Abstract
LDL cholesterol is by far the best established “causal” cardiovascular risk. It is distributed normally, and the mean value ranges around 100∼120 mg/dl. In terms of preventive cardiology, we now know very well that the lower the LDL cholesterol, the better. Clinical usefulness of aggressive LDL-lowering therapies using statin, ezetimibe, and proprotein convertase subtilisin-kexin type 9 (PCSK9) inhibitors have been shown in primary and in secondary prevention settings. Additionally, the idea, based on recent randomized controlled trials (RCT), that the lower LDL cholesterol the better appears to be true for LDL as low as ∼ 30 mg/dl. According to those data, recent guidelines in Europe and in Japan suggest the lowering of LDL cholesterol level < 70 mg/dl for high-risk patients. However, the attainment rates of such “strict” goals seem to be quite low, probably because most cardiologists still have a sense of anxiety of “low” LDL cholesterol level. But “low” indicates no more than “lower” than the “average” range, which is not always implying the optimal range. Additionally, Mendelian randomization studies focusing on individuals exhibiting “low” LDL cholesterol suggest that “normal” LDL cholesterol levels might be too much for us. Moreover, LDL cholesterol levels of other primates are substantially lower than those in humans. In this review article, based on a series of evidence from clinical trials, human genetics, and biology, we provide the idea that we need to rethink what is the optimal range of LDL cholesterol level, instead of “normal” or “average” range.
Keywords: Lipoproteins, LDL, Cholesterol, Genetics, PCSK9
1. Introduction
Based on a series of evidences, cholesterol has been established as a causal factor for atherosclerosis. Firstly, cholesterol is deposited in coronary atherosclerotic plaque1). Secondly, cholesterol-fed animals develop atherosclerotic plaque2). Thirdly, epidemiological studies have shown the positive relationship between cholesterol and atherosclerotic diseases3). Fourthly, familial hypercholesterolemia has been shown to accompany premature coronary artery disease4); on the other hand, familial hypobetalipoproteinemia has been shown as having less of a prevalence of such disease5). Finally, lowering (LDL) cholesterol via any means has been shown to reduce atherosclerotic cardiovascular diseases (ASCVD) regardless of the patients' backgrounds6–9). When we think about LDL cholesterol value, the “normal” range is typically set to 70 to 139 mg/dl based on the distribution. We feel safe to see if the value is around 110 mg/dl because it is nearly the “mean” value. If the value is below 70 mg/dl, the value is reported as “abnormally low” to us even under the secondary prevention settings. This situation doesn't make sense, since there are plenty of evidences suggesting that such an “abnormally” low LDL cholesterol level is associated with better clinical outcomes, especially among the patients with ASCVD, and the current clinical guidelines are accepting this fact10, 11). In the current era where we can use statins, ezetimibe, and proprotein convertase subtilisin/kexin type 9 (PCSK9) inhibitors, LDL cholesterol level can be reduced far greater than in past decades, raising a critical question: How low the LDL cholesterol can be? Most of the cardiologists have a kind of hesitation to further reduce their LDL cholesterol level despite the clinical guidelines stating that LDL cholesterol should be lower than 70 mg/dl in a portion of high-risk patients12). In this review article, we would like to provide lines of evidence clearly showing that “super-aggressive” LDL cholesterol lowering is not always considered as such. Rather, we need to rethink about the optimal range of LDL cholesterol level, instead of “normal” or “average” range.
2. Considerations from Extreme Cases
It is quite easy to understand the fact that LDL cholesterol is associated with ASCVD when we have a chance to see only a single case with homozygous FH. The untreated patients with homozygous FH whose LDL cholesterol levels are quite high are exhibiting premature ASCVD without exception. Interestingly, a simple treatment; namely, LDL cholesterol lowering, regardless of strategies, has been shown to literally save their lives13). Additionally, we experience brothers with compound heterozygous FH where the older brother who had started treatment at the age of 23 exhibited repeated coronary events, whereas, the younger brother who had been treated since the age of 15 had been event-free for a long period, despite the similar LDL cholesterol levels based on the same mutations (NM_000527.4(LDLR):c.2054C > T (p.Pro685Leu)/NM_000527.4(LDLR):c.2431A > T (p.Lys811Ter)) (Fig. 1). The phenotypic difference between them clearly indicates that earlier intervention for LDL cholesterol can be quite beneficial even for such extreme cases. Moreover, several phenocopies of this situation with extremely high LDL cholesterol, including autosomal recessive hypercholesterolemia (ARH)14) and sitosterolemia15) caused by different genetic mutations, exhibit similar phenotypes, including tendon/cutaneous xanthomas, and premature ASCVD, similar to those observed in homozygous FH16). Those cases simply indicate that LDL cholesterol is the causal factor of this situation regardless of genetic etiology. On the other hand, findings from the patients exhibiting extremely low LDL cholesterol with any genetic backgrounds could also tell us a lot about the relationship between LDL cholesterol and ASCVD. Our patient with abetalipoproteinemia (ABL) caused by microsomal triglyceride transfer protein (MTTP) mutations (LDL cholesterol = 0 mg/dl) did not exhibit any coronary plaque nor aortic calcifications at the age of 51 (Fig. 2), although he suffers from spinocerebellar ataxia, and retinal pigmentary degeneration due to lack of fat-soluble vitamin17). On the contrary, we have shown an interesting case of homozygous familial hypobetalipoproteinemia (FHBL) whose LDL cholesterol was as low as 1 mg/dl18). The patient did not exhibit any complications relating to fat-soluble vitamin deficiency, as described above, probably due to his preserved HDL cholesterol (HDL cholesterol ∼60 mg/dl) level containing fat-soluble vitamins It would be important to see that none of the family members whose LDL cholesterol was quite low had atherosclerotic diseases. Moreover, we experience sisters working as nurse practitioners whose LDL cholesterol levels are ∼40 mg/dl, caused by a loss-of function of PCSK9 gene19). All of the mutation carriers exhibiting low LDL cholesterol do not have any ASCVD, or any other clinical complications, including liver dysfunction. Those individuals carrying those mutations simply showed us that very low LDL cholesterol level over a long period is not so harmful, but rather, is beneficial for their preventive cardiology. Also, it would be quite interesting to understand that novel pharmacological interventions for LDL-lowering have been developed based on the findings obtained from those extreme cases5, 20–23) (Table 1).
