Abstract
Sensorineural hearing loss and vestibular dysfunction are caused by damage to neurons and mechanosensitive hair cells, which do not regenerate to any clinically relevant extent in humans. Several protocols have been devised to direct pluripotent stem cells (PSCs) into inner ear hair cells and neurons, which display many properties of their native counterparts. The efficiency, reproducibility, and scalability of these protocols are enhanced by incorporating knowledge of inner ear development. Modeling human diseases in vitro through genetic manipulation of PSCs is already feasible, thereby permitting the elucidation of mechanistic understandings of a wide array of disease etiologies. Early studies on transplantation of PSC-derived otic progenitors have been successful in certain animal models, yet restoration of function and long-term cell survival remain unrealized. Through further research, PSC-based approaches will continue to revolutionize our understanding of inner ear biology and contribute to the development of therapeutic treatments for inner ear disorders.
Keywords: disease modeling, inner ear, organoid, stem cells, therapy
Tang and colleagues reviewed the use of pluripotent stem cells (PSCs) in the treatment and study of inner ear disorders. Precisely timed small-molecule treatments of PSCs generate inner ear hair cells, and support cells and neurons. Stem cell-based transplantation studies have shown successful engraftment in the inner ear and disease mechanisms can be studied in vitro using PSC-derived inner ear tissues.
Main Text
Introduction
It is estimated that 15% of the world's population has some degree of hearing loss (WHO, https://www.who.int/pbd/deafness/estimates/en/). In the US, approximately 35% of individuals over the age of 40 years have balance dysfunction and the prevalence increases with age (Agrawal et al., 2013). Hearing loss and vestibular disorders can stem from various causes, including genetic defects, environmental exposures, aging, and ototoxic medications. The inner ear is composed of the cochlea and vestibular organs (utricle, saccule, and three semicircular canals) and each harbors mechanosensory hair cells that convert mechanical stimuli (i.e., sound or head motion) into electrical signals in afferent sensory neurons through synaptic transmission. These electrical signals in the vestibulocochlear nerve are then transmitted to the brainstem and subsequently the auditory cortex in the brain. Injury to the sensory hair cells, the vestibulocochlear nerve, or the synaptic connections between them can result in hearing loss and/or vertigo. The lack of meaningful regenerative capacity of the inner ear sensory cells of mammals means loss of function is permanent.
Extensive studies using animal models (e.g., frog, zebrafish, birds, and mammals) have advanced our knowledge of the human inner ear function and disease. Access to human inner ear tissues is severely limited since tissue sampling is technically challenging and leads to irreparable damage. Non-invasive clinical imaging techniques, such as computed tomography or MRI, currently do not provide sufficient resolution to determine most pathologies of the inner ear (Kayyali et al., 2018). Pathologies of many human inner ear disorders can currently only be acquired by postmortem examinations.
In addition, access to inner ear tissues in mammalian animal models is also limited, which has slowed progress in the field. The sensory structures of the mammalian inner ear are surrounded by rigid bone and opening this rigid bone can lead to damage of the delicate cellular structures. In addition, cochlear explant cultures from mice are only technically feasible before postnatal day 7 (P7) (Ogier et al., 2019) and cannot be maintained for longer than 2 weeks (Parker et al., 2010, Sobkowicz et al., 1993). Although other animal models (e.g., zebrafish) have more accessible hair cells, there are significant innate differences between non-mammalian vertebrate and mammalian models, such as the capacity for spontaneous hair cell regeneration (Kniss et al., 2016, Warchol and Corwin, 1996). To overcome these limitations associated with the current animal models, alternative model systems that are sustainable, easily accessible, and easily manipulated in the laboratory are needed for furthering our understanding of the inner ear biology and pathology.
Over the past few decades, stem cell biology has accelerated research into the pathologic mechanisms of Parkinson disease and cardiac tissue regeneration (Beltrami et al., 2003, Johnson and Hockemeyer, 2015). Currently, the mature mammalian cochlea completely lacks the capacity to spontaneously proliferate or regenerate hair cells and there is very limited regeneration potential in the vestibular system (Forge et al., 1993, Warchol et al., 1993). Activation of quiescent progenitor cells residing in the sensory epithelium of the inner ear or replacing missing hair cells with new hair cells derived from pluripotent stem cells (PSCs) could be a potential cure. Despite the identification of multipotent cell populations in the human fetal cochlea and in the adult human spiral ganglion, no proliferative cell populations with the capacity to give rise to hair cells have been reported in adult humans (Senn et al., 2020).
Scientists have successfully derived inner ear progenitors and inner ear sensory cells from both multipotent and PSCs. Multipotent stem cells (i.e., somatic stem cells) exhibit limited self-renewal capacity and can differentiate only into cell types within an organ in which those stem cells reside. In contrast, PSCs (embryonic stem cells [ESCs] and induced pluripotent stem cells [iPSCs]) have infinite self-renewal capacity and the ability to differentiate into any cell type of the three major germ layers. Somatic stem cells, such as mesenchymal stem cells and neural stem cells, have been used to generate inner ear progenitors and restore damaged spiral ganglia (Boddy et al., 2012, Mittal et al., 2020, Xu et al., 2016). However, there are a limited number of sources for these somatic stem cells and they exhibit a restricted capacity for differentiation (Clevers, 2015). On the contrary, PSCs are relatively easy to be maintained in culture and can differentiate into essentially any cell types (Rossant and Papaioannou, 1984, Yilmaz and Benvenisty, 2019). Furthermore, genetically reprogramed iPSCs (Takahashi et al., 2007, Takahashi and Yamanaka, 2006) offer unique opportunities for personalized medicine, because the iPSCs can be derived from patients' own cells and they are resistant to immunocompatibility issues (Johnson and Hockemeyer, 2015, Yilmaz and Benvenisty, 2019).
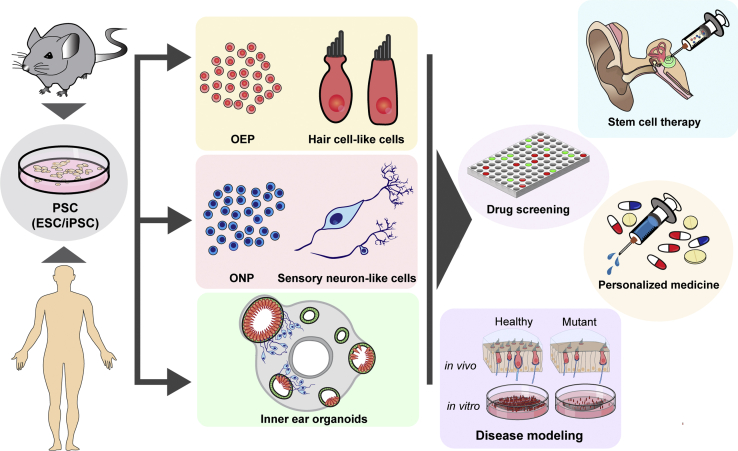
Here, we review the progress of in vitro derivation of PSC-derived inner ear hair cells, neurons, and organoids (Figure 1). We highlight the use of these PSC-derived tissues to understand the mechanisms of inner ear diseases and to restore inner ear function in early therapeutic trials (Figure 1).
Figure 1.
Applications of PSCs in the Inner Ear
Mouse- and human-derived ESCs and iPSCs can be induced to form otic epithelial progenitors (OEPs), hair cells, sensory neurons, and 3D organoids, which have scientific and therapeutic applications. ONP, otic neural progenitor.
Derivation of Inner Ear Tissues from PSCs
Understanding the complex sequence of transcriptional changes and signaling pathways that underlie in vivo inner ear development is critical to the successful differentiation of PSCs into inner ear tissues, such as hair cells, supporting cells, and neurons in vitro (Czajkowski et al., 2019, Roccio and Edge, 2019).
