Abstract
Background.
Childhood traumatic events are risk factors for psychotic-like experiences (PLEs). However, the mechanisms explaining how trauma may contribute to the development of PLEs are not fully understood. In our study, we investigated whether cannabis use and cognitive biases mediate the relationship between early trauma and PLEs.
Methods.
A total sample of 6,772 young adults (age 26.6 ± 4.7, 2,181 male and 3,433 female) was recruited from the general population to participate in an online survey. We excluded 1,158 individuals due to a self-reported lifetime diagnosis of any mental disorder. The online survey included selected items from the following questionnaires: Traumatic Experience Checklist (TEC, 3 items), Childhood Experience of Care and Abuse Questionnaire (CECA.Q, 3 items), Cannabis Problems Questionnaire (CPQ, 10 items), Davos Assessment of Cognitive Biases Scale (DACOBS-18, 9 items), and Prodromal Questionnaire-16 (PQ-16). Mediation analyses were performed with respect to different categories of traumatic experiences (emotional, physical and sexual abuse as well as emotional neglect).
Results.
Our results showed significant associations of any time of childhood trauma with higher scores of cannabis use (CPQ), cognitive biases (DACOBS), and PLEs (PQ-16) (p < 0.001). We found a direct effect of childhood trauma on PLEs as well as significant indirect effect mediated through cannabis use and cognitive biases. All models tested for the effects of specific childhood adversities revealed similar results. The percentage of variance in PQ-16 scores explained by serial mediation models varied between 32.8 and 34.2% depending on childhood trauma category.
Conclusion.
Cannabis use and cognitive biases play an important mediating role in the relationship between childhood traumatic events and the development of PLEs in a nonclinical young adult population.
Keywords: Childhood trauma, cognitive bias, psychotic-like experiences
Introduction
Psychotic-like experiences (PLEs) are defined as subclinical psychotic phenomena that include perceptual anomalies and delusion-like experiences in the absence of overt psychotic illness [1]. PLEs have been found to occur in 5–8% of nonclinical populations [2]. They have been associated with impairments in functioning [3], help-seeking behaviors [3,4], psychiatric diagnoses [3,4], self-harm thoughts and behaviors [5,6], as well as increased suicidality [3,7]. Moreover, PLEs have been linked to risk for developing a psychotic disorder and with many of the same risk factors as psychotic disorder, such as exposure to traumatic life events or cannabis use [2].
The association between early traumatic experiences and PLEs has been shown both in population-based studies [8], as well as among help-seeking adolescents and young adults [9]. It has been shown that even after controlling demographic factors and comorbid mental disorders, the relationship between traumatic life events and PLEs is fairly significant with odds ratios ranging from 3 to 11 as presented by a recent meta-analysis [10]. Moreover, prospective studies have shown that trauma exposure predates the onset of psychosis [11], and having a history of trauma is related to a more severe symptomatic manifestation, unfavorable course, and higher rates of treatment resistance [12].
Cannabis use has been increasing over the past decades and age at first use has been decreasing, which is of particular concern since the brain, which continues to develop in the adolescence, may be vulnerable to toxic effects of cannabis [13]. Early trauma [14] and later traumatic life events [15] have been associated with an increased likelihood of cannabis use. Independent contributions of childhood physical and sexual abuse to cannabis use were observed with no effect of witnessing parental violence [16]. Moreover, it has also been shown that childhood trauma predicts the transition from cannabis initiation to cannabis use disorder [17]. Interestingly, the effect of childhood maltreatment and cannabis abuse on pathogenesis of psychosis is neither fully confounded by other risk factors [10] nor can by explained by the gene–environment interactions [18].
Cannabis use has been repeatedly associated with the continuum of psychotic experiences, ranging from subthreshold psychotic symptoms to clinical high risk for psychosis. It has been demonstrated that PLEs are more prevalent among cannabis users in the general population when compared to nonusers [19–24]. Moreover, there is a dose–response relationship between the frequency of cannabis consumption and increased risk for psychosis [25,26]. Moreover, it has been shown using a longitudinal study design that the experience of childhood trauma moderates the association between cannabis and psychosis in a dose-dependent, extra-linear fashion. In two independent population-based samples, it has been found that severe maltreatment was associated with the greatest effect of cannabis in a later expression of psychosis [27]. This is in line with previous animal and human research showing that early life stress may result in an altered behavioral response to dopamine agonists later in life [28,29].
Despite numerous studies showing the association between early trauma and PLEs/psychosis, the mechanisms by which trauma influences the development of psychotic symptoms remain unclear. Several models focusing on psychological and biological mechanisms have been suggested so far and various mediation models have been proposed linking childhood and adolescent trauma with PLEs in nonclinical samples, including different variables such as: perceived stress, external locus of control, negative self-schemas, negative other-schemas [8], cognitive biases [30–32], resilience [32], dissociation [8,33], depressive symptoms [34], self-disturbances [30,31,35], insecure attachment styles [30], borderline personality features [9], and aberrant salience [36].
So far, the significance of cannabis use [13,27,37,38] as well as cognitive biases [30–32] in the relationship between early adversity and PLEs has been examined. However, to the best of our knowledge, to date, there are no studies addressing both risk factors simultaneously. Existing evidence suggests that linear models directly linking a history of childhood trauma, cannabis use and PLEs might be insufficient to understand causal mechanisms. Thus, in our study, we aimed at investigating serial mediation models including the interplay between cannabis use and cognitive biases to provide more detailed explanatory model for the relationship between exposure to early trauma and psychosis proneness.
Methods
Participants
A total sample of 6,772 young adults (age 26.6 ± 4.7; 2,181 male and 3,433 female) was enrolled from the general population to participate in an online survey using the Computer Assisted Web Interview (CAWI) method. Completing the online survey took on average around 20–30 min. We created the Research Consortium between medical universities in three big cities in Poland (Warsaw, Cracow, and Wroclaw) to investigate the relationship between early trauma, cognitive biases, and risk of psychosis. Participants were recruited from these cities with a range of 640,000–1,700,000 inhabitants. Exclusion criteria were as follows: history of substance dependence in the previous 6 months, history of psychotic or neurological disorders, and taking antipsychotic medication. The study was approved by ethics committee of the Medical University of Warsaw. Participants provided their informed consent to participate in the study. Demographic and clinical characteristics of our sample are shown in Table 1.
Table 1.
