Abstract
Dupuytren’s contracture is a common condition that has the potential to be debilitating. It presents in a variety of manners and can be mild or more aggressive in its progression. There are a large number of management options currently available. In this review of the evidence, non-operative and operative management options are examined, with a consideration of post-operative rehabilitation and complications. A summary of the current concepts in the management of Dupuytren’s contracture is presented.
Keywords: Dupuytren’s contracture, Hand, Review, Collagenase, Surgery, Rehabilitation
1. Introduction
Dupuytren’s contracture is a fibroproliferative disease affecting the palmar aponeurosis of the hand. It commonly affects males between the ages of 40 and 80. Risk factors to developing Dupuytren’s disease include excessive alcohol intake, smoking, epilepsy, diabetes, manual labour, and hand trauma.1, 2, 3 Furthermore, there are a number of genes associated with Dupuytren’s contracture, with more aggressive disease patterns in those with a family history. Early onset (before 40), bilateral involvement, radial digit involvement, and involvement in other sites (known as ectopic disease, affecting areas such as the feet or penis) are also associated with aggressive disease (features of Dupuytren’s diathesis). It occurs secondary to myofibroblast proliferation at the affected palmar fascia, resulting in increased type III collagen, which replaces the normal type I collagen. The distinction between Dupuytren’s contracture and disease is that contracture is nomenclature reserved for those patients with affected function.4
1.1. Clinical presentation
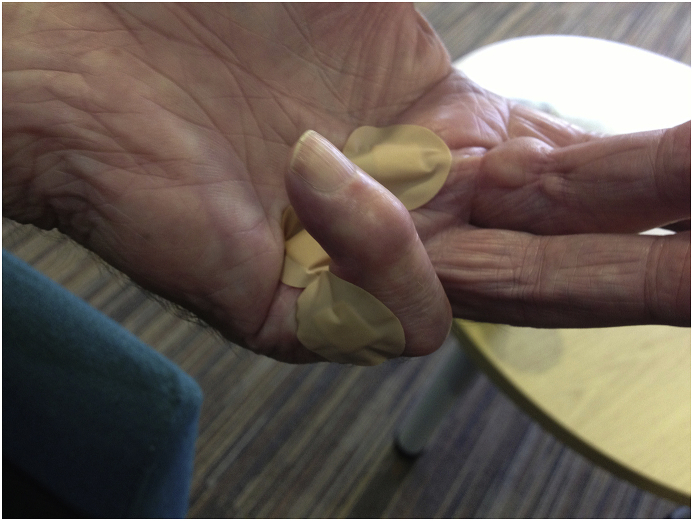
Dupuytren’s contracture presents as pitting and thickening of palmar skin, with the presence of firm, painless nodules, found adherent to skin and deep fascia.3 Cords follow nodules, which contract, leading to fixed flexion deformity of the affected digit (Fig. 1a, Fig. 1b, Fig. 1c). Joints commonly affected are the metacarpophalangeal (MCPJ), and proximal interphalangeal joints (PIPJ), with distal interphalangeal (DIPJ) joints rarely affected. Most common digits affected are the ring and middle, however all digits may be affected.5 In the presence of pain, the differential diagnoses to be excluded include trigger finger or osteoarthritis of the MCPJ and PIPJ.
Fig. 1a.
Dupuytren’s Cord.
Fig. 1b.
Demonstrating Hueston table top test.
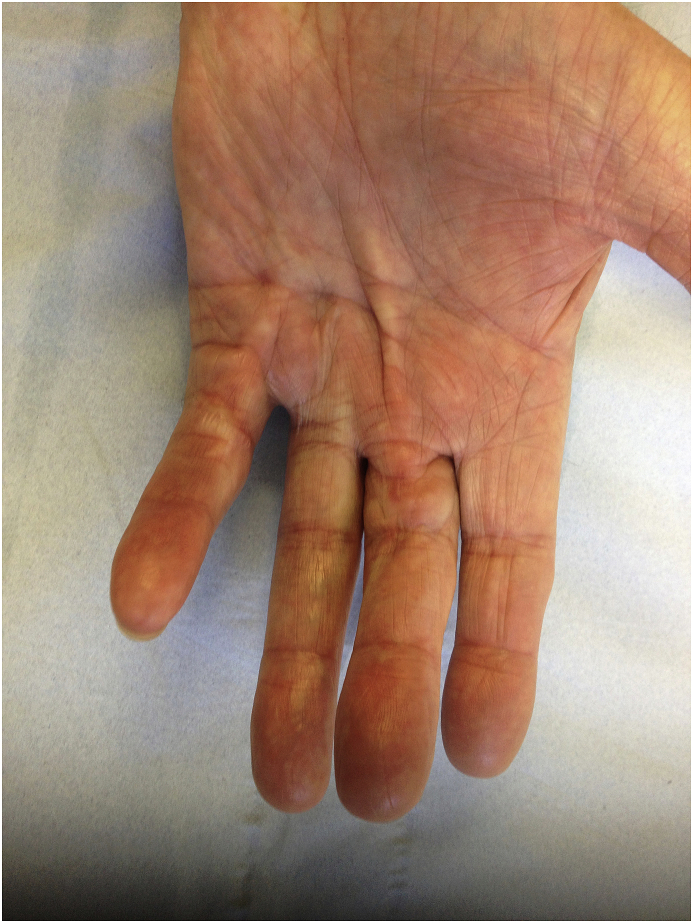
Fig. 1c.
