Abstract
Background
Nesfatin-1 is a novel peptide possessing pleiotropic metabolic effects. No-reflow phenomenon (NR) is a poor prognostic indicator occurring in around 30% of all patients undergoing primary percutaneous coronary interventions (pPCI). Inflammation and complexity of coronary artery disease (CAD) play pivotal roles in the pathogenesis of NR. In this study, we investigated the relationship between admission serum nesfatin-1 level, NR and complexity of CAD assessed by SYNTAX-1 (SS-1) and SYNTAX-2 (SS-2) scores in patients with ST-segment elevation myocardial infarction (STEMI) undergoing pPCI.
Methods
A total of 174 STEMI patients who underwent pPCI were included in the study and divided into NR (n = 36) and normal flow (n = 138) groups. Serum nesfatin-1 was measured by enzyme-linked immunosorbent assay. Seventy-eight consecutive age-, gender- and co-morbidity-matched patients undergoing coronary angiography with < 50% stenosis comprised the control group.
Results
Nesfatin-1 levels were significantly lower in the NR group compared to the normal flow and control groups (10.8 ± 6.6 ng/mL vs. 34.9 ± 24 ng/mL vs. 43.6 ± 23.2 ng/mL, respectively, p < 0.001). Nesfatin-1 was significantly and inversely correlated with SS-1 and SS-2 scores (r = -0.709 and r = -0.655, respectively, both p < 0.001). Multivariate logistic regression analysis showed that nesfatin-1 [odds ratio (OR) = 0.81, 95% confidence interval (CI) = 0.708-0.936, p = 0.004] and glomerular filtration rate (OR = 0.94, 95% CI = 0.892-0.989, p = 0.018) were independently associated with NR. In the receiver operating characteristic analysis, nesfatin-1 < 15.21 ng/mL predicted NR with 78.4% sensitivity and 72.2% specificity (area under the curve = 0.809, 95% CI = 0.701-0.918, p < 0.001).
Conclusions
Admission nesfatin-1 level is a potent predictor of NR in STEMI patients undergoing pPCI. Additionally, nesfatin-1 has a robust and negative correlation with the complexity of CAD.
Keywords: Acute myocardial infarction, Nesfatin-1, No-reflow phenomenon, SYNTAX score
INTRODUCTION
ST-segment elevation myocardial infarction (STEMI) is a significant cause of morbidity and mortality worldwide. A great improvement in short-term mortality has been achieved since the introduction of percutaneous interventional techniques in the coronary vessels; however, the long-term prognosis has significantly improved despite the opening of totally occluded coronary arteries, especially in the setting of STEMI.1 A number of factors have been proposed to explain this situation, including no-reflow (NR) phenomenon.2,3
NR phenomenon is a term that indicates failure of normal reperfusion of the myocardium despite opening of an occluded epicardial coronary vessel,4 and it occurs with varying incidence in all primary percutaneous coronary interventions (pPCI). When encountered in a patient, NR confers a high risk of mortality owing to inadequate healing of the infarct, poor ventricular remodeling, and higher susceptibility to heart failure.2,3,5
Nesfatin-1 is a novel anorexigenic peptide of 82 amino acids which is a derivative nucleobindin-2.6 First isolated in the brain nuclei modulating satiety, nesfatin-1 was then detected in such diverse groups of cells as gastric mucosa, adipose tissue, cardiac cells, and pancreatic cells.6,7 Furthermore, accumulating evidence suggests that nesfatin-1 is involved in thermoregulation, pancreatic insulin recreation, hepatic glucose metabolism, anxiety, gastric function, reproduction, cardiovascular system modulation and inflammation.8,9
Recent studies conducted in patients with acute coronary syndrome have shown associations between disease severity and nesfatin-1 levels.10,11 In 2013, Dai et al.10 assessed serum nesfatin-1 levels in a comparable manner among patients with acute myocardial infarction (AMI), stable angina pectoris (SAP) and control subjects, and detected significantly lower serum nesfatin-1 levels in the AMI patients compared to the SAP patients and controls. Moreover, nesfatin-1 levels were negatively associated with Gensini score. In another study in 2018 by Kuyumcu et al.,11 nesfatin-1 levels were lower in non-ST segment elevation myocardial infarction patients with higher SYNTAX-1 score compared to those with a lower score and controls.
However, the association between serum nesfatin-1 level and NR phenomenon in patients with STEMI has yet to be evaluated. Therefore, in the present study, we investigated the relationship between nesfatin-1 level and NR in STEMI patients, and evaluated its potential association with SYNTAX-1 (SS-1) and SYNTAX-2 (SS-2) scores.
