Abstract
Background
Although the Fontan procedure is associated with a variety of long-term complications, it is the mainstay treatment for congenital heart disease with a functioning single ventricle. Data concerning the epidemiological profile are scarce.
Methods
We investigated the current epidemiological profile using a 2000-2008 nationwide birth cohort from a 2000-2014 database (1,967,991 live births), with complete postnatal data for at least 6 years. We identified 363 patients (2792 patient-years of follow-up) who had received the Fontan procedure, giving an incidence of 0.184/1000 live births.
Results
The overall Fontan surgical survival rate was 81.8%. In post-Fontan patients, the 10-year survival was 0.822 (±0.026). Causes of death included cardiac (43.8%), infection (20.8%), out-of-hospital death (16.7%), sudden death (8.3%), cerebral vascular accident (8.3%) and malignancy (2.1%). The risk of unexpected death (sudden death and out-of-hospital death) was 4.0%, or 0.55% per post-Fontan patient-year. Arrhythmias were common (12.1%). Supraventricular tachycardia was the most common type of arrhythmia, and occurred prior to the Fontan procedure in 22 patients, with a cumulative risk of 2.2%, 6.3%, and 11.6% by the age of 1, 5 and 10 years, respectively. Arrhythmia intervention was performed in 40.9% of those with arrhythmia, including electrophysiological studies/ablation in 12 and device therapy in 6 patients.
Conclusions
In conclusion, the incidence of Fontan patients was 0.184/1000 live births. Their medical complexity included a high risk of supraventricular tachycardia and unexpected death by adolescence.
Keywords: Birth cohort, Fontan procedure, Incidence, Supraventricular tachycardia, Survival
INTRODUCTION
The Fontan procedure was introduced in 1971 as a treatment for patients with tricuspid atresia, and it represented a milestone in congenital heart surgery.1 It provides a means for congenital heart disease (CHD) patients with a single functional ventricle [e.g., tricuspid atresia, heterotaxy syndrome, hypoplastic left heart syndrome (HLHS), and single ventricle] to separate the pulmonary and systemic circulations with fair systemic oxygen saturation. With advancements in perioperative care, the surgical survival rate of first-stage palliation prior to the Fontan procedure and Fontan procedure per se can reach 80%-90%.2,3 Therefore, the population of patients who have received the Fontan procedure (Fontan patients and post-Fontan patients) will increase over time. Prior to the Fontan operation or in early follow-up during childhood, Fontan patients are already at a risk of arrhythmia from the associated conduction system abnormalities.4-6 However, the impact from the arrhythmias and the relevant treatment strategies are still unclear. The medical needs of the Fontan and post-Fontan population are likely to become a formidable challenge; however, it remains ill defined. Thus far, only 1 study, based on a database from a tertiary care hospital, has estimated the incidence of the Fontan procedure from the served population at 0.10/1000.7
In Taiwan, the National Health Insurance (NHI) program, which currently covers over 99% of the population (adult and pediatric populations of approximately 23 and 5 million, respectively), was established in 1995. The NHI waives copayments for patients with CHD and provides affordable care for these children from birth. Consequently, nearly every cardiac patient can receive complete medical services. The child health indices in Taiwan are similar to those in the United States.8 The pediatric cardiac program was started in the 1950s in Taiwan, and the first Fontan procedure was performed in 1985. More than 10 medical centers have provided advanced cardiac care for the past decade, including cardiac surgery and transcatheter interventions. Therefore, a nationwide birth cohort from Taiwan with complete postnatal data for ≥ 6 years is suitable for investigating the current epidemiological profile, including the risk of arrhythmia, of Fontan patients.
METHODS
The Institutional Review Board of National Taiwan University Hospital, Taipei, Taiwan approved the study (IRB no: 201610006RIND).
