Abstract
Acute myocardial infarction (AMI) and the heart failure (HF) that often follows are among the leading causes of death and disability worldwide. As such, new treatments are needed to protect the myocardium against the damaging effects of the acute ischaemia and reperfusion injury (IRI) that occurs in AMI, in order to reduce myocardial infarct (MI) size, preserve cardiac function, and improve patient outcomes. In this regard, cardiac mitochondria play a dual role as arbiters of cell survival and death following AMI. Therefore, preventing mitochondrial dysfunction induced by acute myocardial IRI is an important therapeutic strategy for cardioprotection. In this article, we review the role of mitochondria as key determinants of acute myocardial IRI, and we highlight their roles as therapeutic targets for reducing MI size and preventing HF following AMI. In addition, we discuss the challenges in translating mitoprotective strategies into the clinical setting for improving outcomes in AMI patients.
Keywords: Ischaemic heart disease, Acute myocardial infarction, Ischaemia-reperfusion injury, Mitochondria, Oxidative stress, Calcium overload, Cardioprotection
1. Introduction
Ischaemic heart disease (IHD) is the leading cause of death worldwide, accounting for 9 million deaths each year [1]. It can present emergently as an acute myocardial infarction (AMI), in which rupture of a coronary atheromatous plaque, causes an acute thrombotic occlusion of the coronary artery, severely restricting or completely blocking blood flow to the myocardium, thereby depriving cardiomyocytes of oxygen and nutrients (termed acute myocardial ischaemia), and resulting in cardiomyocyte death. The treatment of choice for AMI is to remove the thrombotic occlusion and restore coronary blood flow (termed acute myocardial reperfusion) as soon as possible using coronary angioplasty and stenting (termed percutaneous coronary intervention [PCI]), in order to reduce acute ischaemic injury to the heart. Despite timely PCI, AMI patients still experience significant mortality and morbidity, and therefore new treatments are needed to protect the myocardium from the detrimental effects of acute myocardial ischaemia and reperfusion injury (IRI) in order to limit myocardial infarct (MI) size, preserve cardiac function, and prevent the onset of heart failure (HF), a significant cause of disability, in terms of symptoms, and hospital re-hospitalisation.
Mitochondrial dysfunction during acute IRI is a critical determinant of cell death following AMI, given the crucial role that mitochondria play in generating the 6 kg/day of ATP required to maintain normal heart contractile function, and allow the heart to beat 100,000 times a day [2]. Therefore, preventing mitochondrial dysfunction induced by acute myocardial IRI is an important therapeutic strategy for cardioprotection. In this article, we provide an overview of mitochondria in acute myocardial IRI, and highlight their role as therapeutic targets for reducing MI size and preventing HF following AMI. Recent attempts to translate cardioprotective strategies that target mitochondria, into the clinical setting for the benefit of AMI patients, have been hugely disappointing, and we discuss in this article the challenges facing the clinical translation of mitoprotective therapies, and the potential solutions for overcoming this.
2. The role of mitochondria in acute myocardial ischaemia/reperfusion injury
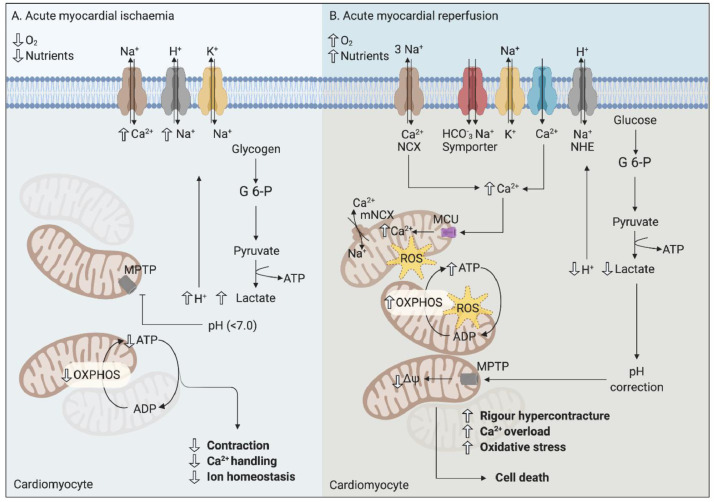
The deprivation of oxygen and nutrients supply to cardiomyocytes at the onset of acute myocardial ischaemia in AMI patients, triggers a series of severe biochemical and metabolic perturbations in the cardiomyocyte, many of which impact adversely on mitochondrial function and ATP production [3]. Cellular metabolism switches from mitochondrial oxidative phosphorylation to anaerobic glycolysis resulting in the intracellular build-up of lactate and accumulation of protons, which lowers intracellular pH to <7.0 during acute myocardial ischaemia (Fig. 1). The build-up of intracellular protons activates the Na+/H+ ion exchanger, which in turn extrudes protons from the cell, in exchange for Na+ entry, and together with a reduction in Na+/K+ ATPase activity due to ATP depletion, intracellular Na+ overload ensues. As a result, the Na+/Ca2+ ion exchanger acts in reverse mode in an attempt to remove surplus Na+, but this results in intracellular and subsequent mitochondrial Ca2+ overload as the cell tries to extrude Na+ [3].
Fig. 1.
Biochemical and metabolic perturbations during acute myocardial ischaemia and reperfusion injury (A) During acute myocardial ischaemia, the absence of oxygen and nutrients switches cell metabolism to anaerobic glycolysis which leads to the production of lactate, accumulation of protons, and a fall in pH (which inhibits MPTP opening). This in turn, results in intracellular sodium and calcium overload. (B) At myocardial reperfusion, the availability of oxygen and nutrients allows mitochondrial re-energisation leading to further mitochondrial calcium overload, and production of oxidative stress which together with the rapid correction of pH, induces opening of the MPTP, rigour hypercontracture, and cell death. Glucose 6-phosphate, G 6-P; oxidative phosphorylation, OXPHOS; the sodium-calcium exchanger, NCX; mitochondrial sodium-calcium exchanger, mNCX; Na+/H+ exchanger, NHE; mitochondrial permeability transition pore, MPTP; reactive oxygen species, ROS.
In the first few minutes of acute myocardial reperfusion, further biochemical and metabolic changes occur that include further mitochondrial Ca2+ overload, oxidative stress, rapid pH correction, and opening of the mitochondrial permeability transition pore (MPTP) [3]. These changes compound the detrimental effects induced by acute myocardial ischaemia, and act in concert to induce mitochondrial dysfunction and cardiomyocyte death – a phenomenon which has been termed acute myocardial reperfusion injury and has been shown to contribute to final MI size (Fig. 1) [3]. Reperfusion induces further intracellular and mitochondrial Ca2+ overload due to disruption of the plasma membrane, oxidative stress-induced damage to the sarcoplasmic reticulum, and mitochondrial re-energisation, which allows the recovery of the mitochondrial membrane potential to drive the entry of Ca2+ into mitochondria via the mitochondrial Ca2+ uniporter (MCU). The molecular identification of the MCU [4], and the mitochondrial Na+/Ca2+ exchanger (NCX), which mediates mitochondrial calcium extrusion [5], may result in the discovery of a new class of specific inhibitors for reducing acute myocardial IRI in AMI.
At the onset of reperfusion, a burst of oxidative stress, is produced by the re-energisation of mitochondria, which induces cardiomyocyte death through a number of different mechanisms including MPTP opening [3]. Experimental and clinical studies have reported mixed results with anti-oxidant therapy administered at the onset of myocardial reperfusion, potential reasons for which include the inability of anti-oxidants to enter the cell and reach mitochondria. In this regard, the discovery of mitochondria-targeting anti-oxidants such as MitoQ which has been shown to reduce MI size following acute myocardial IRI may be a more effective approach to cardioprotection in the clinical setting [6]. MitoQ has been shown to reduce reactive oxygen species (ROS) production at the onset of reperfusion in experimental studies of acute myocardial IRI [7], and to protect the heart during transplantation-induced IRI in a mouse model [8]. In the clinical setting, MitoQ has been shown to have some beneficial effects in patients with hepatitis C, another medical condition characterised by oxidative stress [9]. Experimental studies have reported that the citric acid cycle intermediate, succinate, accumulates during acute myocardial ischaemia, and metabolism of succinate at the onset of reperfusion via reverse transport through mitochondrial complex I, generates ROS, making succinate a potential target for cardioprotection [10]. Interestingly, patients presenting with AMI have been shown to have elevated plasma levels of succinate, confirming the relevance of ischaemic accumulation of succinate in the clinical setting [11]. Chouchani et al. [12] have also shown that mitochondria-selective S-nitrosating agent, MitoSNO, reduced MI size in mice by selective S-nitrosation of Cys39 on the ND3 subunit of mitochondrial complex I, and slowing the reactivation of mitochondrial complex I at the onset of reperfusion, thereby decreasing ROS production.
