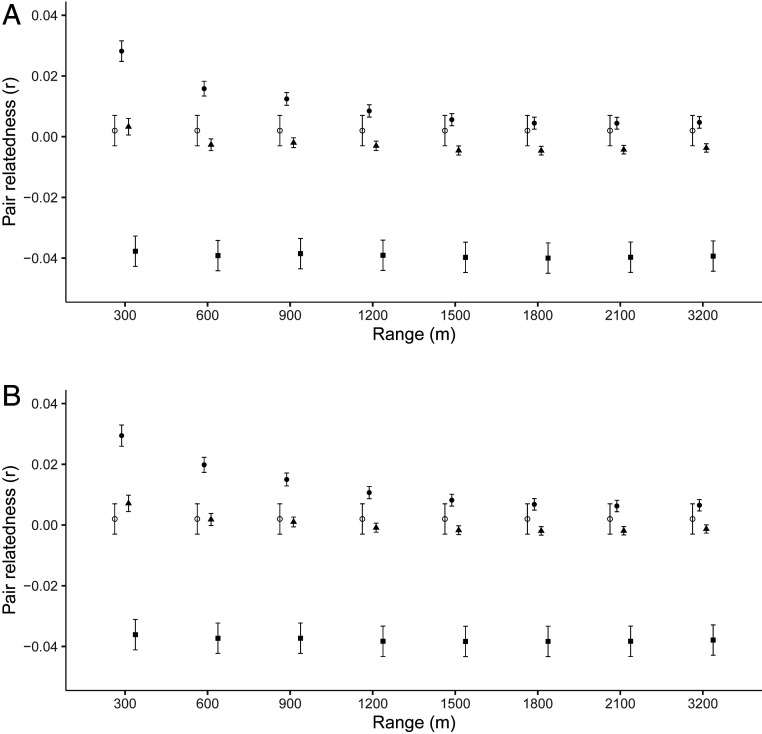
The relatedness of observed pairs was compared with that expected under a series of mate-choice models that assumed that all first-year, widowed, or divorced opposite-sex breeders present in the same year were available as potential partners, within ranges of 300-m, 600-m, and further 300-m increments up to 2,100 m. Mean rQG to a chosen partner was significantly lower than that expected for females selecting partners at random from within 300 m (generalized linear mixed-effects model [GLMM]: n = 2,420, t = 7.23, P < 0.001), 600 m (GLMM: n = 2,433, t = 3.93, P < 0.001), 900 m (GLMM: n = 2433, t = 3.03, P < 0.01), but not 1,200 m (n = 2,433, t = 1.9, P = 0.06) (Fig. 3A). Mean rQG to a chosen partner was lower than that predicted for males selecting mates from within 300 m (n = 2,416, t = 7.84, P < 0.001), 600 m (n = 2,432, t = 5.14, P < 0.001), 900 m (n = 2,432, t = 3.79, P < 0.001), and 1,200 m (n = 2432, t = 2.54, P = 0.01) (Fig. 3B). These results demonstrate strong discrimination against kin as partners within the range within which mates are normally chosen, suggesting that inbreeding depression may be sufficiently strong to cause selection for inbreeding avoidance.
Fig. 3.
Mean genetic relatedness of breeding pairs formed within increasing ranges (open circles) and the expected relatedness if (A) females (n = 445) or (B) males (n = 412) selected mates at random with respect to kinship (closed circles), avoided kin with rQG > 0.375 (closed triangles), and avoided kin with rQG > 0.125 (closed squares). Expected relatedness was the mean relatedness of focal birds to all opposite-sex available breeders within each range under each mate-choice model. Error bars represent the SE around the mean.
To identify a plausible discrimination rule for incest avoidance, we compared observed and expected pair rQG assuming either avoidance of first-order kin (rQG ≥ 0.375) or avoidance of first- and second-order kin (rQG ≥ 0.125) by removal of these kin from the pool of potential partners at pairing ranges within 1,200 m. When first-order kin were removed, observed and expected pair rQG did not differ significantly if females selected mates within 300 m (GLMM: n = 2,420, t = 0.36, P = 0.72), 600 m (n = 2,433, t = −1.32, P = 0.18), 900 m (n = 2,433, t = −1.15, P = 0.25), and 1,200 m (n = 2433, t = −1.46, P = 0.14) (Fig. 3A). The same was true for males when they were assumed to select mates from within 300 m (GLMM: n = 2,416, t = 1.47, P = 0.14), 600 m (n = 2,432, t = −0.05, P = 0.96), 900 m (n = 2,432, t = −0.29, P = 0.77), and 1,200 m (n = 2,432, t = −0.84, P = 0.39) (Fig. 3B). In contrast, when both first- and second-order kin were removed, observed pair rQG was higher than expected at all ranges for both females (GLMM: 300 m—n = 2,420, t = −9.9, P < 0.001; 600 m—n = 2,433, t = −11.46, P < 0.001; 900 m—n = 2,433, t = −11.52, P < 0.001; and 1,200 m—n = 2,433, t = −11.8, P < 0.001) (Fig. 3A) and males (GLMM: 300 m—n = 2,416, t = −9.16, P < 0.001; 600 m—n = 2,432, t = −11.04, P < 0.001; 900 m—n = 2,432, t = −11.19, P < 0.001 and 1,200 m—n = 2,432, t = 11.54, P < 0.001) (Fig. 3B). Thus, the observed relatedness of breeding pairs closely matches the pattern expected by avoidance of first-order kin as mates. This degree of discrimination can effectively reduce inbreeding because first-order relatives are the category of kin most likely to be encountered nearby in long-tailed tit populations (36), although the substantially lower risk of pairing with second-order and more distant kin remains.