Fig. 1.
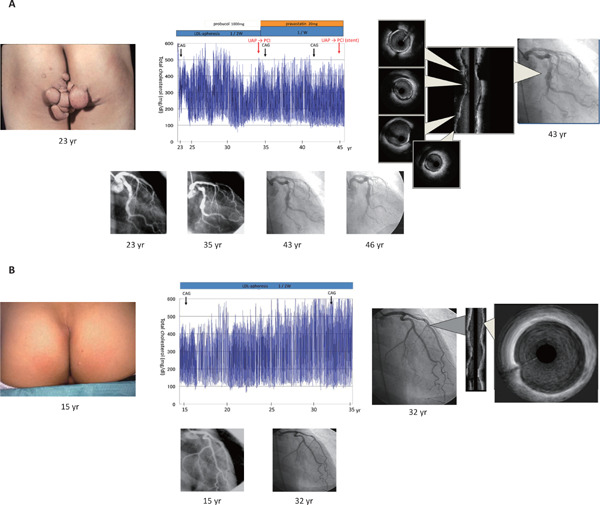
Clinical course of brothers with compound heterozygous FH
A. Clinical course of older brother
Picture of buttocks with huge xanthoma is illustrated on the left. Clinical course is illustrated in the middle. Blue line indicates total cholesterol level (mg/dl). Images obtained through intravascular ultrasound are illustrated in the right.
B. Clinical course of younger brother
Picture of buttocks with small xanthoma is illustrated on the left. Clinical course is illustrated in the middle. Blue line indicates total cholesterol level (mg/dl). Images obtained through intravascular ultrasound are illustrated in the right.
UAP: unstable angina pectoris; CAG: coronary artery disease; PCI: percutaneous coronary intervention
Fig. 2.
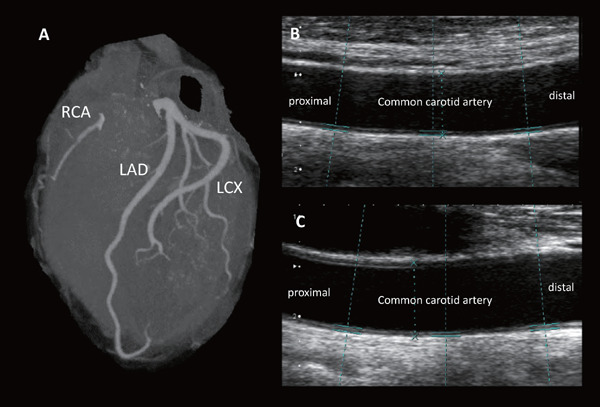
Images of coronary computed tomography and carotid ultrasound in a patient with ABL
A. Coronary computed tomography obtained in a patient with ABL. There are no stenotic lesions nor any calcifications identified in coronary arteries.
B. Carotid ultrasound image obtained in a patient with ABL. There are no stenotic lesions or intima-media thickness in right common carotid artery.
C. Carotid ultrasound image obtained in a patient with ABL. There are no stenotic lesions or intima-media thickness in left common carotid artery.
ABL, abetalipoproteinemia; RCA, right coronary artery; LAD, Left anterior descending coronary artery; LCX, Left circumflex coronary artery
Table 1. Novel pharmacological interventions for LDL-lowering.
| Target | Deficiency or carriers of PTV | Compounds | Randomized controlled trials | Mendelian randomization |
|---|---|---|---|---|
| NPCL1 | Heterozygous carriers (1 in 650 individuals) | Ezetimibe | IMPROVE-IT | Ref 20 |
| PCSK9 | Familial hypobetalipoproteinemia | Evolocumab Alirocumab |
FOURIER ODYSSEY OUTCOMES |
Ref 23 |
| MTTP | Abetalipoproteinemia | Lomitapide | NA | NA |
| APOB | Familial hypobetalipoproteinemia | Mipomersen | NA | Ref 5 |
| ANGPTL3 | Familial combined hypolipoproteinemia | Evinacumab | NA | Ref 22 |
| ACLY | NA | Bempedoic Acid | NA | Ref 21 |
NPC1L1, Niemann-Pick C1-Like 1; PCSK9, proprotein convertase subtilisin-kexin type 9; MTTP, microsomal triglyceride transfer protein; APOB, apolipoprotein B; ANGPTL3, Angiopoietin-like 3; Ref, reference, NA, not available; ACLY, ATP citrate lyase.
3. Considerations from Human Genetics
As stated above, rare genetic variations, for example, mutation(s) involving loss of function in LDL receptor gene (namely, FH), are robustly associated with elevated LDL cholesterol level and ASCVD risk. On the other hand, loss of function mutation(s) in apolipoprotein B (APOB) gene (namely, FHBL) are robustly associated with reduced LDL cholesterol level and ASCVD risk. The same situations are applicable to ATP-binding cassette sub-family G member 5 (ABCG5) (both elevated)24), angiopoietin-like 3 (ANGPTL3) (both reduced)22), and PCSK9 (both reduced)23). It is interesting to note that LDL cholesterol levels are positively associated with ASCVD regardless of genes and diseases. In addition to such rare genetic variations associated with Mendelian LDL disorders, common genetic variations associated with LDL cholesterol appear to be related with ASCVD. The magnitude of the effect on ASCVD is associated with LDL cholesterol level, and also such magnitude observed in genetic studies is far greater than that observed in clinical trials, suggesting that earlier intervention on LDL cholesterol may have a greater effect for preventive ASCVD. On the other hand, genetic variants associated with HDL cholesterol were not associated with ASCVD25), consistent with negative results of RCT targeting lower HDL cholesterol26–28).
