Abstract
Avascular necrosis of the lunate, commonly known as Kienböck’s disease is a disorder that can lead to carpal collapse and the need for surgeries, which can stabilize the wrist. There are different associations with the disease but the exact etiology is unknown. Kienböck’s disease is believed to result from mechanical and vascular factors in genetically predisposed individuals. The newer classification based on advanced wrist arthroscopy and MRI help in a better understanding of the disease, early diagnosis, and treatment. A review of recent literature regarding newer treatment options has shown good results in the early stages of osteonecrosis. This article intends to review an update on the etiopathogenesis, classification, and the current advanced treatment options.
Keywords: Lunate, Kienböck, Avascular necrosis, Semilunar
1. Introduction
In 1910, Robert Kienböck, an Austrian radiologist published the radiological findings of collapse lunate bone, and he named it “lunatomalacia”.1 In 1920, Müller was first to observe that the repetitive microtrauma from occupation activity might cause Kienbock’s disease (KD).2 The disease was considered to be multifactorial and several mechanical, anatomical and vascular factors were thought to contribute to the pathogenesis of KD.3, 4, 5, 6, 7, 8,16,20,27
Numerous theories have been proposed so far for the etiopathogenesis of KD over the last 100 years.3,4,9, 10, 11 KD or avascular necrosis of the lunate carpal bone is an uncommon disease of young adults. Irisarriet al. described the two divisions of KD in children as Infantile and Juvenile lunatomalacia.12 The infantile subgroup has been seen in children less than 12yrs of age with a good prognosis with conservative management. Juvenile lunatomalacia affects children above 13 years old to the end of skeletal maturity. Conservative treatment leads to good outcomes in the early stages of juvenile lunatomalacia. Surgical procedure, usually radial shortening, may be required to treat advanced stages. Like conventional KD, the disease in elderly patients is more prevalent in the hand of manual workers but it may differ in etiology. The elderly group has a low frequency of negative ulnar variance and KD is more often in elderly women than in elderly men. The functional outcomes without surgery have been reported good or excellent even, in advanced stages of Kienböck’s disease in these patients.13,14 This may be related with low functional demand in elderly individuals.
In young adults, the disease is predominantly seen in a unilateral, dominant wrist and it rarely involves bilateral wrist. In a study by Yazakiet al,15 out of the251 Kienböck’s disease patients, 11 patients were found to be bilaterally involved. But they did not find any risk factor for bilateral, as opposed to unilateral Kienböck’s disease.
2. Etiology
2.1. Mechanical factors
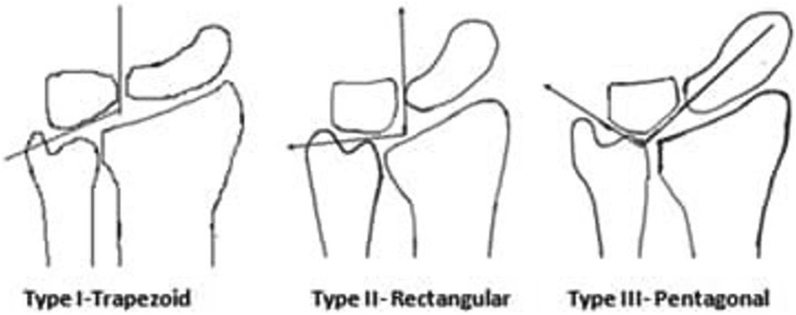
In 1928, Hultén observed a relationship between negative ulnar variance KD.16 He described the abnormal load transmission across the radiolunate joint in negative ulnar variance which subsequently predisposed to stress fracture in the lunate bone. However, D’Hoore, Nakamura, Stahl S et al. did not find a causal relationship between negative ulnar variance and KD.17, 18, 19 In a metanalysis, it was revealed that there is inadequate data to support the association between negative ulnar variance and Kienböck’s disease.20 Antuña-Zapico observed the trabecular pattern in different lunate bones (Fig. 1).21 He noted three types of lunate morphology in their series. He observed that the trapezoidal shape and trabecular angulation of more than 135° (type 1 lunate) have higher chances of collapse and type 1 lunates are more commonly associated with negative ulnar variance. This trapezoidal shape of lunate along with negative ulnar variance predisposes it to abnormal loading forces and shear fractures. Pentagonal lunates (Type 3 lunates) are commonly associated with ulnar positive variance. Tsuge and Nakamura pointed out that patients having lunate that are smaller, more radially inclined and having a flatter radial inclination may be the predisposing factor for KD.22
Fig. 1.
Antuña-Zapico types of lunate morphology.
Viegas recognized two types of lunate morphology based on a medial (hamate) facet on the lunate.23 Type I, having no medial facet in 34.5% and Type II, with a medial facet in 65.5% of the cases. They found the significant chondral erosions at the proximal pole of hamate in type II lunates specimens. They stated that type II lunate bone may be the cause of unidentified ulnar sided wrist pain. However, the association of type 2 lunate with KD is doubtful. Tatebe et al. did the arthroscopic analysis of lunate morphology to find out the impact of lunate morphology on KD and they found that lower proportion of type 2 lunates in KD than for traumatic and in degenerative conditions.24 The difference was attributed to preferential loading across the radiolunate joint in type 1 lunate.
Xionget al. studied the trabecular pattern in normal and stage III KD lunates using micro-computed tomography.25 They found the bony disruptions in the palmar or dorsal region of the distal articular surface of diseased lunates because of compact trabecular bone in the central region in comparison to weaker dorsal and volar region. This might be the reason for fracture and eventually collapse of lunate in KD.
2.2. Vascular factors
The initial studies by Cordes and Stahl had suggested a single blood vessel supply of lunate by either palmar or dorsal vessel.19,26 Lee observed the intraosseous pattern of the blood supply of the lunate bone by microradiography and Spalteholz preparations.27 He described the three main patterns of blood supply. 66.5% of cases had a dual supply of lunate by the palmar and dorsal vessels that anastomose within the bone.7.5% of cases had dual blood supply without anastomosis within the bone. 26% of cases had a single blood supply by palmar or dorsal vessel. The anastomosis lies in the center or slightly distal to the center of the bone, supplying major branches to the various parts of the bone. Gelbermanet et al. described the extraosseous and intraosseous vascularity of the lunate bone in 3 cadaveric limbs.28 They noted the profuse extraosseous blood supply by 2–3 dorsal vessels and 3–4 volar vessels and intraosseous vascularity formed three consistent patterns (Y, X, and I) with anastomosis of dorsal and volar vessels. Poor venous drainage has also been thought to play a critical role in the pathogenesis of avascular necrosis of the lunate. Stress fractures may lead to localized venous obstruction. This can lead to an increase in intraosseous pressure producing, local edema, necrosis and subsequent collapse of the lunate.29,30
Recently Kim et al. showed the wrist position-dependent perfusion of the lunate using super-selective angiography of the radial, ulnar, and interosseous artery.31 They found the maximum blood flow during neutral wrist position, which decreased with extension or flexion of the wrist. They concluded that prolonged wrist flexion or extension in pain or splinting, occupation might contribute to the pathogenesis of the disease.
The association of KD with several multisystem disorders like systemic lupus erythematosus, sickle cell anemia, scleroderma, rheumatoid arthritis, dermatomyositis and gout is well established.32, 33, 34, 35, 36, 37 Vasculitis is the characteristic pathological feature in such cases, which has emphasized the role of vascular factors for the development of the disease.32, 33, 34, 35, 36, 37
Considering these different causation theories, Kienböck’s disease appears to be multifactorial. Patients with risk factorsforKienböck’s disease include single vessel in the lunate, poor intraosseous anastomoses, hypercoagulopathy, small lunate size, flatter radius inclination, negative ulnar variance, and trapezoidal lunate shape. Repetitive microtrauma in predisposed individuals leads to hypoperfusion and ischemia of the lunate.