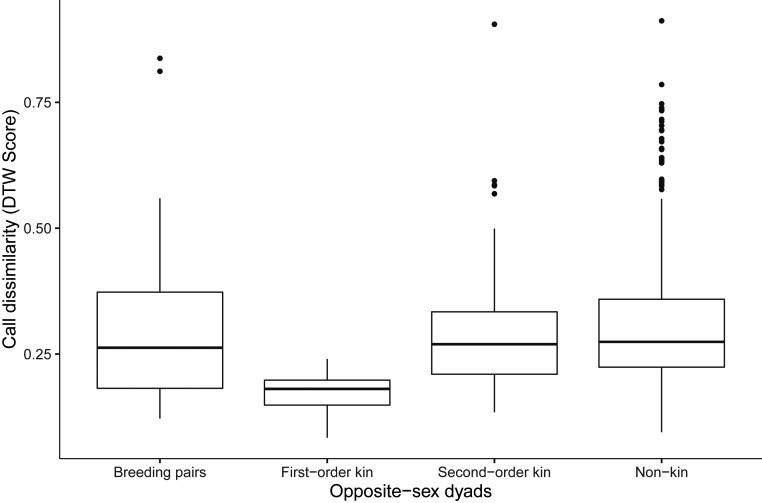
Long-tailed tits can discriminate kin from nonkin using learned vocal cues (41), a mechanism that is consistent with helpers preferentially aiding close kin (37, 40). We investigated whether the same mechanism may enable inbreeding avoidance. Our analyses focused on the churr call, a short-range contact call that is highly repeatable within individuals through time (46). The similarity of the churr calls of opposite-sex breeders varied with relatedness: first-order kin (n = 20 dyads) had more similar calls than second-order kin (GLMM: n = 249 dyads, t = −3.02, P = 0.002) or nonkin (GLMM: n = 1,078 dyads, t = −3.62, P < 0.001). Crucially, the calls of males and females within breeding pairs were significantly less similar than those of opposite-sex first-order kin within pairing range (Fig. 4). In contrast, there was no significant difference in vocal similarity between observed pairs and second-order kin or nonkin (Fig. 4). These results suggest that vocal similarity provides a plausible mechanism for avoidance of first-order kin as partners, although we cannot exclude the possibility that other phenotypic cues are also involved.
Fig. 4.
Dissimilarity of churr calls among groups of opposite-sex dyads: breeding pairs (n = 51); first-order kin (n = 11); second-order kin (n = 155); nonkin (n = 735). Dyads that were not breeding pairs comprised available breeders within pairing range (≤1,350 m, 95% pairs) present in the same breeding year. Call dissimilarity was measured using DTW analysis. Call dissimilarity within breeding pairs was higher than that within potential pairs of first-order kin (GLMM: n = 952, t = 2.87, P = 0.004) but not second-order (t = 0.06, P = 0.94) or nonkin (t = −1.63, P = 0.10). Boxplots represent median ± 1.5 × IQR.
Discussion
We have shown that inbreeding carries long-term fitness costs in long-tailed tits, but have detected no short-term cost on recruitment. Inbreeding depression may be masked in the short-term because external factors such as nest predation have large impacts on offspring fitness in early life. Alternatively, inbreeding depression may affect embryo development or chick survival during the first few days after hatching (47). We genotyped chicks at 11 d old, so inbred individuals would be a missing fraction in our data if inbreeding depression occurs prior to this age. Furthermore, the probability of both individual recruitment and recruit production are likely to be largely governed by stochastic events, such as predation, whereas hatching success and direct fitness may have a stronger genetic component. The presence of helpers may also mitigate some of the fitness consequences of inbreeding depression. Maternal care buffers inbreeding depression in the burying beetle (Nicrophorus vespilloides) (48) and, in long-tailed tits, both the probability that an individual recruits and its own production of recruits are correlated with helper number (49). Investigation into the heritability of life-history traits such as hatching success would further elucidate the mechanism by which inbreeding reduces fitness.
Long-tailed tits actively avoid close inbreeding, despite the substantial risk of incest, by avoidance of first-order kin as mates. By contrast, the observed frequency of pairings between second-order kin was relatively high (15.4% of pairs) when using genetic relatedness estimates, although not when using the pedigree (0.3% of pairs). The kin structure of long-tailed tit populations means that, after excluding first-order kin, the proportion of birds (of either sex) that are second-order kin within 600 m is 14.7% using genetic relatedness estimates and 2.7% of birds using pedigrees (37). Thus, our observed frequencies of second-order kin pairings are close to what would be expected from random pairing among birds that are not first-order kin, further supporting our proposed rule for kin discrimination during mate choice (Fig. 3). Together, the significant inbreeding depression and pattern of inbreeding avoidance observed support the hypothesis that there is selection for inbreeding avoidance.