General characteristics of the sample.
| Mean ± SD or n (%) | |
|---|---|
| Age | 26.6 ± 4.7 |
| Sex | |
| Male | 2,181 (38.8%) |
| Female | 3,433 (61.2%) |
| Professional situation | |
| Study | 1,924 (34.3%) |
| Work | 4,126 (73.5%) |
| Unemployed | 329 (5.9%) |
| Rent | 26 (0.5%) |
| Education | |
| Primary | 154 (2.7%) |
| Secondary | 1,837 (32.7%) |
| Vocational | 196 (3.5%) |
| Incomplete higher | 884 (15.7%) |
| Higher | 2,543 (45.3%) |
| Type of childhood adversity | |
| Emotional abuse | 2,060 (36.7%) |
| Emotional neglect | 2,558 (45.6%) |
| Physical abuse | 2,880 (51.3%) |
| Sexual abuse | 757 (13.5%) |
| Any childhood adversities | 3,919 (69.8%) |
| Questionnaires | |
| CPQ | 2.6 ± 2.3 |
| PQ-16 | 8.4 ± 5.9 |
| DACOBS | 26.0 ± 8.5 |
Abbreviations: CPQ, Cannabis Problems Questionnaire; DACOBS, Davos Assessment of Cognitive Biases Scale; PQ-16, Prodromal Questionnaire.
Measures
Childhood trauma
Early exposure to trauma was assessed using selected items from the Traumatic Events Checklist (TEC) [39] and from the Childhood Experience of Care and Abuse Questionnaire (CECA.Q) [40]. We assessed emotional abuse, emotional neglect, and bullying with three items from the TEC, while sexual harassment and sexual abuse were assessed by three items from the CECA.Q. A detailed description of selected items was provided elsewhere [5]. Sample questions from the TEC are: “When you were a child or a teenager, have you ever felt emotionally neglected (e.g., being left alone, insufficient affection) by your parents, brothers, or sisters?” or “When you were a child or a teenager, have you ever felt emotionally abused (e.g., being belittled, teased, called names, threatened verbally, or unjustly punished) by your parents, brothers, or sisters?” Sample questions from the CECA.Q are: “When you were a child or a teenager, did you have any unwanted sexual experiences?” or “Can you think of any upsetting sexual experiences before age 17 with a related adult or someone in authority, for example, teacher?” We used the Polish version of the items that were prepared using the back-translation procedure. In our sample, the Cronbach’s alpha for selected items was 0.66.
Cognitive biases
Cognitive biases were measured with the Davos Assessment of Cognitive Biases Scale (DACOBS-18) [41]. We included two subscales that assess attention to threat biases (items 6, 7, 24, and 29) and safety behaviors—behavioral coping strategies (items 27, 31, 33, 34, and 35). These subscales have been proven to be best predictors of psychosis risk (for more detailed information about the chosen items see [5]). Sample questions from the DACOBS-18 are: “People cannot be trusted,” “Things went wrong in my life because of other people,” “People make my life miserable,” “People treat me badly for no reason,” “People I do not know are dangerous,” or “I do not go out after dark,” “I do not answer phone calls, to be on the safe side,” “I always sit near the exit to be safe,” “I do not answer phone calls, to be on the safe side,” “There is usually only one explanation for a single event.” We used the Polish version of the DACOBS-18 [42]. In our sample, the Cronbach alpha was 0.81.
Psychotic-like experiences (PLEs)
The Prodromal Questionnaire-16 (PQ-16) [43] was used to screen for the risk of psychosis operationalized as a presence of PLEs. It is a 16-item self-report questionnaire that consists of 9 items of the perceptual abnormalities/hallucinations subscale, 5 items referring to unusual thought content/delusional ideas/paranoia, and 2 negative symptoms. In our study, we used only the items related to the attenuated positive symptoms and we excluded two items associated with depression and anxiety symptoms. Sample questions from PQ-16 are: “I felt as if I had no control over my own ideas and/or thoughts.” In our study, the Polish version of PQ-16 was used that was prepared using a back-translation procedure and was used previously [30]. The Cronbach’s alpha in our sample was 0.87.
Cannabis use
Cannabis use was assessed using the CPQ [44]. We included the following 10 questions out of 16 questions from the CPQ referring to experiences with cannabis use in the preceding 12 months: (a) “Have you tended to smoke more on your own than you used to?”; (b) “Have you been neglecting yourself physically?”; (c) “Have you felt depressed for more than a week?”; (d) “Have you been so depressed you felt like doing away with yourself?”; (e) “Have you given up recreational activities you once enjoyed for smoking?”; (f) “Do you find it hard to get the same enjoyment from your usual interests?”; (g) “Have you felt more antisocial after smoking?”; (h) “Have you been concerned about a lack of motivation?”; (i) “Have you worried about feelings of personal isolation or detachment?”; and (j) “Do you usually have a smoke in the morning, to get yourself going?” The Cronbach’s alpha in our sample was 0.77.
Data analysis
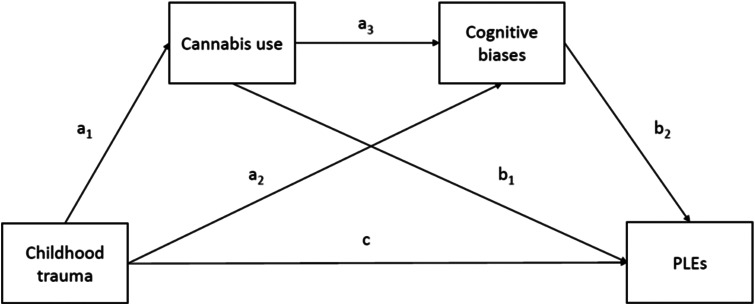
Before data analysis, given the fact that we were interested in nonclinical PLEs, individuals with a self-reported lifetime diagnosis of any mental disorders were excluded. Distribution of the PQ-16, DACOBS, and CPQ scores was non-normal according to the Kolmogorov–Smirnov test. Therefore, correlations between these variables were assessed using the Spearman rank correlation coefficients. Similarly, bivariate comparisons of these scores between individuals with and without any type or specific childhood adversities were performed using the Mann–Whitney U test. The alpha criterion level was set at 0.05 in bivariate analyses. The PROCESS macro was used to perform serial mediation analysis (Model 6) [45]. The bootstrap estimation with 5,000 samples was applied to test indirect effects. The model tested in our study was presented in Figure 1. The history of specific childhood adversities and history of any childhood traumatic events was implemented as an independent variable, while the PQ-16 score was used as a dependent variable. The DACOBS score and the CPQ score were implemented as mediators. Age, sex, and education level were added as covariates. Mediation was considered significant if the 95% confidence intervals (CI) did not include zero [46]. Statistical analysis was performed using the Statistical Package for Social Sciences, version 20 (SPSS Inc., Chicago, IL).