During acute myocardial ischaemia, intracellular pH decreases to <7.0, whereas at reperfusion, physiological pH is rapidly restored by the wash-out of lactate and the activation of the Na+/H+ exchanger and the Na+/HCO− symporter [3]. The rapid pH correction at reperfusion contributes to the cardiomyocyte death of myocardial reperfusion injury by permitting MPTP opening, and inducing cardiomyocyte rigour hypercontracture in the first few minutes of reperfusion (Fig. 1). The normalisation of physiological pH as a cardioprotective strategy to prevent acute myocardial reperfusion injury can be achieved by reperfusion of ischaemic animal hearts with acidic buffer [13], using pharmacological inhibitors of the Na+/H+ exchanger [14], or by interrupting myocardial reperfusion with the endogenous cardioprotective strategy of ischaemic postconditioning (see later section) [15].
The presence of factors (such as age) and co-morbidities present in AMI patients (such as obesity, metabolic syndrome and diabetes) are known to affect cardiac mitochondrial function, and impact on both the susceptibility to acute myocardial IRI, and the efficacy of cardioprotective therapies (see later sections) [16]. Importantly, many of the factors responsible for myocardial reperfusion injury such as mitochondrial calcium overload, oxidative stress, ATP depletion and rapid pH correction converge on the MPTP, making the latter a critical target for cardioprotection.
3. Targeting the mitochondrial permeability transition pore for cardioprotection
The MPTP is a large non-selective channel, that on opening at reperfusion, allows ions and solutes of up to 1.5 kDa to cross the inner mitochondrial membrane (IMM), resulting in mitochondrial swelling, mitochondrial membrane depolarisation, uncoupling of oxidative phosphorylation, ATP depletion, and cell death, primarily by necrosis [17]. Current evidence suggests that the F0F1ATPase may be directly involved in MPTP formation, with two different models being proposed – one based on the c subunit of F0F1ATPase [18], and the other, positing a role for F0F1ATPase oligomers and dimers in MPTP formation [19].
In the setting of acute myocardial IRI, the MPTP has been shown to remain closed during ischaemia due to the acidic conditions that prevail in cardiomyocytes at this time. MPTP inhibition by H+ appears to be mediated by the highly conserved histidyl residue (H112) of the oligomycin sensitive protein subunit of mitochondrial F0F1ATPase [20]. The MPTP only opens in the first few minutes of reperfusion, in response to mitochondrial Ca2+ overload, oxidative stress, ATP depletion, and rapid pH correction [17]. Therefore, therapeutic strategies that target these MPTP-inducing factors during acute myocardial IRI can indirectly inhibit MPTP opening at the time of reperfusion and limit MI size. In aged and obese animal models, it has been shown that disturbances in mitochondrial function and increased susceptibility to MPTP opening act to increase MI size following acute myocardial IRI [21,22].
The administration of known pharmacological MPTP inhibitors (such as cyclosporine-A, [CsA] which targets cyclophilin D) at the onset of myocardial reperfusion has been reported in experimental studies to reduce MI size by 40–50% in animal MI models, although not all experimental studies have been positive [17]. Further studies are needed to identify the molecular components of the MPTP, in order that more potent and specific MPTP inhibitors can be discovered and tested as new mitoprotective therapies. In summary, experimental studies support a role for the MPTP as an important therapeutic target for preventing lethal myocardial reperfusion injury. However, although inhibiting MPTP opening using CsA at the time of reperfusion has been tested in AMI patients undergoing PCI, the results have been disappointing (see later section) [23,24]. MPTP opening at reperfusion can also be indirectly inhibited by improving cellular bioenergetics and limiting oxidative stress, by elevating levels of creatine and phosphocreatine levels to increase ATP availability, which has been demonstrated in mice over-expressing creatine transporter within the heart [25].
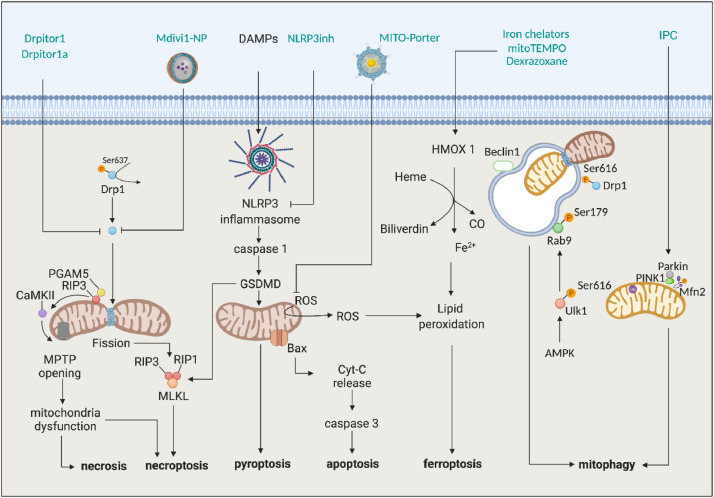
In addition to MPTP-induced cell death, a number of other mitochondria-dependent cell death pathways have been shown to contribute to cardiomyocyte death during acute myocardial IRI, including apoptosis, mitophagy, necroptosis, pyroptosis and ferroptosis, providing critical targets for cardioprotection (Fig. 2) [26]. Mitochondrial outer membrane permeabilisation (MOMP) at the time of reperfusion results in mitochondrial cytochrome C-mediated apoptotic cell death, which has been shown to occur primarily during reperfusion. Inhibiting mitochondrial cytochrome C release and caspase activation has been shown to limit MI size [26]. Necroptosis, a form of regulated necrotic cell death mediated by the receptor-interacting serine/threonine-protein kinase 3 (RIP3)-phosphoglycerate mutase family member 5 (PGAM5) pathway, contributes to acute myocardial IRI, and mediates cell death through Ca2+-calmodulin-dependent protein kinase (CaMKII)-induced MPTP opening [27], and Drp1-dependent mitochondrial fission [28]. Pharmacological inhibition of RIP1 and RIP3 have been shown to be cardioprotective in animal MI models [29]. Pyroptosis is a pro-inflammatory cell death program that occurs in response to the release of damage-associated molecular patterns (DAMPs) such as mitochondrial DNA [30] which results in the assembly of the intracellular NLRP3 inflammasome complex, and contributes to acute myocardial IRI [31]. Inhibition of the NLRP3 inflammasome by pharmacological agents, genetic ablation, and M2 macrophage-derived exosomes, has been shown to reduce MI size in small animal MI models [32,33]. Ferroptosis is a regulated cell death program that occurs in response to accumulation of iron-dependent lipid peroxidation that occurs in response to acute myocardial IRI due to mitochondrial accumulation of iron and oxidative stress, providing a novel mitochondrial target for cardioprotection following AMI [34]. Genetic (cardiac-specific overexpression of a mitochondrial iron export protein) and pharmacological strategies (such as iron chelators and MitoTEMPO) for lowering mitochondrial iron content and oxidative stress have been shown to protect the heart against ferroptosis-induced cell death following acute myocardial IRI [35].
Fig. 2.
Targeting mitochondria-dependent cell death pathways for cardioprotection. Scheme showing mitochondria-dependent cell death pathways that contribute to acute myocardial IRI, and therefore provide new targets for cardioprotection. Receptor-interacting serine/threonine-protein kinase 3, RIP3; Receptor-interacting serine/threonine-protein kinase 1, RIP1; serine/threonine-protein phosphatase, PGAM5; cytochrome C, Cyt-C; 5′ AMP-activated protein kinase, AMPK; Gasdermin D, GSDMD; nucleotide-binding domain, leucine-rich-repeat containing family, pyrin domain-containing 3, NLRP3; NLRP3 inflammasome inhibitors, NLRP3inh; Unc-51 like autophagy activating kinase 1, Ulk1; Ras-related protein 9, Rab9; PTEN-induced kinase 1, PINK1; mixed lineage kinase domain-like, MLKL; Calcium/calmodulin-dependent protein kinase type II alpha chain, CaMKII; ischaemic preconditioning, IPC; Mitochondrial division inhibitor 1 with nanoparticles, Mdivi1-NP; Dynamin related protein 1, Drp1; mitochondrial permeability transition pore, MPTP; damage-associated molecular patterns DAMPs; Apoptosis regulator BAX, Bax; Mitofusin-2, Mfn2; hemeoxygenase-1, HMOX1; mitoTEMPO 2-(2,2,6,6-Tetramethylpiperidin-1-oxyl-4-ylamino)-2-oxoethyl) triphenylphosphonium chloride; carbon monoxide, CO; Ferrous ion, Fe2+.