3. Staging & classifications
Earlier classifications (Stahl (1947) were based on plain radiographic characteristics.19 In 1977, Lichtman et al. modified the Sta°hl’s original classification and describe the four-stage (I-IV) classification of KD.38 Later on, they introduced stage 0 to represent intermittent or stress-induced ischemia of lunate bone in MRI negative individuals and stage IIIC in cases of lunate fracture in coronal plane irrespective of lunate morphology and carpal instability.39
Lichtman classification39
Stage 0- Intermittent ischemia
Stage I- normal X-ray with positive bone scan
Stage II- lunate sclerosis on radiography with normal shape and size
Stage IIIA-lunate collapse with preserved carpal alignment and height
Stage IIIB- Carpal collapse with fixed scaphoid rotation, proximal capitate migration
Stage IIIC-lunate fracture (coronal plane)
Stage IV- Radiocarpal Arthritis
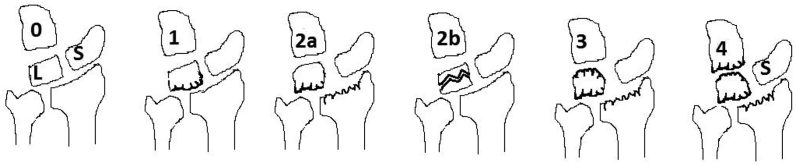
Bain and Begg proposed grading of KD based on arthroscopic findings of non-functional lunate articular surfaces.40 They define the functional articular surface as having a normal glistening appearance with hard bone on probing (Fig. 2). A non-functional articular surface is defined as having any one of the following: extensive fibrillation, fissuring, localized or extensive cartilage loss, a floating articular surface, or fracture. Synovitis is not considered to specify the grade of Kienböck’s disease.
Fig. 2.
Bain and Begg arthroscopic classification.
Bain and Begg arthroscopic classification40
-
0
Articular surfaces are normal
-
1
Proximal surface of lunate abnormal 2A- Proximal surface of the lunate and lunate fossa of radius abnormal.
-
2
B- frontal fracture of lunate.
-
3
Lunate fossa of radius and proximal and distal surfaces of lunate abnormal.
-
4
Lunate fossa of radius and proximal and distal surfaces of the lunate and the proximal surface of capitate abnormal.
Schmitt et al. describe the gadolinium-enhanced MRI based classification to delineate the pattern of osteonecrosis in different parts of the lunate bone.41,42 It includes proximal necrotic zone, middle reparative zone, and distal normal viable lunate bone. Based on signal intensity in different zones of lunate on MRI, Schmitt et al. classified 3 stages of Kienböck’s disease.
Schmitt and Lanz MRI patterns41,42
N- Normal signal
A- Marrow edema with viable and intact bony trabeculae
B- Early marrow necrosis with fibro-vascular reparative tissue
C- Necrotic bone marrow with collapse
Stress-induced ischemia (axial loading of the wrist in an extended wrist) followed by Gadolinium-enhanced MRI will show Schmitt and Lanz pattern A pattern (bone marrow edema). It is recommended in patients having transient lunate ischaemic episodes (stage 0) with negative radiographic or MRI images.
4. Treatment algorithm
Recently, Lichtmanand Bain have proposed a newer classification for Kienböck’s disease taking into consideration the osseous–Lichtman,vascular–Schmitt, cartilage–Bain classification.43 The present classification takes in to account the patient’s age, the status of lunate and wrist, surgeons’ capability, and patient’s preference according to lifestyle. Although comprehensive, present classification provides new insight into the future directions of treatment. In further discussion, we have elaborated and reviewed the newer or advanced treatment options according to this newer classification. The lunate is defined as B1—Lunate intact (functional articular surfaces), B2—Lunate Compromised (nonfunctional proximal lunate articular Surface), or B3-not reconstructable (nonfunctional lunate articular surfaces). The status of the wrist can be defined as C1- compromised central column articulations (C1a-compromised radiolunate articulations, C1b-compromised Radiolunate and midcarpal articulations), C2- Carpal collapse with intact radioscaphoid articulation and C3-non reconstructable wrist.
(A) Age
Patients presented at a younger age have different natural histories from adults. The good revascularization potential and remodeling capacity of the lunate in skeletally immature patients preclude the operative management as the first line of treatment.
A1 < 15 years-treat conservatively with immobilization
A2 16–20 years-joint leveling procedures offered in case of unresponsive to non –operative management for symptoms >3 months. Radial shortening osteotomy has been most frequently described. However, there is a risk of radial overgrowth after the procedure. Alternatively, distal radius epiphysiodesis can also be done.44
A3 > 70 years-consider surgical management if symptoms persist more than 6 months
From, 21–69 years follow section B or C.
B1: Lunate Intact—Lunate Protection
In this stage, the all the articular surfaces of the lunate bone are functional on arthroscopy (Bain 0), without any features of collapse (Lichtman stage 0, I, and II) and well perfusion on gadolinium-enhanced MRI (Schmitt stage A).
4.1. Lunate unloading procedures
Radial shortening osteotomy is the classical technique described to offload the lunate bone by reducing the mechanical forces across it. It is indicated especially in case of ulnar negative variance or neutral. This procedure is well accepted in reducing pain and in improving the function, range of wrist motion, and grip strength. Good results have been reported by combining radial shortening with revascularization procedure.45Although reported results have been encouraging in terms of long-lasting symptomatic relief after radial osteotomy, unable to change the natural course of the disease.46 A systematic review showed that radial osteotomy did not alter the radiological progression of Kienböck’s disease compared with nonoperative treatment in terms of the Lichtman stage but found to be superior in terms of clinical outcomes.47 Botelheiro et al. have shown long term good clinical results recently with radius shortening osteotomy in Lichtman IIIB stage and concluded that advanced disease with carpal collapse should not be considered as a contraindication to carpal sparing radial shortening osteotomy.48
There is a risk of DRUJ incongruity after shortening or wedge osteotomy of the radius. Various modifications have been described later on.
Very distal radius wedge osteotomy is a newer modification where osteotomy is done with its apex distally contrary to convention closing wedge osteotomy. It reduces the radial inclination angle and increases the lunate covering ratio without affecting DRUJcongruity.49
Selective shortening wedge osteotomy of the radius (Camembert osteotomy) facing the lunate can be done.50 They decompress the lunate bone without decompressing the scaphoid as seen in whole radius shortening osteotomy. It is performed in conjunction with Sennwald’s ulnar shortening osteotomy in cases with positive ulnar variance or if there is distal radioulnar joint (DRUJ) discontinuity after shortening.
Partial capitate shortening can also be done as an alternative procedure in cases of ulnar positive or neutral variance.51 Several biomechanical studies have shown that capitate shortening osteotomy results in the largest reduction of the loading forces across the radiolunate joint among all unloading procedures when combined with capitohamate fusion.52, 53, 54 This surgical technique diminishes the radiolunate load without disrupting the distal radioulnar, scaphocapitate, radiocarpal, and midcarpal relationship. Most series reported improvement in both clinical and radiological parameters.55 However, large studies evaluating long-term results are needed to predict future mid carpal arthrosis.
Metaphyseal core decompression (MCD) of the radius is an extraarticular curettage of cancellous bone from the distal radius based on the induction of physiologic fracture healing response that helps in increasing perfusion of the wrist.57,58
Lunate forage is a minimally invasive option based on the concept of reducing intraosseous hypertension that involves arthroscopically assisted drilling of the lunate bone in combination with synovectomy.59,60
Vascularized bone grafts- Various vascular grafting (4 + 5 extensor compartment artery (ECA) based pedicle bone graft, Second or third metacarpal base bone grafts, free vascularized iliac bone or pisiform graft or graft from volar radius procedures have been described for revascularization of the intact lunate bone.61, 62, 63, 64, 65 4 + 5 extensor compartment artery (ECA) based pedicle bone graft that is commonly used due to its wider diameter of the vascular pedicle. Vascular bone graft has also been described in combination with temporary scaphotrapezio-trapezoidal pinning with k wire to bypass the mechanical stress across the lunate till it gains vascularity.66
B2-Lunate Compromised (LichtmanStage IIIA, Schmitt Stage B, and Bain Grade 1)
Lunate reconstruction is the treatment of choice in this stage compromising the proximal articular pole of the lunate.