Figure 1.
Model for serial mediation (direct and Indirect effects).
Results
Out of 6,772 participants, we excluded 1,158 individuals (17.1%) due to a self-reported lifetime diagnosis of any mental disorders. We analyzed data from all individuals who completed the online survey. We included patients with and without traumatic life events and we did not apply any threshold criteria for any questionnaires used in the study. General characteristics of the sample were provided in Table 1. The majority of participants were females, had higher education and were employed on the day of assessment.
The following correlations appeared to be significant (p < 0.001): (a) between the CPQ and DACOBS scores (r = 0.375); (b) between the CPQ and PQ-16 scores (r = 0.465); and (c) between the PQ-16 and the DACOBS scores (r = 0.435). Similarly, a history of all specific childhood adversities and any type of childhood trauma were associated with higher scores of CPQ, DACOBS, and PQ-16 (Table 2).
Table 2.
The level of cannabis use, cognitive biases and psychotic-like experiences with respect to a history of childhood adversities.
| Trauma(+) | Trauma(−) | p | |
|---|---|---|---|
| Emotional abuse | n = 2,060 | n = 3,554 | |
| CPQ | 3.2 ± 2.3 | 2.2 ± 2.1 | <0.001 |
| DACOBS | 28.3 ± 8.45 | 24.6 ± 8.2 | <0.001 |
| PQ-16 | 10.8 ± 6.2 | 7.1 ± 5.3 | <0.001 |
| Emotional neglect | n = 2,558 | n = 3,056 | |
| CPQ | 3.15 ± 2.3 | 2.0 ± 2.1 | <0.001 |
| DACOBS | 27.9 ± 8.4 | 24.3 ± 8.2 | <0.001 |
| PQ-16 | 10.4 ± 5.9 | 6.8 ± 5.4 | <0.001 |
| Physical abuse | n = 2,880 | n = 2,734 | |
| CPQ | 3.1 ± 2.3 | 2.0 ± 2.0 | <0.001 |
| DACOBS | 27.4 ± 8.5 | 24.5 ± 8.3 | <0.001 |
| PQ-16 | 10.1 ± 6.7 | 6.7 ± 5.4 | <0.001 |
| Sexual abuse | n = 757 | n = 4,857 | |
| CPQ | 3.2 ± 2.4 | 2.5 ± 2.2 | 0.001 |
| DACOBS | 29.7 ± 9.2 | 25.4 ± 8.2 | <0.001 |
| PQ-16 | 11.7 ± 6.8 | 7.9 ± 5.6 | <0.001 |
| Any type of trauma | n = 3,919 | n = 1,695 | |
| CPQ | 2.9 ± 2.3 | 1.7 ± 1.9 | <0.001 |
| DACOBS | 27.1 ± 8.3 | 23.5 ± 8.4 | <0.001 |
| PQ-16 | 9.8 ± 5.8 | 5.3 ± 5.0 | <0.001 |
Abbreviations: CPQ, Cannabis Problems Questionnaire; DACOBS, Davos Assessment of Cognitive Biases Scale; PQ-16, Prodromal Questionnaire.
The results of serial mediation analysis were shown in Table 3 and Figure 1. All models testing for the effects of specific childhood adversities or a history of any childhood trauma revealed similar results and included age, sex, and education level as covariates.
Table 3.
Serial mediation analysis to identify direct and indirect effects of childhood trauma on psychotic-like experiences.
| Effect | Path | Coeff. | SE | 95% CI | |
|---|---|---|---|---|---|
| LLCI | ULCI | ||||
| Emotional abuse | |||||
| Direct effect of CT on cannabis use | a1 | 0.9479*** | 0.1496 | 0.6543 | 1.2414 |
| Direct effect of CT on cognitive biases | a2 | 2.4549*** | 0.5144 | 1.4454 | 3.4645 |
| Direct effect of cannabis use on cognitive biases | a3 | 1.2220*** | 0.1135 | 0.9993 | 1.4448 |
| Direct effect of cannabis use on PLEs | b1 | 0.7566*** | 0.0747 | 0.6100 | 0.9031 |
| Direct effect of cognitive biases on PLEs | b2 | 0.2133*** | 0.0209 | 0.1723 | 0.2543 |
| Direct effect of CT on PLEs | c | 1.6438*** | 0.3221 | 1.0115 | 2.2760 |
| Total indirect effect | ab | 1.4879 | 0.2149 | 1.0776 | 1.9226 |
| Indirect effect through cannabis use | a1b1 | 0.7171 | 0.1410 | 0.4546 | 1.0079 |
| Indirect effect through cognitive biases | a2b2 | 0.5237 | 0.1264 | 0.2902 | 0.7826 |
| Indirect effect through cannabis use and cognitive biases | a1a3b2 | 0.2471 | 0.0553 | 0.1490 | 0.3630 |
| Emotional neglect | |||||
| Direct effect of CT on cannabis use | a1 | 1.1336*** | 0.1492 | 0.8408 | 1.4265 |
| Direct effect of CT on cognitive biases | a2 | 2.2282*** | 0.5243 | 1.1993 | 3.2571 |
| Direct effect of cannabis use on cognitive biases | a3 | 1.2140*** | 0.1149 | 0.9886 | 1.4395 |
| Direct effect of cannabis use on PLEs | b1 | 0.7614*** | 0.0758 | 0.6126 | 0.9101 |
| Direct effect of cognitive biases on PLEs | b2 | 0.2199*** | 0.0210 | 0.1787 | 0.2611 |
| Direct effect of CT on PLEs | c | 1.1437** | 0.3292 | 0.4977 | 1.7898 |
| Total indirect effect | ab | 1.6558 | 0.2189 | 1.2525 | 2.0962 |
| Indirect effect through cannabis use | a1b1 | 0.8631 | 0.1504 | 0.5901 | 1.1825 |
| Indirect effect through cognitive biases | a2b2 | 0.4900 | 0.1276 | 0.2543 | 0.7553 |
| Indirect effect through cannabis use and cognitive biases | a1a3b2 | 0.3027 | 0.0613 | 0.1933 | 0.4351 |
| Physical abuse | |||||
| Direct effect of CT on cannabis use | a1 | 1.0117*** | 0.1508 | 0.7158 | 1.3076 |
| Direct effect of CT on cognitive biases | a2 | 2.0724** | 0.5233 | 1.0453 | 3.0995 |
| Direct effect of cannabis use on cognitive biases | a3 | 1.2354*** | 0.1142 | 1.0112 | 1.4596 |
| Direct effect of cannabis use on PLEs | b1 | 0.7567*** | 0.0752 | 0.6092 | 0.9043 |
| Direct effect of cognitive biases on PLEs | b2 | 0.2181*** | 0.0209 | 0.1771 | 0.2591 |
| Direct effect of CT on PLEs | c | 1.4384*** | 0.3264 | 0.7978 | 2.0789 |
| Total indirect effect | ab | 1.4901 | 0.2029 | 1.1149 | 1.9039 |
| Indirect effect through cannabis use | a1b1 | 0.7656 | 0.1415 | 0.5060 | 1.0593 |
| Indirect effect through cognitive biases | a2b2 | 0.4520 | 0.1216 | 0.2274 | 0.7045 |
| Indirect effect through cannabis use and cognitive biases | a1a3b2 | 0.2726 | 0.0573 | 0.1690 | 0.3962 |