4. Targeting mitochondrial fission and fusion proteins for cardioprotection
Mitochondria are dynamic organelles that continually change their shape, by undergoing fission to generate fragmented disconnected mitochondria (which is required for cell division and for removal of damaged mitochondria by mitophagy), and fusion to generate an elongated interconnected phenotype (which is required to replace damaged DNA and maintain normal mitochondrial respiratory function) [3]. These two opposing processes are coordinated by the mitochondrial fission proteins (dynamin-related protein 1 [Drp1], human fission protein 1 [hFis1], and mitochondrial fission factor [Mff]), and the mitochondrial fusion proteins (Optic Atrophy Protein 1 (OPA1), Mitofusin 1 [Mfn1] and Mitofusin 2 [Mfn2]), respectively. An imbalance in mitochondrial fusion and fission can impact on mitochondrial respiratory function, mitochondrial quality control, and susceptibility to cell death in acute myocardial IRI, positioning the mitochondrial fusion and fission proteins as important targets for cardioprotection) [3].
In the adult heart, most cardiac mitochondria are fragmented in morphology and are tightly packed into three intracellular locations that restricts mitochondrial movement: alongside the myofibrils, beneath the sarcolemmal membrane, and adjacent to the nucleus. As such the physiological relevance of mitochondrial morphology and dynamics to the adult heart has been questioned. However, studies have shown that the mitochondrial fission and fusion proteins are expressed in the heart, and genetic ablation of the mitochondrial fission (Drp1, Mff) or fusion proteins (Mfn2, OPA1) induces changes in mitochondrial morphology, impairs mitochondrial respiration, and results in a dilated cardiomyopathy, confirming that these proteins are essential for normal cardiac function [3].
Experimental studies have demonstrated that cardiac mitochondria undergo fission in response to acute myocardial IRI, and this, in turn, induces mitochondrial dysfunction and results in cardiomyocyte death [36]. The mechanisms underlying IRI-induced mitochondrial fission are unclear, but may relate to calcium overload and the production of oxidative stress, which again results in MPTP opening. Calcium accumulation during acute myocardial ischaemia has been demonstrated to activate calcineurin, which dephosphorylates Drp1, at Ser637, which otherwise prevents the mitochondrial translocation of Drp1 to initiate fission [37]. In support of this mechanism, it has been shown that pharmacological inhibition of calcineurin prevented dephosphorylation of Drp1 at Ser637, inhibited IRI-induced mitochondrial fission, reduced MI size and preserved cardiac function following acute myocardial IRI [38].
Genetic and pharmacological inhibition of Drp1-induced mitochondrial fission during acute IRI have been reported to limit MI size [36], highlighting IRI-induced fission as an important target for cardioprotection. Acute pharmacological inhibition of IRI-induced mitochondrial fission using mitochondrial division inhibitor 1 [mdivi-1] (a putative small molecule Drp1 inhibitor) [36], or P110 (a peptide inhibitor that inhibits the interaction between Drp1 and hFis [39]) has been reported to reduce MI size in rodents [4], but not in the clinically-relevant pig heart model of acute IRI [40]. Interestingly, it has been demonstrated that nanoparticle delivery of mdivi-1 to the ischaemic heart enhanced its cardioprotective effect in terms of MI size reduction in the murine AMI model [41], opening up the possibility of using nanocarriers to improve the bioavailability and delivery of mitoprotective therapies to the ischaemic heart. Recent studies suggest that mdivi-1 has off-target Drp1-independent mitochondrial effects [42], and new, more specific, Drp1 inhibitors are needed. In this regard, Drpitor1 and Drpitor1a, new inhibitors of Drp1, have been discovered which are more potent and specific than mdivi-1 in terms of inhibiting Drp1 GTPase activity, and have been shown to confer cardioprotection in the rat heart [43]. Although acute inhibition of mitochondrial fission has been shown to be cardioprotective, long-term genetic deletion of cardiac Drp142 has been reported to increase susceptibility to acute myocardial IRI and the development of cardiomyopathy. This has been attributed to the suppression of mitophagy, and the accumulation of damaged mitochondria, findings which again underscore the importance of balancing mitochondrial fusion and fission for normal cardiac function. In contrast with these findings, it has been reported in obese rats (fed high-fat diet) that chronic pharmacological inhibition of mitochondrial fission (over 2 weeks), using mdivi-1, normalised mitochondrial morphology and improved cardiac mitochondrial and contractile function, although the effect on susceptibility to acute myocardial IRI was not tested in this study [22]. Stimulation of the vagus nerve has also been shown to reduce MI size in animal models by inhibiting mitochondrial fission, preservation of mitochondrial function, and suppression of MPTP opening [45]. The presence of diabetes has been shown to induce mitochondrial fission and dysfunction in a mouse model, and increase MI size following acute myocardial IRI, effects which have been linked to reduced myocardial levels of sirtuin 1 and Akt and enhanced expression of Drp1 [46].
In the adult rodent heart, targeting mitofusins as a cardioprotective strategy has produced unexpected effects. This most likely relates to their pleiotropic non-fusion effects, with the mitofusins playing critical roles in mitophagy, autophagy and tethering mitochondria to the sarcoplasmic reticulum (SR) [3]. Cardiomyocyte specific dual ablation of Mfn1 and Mfn2 has been shown to inhibit MPTP opening and reduce cardiomyocyte death following acute myocardial IRI, an unexpected cardioprotective effect that has been attributed to mitochondria and SR no longer being in close proximity (given the tethering role of mitochondria to SR), thereby protecting mitochondria from calcium overload during acute IRI [47]. Therefore, targeting Mfn2 during acute myocardial IRI to transiently disassociate mitochondria from SR, using newly engineered cell-permeant mini-peptides which either inhibit or activate Mfn2, may provide a novel therapeutic strategy for cardioprotection [48].
In contrast to the mitofusins, genetic ablation of OPA1 has been shown to increase the susceptibility of hearts to acute IRI, suggesting a cardioprotective role of OPA1 [49]. Consistent with this finding, genetic overexpression of OPA1 was also shown to be cardioprotective with preservation of mitochondria cristae, prevention of apoptosis, and improved respiratory function via known non-fusion pleiotropic effects of OPA1. Upregulation of myocardial OPA1 levels by genetic ablation of its protease, OMA1, has also been shown to be cardioprotective [50], providing the opportunity to pharmacologically inhibit OMA1, using newly discovered OMA1 inhibitors (such as epigallocatechin gallate) [51] as a future cardioprotective strategy.
Mitochondrial fusion and fission proteins are also known to impact on mitochondrial quality control by modulating mitophagy and the mitochondrial unfolded protein response (UPRmt). Mitophagy is activated during acute myocardial IRI, where it plays a cardioprotective role to preserve energy substrates, remove damaged mitochondria, and attenuate oxidative stress. Drp1-mediated mitochondrial fission is essential for mitophagy, with mice deficient in cardiomyocyte Drp1 accumulating damaged mitochondria, being more susceptible to acute IRI, and developing a dilated cardiomyopathy [44]. Activation of mitophagy prior to acute myocardial ischaemia, by known cardioprotective strategies such as ischaemic preconditioning [52] has been shown to limit MI size. The mitochondrial fusion protein, Mfn2, has been shown to play a key role in mitophagy by recruiting Parkin to mitochondria to activate the Parkin-PINK1 mitophagy pathway [53]. Mice deficient in Parkin have been shown to accumulate damaged mitochondria and are more susceptible to acute myocardial IRI, with overexpression of Parkin being cardioprotective [54]. Similarly, mice deficient in PINK1 have been shown to sustain small MI size following acute IRI [55], confirming the cardioprotective effects of the PINK-Parkin mitophagy pathway in the setting of acute myocardial IRI. Recently, a novel PINK/Parkin-independent pathway of mitophagy involving a novel Ulk1/Rab9/Rip1/Drp1 mitophagy pathway was shown to protect the heart against acute IRI by preserving mitochondrial function [56].