METHODS
Study population
We prospectively enrolled 174 consecutive patients admitted to our hospital with STEMI and underwent pPCI between February 2018 and December 2018. The enrolled patients were further subdivided into two subgroups: the NR group (n = 36, mean age 57.1 ± 9.5 years) and normal flow group (n = 138, mean age 57.8 ± 12.5 years). Subjects with clinically suspected severe coronary artery disease (CAD) with multiple risk factors but in whom minor CAD (< 50% luminal stenosis by visual estimation) was detected on coronary angiography comprised the control group. STEMI was defined on the basis of the relevant guidelines,12 and included a rise and/or fall in cardiac troponins with at least one value above the 99th percentile of the upper reference limit with at least one of the following features: ischemia-related symptoms; new or presumably new ST segment elevation in ≥ 2 contiguous leads with a cutoff point of ≥ 0.2 mV in the anterior leads or new left bundle branch block; development of pathological Q waves in the electrocardiogram; imaging evidence of new loss of viable myocardium, or new regional wall motion abnormality; and identification of an intracoronary thrombus by angiography.
The exclusion criteria were recent myocardial infarction or cardiac surgery, administration of thrombolytic therapy before PCI, acute or chronic inflammation, severe hepatic, renal, or hematological disease, history of psychiatric or neurological disorders, and history of cardiomyopathy.
A detailed medical history was taken and a thorough physical examination was performed in all of the patients, and baseline demographic features including age, sex, hypertension (HT), chronic obstructive pulmonary disease, peripheral artery disease (PAD), smoking habit, diabetes mellitus (DM), and CAD were recorded. Left ventricular ejection fraction (LVEF) was measured using the modified Simpson’s rule, as suggested by the American Society of Echocardiography.13
This study complied with the principals of the Declaration of Helsinki, and the local ethics committee approved the study protocol. All of the patients provided informed consent.
Coronary angiography, percutaneous coronary intervention and definition of angiographic no-reflow
All patients were treated according to the recommendations of the relevant STEMI guidelines.14 Once written informed consent for cardiac catheterization had been obtained, emergency coronary angiography was performed in all patients using standard techniques. After wiring of the infarct related artery (IRA), a glycoprotein IIb/IIIa inhibitor (tirofiban) or thrombus aspiration was given to the patients in the catheterization laboratory at the operator’s discretion. Direct stenting of the IRA was attempted whenever possible, and balloon pre-dilatation was performed in the remaining cases. Primary PCI of the IRA was performed using standard clinical practice, and the choice of drug-eluting stent or bare metal stent was at the operator’s discretion. Baseline and post-PCI thrombolysis in myocardial infarction (TIMI) flow grade of the IRA in each patient was evaluated by two cardiologists blinded to the study. TIMI flow grade 3 for the IRA with residual stenosis < 20% was considered to be normal flow.15 No-reflow was defined as a TIMI flow grade of ≤ 2 following stenting of the IRA with no evidence of coronary dissection or a reduction in TIMI flow after restoration of TIMI flow grade 3.15
Calculation of the SYNTAX-1 and SYNTAX-2 scores
Assessment of the cineangiographic views was performed using an Axiom (Siemens Medical Solution, Erlangen, Germany) workstation by two experienced cardiologist blinded to the study. Each lesion with a diameter stenosis ≥ 50% in coronary vessels ≥ 1.5 mm in diameter was scored using the online SYNTAX score calculator (http://www.syntaxscore.com). If the cardiologists disagreed about the lesions, the final score was decided by averaging the scores calculated by each cardiologist. SS-1 and SS-2 scores were obtained for each patient.
Laboratory analysis
Blood samples were obtained from every patient through venipuncture on admission to the emergency department. The samples were then immediately transferred to the laboratory. The collected blood samples were centrifuged at 1500 g for 10 min to separate the serum.
Serum was stored at -80 °C until analysis for nesfatin-1. Serum nesfatin-1 levels were measured using a commercial enzyme-linked immunosorbent assay kit (Rel Assay Diagnostics, Mega Tıp, Gaziantep, Turkey), which had a sensitivity of 0.32 ng/ml. Routine serum biochemical parameters were measured using an automated clinical chemistry analyzer (Roche Hitachi Cobas c8000 autoanalyzer, Roche Diagnostic Corp., Mannheim, Germany).
Statistical analysis
Statistical analysis of the study data was performed using SPSS software for Windows (IBM SPSS Statistics for Windows, Version 22.0. Armonk, NY: IBM Corp., USA). Quantitative variables were tested for normality using Kolmogorov-Smirnov and Shapiro-Wilk tests. Univariate analysis of the study parameters, on the basis of their types and the fulfillment of presumptions, was performed using the chi-square test, Fisher’s exact test, Mann-Whitney U test, independent t test, one-way ANOVA, and Kruskal-Wallis test. Descriptive variables were expressed as mean ± standard deviation, median (interquartile range at the 25th and 75th percentiles, IQR), and percentages (%), where appropriate. The variables that were significantly different between groups on the basis of the univariate analysis were further subjected to multivariate logistic regression analysis. Correlations between the variables were assessed using Pearson correlation analysis. Moreover, receiver operating curve (ROC) analysis was performed to define possible diagnostic cut-off values of the variables with significant contribution to the multivariate logistic regression model. A p value < 0.05 was considered to be statistically significant.