| Sexual abuse | |||||
| Direct effect of CT on cannabis use | a1 | 0.7357** | 0.2032 | 0.3369 | 1.1344 |
| Direct effect of CT on cognitive biases | a2 | 3.3727*** | 0.6773 | 2.0433 | 4.7021 |
| Direct effect of cannabis use on cognitive biases | a3 | 1.2697*** | 0.1117 | 1.0487 | 1.4870 |
| Direct effect of cannabis use on PLEs | b1 | 0.7992*** | 0.0748 | 0.6524 | 0.9460 |
| Direct effect of cognitive biases on PLEs | b2 | 0.2208*** | 0.0211 | 0.1794 | 0.2623 |
| Direct effect of CT on PLEs | c | 1.1598* | 0.4297 | 0.3165 | 2.0031 |
| Total indirect effect | ab | 1.5387 | 0.1861 | 0.4064 | 1.1234 |
| Indirect effect through cannabis use | a1b1 | 0.5879 | 0.1740 | 0.2704 | 0.9497 |
| Indirect effect through cognitive biases | a2b2 | 0.7448 | 0.1861 | 0.4064 | 1.1234 |
| Indirect effect through cannabis use and cognitive biases | a1a3b2 | 0.2060 | 0.0649 | 0.0917 | 0.3431 |
| Any childhood adversities | |||||
| Direct effect of CT on cannabis use | a1 | 1.2001*** | 0.1908 | 0.8256 | 1.5746 |
| Direct effect of CT on cognitive biases | a2 | 2.4432** | 0.6618 | 1.1442 | 3.7421 |
| Direct effect of cannabis use on cognitive biases | a3 | 1.2828*** | 0.1143 | 1.0584 | 1.5072 |
| Direct effect of cannabis use on PLEs | b1 | 0.7717*** | 0.0755 | 0.6235 | 0.9198 |
| Direct effect of cognitive biases on PLEs | b2 | 0.2250*** | 0.0208 | 0.1841 | 0.2658 |
| Direct effect of CT on PLEs | c | 1.6244*** | 0.4119 | 0.8160 | 2.4328 |
| Total indirect effect | ab | 1.8221 | 0.2546 | 1.3317 | 2.3237 |
| Indirect effect through cannabis use | a1b1 | 0.9261 | 0.1687 | 0.6262 | 1.2840 |
| Indirect effect through cognitive biases | a2b2 | 0.5496 | 0.1471 | 0.2758 | 0.8569 |
| Indirect effect through cannabis use and cognitive biases | a1a3b2 | 0.3463 | 0.0704 | 0.2200 | 0.4958 |
Abbreviations: CI, confidence intervals; CT, childhood trauma; PLE, psychotic-like experiences; SE, standard error.
p < 0.01.
p < 0.001.
p < 0.0001.
There were significant direct effects of childhood trauma history, CPQ scores and DACOBS scores on the PQ scores. Similarly, the direct effects of childhood trauma history and CPQ scores on the DACOBS scores appeared to be significant in all models. Moreover, there were significant effects of childhood adversities on the CPQ scores. The total and indirect effects of childhood traumatic events on PQ-16 scores were also significant in all models. The percentage of variance in PQ-16 scores explained by serial mediation models varied between 32.8 and 34.2 (emotional neglect: R 2 = 0.3315, emotional abuse: R 2 = 0.3418, physical abuse: R 2 = 0.3370, sexual abuse: R 2 = 0.3279, any childhood adversities: R 2 = 0.3349).
The results of our study did not differ significantly when we performed analysis including individuals with a self-reported lifetime diagnosis of any mental disorder. The table with results will be included in Supplementary Materials (Table S1).
Discussion
In our study, we found that the effect of childhood trauma on the level of PLEs is mediated by cannabis use and cognitive biases. The direct effect of childhood trauma history on the level of PLEs was also tested significant, suggesting that the mediation investigated in our study is partial. This finding is in line with results of previous studies [8,47]. When we analyzed different types of childhood trauma separately, the relationship with PLEs appeared to be most significant for emotional and physical abuse, which is in line with previous findings showing a different impact of specific traumatic events on the risk of psychosis [48]. Recent systematic review and meta-analysis on the association between childhood trauma with psychotic symptoms has shown that the early adversity is connected to severity of experienced symptomatology [49] There are also studies suggesting differential impact of particular types of childhood trauma and positive but not negative symptoms. Specifically, most consistently physical and sexual abuse has been associated with auditory hallucinations, but not delusions. Moreover, a number of studies have shown that childhood adversities are also related to the content of psychotic symptoms (for review see [50,51]).
We have shown the mediating role of cannabis use between trauma and PLEs. As presented by the recent systematic review of nonclinical populations, cannabis use is a risk factor for the development of PLEs [52]. It has been shown that similarly to the development of psychotic disorders [53], younger age at first use and higher frequency of cannabis use are associated with higher risk of PLEs in the general population [54,55]. Although evidence that cannabis use precedes the onset of psychotic symptoms and a dose–response relationship argue for a causal relationship, there are reports suggesting reversed causality. Indeed, experiencing psychotic symptoms may increase the risk for cannabis use. However, in the most recent population-based study on young adult twins, authors showed that there was a stronger support for a causal pathway from cannabis use to PLEs when compared to the opposite or reciprocal pathways after controlling for genetic and environmental factors [22]. Nevertheless, it should be noted that linear causality in psychiatry is unlikely and most psychic phenomena have complex causality with interdependent feedback loops.