Garrods pad.
[Fig. 1a, Fig. 1b, Fig. 1ca, b, 1c: Garrod’s pads & clinical picture of Dupuytren’s contracture and Table Top Test of Hueston].
1.2. Evaluation
Dupuytren’s contracture is a diagnosis made entirely clinically with patient history focussing on function and disruption of activities of daily living (such as difficulty washing, dressing and performing tasks in narrow spaces), family history, and key elements of social history (such as smoking and alcohol history). History of factors leading to a more aggressive disease pattern may help the clinician counsel the patient as to risk of recurrence following intervention.
Examination identifies the key features outlined before: cords, nodules, and digit(s) in fixed flexion. [Fig. 1a]. Examination of the MCPJ deformity is best achieved by flexing the PIPJ. Examination of the PIPJ deformity is best achieved by flexing the MCPJ. Hueston’s table top test [Fig. 1b] can highlight contractures, and is positive when the palm is unable to be placed flat on the table and may indicate the need for treatment.6 Another possible indication to begin treatment is a contracture that is greater than 30° at the MCPJ.7 Several authors cite that no amount of flexion deformity is acceptable at the interphalangeal joints (IPJ’s) due to the risk of lateral band contractures.8
Further elements of patient examination may uncover associated pathologies, such as Garrod’s pads causing calluses on the dorsal aspect of the IPJ’s of the hand [Fig. 1c], Lederhose disease resulting in hardening of the plantar fascia, and Peyronie’s disease causing curvature of the penis.
1.3. Pathoanatomy in Dupuytren’s contracture
In Dupuytren’s disease it is the normal anatomical facial bands of the palm that become diseased. Diseased bands become nodules and cords with nodules usually preceding the cords. Fascia in different regions of the hand is responsible for the formation of the different types of pathological cords; palmar fascia (pretendinous cords), palmodigital fascia (natatory and spiral cords) and digital fascia (central, lateral, digital and retrovascular cords). Shortened cords lead to joint deformity and flexion contractions.
Pretendinous cords are the most common cords and result in flexion contractures at the MCPJ. These cords are often continuous with digital cords.
The spiral cord is the most involved in digital disease resulting in PIPJ deformity. It is most commonly encountered in the little finger and less often the ring finger. Significantly, this cord is superficial to the neurovascular structures in the palm but as it passes the MCPJ it courses deep to these structures and insert into the lateral digital sheet. This is critical as it displaces the neurovascular structures palmar and centrally which makes them susceptible to injury during surgery.
The natatory cord develops from the natatory ligament. It is responsible for transformations of the U shaped webspace fibres into V shaped contractions within the 2nd, 3rd and 4th web-spaces.
The central cord continues from the pretendinous cord of the palm. It travels in the midline of the digits and attaches to the flexor tendon sheath over the PIPJ or the periosteum of the middle phalanx.
The lateral cord is derived from the lateral sheet and attaches to the flexor tendon or skin near Grayson’s ligament. Contraction of the lateral cord primarily causes flexion of the PIPJ but can also have an effect at the DIPJ. This cord can have a large cross-section and therefore displaces the neurovascular bundle.
The digital cord, also known as the adductor digiti minimi cord is an isolated digital cord which arises from the digiti minimi tendon. It travels superficial to the neurovasculature which can displace the bundle medially. It inserts in the majority of cases into the base of the base of the middle phalanx and occasionally the base of the distal phalanx causing flexion at the DIPJ.
The retrovascular cord is less well defined compared to the other cords. It is located deep to the neurovascular bundle and thought to arise from the retrovascular band of Thomine. Diseased retro-vascular tissue does not cause flexion deformities at the joints per se, however incomplete removal of this tissue may result in incomplete correction of PIPJ deformity.