Vascularized Medial Femoral Trochlea Graft- This is particularly useful in the case of proximal pole necrosis of lunate. The osteochondral free graft from medial femoral condyle can be used for the replacement of the proximal necrosed articular surface of the lunate bone.67
4.2. Proximal row carpectomy (PRC)
PRC is an alternative procedure that can be done provided articular surfaces of the lunate fossa of radius and capitate are functional.68 This procedure is relatively contraindicated in patients who are younger than 35 years and who still want to be involved in high demanding activities.69 Although technically easier, it carries a risk of developing arthritis between capitates head and radius. In a study of thirteen patients of Kienböck’s disease managed with proximal row carpectomy with an average 15-year follow-up, Lumsden et al. found degenerative changes, localized to the radiocapitate articulation in all thirteen patients and Twelve of 13 patients demonstrated excellent or good clinical outcomes.70 However, the literature fails to provide any correlation between radiographic and clinical outcomes after proximal row carpectomy.71, 72, 73
B3-Lunate Not Reconstructable: Lunate Salvage (LichtmanStage IIIC, Schmitt Stage C, and Bain Grade 2b)
The stage represents the coronal splits fractures of lunate bone that loses its ability to vascularize by decompression or revascularization methods. It requires the lunate salvage procedure (excision) with replacement or radioscapholunate (RSL) fusion or scaphocapitate fusion.
Lunate excision and its replacement with tendon interposition, silicon, pyrocarbon, polyethylene-based prosthesis have been described.74, 75, 76, 77, 78, 79 Titanium lunate arthroplasty (TLA) has also shown promising results in long term.80 If the articular surface of the capitate and lunate facet are functional, PRC can be done.
Recently coronal splits fractures of the lunate have been salvaged by internal fixation provided intact cortical rim in absence of fracture comminution, sclerosis or collapse and without arthrosis of radiolunate and lunocapitate joints on computer tomography.81
- C1. Compromised Central Column Articulation (Lichtman Stage IIIA or C, Schmitt Stage B, and BainGrade 2a, 3, or 4)
- C1a.CompromisedRadiolunate Joint
If the lunocapitate joint is functional a radioscapholunate arthrodesis can be done at this stage.82,83
C1b—Compromised Radiolunate and Midcarpal Joints
With the involvement of both radiolunate and capitolunate joint, the scaphocapitate fusion can be performed but the prerequisite is intact radioscaphoid articulation.84
C2—Carpal Collapse-Intact Radioscaphoid Articulation (Lichtman Stage IIIB, Schmitt Stage B, and Bain Grade 2–4)
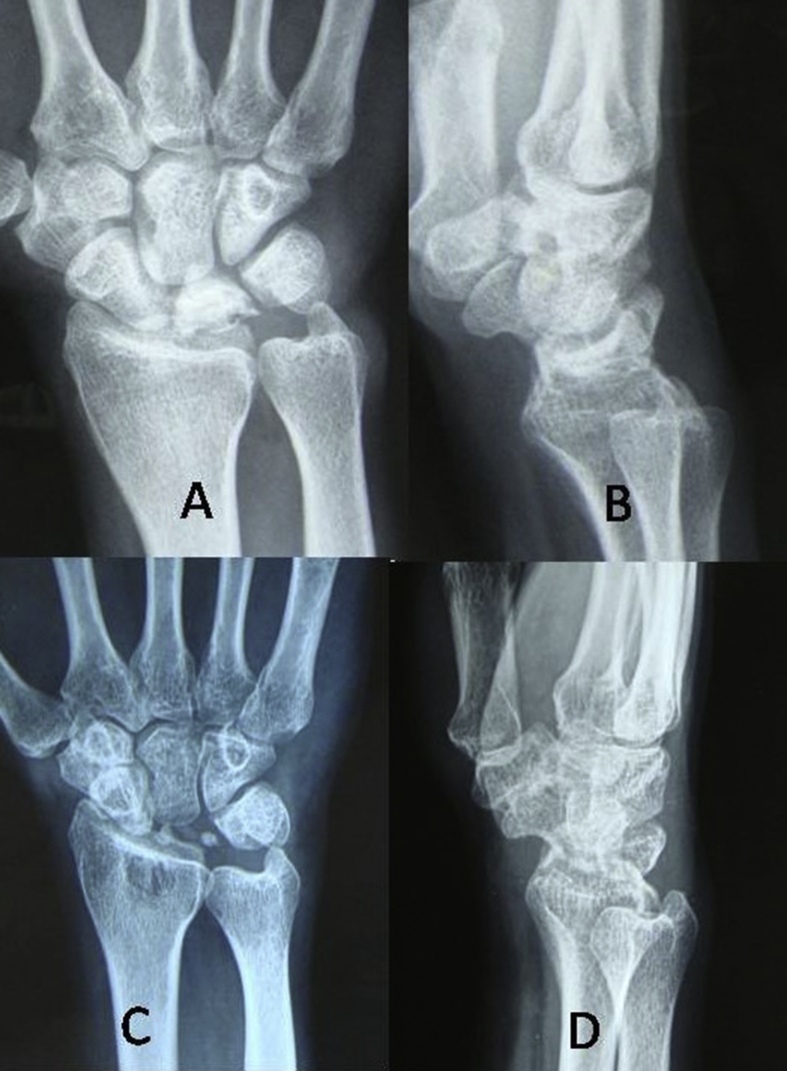
With further collapse and degeneration of the central column, the radioscaphoid articulations are initially functional. So scaphocapitate is a good surgical option (Fig. 6). Alternatively scaphotrapeziotrapezoid [STT]) arthrodesis can also be performed.85,86
Fig. 6.
Preoperative radiographs PA (A) and lateral (B) views show Stage IIIB Kienböck’s disease. Lunate Excision with Triscaphae Arthrodesis was done. Postoperative radiographs(C and D) showed a union at 06 months.
Lunate excision, capitate osteotomy, and its proximal transposition in combination with intercarpal arthrodesis (Graner procedure) have also been described for advanced disease.87 The recent modification includes vascularized capitate transposition with the incorporation of iliac or distal radius bone graft in place of transposed capitates shown good results in stage III.88,89
C3-Wrist Not Reconstructable (Lichtman Stage4, Schmitt Stage C, and Bain Grade 4)
With further collapse or degeneration of the radioscaphoid joint, the wrist can not be reconstructed and arthrodesis or arthroplasty can be done depending upon the patient’s needs.
What Can the Surgeon Offer (D) and What Does the Patient Want? (E).
Depending upon the surgeon’s ability, skills, experience, and working set up, different surgical options can be offered to the patients with their pros and cons. Accordingly option of surgery should be offered as per the patient’s needs and activity.