Hair Cells and Sensory Epithelia
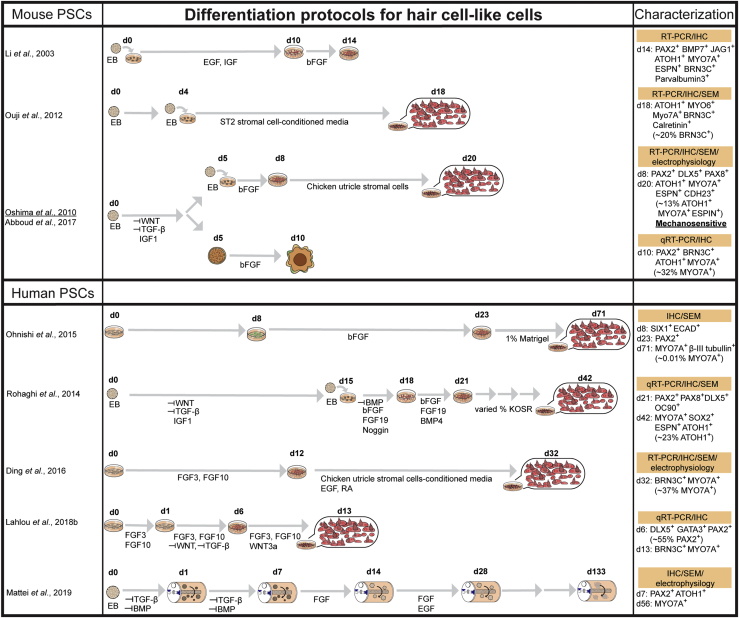
Numerous induction protocols have been developed to differentiate PSCs into hair cell-like cells (Figures 2 and 3). Many of these protocols start with the generation of floating embryoid bodies (i.e., aggregated cell masses) followed by stepwise treatments of a combination of small molecules and/or recombinant proteins that become adhesive 2D cultures after approximately 5 days (Abboud et al., 2017, Li et al., 2003, Oshima et al., 2010, Ouji et al., 2012, Ronaghi et al., 2014). Other protocols are carried out entirely in 2D culture, which can provide a homogeneous cell population (Chen et al., 2012, Ding et al., 2016, Lahlou et al., 2018a, Ohnishi et al., 2015). In contrast, 3D organoid systems contain multiple cell types and more precisely recapitulate in vivo composition of an organ. In 2013, the first 3D inner ear organoid system was developed using mESCs (Figure 3) (Koehler et al., 2013).
Figure 2.
Hair Cell-like Cells Derived from PSCs
Summary of induction strategies and characterization of both mouse and human PSC-derived hair cell-like cells. Petri dishes reflect adhesive 2D cnuultures, cell aggregates represent 3D floating cultures, and capsules represent rotary cultures. Characterization method highlighted in orange, key molecular markers (percentage of cells expressing) and mechanosensitivity are indicated. EB, embryonic body; d, day in culture; KOSR, knockout serum replacement; RA, retinoic acid.
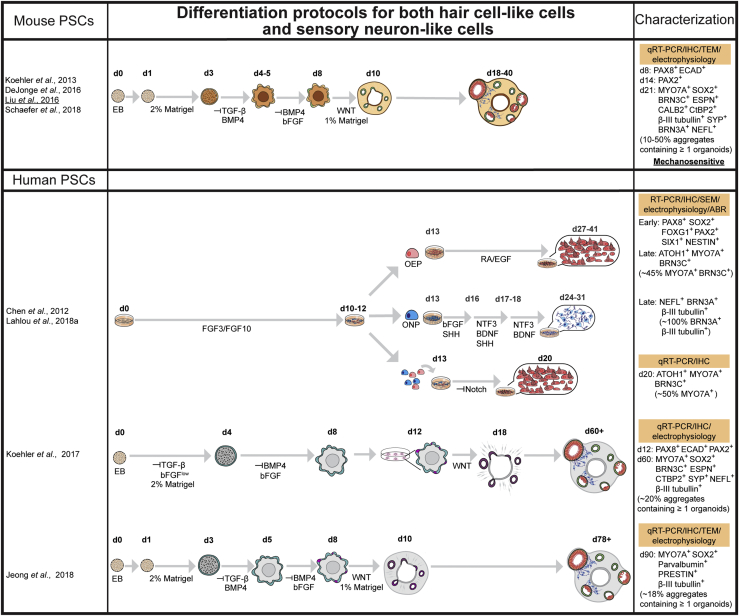
Figure 3.
Organoids Derived from PSCs
Summary of induction strategies and characterization of both mouse and human PSC-derived organoids. Cell aggregates represent 3D floating cultures. Characterization method highlighted in orange, key molecular markers (percentage of cells expressing) and mechanosensitivity are indicated. ABR, auditory brainstem response; d, culture day; EB, embryonic body; RA, retinoic acid.
The initial steps of inner ear development require the formation of the ectodermal germ layer followed by formation of the pre-placodal ectoderm (PPE). Protocols first inhibit transforming growth factor β and WNT signaling and activate BMP to promote non-neural ectodermal development, while reducing mesoderm development (Figures 2 and 3) (Abboud et al., 2017, Koehler et al., 2013, Koehler et al., 2017, Lahlou et al., 2018a, Oshima et al., 2010, Ronaghi et al., 2014). Insulin-like growth factor 1 promotes the fate of the anterior ectoderm, where the PPE emerges (Figure 2) (Li et al., 2003, Ronaghi et al., 2014). The PPE gives rise to most of the cranial placodes, including the otic placode. Fibroblast growth factor (FGF) signaling (FGF3/10 in mouse) is the main inducer of the posterior domain of the PPE that gives rise to otic placode. WNT activation along with FGF signaling facilitates otic placode induction (Singh and Groves, 2016) (Figures 2 and 3).
Physical environmental cues provided by extracellular matrices, such as Matrigel, improve the efficiency of differentiation, as well as the resulting cellular assemblage (Jeong et al., 2018, Koehler et al., 2013, Koehler et al., 2017, Mattei et al., 2019). For example, addition of Matrigel in the 3D inner ear organoid systems established by Koehler et al., 2013, Koehler et al., 2017 facilitated formation of fluid-filled vesicles containing hair cells and supporting cell-like cells (Figure 3). However, vestibular tissue-like organoids derived from hPSCs using the rotary cell culture system form hair cell-like cells on the surface of the organoids (Figure 2) (Mattei et al., 2019). At present, detailed mechanisms as to how extracellular matrices influence cell differentiation and survival are still unclear.
Specific PSC-derived cells along the developmental otic lineage are verified via expression of molecular markers, morphological examinations, and/or electrophysiological measurements (Chen et al., 2012, Koehler et al., 2017, Li et al., 2003, Oshima et al., 2010). SIX1 and E-cadherin expression are used to identify cells within the PPE (Ohnishi et al., 2015, Tchieu et al., 2017). PAX8 and PAX2 mark the posterior PPE (i.e., otic-epibranchial placodal domain, Figures 2 and 3) and are used to identify the otic progenitor cells within PSC-derived inner ear tissues (Chen et al., 2012, Jeong et al., 2018, Lahlou et al., 2018a). Induction of hair cells from the posterior PPE requires either endogenous WNT or supplementation with a WNT activator. Hair cell-like cells are verified by the expression of BRN3C (also known as POU4F3), MYO7A, and ATOH1 (Abboud et al., 2017, Koehler et al., 2013, Koehler et al., 2017, Oshima et al., 2010). Because native hair cells exhibit unique mechanosensitivity, patch-clamp recordings have been used to assess functional properties of PSC-derived hair cells. While the net potassium voltage gradient is most commonly recorded (Chen et al., 2012, Ding et al., 2016, Koehler et al., 2017), only a few studies have successfully recorded mechanotransduction currents from PSC-derived hair cells by deflecting hair bundles (Liu et al., 2016, Oshima et al., 2010). To date, PSC-derived hair cell-like cells display molecular markers and electrophysiological properties of vestibular hair cells and not cochlear hair cells. Discovery of alternative small molecules or culture conditions to generate cochlear hair cells from PSCs is still needed.