RESULTS
The clinical and procedural characteristics of all STEMI patients are shown in Table 1, and the baseline demographic and clinical features of the study population are shown in Table 2. Of the 174 STEMI patients, NR was observed in 36 (mean age 57.1 ± 9.5 years), and normal flow was achieved in 138 (mean age 57.8 ± 12.5 years). There were no significant differences between the groups in DM, CAD, HT, hyperlipidemia (HL), smoking habit, and obesity (p > 0.05). LVEF was significantly lower in the STEMI patients compared to the controls; however, a pair-wise comparison of LVEF between the normal flow and NR groups did not reveal a statistically significant difference. The STEMI patients with NR had significantly higher SS-1 and SS-2 scores compared to those with normal flow (27.2 ± 8.3 vs. 14.8 ± 7.3, respectively for SS-1, p < 0.001; 42.38 ± 5.9 vs. 21.70 ± 9.44, respectively for SS-2, p < 0.001).
Table 1. Clinical and procedural characteristics of all STEMI patients.
| Variables | Value |
| Age (years) | 57.6 ± 12.6 |
| Female gender, n (%) | 45 (25.8) |
| LVEF (%) | 43.7 ± 7.7 |
| MI region | |
| Anterior MI, n (%) | 54 (31.0) |
| Septal MI, n (%) | 27 (15.5) |
| Lateral MI, n (%) | 23 (13.2) |
| Inferior MI, n (%) | 48 (27.5) |
| Posterior MI, n (%) | 18 (10.3) |
| Right ventricle MI, n (%) | 4 (2.2) |
| KILLIP class, n (%) | |
| I | 72 (41.3) |
| II | 53 (30.4) |
| III | 20 (11.4) |
| IV | 29 (16.6) |
| Drug usage, n (%) | |
| Beta-blockers | 40 (22.9) |
| Calcium channel blockers | 28 (16.0) |
| ACEIs/ARBs | 58 (33.3) |
| ASA | 16 (9.1) |
| Clopidogrel/prasugrel/ticagrelor | 9 (5.1) |
| Warfarin/NOACs | 6 (3.4) |
| Statins | 23 (13.2) |
| Co-medication for MI, n (%) | |
| Dual antiplatelet therapy, n(%) | |
| ASA plus clopidogrel | 130 (74.7) |
| ASA plus ticagrelor | 35 (20.1) |
| ASA plus prasugrel | 9 (5.1) |
| Glycoprotein IIb/IIIa inhibitors (tirofiban) | 60 (34.4) |
| Thrombus aspiration | 23 (13.2) |
| Intra-aortic balloon pumping | 7 (4.0) |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; ASA, acetylsalicylic acid; LVEF, left ventricular ejection fraction; MI, myocardial infarction; NOACs, novel oral anticoagulant drugs; STEMI, ST-segment elevation myocardial infarction.
Table 2. Demographic and clinical characteristics of the study groups.
| Variables | Normal flow group (n = 138) | No-reflow group (n = 36) | Controls (n = 78) | p value |
| Age (years) | 57.8 ± 12.5 | 57.1 ± 9.5 | 55.2 ± 13.9 | 0.662 |
| Female gender, n (%) | 36 (26) | 9 (25) | 20 (25.6) | 0.642 |
| DM, n (%) | 8 (5.7) | 2 (5.5) | 4 (5.1) | 0.611 |
| CAD, n (%) | 14 (10.1) | 6 (16.6) | 18 (23) | 0.192 |
| HT, n (%) | 58 (42) | 10 (27.7) | 24 (30.7) | 0.358 |
| HL, n (%) | 38 (27.5) | 4 (11.1) | 12 (15.3) | 0.173 |
| Smoking, n (%) | 58 (42) | 12 (33.3) | 18 (23) | 0.138 |
| Obesity, n (%) | 16 (11.5) | 6 (16.6) | 12 (15.3) | 0.783 |
| LVEF (%) | 44.1 ± 7.4 | 42.4 ± 5.9 | 59.3 ± 4.2 | < 0.001 |
| SS-1 | 14.8 ± 7.3 | 27.2 ± 8.3 | - | < 0.001 |
| SS-2 | 21.70 ± 9.44 | 42.38 ± 5.9 | - | < 0.001 |
| Drug usage, n (%) | ||||
| Beta-blockers | 33 (23.9%) | 7 (19.4%) | 12 (15.3%) | 0.256 |
| Calcium channel blockers | 22 (15.9%) | 6 (16.6%) | 8 (10.2%) | 0.442 |
| ACEIs/ARBs | 47 (34.0%) | 11 (30.5%) | 23 (29.4%) | 0.991 |
| ASA | 11 (7.9%) | 5 (13.8%) | 14 (17.9%) | 0.155 |
| Clopidogrel/prasugrel/ticagrelor | 6 (4.3%) | 3 (8.3%) | 6 (7.6%) | 0.323 |
| Warfarin/NOACs | 4 (2.8%) | 2 (5.5%) | 2 (2.5%) | 0.568 |
| Statins | 20 (14.4%) | 3 (8.3%) | 8 (10.2%) | 0.189 |
ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; ASA, acetylsalicylic acid; CAD, coronary artery disease; DM, diabetes mellitus; HL, hyperlipidemia; HT, hypertension; LVEF, left ventricular ejection fraction; NOACs, novel oral anticoagulant drugs; SS-1, SYNTAX-1 score; SS-2, SYNTAX-2 score.