Patient cohort
Complete health care records of subjects who were born between January 1, 2000 and December 31, 2008 were retrieved from the NHI Database from January 1, 2000 to December 31, 2014. CHD patients were selected based on International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) codes (ICD-9 codes: 745.0-747.42).9 These subjects constituted a 2000-2008 birth cohort with complete data on postnatal follow-up for at least 6 years. To avoid errors from tentative diagnoses, we excluded patients who had made fewer than three outpatient clinic visits for a major diagnosis of CHD. To elucidate the risk of arrhythmias from structural anomalies of CHD, we further defined a major CHD by excluding those with simple left-to-right shunt and obstruction.10 Fontan patients were identified using the reimbursement codes for Fontan-type procedures (68033A, 68033B, and 68046B) which represented the creation of a cavopulmonary connection, including either Glenn shunt creation or total cavopulmonary connection. Each health record had a scrambled identification number and consisted of information such as the date of birth, date of visit, sex, type of admission or outpatient department visit, diagnosis and treatment codes, reimbursement fees, and survival status at discharge. Cardiac surgical death was defined as death at discharge from the admission in which the cardiac surgery was performed. The survival status was further validated by the health insurance status on December 31, 2014.
Statistics
We used the Statistical Package for the Social Sciences (SPSS, Version 20.0, SPSS Inc., Chicago, IL, USA) or Statistics Analysis System (SAS, Version 9.4, SAS Institute Inc., Cary, NC, USA) for analysis. We used the chi-squared test to analyze the associations between categorical variables. Multivariate logistic regression was subsequently applied to identify predictors. The trend of Fontan operation incidence with year was then examined using the Poisson regression model which used the numbers of live births each year as the “offsets”. Kaplan-Meier analysis was used to estimate the overall event-free survival. Population and birth statistics data were obtained from the Taiwan National Statistical Yearbook of the Ministry of the Interior (http://sowf.moi.gov.tw/stat/year/list.htm). All p values were 2-sided, and statistical significance was defined as p < 0.05.
RESULTS
From 2000 to 2008, 1,967,991 live births were recorded, and we identified 30241 CHD patients.
Fontan patient cohort
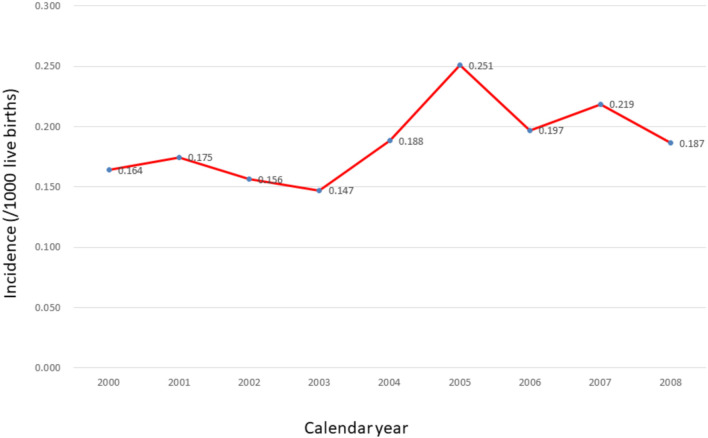
A total of 363 (57.8% male) patients who had undergone a Fontan procedure constituted the Fontan patient cohort, accounting for 1.2% of all cases of CHD. The incidence of Fontan patients from this birth cohort was 0.184/1000 live births (male 0.206/1000 and female 0.164/1000). The underlying diagnoses of these Fontan patients included tricuspid atresia in 52 (14.3%), HLHS in 51 (14.0%), complex CHD in 203 (55.9%), congenitally corrected transposition of great arteries in 9 (2.5%), Ebstein’s anomaly in 6 (1.7%), and others in 42 (11.6%). We defined those with more than 3 underlying cardiac diagnoses as having complex CHD. This mostly involved heterotaxy syndrome, which is composed of right atrial isomerism, endocardial cushion defect, double-outlet right ventricle, total anomalous pulmonary venous connection, pulmonary stenosis and/or pulmonary atresia. The incidence of Fontan procedure stratified by birth year revealed an increasing trend over time; however, the increase was not statistically significant (p = 0.066) (Figure 1).