Our results also indicate that cannabis use mediates the relationship between childhood and adolescent traumatic events and PLEs. This is in line with other studies that support our findings [13,27,37,38]. It has been shown that childhood adversities, in addition to cannabis use, may increase the risk of psychotic disorders [56,57] and that exposure to abuse and other life adversities together with cannabis use is associated with up to fourfold increased odds of reporting psychotic experiences [38]. Interestingly, a recent study showed that neither solely lifetime cannabis use nor reported exposure to childhood abuse was associated with increased risk of psychotic disorder, while the combination of the two risk factors substantially raised the likelihood of experiencing psychosis [58]. Moreover, exposure to childhood trauma and cannabis use were found to increase associations between hallucination and delusion in healthy and in genetically at risk populations thus increasing risk of transition to psychosis [59,60].
We found that early traumatic events as well as cannabis use are associated with cognitive biases that in turn contribute to the increased risk for psychotic experiences. It could be hypothesized that the additive interaction between early trauma and lifetime cannabis use produces alterations in salience processing, that is aberrant salience, and thus contributes to the development of cognitive biases that may in turn increase a risk of PLEs [30–32]. Two mechanisms of the emergence of aberrant salience have been proposed, both linked to stress and cannabis use. First, the role of cross-sensitization between stress and cannabis involving increased dopaminergic signaling in shaping the risk of psychotic outcomes has been suggested [61]. Second, it has been shown that both higher levels of cannabis use or childhood trauma compromise brain connectivity over the course of psychotic illness [62]. Both excessive dopamine signaling [63,64] and reduced cortico-striatal connectivity [65] have been associated with alterations in salience processing.
The aberrant salience model of psychosis proposes that chaotic brain dopamine transmission leads to the attribution of significance to stimuli that would normally be considered irrelevant [64]. Cognitive interpretation of these excessively salient stimuli can lead to the formation of biased cognitive schema that result in the formation of psychotic symptoms [66]. Hyperactivation in attentional systems and the biased schema, in turn, result in the excessively salient stimuli being interpreted as threatening and in the orientation of attention towards particularly threatening or anxiety-provoking environmental stimuli [67]. This process can give rise to attentional biases and as a result increase tendency for safety behaviors. For the review on the effect of aberrant signaling in dopaminergic neurotransmission on the development of cognitive biases see Broyd [67].
Aberrant salience has been shown to mediate the relationship between early trauma and PLEs [36,68]. It has been shown that individuals at ultra-high risk of psychosis demonstrate aberrant salience, the degree of which relates to the severity of delusion-like symptoms [69], while cannabis users show aberrant salience processing that is related to a severity of cannabis-induced psychotic symptoms [70]. Moreover, the positive relationship between aberrant salience and delusional symptoms has been described in schizophrenia patients [71].
Our study replicated the importance of cognitive biases for the emergence of PLEs. The role of cognitive biases in the relationship between early traumatic life events and PLEs has been reported previously [5,30,31,34]. The exposure to childhood adversities may cause deprivation of early social interactions, cognitive biases and disrupted attachment, which in turn may affect the development of neurocognition and social cognition observed in psychotic disorders [72].
Concluding, our results further support the hypothesis of childhood trauma having effect on psychosis proneness in the general nonclinical population. We have shown that most significant association can be observed between emotional and physical abuse and PLEs. Important mediators of the relationship between early trauma and PLEs are cannabis use and cognitive biases. Early traumatic events as well as cannabis use are associated with cognitive biases that in turn contribute to the increased risk for psychotic experiences. Although there are no studies showing the relationship between cannabis use and cognitive biases as assessed in our study, it can be speculated that trauma and cannabis use through increased dopamine synthesis and altered brain connectivity can promote aberrant salience that in turn may lead to higher occurrence of cognitive biases and PLEs.
Limitations
There are some limitations of our study that need to be discussed. First, the questionnaires used in our study were shortened and the items were selected arbitrarily for the online screening purposes. Second, our study includes only self-report measures, that is measures that rely on the individual’s own report of their symptoms, beliefs, behaviors, or attitudes. Collecting information through self-report has its well-known limitations and disadvantages such as for example bias of how people feel at the time they fill out the questionnaire, recall bias and forgetting, or desirability bias. However, results from studies on trauma using self-report have been often validated by studies using other data methods that show that responses measure what they claim that they measure [51,73]. It has been shown that retrospective self-report can be used reliably to assess childhood trauma in people experiencing acute psychotic symptoms and that although the severity of childhood trauma reports can fluctuate between assessments, there are rarely complete retractions of severe abuse claims [73]. Moreover, given the cross-sectional nature of our study, we were unable to confirm causality between selected variables, thus limiting the clinical implications of our results. Prospective, longitudinal studies are required to examine the temporal course of trauma exposure, cannabis use, cognitive biases, and PLEs. Inclusion of biological measures that allow recording dopamine release or altered brain connectivity could provide further insights into our mediation model.
Financial Support
This study was supported by OPUS grant from the National Science Centre, Poland (ŁG, 2016/21/B/HS6/03210).
Conflict of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Data Availability Statement
The data that support the findings of this study are openly available on request to the all authors. Please contact corresponding author.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1192/j.eurpsy.2020.31.