The UPRmt is a cytoprotective signalling pathway triggered by the mitochondrial accumulation of toxic unfolded proteins under conditions of cellular stress such as acute myocardial IRI that acts to restore mitochondrial proteostasis and respiratory function [57]. Pharmacological induction of the UPRmt using either oligomycin or doxycycline has been reported to reduce MI size in mice [58]. The mitochondrial protease, LonP1, which contributes to mitochondrial proteostasis and regulates adaptive responses to cell stress, has been shown to contribute to the cardioprotection elicited by ischaemic preconditioning [59]. The ubiquitin-proteasome system (UPS) eliminates misfolded or damaged proteins in the heart via selective polyubiquitination and subsequent degradation by the proteasome, and pharmacological inhibition of the proteasome, using MG132, has been shown to protect the isolated perfused rat heart against acute myocardial IRI by preserving myocardial Mfn2 levels, maintaining mitochondrial mass, and inhibiting mitochondrial fission [60].
5. Targeting mitochondria using endogenous ischaemic conditioning strategies
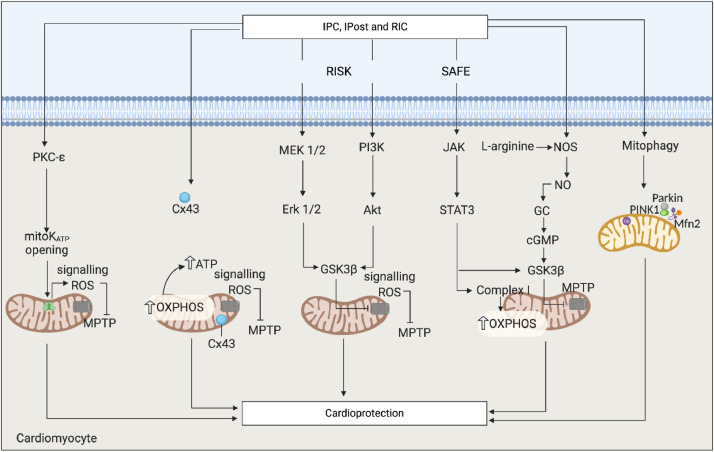
The dual roles of mitochondria as key determinants of cell death induced by acute myocardial IRI, and as key targets for cardioprotection are exemplified by the central role they play in endogenous cardioprotective strategies for limiting MI size such as ‘ischaemic conditioning’ [3]. Cardiomyocytes can be rendered resistant to IRI-induced cell death by subjecting the heart itself (‘ischaemic conditioning’) or an organ or tissue (such as the limb) away from the heart (‘remote ischaemic conditioning’ or RIC) to brief cycles (usually 1–3 cycles) of non-lethal ischaemia and reperfusion (usually 5 min in duration). The ‘conditioning’ stimuli can be applied either prior to (‘preconditioning’) or during (‘perconditioning’) the lethal episode of acute myocardial ischaemia, or even at the onset of reperfusion (‘postconditioning’) to limit MI size. The mechanisms through which ischaemic conditioning confers cardioprotection is not clear, although a large number of signalling pathways have been implicated. Mitochondria have been shown to play a central role in both triggering ischaemic conditioning cardioprotection, and acting as a key end-effector of cardioprotection in terms of MPTP inhibition at time of reperfusion (Fig. 3) [61]. The elucidation of the signalling pathways underlying ischaemic conditioning have identified a number of mitochondrial proteins that can be targeted using pharmacological agents to mimic the cardioprotective effects of ischaemic conditioning [3].
Fig. 3.
Targeting mitochondria using endogenous cardioprotective strategies. Scheme showing the recruitment of signalling pathways by ischaemic conditioning that converge on mitochondria to confer cardioprotection. Many of these signaling pathways reduce myocardial infarct size by inhibiting MPTP opening at the onset of reperfusion through the opening of mitochondrial channels (such as mitoK and Cx43), the activation of cytoprotective kinase cascades (MEK1/2-Erk1/2 and PI3K-Akt of the RISK pathway, and JAK-STAT3 of the SAFE pathway), or the release of nitric oxide and activation of cGMP. In addition, the PINK1-Parkin-Mfn2 mitophagy pathway is also activated by these endogenous cardioprotective strategies. Ischaemic preconditioning, IPC; ischaemic postconditioning, IPost, remote ischaemic conditioning, RIC; reperfusion injury salvage kinase, RISK; survival activating factor enhancement, SAFE; phosphatidylinositol 3 kinase, PI3K; nitric oxide synthase, NOS; nitric oxide, NO; protein kinase G, PKG; protein kinase C, PKC; extracellular regulated kinase 1 and 2, Erk 1/2; glycogen synthase kinase 3β, GSK3ß; Janus-activated kinase, JAK; signal transducer and activator of transcription, STAT; mitochondrial permeability transition pore, MPTP; oxidative phosphorylation, OXPHOS; connexin 43, Cx43.
Signalling to mitochondria has been shown to play a key role in triggering ischaemic conditioning protection, through the activation of cardioprotective pathways such as mitochondrial PKC-ε which opens the mitochondrial KATP channel (mitoKATP) and produces mitochondrial signalling ROS, which in turn acts to inhibit MPTP opening [62]. Recently, the molecular identity of the mitoKATP has been shown to comprise pore-forming (MITOK) and ATP-binding subunits (MITOSUR) [63], thereby providing novel therapeutic targets for cardioprotection. Pharmacological activation of the ‘big’ conductance calcium-sensitive (BKCa) channel has also been shown to limit MI size in animal models of acute IRI, through a variety of mitoprotective effects [64]. Interestingly, the MI-limiting effects of IPC, IPost, and limb RIC have all been shown to be abrogated in the presence of a pharmacological BKCa blocker, suggesting that opening of the BKCa channel is also required for ischaemic conditioning cardioprotection [3]. Another mitochondrial channel that has been implicated as a mediator of IPC-cardioprotection is the gap junction protein, connexin-43 (Cx43), which has been shown to regulate mitochondrial oxygen consumption and ATP production [65], mitochondrial Ca2+ uptake [66], and MPTP opening,[66] factors which are known to modify mitoprotection [67].
Mitochondria also play a critical role in mediating the cardioprotective effects of ischaemic conditioning through a number of signalling pathways recruited at the time of reperfusion including the Reperfusion Injury Salvage Kinase (RISK, comprising Akt and Erk1/2), Survivor Activator Factor Enhancement (SAFE, compromising TNF-α and STAT3), and the nitric oxide-cGMP-PKG pathways, the activation of which terminate on mitochondria to prevent mitochondrial dysfunction, inhibit MPTP opening and reduce cardiomyocyte death following acute IRI (Fig. 3) [68]. Therefore, these endogenous ischaemic conditioning strategies are able to indirectly confer mitoprotective effects against acute myocardial IRI in animal models and have also been tested in AMI patients.
6. Mitoprotective strategies in AMI patients
The translation of mitoprotective therapeutic strategies into the clinical setting for the benefit of AMI patients has been extremely challenging, and the results have been overwhelmingly disappointing. The most promising mitochondrial target for cardioprotection, had been the MPTP, given the substantial experimental data demonstrating MI size reduction using CsA to target MPTP opening at reperfusion [61]. An initial small proof-of-concept clinical study in AMI patients had demonstrated a significant reduction in MI size (measured by serum cardiac biomarkers and cardiac MRI) with CsA administered at time of PCI compared to control [23]. However, the subsequent large randomised controlled CIRCUS trial, failed to demonstrate any benefit with CsA on either MI size reduction or clinical outcomes in AMI patients [24]. Potential reasons for the failure to translate MPTP inhibition into clinical benefit include: insufficient delivery of CsA to ischaemic cardiomyocytes, and the presence of factors which are known to confound cardioprotection, such as co-morbidities (e.g. age, diabetes), and co-medications (e.g. platelet P2Y12 inhibitors) (see below). In this regard, nanoparticles have been used to improve the bioavailability and delivery of CsA to ischaemic cardiomyocytes and target mitochondria in animal models [69,70], and this approach may have therapeutic potential in future clinical studies. Alternatively, MITO-Porter (a liposome-based carrier system), which promotes both its fusion with the mitochondrial membrane and the release of its cargo into the mitochondrial matrix, may provide mitochondria-targeted delivery of both small and large therapeutic molecules and mitochondrial RNA [71]. It has been used to activate cardiac progenitor cells by delivering resveratrol to mitochondria, and intramyocardial injection of the activated progenitor cells was shown to protect against doxorubicin cardiomyopathy [72]. MITO-Porter could potentially be used to deliver mitoprotective agents such as CsA to cardiac mitochondria in ischaemic cardiomyocytes following AMI. Novel MPTP inhibitors that are more specific and efficacious than CsA may be more successful in translating MPTP inhibition as a cardioprotective strategy in AMI patients [73]. A number of other therapeutic strategies aimed at targeting mitochondria to reduce MI size have also been tried, but these have also failed in AMI patients including elamipretide (a cell-permeable peptide postulated to preserve mitochondrial cardiolipin) [74], and TRO40303 (suggested to inhibit the TSPO) [75].