These findings are consistent with previous studies demonstrating recognition of first-order kin in a cooperative context (37, 40). They are also consistent with the idea that kin recognition in long-tailed tits requires a period of association during development, when vocalizations are learned (41). It is very likely that first-order kin (siblings, parents, and offspring) associate during rearing, whereas second-order kin are likely to be reared apart. Consequently, vocalizations are more similar among first-order relatives than among second-order or nonkin (50). There are two instances in which this is not the case: extrapair paternity and when pair bonds last more than 1 y so that full siblings are produced in different nests. However, long-tailed tits are not very promiscuous (44), and their low mate fidelity across seasons (38), high annual mortality, and low chance of successful reproduction (51) mean that the probability of either instance is low. Avoidance of first-order, but not second-order kin as mates therefore supports familiarity as the mechanism of kin recognition. However, because long-tailed tits do not live in stable kin groups throughout their life, recognition of familiar individuals still relies on phenotypic rather than spatial cues.
Our results suggest that a single kin-discrimination rule may explain inbreeding avoidance and kin preference in helping in long-tailed tits, with observational evidence showing that vocal cues offer a plausible mechanism for kin recognition. However, there is an intriguing contrast between the observations that, while distant and nonkin are frequently helped (35), close inbreeding is extremely rare. A single recognition mechanism can produce variable outcomes depending on the position of the acceptance threshold, which may shift according to the relative fitness costs and benefits associated with acceptance and rejection errors (52, 53). These in turn will be determined by the probability of encountering a relative and the fitness consequences of the associated behavior. Assuming that there is some overlap in the similarity of cues produced by close kin and by distant or nonkin (50) (Fig. 4), an acceptance threshold that includes most close kin, but also some distant or nonkin, would explain the observed pattern of helping (35). The same recognition threshold could also operate during mate choice but with the reverse effect that almost all close kin, and presumably some distant or nonkin, are rejected as partners, resulting in the infrequent close inbreeding that we observed. A recognition threshold that is generous in the context of helping and stringent in the context of mate choice makes intuitive sense in long-tailed tits. Redirected helping by failed breeders is likely to incur little cost but potentially substantial benefit when kin-directed (35). In contrast, inbreeding depression (Fig. 1) suggests selection for strict avoidance of close kin as partners. Therefore, we conclude that a single kin-discrimination mechanism has evolved to serve two functions, driving kin association in one context and kin avoidance in the other.
Materials and Methods
Study Population.
A population of 17 to 72 (mean ∼0.50) pairs of long-tailed tits was studied during the breeding seasons (February to June) between 1994 and 2017 in the Rivelin Valley, Sheffield, United Kingdom (53°38′N 1°56′W). The site is ∼2.5 km2 and comprises predominantly deciduous woodland and scrub. The population is open: ∼40% of breeders hatched in the study site and are referred to as native, while the remaining immigrant adults are assumed to have dispersed into the study site during their first year, based on the observation that individuals have high site fidelity following their first breeding year (49). Each year, almost all individuals (>95%) were marked with a British Trust for Ornithology (BTO) ring and a unique combination of two color rings. Native birds were ringed as 11-d-old nestlings, and immigrant adults were captured in mist nests under BTO license before or during their first breeding season. When ringed, a sample of 5 to 30 µL of blood was taken by brachial venipuncture under a UK Home Office license. All breeding attempts were closely monitored and Global Positioning System coordinates were taken for each nest (n = 1,461); a Universal Transverse Mercator (UTM) coordinate system was used to describe geographic distance between nests. An ethical review of licensed procedures was undertaken by the University of Sheffield's Administration Ethical Review Process (Project Applications and Amendments Subcommittee).
Social Pedigree.
We used the social pedigree to predict the correlation between heterozygosity and individual inbreeding coefficients and to identify matings among known kin in our population. The pedigree was created using 23 y of field observations (1994 to 2017; n = 3,068 birds). For further details on pedigree construction, see ref. 37. To calculate social relatedness (r) among dyads, an additive relationship matrix was generated from the pedigree in R (version 3.5.0, 2018) using the nadiv package (54). Six breeding birds in our study population (0.2%) were from cross-fostered broods from 1996 to 1998, but, given that birds raised together treat each other as kin (41), we included them in the social pedigree. For the same reason, while there is a low rate of extrapair paternity (11% of chicks in 30% of nests) in long-tailed tits (44), it has not been corrected for in the social pedigree.
Inbreeding Coefficients.
Inbreeding coefficients were calculated from the social pedigree. It was possible to infer reliable f values from the pedigree for 129 birds (native individuals with all grandparents known). The f values from an additional nine birds that were offspring of presumed immigrant siblings, based on genetic sibship reconstruction, were also included. As more distant shared ancestors than grandparents, if known, would cause individual inbreeding coefficients to increase, f values are likely to be underestimated based on incomplete pedigree information.