Fig. 2: Cords and nodules causing Dupuytren’s contracture (author BS’s illustration).
Fig. 2.
Cords and nodules in Hand.
1.4. Classification
Luck’s classification attempts to classify Dupuytren’s disease based on its histological process and comprises of three stages: the proliferative stage (hypercellular, large myofibroblasts, immature fibroblasts, vascular, minimal extracellular matrix), involutional stage (dense network of myofibroblasts which align along tension lines, producing more collagen, an increased ratio of type 3 to type 1 collagen), and the residual stage (myoblasts no longer seen, and fibroblasts the predominant cell type, collagen dense tissue).
2. Management
2.1. Principles
There is no absolute cure for Dupuytren’s contracture. The primary aim of management is to control and correct the deformity and prevent neurovascular injury. This may be achieved by excision, breakage, or dissolution of the fibrous cord, allowing extension of the affected digit and thereby improving hand function. Unfortunately, not all Dupuytren’s tissue is possible to be excised, and thus there is always a risk of disease recurrence, either at the same site, or in another site altogether.7
The management is based on the severity of deformity, level of deformity, digits involved and the patient’s general condition.
2.2. Non-operative management
2.2.1. Observation
Dupuytren’s contracture is essentially benign, and so observation may be a treatment option, in light of benefits and risks of treatment, and the patient’s decision based on their function. Ideally management is undertaken before the disease process is too severe (usually an MCPJ contracture of >30° or PIPJ contracture >15°),7 resulting in finger contractures unlikely to recover despite invasive surgery.
2.2.2. Physiotherapy
Options include night or extension splinting, stretching exercises, and friction massage. There have been a number of studies examining the effect of physiotherapy on disease modification both in isolation, and as an adjunct to surgery. One study showed an improvement 80% of their patients with night extension splints,9 ranging from 2 to 12° improved range. A further study applied splinting and soft tissue mobilisation techniques to 12 patients with PIPJ contractures specifically, showing improvement in range of motion (5°-25°) over approximately 13 months of therapy.10
2.2.3. Pharmacological therapies
Several pharmacological therapies have been discussed in the literature. These include steroids, vitamin E, Aminosyn®, and hyperbaric oxygen. None of these have shown to provide sustainable correction.
One study assessed the effect of the steroid triamcinolone acetonide on 75 hands over a 4-year period.11 Patients with early disease (MCPJ <15°, normal IPJ) were selected. Steroid was injected directly into lesions, with 97% disease regression found following an average of 3.2 injections. However, 50% of patients experienced recurrence within 3 years.
Vitamin E was hypothesised to downregulate fibroblast activity converting type I collagen into pathological type III collagen.12 Despite this, there is no recent evidence to support the use of vitamin E by itself to treat or prevent Dupuytren’s contracture.
It has been hypothesised that local ischaemic changes can affect the palmar fascia, leading to free radical formation, and the conversion of fibroblasts to myofibroblasts.13 On this basis, a histological study examining the effect of different oxygen environments on both healthy and Dupuytren’s tissue samples.14 No difference was found between the different oxygen environments on the tissue samples (hypoxic, normoxic, hyperoxic).
2.2.4. Radiotherapy
Radiotherapy has been hypothesised to inhibit fibroblast proliferation and thus have an anti-inflammatory effect, preventing worsening of the pathological process.15 A recent review identified six studies, with 698 patients in total, and 770 irradiated hands.16 Majority of radiotherapy dosage was 30 Gy, with number of factions ranging from 2 to 14.93% of patients were Tubiana stage N or I. A statistically significant number had improvements in subjective and objective measure (skin fixation and retraction, extension deficit, progression rates) in the short term (up to 1 year). 3–10% required further surgery. This suggests that radiotherapy may be beneficial in early stages of disease, however there is limited long-term follow-up and a lack of control groups. It should be noted also that radiotherapy is associated with a high incidence of acute (erythema; 20–40%) and chronic (skin atrophy; less than 10%) side effects, and a low incidence of malignancy (0.02%).16
2.2.5. Collagenase
Clostridium histolyticum is a gram-positive bacterium, which produces a number of toxins, amongst which includes collagenase. It causes lysis of pathological type III collagen in cords, thus reversing the effect of contractures (Fig. 3a, Fig. 3b, Fig. 3c, Fig. 3d).17 The use of collagenase Clostridium histolyticum to treat Dupuytren’s increased after the results of the POINT X study,18 which looked at 254 European patients. 93% of fingers treated were little or ring. Mean improvement was 34° by day 1, and 42° by day 7. This improvement was maintained at 6 months, with an average of 1.2 injections required per patient, and median recovery of 4 days. Patient satisfaction was reported as 87%. A further high-quality study with 66 patients compared a collagenase injection with a placebo found significant improvement (improvement to within 0–5°of normal in all joints) in the collagenase group.19 Only one study describes long-term results: the CORDLESS study (5-year follow up).20 This showed 291 of 623 (47%) initially treated patients had a recurrence of symptoms (defined as worsening of contracture of 20° or greater). Although high, this is comparable to recurrence rates post-surgery. The main adverse effect of collagenase treatment is skin atrophy [Fig. 3a, Fig. 3b, Fig. 3c, Fig. 3da, b, 3c, 3d: Post collagenase injections, manipulation under anaesthetic, post healing and correction].