Figure 1.
The incidence of patients (/1000 live births) who had ever received Fontan operation by calendar year.
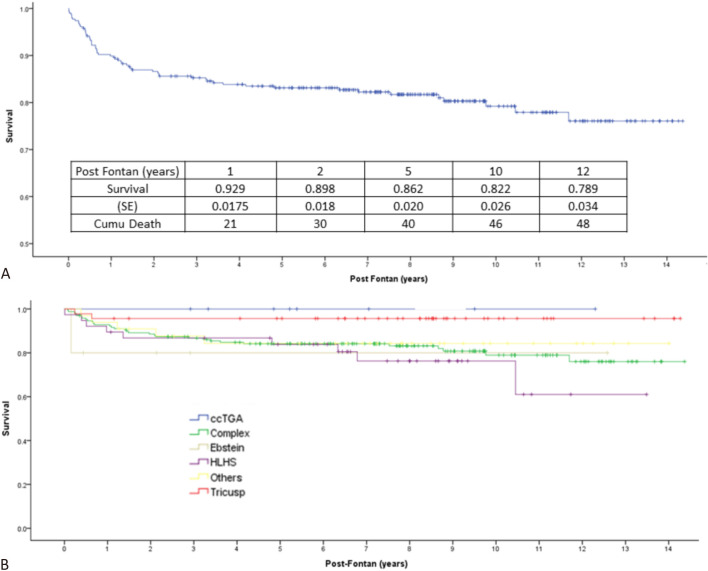
The total postnatal follow-up of the Fontan patients was 2792 patient-years (average: 7.69 patient-years). The initial Fontan-type procedure in the 363 patients was performed at a median age of 14 (24 ± 27) months. Surgical survival was defined as survival to discharge from the surgical admission, regardless of whether the cause of death was the operation itself or other complications. Survival to discharge from the initial Fontan-type procedure was 86.0%. A subsequent staged Fontan procedure was performed in 177 patients, with surgical survival of 92.0%. The overall Fontan surgical survival rate was 81.8%. In those who survived the Fontan procedure, the survival was 0.929 (±0.015), 0.862 (±0.020), 0.822 (±0.026), and 0.789 (±0.034) at 1, 5, 10, and 12 years after the Fontan procedure, respectively (Figure 2A). The causes of death were cardiac in 21 (43.8%), out-of-hospital death in 8 (16.7%), sudden death in 4 (8.3%), infection in 10 (20.8%; sepsis in 8, brain abscess in 1, and endocarditis in 1), cerebral vascular accident in 4 (8.3%), and malignancy in 1 (2.1%). Overall, unexpected death, including sudden death and out-of-hospital death, accounted for 25% of the deaths. The incidence of unexpected death in the post-Fontan patients was 4.0%, or 0.55% per patient-year. Two patients received heart transplant at the ages of 10 and 13 years, respectively. In the post-Fontan patients, the freedom from either death or transplant was 0.929 (±0.015), 0.862 (±0.020), 0.815 (±0.026), and 0.783 (±0.034) at 1, 5, 10, and 12 years after the Fontan procedure, respectively.
Figure 2.
(A) The Kaplan-Meier survival curve of the 297 patients who survived the Fontan procedure. (B) The Kaplan-Meier survival curve of the 297 patients who survived the Fontan procedure grouped according to the diagnosis of underlying congenital heart disease. ccTGA, congenitally corrected transposition of great arteries; Complex, complex congenital heart disease; Ebstein, Ebstein’s anomaly; HLHS, hypoplastic left heart syndrome; Tricuspid, tricuspid atresia.