Of the endogenous cardioprotective strategies which are known to limit MI size in small and large animal models of acute myocardial IRI, IPost and limb RIC have been tested in AMI patients. IPost can be applied in AMI patients during PPCI, by applying serial inflations and deflations (of 30 to 60 s duration) of the coronary angioplasty balloon, immediately following opening of the infarct-related coronary artery, in order to interrupt reperfusion, a manoeuvre which has been shown to reduce MI size in AMI patients in small clinical studies [76], but not all [77]. Unfortunately, the large 1234 patient DANAMI-3 clinical trial failed to find any improvement in clinical outcomes (death and HF at median follow-up of 38 months) in AMI patients treated by IPost, although this study was underpowered and used a suboptimal IPost protocol [78]. Limb RIC has the advantage over IPost in that it can be applied non-invasively by simply inflating and deflating a pneumatic cuff placed on either the arm or leg to induce three to four-5 min cycles of brief ischaemia and reperfusion to the arm or leg. Again, although small proof-of-concept studies [79,80], but not all,[81] reported limitation of MI size in AMI patients treated by either thrombolysis or PPCI, limb RIC failed to improve clinical outcomes (death and HF hospitalisation at one year) in the 5400 patient CONDI-2/ERIC-PPCI trial [82], the reasons for which have been discussed in recent commentaries and are discussed in the next section [83].
Large clinical outcome studies have reported SGLT2 inhibitors such as empagliflozin [84] to reduce cardiovascular death and hospitalisation for heart failure in diabetic patients, although the mechanisms underlying this beneficial effect remain unclear. Interestingly, animal studies have shown that treatment with empagliflozin protected the diabetic rat heart following AMI, as evidenced by less IRI-induced mitochondrial fission, attenuated oxidative stress and enhanced mitophagy, although the effect on MI size and cardiac function was not evaluated [85]. Intriguingly, experimental animal studies have shown that injection of viable mitochondria into the ischaemic heart following AMI, was cardioprotective as evidenced by increased myocardial ATP levels, upregulated proteomic pathways for mitochondrial function, and replaced damaged mitochondrial DNA [86]. In the clinically relevant pig model, it has been demonstrated that intracoronary injection of autologous mitochondria at the onset of myocardial reperfusion (after 30 min of coronary artery ligation) reduced MI size and preserved cardiac function [86], demonstrating potential feasibility for clinical application in AMI patients undergoing myocardial reperfusion by primary PCI. In this regard, a small feasibility pilot study of 5 paediatric patients (who had sustained significant acute myocardial ischaemic injury during cardiac surgery) reported that intramyocardial injection of autologous mitochondria (harvested from skeletal tissue) was safe and improved cardiac function assessed by echocardiography [87].
7. Challenges in translating mitoprotective strategies into the clinical setting
The failure to translate cardioprotective strategies identified in experimental studies into the clinical setting has been an extensively discussed topic in the literature, and has been attributed to different factors: (1) The majority of animal models employed to test novel cardioprotective therapies have used healthy juvenile animals, that do not recapitulate the typical middle-aged AMI patient with co-morbidities (such as diabetes, hypertension and hyperlipidaemia), the presence of which may confound cardioprotection [16]; (2) Many clinical cardioprotection studies have tested novel cardioprotective therapies which have either failed to demonstrate consistent cardioprotection in animal studies or have not been rigorously tested in animal studies (e.g. not tested in large animal models prior to clinical testing) [88,89]; (3) The design of the clinical study in terms of the patient population (higher risk AMI patients with fully occluded coronary arteries and large infarcts are more likely to benefit from cardioprotection) [90]; (4) Many cardioprotection studies have focused on a single cardioprotective agent directed to a single therapeutic target within the cardiomyocyte, but given the multiple components (mitochondrial dysfunction, calcium overload, oxidative stress) and players (cardiomyocytes, endothelial cells, inflammatory cells, fibroblasts, cardiac innervation) in acute myocardial IRI, a multi-component multi-targeted approach to cardioprotection may be more effective [91]; and (5) To be effective against the mitochondrial dysfunction that occurs in the first few minutes of myocardial reperfusion, the cardioprotective therapy has to be administered prior to the onset of reperfusion, with delayed administration after reperfusion has already taken place, being ineffective [90]. Similarly, some cardioprotective therapies (such as hypothermia, sodium hydrogen exchanger inhibitors, SGLT2 inhibitors, insulin) may only be effective when administered prior to the index acute myocardial ischaemic event, which is not feasible in the clinical setting, where AMI patients present after the onset of acute myocardial ischaemia [90].
In summary, despite there being substantial experimental data supporting the targeting of mitochondria as a therapeutic strategy to reduce MI size, the translation to the clinical setting for patient benefit has been hugely disappointing, and innovative approaches and new therapeutic targets are needed.
8. Conclusions and future perspectives
Given the essential role cardiac mitochondria play in providing the energy requirements for normal cardiac contractile function, preventing mitochondrial dysfunction during AMI is an important therapeutic strategy for cardioprotection. However, a number of pharmacological and endogenous cardioprotective strategies (such as ischaemic conditioning), which have been shown to target mitochondria and reduce MI size in animal models, have failed to be translated into the clinical setting for the benefit of AMI patients. Potential strategies for improving the translation of mitoprotective therapies for the benefit for AMI patients include: (1) More rigorous pre-clinical selection of novel cardioprotective strategies before embarking on clinical trials; (2) The use of combination multi-targeted therapies (aimed at different mitochondrial death pathways, and non-cardiomyocyte cells such as the coronary endothelial and inflammatory cells); (3) Use of nanocarriers to more effectively target mitoprotective therapeutics to the ischaemic heart; (4) Discovery of novel mitochondrial targets for cardioprotection (such as the mitochondrial fusion and fission proteins); and (5) the discovery of more specific and efficacious MPTP inhibitors.
9. Outstanding questions
The reasons why several mitoprotective therapies have failed to improve clinical outcomes in AMI patients despite demonstrating benefit in animal models of acute myocardial IRI, is not clear. As such, innovative strategies directed to new mitochondrial targets both within and outside the cardiomyocyte are needed to improve the translation of mitoprotective therapies into the clinical setting for the benefit of AMI patients. Cardioprotective efficacy may be improved by targeting mitoprotective therapies to the ischaemic heart using nanoparticles, and mitochondria by chemical modification (such as MitoQ and MitoSNO). Furthermore, the identity of the MPTP and other mitochondrial channels (such as the MCU and NCX) may result in the discovery of novel mitoprotective therapies. Finally, the effect of aging and co-morbidities (such as diabetes, obesity, and left ventricular hypertrophy) on the efficacy of mitoprotective therapies needs to be investigated.
10. Search strategy and selection criteria
Data for this Review were identified by searches of PubMed and references from relevant article using the search terms “Ischemic heart disease”, “Ischemia”, “Reperfusion”, “Infarction” and “Mitochondria”. All impactful studies were considered, irrespective of the published date in order to reflect the progress made in understanding the role of mitochondrial in acute myocardial infarction and cardioprotection.
Author contributions
CJAR, SHR, GEC, YL, and DJH performed the literature search, prepared the figures, and wrote the manuscript.
Declaration of Competing Interest
Author declare no conflicts of interest.
Acknowledgments
Acknowledgements
Chrishan Ramachandra is supported by the Singapore Ministry of Health's National Medical Research Council under its Open Fund-Young Individual Research Grant (NMRC/OFYIRG/0073/2018), the National Health Innovation Centre Singapore under its Innovation to Develop Grant (NHIC-I2S-1811007) and the SingHealth Duke-NUS Academic Medical Centre under its SingHealth Duke-NUS Academic Medicine Research Grant (AM/TP033/2020 [SRDUKAMR2033]). Sauri Hernandez-Resendiz is supported by the Singapore Ministry of Health's National Medical Research Council under its Open Fund-Young Individual Research Grant (OF-YIRG)–[NMRC/OFYIRG/0078/2018]. Derek J Hausenloy was supported by the British Heart Foundation (CS/14/3/31002), Duke-National University Singapore Medical School, Singapore Ministry of Health's National Medical Research Council under its Clinician Scientist-Senior Investigator scheme (NMRC/CSA-SI/0011/2017) and Collaborative Centre Grant scheme (NMRC/CGAug16C006), and the Singapore Ministry of Education Academic Research Fund Tier 2 (MOE2016-T2-2-021). This article is based upon work from COST Action EU-CARDIOPROTECTION CA16225 supported by COST (European Cooperation in Science and Technology). The funders had no role in the writing of this article.