Fig. 3a.
After collagenase injection.
Fig. 3b.
After collagenase injection.
Fig. 3c.
After manipulation showing skin tear.
Fig. 3d.
8 weeks post injection and manipulation showing good skin healing.
2.3. Operative management
2.3.1. Needle fasciotomy
This procedure involves percutaneous division of fascial bands using a hypodermic needle. Advantages of this procedure are that it can be done under local anaesthetic in an outpatient setting making it very cost effective and as it is minimally invasive.21 Disadvantages are that it does not remove the pathological tissue and it is not effective in severe disease.20 Due to the percutaneous nature of the procedure neurovascular bundles are at risk of injury however one recent study has reported no significant neurovascular damage in 54 patients and only one transient dysesthesia.22 The main issue for this technique is the significantly high recurrence rate ranging from 9 to 75% at five years.23,24
Needle fasciotomy is generally agreed to have a role in managing MCPJ contractures, however it is less favorable in cords causing PIPJ contractures due to inability to reproducibly divide these cords and their proximity to the digital nerves.7
One study compared the early and the late outcomes of needle fasciotomy versus open fasciectomy.23,25 It found that patient recorded outcome measure were greater in the short term in the needle fasciectomy group compared to the open fasciotomy. It also found in the needle fasciectomy group had better correction of milder contractures.
2.3.2. Limited fasciectomy
This is a widely used open procedure done in theatre usually under general anaesthetic or regional anaesthesia. The aim is to remove all of the cord causing the deformity. There is a significant risk of recurrence of the disease quoted up to 20% as well as stiffness of diseased and un-diseased digits.7,23 Intensive and prolonged rehabilitation can go some way to negating these risks.
A segmental fasciectomy technique has been described by Moermans whereby small segments of the cords are excised via a series of small incisions allowing the digit to extend.26 Advantages of this technique are that it is a quick procedure, less invasive and requires a short rehabilitation period. However, a recurrence rate of up to 38% has been reported long term.27 It is more effective in releasing MCPJ contractures than PIPJ contractures.7
When compared to needle fasciotomy, fasciectomy is shown to have reduced recurrence rates, 20.9% vs 84.9% at 5 years, better correction of more severe deformities, higher patient recorded outcome measures at 5 years and better hand function.24,26
There is no evidence that surgical approach; either Brunner’s or Z-plasty with this technique has an effect on rates of recurrence, extension or complication rates at 2 years post-surgery.28
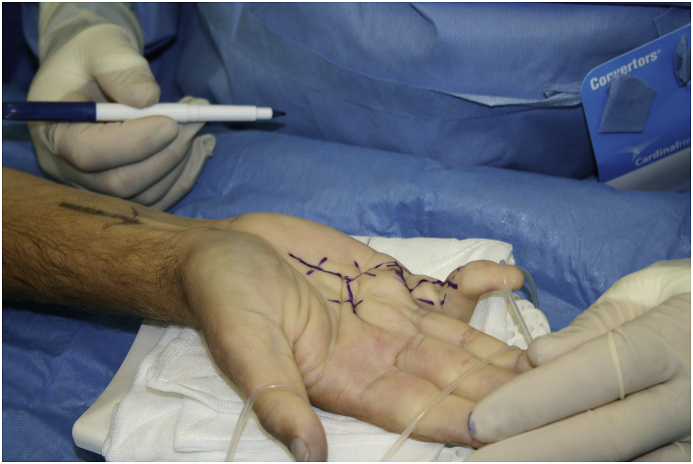
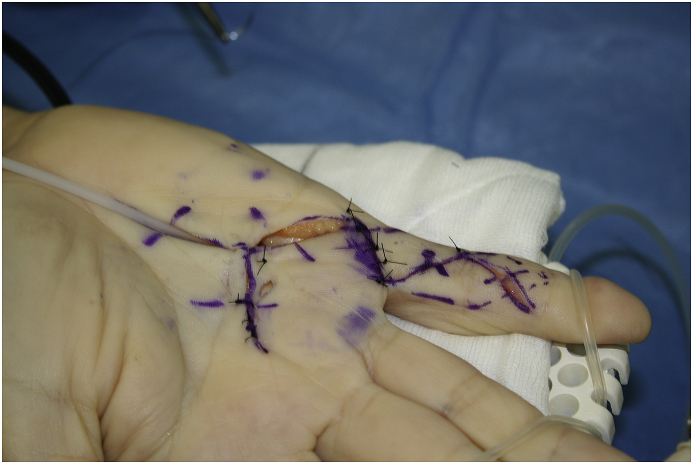
[Fig. 4a, Fig. 4b, Fig. 4ca, b, 4c: Pre, intra, and post-operative images, limited fasciectomy.]