4. Considerations from Clinical Trials Aiming to Reduce LDL Cholesterol Aggressively
Since the establishment of clinical usefulness of statins, there are debates regarding super-aggressive LDL cholesterol lowering therapies, including targeting cholesterol levels much lower than 100 mg/dl, as well as the additional drugs on top of statins. Regarding the first matter, a RCT named EMPATHY study, targeting LDL cholesterol level < 70 mg/dl using mainly statins among high-risk Japanese diabetic patients with primary prevention setting, revealed beneficial effect29). In this study, patients receiving aggressive LDL cholesterol lowering therapies (mean LDL cholesterol level was 76.5 mg/dl) exhibited significantly lower ischemic stroke events than those with standard care (mean LDL cholesterol level was 104.1 mg/dl). Moreover, high-dose statin therapy reaching to LDL cholesterol level at 76.6 mg/dl has been shown to be better than low-dose statin therapy reaching to LDL cholesterol level at 91 mg/dl among Japanese secondary prevention patients30). As for the second matter, recent mega RCT using ezetimibe, PCSK9 inhibitors, and a cholesteryl ester transfer protein (CETP) inhibitor on top of statins consistently revealed that additional beneficial effects could be obtained through such super-aggressive LDL cholesterol lowering therapies in proportion to the absolute degree of LDL cholesterol lowering7–9, 31). It is of note that ASCVD events seemed to decline with achieved LDL cholesterol, to a level of approximately 30 mg/dl in ODYSSEY OUTCOMES (using alirocumab)32), and to a level of approximately 10 mg/dl in FOURIER (using evolocumab)33). Those observations collectively make us confident that the lower the LDL cholesterol, the better could be applicable, at least at the range of LDL cholesterol ∼30 mg/dl in patients with ASCVD. Moreover, Table 2 summarizing the results obtained through RCT and Mendelian randomization studies focusing on protein truncating variants (extreme situations) clearly indicates that super aggressive as well as earlier LDL-C lowering should be beneficial.
Table 2. Effects of randomized controlled trials and Mendelian randomization study in PTV on LDL-C and on ASCVD.
| Gene | RCT |
Mendelian randomization study in PTV |
|||
|---|---|---|---|---|---|
| Trial name | LDL cholesterol reduction (mg/dl) | ASCVD reduction (%) | LDL cholesterol reduction (mg/dl) | ASCVD reduction (%) | |
| APOB | NA | NA | NA | 43 | 72 |
| CETP | REVEAL | 26 | 9 | 12 | 30 |
| NPC1L1 | IMPROVE-IT | 17 | 6 | 12 | 53 |
| PCSK9 | FOURIER/ODYSSEY | 62/48 | 15/15 | 21 | 88 |
RCT, randomized controlled trial; PTV, protein truncating variant; ASCVD, atherosclerotic cardiovascular disease; APOB, apolipoprotein B; CETP, cholesteryl ester transfer protein; NPC1L1, Niemann-Pick C1-Like 1; PCSK9, proprotein convertase subtilisin-kexin type 9; NA, not available.
5. Lessons from Professors. Brown and Goldstein
In addition to the observations from those RCT, professors Brown and Goldstein, both of whom are Nobel laureates, suggested that the levels of cholesterol in our industrialized societies are inappropriately high34). This comment was derived from three different important aspects of nature: 1) a level of LDL cholesterol in serum of 25 mg/dl would be sufficient to nourish body cells with cholesterol, estimated by the experimental studies showing that LDL receptor binds LDL optimally when the lipoprotein is present at a cholesterol concentration of 2.5 mg/dl. And it has been shown that there is a 10-to-1 gradient between concentrations of LDL in plasma and interstitial fluid; 2) plasma LDL cholesterol levels of other mammals without development of atherosclerosis are generally less than 80 mg/dl; 3) LDL cholesterol level in newborn humans is approximately 30 mg/dl; 4) when humans are raised on a low fat diet, the plasma LDL cholesterol levels tend to stay in the range of 50 to 80 mg/dl.
6. Lessons from Monkeys, our Estimable Ancestors
Let me remind you that LDL cholesterol levels of monkeys, who are our estimable ancestors, have been shown to be as low as ∼30 mg/dl35). Japanese macaque, whose life span is around 20 to 30 years, hardly exhibit ASCVD35, 36). Typically, wild monkeys have to survive in a natural field, requiring LDL cholesterol because of the incident of bleedings and/or infections. Accordingly, it could be skeptical that humans, especially, those living in industrialized societies, need a LDL cholesterol level as high as ∼100 mg/dl. In this regard, “standard” levels are usually determined based on “average” values, not bade on “healthy” values in any biomarkers, including LDL cholesterol. Thus, it would be better to rethink “standard” levels of cholesterol.
7. Potential Concerns for Low LDL Cholesterol
In spite of a series of beneficial evidences as stated above, there are still many cardiologists who have some concerns about low LDL cholesterol, such as Alzheimer's disease, dementia, Parkinson's disease, and hemorrhagic stroke. In this regard, recent Mendelian randomization studies have suggested that low LDL cholesterol levels due to PCSK9 and hydroxymethylglutaryl-CoA reductase (HMGCR) variants had no causal effect on high risk of Alzheimer's disease, vascular dementia, any dementia, or Parkinson's disease; instead, low LDL cholesterol levels may have a causal effect in reducing the risk of Alzheimer's disease37). On the other hand, another study showed strong positive associations of LDL cholesterol with ischemic stroke and inverse associations with hemorrhagic stroke; however, lowering LDL cholesterol appears to have net benefit for prevention of overall vascular events38).
8. Precision Medicine of LDL Cholesterol Lowering
As stated, LDL cholesterol, as an important causal factor for ASCVD should be reduced as much as possible, especially in the secondary prevention settings. However, there is an emerging concept of precision medicine in almost all fields of medicine, including preventive cardiology. In the statin era, it has been shown that the effectiveness of this drug seems to be equal among a set of clinical subgroups, such as hypertension, diabetes, smoking, and so on6). On the other hand, there are a series of patients who exhibit greater responsiveness to ezetimibe, including, patients with diabetes, and those with ABCG5 or ABCG8 genetic mutation(s)39–41). Moreover, sub-analyses from recent clinical trials using PCSK9 inhibitors have suggested that there are several types of groups of patients who had greater benefit via this costly drug, including those with peripheral artery disease, elevated Lp(a) levels, or with high polygenic risk42–45).