click here to view supplementary material
References
- [1].Os J, Hanssen M, Bijl RV, Ravelli A. Strauss (1969) revisited: a psychosis continuum in the general population? Schizophr Res. 2000;45(1–2):11–20. [DOI] [PubMed] [Google Scholar]
- [2].Os J, Linscott RJ, Myin-Germeys I, Delespaul P, Krabbendam L. A systematic review and meta-analysis of the psychosis continuum: evidence for a psychosis proneness-persistence-impairment model of psychotic disorder. Psychol Med. 2009;39(2):179–95. [DOI] [PubMed] [Google Scholar]
- [3].Kelleher I, Devlin N, Wigman JT, Kehoe A, Murtagh A, Fitzpatrick C, et al. Psychotic experiences in a mental health clinic sample: implications for suicidality, multimorbidity and functioning. Psychol Med. 2014;44(8):1615–1624. [DOI] [PubMed] [Google Scholar]
- [4].DeVylder JE, Oh HY, Corcoran CM, Lukens EP. Treatment seeking and unmet need for care among persons reporting psychosis-like experiences. Psychiatr Serv. 2014;65(6):774–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Gawęda Ł, Pionke R, Krężołek M, Frydecka D, Nelson B, Cechnicki A. The interplay between childhood trauma, cognitive biases, psychotic-like experiences and depression and their additive impact on predicting lifetime suicidal behavior in young adults. Psychol Med. 2020. Jan;50(1) 116–124. doi: 10.1017/S0033291718004026. [DOI] [PubMed] [Google Scholar]
- [6].Kelleher I, Cederlof M, Lichtenstein P. Psychotic experiences as a predictor of the natural course of suicidal ideation: a Swedish cohort study. World Psychiatry. 2014;13(2):184–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Honings S, Drukker M, van Nierop M, van Winkel R, Wittchen HU, Lieb R, et al. Psychotic experiences and incident suicidal ideation and behaviour: disentangling the longitudinal associations from connected psychopathology. Psychiatry Res. 2016;245:267–75. [DOI] [PubMed] [Google Scholar]
- [8].Gibson LE, Reeves LE, Cooper S, Olino TM, Ellman LM. Traumatic life event exposure and psychotic-like experiences: a multiple mediation model of cognitive-based mechanisms. Schizophr. Res. 2019;205:15–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sengutta M, Gaweda L, Moritz S, Karow A. The mediating role of borderline personality features in the relationship between childhood trauma and psychotic-like experiences in a sample of help-seeking non-psychotic adolescents and young adults. Eur Psychiatry. 2019;56:84–90. [DOI] [PubMed] [Google Scholar]
- [10].Varese F, Smeets F, Drukker M, Lieverse R, Lataster T, Viechtbauer W, et al. Childhood adversities increase the risk of psychosis: a meta-analysis of patient-control, prospective- and cross-sectional cohort studies. Schizophr Bull. 2012;38(4):661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Alemany S, Goldberg X, Winkel R, Gasto C, Peralta V, Fananas L. Childhood adversity and psychosis: examining whether the association is due to genetic confounding using a monozygotic twin differences approach. Eur Psychiatry. 2013;28(4):207–12. [DOI] [PubMed] [Google Scholar]
- [12].Hassan AN, De Luca V. The effect of lifetime adversities on resistance to antipsychotic treatment in schizophrenia patients. Schizophr Res. 2015;161(2–3):496–500. [DOI] [PubMed] [Google Scholar]
- [13].Harley M, Kelleher I, Clarke M, Lynch F, Arseneault L, Connor D, et al. Cannabis use and childhood trauma interact additively to increase the risk of psychotic symptoms in adolescence. Psychol Med. 2010;40(10):1627–1634. [DOI] [PubMed] [Google Scholar]
- [14].Mandavia A, Robinson GG, Bradley B, Ressler KJ, Powers A. Exposure to childhood abuse and later substance use: indirect effects of emotion dysregulation and exposure to trauma. J Trauma Stress. 2016;29(5):422–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kevorkian S, Bonn-Miller MO, Belendiuk K, Carney DM, Roberson-Nay R, Berenz EC. Associations among trauma, posttraumatic stress disorder, cannabis use, and cannabis use disorder in a nationally representative epidemiologic sample. Psychol Addict Behav. 2015;29(3):633–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Meyers JL, Sartor CE, Werner KB, Koenen KC, Grant BF, Hasin D. Childhood interpersonal violence and adult alcohol, cannabis, and tobacco use disorders: variation by race/ethnicity? Psychol Med. 2018;48(9):1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Werner KB, McCutcheon VV, Agrawal A, Sartor CE, Nelson EC, Heath AC, et al. The association of specific traumatic experiences with cannabis initiation and transition to problem use: differences between African-American and European-American women. Drug Alcohol Depend. 2016;162:162–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fisher HL, McGuffin P, Boydell J, Fearon P, Craig TK, Dazzan P, et al. Interplay between childhood physical abuse and familial risk in the onset of psychotic disorders. Schizophr Bull. 2014;40(6):1443–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Duman B, Sedes N, Baskak B. Additive effects of former methylenedioxymethamphetamine and cannabis use on subclinical psychotic symptoms. Noro Psikiyatr Ars. 2017;54(1):38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Griffith-Lendering MF, Wigman JT, Prince van Leeuwen A, Huijbregts SC, Huizink AC, Ormel J, et al. Cannabis use and vulnerability for psychosis in early adolescence—a TRAILS study. Addiction. 2013;108(4):733–740. [DOI] [PubMed] [Google Scholar]
- [21].Karcher NR, Barch DM, Demers CH, Baranger DAA, Heath AC, Lynskey MT, et al. Genetic predisposition vs individual-specific processes in the association between psychotic-like experiences and cannabis use. JAMA Psychiatry. 2019;76(1):87–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Nesvag R, Reichborn-Kjennerud T, Gillespie NA, Knudsen GP, Bramness JG, Kendler KS, et al. Genetic and environmental contributions to the association between cannabis use and psychotic-like experiences in young adult twins. Schizophr Bull. 2017;43(3):644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Shakoor S, Zavos HM, McGuire P, Cardno AG, Freeman D, Ronald A. Psychotic experiences are linked to cannabis use in adolescents in the community because of common underlying environmental risk factors. Psychiatry Res. 2015;227(2–3):144–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].van Gastel WA, Wigman JT, Monshouwer K, Kahn RS, van Os J, Boks MP, et al. Cannabis use and subclinical positive psychotic experiences in early adolescence: findings from a Dutch survey. Addiction. 2012;107(2):381–7. [DOI] [PubMed] [Google Scholar]