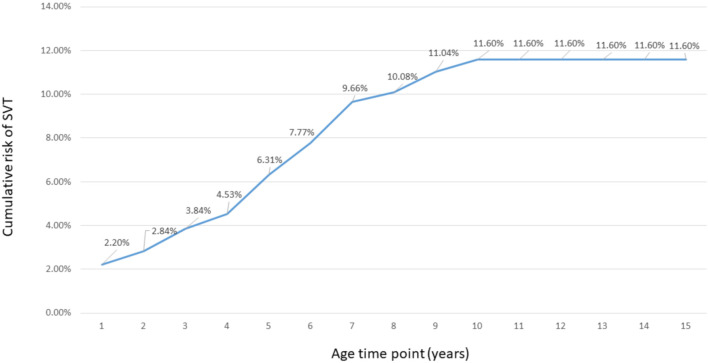
Arrhythmias were noted in 44 (12.1%) patients: tachyarrhythmia in 38 and bradyarrhythmia in 6. Supraventricular tachycardia (SVT) was the most common type of arrhythmia (33 patients, 9.09% of the Fontan patients). In the underlying CHD subgroups, the chance of SVT occurrence was highest in those with Ebstein’s anomaly (2/6, 33.33%) compared to the others (congenitally corrected transposition of great arteries 11.11%, complex 10.84%, tricuspid atresia 7.69%, HLHS 5.88% and others 2.38%), but there was no statistical significance (p = 0.15), probably due to the small number of cases of the subgroup. Two-thirds (22 patients) of these patients experienced the first SVT prior to the Fontan procedure. The cumulative risk of SVT developing in the patients overall was 2.20%, 6.31%, 11.60% and 11.60% by the age of 1, 5, 10 and 15 years, respectively (Figure 3). Electrophysiological studies and radiofrequency ablation were performed in 15 SVT patients at a median age of 6 years (range 3-9 years): 10 prior to the Fontan procedure and 5 after the procedure. Atrial flutter or fibrillation was noted in 4 patients, 2 prior to the Fontan procedure and 2 after the Fontan procedure. Ventricular tachyarrhythmia occurred in 2 patients prior to the Fontan procedure, and in 1 patient after the operation. This patient also received an implantable cardioverter defi-brillator. Six patients experienced multiple forms of tachyarrhythmia. Pacemaker therapy was provided to 5 of the 6 patients with bradycardia (atrioventricular block in 3 patients and sick sinus syndrome plus atrioventricular block in 2).
Figure 3.
From the nationwide birth cohort from 2000 to 2008, the cumulative risk of developing supraventricular tachycardia by age in the Fontan patients (363 patients) (blue line). CHD, congenital heart disease.
As shown in Table 1 and Table 2, the presence of arrhythmias, overall and in each type, was not associated with Fontan surgical survival or post-Fontan survival. Only the underlying CHD group was a significant predictor for post-Fontan survival. Patients with tricuspid atresia had the best post-Fontan survival. Patients with HLHS [p = 0.014, hazard ratio 6.696 (95% confidence interval (CI): 1.466-30.576)] and complex CHD [p = 0.036, hazard ratio 4.611 (95% CI: 1.105-19.242)] had lower post-Fontan survival compared to the patients with tricuspid atresia (Figure 2B). However, the underlying CHD group was not associated with Fontan surgical survival (Table 1, Table 2).
Table 1. Demographic data and risk analysis of Fontal surgical results for the 363 Fontan patients from a birth cohort 2000-2008, derived from Taiwan national database 2000-2014.
| Variable | Fontan surgical results | ||
| Dead (n = 64) | Alive (n = 299) | X2 test | |
| p value | |||
| Male | 34 (53%) | 175 (59%) | 0.486 |
| Arrhythmias | 4 (6%) | 36 (12%) | 0.269 |
| Cardiac diagnosis | |||
| Tricuspid atresia | 6 | 46 | 0.213 |
| Complex | 35 | 168 | 0.826 |
| Ebstein | 1 | 5 | 0.950 |
| HLHS | 12 | 39 | 0.233 |
| ccTGA | 1 | 8 | 0.603 |
| Others | 9 | 33 | 0.492 |
Abbreviations are in Figure 2.