Fig. 4a.
Pre surgery.
Fig. 4b.
Brunner’s Type skin incision.
Fig. 4c.
After excision of Dupuytren’s Tissue.
2.3.3. Dermofasciectomy
This is the most involved on the listed procedures, whereby all the pathological cord is excised as well as the subcutaneous fat and skin on the volar surface of the hand overlying the cord. The remaining tendon and digital neurovascular bundles are then covered using a full thickness skin graft generally harvested from the forearm or upper arm. Theoretical advantage of this approach is that because the overlying diseased tissue and pathological cells are removed therefore the risk of recurrence is less.29 Indications for this procedure include preferential use in young patients with Dupuytren’s diathesis. Disadvantages include donor and recipient graft site morbidity, longer rehabilitation and chronic regional pain syndrome (CRPS).7
Ullah compared results of fasciectomy alone to firebreak dermofasciectomy. Results showed that no significant difference between the procedures in regard to patient recorded outcome measures, grip strength, angular deformities or recurrence. Adverse effects were again similar with no differences in wound complications or CRPS. It was noted that the fasciectomy group experienced significantly less ulnar sided hypoparaesthesia.30
2.3.4. Salvage
In a patient in whom there is advanced or recurring disease and further surgery carries a risk of poor outcome or unacceptable risk, salvage procedures such as digital amputation or arthrodesis may also be considered. This results in shortening of the finger but avoids recurrence.31
3. Rehabilitation following surgery
There still remains some debate as to the best postoperative protocol for patients undergoing surgery for Dupuytren’s contracture. There is limited high quality evidence in this area. Rehabilitation goals are to reduce swelling, increase function and reduce recurrence in the long term.
Studies have looked at different types of post-operative dressings. At seven days post-op intermittent compression dressings have been shown to reduce hand swelling and oedema when compared to a continuous compression using a boxing glove type dressing and roller towel elevation.32 Other advantages of intermittent compression included decreased analgesia requirements and earlier return to normal hand function.
Evidence supporting the use of splints following surgery is equivocal. Most of the evidence supporting the use of splints is historic. Three RCTs look at the use of postoperative splinting. Kemler investigated hand therapy and splinting versus hand therapy alone and found that there was no evidence that splinting reduced recurrence and there was a trend toward decreased finger flexion due to immobilisation.33 The decreased flexion was hypothesised to be due to an abnormal pattern of scarring when the fingers are held in extension compared to more functional laying down of scar tissue when the fingers are allowed to mobilise. The main reported adverse effect of splinting was that of patient discomfort. Collis investigated night splinting and hand therapy versus hand therapy alone at 3 months post op.34 This group found no difference in maintaining finger extension and no difference in secondary outcome measures such as total finger flexion between the two treatment arms. A similar study by Jerosch-Herold showed no difference between DASH scores or patient satisfaction at 12 months when splinting was implemented.35 Splinting is now falling out of favour in many rehab protocols as evidence behind their use is limited. However, it should be noted that none of these studies had prolonged follow up periods.
3.1. Complications of surgery
Bleeding/haematoma, delayed wound healing, neuropraxia affecting digits, nerve, tendon, and vessel injury, infection, disease recurrence, and CRPS have all been described.36 A recent review found an overall complication rate of 17% in 71 fasciectomy patients, 19% in 16 needle fasciotomy patients, and 12% in 11 dermofasciectomy patients.36 Greatest numbers of nerve and vessel injuries were seen in fasciectomy patients with late stage disease. Lowest recurrence was found following dermofasciectomy and skin grafting, and higher rates after collagenase injections, and partial fasciectomy.