Induction protocols require optimization across PSC lines and across species. For example, Jeong and colleagues adapted the protocol for mouse ESC inner ear organoid (Koehler et al., 2013) to generate inner ear organoids from human PSCs (Figure 3) (Jeong et al., 2018). Temporal and spatial differences in gene expression across PSC lines likely contributes to known variability and efficacy of hair cell generation. Future improvements will occur as we advance our understanding of hair cell development or culture conditions.
Supporting cells of the sensory epithelia play an active role in ion metabolism necessary for hair cell function. The connexin proteins are the most abundant gap junction proteins expressed in the supporting cells. Mutations in GJB2, which encodes connexin 26 (CX26), are the most common cause of autosomal recessive non-syndromic sensorineural hearing loss (Kenna et al., 2010). In mouse 3D inner ear organoids, removing day 4 treatments (i.e., inhibition of BMP and activation of FGF) leads to increased expression of CX26 in day 7 aggregates (Fukunaga et al., 2016). Co-culturing the isolated small vesicles from day 7 aggregates with mouse cochlear feeder cells in 2D conditions resulted in hemichannel formation that was disrupted in CX26-deficient cell lines (Fukunaga et al., 2016). This study highlights the use of PSCs to study otic supporting cells.
Neurons and Glia
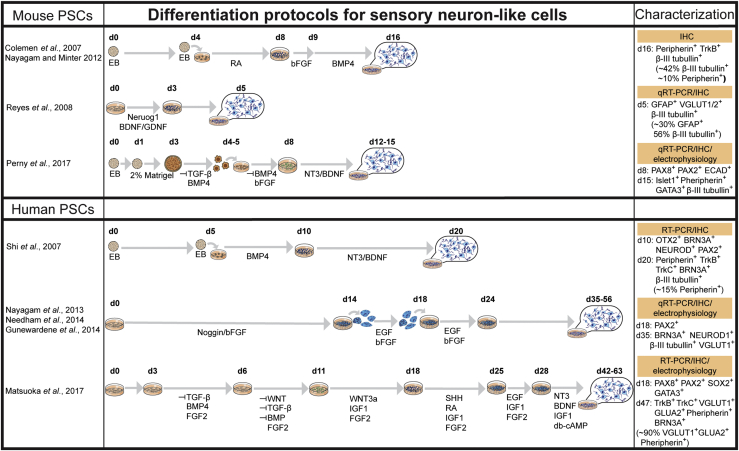
Early neural induction protocols from PSCs involved using stromal cells derived from skull bone marrow, which resulted in efficient dopaminergic neuron production (Kawasaki et al., 2000, Ying et al., 2003). These protocols have been modified to derive inner ear sensory neurons and glial cells from PSCs (Figure 4). For example, mESCs treated with retinoic acid (RA), basic FGF (bFGF), and BMP4 were shown to give rise to β-III tubulin+ and peripherin+ neurons (Coleman et al., 2007, Nayagam and Minter, 2012). However, many of the available protocols are tailored to generate dopaminergic neurons due to their role in neurodegenerative disorders, such as Parkinson disease (Chen et al., 2016b). In contrast, glutamate is the primary neurotransmitter for the synaptic transmission between hair cells and afferent sensory neurons within the inner ear (Oestreicher et al., 2002). Thus, the derivation of glutamatergic neurons was a key step for recapitulating afferent neural transmission in the inner ear. Reyes et al. (2008) used a mESC line that transiently expressed Neurog1 along with supplementation of brain-derived neurotrophic factor (BDNF) and glia cell line-derived neurotrophic factor to promote the differentiation of mESCs into glutamatergic neurons both in vitro and in vivo. Forced expression of Tlx3, a genetic switch capable of conferring a glutamatergic over a GABAergic transmitter phenotype, promotes the glutamatergic neuronal specification from mESCs (Kondo et al., 2008).
Figure 4.
Sensory Neuron-like Cells Derived from PSCs
Summary of induction strategies and characterization of mouse and human PSC-derived neuron-like cells. Petri dishes reflect adhesive 2D cultures and cell aggregates represent 3D floating cultures. Characterization method highlighted in orange and key molecular markers (percentage of cells expressing) are indicated. EB, embryonic body; d, culture day; RA, retinoic acid.
As with the sensory epithelia in general, inner ear sensory neurons are derived from the otic placode (Steventon et al., 2014). Therefore, some of the early induction steps for these sensory neurons were designed based on known developmental trajectories of the otic placode. Small molecules, such as FGFs, BMP, sonic hedgehog, and noggin are used to support neuronal outgrowth from the otic placode (Gunewardene et al., 2014, Matsuoka et al., 2017, Nayagam et al., 2013, Needham et al., 2014, Perny et al., 2017). BDNF and NT3 provide critical trophic support for inner ear sensory neuron afferents in vivo (Fritzsch et al., 2004) and are also used to support survival and growth of PSC-derived neurons (Matsuoka et al., 2017, Perny et al., 2017, Shi et al., 2007). Peripherin (which is specifically expressed by type II spiral ganglion neurons [Huang et al., 2007]), BRN3A (also known as POU4F1), and β-III tubulin are commonly used to verify the formation of neurons, with glutamate receptors and transporters subsequently exploited to confirm subtype-specific derivatives (Figure 4). However, not all ESC-derived neurons within the same protocol express the same markers. Matsuoka et al. (2017) showed that ∼90% of neuron-like cells were peripherin+ and β-III tubulin+, but only ∼46% were BRN3A+. Alternatively, synaptic contacts and neural innervation between co-existing hair cell-like cells and sensory neuron-like cells will provide more convincing evidence as to the identity of the derived cells.
To more precisely recapitulate the peripheral neuro-circuitry in vitro, a model that contains both sensory epithelia and innervating neurons is needed. Currently, there are two systems that potentially generate both tissues simultaneously (Figure 3). Treatment of hESCs with FGF3 and FGF10 for 10–12 days in 2D culture gives rise to both otic epithelial progenitors (OEPs) and otic neural progenitors (ONPs), which can be distinguished based on their morphology (Chen et al., 2012). Hair cell-like cells can be derived from OEP after the inhibition of Notch and supplementation with RA and epidermal growth factor (Chen et al., 2012, Lahlou et al., 2018b). Sensory neuron-like cells can be derived from ONPs using bFGF and sonic hedgehog followed by BDNF and NT3 supplementation (Chen et al., 2012). Co-culture of these derived hair cells and sensory neurons results in formation of neural connections in vitro (Chen et al., 2018). However, this method required separate induction protocols before the co-culture of derived hair cells and sensory neurons.
On the other hand, 3D inner ear organoids contain both sensory epithelia (hair cells and supporting cells) and sensory neuron-like cells (Figure 3) (Jeong et al., 2018, Koehler et al., 2013, Koehler et al., 2017). Furthermore, several studies have replicated these protocols (DeJonge et al., 2016, Schaefer et al., 2018) and used the systems for different applications, such as electrophysiological studies and disease modeling (Liu et al., 2016, Tang et al., 2019). Although further characterization of sensory neuron-like cells is still in need, the inner ear organoid system provides a powerful tool to study peripheral sensory neural networks in the inner ear in vitro. Human inner ear organoids derived from hPSCs also offer the opportunity to explore developmental biology of the human inner ear and understand the differences that exist between mice and human inner ear development.
Stem Cell Therapy
Cell-based therapy aims at replacing lost or damaged tissues via engraftment of viable transplanted cells or via stimulation of endogenous self-healing pathways by trophic factors (Jain, 2011). Recent advances in stem cell engineering have made it possible to use cell-based therapy in combatting disorders, such as cancer, cardiovascular disease, and neurodegeneration (Chopra et al., 2019, Goradel et al., 2018, Vasic et al., 2019). Because mechanosensory hair cells and their associated neurons in the mammalian inner ears lack the capacity for regeneration (Kujawa and Liberman, 2009), cell-based therapy may offer a viable treatment option for inner ear disorders. Current challenges for cellular transplantation include cell survival and differentiation after transplantation, particularly in the scala media of the cochlea, which contains high potassium endolymph. In addition, proper arrangement of nascent sensory cells is critical since engraftment of supernumerary hair cells in the cochlea does not restore function (Chen and Segil, 1999). The challenges to neuronal transplantation include establishing synaptic connections with both the inner ear sensory cells and the brainstem.