Table 2. Demographic data and risk analysis of Post-Fontal survival for the 363 Fontan patients from a birth cohort 2000-2008, derived from Taiwan national database 2000-2014.
| Variable | Post-Fontan survival analysis | |||||
| Death (n = 50) | Survival (n = 249) | Log rank test | Cox regression multivariable analysis | |||
| p value | Hazard ratio | 95% CI | p value | |||
| Male | 33 (66%) | 142 (57%) | 0.263 | NA | NA | NA |
| Arrhythmias | 5 (10%) | 31 (12%) | 0.624 | NA | NA | NA |
| Cardiac diagnosis | ||||||
| Tricuspid atresia | 2 | 44 | 0.019 | Ref. | Ref. | Ref. |
| Complex | 32 | 136 | 0.284 | 4.611 | 1.105-19.242 | 0.036 |
| Ebstein | 1 | 4 | 0.544 | 7.238 | 0.668-81.665 | 0.103 |
| HLHS | 10 | 29 | 0.090 | 6.696 | 1.466-30.576 | 0.014 |
| ccTGA | 0 | 8 | 0.242 | 0 | 0.977 | |
| Others | 5 | 28 | 0.769 | 3.597 | 0.698-18.544 | 0.126 |
Abbreviations are in Figure 2.
DISCUSSION
This is the first study on Fontan patients conducted using a nationwide birth cohort with complete postnatal medical data for at least 6 years. This study provides some novel findings. First, the incidence of CHD requiring the Fontan procedure was 0.184/1000 live births. Second, SVT was common in Fontan patients, with two-thirds of cases occurring prior to the Fontan procedure. The postnatal cumulative risk of SVT was 11.6% by the age of 10 years, which was 145-fold greater than that in the non-Fontan major CHD and general pediatric populations. Third, in those who survived the Fontan operation, survival at 10 years after the Fontan procedure was 82.2%. The majority of deaths were due to cardiac causes, but the incidence of unexpected death was relatively high and was 0.55% per patient-year by adolescence.
In the current nationwide birth cohort 2000-2008, the incidence of CHD patients who required the Fontan procedure was 0.184/1000 live births. This incidence is slightly higher than an earlier estimate that was derived from a tertiary care center and the served population in Australia from 1995-1999 (0.10/1000).7 The Fontan procedure can be offered to a heterogeneous group of CHD patients. Using the sum of incidence data of single ventricle, tricuspid atresia, and HLHS, the median estimate for the number of potential Fontan candidates would be 0.403 (0.248-0.533)/1000.9,11 Nevertheless, these patients must receive and survive the neonatal or infant palliation procedures tailored to each CHD to receive a Fontan procedure. Therefore, the number of Fontan candidates is lower than the sum of the incidence data of CHDs that require the Fontan procedure as the final treatment. With surgical advancements in neonatal palliation surgery prior to the Fontan procedure, such as the Norwood procedure, the number of Fontan candidates may continue to increase. An increasing trend from 1995 to 1998 was reported.7 Accordingly, the incidence of patients requiring the Fontan procedure currently lies in the range of 0.1-0.2/1000 live births, and this may increase over time in countries with structured CHD programs. Such data will help to estimate the future need for the Fontan procedure and associated long-term care. For example, there may be 40 and 800 new Fontan patients in countries with 200000 (e.g., Taiwan) and 4 million (e.g., United States) live births, respectively.