4.3. Authors’ preferred treatment
The treatment algorithm suggested by Lichtman and Bain is very comprehensive and useful.43 We try to follow the above mentioned treatment protocol with our own modifications. On presentation, we assess the following: (1) the intensity of the pain (2) active range of motion of the patient (3) how it is affecting the life style of the patient (3) duration of the disease (4) age of the patient, (5) ulnar variance and (6) radiographic classification as suggested by Lichtmanclassification.39 All the patients are explained about the disease and that multiple procedures may be required in future. The very young kids (less than 15 years) are given plaster of Paris (POP) cast of 8–12 weeks and they are reassessed. Usually, these kids have relief in pain. They rarely require surgery, if pain persists or there is deterioration in stage of the disease then the patient is offered surgery. Radial shortening in ulnar positive or ulnar neutral variance is the most common procedure, which is suggested to these patients, irrespective of the stage of the disease. The results of this surgery have been good to excellent in these patients. In young individuals (20–40 years), all the patients are advised surgery and wrist arthroscopy has been incorporated as essential tool for these patients. The patients with severe pain have synovitis around the lunate, in reaction to the dead bone. Wrist arthroscopy helps not only in assessment of articular surfaces of the carpal bone but synovectomy is uniformly performed in all cases. The synovectomy around the radio-lunate and luno-capitate area helps in early pain relief. The patients who present early and who are in Litchman stage1 or stage2 with ulnar positive variance are suggested radial shortening after doing arthroscopic synovectomy of the wrist. The radiographic results have been variable and sometimes, the vascularity of the lunate returns (Fig. 3). In literature, the best results have been with radial shortening in ulnar positive variance cases. If there is ulnar negative variance then capitate shortening is performed. The 4,5 ICSRA based graft procedure is reserved for patients of Litchman stage1 with neutral or negative ulnar variance (Fig. 4). Vascularized bone procedure requires violation of the wrist capsule and sometimes this leads to fibrosis and restriction of range of motion of the wrist. We do scapho-capitate pinning with temporary K wire to unload the lunate. The pin is removed after four months. The patients who have limited range of motion, usually, they have guarded prognosis, irrespective of the procedure performed. These patients usually present late and they are in advanced stages of the disease. In our practice, arthroscopic scapho-capitate fusion and excision of the lunate is the most common procedure in the patients who present late in Litchman stage3a or Litchman stage3b (Fig. 5). Occasionally, we perform radial shortening in patients with Litchman stage3a, but, the patient is informed that he may require additional procedure (scapho-capitate fusion) in future. Triscaphe fusion is another procedure, which we used to perform in Litchman stage3b (Fig. 6). The range of motion of the wrist in patients with triscpahe fusion has been a little less then in patients who have undergone scaphocapitate fusion. The future requirement of radial styloidectomy is another concern in patients of triscaphe fusion. Although there are no comparative studies, there has been a shift from triscaphae fusion to scapho-capitate fusion in our practice. Proximal row carpectomy (PRC) is not an unusual surgical procedure in our hand and we reserve this procedure for the patients who are more than 40 years old (Fig. 7). If there are changes, as the articular defects in the cartilage of the radius and capitate then we perform capsular interposition along with PRC. The elderly individuals (more than 70 years old) are offered one time procedure in term of PRC or PRC with capsular interposition. Wrist arthrodesis is highly reserved procedure for KD. It has been reserved for the patients when all the motion-preserving options have failed but we are yet to find such patients.
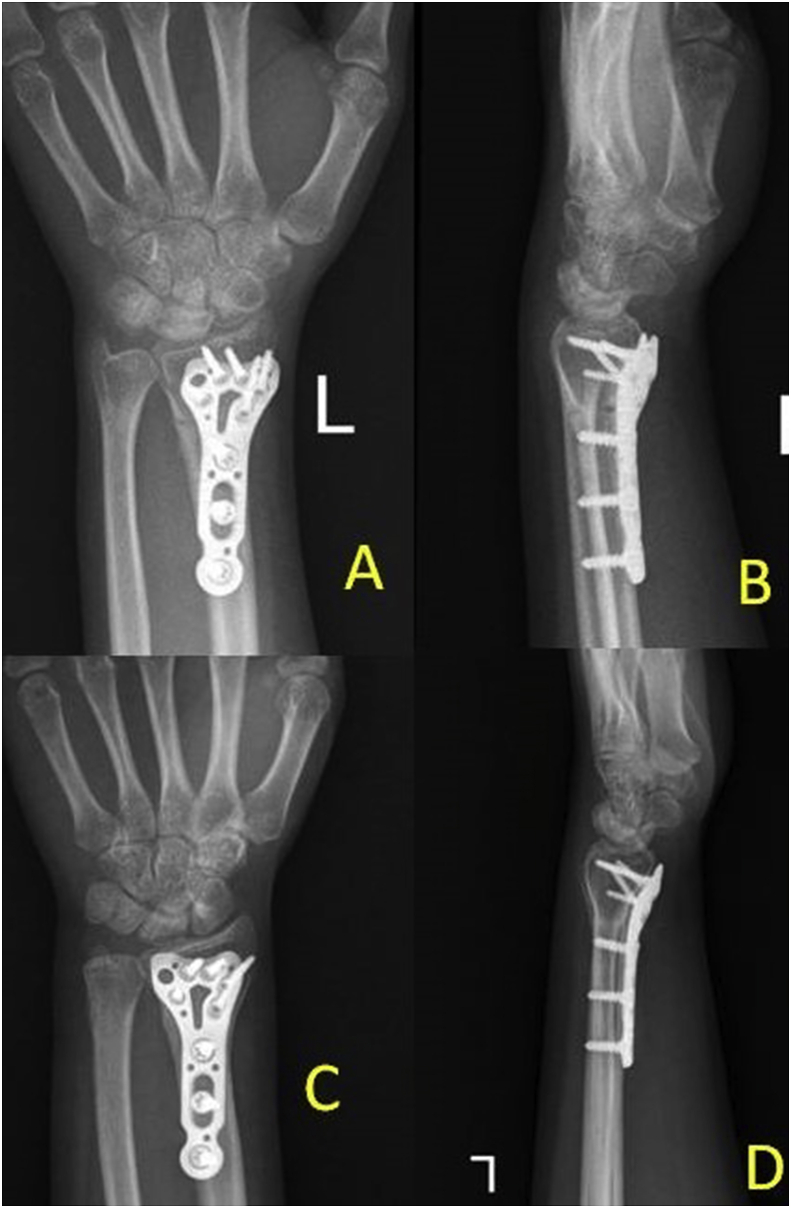
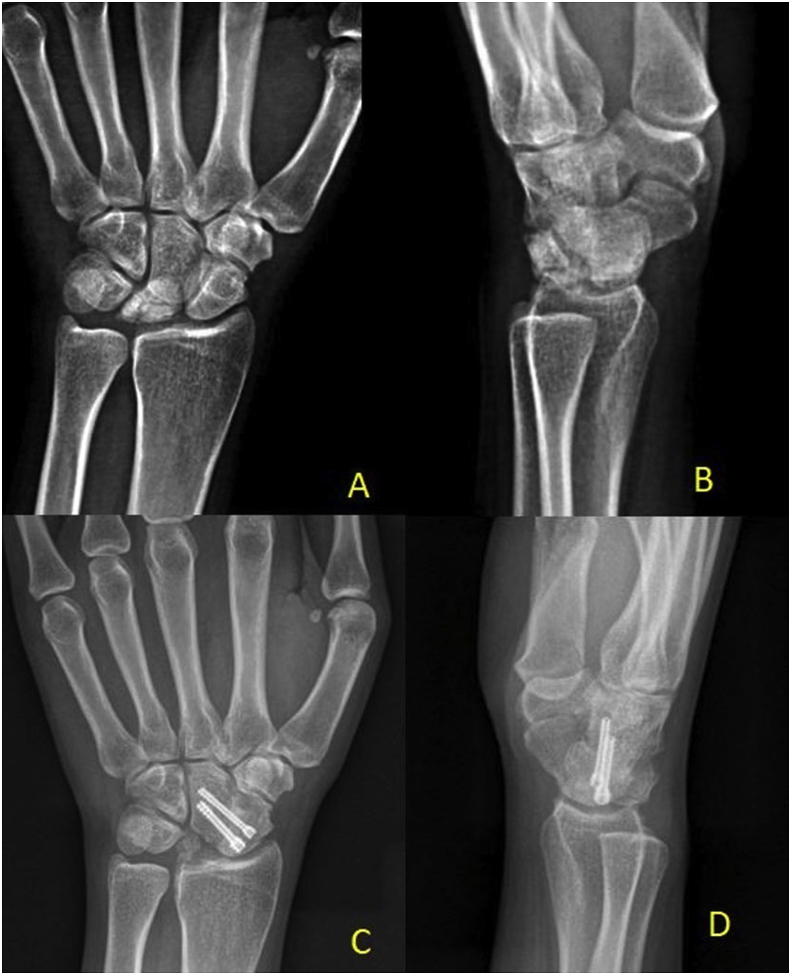
Fig. 3.