On the other hand, when we try to reduce LDL cholesterol among the patients with FH, we typically use the multiple LDL-lowering therapies listed above. However, there is an emerging concept of “cholesterol burden” in these particular patients. Namely, the integrated, accumulated sum of LDL cholesterol burden appears to lead them for their premature ASCVD. In other words, target LDL cholesterol level should be quite low if the patients with FH started treatment too late, whereas, that can be moderate under the situation where LDL cholesterol lowering is started early enough. To support this notion, a recent study showed that the patients with FH who had been treated moderately (LDL cholesterol level from 237 mg/dl to 160 mg/dl) since the mean age of 13 years exhibited far better prognoses compared with their age-matched relatives with FH46). Accordingly, “the earlier, the better” concept seems to be applicable to this extreme case, and we believe that it should also be true for non-FH hyper LDL cholesterolemia47).
Moreover, we know that there are large variations of severity of disease (susceptibility to ASCVD) even among the patients with FH47–51). At least a part of it has been explained by their genetic status of FH and their physical signs of FH52). Another study has shown that accumulated effects of common genetic variations, in addition to rare mutation(s), which lead them to FH are contributing to their phenotypic variability53). Accordingly, the ideal strategy of LDL cholesterol lowering should be quite individual-specific, including genetic backgrounds, and the timing of treatments (Fig. 3).
Fig. 3.
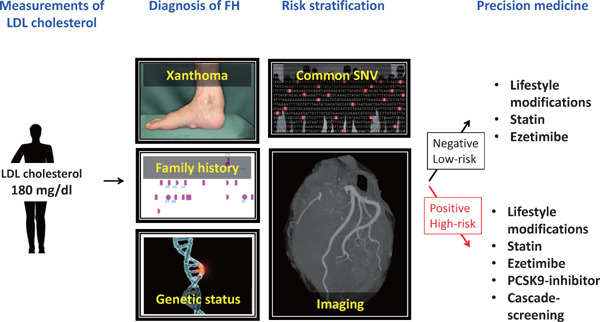
Precision medicine for FH
When we encounter an individual whose LDL cholesterol level is ≥ 180 mg/dl, then we have to consider a clinical as well as genetic diagnosis of FH. Additionally, additional risk stratification can be considered based on their common genetic variations, and their imaging. According to this information, we can select the best approach for their LDL cholesterol reduction.
FH: familial hypercholesterolemia; SNV: single nucleotide variation
9. Conclusion
In this paper, we have repeatedly emphasized that LDL cholesterol is a causal risk factor for ASCVD. Also we have learned from lines of evidence that super-aggressive LDL cholesterol lowering therapies, at least at around 30 mg/dl, are safe. We need to rethink what is the optimal range of LDL cholesterol level, instead of “normal”, or “average” range, based on a series of evidences from clinical trials, human genetics, and biology.
Acknowledgements and Notice of Grant Support
None.
Conflict of Interest Disclosures
None.
References
- 1). Wissler RW. Update on the pathogenesis of atherosclerosis. Am J Med, 1991; 91: 3S-9S [DOI] [PubMed] [Google Scholar]
- 2). Friedman M. Pathogenesis of the spontaneous atherosclerotic plaque. A study on the A/Friedman M: Pathogenesis of the spontaneous atherosclerotic plaque. A study on the cholesterol-fed rabbit. Arch Pathol, 1963; 76: 318-329 [PubMed] [Google Scholar]
- 3). Kannel WB, Castelli WP, Gordon T, McNamara PM. Serum cholesterol, lipoproteins, and the risk of coronary heart disease. The Framingham study. Ann Intern Med, 1971; 74: 1-12 [DOI] [PubMed] [Google Scholar]
- 4). Mabuchi H. Half a Century Tales of Familial Hypercholesterolemia (FH) in Japan. J Atheroscler Thromb, 2017; 24: 189-207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Peloso GM, Nomura A, Khera AV, Chaffin M, Won HH, Ardissino D, Danesh J, Schunkert H, Wilson JG, Samani N, Erdmann J, McPherson R, Watkins H, Saleheen D, McCarthy S, Teslovich TM, Leader JB, Lester Kirchner H, Marrugat J, Nohara A, Kawashiri MA, Tada H, Dewey FE, Carey DJ, Baras A, Kathiresan S. Rare Protein-Truncating Variants in APOB, Lower Low-Density Lipoprotein Cholesterol, and Protection Against Coronary Heart Disease. Circ Genom Precis Med, 2019; 12: e002376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6). Cholesterol Treatment Trialists' (CTT) Collaboration1. Baigent C, Blackwell L, Emberson J, Holland LE, Reith C, Bhala N, Peto R, Barnes EH, Keech A, Simes J, Collins R. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet, 2010; 376: 1670-1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7). Cannon CP, Blazing MA, Giugliano RP, McCagg A, White JA, Theroux P, Darius H, Lewis BS, Ophuis TO, Jukema JW, De Ferrari GM, Ruzyllo W, De Lucca P, Im K, Bohula EA, Reist C, Wiviott SD, Tershakovec AM, Musliner TA, Braunwald E, Califf RM, IMPROVE-IT Investigators Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med, 2015; 372: 2387-2397 [DOI] [PubMed] [Google Scholar]
- 8). Sabatine MS, Giugliano RP, Keech AC, Honarpour N, Wiviott SD, Murphy SA, Kuder JF, Wang H, Liu T, Wasserman SM, Sever PS, Pedersen TR, FOURIER Steering Committee and Investigators Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med, 2017; 376: 1713-1722 [DOI] [PubMed] [Google Scholar]