Fontan patients are at a higher risk of SVT due to associated cardiac conduction system anomalies.4-6,12-15 The twin atrioventricular nodes, the so called "Monckeberg sling" are common in many CHDs, including right atrial isomerism, congenitally corrected transposition of the great arteries, atrioventricular discordance with a malaligned atrioventricular defect, and may result in SVT.4,5,15-17 However, the postnatal cumulative risk of SVT has never been described. In the current study, SVT appeared early, and two-thirds of the cases occurred prior to the Fontan procedure. The cumulative risks of SVT had already reached 6.3% and 11.6% by the age of 5 years and 10 years, respectively, which is much higher than that in the general population.10,18,19 In the same birth cohort, the cumulative incidence of SVT in the general population was only 0.08% by the age of 10 years.10 Ebstein’s anomaly is known to be associated with a higher probability (20-30%) of SVT from right-sided accessory pathways.20 The reported probability of being free from tachyarrhythmia by 20 years of age is 76%.20 Only severe forms of Ebstein’s anomaly need to be treated with the Fontan procedure. In this birth cohort, the rates of Ebstein’s anomaly in the Fontan and non-Fontan patients were low at 1.7% and 3.3%, respectively, and this would not result in a higher risk of SVT in the Fontan patients. Although the hemodynamic compromise from SVT in patients with single ventricle physiology is significant, the occurrence of SVT was not associated with Fontan surgical mortality or post-Fontan survival in this birth cohort.
In the 2016 update of the Society of Thoracic Surgeons Congenital Heart Surgery Database, the surgical mortality rate of the Fontan procedure ranged from 0% to 20%.2 The surgical mortality rate in the current study was 18%, which is close to the high end and may be related to our non-selection study bias. We selected cases from all institutions regardless of the patient volume. In our post-Fontan patients, the survival rates at 1, 5, and 10 years after the Fontan procedure were 92.9%, 86.2% and 82.2%, respectively. These data are close to those from a previous institutional study from Taiwan, with estimated event-free survival rates at 1 year, 5 years, and 10 years of 90.6%, 89.3%, and 77.2%, respectively.21 With advancements in medical care, the 10-year survival of post-Fontan patients has greatly improved, with the best results ranging from 83.4% to 92%.22-25 Compared with Western Fontan cohorts, our cohort had fewer patients with HLHS, which may be due to a lower incidence of HLHS in Eastern populations than in Western populations.9,11 Apart from cardiac causes of death, sudden death and out-of-hospital death accounted for 8.3% and 16.7%, respectively, of the deaths in the post-Fontan patients. Overall, unexpected death (including sudden death and out-of-hospital death) accounted for 25% of all deaths. The incidence of unexpected death in the post-Fontan patients, including sudden death and out of hospital death, was already 4.0%, or 0.55% per patient-year by adolescence. A previous study reported that sudden death was a major cause of late death in Fontan patients, occurring in 5% of the patients at late follow-up (mean age of 20.5 ± 10.1 years).26 A more recent large nationwide study found that 13% of deaths were due to sudden cardiac death, and they were presumed to be caused by arrhythmia.27 An atrioventricular valve replacement at the time of the Fontan procedure and a post-bypass Fontan pressure > 20 mmHg are considered to be risk factors for late sudden death, however the underlying mechanisms remain unclarified.26 The mechanisms of unexpected death in young Fontan patients need to be elucidated in future studies.
CONCLUSIONS
From a nationwide birth cohort, the incidence of CHD that required a Fontan procedure was 0.184/1000 live births. These patients were at a high risk of SVT from infancy, probably due to associated cardiac conduction system anomalies, and two-thirds of the cases of SVT occurred prior to the Fontan procedure. The postnatal cumulative risk of SVT was already 11.6% by the age of 10 years, which is 145-fold greater than that in the age-comparable general population. The survival at 10 years after the Fontan procedure was 82.2%, with major causes of death from cardiac causes and unexpected death. The long-term medical needs of post-Fontan patients will be a major issue if surgical alternatives to rescue patients with a single functional ventricle are not developed.
Limitations
Our findings are robust despite certain limitations. First, the birth cohort was extracted from the NHI Database, and we could not directly assess the details of cardiac morphology and hemodynamic data. We therefore could not perform further analysis. Second, we could not examine the electrocardiograms and validate the diagnosis of the type of arrhythmia. Third, the age distribution of the cohort may have slightly underestimated the number of children who developed arrhythmias after 10 years of age. Finally, patients who suddenly died before reaching the hospital may not have had an accurate diagnosis, which may have resulted in an underestimation of sudden death. These patients may have been diagnosed as out-of-hospital death.