Transplantation of Undifferentiated Stem Cells
Cellular transplantation of undifferentiated mESCs were initially tested by injecting them directly into the healthy rat cochlea (Hu et al., 2004). Migration of transplanted cells along the auditory nerve toward the brain stem was detected, yet only ∼1% of the transplanted cells survived (Hu et al., 2004). When using animals with injured vestibulocochlear nerves, the survival rate of implanted mESCs increased to over 60% 2 weeks after injection, but the survival rate drastically decreased to 25% 4 weeks after injection (Regala et al., 2005). During transplantation, the use of BDNF improved the survival of donor cells and promoted the neural differentiation, while the use of the extracellular matrix enzyme ChABC (chondroitinase ABC enzyme) improves donor cell migration (Palmgren et al., 2012).
Most transplanted cells have been detected in the perilymph spaces (Lang et al., 2008, Regala et al., 2005), while a far lower percentage localized to the endolymph, where the sensory epithelia reside (Lang et al., 2008). While the majority of transplantation studies were carried out via intra-cochlear sites, the feasibility of injecting mESCs into the utricle was tested and a population of the injected mESCs traveled to the perilymph space, with observation of some cells in the vicinity of the cochlear sensory epithelia and spiral limbus (Praetorius et al., 2008). When mESCs were injected into the modiolus of the cochlea via the scala tympani, transplanted cells were detected along the vestibulocochlear nerve toward the brainstem and in the endolymph compartment (Palmgren et al., 2012, Zhao et al., 2013). Collectively, the site of injection appears to determine the trajectory and final destination of the transplanted ESCs.
In addition to cell survival and engraftment, it is important to verify whether implanted PSCs can further differentiate into inner ear cell types. Sakamoto and colleagues injected EGFP reporter-tagged mESCs into adult mouse inner ears and after 4 weeks observed differentiation of the transplanted cells marked by E-cadherin and neural cell adhesion molecule, markers for the ectodermal lineage. Nevertheless, these transplanted cells did not differentiate further into hair cells or neurons (Sakamoto et al., 2004). Overall, many studies over the past 15 years have documented the engraftments of PSCs and improved the transplantation procedures. Despite this success, the restoration in hearing function has not yet been achieved using undifferentiated PSCs as donor cells (Gokcan et al., 2016). In addition, the concern remains with transplantation of undifferentiated PSCs and the development of teratomas (Lee et al., 2013).
Transplantation of Partially Differentiated Progenitors
Transplantation of partially differentiated progenitors from PSCs could be safer and more efficacious since this approach is less likely to result in teratoma formation when compared with undifferentiated PSCs (Chen et al., 2018, Fukuda et al., 2006) and is more likely to generate terminally differentiated hair cells or neurons.
Neural progenitors derived from mESCs were first tested in mouse cochleae where the transplanted progenitor cells survived and underwent differentiation (Corrales et al., 2006, Okano et al., 2005). Outgrowth of neurites from transplanted cells toward damaged sensory epithelia was observed, but restoration of function was not detected (Coleman et al., 2006, Corrales et al., 2006, Okano et al., 2005). Synaptic connectivity between neurons and hair cells is critical for proper inner ear function. Matsumoto et al. (2008) co-cultured ESC-derived neurons and cochlear explants and observed establishment of synaptic contacts with the inner hair cells in vitro. Similar re-integration between differentiated neurons and native hair cells was confirmed using iPSC-derived progenitors (Nishimura et al., 2009).
Many of the aforementioned early studies used the “stroma cell-derived inducing activity” protocol, which was originally developed for the induction of dopaminergic neurons (Kawasaki et al., 2000), to derive neural progenitors before transplantation to the inner ear (Matsumoto et al., 2008, Okano et al., 2005, Sakamoto et al., 2004). The lack of glutamatergic phenotype may explain the failed recovery of function in these studies even though the synaptic connections were re-formed. More recently, neural induction of iPSCs in a 3D collagen matrix followed by transplantation into the scala tympani of the guinea-pig cochleae resulted in glutamate transporter (VGlut1) expression in >50% of the transplanted neurons, yet only ∼2% of cells survived 2 weeks post-transplantation (Ishikawa et al., 2017).
Transplantation of hESC-derived ONPs into gerbils with selective loss of spiral ganglion neurons has led to improvement in the auditory brainstem response 10 weeks after transplantation. Neural cell bodies with processes extending to both cochlear hair cells and the cochlear nucleus of the brainstem were observed (Chen et al., 2012). Human iPSC-derived OEPs have been injected into the modiolus of gerbil cochleae and the migration, engraftment, and differentiation of transplanted progenitor cells have been observed (Chen et al., 2018, Lopez-Juarez et al., 2019). Furthermore, immunosuppression with cyclosporine improved the quantity of engrafted cells that migrated beyond the basilar membrane toward the scala media (Lopez-Juarez et al., 2019). However, these transplanted cells still failed to differentiate into hair cells (Chen et al., 2018).
In summary, when compared with undifferentiated PCSs, partially differentiated progenitors show more promising capacity for further differentiation, migration, and engraftment after transplantation and the risk of teratomas appears to be lower (Nishimura et al., 2012). Despite the fact that restoration of auditory function has not been consistently realized, significant progress has been made with inner ear transplantation methodologies and survival of transplanted cells. Further studies to understand how to tonotopically organize transplanted cells, the mechanistic role of extracellular matrix components in cell survival, and the presence and proliferation of progenitor populations within the inner ear are needed to realize stem cell therapy within the auditory and vestibular system.
Modeling Inner Ear Disorders with PSCs
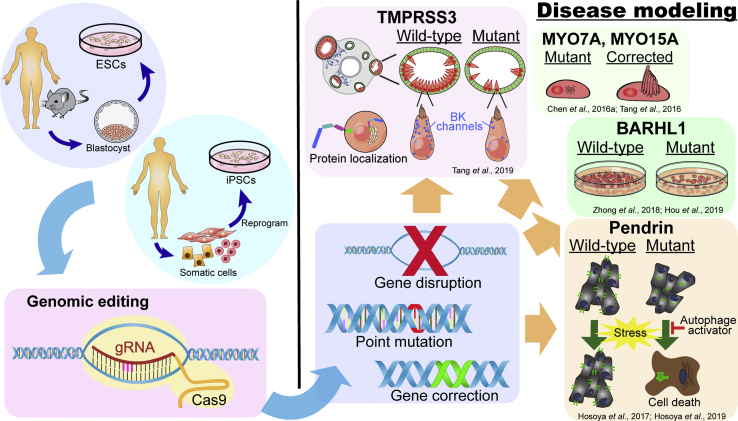
Modeling human inner ear disorders has relied heavily on insights obtained from rodent models (Nist-Lund et al., 2019, Straka et al., 2016, Wangemann, 2013). In addition, the use of mammalian cochlear explants are generally limited to neonatal mice due to the aforementioned technical difficulties associated with inner ear tissues at later developmental ages (Ogier et al., 2019). Another limiting factor with primary cochlear explants is that cultures cannot be maintained for longer than ∼14 days (Parker et al., 2010, Sobkowicz et al., 1993). In contrast, inner ear tissues generated from human and mouse PSCs can be maintained for many weeks under proper culture conditions. Furthermore, patient-specific, disease-associated mutations can be introduced in the genome of PSCs using CRISPR/Cas9 genome editing technology to study the molecular mechanisms of genetic hearing loss.
Hundreds of genetic mutations are associated with hearing loss, yet the specific mechanisms of deafness are unknown for most genes. Barhl1 is homeobox gene expressed in hair cells and important for hair cell maintenance. Constitutive expression of mutant Barhl1 in mESC-derived hair cells disrupts the derivation of hair cells without altering the induction of early otic progenitors (Zhong et al., 2018). It should be noted that the mESCs were co-cultured with utricular stromal cells, which can result in high variability across cultures. Subsequent RNA sequencing (RNA-seq) analysis revealed that the integrity of the 3′ enhancer region of Barh1 contains a plausible binding site for ATOH1 and is critical for proper hair cell differentiation (Hou et al., 2019) (Figure 5).