- 9). Schwartz GG, Steg PG, Szarek M, Bhatt DL, Bittner VA, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Pordy R, Quintero K, Roe MT, Sasiela WJ, Tamby JF, Tricoci P, White HD, Zeiher AM, ODYSSEY OUTCOMES Committees and Investigators Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N Engl J Med, 2018; 379: 2097-2107 [DOI] [PubMed] [Google Scholar]
- 10). Kinoshita M, Yokote K, Arai H, Iida M, Ishigaki Y, Ishibashi S, Umemoto S, Egusa G, Ohmura H, Okamura T, Kihara S, Koba S, Saito I, Shoji T, Daida H, Tsukamoto K, Deguchi J, Dohi S, Dobashi K, Hamaguchi H, Hara M, Hiro T, Biro S, Fujioka Y, Maruyama C, Miyamoto Y, Murakami Y, Yokode M, Yoshida H, Rakugi H, Wakatsuki A, Yamashita S, Committee for Epidemiology and Clinical Management of Atherosclerosis Japan Atherosclerosis Society (JAS) Guidelines for Prevention of Atherosclerotic Cardiovascular Diseases 2017. J Atheroscler Thromb, 2018; 25: 846-984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11). Mach F, Baigent C, Catapano AL, Koskinas KC, Casula M, Badimon L, Chapman MJ, De Backer GG, Delgado V, Ference BA, Graham IM, Halliday A, Landmesser U, Mihaylova B, Pedersen TR, Riccardi G, Richter DJ, Sabatine MS, Taskinen MR, Tokgozoglu L, Wiklund O, ESC Scientific Document Group 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J, 2020; 41: 111-188 [DOI] [PubMed] [Google Scholar]
- 12). Tada H, Kawashiri MA, Nohara A, Inazu A, Kobayashi J, Yasuda K, Mabuchi H, Yamagishi M, Hayashi K. Lipid Management in a Japanese Community: Attainment Rate of Target Set by the Japan Atherosclerosis Society Guidelines for the Prevention of Atherosclerotic Cardiovascular Diseases 2012. J Atheroscler Thromb, 2017; 24: 338-345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Makino H, Koezuka R, Tamanaha T, Ogura M, Matsuki K, Hosoda K, Harada-Shiba M. Familial Hypercholesterolemia and Lipoprotein Apheresis. J Atheroscler Thromb, 2019; 26: 679-687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14). Tada H, Kawashiri MA, Ikewaki K, et al. Altered metabolism of low-density lipoprotein and very-low-density lipoprotein remnant in autosomal recessive hypercholesterolemia: results from stable isotope kinetic study in vivo. Circ Cardiovasc Genet, 2012; 5: 35-41 [DOI] [PubMed] [Google Scholar]
- 15). Tada H, Nohara A, Inazu A, et al. Sitosterolemia, Hypercholesterolemia, and Coronary Artery Disease. J Atheroscler Thromb, 2018; 25: 783-789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16). Tada H, Kawashiri MA, Yamagishi M. Clinical Perspectives of Genetic Analyses on Dyslipidemia and Coronary Artery Disease. J Atheroscler Thromb, 2017; 24: 452-461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Yang XP, Inazu A, Yagi K, Kajinami K, Koizumi J, Mabuchi H. Abetalipoproteinemia caused by maternal isodisomy of chromosome 4q containing an intron 9 splice acceptor mutation in the microsomal triglyceride transfer protein gene. Arterioscler Thromb Vasc Biol, 1999; 19: 1950-1955 [DOI] [PubMed] [Google Scholar]
- 18). Kawashiri MA, Tada H, Hashimoto M, Taniyama M, Nakano T, Nakajima K, Inoue T, Mori M, Nakanishi C, Konno T, Hayashi K, Nohara A, Inazu A, Koizumi J, Ishihara H, Kobayashi J, Hirano T, Mabuchi H, Yamagishi M. Extreme Contrast of Postprandial Remnant-Like Particles Formed in Abetalipoproteinemia and Homozygous Familial Hypobetalipoproteinemia. JIMD Rep, 2015; 22: 85-94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19). Tada H, Okada H, Nomura A, Nohara A, Takamura M, Kawashiri MA. A Healthy Family of Familial Hypobetalipoproteinemia Caused by a Protein-truncating Variant in the PCSK9 Gene. Intern Med, 2020. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Myocardial Infarction Genetics Consortium Investigators. Stitziel NO, Won HH, Morrison AC, Peloso GM, Do R, Lange LA, Fontanillas P, Gupta N, Duga S, Goel A, Farrall M, Saleheen D, Ferrario P, König I, Asselta R, Merlini PA, Marziliano N, Notarangelo MF, Schick U, Auer P, Assimes TL, Reilly M, Wilensky R, Rader DJ, Hovingh GK, Meitinger T, Kessler T, Kastrati A, Laugwitz KL, Siscovick D, Rotter JI, Hazen SL, Tracy R, Cresci S, Spertus J, Jackson R, Schwartz SM, Natarajan P, Crosby J, Muzny D, Ballantyne C, Rich SS, O'Donnell CJ, Abecasis G, Sunaev S, Nickerson DA, Buring JE, Ridker PM, Chasman DI, Austin E, Kullo IJ, Weeke PE, Shaffer CM, Bastarache LA, Denny JC, Roden DM, Palmer C, Deloukas P, Lin DY, Tang ZZ, Erdmann J, Schunkert H, Danesh J, Marrugat J, Elosua R, Ardissino D, McPherson R, Watkins H, Reiner AP, Wilson JG, Altshuler D, Gibbs RA, Lander ES, Boerwinkle E, Gabriel S, Kathiresan S. Inactivating mutations in NPC1L1 and protection from coronary heart disease. N Engl J Med, 2014; 371: 2072-2082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21). Ference BA, Ray KK, Catapano AL, Ference TB, Burgess S, Neff DR, Oliver-Williams C, Wood AM, Butterworth AS, Di Angelantonio E, Danesh J, Kastelein