Acknowledgments
The authors acknowledge statistical assistance provided by Department of Medical Research in National Taiwan University Hospital.
FUNDING STATEMENT
This work was supported by the Ministry of Science and Technology, Taiwan (ROC) [grant numbers 106-2314-B-002-175-MY3, 103-2314-B-002-054-MY3].
CONFLICT OF INTEREST
All the authors declare no conflict of interest.
REFERENCES
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jacobs JP, Mayer JE, Jr., Mavroudis C, et al. The Society of Thoracic Surgeons Congenital Heart Surgery Database: 2016 update on outcomes and quality. Ann Thorac Surg. 2016;101:850–862. doi: 10.1016/j.athoracsur.2016.01.057. [DOI] [PubMed] [Google Scholar]
- 3.Dabal RJ, Kirklin JK, Kukreja M, et al. The modern Fontan operation shows no increase in mortality out to 20 years: a new paradigm. J Thorac Cardiovasc Surg. 2014;148:2517–2523, e1. doi: 10.1016/j.jtcvs.2014.07.075. [DOI] [PubMed] [Google Scholar]
- 4.Wu MH, Wang JK, Lin JL, et al. Supraventricular tachycardia in patients with right atrial isomerism. J Am Coll Cardiol. 1998;32:773–779. doi: 10.1016/s0735-1097(98)00307-6. [DOI] [PubMed] [Google Scholar]
- 5.Epstein MR, Saul JP, Weindling SN, et al. Atrioventricular reciprocating tachycardia involving twin atrioventricular nodes in patients with complex congenital heart disease. J Cardiovasc Electrophysiol. 2001;12:671–679. doi: 10.1046/j.1540-8167.2001.00671.x. [DOI] [PubMed] [Google Scholar]
- 6.Dickinson DF, Wilkinson JL, Anderson KR, et al. The cardiac conduction system in situs ambiguus. Circulation. 1979;59:879–885. doi: 10.1161/01.cir.59.5.879. [DOI] [PubMed] [Google Scholar]
- 7.Iyengar AJ, Shann F, Cochrane AD, et al. The Fontan procedure in Australia: a population-based study. J Thorac Cardiovasc Surg. 2007;134:1353–1354. doi: 10.1016/j.jtcvs.2007.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Wu MH, Chen HC, Wang JK, et al. Population-based study of pediatric sudden death in Taiwan. J Pediatr. 2009;155:870–874, e2. doi: 10.1016/j.jpeds.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 9.Wu MH, Chen HC, Lu CW, et al. Prevalence of congenital heart disease at live birth in Taiwan. J Pediatr. 2010;156:782–785. doi: 10.1016/j.jpeds.2009.11.062. [DOI] [PubMed] [Google Scholar]
- 10.Wu MH, Chen HC, Kao FY, Huang SK. Postnatal cumulative incidence of supraventricular tachycardia in a general pediatric population: a national birth cohort database study. Heart Rhythm. 2016;13:2070–2075. doi: 10.1016/j.hrthm.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–1900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 12.Chiu SN, Wang JK, Lu CW, et al. Electrophysiology study for complex supraventricular tachycardia in congenital heart disease patients with single-ventricle physiology. J Am Heart Assoc. 2016;5 doi: 10.1161/JAHA.116.004504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carins TA, Shi WY, Iyengar AJ, et al. Long-term outcomes after first-onset arrhythmia in Fontan physiology. J Thorac Cardiovasc Surg. 2016;152:1355–1363, e1. doi: 10.1016/j.jtcvs.2016.07.073. [DOI] [PubMed] [Google Scholar]
- 14.Smith A, Ho SY, Anderson RH, et al. The diverse cardiac morphology seen in hearts with isomerism of the atrial appendages with reference to the disposition of the specialised conduction system. Cardiol Young. 2006;16:437–454. doi: 10.1017/S1047951106000382. [DOI] [PubMed] [Google Scholar]