Immediate postoperative radiographs (A and B) of a 23 years female shows stage II Kienböck’s disease treated with radial shortening osteotomy. After a 1yearfollow-up, PA radiograph (C) and lateral radiograph (D) showed resolution of sclerosis and restoration of lunate height.
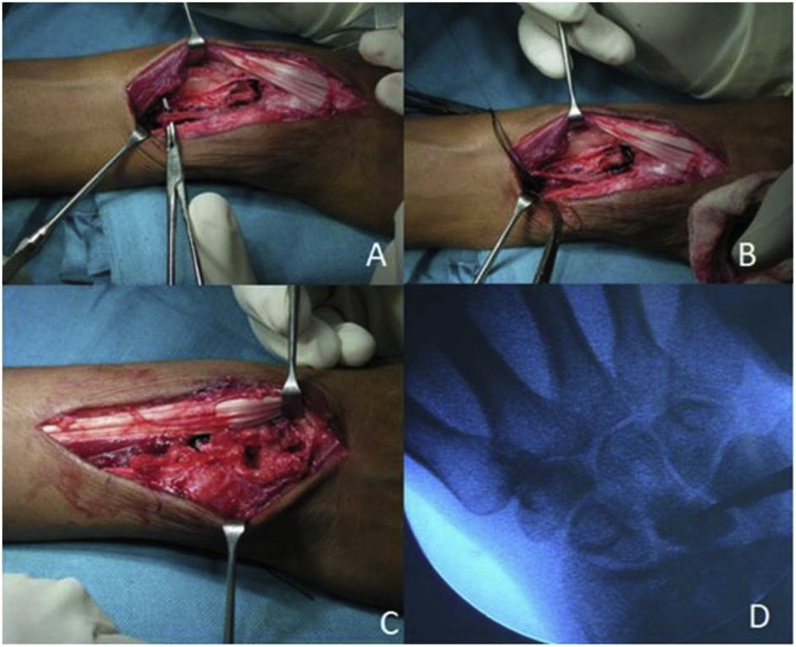
Fig. 4.
Intraoperative images show exposure and harvesting of 4-5thextensor compartment artery (ECA) based pedicle bone graft(A-C). Placement of graft to the necrotic lunate under fluoroscopic guidance (D).
Fig. 5.
Preoperative radiographs PA (A) and lateral (B) views show Stage IIIB Kienböck’s disease. Lunate Excision with Scaphocapitate Arthrodesis was done as cartilage changes were present in the capitate head. Postoperative radiographs(C and D) showed a union without arthritis at two years.
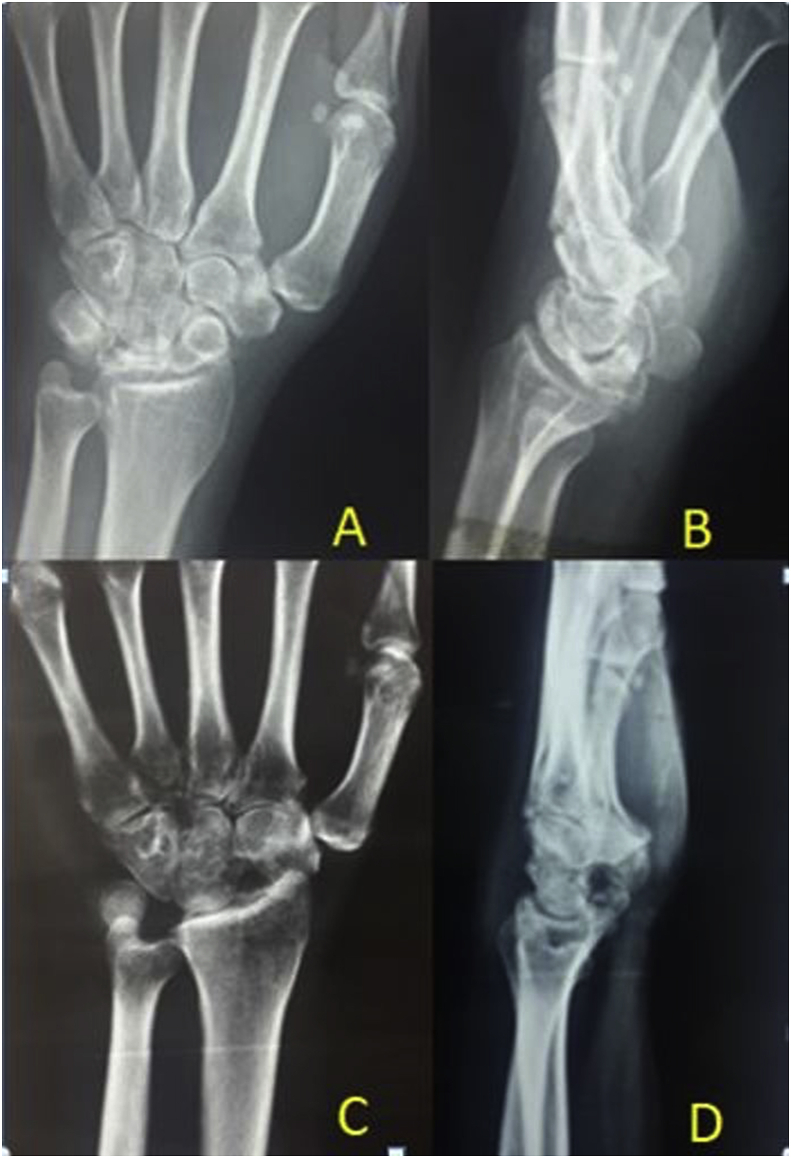
Fig. 7.
Preoperative radiographs, PA (A) and lateral (B) show Stage IIIB Kienböck’s disease. Proximal row carpectomy was done as capitates head was not arthritic. Radiographs (C and D) 2 years after surgery showed well alignment of the radio-capitate joint without degeneration.
To summarize, historically, Kienböck’s disease has been treated with radiology based on Lichtman’s classification. Guided by Osseous–Lichtman and vascular–Schmitt classification, we should use imaging modalities to plan the surgical treatment (Table 1), with the goals being healthy articular surface articulating with an acceptable range of motion.
Table 1.
Treatment of Kienböck’s disease.
| Osseous (Lichtman) | Vascular (Schmitt) | Principle | Technique |
|---|---|---|---|
| 0 I, II,IIIA | A,B | Unload Radiolunate | Immobilization, Joint leveling procedures Radial shortening osteotomy, Ulnar lengthening, capitate shortening |
| Revascularization | Vascularized bone graft core decompression, Radius Metaphyseal core decompression (MCD) | ||
| IIIB | B | Unload Radiolunate | Radial shortening osteotomy |
| Lunate excision + Bypass disease column/or proximal shift of capitates (restore lunate function) | SC fusion, STT fusion capitate osteotomy and transposition |
||
| fuse disease column | RSL fusion | ||
| proximal row carpectomy | |||
| IIIC | C | Lunate Salvage | Internal fixation |
| Lunate excision | Lunate replacement and interposition (tendon, silicon, pyrocarban prosthesis) | ||
| Lunate excision + Bypass disease column/or proximal shift of capitates (restore lunate function) | SC fusion, STT fusion capitate osteotomy and transposition |
||
| fuse disease column | RSL fusion | ||
| proximal row carpectomy | |||
| IV | C | Wrist Salvage | Wrist arthrodesis, wrist arthroplasty |
Funding statement
The authors received no financial support for research, authorship, and/or publication of this article.
Declaration of competing interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Acknowledgements
Nil.