References
- 1.Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016. Geneva, Switzerland: World Health Organization; 2018. https://www.who.int/healthinfo/global_burden_disease/estimates/en/.
- 2.Neubauer S. The failing heart–an engine out of fuel. N Engl J Med. 2007;356:1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Resendiz S, Prunier F, Girao H, Dorn GW, Hausenloy DJ. Targeting mitochondrial fusion and fission proteins for cardioprotection. J Cell Mol.Med. 2020 doi: 10.1111/jcmm.15384. May 14Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De SD, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luongo TS, Lambert JP, Gross P, Nwokedi M, Lombardi AA, Shanmughapriya S. The mitochondrial Na(+)/Ca(2+) exchanger is essential for Ca(2+) homeostasis and viability. Nature. 2017;545:93–97. doi: 10.1038/nature22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adlam VJ, Harrison JC, Porteous CM, James AM, Smith RA, Murphy MP. Targeting an antioxidant to mitochondria decreases cardiac ischemia-reperfusion injury. FASEB J. 2005;19:1088–1095. doi: 10.1096/fj.05-3718com. [DOI] [PubMed] [Google Scholar]
- 7.Hansson MJ, Llwyd O, Morin D, De PD, Arnoux T, Gouarne C. Differences in the profile of protection afforded by TRO40303 and mild hypothermia in models of cardiac ischemia/reperfusion injury. Eur J Pharmacol. 2015;760:7–19. doi: 10.1016/j.ejphar.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Dare AJ, Logan A, Prime TA, Rogatti S, Goddard M, Bolton EM. The mitochondria-targeted anti-oxidant MitoQ decreases ischemia-reperfusion injury in a murine syngeneic heart transplant model. J Heart Lung Transplant. 2015;34:1471–1480. doi: 10.1016/j.healun.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gane EJ, Weilert F, Orr DW, Keogh GF, Gibson M, Lockhart MM. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver Int. 2010;30:1019–1026. doi: 10.1111/j.1478-3231.2010.02250.x. [DOI] [PubMed] [Google Scholar]
- 10.Chouchani ET, Pell VR, Gaude E, Aksentijevic D, Sundier SY, Robb EL. Ischaemic accumulation of succinate controls reperfusion injury through mitochondrial ROS. Nature. 2014;515:431–435. doi: 10.1038/nature13909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kohlhauer M, Dawkins S, Costa ASH, Lee R, Young T, Pell VR. Metabolomic profiling in acute ST-segment-elevation myocardial infarction identifies succinate as an early marker of human ischemia-reperfusion injury. J Am Heart Assoc. 2018;7 doi: 10.1161/JAHA.117.007546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chouchani ET, Methner C, Nadtochiy SM, Logan A, Pell VR, Ding S. Cardioprotection by S-nitrosation of a cysteine switch on mitochondrial complex I. Nat Med. 2013;19:753–759. doi: 10.1038/nm.3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inserte J, Barba I, Hernando V, Abellan A, Ruiz-Meana M, Rodriguez-Sinovas A. Effect of acidic reperfusion on prolongation of intracellular acidosis and myocardial salvage. Cardiovasc Res. 2008;77:782–790. doi: 10.1093/cvr/cvm082. [DOI] [PubMed] [Google Scholar]
- 14.Avkiran M, Marber MS. Na(+)/H(+) exchange inhibitors for cardioprotective therapy: progress, problems and prospects. J Am Coll Cardiol. 2002;39:747–753. doi: 10.1016/s0735-1097(02)01693-5. [DOI] [PubMed] [Google Scholar]
- 15.Fujita M, Asanuma H, Hirata A, Wakeno M, Takahama H, Sasaki H. Prolonged transient acidosis during early reperfusion contributes to the cardioprotective effects of postconditioning. Am J Physiol Heart Circ Physiol. 2007;292:H2004–H2008. doi: 10.1152/ajpheart.01051.2006. [DOI] [PubMed] [Google Scholar]
- 16.Ferdinandy P, Hausenloy DJ, Heusch G, Baxter GF, Schulz R. Interaction of risk factors, comorbidities, and comedications with ischemia/reperfusion injury and cardioprotection by preconditioning, postconditioning, and remote conditioning. Pharmacol Rev. 2014;66:1142–1174. doi: 10.1124/pr.113.008300. [DOI] [PubMed] [Google Scholar]
- 17.Ong SB, Samangouei P, Kalkhoran SB, Hausenloy DJ. The mitochondrial permeability transition pore and its role in myocardial ischemia reperfusion injury. J Mol Cell Cardiol. 2015;78C:23–34. doi: 10.1016/j.yjmcc.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 18.Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbani A, Giorgio V, Carrer A, Franchin C, Arrigoni G, Jiko C. Purified F-ATP synthase forms a Ca(2+)-dependent high-conductance channel matching the mitochondrial permeability transition pore. Nat Commun. 2019;10:4341. doi: 10.1038/s41467-019-12331-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Antoniel M, Jones K, Antonucci S, Spolaore B, Fogolari F, Petronilli V. The unique histidine in OSCP subunit of F-ATP synthase mediates inhibition of the permeability transition pore by acidic pH. EMBO Rep. 2018;19:257–268. doi: 10.15252/embr.201744705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Littlejohns B, Pasdois P, Duggan S, Bond AR, Heesom K, Jackson CL. Hearts from mice fed a non-obesogenic high-fat diet exhibit changes in their oxidative state, calcium and mitochondria in parallel with increased susceptibility to reperfusion injury. PLoS One. 2014;9 doi: 10.1371/journal.pone.0100579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maneechote C, Palee S, Apaijai N, Kerdphoo S, Jaiwongkam T, Chattipakorn SC. Mitochondrial dynamic modulation exerts cardiometabolic protection in obese insulin-resistant rats. Clin Sci (Lond) 2019;133:2431–2447. doi: 10.1042/CS20190960. [DOI] [PubMed] [Google Scholar]
- 23.Piot C, Croisille P, Staat P, Thibault H, Rioufol G, Mewton N. Effect of cyclosporine on reperfusion injury in acute myocardial infarction. N Engl J Med. 2008;359:473–481. doi: 10.1056/NEJMoa071142. [DOI] [PubMed] [Google Scholar]
- 24.Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–1031. doi: 10.1056/NEJMoa1505489. [DOI] [PubMed] [Google Scholar]
- 25.Lygate CA, Bohl S, ten HM, Faller KM, Ostrowski PJ, Zervou S. Moderate elevation of intracellular creatine by targeting the creatine transporter protects mice from acute myocardial infarction. Cardiovasc Res. 2012;96:466–475. doi: 10.1093/cvr/cvs272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson SM, Adameova A, Barile L, Cabrera-Fuentes HA, Lazou A, Pagliaro P. Mitochondrial and mitochondrial-independent pathways of myocardial cell death during ischaemia and reperfusion injury. J Cell Mol Med. 2020;24:3795–3806. doi: 10.1111/jcmm.15127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang T, Zhang Y, Cui M, Jin L, Wang Y, Lv F. CaMKII is a RIP3 substrate mediating ischemia- and oxidative stress-induced myocardial necroptosis. Nat Med. 2016;22:175–182. doi: 10.1038/nm.4017. [DOI] [PubMed] [Google Scholar]
- 28.Wang Z, Jiang H, Chen S, Du F, Wang X. The mitochondrial phosphatase PGAM5 functions at the convergence point of multiple necrotic death pathways. Cell. 2012;148:228–243. doi: 10.1016/j.cell.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 29.Oerlemans MI, Liu J, Arslan F, den OK, van Middelaar BJ, Doevendans PA. Inhibition of RIP1-dependent necrosis prevents adverse cardiac remodeling after myocardial ischemia-reperfusion in vivo. Basic Res Cardiol. 2012;107:270. doi: 10.1007/s00395-012-0270-8. [DOI] [PubMed] [Google Scholar]
- 30.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469:221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 31.Zuurbier CJ, Abbate A, Cabrera-Fuentes HA, Cohen MV, Collino M, de Kleijn DPV. Innate immunity as a target for acute cardioprotection. Cardiovasc Res. 2019;115:1131–1142. doi: 10.1093/cvr/cvy304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Toldo S, Marchetti C, Mauro AG, Chojnacki J, Mezzaroma E, Carbone S. Inhibition of the NLRP3 inflammasome limits the inflammatory injury following myocardial ischemia-reperfusion in the mouse. Int J Cardiol. 2016;209:215–220. doi: 10.1016/j.ijcard.2016.02.043. [DOI] [PubMed] [Google Scholar]