Hematological, biochemical and serum nesfatin-1 measurements of the 3 study groups are given in Table 3. White blood cell (WBC) (p = 0.011), neutrophil (p = 0.005), and monocyte (p = 0.002) counts were significantly higher in the NR group compared to the normal flow and control groups. On the other hand, glomerular filtration rate (GFR) (p = 0.001), serum albumin level (p < 0.001), and platecrit (PCT) (p = 0.036) were significantly lower in the NR group compared to the normal flow and control groups. Moreover, serum nesfatin-1 level was found to be significantly lower in the NR group compared to the normal flow and control groups (10.8 ± 6.6 ng/mL, p < 0.001); however, no statistically significant difference was detected in a pair-wise comparison between the normal flow and control groups (34.9 ± 24 ng/mL vs. 43.6 ± 23.2 ng/mL, respectively, p > 0.05).
Table 3. Hematological and biochemical measures of the study groups.
| Variables | Normal flow group (n = 138) | No-reflow group (n = 36) | Controls (n = 78) | p value |
| Glucose (mg/dL) | 120.5 ± 54 | 117.2 ± 51 | 100.4 ± 18.6 | 0.089 |
| GFR (mL/min/1.73 m2) | 86.1 ± 19.9a | 77.4 ± 22.4b | 92.4 ± 10.2a | 0.001 |
| TG (mg/dL) | 175.8 ± 100 | 152.1 ± 93.6 | 170.8 ± 91.4 | 0.835 |
| T-C (mg/dL) | 171.9 ± 47.9 | 158.8 ± 52.6 | 196.3 ± 85.3 | 0.113 |
| LDL-C (mg/dL) | 96.8 ± 41 | 88 ± 38.4 | 121.2 ± 76.3 | 0.063 |
| HDL-C (mg/dL) | 40.1 ± 11.4 | 39 ± 8.1 | 44.4 ± 10.1 | 0.110 |
| Calcium (mg/dL) | 9.3 ± 0.6 | 9.2 ± 0.7 | 9.6 ± 0.4 | 0.135 |
| Albumin (mg/dL) | 4.03 ± 0.4a | 3.4 ± 0.5a | 4.5 ± 0.2b | < 0.001 |
| WBC (×109/L) | 9.22 ± 4.3ab | 10.5 ± 3.6a | 8.46 ± 1.3b | 0.011 |
| Hb (gr/dL) | 14.23 ± 1.74 | 13.78 ± 2.14 | 13.79 ± 2.53 | 0.656 |
| Plt (×109/L) | 267.31 ± 73.07 | 230.22 ± 106.89 | 293.00 ± 129.87 | 0.144 |
| Neutrophil (×109/L) | 6.09 ± 4.5ab | 7.00 ± 3a | 5.04 ± 1.2b | 0.005 |
| Lymphocyte (×109/L) | 2.48 ± 1.17 | 2.16 ± 0.96 | 2.57 ± 0.39 | 0.619 |
| Monocyte (×109/L) | 0.74 ± 0.22a | 0.80 ± 0.27a | 0.60 ± 0.26b | 0.002 |
| MPV (fL) | 10.28 ± 0.9 | 10.57 ± 0.86 | 10.28 ± 0.91 | 0.807 |
| PDW (%) | 12.17 ± 2 | 12.47 ± 1.8 | 12.09 ± 2.1 | 0.904 |
| PCT (%) | 0.27 ± 0.06ab | 0.22 ± 0.06a | 0.30 ± 0.11b | 0.036 |
| C-RP (mg/dL) | 0.43 (0.21-1.58) | 0.55 (0.32-1.01) | 0.37 (0.22-0.79) | 0.131 |
| Troponin (pg/mL) | 1201.00 (87.00-3759.0) | 1451.50 (29.85-5859.00) | - | 0.364 |
| CK-MB (ng/mL) | 16.00 (3.05-71.75) | 21.81 (2.82-96.50) | - | 0.344 |
| Nesfatin-1 (ng/mL) | 34.9 ± 24a | 10.8 ± 6.6b | 43.6 ± 23.2a | < 0.001 |
* There is no statistically significant difference between the pairs marked with the same letter within the same line (p > 0,05).