Molecular Genetics.
Individuals were genotyped at 17 microsatellite loci (55). Population allele frequencies were generated in CERVUS (version 3.0.7, 2007). All available genotypes were used (1994 to 2017; n = 3,304 birds) to maximize accuracy and ensure nonzero estimates for all alleles. The genetic relatedness of dyads, rQG, is a genetic estimate of the coefficient of relatedness based on genetic markers (43), calculated in SPAGeDi (version 1.1.5, 2002). This estimate is reliable when tested against our social pedigree (56).
Inbreeding.
Inbreeding cases were identified using the social pedigree and genetic relatedness estimates. Genetic (rQG) and social (r) relatedness of all breeding pairs from 1994 to 2016 in which both adults were ringed and genotyped were calculated. Measurements were taken from distinct pairs. Occasionally, long-tailed tits swap partners within a breeding season, in which case the first pairing of that year was used. Individuals often breed in multiple years, either with the same partner or a new partner. The dataset used in this study contained 609 pairs made up of 445 females and 412 males observed from 1994 to 2016. Pairs were considered closely or moderately inbred if they comprised known first-order (r = 0.5) or second-order (r = 0.25) kin, respectively. As incomplete social pedigrees may underestimate incest rates in open populations, inbreeding was also quantified using genetic relatedness estimates (rQG). The rQG estimate of known first-order kin (r = 0.5) was 0.468 ± 0.136 (mean ± SD, n = 500 dyads). For known second-order kin (r = 0.25), rQG was 0.241 ± 0.179 (mean ± SD, n = 338 dyads). The rQG estimate of all other dyads of known parentage (r < 0.25) was 0.004 ± 0.133 (mean ± SD, n = 25,638 dyads). The distribution of rQG estimates among known first-order, second-order, and nonkin are shown in SI Appendix, Fig. S1. Based on these distributions, a lower rQG threshold of 0.375 was set to approximate first-order kin (mean rQG ± SD = 0.502 ± 0.094, n = 1,148) and 0.125 to approximate second-order kin (mean rQG ± SD = 0.197 ± 0.059, n = 9,926). The mean rQG of observed pairs was 0.002 ± 0.123 (mean ± SD, n = 609).
Mate Choice Models.
For each focal breeder, their rQG to their chosen partner was compared with their mean rQG to all potential partners within each breeding year (1994 to 2016) under the pairing constraints of a series of mate-choice models assuming that all first-year, widowed, or divorced opposite-sex breeders present in the same year were available as potential partners within concentric ranges of radius 300-m, 600-m, 900-m, and further 300-m increments up to 2,100 m.
Heterozygosity-Fitness Correlations.
Pedigree-derived inbreeding coefficients can be estimated only when parentage can be traced back at least two generations, but both sets of grandparents were known for only 5.3% of native birds (n = 138). Therefore, standardized multilocus H was estimated for all genotypes (1994 to 2016; n = 3,182). Heterozygosity is standardized by dividing the proportion of typed loci for which an individual was heterozygous by the mean heterozygosity of those loci at which the individual was typed (57). Heterozygosity-fitness correlations can be regarded as providing evidence for inbreeding depression only if heterozygosity is a predictor of individual inbreeding coefficients. We used the analytical derivations outlined in ref. 39 to predict the correlation between heterozygosity and f in our population as r(H, f) = −0.43 (n = 138, mean f = 0.03, variance in f = 0.004, number of loci = 17, mean heterozygosity of loci = 0.759). This value is relatively large compared to other studies predicting the relationship between inbreeding coefficient and heterozygosity, including populations in which inbreeding depression has been demonstrated. For example, the correlation coefficient r(H, f) in red deer (Cervus elaphus) (58) and song sparrows (Melospiza melodia) (59) are −0.25 and −0.22, respectively (39). Thus, genetic diversity at marker loci reflects genetic diversity throughout the genome, including at unknown loci that affect trait variation; i.e., marker and fitness loci are in identity disequilibrium (42). This validates the use of heterozygosity as a proxy for inbreeding coefficient in our study. Measurements were taken from distinct samples.
Direct Fitness.
Direct fitness was calculated as lifetime reproductive success quantified in terms of genetic offspring equivalents and corrected for extrapair paternity and the offspring gained by having helpers. The fraction of recruits in a brood that was attributable to helpers was estimated using a mixed-effects model of the effect of helper number on recruitment (49). This fraction was subtracted from the total number of recruits produced over an individual’s lifetime. The remaining fraction was halved to reflect the relatedness between a single parent and its offspring. The assumption that parents and their offspring have a relatedness coefficient of 0.5 does not account for higher relatedness of inbred offspring to their parents (60). However, the almost complete absence of close inbreeding and the low incidence of inbreeding among more distant relatives indicate that errors in our estimation of direct fitness introduced by this simplifying assumption will be small.
Acoustic Recordings.