JJP, Nicholls SJ. Mendelian Randomization Study of ACLY and Cardiovascular Disease. N Engl J Med, 2019; 380: 1033-1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22). Stitziel NO, Khera AV, Wang X, Bierhals AJ, Vourakis AC, Sperry AE, Natarajan P, Klarin D, Emdin CA, Zekavat SM, Nomura A, Erdmann J, Schunkert H, Samani NJ, Kraus WE, Shah SH, Yu B, Boerwinkle E, Rader DJ, Gupta N, Frossard PM, Rasheed A, Danesh J, Lander ES, Gabriel S, Saleheen D, Musunuru K, Kathiresan S, PROMIS and Myocardial Infarction Genetics Consortium Investigators ANGPTL3 Deficiency and Protection Against Coronary Artery Disease. J Am Coll Cardiol, 2017; 69: 2054-2063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23). Benn M, Nordestgaard BG, Grande P, Schnohr P, Tybjaerg-Hansen A. PCSK9 R46L, low-density lipoprotein cholesterol levels, and risk of ischemic heart disease: 3 independent studies and meta-analyses. J Am Coll Cardiol, 2010; 55: 2833-2842 [DOI] [PubMed] [Google Scholar]
- 24). Nomura A, Emdin CA, Won HH, Peloso G, Natarajan P, Ardissino D, Danesh J, Schunkert H, Correa A, Bown M, Samani N, Erdmann J, McPherson R, Watkins H, Saleheen D, Elosua R, Kawashiri MA, Tada H, Gupta N, Shah S, Rader DJ, Gabriel S, Khera AV, Kathiresan S. Heterozygous ATP-binding Cassette Transporter G5 Gene Deficiency and Risk of Coronary Artery Disease. Circ Genom Precis Med, 2020. in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Do R, Willer CJ, Schmidt EM, Sengupta S, Gao C, Peloso GM, Gustafsson S, Kanoni S, Ganna A, Chen J, Buchkovich ML, Mora S, Beckmann JS, Bragg-Gresham JL, Chang HY, Demirkan A, Den Hertog HM, Donnelly LA, Ehret GB, Esko T, Feitosa MF, Ferreira T, Fischer K, Fontanillas P, Fraser RM, Freitag DF, Gurdasani D, Heikkilä K, Hyppönen E, Isaacs A, Jackson AU, Johansson A, Johnson T, Kaakinen M, Kettunen J, Kleber ME, Li X, Luan J, Lyytikäinen LP, Magnusson PK, Mangino M, Mihailov E, Montasser ME, Müller-Nurasyid M, Nolte IM, O'Connell JR, Palmer CD, Perola M, Petersen AK, Sanna S, Saxena R, Service SK, Shah S, Shungin D, Sidore C, Song C, Strawbridge RJ, Surakka I, Tanaka T, Teslovich TM, Thorleifsson G, Van den Herik EG, Voight BF, Volcik KA, Waite LL, Wong A, Wu Y, Zhang W, Absher D, Asiki G, Barroso I, Been LF, Bolton JL, Bonnycastle LL, Brambilla P, Burnett MS, Cesana G, Dimitriou M, Doney AS, Döring A, Elliott P, Epstein SE, Eyjolfsson GI, Gigante B, Goodarzi MO, Grallert H, Gravito ML, Groves CJ, Hallmans G, Hartikainen AL, Hayward C, Hernandez D, Hicks AA, Holm H, Hung YJ, Illig T, Jones MR, Kaleebu P, Kastelein JJ, Khaw KT, Kim E, Klopp N, Komulainen P, Kumari M, Langenberg C, Lehtimäki T, Lin SY, Lindström J, Loos RJ, Mach F, McArdle WL, Meisinger C, Mitchell BD, Müller G, Nagaraja R, Narisu N, Nieminen TV, Nsubuga RN, Olafsson I, Ong KK, Palotie A, Papamarkou T, Pomilla C, Pouta A, Rader DJ, Reilly MP, Ridker PM, Rivadeneira F, Rudan I, Ruokonen A, Samani N, Scharnagl H, Seeley J, Silander K, Stančáková A, Stirrups K, Swift AJ, Tiret L, Uitterlinden AG, van Pelt LJ, Vedantam S, Wainwright N, Wijmenga C, Wild SH, Willemsen G, Wilsgaard T, Wilson JF, Young EH, Zhao JH, Adair LS, Arveiler D, Assimes TL, Bandinelli S, Bennett F, Bochud M, Boehm BO, Boomsma DI, Borecki IB, Bornstein SR, Bovet P, Burnier M, Campbell H, Chakravarti A, Chambers JC, Chen YD, Collins FS, Cooper RS, Danesh J, Dedoussis G, de Faire U, Feranil AB, Ferrières J, Ferrucci L, Freimer NB, Gieger C, Groop LC, Gudnason V, Gyllensten U, Hamsten A, Harris TB, Hingorani A, Hirschhorn JN, Hofman A, Hovingh GK, Hsiung CA, Humphries SE, Hunt SC, Hveem K, Iribarren C, Järvelin MR, Jula A, Kähönen M, Kaprio J, Kesäniemi A, Kivimaki M, Kooner JS, Koudstaal PJ, Krauss RM, Kuh D, Kuusisto J, Kyvik KO, Laakso M, Lakka TA, Lind L, Lindgren CM, Martin NG, März W, McCarthy MI, McKenzie CA, Meneton P, Metspalu A, Moilanen L, Morris AD, Munroe PB, Njølstad I, Pedersen NL, Power C, Pramstaller PP, Price JF, Psaty BM, Quertermous T, Rauramaa R, Saleheen D, Salomaa V, Sanghera DK, Saramies J, Schwarz PE, Sheu WH, Shuldiner AR, Siegbahn A, Spector TD, Stefansson K, Strachan DP, Tayo BO, Tremoli E, Tuomilehto J, Uusitupa M, van Duijn CM, Vollenweider P, Wallentin L, Wareham NJ, Whitfield JB, Wolffenbuttel BH, Altshuler D, Ordovas JM, Boerwinkle E, Palmer CN, Thorsteinsdottir U, Chasman DI, Rotter JI, Franks PW, Ripatti S, Cupples LA, Sandhu MS, Rich SS, Boehnke M, Deloukas P, Mohlke KL, Ingelsson E, Abecasis GR, Daly MJ, Neale BM, Kathiresan S. Common variants associated with plasma triglycerides and risk for coronary artery disease. Nat Genet, 2013; 45: 1345-1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). Hulley S, Grady D, Bush T, Furberg C, Herrington D, Riggs B, Vittinghoff E. Randomized trial of estrogen plus progestin for secondary prevention of coronary heart disease in postmenopausal women. Heart and Estrogen/progestin Replacement Study (HERS) Research Group. JAMA, 1998; 280: 605-613 [DOI] [PubMed] [Google Scholar]