CK-MB, MB fraction of creatin kinase; C-RP, C-reactive protein; GFR, glomerular filtration rate; Hb, hemoglobin; HDL-C, high-density lipoprotein cholesterol; LDL-C; low-density lipoprotein cholesterol; MPV, mean platelet volume; PCT, platecrit; PDW, platelet distribution width; Plt, platelet count; T-C, total cholesterol; TG, triglyceride; WBC, white blood cell count.
Bivariate correlations are shown in Table 4. According to the correlation analysis, serum nesfatin-1 levels were significantly negatively correlated with SS-1 and SS-2 scores (r = -0.709 and r = -0.655, respectively, both p < 0.001). Furthermore, SS-2 scores were significantly and positively correlated with SS-1 scores (r = 0.919, p < 0.001). The other variables with statistically significant differences between the 3 groups did not show significant correlations between the groups (p > 0.05).
Table 4. Bivariate correlations between the study variables.
| GFR | Albumin | WBC | Neutrophil | Monocyte | SS-1 | SS-2 | PCT | Nesfatin-1 | |
| GFR | 1 | 0.288* | -0.194* | -0.250* | -0.093 | -0.178 | -0.165 | 0.073 | 0.038 |
| Albumin | 1 | -0.039 | -0.140 | -0271* | -0.072 | -0.089 | 0.037 | 0.127 | |
| WBC | 1 | 0.937* | 0.592* | 0.043 | 0.048 | 0.108 | -0.068 | ||
| Neutrophil | 1 | 0.465* | 0.038 | 0.050 | 0.088 | -0.100 | |||
| Monocyte | 1 | 0.002 | -0.019 | -0.037 | 0.062 | ||||
| SS-1 | 1 | 0.919* | 0.085 | -0.709* | |||||
| SS-2 | 1 | 0.090 | -0.655* | ||||||
| PCT | 1 | -0.033 | |||||||
| Nesfatin-1 | 1 |
* Correlation is significant at the 0.01 level. Abbreviations are in Table 3.
Multivariate logistic regression analysis showed that nesfatin-1 [odds ratio (OR) = 0.81, 95% confidence interval (CI) = 0.708-0.936, p = 0.004] and GFR (odds ratio = 0.94, 95% CI = 0.892-0.989, p = 0.018) were independent predictors of NR in the STEMI patients. However, neutrophils and albumin were not statistically significant independent predictors of NR. Coefficients of the predictors in the logistic model are shown in Table 5.
Table 5. Results of multivariate logistic regression analysis.
| Variables | β | p value | Odds ratio | 95%C.I. for OR |
| Lower-upper | ||||
| GFR | -0.062 | 0.018 | 0.940 | 0.892-0.989 |
| Neutrophil | -0.202 | 0.150 | 0.817 | 0.621-1.076 |
| Nesfatin-1 | -0.206 | 0.004 | 0.814 | 0.708-0.936 |
| Albumin | 0.594 | 0.556 | 1.812 | 0.250-13.102 |
CI, confidence interval; GFR, glomerular filtration rate; OR, odds ratio.
In the ROC analysis, the optimal cut-off value of nesfatin-1 to predict the occurrence of NR with 78.4% sensitivity and 72.2% specificity was 15.21 ng/mL [area under the curve (AUC) = 0.809, 95% CI = 0.701-0.918, p < 0.001] (Figure 1).
Figure 1.
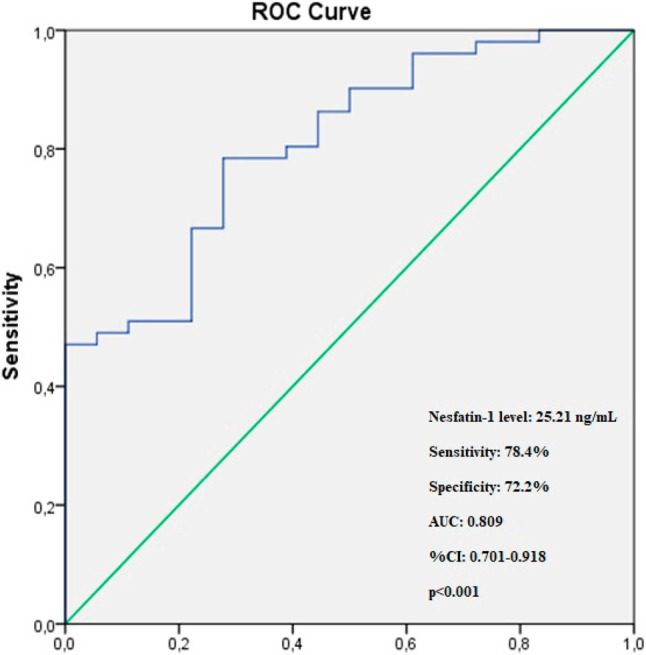
The ROC curve analysis of serum nesfatin-1 for predicting the occurence of no-reflow in STEMI patients. ROC, receiver operating characteristic; STEMI, ST-segment elevation myocardial infarction.