- 33.Dai Y, Wang S, Chang S, Ren D, Shali S, Li C. M2 macrophage-derived exosomes carry microRNA-148a to alleviate myocardial ischemia/reperfusion injury via inhibiting TXNIP and the TLR4/NF-kappaB/NLRP3 inflammasome signaling pathway. J Mol Cell Cardiol. 2020;142:65–79. doi: 10.1016/j.yjmcc.2020.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Yan HF, Tuo QZ, Yin QZ, Lei P. The pathological role of ferroptosis in ischemia/reperfusion-related injury. Zool Res. 2020;41:220–230. doi: 10.24272/j.issn.2095-8137.2020.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chang HC, Wu R, Shang M, Sato T, Chen C, Shapiro JS. Reduction in mitochondrial iron alleviates cardiac damage during injury. EMBO Mol Med. 2016;8:247–267. doi: 10.15252/emmm.201505748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ong SB, Subrayan S, Lim SY, Yellon DM, Davidson SM, Hausenloy DJ. Inhibiting mitochondrial fission protects the heart against ischemia/reperfusion injury. Circulation. 2010;121:2012–2022. doi: 10.1161/CIRCULATIONAHA.109.906610. [DOI] [PubMed] [Google Scholar]
- 37.Cereghetti GM, Stangherlin A, dB Martins, Chang CR, Blackstone C, Bernardi P. Dephosphorylation by calcineurin regulates translocation of Drp1 to mitochondria. Proc Natl Acad Sci U S A. 2008;105:15803–15808. doi: 10.1073/pnas.0808249105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharp WW, Fang YH, Han M, Zhang HJ, Hong Z, Banathy A, et al. Dynamin-related Protein 1 (Drp1)-`mediated Diastolic Dysfunction in Myocardial Ischemia-Reperfusion Injury: Therapeutic Benefits of Drp1 Inhibition to Reduce Mitochondrial Fission. FASEB J 2014;28:316–26. [DOI] [PMC free article] [PubMed]
- 39.Disatnik MH, Ferreira JC, Campos JC, Gomes KS, Dourado PM, Qi X. Acute inhibition of excessive mitochondrial fission after myocardial infarction prevents long-term cardiac dysfunction. J Am Heart Assoc. 2013;2 doi: 10.1161/JAHA.113.000461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ong SB, Kwek XY, Katwadi K, Hernandez-Resendiz S, Crespo-Avilan GE, Ismail NI. Targeting Mitochondrial Fission Using Mdivi-1 in A Clinically Relevant Large Animal Model of Acute Myocardial Infarction: A Pilot Study. Int J Mol Sci. 2019;20 doi: 10.3390/ijms20163972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ishikita A, Matoba T, Ikeda G, Koga J, Mao Y, Nakano K. Nanoparticle-mediated delivery of mitochondrial division inhibitor 1 to the myocardium protects the heart from ischemia-reperfusion injury through inhibition of mitochondria outer membrane permeabilization: a new therapeutic modality for acute myocardial infarction. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bordt EA, Clerc P, Roelofs BA, Saladino AJ, Tretter L, Adam-Vizi V. The putative Drp1 inhibitor mdivi-1 is a reversible mitochondrial complex I inhibitor that modulates reactive oxygen species. Dev Cell. 2017;40:583–594. doi: 10.1016/j.devcel.2017.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu D, Dasgupta A, Chen KH, Neuber-Hess M, Patel J, Hurst TE. Identification of novel dynamin-related protein 1 (Drp1) GTPase inhibitors: therapeutic potential of Drpitor1 and Drpitor1a in cancer and cardiac ischemia-reperfusion injury. FASEB J. 2020;34:1447–1464. doi: 10.1096/fj.201901467R. [DOI] [PubMed] [Google Scholar]
- 44.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res. 2015;116:264–278. doi: 10.1161/CIRCRESAHA.116.303356. [DOI] [PubMed] [Google Scholar]
- 45.Nuntaphum W, Pongkan W, Wongjaikam S, Thummasorn S, Tanajak P, Khamseekaew J. Vagus nerve stimulation exerts cardioprotection against myocardial ischemia/reperfusion injury predominantly through its efferent vagal fibers. Basic Res Cardiol. 2018;113:22. doi: 10.1007/s00395-018-0683-0. [DOI] [PubMed] [Google Scholar]
- 46.Tao A, Xu X, Kvietys P, Kao R, Martin C, Rui T. Experimental diabetes mellitus exacerbates ischemia/reperfusion-induced myocardial injury by promoting mitochondrial fission: role of down-regulation of myocardial Sirt1 and subsequent Akt/Drp1 interaction. Int J Biochem Cell Biol. 2018;105:94–103. doi: 10.1016/j.biocel.2018.10.011. [DOI] [PubMed] [Google Scholar]
- 47.Hall AR, Burke N, Dongworth RK, Kalkhoran SB, Dyson A, Vicencio JM. Hearts deficient in both Mfn1 and Mfn2 are protected against acute myocardial infarction. Cell Death Dis. 2016;7:e2238. doi: 10.1038/cddis.2016.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Franco A, Kitsis RN, Fleischer JA, Gavathiotis E, Kornfeld OS, Gong G. Correcting mitochondrial fusion by manipulating mitofusin conformations. Nature. 2016;540:74–79. doi: 10.1038/nature20156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chen L, Gong Q, Stice JP, Knowlton AA. Mitochondrial OPA1, apoptosis, and heart failure. Cardiovasc Res. 2009;84:91–99. doi: 10.1093/cvr/cvp181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xiao X, Hu Y, Quiros PM, Wei Q, Lopez-Otin C, Dong Z. OMA1 mediates OPA1 proteolysis and mitochondrial fragmentation in experimental models of ischemic kidney injury. Am J Physiol Renal Physiol. 2014;306:F1318–F1326. doi: 10.1152/ajprenal.00036.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nan J, Nan C, Ye J, Qian L, Geng Y, Xing D. EGCG protects cardiomyocytes against hypoxia-reperfusion injury through inhibition of OMA1 activation. J Cell Sci. 2019;132 doi: 10.1242/jcs.220871. [DOI] [PubMed] [Google Scholar]
- 52.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One. 2011;6:e20975. doi: 10.1371/journal.pone.0020975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chen Y, Dorn GW. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem. 2013;288:915–926. doi: 10.1074/jbc.M112.411363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Siddall HK, Yellon DM, Ong SB, Mukherjee UA, Burke N, Hall AR. Loss of PINK1 increases the heart's vulnerability to ischemia-reperfusion injury. PLoS One. 2013;8:e62400. doi: 10.1371/journal.pone.0062400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saito T, Nah J, Oka SI, Mukai R, Monden Y, Maejima Y. An alternative mitophagy pathway mediated by Rab9 protects the heart against ischemia. J Clin Invest. 2019;129:802–819. doi: 10.1172/JCI122035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fiorese CJ, Schulz AM, Lin YF, Rosin N, Pellegrino MW, Haynes CM. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr Biol. 2016;26:2037–2043. doi: 10.1016/j.cub.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang YT, Lim Y, McCall MN, Huang KT, Haynes CM, Nehrke K. Cardioprotection by the mitochondrial unfolded protein response requires ATF5. Am J Physiol Heart Circ Physiol. 2019;317:H472–H478. doi: 10.1152/ajpheart.00244.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venkatesh S, Li M, Saito T, Tong M, Rashed E, Mareedu S. Mitochondrial LonP1 protects cardiomyocytes from ischemia/reperfusion injury in vivo. J Mol Cell Cardiol. 2019;128:38–50. doi: 10.1016/j.yjmcc.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 60.Olmedo I, Pino G, Riquelme JA, Aranguiz P, Diaz MC, Lopez-Crisosto C. Inhibition of the proteasome preserves Mitofusin-2 and mitochondrial integrity, protecting cardiomyocytes during ischemia-reperfusion injury. Biochim Biophys Acta Mol Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2019.165659. [DOI] [PubMed] [Google Scholar]
- 61.Hausenloy DJ, Maddock HL, Baxter GF, Yellon DM. Inhibiting mitochondrial permeability transition pore opening: a new paradigm for myocardial preconditioning. Cardiovasc Res. 2002;55:534–543. doi: 10.1016/s0008-6363(02)00455-8. [DOI] [PubMed] [Google Scholar]
- 62.Costa AD, Jakob R, Costa CL, Andrukhiv K, West IC, Garlid KD. The mechanism by which the mitochondrial ATP-sensitive K+ channel opening and H2O2 inhibit the mitochondrial permeability transition. J Biol Chem. 2006;281:20801–20808. doi: 10.1074/jbc.M600959200. [DOI] [PubMed] [Google Scholar]