A short-distance contact call, the churr, was recorded from adults using a Sennheiser ME67/K6 shotgun microphone fitted with a Rycote windjammer. Recordings were made onto a Roland R-05 version 1.03 WAV/MP3 recorder with a 6GB SanDisk memory card, set to a sample rate of 48 kHz with WAV-16bit accuracy. The microphone input level was set to 60 db with a low-cut frequency of 400 Hz. All recordings were made between 6:00 AM and 6:00 PM British summer time. Birds were recorded at a distance of ∼3 to 15 m to minimize sound degradation and reverberation. Birds were recorded at the nest and identified by their unique color ring combinations. If more than one bird was present, vocalizations were assigned to individuals by observing movements of the bill and throat feathers. At the start of each recording, date, time, nest number, and recording number were dictated into the microphone. When caller identification (ID) could be identified with certainty, this was dictated into the microphone after each call. In total, 213 recordings were made from 2015 to 2017, containing 1,116 churr calls from 98 birds (mean ± SD = 11.39 ± 10.24 per bird; range 1 to 42).
Acoustic Analysis.
The sampling frequency was converted to 22.05 kHz, and recordings were visualized spectrographically to assess call quality, with a frequency resolution of 188 Hz and a time resolution of 2.7 ms in Avisoft SAS-Lab Pro version 4.52 (Avisoft Bioacoustics). Recordings with extreme background noise were excluded. All usable calls were isolated, stored, and measured in Luscinia (version 2.16.10.29.01, https://rflachlan.github.io/Luscinia/). Vocal similarity was assessed by dynamic time-warping analysis (DTW) implemented in Luscinia. DTW analysis generates a score representing the amount of warping required to match one signal to another. The acoustic features used in the DTW analysis were weighted as the following: time = 1, fundamental frequency = 2, change in fundamental frequency = 2, compression factor = 0.1, minimum element length = 10, time SD weighting = 1, ArcTan transform weight for frequency slope = 0.02, and maximum warp = 100%. These settings generated a DTW algorithm that correctly matched visually similar vocalizations, assessed using a dendrogram and multidimensional scaling plot. The low compression factor optimizes the capture of acoustic complexity. This increased weighting of frequency parameters to time is also in line with previous studies suggesting that frequency parameters show greater individuality than temporal parameters and are particularly important for kin recognition in this species (46).
Call Similarity and Pairing.
Among the breeding pairs for which we had recordings of both breeders (n = 51), there were no cases of pairing among known first-order or second-order kin, based on the social pedigree. Based on genetic relatedness estimates, there were no cases of pairing among first-order kin (rQG ≥ 0.375) and 13 (25.5%) cases of pairing among second-order kin (rQG ≥ 0.125). Dyadic vocal similarity (DTW score) was compared among the following: breeding pairs; potential pairs of first-order kin (rQG ≥ 0.375); potential pairs of second-order kin (0.375 > rQG ≥ 0.125); and potential pairs of nonkin (rQG < 0.125) within 1,350 m, the range within which 95% pairs are formed. Genetic estimates of pedigree relationships were used for consistency with our analysis of putative discrimination rules. Potential pairings were dyads of opposite-sex first-year, widowed, or divorced breeders present in the breeding population in the same year. The distance between adults was based on the location of an individual’s first breeding attempt in a given year.
Statistical Analysis.
All statistical analyses were carried out in R (version 3.5.0, 2018). Heterozygosity-fitness correlations (HFCs) were tested using generalized linear mixed-effects models in the lme4 package. Recruitment was modeled as a binary response variable with a binomial error distribution and logit link. The fixed effects were the following: H, sex (to control for male-biased philopatry), and fledge date (days since March 1) because offspring that fledge earlier in the year have a greater probability of recruitment (61) and number of helpers at the natal nest, as helper number has been shown to increase recruitment probability (51). Hatching success was modeled as a proportional response variable with a binomial error distribution and logit link. The fixed effects were the following: H, lay date, and female mass as a nestling. The probability of producing recruits was modeled as a binary response variable with a binomial error distribution and logit link. The fixed effects were the following: H and fledgling sex ratio (proportion of male fledglings produced, to control for male philopatry). Direct fitness was modeled as a continuous response variable with a Gamma error distribution and inverse link, with H, sex, and fledgling sex ratio fitted as fixed effects. In all HFC models, genetic brood was fitted as a random effect to avoid pseudoreplication of H estimates and control for seasonal differences. In hatching success models, breeding year was also fitted as a random effect.
Analyses of the mating options available to males and females were conducted in separate mate-choice models. As the same allele frequencies are used to calculate rQG across years, the rQG of unique dyads across years is consistent. However, due to demographic factors such as divorce, migration, birth, death, and dispersal, the mean rQG of focal breeders to their potential partners under each mate-choice model will vary across years. To quantify inbreeding avoidance and identify a putative decision rule with regard to kinship, we fitted linear mixed-effects models with restricted maximum likelihood. For focal males and females, we compared rQG to the chosen partner with 1) mean rQG to potential partners under random mate choice (with respect to kinship), 2) mean rQG to potential partners after the removal of close kin, and 3) mean rQG to potential partners after the removal of close and distant kin, within pairing ranges of 300, 600, 900, and 1,200 m. When pairs persisted across years, the first year in which a pair was observed was used in the analysis. The breeding year nested within the focal bird ID was fitted as a random effect to generate comparisons within individuals in a given year.