Needle fasciotomy has been shown to have a higher re-operation rate than limited fasciectomy or dermofasciectomy, however overall fewer complications.37 It should be noted that re-operation is not a surrogate for recurrence – the patient would require to be suitable for further surgery, thus suggesting that less invasive methods of initial treatment may be more amenable to surgery for recurrence.
4. Conclusions
Dupuytren’s disease is a common disease that can have significant impact on function. The treatment options are varied, and the decision should be based on patient-factors and expectations. Amongst the many non-operative options available, collagenase treatment appears to have the most robust evidence for success. There is a lack of high-quality evidence to support use of corticosteroid injections, radiotherapy, and other methods of non-operative management alone.
Needle fasciotomy has a role in MCPJ mild disease and may be chosen by the patient who wishes to take a smaller risk for post-operative complications, in the knowledge of a high recurrence rate. Partial fasciectomy remains the surgical treatment of choice but will be rivalled by collagenase injections as the evidence base grows for the latter, mainly due to a lower risk profile.
There is a lack of high-quality evidence regarding post-operative rehabilitation, however the trend is towards intermittent compression and active mobilisation, and away from splinting. Night-time splinting does not improve long term outcomes.
Regarding complications of surgery, quantifying complications amongst large collated patient groups is limited: studies use differing definitions of what constitutes a specific complication, and many don’t report recurrence, it being a long-term complication. Furthermore, patient satisfaction may not be affected by objective measures, such as degrees of finger extension, and function and patient lifestyle-factors are key to assess.
Thus doctor-patient discussions on Dupuytren’s contracture management should discuss options, certainly those with more of an evidence-base, but factor in patient wishes regarding how much function they expect to regain or maintain, how invasive they would accept their treatment to be, and the risks and rates of recurrence they would accept. Patients may choose their management based on the information currently available to optimise outcomes. Meanwhile, further trials with collaborative groups are likely to produce the high-power studies required to direct clinicians with more confidence in available management options.
CRediT authorship contribution statement
Agneish Dutta: Writing - original draft, Writing - review & editing. Gihan Jayasinghe: Writing - original draft, Writing - review & editing. Saurabh Deore: Writing - original draft. Karim Wahed: Writing - original draft. Kavynash Bhan: Writing - original draft. Nik Bakti: Supervision, Writing - review & editing. Bijayendra Singh: Supervision, Writing - review & editing.
Declaration of competing interest
All authors declare no conflict of interest relating to the article: ‘Dupuytren’s Contracture – Current Concepts’.
Contributor Information
Agneish Dutta, Email: agneishd@gmail.com.
Gihan Jayasinghe, Email: gihan.jayasinghe@nhs.net.
Saurabh Deore, Email: sdsaurabhdeore@gmail.com.
Karim Wahed, Email: karim.wahed4@gmail.com.
Kavynash Bhan, Email: kavyansh.bhan@nhs.net.
Nik Bakti, Email: nik.bakti@gmail.com.
Bijayendra Singh, Email: bijayendra.singh@nhs.net, bijayendrasingh@gmail.com.
References
- 1.Geoghegan J.M., Forbes J., Clark D.I., Smith C., Hubbard R. Dupuytren’s disease risk factors. J Hand Surg. 2004;29(5):423–426. doi: 10.1016/j.jhsb.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Godtfredsen N.S., Lucht H., Prescott E., Sørensen T.I., Grønbæk M. A prospective study linked both alcohol and tobacco to Dupuytren’s disease. J Clin Epidemiol. 2004;57(8):858–863. doi: 10.1016/j.jclinepi.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Townley W.A., Baker R., Sheppard N., Grobbelaar A.O. Dupuytren’s contracture unfolded. BMJ. 2006;332(7538):397–400. doi: 10.1136/bmj.332.7538.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nordenskjöld J., Englund M., Zhou C., Atroshi I. Prevalence and incidence of doctor-diagnosed Dupuytren’s disease: a population-based study. J Hand Surg. 2017;42(7):673–677. doi: 10.1177/1753193416687914. [DOI] [PubMed] [Google Scholar]
- 5.Mikkelsen O.A. Dupuytren’s disease—a study of the pattern of Distribution and stage of contracture in the hand. Hand. 1976;8(3):265–271. doi: 10.1016/0072-968x(76)90013-9. [DOI] [PubMed] [Google Scholar]
- 6.Hueston J.T. The tabletop test. Hand. 1982;(1):100–103. doi: 10.1016/s0072-968x(82)80053-3. [DOI] [PubMed] [Google Scholar]
- 7.Rodrigues J.N., Becker G.W., Ball C. Surgery for Dupuytren’s contracture of the fingers. Cochrane Database Syst Rev. 2015;12 doi: 10.1002/14651858.CD010143.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.British Society for Surgery of the Hand. BSSH Evidence for Surgical Treatment (BEST). 1: Dupuytren’s Disease. http://www.bssh.ac.uk/education/guidelines/dd_guidelines.pdf accessed 29 December 2019.