- 63.Paggio A, Checchetto V, Campo A, Menabo R, Di MG, Di LF. Identification of an ATP-sensitive potassium channel in mitochondria. Nature. 2019;572:609–613. doi: 10.1038/s41586-019-1498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Goswami SK, Ponnalagu D, Hussain AT, Shah K, Karekar P, Gururaja RS. Expression and activation of BKCa channels in mice protects against ischemia-reperfusion injury of isolated hearts by modulating mitochondrial function. Front Cardiovasc Med. 2018;5:194. doi: 10.3389/fcvm.2018.00194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Boengler K, Ruiz-Meana M, Gent S, Ungefug E, Soetkamp D, Miro-Casas E. Mitochondrial connexin 43 impacts on respiratory complex I activity and mitochondrial oxygen consumption. J Cell Mol Med. 2012;16:1649–1655. doi: 10.1111/j.1582-4934.2011.01516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gadicherla AK, Wang N, Bulic M, Agullo-Pascual E, Lissoni A, De SM. Mitochondrial Cx43 hemichannels contribute to mitochondrial calcium entry and cell death in the heart. Basic Res Cardiol. 2017;112:27. doi: 10.1007/s00395-017-0618-1. [DOI] [PubMed] [Google Scholar]
- 67.Hausenloy DJ, Schulz R, Girao H, Kwak BR, De Stefani D, Rizzuto R. Mitochondrial ion channels as targets for cardioprotection. J Cell Mol.Med. 2020 doi: 10.1111/jcmm.15341. Jun 3.Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxid Redox Signal. 2011;14:893–907. doi: 10.1089/ars.2010.3360. [DOI] [PubMed] [Google Scholar]
- 69.Ikeda G, Matoba T, Nakano Y, Nagaoka K, Ishikita A, Nakano K. Nanoparticle-mediated targeting of cyclosporine A enhances cardioprotection against ischemia-reperfusion injury through inhibition of mitochondrial permeability transition pore opening. Sci Rep. 2016;6:20467. doi: 10.1038/srep20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang CX, Cheng Y, Liu DZ, Liu M, Cui H, Zhang BL. Mitochondria-targeted cyclosporin A delivery system to treat myocardial ischemia reperfusion injury of rats. J Nanobiotechnol. 2019;17:18. doi: 10.1186/s12951-019-0451-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kawamura E, Maruyama M, Abe J, Sudo A, Takeda A, Takada S. Validation of gene therapy for mutant mitochondria by delivering mitochondrial RNA using a MITO-porter. Mol Ther Nucleic Acids. 2020;20:687–698. doi: 10.1016/j.omtn.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Abe J, Yamada Y, Takeda A, Harashima H. Cardiac progenitor cells activated by mitochondrial delivery of resveratrol enhance the survival of a doxorubicin-induced cardiomyopathy mouse model via the mitochondrial activation of a damaged myocardium. J Control Release. 2018;269:177–188. doi: 10.1016/j.jconrel.2017.11.024. [DOI] [PubMed] [Google Scholar]
- 73.Antonucci S, Di SM, Sileikyte J, Deveraux J, Bauer T, Bround MJ. A novel class of cardioprotective small-molecule PTP inhibitors. Pharmacol Res. 2020;151 doi: 10.1016/j.phrs.2019.104548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gibson CM, Giugliano RP, Kloner RA, Bode C, Tendera M, Janosi A. EMBRACE STEMI study: a Phase 2a trial to evaluate the safety, tolerability, and efficacy of intravenous MTP-131 on reperfusion injury in patients undergoing primary percutaneous coronary intervention. Eur Heart J. 2016;37:1296–1303. doi: 10.1093/eurheartj/ehv597. [DOI] [PubMed] [Google Scholar]
- 75.Atar D, Arheden H, Berdeaux A, Bonnet JL, Carlsson M, Clemmensen P. Effect of intravenous TRO40303 as an adjunct to primary percutaneous coronary intervention for acute ST-elevation myocardial infarction: MITOCARE study results. Eur Heart J. 2015;36:112–119. doi: 10.1093/eurheartj/ehu331. [DOI] [PubMed] [Google Scholar]
- 76.Staat P, Rioufol G, Piot C, Cottin Y, Cung TT, L'Huillier I. Postconditioning the human heart. Circulation. 2005;112:2143–2148. doi: 10.1161/CIRCULATIONAHA.105.558122. [DOI] [PubMed] [Google Scholar]
- 77.Freixa X, Bellera N, Ortiz-Perez JT, Jimenez M, Pare C, Bosch X. Ischaemic postconditioning revisited: lack of effects on infarct size following primary percutaneous coronary intervention. Eur Heart J. 2012;33:103–112. doi: 10.1093/eurheartj/ehr297. [DOI] [PubMed] [Google Scholar]
- 78.Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Clemmensen P. Effect of ischemic postconditioning during primary percutaneous coronary intervention for patients with ST-segment elevation myocardial infarction: a randomized clinical trial. JAMA Cardiol. 2017;2:490–497. doi: 10.1001/jamacardio.2017.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ. Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet. 2010;375:727–734. doi: 10.1016/S0140-6736(09)62001-8. [DOI] [PubMed] [Google Scholar]
- 80.White SK, Frohlich GM, Sado DM, Maestrini V, Fontana M, Treibel TA. Remote ischemic conditioning reduces myocardial infarct size and edema in patients with ST-segment elevation myocardial infarction. JACC Cardiovasc Interv. 2015;8:178–188. doi: 10.1016/j.jcin.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 81.Verouhis D, Sorensson P, Gourine A, Henareh L, Persson J, Saleh N. Effect of remote ischemic conditioning on infarct size in patients with anterior ST-elevation myocardial infarction. Am Heart J. 2016;181:66–73. doi: 10.1016/j.ahj.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 82.Hausenloy DJ, Kharbanda RK, Moller UK, Ramlall M, Aaroe J, Butler R. Effect of remote ischaemic conditioning on clinical outcomes in patients with acute myocardial infarction (CONDI-2/ERIC-PPCI): a single-blind randomised controlled trial. Lancet. 2019;394:1415–1424. doi: 10.1016/S0140-6736(19)32039-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hausenloy DJ, Botker HE. Why did remote ischaemic conditioning not improve clinical outcomes in acute myocardial infarction in the CONDI-2/ERIC-PPCI trial. Cardiovasc Res. 2019;115:e161–e163. doi: 10.1093/cvr/cvz242. [DOI] [PubMed] [Google Scholar]
- 84.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 85.Mizuno M, Kuno A, Yano T, Miki T, Oshima H, Sato T. Empagliflozin normalizes the size and number of mitochondria and prevents reduction in mitochondrial size after myocardial infarction in diabetic hearts. Physiol Rep. 2018;6:e13741. doi: 10.14814/phy2.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shin B, Saeed MY, Esch JJ, Guariento A, Blitzer D, Moskowitzova K. A novel biological strategy for myocardial protection by intracoronary delivery of mitochondria: safety and efficacy. JACC Basic Transl Sci. 2019;4:871–888. doi: 10.1016/j.jacbts.2019.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Emani SM, Piekarski BL, Harrild D, Del Nido PJ, McCully JD. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 2017;154:286–289. doi: 10.1016/j.jtcvs.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 88.Lecour S, Botker HE, Condorelli G, Davidson SM, Garcia-Dorado D, Engel FB. ESC working group cellular biology of the heart: position paper: improving the preclinical assessment of novel cardioprotective therapies. Cardiovasc Res. 2014;104:399–411. doi: 10.1093/cvr/cvu225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Botker HE, Hausenloy D, Andreadou I, Antonucci S, Boengler K, Davidson SM. Practical guidelines for rigor and reproducibility in preclinical and clinical studies on cardioprotection. Basic Res Cardiol. 2018;113:39. doi: 10.1007/s00395-018-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hausenloy DJ, Erik BH, Condorelli G, Ferdinandy P, Garcia-Dorado D, Heusch G. Translating cardioprotection for patient benefit: position paper from the Working Group of Cellular Biology of the Heart of the European Society of Cardiology. Cardiovasc Res. 2013;98:7–27. doi: 10.1093/cvr/cvt004. [DOI] [PubMed] [Google Scholar]
- 91.Davidson SM, Ferdinandy P, Andreadou I, Botker HE, Heusch G, Ibanez B. Multitarget strategies to reduce myocardial ischemia/reperfusion injury: JACC review topic of the week. J Am Coll Cardiol. 2019;73:89–99. doi: 10.1016/j.jacc.2018.09.086. [DOI] [PubMed] [Google Scholar]