DISCUSSION
The main findings of this study can be summarized as follows: 1) Admission serum nesfatin-1 levels were significantly lower in the STEMI patients treated with pPCI in whom NR developed compared to those in whom normal flow was achieved. 2) Admission nesfatin-1 levels were a strong and independent predictor of NR in the STEMI patients treated with pPCI. GFR was also an independent predictor. 3) The optimal diagnostic cut-off value of nesfatin-1 calculated by the ROC analysis for the prediction of NR was 15.21 ng/mL (AUC = 0.809, 95% CI = 0.701-0.918, p < 0.001). To the best of our knowledge, this is the first study to demonstrate an independent association between nesfatin-1 levels and angiographic NR in STEMI patients undergoing pPCI.
Older studies have reported an incidence of NR of 11.5% after all PCIs.16 However, the incidence of angiographic NR has been suggested to be up to 32.8% in STEMI patients treated with pPCI in more recent studies,17 and 27.6% in patients with anterior STEMI undergoing pPCI.18 In our study, the incidence of angiographic NR was 20.6%, which is lower than these recent studies. The mechanism by which NR occurs is multifactorial and encompasses various events including distal thrombus embolization, microvascular spasm, reperfusion and ischemic injuries, and inflammation.19,20
Inflammation plays a pivotal role in each step of the extent of atherosclerosis and atherosclerotic plaque rupture and occurrence of NR.21,22 Interestingly, systemic endothelial dysfunction, a surrogate marker of systemic inflammation, has been shown to not be associated with the occurrence of NR during pPCI.15 Furthermore, the extent and complexity of CAD, as calculated by SS-1 and SS-2 scores, has also been suggested to be strongly associated with angiographic NR in STEMI patients undergoing pPCI.17,23 Yesin et al.23 reported higher SS-1 (28.3 ± 5.5) and SS-2 (42.5 [22.1-58.5]) scores in STEMI patients treated with PCI in whom NR occurred, compared to those in whom normal flow was achieved. They also reported a cut-off value of > 32.3 with 88% sensitivity and 80% specificity for SS-2 score by ROC analysis to predict NR in STEMI patients. The SS-1 and SS-2 scores in our NR group were similar to the findings of their study. Additionally, a significant association between the extent and complexity of CAD calculated by SS-111 and Gensini score10 with serum nesfatin-1 levels was reported in two recent studies. Our study also demonstrated a significant and negative correlation between serum nesfatin-1 levels and SS-1 (r = -0.709, p < 0.001) and SS-2 (r = -0.655, p < 0.001) scores.
Nesfatin-1 was first reported in 2006 by Oh-I et al.6 as an anorexigenic molecule secreted by the brain. Nesfatin-1 plays a pivotal role in anti-inflammation. Previous studies have suggested that nesfatin-1 exerts anti-inflammatory and anti-apoptotic effects during brain injury in experimental rat models.24,25 Although few studies have investigated the relationship between nesfatin-1 and atherosclerotic cardiovascular diseases (ASCVD), the majority of them have suggest that nesfatin-1 plays a protective role against cardiovascular diseases.9-11,26-29 Kuyumcu et al.27 found significantly lower serum nesfatin-1 levels in patients with > 60% carotid artery stenosis (CAS) compared to those with < 60% CAS. Moreover, serum nesfatin-1 levels were negatively correlated with the rate of CAS in their study. In addition, Robinson et al.29 reported that serum nesfatin-1 levels were associated with reduced carotid atherosclerosis and increased plaque stability in patients with rheumatoid arthritis. They further reported an inverse association between nesfatin-1 and carotid intima-media thickness. Ding et al.30 reported significantly lower nesfatin-1 levels in type 2 DM patients with PAD compared to type 2 DM patients without PAD. In logistic regression analysis, they also found an inverse association between nesfatin-1 levels and the development of PAD. In another study by Kuyumcu et al.,28 serum nesfatin-1 levels were significantly lower in patients with slow coronary flow (SCF) than in those with normal coronary flow. They also suggested an independent association between nesfatin-1 levels and SCF. Only one study, however, has reported higher levels of serum nesfatin-1 in patients with CAD compared to those without CAD, and showed that higher nesfatin-1 levels were associated with the severity of CAD.31 In addition, few studies have investigated the relationship between nesfatin-1 and AMI, but all have revealed consistently significantly lower levels of nesfatin-1 as well as independent and inverse associations between nesfatin-1 and the severity of AMI compared to the control subjects.10,11 In our study, we found significantly lower serum nesfatin-1 levels in the STEMI patients with NR compared to those with normal flow and the controls. Our findings are supported by the logistic regression analysis and are compatible with those of the previous studies. In addition, since most of the afore-mentioned studies reported an inverse association between nesfatin-1 and the presence and extent of stable and/or unstable ASCVD, it is prudent to assume that NR is more common in STEMI patients with lower admission serum nesfatin-1 levels due to the close association between this phenomenon and the severity of atherosclerotic plaque burden and vulnerability.