- 27). AIM-HIGH Investigators. Boden WE, Probstfield JL, Anderson T, Chaitman BR, Desvignes-Nickens P, Koprowicz K, McBride R, Teo K, Weintraub W. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med, 2011; 365: 2255-2267 [DOI] [PubMed] [Google Scholar]
- 28). Forrest MJ, Bloomfield D, Briscoe RJ, Brown PN, Cumiskey AM, Ehrhart J, Hershey JC, Keller WJ, Ma X, McPherson HE, Messina E, Peterson LB, Sharif-Rodriguez W, Siegl PK, Sinclair PJ, Sparrow CP, Stevenson AS, Sun SY, Tsai C, Vargas H, Walker M, 3rd, West SH, White V, Woltmann RF. Torcetrapib-induced blood pressure elevation is independent of CETP inhibition and is accompanied by increased circulating levels of aldosterone. Br J Pharmacol, 2008; 154: 1465-1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29). Itoh H, Komuro I, Takeuchi M, Akasaka T, Daida H, Egashira Y, Fujita H, Higaki J, Hirata KI, Ishibashi S, Isshiki T, Ito S, Kashiwagi A, Kato S, Kitagawa K, Kitakaze M, Kitazono T, Kurabayashi M, Miyauchi K, Murakami T, Murohara T, Node K, Ogawa S, Saito Y, Seino Y, Shigeeda T, Shindo S, Sugawara M, Sugiyama S, Terauchi Y, Tsutsui H, Ueshima K, Utsunomiya K, Yamagishi M, Yamazaki T, Yo S, Yokote K, Yoshida K, Yoshimura M, Yoshimura N, Nakao K, Nagai R, EMPATHY Investigators Intensive Treat-to-Target Statin Therapy in High-Risk Japanese Patients With Hypercholesterolemia and Diabetic Retinopathy: Report of a Randomized Study. Diabetes Care, 2018; 41: 1275-1284 [DOI] [PubMed] [Google Scholar]
- 30). Taguchi I, Iimuro S, Iwata H, Takashima H, Abe M, Amiya E, Ogawa T, Ozaki Y, Sakuma I, Nakagawa Y, Hibi K, Hiro T, Fukumoto Y, Hokimoto S, Miyauchi K, Yamazaki T, Ito H, Otsuji Y, Kimura K, Takahashi J, Hirayama A, Yokoi H, Kitagawa K, Urabe T, Okada Y, Terayama Y, Toyoda K, Nagao T, Matsumoto M, Ohashi Y, Kaneko T, Fujita R, Ohtsu H, Ogawa H, Daida H, Shimokawa H, Saito Y, Kimura T, Inoue T, Matsuzaki M, Nagai R. High-Dose Versus Low-Dose Pitavastatin in Japanese Patients With Stable Coronary Artery Disease (REAL-CAD): A Randomized Superiority Trial. Circulation, 2018; 137: 1997-2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31). HPS3/TIMI55-REVEAL Collaborative Group. Bowman L, Hopewell JC, Chen F, Wallendszus K, Stevens W, Collins R, Wiviott SD, Cannon CP, Braunwald E, Sammons E, Landray MJ. Effects of Anacetrapib in Patients with Atherosclerotic Vascular Disease. N Engl J Med, 2017; 377: 1217-1227 [DOI] [PubMed] [Google Scholar]
- 32). Steg PG, Szarek M, Bhatt DL, Bittner VA, Brégeault MF, Dalby AJ, Diaz R, Edelberg JM, Goodman SG, Hanotin C, Harrington RA, Jukema JW, Lecorps G, Mahaffey KW, Moryusef A, Ostadal P, Parkhomenko A, Pordy R, Roe MT, Tricoci P, Vogel R, White HD, Zeiher AM, Schwartz GG. Effect of Alirocumab on Mortality After Acute Coronary Syndromes. Circulation, 2019; 140: 103-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Giugliano RP, Pedersen TR, Park JG, De Ferrari GM, Gaciong ZA, Ceska R, Toth K, Gouni-Berthold I, Lopez-Miranda J, Schiele F, Mach F, Ott BR, Kanevsky E, Pineda AL, Somaratne R, Wasserman SM, Keech AC, Sever PS, Sabatine MS, FOURIER Investigators Clinical efficacy and safety of achieving very low LDL-cholesterol concentrations with the PCSK9 inhibitor evolocumab: a prespecified secondary analysis of the FOURIER trial. Lancet, 2017; 390: 1962-1971 [DOI] [PubMed] [Google Scholar]
- 34). Brown MS, Goldstein JL. A receptor-mediated pathway for cholesterol homeostasis. Science, 1986; 232: 34-47 [DOI] [PubMed] [Google Scholar]
- 35). Takenaka A, Matsumoto Y, Nagaya A, Watanabe K, Goto S, Suryobroto B, Takenaka O. Plasma cholesterol levels in free-ranging macaques compared with captive macaques and humans. Primates, 2000; 41: 299-309 [DOI] [PubMed] [Google Scholar]
- 36). Fedigan L, 1991. Lifespan and reproduction in Japanese macaque females. In: The Monkeys of Arashiyama: 35 Years of Research in Japan and the West, Fedigan L. M., Asquith P. J. (eds.), State Univ. of New York Press, Albany, New York, pp. 140-154 [Google Scholar]
- 37). Benn M, Nordestgaard BG, Frikke-Schmidt R, Tybjærg-Hansen A. Low LDL cholesterol, PCSK9 and HMGCR genetic variation, and risk of Alzheimer's disease and Parkinson's disease: Mendelian randomisation study. BMJ, 2017; 357: j1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38). Sun L, Clarke R, Bennett D, Guo Y, Walters RG, Hill M, Parish S, Millwood IY, Bian Z, Chen Y, Yu C, Lv J, Collins R, Chen J, Peto R, Li L, Chen Z, China Kadoorie Biobank Collaborative Group; International Steering Committee; International Co-ordinating Centre, Oxford; National Co-ordinating Centre, Beijing; Regional Coordinating Centres Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med, 2019; 25: 569-574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39). Giugliano RP, Cannon CP, Blazing MA, Nicolau JC, Corbalán R, Špinar J, Park JG, White JA, Bohula EA, Braunwald E, IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial) Investigators Benefit of Adding Ezetimibe to Statin Therapy on Cardiovascular Outcomes and Safety in Patients With Versus Without Diabetes Mellitus: Results From IMPROVE-IT (Improved Reduction of Outcomes: Vytorin Efficacy International Trial). Circulation, 2018; 137: 1571-1582 [DOI] [PubMed] [Google Scholar]