Figure 5.
PSCs in Inner Ear Disease Modeling
Human and mouse ESCs and iPSCs in combination with genomic editing technologies (e.g., CRISPR/Cas9), provide powerful tools for investigating disease mechanisms.
In lieu of the 2D culture system, mESC-derived inner ear 3D organoids have been used to study hair cell degeneration resulting from defective Tmprss3 (Figure 5). The type II transmembrane serine protease, TMPRSS3, is required for maintenance of hair cells in mice and as nonsense mutation (Tmprss3Y260X) leads to rapid hair cell degeneration at P12 in mice (Fasquelle et al., 2011). Mutations in TMPRSS3 cause congenital or early-onset hearing loss in humans (Scott et al., 2001), yet the functional role of TMPRSS3 in the inner ear is not fully understood. ESC-derived organoids from Tmprss3Y260X mice show normal hair cell development followed by hair cell degeneration at differentiation day 38 (equivalent to ∼P14 in mouse) (Tang et al., 2019). These observations are comparable with phenotypes observed in Tmprss3Y260X mice. Furthermore, hair cells derived from CRISPR/Cas9-generated Tmprss3 knockout (KO) mESCs showed more severe degeneration than those derived from Tmprss3Y260X mESCs (Figure 5). These results demonstrate that the extent of degeneration varies across type of mutations (KO versus truncation). The potential roles of TMPRSS3, including the maintenance of intracellular ion homeostasis and extracellular matrix organization, in hair cells were inferred by differential gene expression analyses using single-cell RNA-seq (scRNA-seq) between wild-type and Tmprss3-KO organoid hair cells (Tang et al., 2019). Together, this work highlights the utility of PSC-derived organoid systems for investigating the molecular mechanisms underlying genetic inner ear disorders and will be vital for confronting the high levels of genetic heterogeneity that have hindered our understanding of the link between genetic mutations and hearing loss (Van Camp et al., 1997).
In addition to recapitulating inner ear pathology caused by genetic mutations, PSCs are also a powerful tool for examining the effects of “genetic correction” in gene therapy. Mutations in MYO7A and MYO15A are known human deafness genes (Shearer et al., 1993). Hair cells derived from patient-specific iPSC lines carrying MYO7A and MYO15A mutations harbor abnormal stereocilia (Chen et al., 2016a, Tang et al., 2016). After correction of genetic mutations using CRISPR/Cas9 technology, derived hair cells showed restored structure of stereocilia-like protrusions and voltage-dependent currents and current densities similar to controls (Figure 5) (Chen et al., 2016a, Tang et al., 2016).
As an alternative to CRISPR-edited PSCs, patient-derived iPSCs offer personalized human models for studying genetic-associated inner ear disorders. iPSCs derived from Pendred syndrome patients with biallelic SLC26A4 point mutations have provided novel insights into how pendrin protein dysfunction leads to hearing loss (Figure 5) (Hosoya et al., 2017, Hosoya et al., 2019). Pendrin is an anion exchange protein encoded by the gene SLC26A4 and a primary cause of hair cell dysfunction was believed to stem from defects in anion exchange (Hosoya et al., 2017). Outer sulcus cells generated from these SLC26A4 mutant iPSCs demonstrated aggregated mutant pendrin proteins within the cytoplasm and exhibited decreased survival in response to cell stress induced with proteasome inhibition. These data suggest that hair cell dysfunction in Pendred syndrome may result from aggregation of mutant pendrin protein that results in susceptibility of cells to stress and cell death. Furthermore, by testing patient-derived iPSCs, they demonstrated that treatment with rapamycin, an autophagy activator, increases cell survival and may attenuate the progression of hearing loss in patients with Pendred syndrome (Figure 5) (Hosoya et al., 2017, Hosoya et al., 2019).
In summary, PSC-derived in vitro inner ear models offer valuable tools for elucidating mutant genotype-associated phenotypes, examining the effects of gene correction, and investigating the molecular mechanisms underlying the pathologies.
Conclusions and Future Perspectives
Significant progress has been made over the past 15 years in applications of PSCs in inner ear therapies. Inner ear hair cells, supporting cells, and neurons can now be generated from both mouse and human PSCs, although reproducibility and scalability still pose challenges. Better understandings of inner ear development and incorporation of such knowledge into the induction protocols will greatly improve efficiency and reproducibility. PSC-derived otic progenitors have shown the capacity for differentiation, migration, and engraftment with native tissues after transplantation in rodent models (Figure 5). However, there remains the risk of the formation of teratomas. Reduction of this potential side effect has been demonstrated using partially differentiated PSCs, yet discovery of novel methods to regulate off target differentiation in vivo is needed.
PSC-derived inner ear tissues, especially those from patient-specific iPSCs, provide powerful tools for unveiling disease mechanisms that were previously difficult, if not impossible, to examine. Stem cell-derived inner ear organoids along with scRNA-seq can provides mechanistic insights into genetic deafness (Figure 5). Human PSC-based organoid studies in vitro may be directly translatable to patients in vivo. Current limitations to the use of PSCs to study and treat inner ear disorders include labor-intensive culture protocols, variable efficiency of tissue derivation, inability to derive cochlear tissues in 3D organoids, limited differentiation and integration in host tissues, and inability to study age-related disorders. Opportunities for future translation in the field lie in optimization of gene therapies, personalized cell transplantation therapeutics, and drug screening for hair cell regeneration or ototoxicity.
Author Contributions
P.-C.T. performed literature search and wrote the manuscript. E.H. and R.F.N. conceived the project and revised the manuscript. All authors reviewed the manuscript and all approved of the final version.
Acknowledgments
This work was supported by the NIH (K08-DC016034 to R.F.N., R01-DC015788 to E.H.) and the Triological Society and American College of Surgeons (Clinician Scientist Development Award to R.F.N.). We thank Dr. Anderson Mayfield for critically reviewing the manuscript.