There are some limitations to this study. This was a single-center study comprising a relatively small population. In addition, we did not correlate admission nesfatin-1 levels with long-term major adverse cardiovascular events. Moreover, we did not include the time interval between the onset of symptoms and hospital admission, which is likely to predispose to the development of NR in the setting of STEMI.
CONCLUSION
Our study findings showed that admission serum nesfatin-1 level is an independent predictor for NR in patients with STEMI undergoing pPCI. Additionally, nesfatin-1 level was inversely correlated with SS-1 and SS-2 scores. Our findings may help to elucidate the implications of nesfatin-1 on the pathogenesis of atherosclerotic plaque extent and vulnerability in STEMI patients. However, further multicenter studies with a larger patient cohort are warranted to confirm our findings.
CONFLICTS OF INTEREST
All authors declare no conflicts of interest.
FUNDING
There was no funding for this study.
AUTHOR’S CONTRIBUTION
S.S, study concept, design, data collection; E.S, data collection, design, drafting the manuscript, critical revision; M.Ç, analysis and interpretation of data; K.G, design, analysis and interpretation of data.
REFERENCES
- 1.Roger VL, Weston SA, Gerber Y, et al. Trends in incidence, severity, and outcome of hospitalized myocardial infarction. Circulation. 2010;121:863–869. doi: 10.1161/CIRCULATIONAHA.109.897249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bolognese L, Carrabba N, Parodi G, et al. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–1126. doi: 10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- 3.Ndrepepa G, Tiroch K, Fusaro M, et al. 5-year prognostic value of no-reflow phenomenon after percutaneous coronary intervention in patients with acute myocardial infarction. J Am Coll Cardiol. 2010;55:2383–2389. doi: 10.1016/j.jacc.2009.12.054. [DOI] [PubMed] [Google Scholar]
- 4.Kloner RA. No-reflow phenomenon:maintaining vascular integrity. J Cardiovasc Pharmacol Ther. 2011;16:244–250. doi: 10.1177/1074248411405990. [DOI] [PubMed] [Google Scholar]
- 5.Rezkalla SH, Stankowski RV, Hanna J, Kloner RA. Management of no-reflow phenomenon in the catheterization laboratory. JACC Cardiovasc Interv. 2017;10:215–223. doi: 10.1016/j.jcin.2016.11.059. [DOI] [PubMed] [Google Scholar]
- 6.Oh IS, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–712. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 7.Schalla MA, Stengel A. Current understanding of the role of nesfatin-1. J Endocr Soc. 2018;2:1188–1206. doi: 10.1210/js.2018-00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aydin S. Multi-functional peptide hormone NUCB2/nesfatin-1. Endocrine. 2013;44:312–325. doi: 10.1007/s12020-013-9923-0. [DOI] [PubMed] [Google Scholar]
- 9.Feijoo-Bandin S, Rodriguez-Penas D, Garcia-Rua V, et al. Nesfatin-1: a new energy-regulating peptide with pleiotropic functions. Implications at cardiovascular level. Endocrine. 2016;52:11–29. doi: 10.1007/s12020-015-0819-z. [DOI] [PubMed] [Google Scholar]
- 10.Dai H, Li X, He T, et al. Decreased plasma nesfatin-1 levels in patients with acute myocardial infarction. Peptides. 2013;46:167–171. doi: 10.1016/j.peptides.2013.06.006. [DOI] [PubMed] [Google Scholar]
- 11.Serdar Kuyumcu M, Kuyumcu A, Yayla C, et al. The relationship between nesfatin-1 levels and SYNTAX score in patients with non-ST segment elevation myocardial infarction. Acta Cardiol Sin. 2018;34:386–393. doi: 10.6515/ACS.201809_34(5).20180423A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thygesen K, Alpert JS, Jaffe AS, et al. Third universal definition of myocardial infarction. Circulation. 2012;126:2020–2035. doi: 10.1161/CIR.0b013e31826e1058. [DOI] [PubMed] [Google Scholar]
- 13.Quinones MA, Otto CM, Stoddard M, et al. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 14.Ibanez B, James S, Agewall S, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–177. doi: 10.1093/eurheartj/ehx393. [DOI] [PubMed] [Google Scholar]