Churr call dissimilarity was compared among four groups of individuals (breeding pairs, potential breeding pairs of first-order kin, potential breeding pairs of second-order kin, and potential breeding pairs of nonkin) using generalized linear mixed-effects models. DTW score was modeled as a continuous response variable with a Gamma distribution and inverse link function. The fixed effect was group with both male ID and female ID fitted as random effects. The relationship between churr call dissimilarity and kinship was tested using a separate model that included all genotyped breeders, irrespective of pairing status. DTW score was modeled as a continuous response variable with a Gamma distribution and inverse link function. The fixed effect was kinship with both male ID and female ID fitted as random effects.
Data Availability.
Source datasets and code for this paper have been deposited in the Dryad Digital Repository, https://doi.org/10.5061/dryad.k6djh9w49 (62).
Supplementary Material
Acknowledgments
Molecular analyses were conducted at the Natural Environment Research Council Biomolecular Analysis Facility at the University of Sheffield with support from Terry Burke, Deborah Dawson, Natalie dos Remedios, and Maria-Elena Mannarelli. We thank all those who have contributed to the long-tailed tit project and Tim Clutton-Brock and René van Dijk for discussion. The Sheffield City Council, Yorkshire Water, Hallamshire Golf Club, and private landowners of the Rivelin Valley allowed access to their land, and the Sorby Breck Ringing Group provided logistical support. This work was funded by the National Environment Research Council of the United Kingdom (Awards 1517208 and NE/I027118/1).
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
Data deposition: Source datasets and code for this paper have been deposited in the Dryad Digital Repository, https://doi.org/10.5061/dryad.k6djh9w49.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1918726117/-/DCSupplemental.
References
- 1.Charlesworth B., Charlesworth D., The genetic basis of inbreeding depression. Genet. Res. 74, 329–340 (1999). [DOI] [PubMed] [Google Scholar]
- 2.Keller L. F., Waller D. M., Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241 (2002). [Google Scholar]
- 3.Jamieson I. G., Taylor S. S., Tracy L. N., Kokko H., Armstrong D. P., Why some species of birds do not avoid inbreeding: Insights from New Zealand robins and saddlebacks. Behav. Ecol. 20, 575–584 (2009). [Google Scholar]
- 4.Wang C., Lu X., Female ground tits prefer relatives as extra-pair partners: Driven by kin-selection? Mol. Ecol. 20, 2851–2863 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Kokko H., Ots I., When not to avoid inbreeding. Evolution 60, 467–475 (2006). [PubMed] [Google Scholar]
- 6.Parker G. A., Sexual conflict over mating and fertilization: An overview. Philos. Trans. R. Soc. Lond. B Biol. Sci. 361, 235–259 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duthie A. B., Reid J. M., Evolution of inbreeding avoidance and inbreeding preferences through mate choice among interacting relatives. Am. Nat. 188, 651–667 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Lehmann L., Perrin N., Inbreeding avoidance through kin recognition: Choosy females boost male dispersal. Am. Nat. 162, 638–652 (2003). [DOI] [PubMed] [Google Scholar]
- 9.Pusey A., Wolf M., Inbreeding avoidance in animals. Trends Ecol. Evol. (Amst.) 11, 201–206 (1996). [DOI] [PubMed] [Google Scholar]
- 10.Riehl C., Stern C. A., How cooperatively breeding birds identify relatives and avoid incest: New insights into dispersal and kin recognition. BioEssays 37, 1303–1308 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Greenwood P. J., Mating systems, philopatry and dispersal in birds and mammals. Anim. Behav. 28, 1140–1162 (1980). [Google Scholar]
- 12.Du Plessis M. A., Obligate cavity-roosting as a constraint on dispersal of green (red-billed) woodhoopoes: Consequences for philopatry and the likelihood of inbreeding. Oecologia 90, 205–211 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Cockburn A., Osmond H. L., Mulder R. A., Green D. J., Double M. C., Divorce, dispersal and incest avoidance in the cooperatively breeding superb fairy-wren Malurus cyaneus. J. Anim. Ecol. 72, 189–202 (2003). [Google Scholar]
- 14.Stacey P., Ligon J., Territory quality and dispersal options in the acorn woodpecker, and a challenge to the habitat-saturation model of cooperative breeding. Am. Nat. 130, 654–676 (1987). [Google Scholar]
- 15.Hatchwell B. J., The evolution of cooperative breeding in birds: Kinship, dispersal and life history. Philos. Trans. R. Soc. Lond. B Biol. Sci. 364, 3217–3227 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brooker M. G., Rowley I., Adams M., Baverstock P. R., Promiscuity: An inbreeding avoidance mechanism in a socially monogamous species? Behav. Ecol. Sociobiol. 26, 191–199 (1990). [Google Scholar]