References
- Abboud N., Fontbonne A., Watabe I., Tonetto A., Brezun J.M., Feron F., Zine A. Culture conditions have an impact on the maturation of traceable, transplantable mouse embryonic stem cell-derived otic progenitor cells. J. Tissue Eng. Regen. Med. 2017;11:2629–2642. doi: 10.1002/term.2163. [DOI] [PubMed] [Google Scholar]
- Agrawal Y., Ward B.K., Minor L.B. Vestibular dysfunction: prevalence, impact and need for targeted treatment. J. Vestib. Res. 2013;23:113–117. doi: 10.3233/VES-130498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrami A.P., Barlucchi L., Torella D., Baker M., Limana F., Chimenti S., Kasahara H., Rota M., Musso E., Urbanek K. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell. 2003;114:763–776. doi: 10.1016/s0092-8674(03)00687-1. [DOI] [PubMed] [Google Scholar]
- Boddy S.L., Chen W., Romero-Guevara R., Kottam L., Bellantuono I., Rivolta M.N. Inner ear progenitor cells can be generated in vitro from human bone marrow mesenchymal stem cells. Regen. Med. 2012;7:757–767. doi: 10.2217/rme.12.58. [DOI] [PubMed] [Google Scholar]
- Chen J., Hong F., Zhang C., Li L., Wang C., Shi H., Fu Y., Wang J. Differentiation and transplantation of human induced pluripotent stem cell-derived otic epithelial progenitors in mouse cochlea. Stem Cell Res. Ther. 2018;9:230. doi: 10.1186/s13287-018-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J.R., Tang Z.H., Zheng J., Shi H.S., Ding J., Qian X.D., Zhang C., Chen J.L., Wang C.C., Li L. Effects of genetic correction on the differentiation of hair cell-like cells from iPSCs with MYO15A mutation. Cell Death Differ. 2016;23:1347–1357. doi: 10.1038/cdd.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Segil N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development. 1999;126:1581–1590. doi: 10.1242/dev.126.8.1581. [DOI] [PubMed] [Google Scholar]
- Chen W., Jongkamonwiwat N., Abbas L., Eshtan S.J., Johnson S.L., Kuhn S., Milo M., Thurlow J.K., Andrews P.W., Marcotti W. Restoration of auditory evoked responses by human ES-cell-derived otic progenitors. Nature. 2012;490:278–282. doi: 10.1038/nature11415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Xiong M., Dong Y., Haberman A., Cao J., Liu H., Zhou W., Zhang S.C. Chemical control of grafted human PSC-derived neurons in a mouse model of Parkinson's disease. Cell Stem Cell. 2016;18:817–826. doi: 10.1016/j.stem.2016.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra N., Choudhury S., Bhargava S., Wajid S., Ganguly N.K. Potentials of "stem cell-therapy" in pancreatic cancer: an update. Pancreatology. 2019;19:1034–1042. doi: 10.1016/j.pan.2019.09.016. [DOI] [PubMed] [Google Scholar]
- Clevers H. Stem Cells. What is an adult stem cell? Science. 2015;350:1319–1320. doi: 10.1126/science.aad7016. [DOI] [PubMed] [Google Scholar]
- Coleman B., Fallon J.B., Pettingill L.N., de Silva M.G., Shepherd R.K. Auditory hair cell explant co-cultures promote the differentiation of stem cells into bipolar neurons. Exp. Cell Res. 2007;313:232–243. doi: 10.1016/j.yexcr.2006.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman B., Hardman J., Coco A., Epp S., de Silva M., Crook J., Shepherd R. Fate of embryonic stem cells transplanted into the deafened mammalian cochlea. Cell Transplant. 2006;15:369–380. doi: 10.3727/000000006783981819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrales C.E., Pan L., Li H., Liberman M.C., Heller S., Edge A.S. Engraftment and differentiation of embryonic stem cell-derived neural progenitor cells in the cochlear nerve trunk: growth of processes into the organ of Corti. J. Neurobiol. 2006;66:1489–1500. doi: 10.1002/neu.20310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czajkowski A., Mounier A., Delacroix L., Malgrange B. Pluripotent stem cell-derived cochlear cells: a challenge in constant progress. Cell. Mol. Life Sci. 2019;76:627–635. doi: 10.1007/s00018-018-2950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeJonge R.E., Liu X.P., Deig C.R., Heller S., Koehler K.R., Hashino E. Modulation of Wnt signaling enhances inner ear organoid development in 3D culture. PLoS One. 2016;11:e0162508. doi: 10.1371/journal.pone.0162508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Tang Z., Chen J., Shi H., Chen J., Wang C., Zhang C., Li L., Chen P., Wang J. Induction of differentiation of human embryonic stem cells into functional hair-cell-like cells in the absence of stromal cells. Int. J. Biochem. Cell Biol. 2016;81:208–222. doi: 10.1016/j.biocel.2015.11.012. [DOI] [PubMed] [Google Scholar]
- Fasquelle L., Scott H.S., Lenoir M., Wang J., Rebillard G., Gaboyard S., Venteo S., Francois F., Mausset-Bonnefont A.L., Antonarakis S.E. Tmprss3, a transmembrane serine protease deficient in human DFNB8/10 deafness, is critical for cochlear hair cell survival at the onset of hearing. J. Biol. Chem. 2011;286:17383–17397. doi: 10.1074/jbc.M110.190652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forge A., Li L., Corwin J.T., Nevill G. Ultrastructural evidence for hair cell regeneration in the mammalian inner ear. Science. 1993;259:1616–1619. doi: 10.1126/science.8456284. [DOI] [PubMed] [Google Scholar]
- Fritzsch B., Tessarollo L., Coppola E., Reichardt L.F. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog. Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- Fukuda H., Takahashi J., Watanabe K., Hayashi H., Morizane A., Koyanagi M., Sasai Y., Hashimoto N. Fluorescence-activated cell sorting-based purification of embryonic stem cell-derived neural precursors averts tumor formation after transplantation. Stem Cells. 2006;24:763–771. doi: 10.1634/stemcells.2005-0137. [DOI] [PubMed] [Google Scholar]
- Fukunaga I., Fujimoto A., Hatakeyama K., Aoki T., Nishikawa A., Noda T., Minowa O., Kurebayashi N., Ikeda K., Kamiya K. In vitro models of GJB2-related hearing loss recapitulate Ca(2+) transients via a gap junction characteristic of developing cochlea. Stem Cell Reports. 2016;7:1023–1036. doi: 10.1016/j.stemcr.2016.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokcan M.K., Mulazimoglu S., Ocak E., Can P., Caliskan M., Besalti O., Dizbay Sak S., Kaygusuz G. Study of mouse induced pluripotent stem cell transplantation into Wistar albino rat cochleae after hair cell damage. Turk. J. Med. Sci. 2016;46:1603–1610. doi: 10.3906/sag-1510-136. [DOI] [PubMed] [Google Scholar]
- Goradel N.H., Hour F.G., Negahdari B., Malekshahi Z.V., Hashemzehi M., Masoudifar A., Mirzaei H. Stem cell therapy: a new therapeutic option for cardiovascular diseases. J. Cell. Biochem. 2018;119:95–104. doi: 10.1002/jcb.26169. [DOI] [PubMed] [Google Scholar]
- Gunewardene N., Bergen N.V., Crombie D., Needham K., Dottori M., Nayagam B.A. Directing human induced pluripotent stem cells into a neurosensory lineage for auditory neuron replacement. Biores. Open Access. 2014;3:162–175. doi: 10.1089/biores.2014.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya M., Fujioka M., Sone T., Okamoto S., Akamatsu W., Ukai H., Ueda H.R., Ogawa K., Matsunaga T., Okano H. Cochlear cell modeling using disease-specific iPSCs unveils a degenerative phenotype and suggests treatments for congenital progressive hearing loss. Cell Rep. 2017;18:68–81. doi: 10.1016/j.celrep.2016.12.020. [DOI] [PubMed] [Google Scholar]
- Hosoya M., Saeki T., Saegusa C., Matsunaga T., Okano H., Fujioka M., Ogawa K. Estimating the concentration of therapeutic range using disease-specific iPS cells: low-dose rapamycin therapy for Pendred syndrome. Regen. Ther. 2019;10:54–63. doi: 10.1016/j.reth.2018.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou K., Jiang H., Karim M.R., Zhong C., Xu Z., Liu L., Guan M., Shao J., Huang X. A critical E-box in Barhl1 3' enhancer is essential for auditory hair cell differentiation. Cells. 2019;8 doi: 10.3390/cells8050458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Z., Ulfendahl M., Olivius N.P. Central migration of neuronal tissue and embryonic stem cells following transplantation along the adult auditory nerve. Brain Res. 2004;1026:68–73. doi: 10.1016/j.brainres.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Huang L.C., Thorne P.R., Housley G.D., Montgomery J.M. Spatiotemporal definition of neurite outgrowth, refinement and retraction in the developing mouse cochlea. Development. 2007;134:2925–2933. doi: 10.1242/dev.001925. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Ohnishi H., Skerleva D., Sakamoto T., Yamamoto N., Hotta A., Ito J., Nakagawa T. Transplantation of neurons derived from human iPS cells cultured on collagen matrix into guinea-pig cochleae. J. Tissue Eng. Regen. Med. 2017;11:1766–1778. doi: 10.1002/term.2072. [DOI] [PubMed] [Google Scholar]