- [25].Matheson SL, Shepherd AM, Laurens KR, Carr VJ. A systematic meta-review grading the evidence for non-genetic risk factors and putative antecedents of schizophrenia. Schizophr Res. 2011;133(1–3):133–142. [DOI] [PubMed] [Google Scholar]
- [26].Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M, et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet. 2007;370(9584):319–328. [DOI] [PubMed] [Google Scholar]
- [27].Konings M, Stefanis N, Kuepper R, Graaf R, Have M, Os J, et al. Replication in two independent population-based samples that childhood maltreatment and cannabis use synergistically impact on psychosis risk. Psychol Med. 2012;42(1):149–59. [DOI] [PubMed] [Google Scholar]
- [28].Engert V, Joober R, Meaney MJ, Hellhammer DH, Pruessner JC. Behavioral response to methylphenidate challenge: influence of early life parental care. Dev Psychobiol. 2009;51(5):408–416. [DOI] [PubMed] [Google Scholar]
- [29].Rodrigues AJ, Leao P, Carvalho M, Almeida OF, Sousa N. Potential programming of dopaminergic circuits by early life stress. Psychopharmacology (Berl.). 2011;214(1):107–120. [DOI] [PubMed] [Google Scholar]
- [30].Gaweda L, Pionke R, Krezolek M, Prochwicz K, Klosowska J, Frydecka D, et al. Self-disturbances, cognitive biases and insecure attachment as mechanisms of the relationship between traumatic life events and psychotic-like experiences in non-clinical adults—a path analysis. Psychiatry Res. 2018;259:571–578. [DOI] [PubMed] [Google Scholar]
- [31].Gaweda L, Prochwicz K, Adamczyk P, Frydecka D, Misiak B, Kotowicz K, et al. The role of self-disturbances and cognitive biases in the relationship between traumatic life events and psychosis proneness in a non-clinical sample. Schizophr Res. 2018;193:218–224. [DOI] [PubMed] [Google Scholar]
- [32].Mętel D., Arciszewska A., Daren A., Pionke R., Cechnicki A., Frydecka D. & Gawęda, Ł (2020). Mediating role of cognitive biases, resilience and depressive symptoms in the relationship between childhood trauma and psychotic-like experiences in young adults. Early Interv Psychiatry 14(1), 87-96. [DOI] [PubMed] [Google Scholar]
- [33].Yamasaki S, Ando S, Koike S, Usami S, Endo K, French P, et al. Dissociation mediates the relationship between peer victimization and hallucinatory experiences among early adolescents. Schizophr Res Cogn. 2016;4:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Metel D, Arciszewska A, Daren A, Frydecka D, Cechnicki A, Gaweda L. Resilience and cognitive biases mediate the relationship between early exposure to traumatic life events and depressive symptoms in young adults. J Affect Disord. 2019;254:26–33. [DOI] [PubMed] [Google Scholar]
- [35].Koren D, Reznik N, Adres M, Scheyer R, Apter A, Steinberg T, et al. Disturbances of basic self and prodromal symptoms among non-psychotic help-seeking adolescents. Psychol Med. 2013;43(7):1365–1376. [DOI] [PubMed] [Google Scholar]
- [36].Gaweda L, Goritz AS, Moritz S. Mediating role of aberrant salience and self-disturbances for the relationship between childhood trauma and psychotic-like experiences in the general population. Schizophr Res. 2019;206:149–156. [DOI] [PubMed] [Google Scholar]
- [37].Mackie CJ, O'Leary-Barrett M, Al-Khudhairy N, Castellanos-Ryan N, Struve M, Topper L, et al. Adolescent bullying, cannabis use and emerging psychotic experiences: a longitudinal general population study. Psychol Med. 2013;43(5):1033–1044. [DOI] [PubMed] [Google Scholar]
- [38].Morgan C, Reininghaus U, Reichenberg A, Frissa S, Hotopf M, Hatch SL. Adversity, cannabis use and psychotic experiences: evidence of cumulative and synergistic effects. Br J Psychiatry. 2014;204:346–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Nijenhuis E, Van der Hart O, Vanderlinden J. The Traumatic Experiences Checklist (TEC). Somatoform dissociation: phenomenia, measurement, and theoretical issues. Assen, The Netherlands: Van Gorcum, 1999. [Google Scholar]
- [40].Smith N, Lam D, Bifulco A, Checkley S. Childhood Experience of Care and Abuse Questionnaire (CECA.Q). Validation of a screening instrument for childhood adversity in clinical populations. Social Psychiatry Psychiatr Epidemiol. 2002;37(12):572–579. [DOI] [PubMed] [Google Scholar]
- [41].van der Gaag M, Schutz C, Ten Napel A, Landa Y, Delespaul P, Bak M, et al. Development of the Davos assessment of cognitive biases scale (DACOBS). Schizophr Res. 2013;144(1–3):63–71. [DOI] [PubMed] [Google Scholar]
- [42].Gaweda L, Prochwicz K, Krezolek M, Klosowska J, Staszkiewicz M, Moritz S. Self-reported cognitive distortions in the psychosis continuum: a Polish 18-item version of the Davos Assessment of Cognitive Biases Scale (DACOBS-18). Schizophr Res. 2018;192:317–326. [DOI] [PubMed] [Google Scholar]
- [43].Ising HK, Veling W, Loewy RL, Rietveld MW, Rietdijk J, Dragt S, et al. The validity of the 16-item version of the Prodromal Questionnaire (PQ-16) to screen for ultra high risk of developing psychosis in the general help-seeking population. Schizophr Bull. 2012;38(6):1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Copeland J, Gilmour S, Gates P, Swift W. The Cannabis Problems Questionnaire: factor structure, reliability, and validity. Drug Alcohol Depend. 2005;80(3):313–319. [DOI] [PubMed] [Google Scholar]
- [45].Hayes AF. Introduction to mediation, moderation, and conditional process analysis. A regression-based approach. 2nd ed. New York: Guilford Publications; 2017. [Google Scholar]
- [46].Preacher KJ, Hayes AF. Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behav Res Methods. 2008;40(3):879–891. [DOI] [PubMed] [Google Scholar]
- [47].Kelleher I, Keeley H, Corcoran P, Ramsay H, Wasserman C, Carli V, et al. Childhood trauma and psychosis in a prospective cohort study: cause, effect, and directionality. Am J Psychiatry. 2013;170(7):734–741. [DOI] [PubMed] [Google Scholar]
- [48].Sitko K, Bentall RP, Shevlin M, O'Sullivan N, Sellwood W. Associations between specific psychotic symptoms and specific childhood adversities are mediated by attachment styles: an analysis of the National Comorbidity Survey. Psychiatry Res. 2014;217(3):202–209. [DOI] [PubMed] [Google Scholar]