References
- 1.Kienböck’s R. Concerning traumatic malacia of the lunate and its consequences: joint degeneration and compression. FortschGeb Roentgen. 1910;16:77–103. [Google Scholar]
- 2.Über MüllerW. Die Erweichung und Verdichtung des Os lunatum, einetypischeErkrankung des Handgelenks. BeitrKlinChi.r. 1920;119:664. [Google Scholar]
- 3.Irisarri C. Aetiology of Kienböck’s disease. J Hand Surg Br. 2004;29:281–287. doi: 10.1016/j.jhsb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 4.Lluch A., Garcia-Elias M. Etiology of Kienböck disease. Tech Hand Surg. 2011;15:33–37. doi: 10.1097/BTH.0b013e3182107329. [DOI] [PubMed] [Google Scholar]
- 5.Razemon J.P. Kienböck’s disease radiology. Ann Radiol (Paris) 1982;25:353–358. [PubMed] [Google Scholar]
- 6.Therkelsen F., Adersen K. Lunatomalacia. Acta Chir Scand. 1949;97:503–526. [PubMed] [Google Scholar]
- 7.Williams C.S., Gelberman R.H. Vascularity of the lunate: anatomic studies and implications for the development of osteonecrosis. Hand Clin. 1993;9:391–398. [PubMed] [Google Scholar]
- 8.Lamas C., Carrera A., Proubasta I. The anatomy and vascularity of the lunate: considerations applied to Kienböck’s disease. Chir Main. 2007;26:13–20. doi: 10.1016/j.main.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Kienböck R. Concerning traumatic malacia of the lunate and its consequences: degeneration and compression fractures. Clin Orthop Relat Res. 1980;149:4–8. [PubMed] [Google Scholar]
- 10.LAMA. Schuind F., Eslami S., Kienbock’s disease Ledoux P. J Bone Joint Surg(Br) 2008;90(2):133–139. doi: 10.1302/0301-620X.90B2.20112. [DOI] [PubMed] [Google Scholar]
- 11.Bain G.I., MacLean S.B., Yeo C.J., Perilli E., Lichtman D.M. The etiology and pathogenesis of Kienböck disease. J Wrist Surg. 2016 Nov;5(4):248–254. doi: 10.1055/s-0036-1583755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Irisarri C., Kalb K., Ribak S. Infantile and juvenile lunatomalacia. J Hand Surg Eur Vol. 2010;35(7):544–548. doi: 10.1177/1753193410364913. [DOI] [PubMed] [Google Scholar]
- 13.Geutjens G.G. Kienböck’s disease in an elderly patient. J Hand Surg Am. 1995;20(1):42–43. doi: 10.1016/S0363-5023(05)80056-5. [DOI] [PubMed] [Google Scholar]
- 14.Taniguchi Y., Yoshida M., Iwasaki H., Otakara H., Iwata S. Kienböck’s disease in elderly patients. J Hand Surg Am. 2003;28(5):779–783. doi: 10.1016/s0363-5023(03)00299-5. [DOI] [PubMed] [Google Scholar]
- 15.Yazaki N., Nakamura R., Nakao E. Bilateral Kienböck’s disease. J Hand Surg. 2005;30B:133–136. doi: 10.1016/j.jhsb.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 16.Hultén O. Concerning anatomical variations of the carpal bones. ActaRadiol. 1928;9:155–168. [Google Scholar]
- 17.D’Hoore K., De Smet L., Verellen K. Negative ulnar variance is not a risk factor for Kienböck’s disease. J Hand Surg. 1994;19A:229–231. doi: 10.1016/0363-5023(94)90010-8. [DOI] [PubMed] [Google Scholar]
- 18.Nakamura R., Tanaka Y., Imaeda T. The influence of age and sex on ulnar variance. J Hand Surg. 1991;16B:84–88. doi: 10.1016/0266-7681(91)90136-c. [DOI] [PubMed] [Google Scholar]
- 19.Stahl F. On lunatomalacia (Kienböck’s disease): a clinical and roentgenological study, especially on its pathogenesis and the late results of immobilization treatment. Acta Chir Scand. 1947;95(Suppl):3–73. [Google Scholar]
- 20.Chung K.C., Spilson M.S., Kim M.H. Is negative ulnar variance a risk factor for Kienböck’s disease? a meta-analysis. Ann Plast Surg. 2001;47:494–499. doi: 10.1097/00000637-200111000-00004. [DOI] [PubMed] [Google Scholar]
- 21.AntuñaZapico J.M. Kienböck’s disease. Rev Ortop Traumatol. 1993;37IB(suppl I):100–113. [Google Scholar]
- 22.Tsuge S., Nakamura R. Anatomical risk factors for Kienböck’s disease. J Hand Surg. 1993;18:70–75. doi: 10.1016/0266-7681(93)90201-p. [DOI] [PubMed] [Google Scholar]
- 23.ViegasSF, Wagner K., Patterson R., Peterson P. Medial (hamate) facet of the lunate. J Hand Surg Am. 1990;15(4):564–571. doi: 10.1016/s0363-5023(09)90016-8. [DOI] [PubMed] [Google Scholar]
- 24.Tatebe M., Shinohara T., Okui N., Yamamoto M., Kurimoto S., Hirata H. Arthroscopic lunate morphology and wrist disorders. Surg Radiol Anat. 2012;35:79–83. doi: 10.1007/s00276-012-0991-2. [DOI] [PubMed] [Google Scholar]
- 25.Xiong G., Xiao Z., Wang H., Guo S., Tao J. Microstructural study of the lunate in stage III Kienböck’s disease with micro-computed tomography imaging. J Hand Surg Eur. 2017;42(1):71–77. doi: 10.1177/1753193416664502. [DOI] [PubMed] [Google Scholar]
- 26.Cortles E. Uher die Entstehung der subrhondralenOsteonekrosen, A: die Lunatnmnekrose. HeitragezurklinischenChirurgie. 1930;19:28. [Google Scholar]
- 27.Lee M.H.L. The intraosseous arterial pattern of the carpal lunate bone and its relation to avascular necrosis. Acta Orthop Scand. 1963;33:43. doi: 10.3109/17453676308999833. [DOI] [PubMed] [Google Scholar]
- 28.Gelberman R.H., Bauman T.D., Menon J. The vascularity of the lunate bone and Kienböck’s disease. J Hand Surg. 1980;5A:272–278. doi: 10.1016/s0363-5023(80)80013-x. [DOI] [PubMed] [Google Scholar]
- 29.Schiltenwolf M., Martini A.K., Mau H.C., Eversheim S., Brocai D.R.C., Jensen C.H. Further investigations of the intraosseous pressure characteristics in necrotic lunates (Kienböck’s disease) J Hand Surg Am. 1996;21(5):754–758. doi: 10.1016/S0363-5023(96)80187-0. [DOI] [PubMed] [Google Scholar]
- 30.Jensen C.H. Intraosseous pressure in Kienböck’s disease. J Hand Surg Am. 1993;18(2):355–359. doi: 10.1016/0363-5023(93)90375-D. [DOI] [PubMed] [Google Scholar]
- 31.Kim S., Eichenauer F., Asmus A., Mutze S., Eisenschenk A., Honigmann P. Superselective angiography of the wrist in patients with Kienböck’s disease. BMC Musculoskelet Disord. 2019;20:143. doi: 10.1186/s12891-019-2492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Taniguchi Y., Tamaki T., Yoshida M. Kienböck’s disease in systemic lupus erythematosus. Hand Surg. 2002;7:197–200. doi: 10.1142/s0218810402001151. [DOI] [PubMed] [Google Scholar]
- 33.Lanzer W., Szabo R., Gelberman R. A vascular necrosis of the lunate and sickle cell anemia. A case report. Clin Orthop Relat Res. 1984:168–171. [PubMed] [Google Scholar]
- 34.Matsumoto A.K., Moore R., Alli P. Three cases of osteonecrosis of the lunate bone of the wrist in scleroderma. Clin Exp Rheumatol. 1999;17:730–732. [PubMed] [Google Scholar]