- Jain K.K. Nanobiotechnology and personalized medicine. Prog. Mol. Biol. Transl Sci. 2011;104:325–354. doi: 10.1016/B978-0-12-416020-0.00008-5. [DOI] [PubMed] [Google Scholar]
- Jeong M., O'Reilly M., Kirkwood N.K., Al-Aama J., Lako M., Kros C.J., Armstrong L. Generating inner ear organoids containing putative cochlear hair cells from human pluripotent stem cells. Cell Death Dis. 2018;9:922. doi: 10.1038/s41419-018-0967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J.Z., Hockemeyer D. Human stem cell-based disease modeling: prospects and challenges. Curr. Opin. Cell Biol. 2015;37:84–90. doi: 10.1016/j.ceb.2015.10.007. [DOI] [PubMed] [Google Scholar]
- Kawasaki H., Mizuseki K., Nishikawa S., Kaneko S., Kuwana Y., Nakanishi S., Nishikawa S.I., Sasai Y. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Kayyali M.N., Wright A.C., Ramsey A.J., Brant J.A., Stein J.M., O'Malley B.W., Jr., Li D. Challenges and opportunities in developing targeted molecular imaging to determine inner ear defects of sensorineural hearing loss. Nanomedicine. 2018;14:397–404. doi: 10.1016/j.nano.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenna M.A., Feldman H.A., Neault M.W., Frangulov A., Wu B.L., Fligor B., Rehm H.L. Audiologic phenotype and progression in GJB2 (Connexin 26) hearing loss. Arch. Otolaryngol. Head Neck Surg. 2010;136:81–87. doi: 10.1001/archoto.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniss J.S., Jiang L., Piotrowski T. Insights into sensory hair cell regeneration from the zebrafish lateral line. Curr. Opin. Genet. Dev. 2016;40:32–40. doi: 10.1016/j.gde.2016.05.012. [DOI] [PubMed] [Google Scholar]
- Koehler K.R., Mikosz A.M., Molosh A.I., Patel D., Hashino E. Generation of inner ear sensory epithelia from pluripotent stem cells in 3D culture. Nature. 2013;500:217–221. doi: 10.1038/nature12298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehler K.R., Nie J., Longworth-Mills E., Liu X.P., Lee J., Holt J.R., Hashino E. Generation of inner ear organoids containing functional hair cells from human pluripotent stem cells. Nat. Biotechnol. 2017;35:583–589. doi: 10.1038/nbt.3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo T., Sheets P.L., Zopf D.A., Aloor H.L., Cummins T.R., Chan R.J., Hashino E. Tlx3 exerts context-dependent transcriptional regulation and promotes neuronal differentiation from embryonic stem cells. Proc. Natl. Acad. Sci. U S A. 2008;105:5780–5785. doi: 10.1073/pnas.0708704105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kujawa S.G., Liberman M.C. Adding insult to injury: cochlear nerve degeneration after "temporary" noise-induced hearing loss. J. Neurosci. 2009;29:14077–14085. doi: 10.1523/JNEUROSCI.2845-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou H., Lopez-Juarez A., Fontbonne A., Nivet E., Zine A. Modeling human early otic sensory cell development with induced pluripotent stem cells. PLoS One. 2018;13:e0198954. doi: 10.1371/journal.pone.0198954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahlou H., Nivet E., Lopez-Juarez A., Fontbonne A., Assou S., Zine A. Enriched differentiation of human otic sensory progenitor cells derived from induced pluripotent stem cells. Front. Mol. Neurosci. 2018;11:452. doi: 10.3389/fnmol.2018.00452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang H., Schulte B.A., Goddard J.C., Hedrick M., Schulte J.B., Wei L., Schmiedt R.A. Transplantation of mouse embryonic stem cells into the cochlea of an auditory-neuropathy animal model: effects of timing after injury. J. Assoc. Res. Otolaryngol. 2008;9:225–240. doi: 10.1007/s10162-008-0119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A.S., Tang C., Rao M.S., Weissman I.L., Wu J.C. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med. 2013;19:998–1004. doi: 10.1038/nm.3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Roblin G., Liu H., Heller S. Generation of hair cells by stepwise differentiation of embryonic stem cells. Proc. Natl. Acad. Sci. U S A. 2003;100:13495–13500. doi: 10.1073/pnas.2334503100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.P., Koehler K.R., Mikosz A.M., Hashino E., Holt J.R. Functional development of mechanosensitive hair cells in stem cell-derived organoids parallels native vestibular hair cells. Nat. Commun. 2016;7:11508. doi: 10.1038/ncomms11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Juarez A., Lahlou H., Ripoll C., Cazals Y., Brezun J.M., Wang Q., Edge A., Zine A. Engraftment of human stem cell-derived otic progenitors in the damaged cochlea. Mol. Ther. 2019;27:1101–1113. doi: 10.1016/j.ymthe.2019.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M., Nakagawa T., Kojima K., Sakamoto T., Fujiyama F., Ito J. Potential of embryonic stem cell-derived neurons for synapse formation with auditory hair cells. J. Neurosci. Res. 2008;86:3075–3085. doi: 10.1002/jnr.21754. [DOI] [PubMed] [Google Scholar]
- Matsuoka A.J., Morrissey Z.D., Zhang C., Homma K., Belmadani A., Miller C.A., Chadly D.M., Kobayashi S., Edelbrock A.N., Tanaka-Matakatsu M. Directed differentiation of human embryonic stem cells toward placode-derived spiral ganglion-like sensory neurons. Stem Cells Transl. Med. 2017;6:923–936. doi: 10.1002/sctm.16-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattei C., Lim R., Drury H., Nasr B., Li Z., Tadros M.A., D'Abaco G.M., Stok K.S., Nayagam B.A., Dottori M. Generation of vestibular tissue-like organoids from human pluripotent stem cells using the rotary cell culture system. Front. Cell Dev. Biol. 2019;7:25. doi: 10.3389/fcell.2019.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R., Ocak E., Zhu A., Perdomo M.M., Pena S.A., Mittal J., Bohorquez J., Eshraghi A.A. Effect of bone marrow-derived mesenchymal stem cells on cochlear function in an experimental rat model. Anat. Rec. (Hoboken) 2020;303:487–493. doi: 10.1002/ar.24065. [DOI] [PubMed] [Google Scholar]
- Nayagam B.A., Edge A.S., Needham K., Hyakumura T., Leung J., Nayagam D.A., Dottori M. An in vitro model of developmental synaptogenesis using cocultures of human neural progenitors and cochlear explants. Stem Cells Dev. 2013;22:901–912. doi: 10.1089/scd.2012.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nayagam B.A., Minter R.L. A comparison of in vitro treatments for directing stem cells toward a sensory neural fate. Am. J. Otolaryngol. 2012;33:37–46. doi: 10.1016/j.amjoto.2010.12.009. [DOI] [PubMed] [Google Scholar]
- Needham K., Hyakumura T., Gunewardene N., Dottori M., Nayagam B.A. Electrophysiological properties of neurosensory progenitors derived from human embryonic stem cells. Stem Cell Res. 2014;12:241–249. doi: 10.1016/j.scr.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Nakagawa T., Ono K., Ogita H., Sakamoto T., Yamamoto N., Okita K., Yamanaka S., Ito J. Transplantation of mouse induced pluripotent stem cells into the cochlea. Neuroreport. 2009;20:1250–1254. doi: 10.1097/WNR.0b013e32832ff287. [DOI] [PubMed] [Google Scholar]
- Nishimura K., Nakagawa T., Sakamoto T., Ito J. Fates of murine pluripotent stem cell-derived neural progenitors following transplantation into mouse cochleae. Cell Transplant. 2012;21:763–771. doi: 10.3727/096368911X623907. [DOI] [PubMed] [Google Scholar]
- Nist-Lund C.A., Pan B., Patterson A., Asai Y., Chen T., Zhou W., Zhu H., Romero S., Resnik J., Polley D.B. Improved TMC1 gene therapy restores hearing and balance in mice with genetic inner ear disorders. Nat. Commun. 2019;10:236. doi: 10.1038/s41467-018-08264-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oestreicher E., Wolfgang A., Felix D. Neurotransmission of the cochlear inner hair cell synapse—implications for inner ear therapy. Adv. Otorhinolaryngol. 2002;59:131–139. doi: 10.1159/000059245. [DOI] [PubMed] [Google Scholar]