- [49].Bailey T, Alvarez-Jimenez M, Garcia-Sanchez AM, Hulbert C, Barlow E, Bendall S. Childhood trauma is associated with severity of hallucinations and delusions in psychotic disorders: a systematic review and meta-analysis. Schizophr Bull. 2018;44(5):1111–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Misiak B, Krefft M, Bielawski T, Moustafa AA, Sasiadek MM, Frydecka D. Toward a unified theory of childhood trauma and psychosis: a comprehensive review of epidemiological, clinical, neuropsychological and biological findings. Neurosci Biobehav Rev. 2017;75:393–406. [DOI] [PubMed] [Google Scholar]
- [51].Redman SL, Corcoran CM, Kimhy D, Malaspina D. Effects of early trauma on psychosis development in clinical high-risk individuals and stability of trauma assessment across studies: a review. Arch Psychol (Chic). 2017;1(3) 1–21. [PMC free article] [PubMed] [Google Scholar]
- [52].Ragazzi TCC, Shuhama R, Menezes PR, Del-Ben CM. Cannabis use as a risk factor for psychotic-like experiences: a systematic review of non-clinical populations evaluated with the community assessment of psychic experiences. Early Interv Psychiatry. 2018;12(6):1013–1023. [DOI] [PubMed] [Google Scholar]
- [53].Myles H, Myles N, Large M. Cannabis use in first episode psychosis: meta-analysis of prevalence, and the time course of initiation and continued use. Aust N Z J Psychiatry. 2016;50(3):208–219. [DOI] [PubMed] [Google Scholar]
- [54].Reeves LE, Anglin DM, Heimberg RG, Gibson LE, Fineberg AM, Maxwell SD, et al. Anxiety mediates the association between cannabis use and attenuated positive psychotic symptoms. Psychiatry Res. 2014;218(1–2):180–186. [DOI] [PubMed] [Google Scholar]
- [55].Stefanis NC, Delespaul P, Henquet C, Bakoula C, Stefanis CN, Van Os J. Early adolescent cannabis exposure and positive and negative dimensions of psychosis. Addiction. 2004;99(10):1333–1341. [DOI] [PubMed] [Google Scholar]
- [56].Arranz S, Monferrer N, Jose Algora M, Cabezas A, Sole M, Vilella E, et al. The relationship between the level of exposure to stress factors and cannabis in recent onset psychosis. Schizophr Res. 2018;201:352–359. [DOI] [PubMed] [Google Scholar]
- [57].Cougnard A, Marcelis M, Myin-Germeys I, De Graaf R, Vollebergh W, Krabbendam L, et al. Does normal developmental expression of psychosis combine with environmental risk to cause persistence of psychosis? A psychosis proneness-persistence model. Psychol Med. 2007;37(4):513–527. [DOI] [PubMed] [Google Scholar]
- [58].Sideli L, Fisher HL, Murray RM, Sallis H, Russo M, Stilo SA, et al. Interaction between cannabis consumption and childhood abuse in psychotic disorders: preliminary findings on the role of different patterns of cannabis use. Early Interv Psychiatry. 2018;12(2):135–142. [DOI] [PubMed] [Google Scholar]
- [59].Smeets F, Lataster T, Dominguez MD, Hommes J, Lieb R, Wittchen HU, et al. Evidence that onset of psychosis in the population reflects early hallucinatory experiences that through environmental risks and affective dysregulation become complicated by delusions. Schizophr Bull. 2012;38(3):531–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Smeets F, Lataster T, Viechtbauer W, Delespaul P, G.R.O.U.P. Evidence that environmental and genetic risks for psychotic disorder may operate by impacting on connections between core symptoms of perceptual alteration and delusional ideation. Schizophr Bull. 2015;41(3):687–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Collip D, Myin-Germeys I, van Os J. Does the concept of "sensitization" provide a plausible mechanism for the putative link between the environment and schizophrenia? Schizophr Bull. 2008;34(2):220–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Domen P, Michielse S, Peeters S, Viechtbauer W, van Os J, Marcelis M, et al. Childhood trauma- and cannabis-associated microstructural white matter changes in patients with psychotic disorder: a longitudinal family-based diffusion imaging study. Psychol Med. 2019;49(4):628–638. [DOI] [PubMed] [Google Scholar]
- [63].Boehme R, Deserno L, Gleich T, Katthagen T, Pankow A, Behr J, et al. Aberrant salience is related to reduced reinforcement learning signals and elevated dopamine synthesis capacity in healthy adults. J Neurosci. 2015;35(28):10103–10111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kapur S. Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry. 2003;160(1):13–23. [DOI] [PubMed] [Google Scholar]
- [65].McCutcheon RA, Bloomfield MAP, Dahoun T, Mehta M, Howes OD. Chronic psychosocial stressors are associated with alterations in salience processing and corticostriatal connectivity. Schizophr Res. 2018, 213: 56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III—the final common pathway. Schizophr Bull. 2009;35(3):549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Broyd A, Balzan RP, Woodward TS, Allen P. Dopamine, cognitive biases and assessment of certainty: a neurocognitive model of delusions. Clin Psychol Rev. 2017;54:96–106. [DOI] [PubMed] [Google Scholar]
- [68].Anglin DM, Espinosa A, Barada B, Tarazi R, Feng A, Tayler R, et al. Comparing the role of aberrant salience and dissociation in the relation between cumulative traumatic life events and psychotic-like experiences in a multi-ethnic sample. J Clin Med. 2019;8(8), 1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr Bull. 2013;39(6):1328–1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bloomfield MA, Mouchlianitis E, Morgan CJ, Freeman TP, Curran HV, Roiser JP, et al. Salience attribution and its relationship to cannabis-induced psychotic symptoms. Psychol. Med. 2016;46(16):3383–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Roiser JP, Stephan KE, Ouden HE, Barnes TR, Friston KJ, Joyce EM. Do patients with schizophrenia exhibit aberrant salience? Psychol Med. 2009;39(2):199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Mansueto G, Schruers K, Cosci F, Os J, GROUP Investigators. Childhood adversities and psychotic symptoms: the potential mediating or moderating role of neurocognition and social cognition. Schizophr Res. 2019;206:183–93. [DOI] [PubMed] [Google Scholar]
- [73].Simpson S, Phillips L, Baksheev G, Garner B, Markulev C, Phassouliotis C, et al. Stability of retrospective self-reports of childhood trauma in first-episode psychosis. Early Interv Psychiatry. 2019;13(4):908–913. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1192/j.eurpsy.2020.31.
click here to view supplementary material
Data Availability Statement
The data that support the findings of this study are openly available on request to the all authors. Please contact corresponding author.