- 35.Mok C.C., Wong R.W., Lau C.S. Kienböck’s disease in rheumatoid arthritis. Br J Rheumatol. 1998;37:796–797. doi: 10.1093/rheumatology/37.7.796. [DOI] [PubMed] [Google Scholar]
- 36.Kahn S.J., Sherry D.D. Kienböck’s disease-avascular necrosis of the carpal lunate bone--in a 7-year-old girl with dermatomyositis. ClinPediatr (Phila) 1994;33:752–754. doi: 10.1177/000992289403301210. [DOI] [PubMed] [Google Scholar]
- 37.Chouhan D., Shankar V., Ansari M.T. Bilateral Kienböck’s disease concomitant with gouty arthritis. BMJ Case Reports CP. 2020;13 doi: 10.1136/bcr-2019-233725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lichtman D.M., Degnan G.G. Staging and its use in the determination of treatment modalities for Kienböck’s disease. Hand Clin. 1993;9(3):409–416. [PubMed] [Google Scholar]
- 39.Lichtman D.M., Lesley N.E., Simmons S.P. The classification and treatment of Kienböck’s disease: the state of the art and a look at the future. J Hand Surg Eur Vol. 2010;35(7):549–554. doi: 10.1177/1753193410374690. [DOI] [PubMed] [Google Scholar]
- 40.Bain G.I., Begg M. Arthroscopic assessment and classification of Kienböck’s disease. Tech Hand Up Extrem Surg. 2006;10(1):8–13. doi: 10.1097/00130911-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 41.Schmitt R., Kalb K. Imaging in Kienböck’s disease [in German] Handchir Mikrochir Plast Chir. 2010;42(3):162–170. doi: 10.1055/s-0030-1253433. [DOI] [PubMed] [Google Scholar]
- 42.Schmitt R., Heinze A., Fellner F., Obletter N., Strühn R., Bautz W. Imaging and staging of avascular osteonecroses at the wrist and hand. Eur J Radiol. 1997;25(2):92–103. doi: 10.1016/s0720-048x(97)00065-x. [DOI] [PubMed] [Google Scholar]
- 43.Lichtman D.M., Pientka W.F., II, Bain G.I. The future of Kienböck’s disease: a new algorithm. In: Lichtman D.M., Bain G.I., editors. Kienbock’s Disease: Advances in Diagnosis and Treatment. Springer; New York: 2016. pp. 307–320. [Google Scholar]
- 44.Jorge-Mora A., Pretell-Mazzini J., Marti-Ciruelos R., Andres-Esteban E.M., Curto de la Mano A. Distal radius definitive epiphysiodesis for management of Kienböcḱ’s disease in skeletally immature patients. Int Orthop. 2012;36(10):2101–2105. doi: 10.1007/s00264-012-1597-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dehghani M., Moshgelani M.A., Nouraei M.H., Dehghani S., Gholshahi M. Clinical outcomes of radial shortening osteotomy and vascularized bone graft in Kienböck’s disease. Int Sch Res Notices. 2014 Nov 9;2014:956369. doi: 10.1155/2014/956369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takahara M., Watanabe T., Tsuchida H., Yamahara S., Kikuchi N., Ogino T. Long-term follow-up of radial shortening osteotomy for Kienböck’s disease. Surgical technique. J Bone Joint Surg Am. 2009 Oct 1;91(Suppl 2):184–190. doi: 10.2106/JBJS.I.00315. [DOI] [PubMed] [Google Scholar]
- 47.Shin Y.H., Kim J.K., Han M., Lee T.K., Yoon J.O. Comparison of long term outcomes of radial osteotomy and nonoperative treatment for Kienböck’s disease: a systematic review. J Bone Joint Surg Am. 2018 Jul 18;100(14):1231–1240. doi: 10.2106/JBJS.17.00764. [DOI] [PubMed] [Google Scholar]
- 48.Botelheiro J.C., Silverio S., Neto A.L. Treatment of advanced Kienböck’s disease(Lichtman stage IIIB with carpal collapse) by a shortening osteotomy of the radius: 21 cases. J Wrist Surg. 2019;8(4):264–267. doi: 10.1055/s-0039-1688947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okubo H., Futenma C., Sunagawa H., Kinjo M., Kanaya F. Very distal radius wedge osteotomy for Kienböck’s disease: case series. J Hand Surg Asian Pac. 2017 Dec;22(4):490–496. doi: 10.1142/S0218810417500551. [DOI] [PubMed] [Google Scholar]
- 50.Camus E.J., Van Overstraeten L. Evaluation of Kienböck’s disease treated by Camembert osteotomy at seven years. J Wrist Surg. 2019 Jun;8(3):226–233. doi: 10.1055/s-0039-1683931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moritomo H., Murase T., Yoshikawa H. Operative technique of a new decompression procedure for Kienböck’s disease: partial capitates shortening. Tech Hand Up Extrem Surg. 2004;8(2):110–115. doi: 10.1097/01.bth.0000126571.20944.47. [DOI] [PubMed] [Google Scholar]
- 52.Horii E., Garcia-Elias M., An K.N. Effect on force transmission across the carpus in procedures used to treat Kienböck’s disease. J Hand Surg Am. 1990;15:393–400. doi: 10.1016/0363-5023(90)90049-w. [DOI] [PubMed] [Google Scholar]
- 53.Kataoka T., Moritomo H., Omokawa S., Iida A., Wada T., Aoki M. Decompression effect of partial capitate shortening for Kienböck’s disease: a biomechanical study. Hand Surg. 2012;17:299–305. doi: 10.1142/S0218810412500219. [DOI] [PubMed] [Google Scholar]
- 54.Werber K.D., Schmelz R., Peimer C.A., Wagenpfeil S., Machens H.-G., Lohmeyer J.A. Biomechanical effect of isolated capitate shortening in Kienböck’s disease: an anatomical study. J Hand Surg Eur. 2013;38:500–507. doi: 10.1177/1753193412458996. [DOI] [PubMed] [Google Scholar]
- 55.Citlak A., Akgun U., Bulut T., Tahta M., Dirim Mete B., Sener M. Partial capitate shortening for Kienböck’sdisease. J Hand Surg Eur. 2015;40:957–960. doi: 10.1177/1753193414562355. [DOI] [PubMed] [Google Scholar]
- 57.Illarramendi A.A., Schulz C., De Carli P. The surgical treatment of Kienböck’s disease by radius and ulna metaphyseal core decompression. J Hand Surg Am. 2001;26(2):252–260. doi: 10.1053/jhsu.2001.22928. [DOI] [PubMed] [Google Scholar]
- 58.Schulz C.U. Metaphyseal core decompression of the distal radius for early lunate necrosis. J Hand Surg Asian Pac. 2019 Sep;24(3):276–282. doi: 10.1142/S2424835519500346. [DOI] [PubMed] [Google Scholar]
- 59.Bain G.I., Smith M.L., Watts A.C. Arthroscopic core decompression of the lunate in early stage Kienböck’s disease of the lunate. Tech Hand Up Extrem Surg. 2011;15(1):66–69. doi: 10.1097/BTH.0b013e3181e1d2b4. [DOI] [PubMed] [Google Scholar]
- 60.Mehrpour S.R., Kamrani R.S., Aghamirsalim M.R., Sorbi R., Kaya A. Treatment of Kienböck’s disease by lunate core decompression. J Hand Surg Am. 2011;36(10):1675–1677. doi: 10.1016/j.jhsa.2011.06.024. [DOI] [PubMed] [Google Scholar]
- 61.Gabl M., Lutz M., Reinhart C. Stage 3 Kienböck’s disease: reconstruction of the fractured lunate using a free vascularized iliac bone graft and external fixation. J Hand Surg [Br] 2002;27:369–373. doi: 10.1054/jhsb.2002.0766. [DOI] [PubMed] [Google Scholar]