- 17.Tarvin K., Webster M., Tuttle E., Pruett-Jones S., Genetic similarity predicts the level of extra-pair paternity in splendid fairy-wrens. Anim. Behav. 70, 945–955 (2005). [Google Scholar]
- 18.Varian-Ramos C. W., Webster M. S., Extrapair copulations reduce inbreeding for female red-backed fairy-wrens, Malurus melanocephalus. Anim. Behav. 83, 857–864 (2012). [Google Scholar]
- 19.Hajduk G. K.et al., Inbreeding, inbreeding depression, and infidelity in a cooperatively breeding bird. Evolution 72, 1500–1514 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtenauer W., van de Pol M., Cockburn A., Brouwer L., Indirect fitness benefits through extra-pair mating are large for an inbred minority, but cannot explain widespread infidelity among red-winged fairy-wrens. Evolution 73, 467–480 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kokko H., Ekman J., Delayed dispersal as a route to breeding: Territorial inheritance, safe havens, and ecological constraints. Am. Nat. 160, 468–484 (2002). [DOI] [PubMed] [Google Scholar]
- 22.Koenig W. D., Haydock J., “Incest and incest avoidance” in Ecology and Evolution of Cooperative Breeding in Birds, Koenig W. D., Dickinson J. L., Eds. (Cambridge University Press, 2004), pp. 142–156. [Google Scholar]
- 23.Magrath R. D., Heinsohn R. G., Johnstone R. A., “Reproductive skew” in Ecology and Evolution of Cooperative Breeding in Birds, Koenig W. D., Dickinson J. L., Eds. (Cambridge University Press, 2004), pp. 157–176. [Google Scholar]
- 24.Koenig W. D., Stanback M. T., Haydock J., Demographic consequences of incest avoidance in the cooperatively breeding acorn woodpecker. Anim. Behav. 57, 1287–1293 (1999). [DOI] [PubMed] [Google Scholar]
- 25.Koenig W. D., Haydock J., Stanback M. T., Reproductive roles in the cooperatively breeding acorn woodpecker: Incest avoidance versus reproductive competition. Am. Nat. 151, 243–255 (1998). [DOI] [PubMed] [Google Scholar]
- 26.Riehl C., Kinship and incest avoidance drive patterns of reproductive skew in cooperatively breeding birds. Am. Nat. 190, 774–785 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Dickinson J. L., Hatchwell B. J., “Fitness consequences of helping” in Ecology and Evolution of Cooperative Breeding in Birds, Koenig W. D., Dickinson J. L., Eds. (Cambridge University Press, 2004), pp. 48–66. [Google Scholar]
- 28.van Dijk R. E., Covas R., Doutrelant C., Spottiswoode C. N., Hatchwell B. J., Fine-scale genetic structure reflects sex-specific dispersal strategies in a population of sociable weavers (Philetairus socius). Mol. Ecol. 24, 4296–4311 (2015). [DOI] [PubMed] [Google Scholar]
- 29.Cornwallis C. K., West S. A., Griffin A. S., Routes to indirect fitness in cooperatively breeding vertebrates: Kin discrimination and limited dispersal. J. Evol. Biol. 22, 2445–2457 (2009). [DOI] [PubMed] [Google Scholar]
- 30.McRae S. B., Family values: Costs and benefits of communal nesting in the moorhen. Anim. Behav. 52, 225–245 (1996). [Google Scholar]
- 31.Townsend A. K.et al., Disease-mediated inbreeding depression in a large, open population of cooperative crows. Proc. Biol. Sci. 276, 2057–2064 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dickinson J. L., Akçay C., Ferree E. D., Stern C. A., A hierarchical analysis of incest avoidance in a cooperative breeder. Behav. Ecol. 27, 1132–1140 (2016). [Google Scholar]
- 33.Brouwer L., Van De Pol M., Atema E., Cockburn A., Strategic promiscuity helps avoid inbreeding at multiple levels in a cooperative breeder where both sexes are philopatric. Mol. Ecol. 20, 4796–4807 (2011). [DOI] [PubMed] [Google Scholar]
- 34.Blackmore C. J., Heinsohn R., Variable mating strategies and incest avoidance in cooperatively breeding grey-crowned babblers. Anim. Behav. 75, 63–70 (2008). [Google Scholar]
- 35.Hatchwell B. J., Gullett P. R., Adams M. J., Helping in cooperatively breeding long-tailed tits: A test of Hamilton’s rule. Philos. Trans. R. Soc. Lond. B Biol. Sci. 369, 20130565 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharp S. P., Baker M. B., Hadfield J. D., Simeoni M., Hatchwell B. J., Natal dispersal and recruitment in a cooperatively breeding bird. Oikos 117, 1371–1379 (2008). [Google Scholar]
- 37.Leedale A. E., Sharp S. P., Simeoni M., Robinson E. J. H., Hatchwell B. J., Fine-scale genetic structure and helping decisions in a cooperatively breeding bird. Mol. Ecol. 27, 1714–1726 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Hatchwell B. J., Russell A. F., Ross D. J., Fowlie M. K., Divorce in cooperatively breeding long-tailed tits: A consequence of inbreeding avoidance? Proc. Biol. Sci. 267, 813–819 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Slate J.et al., Understanding the relationship between the inbreeding coefficient and multilocus heterozygosity: Theoretical expectations and empirical data. Heredity 93, 255–265 (2004). [DOI] [PubMed] [Google Scholar]