- 40). Yamaguchi J, Kawada-Watanabe E, Koyanagi R, Arashi H, Sekiguchi H, Nakao K, Tobaru T, Tanaka H, Oka T, Endo Y, Saito K, Uchida T, Matsui K, Ogawa H, Hagiwara N. Baseline serum sitosterol level as predictor of adverse clinical events in acute coronary syndrome patients with dyslipidaemia: A sub-analysis of HIJPROPER. Atherosclerosis, 2018; 274: 139-145 [DOI] [PubMed] [Google Scholar]
- 41). Tada H, Okada H, Nomura A, Takamura M, Kawashiri MA. Beneficial effect of ezetimibe-atorvastatin combination therapy in patients with a mutation in ABCG5 or ABCG8 gene. Lipids Health Dis, 2020; 19: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Bonaca MP, Nault P, Giugliano RP, Keech AC, Pineda AL, Kanevsky E, Kuder J, Murphy SA, Jukema JW, Lewis BS, Tokgozoglu L, Somaratne R, Sever PS, Pedersen TR, Sabatine MS. Low-Density Lipoprotein Cholesterol Lowering With Evolocumab and Outcomes in Patients With Peripheral Artery Disease: Insights From the FOURIER Trial (Further Cardiovascular Outcomes Research With PCSK9 Inhibition in Subjects With Elevated Risk). Circulation, 2018; 137: 338-350 [DOI] [PubMed] [Google Scholar]
- 43). Shapiro MD, Minnier J, Tavori H, Kassahun H, Flower A, Somaratne R, Fazio S. Relationship Between Low-Density Lipoprotein Cholesterol and Lipoprotein(a) Lowering in Response to PCSK9 Inhibition With Evolocumab. J Am Heart Assoc, 2019; 8: e010932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44). Damask A, Steg PG, Schwartz GG, Szarek M, Hagström E, Badimon L, Chapman MJ, Boileau C, Tsimikas S, Ginsberg HN, Banerjee P, Manvelian G, Pordy R, Hess S, Overton JD, Lotta LA, Yancopoulos GD, Abecasis GR, Baras A, Paulding C, Regeneron Genetics Center and the ODYSSEY OUTCOMES Investigators Patients with High Genome-Wide Polygenic Risk Scores for Coronary Artery Disease May Receive Greater Clinical Benefit from Alirocumab Treatment in the Odyssey Outcomes Trial. Circulation, 2020; 141: 624-636 [DOI] [PubMed] [Google Scholar]
- 45). Marston NA, Kamanu FK, Nordio F, Gurmu Y, Roselli C, Sever PS, Pedersen TR, Keech AC, Wang H, Lira Pineda A, Giugliano RP, Lubitz SA, Ellinor PT, Sabatine MS, Ruff CT. Predicting Benefit From Evolocumab Therapy in Patients With Atherosclerotic Disease Using a Genetic Risk Score: Results From the FOURIER Trial. Circulation, 2020; 141: 616-623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46). Luirink IK, Wiegman A, Kusters DM, Hof MH, Groothoff JW, de Groot E, Kastelein JJP, Hutten BA. 20-Year Follow-up of Statins in Children with Familial Hypercholesterolemia. N Engl J Med, 2019; 381: 1547-1556 [DOI] [PubMed] [Google Scholar]
- 47). Brandts J, Ray KK. LDL-Cholesterol Lowering Strategies and Population Health - Time to Move to a Cumulative Exposure Model. Circulation, 2020. in press [DOI] [PubMed] [Google Scholar]
- 48). Tada H, Kawashiri MA, Nomura A, Teramoto R, Hosomichi K, Nohara A, Inazu A, Mabuchi H, Tajima A, Yamagishi M. Oligogenic familial hypercholesterolemia, LDL cholesterol, and coronary artery disease. J Clin Lipidol, 2018; 12: 1436-1444 [DOI] [PubMed] [Google Scholar]
- 49). Tada H, Kawashiri MA, Okada H, Teramoto R, Konno T, Yoshimuta T, Sakata K, Nohara A, Inazu A, Kobayashi J, Mabuchi H, Yamagishi M, Hayashi K. Assessment of coronary atherosclerosis in patients with familial hypercholesterolemia by coronary computed tomography angiography. Am J Cardiol, 2015; 115: 724-729 [DOI] [PubMed] [Google Scholar]
- 50). Tada H, Kawashiri MA, Okada H, Nakahashi T, Sakata K, Nohara A, Inazu A, Mabuchi H, Yamagishi M, Hayashi K. Assessments of Carotid Artery Plaque Burden in Patients With Familial Hypercholesterolemia. Am J Cardiol, 2017; 120: 1955-1960 [DOI] [PubMed] [Google Scholar]
- 51). Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M. Assessment of arterial stiffness in patients with familial hypercholesterolemia. J Clin Lipidol, 2018; 12: 397-402 [DOI] [PubMed] [Google Scholar]
- 52). Tada H, Kawashiri MA, Nohara A, Inazu A, Mabuchi H, Yamagishi M. Impact of clinical signs and genetic diagnosis of familial hypercholesterolaemia on the prevalence of coronary artery disease in patients with severe hypercholesterolaemia. Eur Heart J, 2017; 38: 1573-1579 [DOI] [PubMed] [Google Scholar]
- 53). Fahed AC, Wang M, Chaffin M, Bick AG, Patterson C, Natarajan P, Lebo M, Batra P, Ng K, Ellinor PT, Philippakis AA, Kathiresan S, Khera AV. Risk of Myocardial Infarction in Carriers of Familial Hypercholesterolemia Mutations is Modified by Common Variant Genetic Background or Adherence to a Healthy Lifestyle. Circulation, 2019; 140: A15044 [Google Scholar]