- 15.Levi Y, Sultan A, Alemayehu M, et al. Association of endothelial dysfunction and no-reflow during primary percutaneous coronary intervention for ST-elevation myocardial infarction. Cardiovasc Revasc Med. 2016;17:552–555. doi: 10.1016/j.carrev.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Piana RN, Paik GY, Moscucci M, et al. Incidence and treatment of ‘no-reflow’ after percutaneous coronary intervention. Circulation. 1994;89:2514–2518. doi: 10.1161/01.cir.89.6.2514. [DOI] [PubMed] [Google Scholar]
- 17.Sahin DY, Gur M, Elbasan Z, et al. SYNTAX score is a predictor of angiographic no-reflow in patients with ST-elevation myocardial infarction treated with a primary percutaneous coronary intervention. Coronary Artery Disease. 2013;24:148–153. doi: 10.1097/MCA.0b013e32835c4719. [DOI] [PubMed] [Google Scholar]
- 18.Gur M, Turkoglu C, Taskin A, et al. Paraoxonase-1 activity and oxidative stress in patients with anterior ST elevation myocardial infarction undergoing primary percutaneous coronary intervention with and without no-reflow. Atherosclerosis. 2014;234:415–420. doi: 10.1016/j.atherosclerosis.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Bouleti C, Mewton N, Germain S. The no-reflow phenomenon: state of the art. Arch Cardiovasc Dis. 2015;108:661–674. doi: 10.1016/j.acvd.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190:255–266. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Wu MY, Li CJ, Hou MF, Chu PY. New insights into the role of inflammation in the pathogenesis of atherosclerosis. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiao Q, Ke Q, Li W, et al. Effect of inflammatory factor-induced cyclo-oxygenase expression on the development of reperfusion-related no-reflow phenomenon in acute myocardial infarction. Clin Exp Pharmacol Physiol. 2015;42:162–170. doi: 10.1111/1440-1681.12339. [DOI] [PubMed] [Google Scholar]
- 23.Yesin M, Cagdas M, Kalcik M, et al. Comparison of syntax score and syntax score II to predict “no reflow phenomenon” in patients with ST-segment elevation myocardial infarction. Int J Cardiovasc Imaging. 2017;33:1883–1889. doi: 10.1007/s10554-017-1200-5. [DOI] [PubMed] [Google Scholar]
- 24.Tang CH, Fu XJ, Xu XL, et al. The anti-inflammatory and anti-apoptotic effects of nesfatin-1 in the traumatic rat brain. Peptides. 2012;36:39–45. doi: 10.1016/j.peptides.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 25.Ozsavci D, Ersahin M, Sener A, et al. The novel function of nesfatin-1 as an anti-inflammatory and antiapoptotic peptide in subarachnoid hemorrhage-induced oxidative brain damage in rats. Neurosurgery. 2011;68:1699–1708; discussion 1708. doi: 10.1227/NEU.0b013e318210f258. [DOI] [PubMed] [Google Scholar]
- 26.Angelone T, Filice E, Pasqua T, et al. Nesfatin-1 as a novel cardiac peptide: identification, functional characterization, and protection against ischemia/reperfusion injury. Cell Mol Life Sci. 2013;70:495–509. doi: 10.1007/s00018-012-1138-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuyumcu A, Kuyumcu MS, Ozbay MB, Ozeke O. The relationship between nesfatin-1 and carotid artery stenosis. Scand Cardiovasc J. 2019:1–18. doi: 10.1080/14017431.2018.1547840. [DOI] [PubMed] [Google Scholar]
- 28.Kuyumcu MS, Kuyumcu A, Yayla C, et al. Nesfatin-1 levels in patients with slow coronary flow. Kardiol Pol. 2018;76:401–405. doi: 10.5603/KP.a2017.0210. [DOI] [PubMed] [Google Scholar]
- 29.Robinson C, Tsang L, Solomon A, et al. Nesfatin-1 and visfatin expression is associated with reduced atherosclerotic disease risk in patients with rheumatoid arthritis. Peptides. 2018;102:31–37. doi: 10.1016/j.peptides.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 30.Ding S, Qu W, Dang S, et al. Serum nesfatin-1 is reduced in type 2 diabetes mellitus patients with peripheral arterial disease. Med Sci Monit. 2015;21:987–991. doi: 10.12659/MSM.892611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibe S, Kishimoto Y, Niki H, et al. Associations between plasma nesfatin-1 levels and the presence and severity of coronary artery disease. Heart Vessels. 2019 doi: 10.1007/s00380-018-01328-3. [DOI] [PubMed] [Google Scholar]