- 9.Ball C., Nanchahal J. The use of splinting as a non-surgical treatment for Dupuytren’s disease: a pilot study. Br J Hand Ther. 2002;7:76–78. [Google Scholar]
- 10.Larocerie-Salgado J., Davidson J. Nonoperative treatment of PIPJ flexion contractures associated with Dupuytren’s disease. J Hand Surg Eur. 2012;37:722–727. doi: 10.1177/1753193411422680. [DOI] [PubMed] [Google Scholar]
- 11.Ketchum L.D., Donahue T.K. The injection of nodules of Dupuytren’s disease with triamcinolone acetonide. J Hand Surg. 2000;25(6):1157–1162. doi: 10.1053/jhsu.2000.18493. [DOI] [PubMed] [Google Scholar]
- 12.Steinberg C.L. Tocopherols in treatment of primary fibrositis; including Dupuytren’s contracture, periarthritis of the shoulders, and Peyronie’s disease. Arch Surg. 1951;63:824–833. doi: 10.1001/archsurg.1951.01250040840013. [DOI] [PubMed] [Google Scholar]
- 13.Murrell G.A., Francis M.J., Howlett C.R. Dupuytren’s contracture. Fine structure in relation to aetiology. The Journal of bone and joint surgery. British volume. 1989;71(3):367–373. doi: 10.1302/0301-620X.71B3.2722922. [DOI] [PubMed] [Google Scholar]
- 14.Türker T., Murphy E., Kaufman C.L., Kutz J.E., Meister E.A., Hoying J.B. Response of dupuytren’s fibroblasts to different oxygen environments. J Hand Surg. 2013;38(12):2365–2369. doi: 10.1016/j.jhsa.2013.08.122. [DOI] [PubMed] [Google Scholar]
- 15.Arenas M. Anti-inflammatory effects of low-dose radiotherapy: Indications, dose and radiobiological mechanisms involved. Rep Practical Oncol Radiother. 2013;18:S12–S13. doi: 10.1007/s00066-012-0170-8. [DOI] [PubMed] [Google Scholar]
- 16.Kadhum M., Smock E., Khan A., Fleming A. Radiotherapy in Dupuytren’s disease: a systematic review of the evidence. J Hand Surg. 2017;42(7):689–692. doi: 10.1177/1753193417695996. [DOI] [PubMed] [Google Scholar]
- 17.Hurst L.C., Badalamente M.A., Hentz V.R. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361(10):968–979. doi: 10.1056/NEJMoa0810866. [DOI] [PubMed] [Google Scholar]
- 18.Warwick D., Arner M., Pajardi G. Collagenase Clostridium histolyticum in patients with Dupuytren’s contracture: results from POINT X, an open-label study of clinical and patient-reported outcomes. J Hand Surg. 2015;40(2):124–132. doi: 10.1177/1753193413519926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gilpin D., Coleman S., Hall S., Houston A., Karrasch J., Jones N. Injectable collagenase Clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg. 2010;35(12):2027–2038. doi: 10.1016/j.jhsa.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Peimer C.A., Blazar P., Coleman S., Kaplan F.T.D., Smith T., Lindau T. Dupuytren’s contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS [Collagenase Option for Reduction of Dupuytren’s Long-Term Evaluation of Safety Study]): 5-year data. J Hand Surg. 2015;40(8):1597–1605. doi: 10.1016/j.jhsa.2015.04.036. [DOI] [PubMed] [Google Scholar]
- 21.Mella J.R., Guo L., Hung V. Dupuytren’s contracture: an evidence based review. Ann Plast Surg. 2018;81(6S):S97–S101. doi: 10.1097/SAP.0000000000001607. [DOI] [PubMed] [Google Scholar]
- 22.Moog P., Buchner L., Cerny M.K., Schmauss D., Megerle K., Erne H. Analysis of recurrence and complications after percutaneous needle fasciotomy in Dupuytren’s disease. Arch Orthop Trauma Surg. 2019;139(10):1471–1477. doi: 10.1007/s00402-019-03247-y. [DOI] [PubMed] [Google Scholar]