- 40.Russell A. F., Hatchwell B. J., Experimental evidence for kin-biased helping in a cooperatively breeding vertebrate. Proc. Biol. Sci. 268, 2169–2174 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharp S. P., McGowan A., Wood M. J., Hatchwell B. J., Learned kin recognition cues in a social bird. Nature 434, 1127–1130 (2005). [DOI] [PubMed] [Google Scholar]
- 42.Szulkin M., Bierne N., David P., Heterozygosity-fitness correlations: A time for reappraisal. Evolution 64, 1202–1217 (2010). [DOI] [PubMed] [Google Scholar]
- 43.Queller D. C., Goodnight K. F., Estimating relatedness using genetic markers. Evolution 43, 258–275 (1989). [DOI] [PubMed] [Google Scholar]
- 44.Green J. P., Hatchwell B. J., Inclusive fitness consequences of dispersal decisions in a cooperatively breeding bird, the long-tailed tit (Aegithalos caudatus). Proc. Natl. Acad. Sci. U.S.A. 115, 12011–12016 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hatchwell B. J., Ross D. J., Chaline N., Fowlie M. K., Burke T., Parentage in the cooperative breeding system of long-tailed tits, Aegithalos caudatus. Anim. Behav. 64, 55–63 (2002). [Google Scholar]
- 46.Sharp S. P., Hatchwell B. J., Individuality in the contact calls of cooperatively breeding long-tailed tits (Aegithalos caudatus). Behaviour 142, 1559–1575 (2005). [Google Scholar]
- 47.Hemmings N. L., Slate J., Birkhead T. R., Inbreeding causes early death in a passerine bird. Nat. Commun. 3, 863 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Pilakouta N., Jamieson S., Moorad J. A., Smiseth P. T., Parental care buffers against inbreeding depression in burying beetles. Proc. Natl. Acad. Sci. U.S.A. 112, 8031–8035 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGowan A., Hatchwell B. J., Woodburn R. J. W., The effect of helping behaviour on the survival of juvenile and adult long-tailed tits Aegithalos caudatus. J. Anim. Ecol. 72, 491–499 (2003). [Google Scholar]
- 50.Leedale A. E., Lachlan R. F., Robinson E. J. H., Hatchwell B. J., Helping decisions and kin recognition in long-tailed tits: Is call similarity used to direct help towards kin? Philos. Trans. R. Soc. Lond. B Biol. Sci. 375, 20190565 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hatchwell B. J., Sharp S. P., Beckerman A. P., Meade J., Ecological and demographic correlates of helping behaviour in a cooperatively breeding bird. J. Anim. Ecol. 82, 486–494 (2013). [DOI] [PubMed] [Google Scholar]
- 52.Reeve H. K., The evolution of conspecific acceptance thresholds. Am. Nat. 133, 407–435 (1989). [Google Scholar]
- 53.Downs S. G., Ratnieks F. L. W., Adaptive shifts in honey bee (Apis mellifera L.) guarding behavior support predictions of the acceptance threshold model. Behav. Ecol. 11, 326–333 (2000). [Google Scholar]
- 54.Wolak M. E., nadiv: An R package to create relatedness matrices for estimating non-additive genetic variances in animal models. Methods Ecol. Evol. 3, 792–796 (2012). [Google Scholar]
- 55.Simeoni M.et al., Characterization of 20 microsatellite loci in the long-tailed tit Aegithalos caudatus (Aegithalidae, AVES). Mol. Ecol. Notes 7, 1319–1322 (2007). [Google Scholar]
- 56.Nam K.-B., Simeoni M., Sharp S. P., Hatchwell B. J., Kinship affects investment by helpers in a cooperatively breeding bird. Proc. Biol. Sci. 277, 3299–3306 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coltman D. W., Pilkington J. G., Smith J. A., Pemberton J. M., Parasite‐mediated selection against inbred Soay sheep in a free‐living island population. Evolution 53, 1259–1267 (1999). [DOI] [PubMed] [Google Scholar]
- 58.Marshall T. C.et al., Estimating the prevalence of inbreeding from incomplete pedigrees. Proc. Biol. Sci. 269, 1533–1539 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Keller L. F., Inbreeding and its fitness effects in an insular population of sparrows (Melospiza melodia). Evolution 52, 240–250 (1998). [DOI] [PubMed] [Google Scholar]
- 60.Reid J. M.et al., Variation in parent-offspring kinship in socially monogamous systems with extra-pair reproduction and inbreeding. Evolution 70, 1512–1529 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hatchwell B. J.et al., Helpers increase long-term but not short-term productivity in cooperatively breeding long-tailed tits. Behav. Ecol. 15, 1–10 (2004). [Google Scholar]
- 62.Leedale A. E., et al. , Cost, risk, and avoidance of inbreeding in a cooperatively breeding bird. Dryad Digital Repository. 10.5061/dryad.k6djh9w49. Deposited 6 June 2020. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Source datasets and code for this paper have been deposited in the Dryad Digital Repository, https://doi.org/10.5061/dryad.k6djh9w49 (62).