- 62.Tamai S., Yajima H., Ono H. Revascularization procedures in the treatment of Kienbock’s disease. Hand Clin. 1993;9:455–466. [PubMed] [Google Scholar]
- 63.Daecke W., Lorenz S., Wieloch P. Lunate resection and vascularized os pisiform transfer in Kienbock’s disease: an average of 10 years of follow-up study after Saffar’s procedure. J Hand Surg [Am] 2005;30:677–684. doi: 10.1016/j.jhsa.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 64.Saffar P. Vascularized pisiform transfer in place of lunatum for Kienböck’s disease [in French] Chir Main. 2010;29(Suppl 1):S112–S118. doi: 10.1016/j.main.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 65.Moran S.L., Cooney P., Berger R.A., Bishop A.T., Shin A.Y. The use of the 4 + 5 extensor compartmental vascularized bone graft for the treatment of Kienbock’s disease. J Hand Surg [Am] 2005;30:50–58. doi: 10.1016/j.jhsa.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 66.Yajima H., Ono H., Tamai S. Temporary internal fixation of the scaphotrapezio-trapezoidal joint for the treatment of Kienböck’s disease: a preliminary study. J Hand Surg Am. 1998;23(3):402–410. doi: 10.1016/S0363-5023(05)80457-5. [DOI] [PubMed] [Google Scholar]
- 67.Bürger H.K., Windhofer C., Gaggl A.J., Higgins J.P. Vascularized medial femoral trochlea osteocartilaginous flap reconstruction of proximal pole scaphoid nonunions. J Hand Surg Am. 2013;38(4):690–700. doi: 10.1016/j.jhsa.2013.01.036. [DOI] [PubMed] [Google Scholar]
- 68.Lin H.H., Stern P.J. Salvage” procedures in the treatment of Kienbock’s disease. Proximal row carpectomy and total wrist arthrodesis. Hand Clin. 1993;9(3):521–526. [PubMed] [Google Scholar]
- 69.Chim H., Moran S.L. Long-term outcomes of proximal row carpectomy: a systematic review of the literature. J Wrist Surg. 2012;1(2):141–148. doi: 10.1055/s-0032-1329547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lumsden B.C., Stone A., Engber W.D. J Hand Surg Am. 2008 Apr;33(4):493–502. doi: 10.1016/j.jhsa.2007.12.010. Treatment of advanced-stage Kienböck`sdisease with proximal row carpectomy: an average 15-year follow-up. [DOI] [PubMed] [Google Scholar]
- 71.Inglis A.E., Jones E.C. Proximal-row carpectomy for diseases of the proximal row. J Bone Joint Surg. 1977;59A:460–463. [PubMed] [Google Scholar]
- 72.Jorgensen E.C. Proximal-row carpectomy. An end-result study of twenty-two cases. J Bone Joint Surg. 1969;51A:1104–1111. [PubMed] [Google Scholar]
- 73.Tomaino M.M., Delsignore J., Burton R.I. Long-term results following proximal row carpectomy. J Hand Surg. 1994;19A:694–703. doi: 10.1016/0363-5023(94)90284-4. [DOI] [PubMed] [Google Scholar]
- 74.Ueba Y., Nosaka K., Seto Y. An operative procedure for advancedKienböck’s disease. Excision of the lunate and subsequent replacement with a tendon-ball implant. J Orthop Sci. 1999;4(3):207–215. doi: 10.1007/s007760050095. [DOI] [PubMed] [Google Scholar]
- 75.Lichtman D.M., Mack G.R., MacDonald R.I., Gunther S.F., Wilson J.N. Kienböck’s disease: the role of silicone replacement arthroplasty. J Bone Joint Surg Am. 1977;59(7):899–908. [PubMed] [Google Scholar]
- 76.Roca J., Beltran J.E., Fairen M.F., Alvarez A. Treatment of Kienböck’s disease using a silicone rubber implant. J Bone Joint Surg. 1976;58(3):373–376. Am. [PubMed] [Google Scholar]
- 77.Werthel J.D., Hoang D.V., Boyer P., Dallaudière B., Massin P., LoriautP Treatment of Kienböck’s disease using a pyrocarbon implant: case report [in French] Chir Main. 2014;33(6):404–409. doi: 10.1016/j.main.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 78.Bellemère P., Maes-Clavier C., Loubersac T., Gaisne E., KerjeanY Collon S. Pyrocarbon interposition wrist arthroplasty in the treatment of failed wrist procedures. J Wrist Surg. 2012;1(1):31–38. doi: 10.1055/s-0032-1323641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xie Mei-ming, Tang Kang-lai, Yuan Chen-song. 3D printing lunate prosthesis for stage IIIc Kienböck’s disease: a case report. Arch Orthop Trauma Surg. 2018;138(4):447–451. doi: 10.1007/s00402-017-2854-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Viljakka T., Tallroth K., Vastamäki M. Long-term clinical outcome after Titanium lunate arthroplasty for Kienböck’s disease. J Hand Surg Am. 2018 Oct;43(10) doi: 10.1016/j.jhsa.2018.02.009. 945.e1-945.e10. [DOI] [PubMed] [Google Scholar]
- 81.Chou J., Bacle G., Ek ETH. Tham S.K.Y. Fixation of the fractured lunate in Kienböck’s disease. J Hand Surg Am. 2019 Jan;44(1):67.e1–67.e8. doi: 10.1016/j.jhsa.2018.05.008. [DOI] [PubMed] [Google Scholar]
- 82.Bain G.I., Ondimu P., Hallam P. Radioscapholunate arthrodesis - a prospective study. Hand Surg. 2009;14(2- 3):73–82. doi: 10.1142/S021881040900427X. [DOI] [PubMed] [Google Scholar]
- 83.Garcia-Elias M., Lluch A., Ferreres A. Treatment of radiocarpal degenerative osteoarthritis by radioscapholunate arthrodesis and distal scaphoidectomy. J Hand Surg Am. 2005;30(1):8–15. doi: 10.1016/j.jhsa.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 84.Moy O.J., Peimer C.A. Scaphocapitate fusion in the treatment of Kienböck’s disease. Hand Clin. 1993 Aug;9(3):501–504. [PubMed] [Google Scholar]
- 85.Watson H.K., Ryu J., DiBella A. An approach to Kienbock’s disease: triscaphe arthrodesis. J Hand Surg Am. 1985;10(2):179–187. doi: 10.1016/s0363-5023(85)80101-5. [DOI] [PubMed] [Google Scholar]
- 86.Kleinman W.B., Carroll Ct. Scapho-trapezio-trapezoid arthrodesis for treatment of chronic static and dynamic scapho-lunate instability: a 10-year perspective on pitfalls and complications. J Hand Surg Am. 1990;15(3):408–414. doi: 10.1016/0363-5023(90)90051-r. [DOI] [PubMed] [Google Scholar]
- 87.Graner O., Lopes E.I., Carvalho B.C., Atlas S. Arthrodesis of the carpal bones in treatment ofKienböck’s disease, painful united fractures of the navicular and lunate bones with avascular necrosis, and old fractures dislocations of carpal bones. J Bone J Surg Am. 1966;48:767–774. [PubMed] [Google Scholar]
- 88.Lu L., Gong X., Wang K. Vascularized capitate transposition for advanced Kienböck’s disease: application of 40 cases and their anatomy. Ann Plast Surg. 2006;57:637–641. doi: 10.1097/01.sap.0000235425.03914.4f. [DOI] [PubMed] [Google Scholar]
- 89.Li Jianbing, Pan Zhijun, Zhao Yunzhen, Hu Xinlei, Zhao Xiang. Capitate osteotomy and transposition for type III Kienböck’s disease. J Hand Surg Eur. 2018;43(7):708–711. doi: 10.1177/1753193418780552. [DOI] [PubMed] [Google Scholar]