- 23.Van Rijssen A.L., Gerbrandy F.S., Ter Linden H., Klip H., Werker P.M. A comparison of the direct outcomes of percutaneous needle fasciotomy and limited fasciectomy for Dupuytren’s disease: a 6-week follow-up study. J Hand Surg. 2006;31(5):717–725. doi: 10.1016/j.jhsa.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Pereira A., Massada M., Sousa R., Silva C., Trigueiros M., Lemos R. Percutaneous needle fasciotomy in Dupuytren’s contracture: is it a viable technique. Acta Orthop Belg. 2012;78(1):30–34. [PubMed] [Google Scholar]
- 25.Van Rijssen A.L., Ter Linden H., Werker P.M. Five-year results of a randomized clinical trial on treatment in Dupuytren’s disease: percutaneous needle fasciotomy versus limited fasciectomy. Plast Reconstr Surg. 2012;129(2):469–477. doi: 10.1097/PRS.0b013e31823aea95. [DOI] [PubMed] [Google Scholar]
- 26.Moermans J.P. Segmental aponeurectomy in Dupuytren’s disease. J Hand Surg. 1991;16(3):243–254. doi: 10.1016/0266-7681(91)90047-r. [DOI] [PubMed] [Google Scholar]
- 27.Moermans J.P. Long-term results after segmental aponeurectomy for Dupuytren’s disease. J Hand Surg. 1996;21B(6):797–800. doi: 10.1016/s0266-7681(96)80195-1. [DOI] [PubMed] [Google Scholar]
- 28.Citron N., Nunez V. Recurrence after surgery for Dupuytren’s disease: a randomized trial of two skin incisions. J Hand Surg Br. 2005;30:563–566. doi: 10.1016/j.jhsb.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Armstrong J.R., Hurren J.S., Logan A.M. Dermofasciectomy in the management of Dupuytren’s disease. J Bone Joint Surg. 2000;82(1):90–94. doi: 10.1302/0301-620x.82b1.9808. [DOI] [PubMed] [Google Scholar]
- 30.Ullah A.S., Dias J.J., Bhowal B. Does a ’firebreak’ full-thickness skin graft prevent recurrence after surgery for Dupuytren’s contracture? A prospective, randomised trial. J Bone Joint Surg. 2009;91(3):374–378. doi: 10.1302/0301-620X.91B3.21054. [DOI] [PubMed] [Google Scholar]
- 31.Calandruccio J.H. Dupuytren’s contracture. In: Azar F.M., Beaty J.H., Canale S.T., editors. Campbell’s Operative Orthopaedics. Elsevier; St Louis, MO: 2017. pp. 3734–3749. [Google Scholar]
- 32.Hazarika E.Z., Knight M.T., Frazer-Moodie A. The effect of intermit- tent pneumatic compression on the hand after fasciectomy. Hand. 1979;11:309–314. doi: 10.1016/s0072-968x(79)80056-x. [DOI] [PubMed] [Google Scholar]
- 33.Kemler M.A., Houpt P., van der Horst C.M. A pilot study assessing the effectiveness of postoperative splinting after limited fasciectomy for Dupuytren’s disease. J Hand Surg Eur. 2012;37:733–737. doi: 10.1177/1753193412437631. [DOI] [PubMed] [Google Scholar]
- 34.Collis J., Collocate S., Hing W., Kelly E. The effect of night extension orthoses following surgical release of Dupuytren’s contracture: a single-center, randomized, controlled trial. J Hand Surg Am. 2013;38:1285–1294.e2. doi: 10.1016/j.jhsa.2013.04.012. [DOI] [PubMed] [Google Scholar]
- 35.Jerosch-Herold C., Shepstone L., Chojnowski A.J., Larson D., Barrett E., Vaughan S.P. Night-time splinting after fasciectomy or dermo- fasciectomy for Dupuytren’s contracture: a pragmatic, multi-centre, randomised controlled trial. BMC Muscoskel Disord. 2011;12:136. doi: 10.1186/1471-2474-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krefter C., Marks M., Hensler S., Herren D.B., Calcagni M. Complications after treating Dupuytren’s disease. A systematic literature review. Hand surgery and rehabilitation. 2017;36(5):322–329. doi: 10.1016/j.hansur.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 37.Rodrigues J.N., Zhang W., Scammell B.E. Functional outcome and complications following surgery for Dupuytren’s disease: a multi-centre cross-sectional study. J Hand Surg. 2017;42(1):7–17. doi: 10.1177/1753193416660045. [DOI] [PubMed] [Google Scholar]