- 15.Wu MH, Wang JK, Lin JL, et al. Long-term outcome of twin atrioventricular node and supraventricular tachycardia in patients with right isomerism of the atrial appendage. Heart Rhythm. 2008;5:224–229. doi: 10.1016/j.hrthm.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 16.Bae EJ, Noh CI, Choi JY, et al. Twin AV node and induced supraventricular tachycardia in Fontan palliation patients. Pacing Clin Electrophysiol. 2005;28:126–134. doi: 10.1111/j.1540-8159.2005.09450.x. [DOI] [PubMed] [Google Scholar]
- 17.Wu MH, Lin JL, Wang JK, et al. Electrophysiological properties of dual atrioventricular nodes in patients with right atrial isomerism. Br Heart J. 1995;74:553–555. doi: 10.1136/hrt.74.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Balli S, Kucuk M, Orhan Bulut M, et al. Transcatheter cryoablation procedures without fluoroscopy in pediatric patients with atrioventricular nodal reentrant tachycardia: a single-center experience. Acta Cardiol Sin. 2018;34:337–343. doi: 10.6515/ACS.201807_34(4).20180326A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng WC, Wu MH, Lu CW, et al. Zero fluoroscopy during ablation of right-sided supraventricular tachycardia substrates in a pediatric population – initial experience in Taiwan. Acta Cardiol Sin. 2019;35:476–483. doi: 10.6515/ACS.201909_35(5).20190211A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang YM, Wang JK, Chiu SN, et al. Clinical spectrum and long-term outcome of Ebstein’s anomaly based on a 26-year experience in an Asian cohort. Eur J Pediatr. 2009;168:685–690. doi: 10.1007/s00431-008-0820-0. [DOI] [PubMed] [Google Scholar]
- 21.Pan JY, Lin CC, Wu CJ, Chang JP. Early and intermediate-term results of the extracardiac conduit total cavopulmonary connection for functional single-ventricle hearts. J Formos Med Assoc. 2016;115:318–324. doi: 10.1016/j.jfma.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 22.Ohuchi H, Kagisaki K, Miyazaki A, et al. Impact of the evolution of the Fontan operation on early and late mortality: a single-center experience of 405 patients over 3 decades. Ann Thorac Surg. 2011;92:1457–1466. doi: 10.1016/j.athoracsur.2011.05.055. [DOI] [PubMed] [Google Scholar]
- 23.Martin BJ, Ross DB, Aklabi MA, et al. Post-operative outcomes in children undergoing Fontan palliation in a regionalized surgical system. Pediatr Cardiol. 2017 doi: 10.1007/s00246-017-1710-x. [DOI] [PubMed] [Google Scholar]
- 24.Pundi KN, Johnson JN, Dearani JA, et al. 40-year follow-up after the Fontan operation: long-term outcomes of 1,052 patients. J Am Coll Cardiol. 2015;66:1700–1710. doi: 10.1016/j.jacc.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 25.Downing TE, Allen KY, Glatz AC, et al. Long-term survival after the Fontan operation: twenty years of experience at a single center. J Thorac Cardiovasc Surg. 2017;154:243–253, e2. doi: 10.1016/j.jtcvs.2017.01.056. [DOI] [PubMed] [Google Scholar]
- 26.Pundi KN, Pundi KN, Johnson JN, et al. Sudden cardiac death and late arrhythmias after the Fontan operation. Congenit Heart Dis. 2017;12:17–23. doi: 10.1111/chd.12401. [DOI] [PubMed] [Google Scholar]
- 27.Dennis M, Zannino D, du Plessis K, et al. Clinical outcomes in adolescents and adults after the Fontan procedure. J Am Coll Cardiol. 2018;71:1009–1017. doi: 10.1016/j.jacc.2017.12.054. [DOI] [PubMed] [Google Scholar]