- Ogier J.M., Burt R.A., Drury H.R., Lim R., Nayagam B.A. Organotypic culture of neonatal murine inner ear explants. Front. Cell. Neurosci. 2019;13:170. doi: 10.3389/fncel.2019.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi H., Skerleva D., Kitajiri S., Sakamoto T., Yamamoto N., Ito J., Nakagawa T. Limited hair cell induction from human induced pluripotent stem cells using a simple stepwise method. Neurosci. Lett. 2015;599:49–54. doi: 10.1016/j.neulet.2015.05.032. [DOI] [PubMed] [Google Scholar]
- Okano T., Nakagawa T., Endo T., Kim T.S., Kita T., Tamura T., Matsumoto M., Ohno T., Sakamoto T., Iguchi F. Engraftment of embryonic stem cell-derived neurons into the cochlear modiolus. Neuroreport. 2005;16:1919–1922. doi: 10.1097/01.wnr.0000187628.38010.5b. [DOI] [PubMed] [Google Scholar]
- Oshima K., Shin K., Diensthuber M., Peng A.W., Ricci A.J., Heller S. Mechanosensitive hair cell-like cells from embryonic and induced pluripotent stem cells. Cell. 2010;141:704–716. doi: 10.1016/j.cell.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouji Y., Ishizaka S., Nakamura-Uchiyama F., Yoshikawa M. In vitro differentiation of mouse embryonic stem cells into inner ear hair cell-like cells using stromal cell conditioned medium. Cell Death Dis. 2012;3:e314. doi: 10.1038/cddis.2012.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren B., Jiao Y., Novozhilova E., Stupp S.I., Olivius P. Survival, migration and differentiation of mouse tau-GFP embryonic stem cells transplanted into the rat auditory nerve. Exp. Neurol. 2012;235:599–609. doi: 10.1016/j.expneurol.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Parker M., Brugeaud A., Edge A.S. Primary culture and plasmid electroporation of the murine organ of Corti. J. Vis. Exp. 2010 doi: 10.3791/1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perny M., Ting C.C., Kleinlogel S., Senn P., Roccio M. Generation of otic sensory neurons from mouse embryonic stem cells in 3D culture. Front. Cell. Neurosci. 2017;11:409. doi: 10.3389/fncel.2017.00409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius M., Vicario I., Schimmang T. Efficient transfer of embryonic stem cells into the cochlea via a non-invasive vestibular route. Acta Otolaryngol. 2008;128:720–723. doi: 10.1080/00016480701714236. [DOI] [PubMed] [Google Scholar]
- Regala C., Duan M., Zou J., Salminen M., Olivius P. Xenografted fetal dorsal root ganglion, embryonic stem cell and adult neural stem cell survival following implantation into the adult vestibulocochlear nerve. Exp. Neurol. 2005;193:326–333. doi: 10.1016/j.expneurol.2004.12.027. [DOI] [PubMed] [Google Scholar]
- Reyes J.H., O'Shea K.S., Wys N.L., Velkey J.M., Prieskorn D.M., Wesolowski K., Miller J.M., Altschuler R.A. Glutamatergic neuronal differentiation of mouse embryonic stem cells after transient expression of neurogenin 1 and treatment with BDNF and GDNF: in vitro and in vivo studies. J. Neurosci. 2008;28:12622–12631. doi: 10.1523/JNEUROSCI.0563-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roccio M., Edge A.S.B. Inner ear organoids: new tools to understand neurosensory cell development, degeneration and regeneration. Development. 2019;146 doi: 10.1242/dev.177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronaghi M., Nasr M., Ealy M., Durruthy-Durruthy R., Waldhaus J., Diaz G.H., Joubert L.M., Oshima K., Heller S. Inner ear hair cell-like cells from human embryonic stem cells. Stem Cells Dev. 2014;23:1275–1284. doi: 10.1089/scd.2014.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossant J., Papaioannou V.E. The relationship between embryonic, embryonal carcinoma and embryo-derived stem cells. Cell Differ. 1984;15:155–161. doi: 10.1016/0045-6039(84)90068-x. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Nakagawa T., Endo T., Kim T.S., Iguchi F., Naito Y., Sasai Y., Ito J. Fates of mouse embryonic stem cells transplanted into the inner ears of adult mice and embryonic chickens. Acta Otolaryngol. 2004;Suppl:48–52. doi: 10.1080/03655230310016825. [DOI] [PubMed] [Google Scholar]
- Schaefer S.A., Higashi A.Y., Loomis B., Schrepfer T., Wan G., Corfas G., Dressler G.R., Duncan R.K. From otic induction to hair cell production: Pax2(EGFP) cell line illuminates key stages of development in mouse inner ear organoid model. Stem Cells Dev. 2018;27:237–251. doi: 10.1089/scd.2017.0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott H.S., Kudoh J., Wattenhofer M., Shibuya K., Berry A., Chrast R., Guipponi M., Wang J., Kawasaki K., Asakawa S. Insertion of beta-satellite repeats identifies a transmembrane protease causing both congenital and childhood onset autosomal recessive deafness. Nat. Genet. 2001;27:59–63. doi: 10.1038/83768. [DOI] [PubMed] [Google Scholar]
- Senn P., Mina A., Volkenstein S., Kranebitter V., Oshima K., Heller S. Progenitor cells from the adult human inner ear. Anat. Rec. (Hoboken) 2020;303:461–470. doi: 10.1002/ar.24228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer A.E., Hildebrand M.S., Smith R.J.H. Hereditary hearing loss and deafness overview. In: Adam M.P., Ardinger H.H., Pagon R.A., Wallace S.E., Bean L.J.H., Stephens K., Amemiya A., editors. GeneReviews((R)) University of Washington; 1993. [Google Scholar]
- Shi F., Corrales C.E., Liberman M.C., Edge A.S. BMP4 induction of sensory neurons from human embryonic stem cells and reinnervation of sensory epithelium. Eur. J. Neurosci. 2007;26:3016–3023. doi: 10.1111/j.1460-9568.2007.05909.x. [DOI] [PubMed] [Google Scholar]
- Singh S., Groves A.K. The molecular basis of craniofacial placode development. Wiley Interdiscip. Rev. Dev. Biol. 2016;5:363–376. doi: 10.1002/wdev.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobkowicz H.M., Loftus J.M., Slapnick S.M. Tissue culture of the organ of Corti. Acta Otolaryngol. Suppl. 1993;502:3–36. [PubMed] [Google Scholar]
- Steventon B., Mayor R., Streit A. Neural crest and placode interaction during the development of the cranial sensory system. Dev. Biol. 2014;389:28–38. doi: 10.1016/j.ydbio.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straka H., Zwergal A., Cullen K.E. Vestibular animal models: contributions to understanding physiology and disease. J. Neurol. 2016;263(Suppl 1):S10–S23. doi: 10.1007/s00415-015-7909-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K., Tanabe K., Ohnuki M., Narita M., Ichisaka T., Tomoda K., Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Tang P.C., Alex A.L., Nie J., Lee J., Roth A.A., Booth K.T., Koehler K.R., Hashino E., Nelson R.F. Defective Tmprss3-associated hair cell degeneration in inner ear organoids. Stem Cell Reports. 2019;13:147–162. doi: 10.1016/j.stemcr.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z.H., Chen J.R., Zheng J., Shi H.S., Ding J., Qian X.D., Zhang C., Chen J.L., Wang C.C., Li L. Genetic correction of induced pluripotent stem cells from a deaf patient with MYO7A mutation results in morphologic and functional recovery of the derived hair cell-like cells. Stem Cells Transl. Med. 2016;5:561–571. doi: 10.5966/sctm.2015-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchieu J., Zimmer B., Fattahi F., Amin S., Zeltner N., Chen S., Studer L. A modular platform for differentiation of human PSCs into all major ectodermal lineages. Cell Stem Cell. 2017;21:399–410.e7. doi: 10.1016/j.stem.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Camp G., Willems P.J., Smith R.J. Nonsyndromic hearing impairment: unparalleled heterogeneity. Am. J. Hum. Genet. 1997;60:758–764. [PMC free article] [PubMed] [Google Scholar]
- Vasic V., Barth K., Schmidt M.H.H. Neurodegeneration and neuro-regeneration—Alzheimer's disease and stem cell therapy. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20174272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P. Mouse models for pendrin-associated loss of cochlear and vestibular function. Cell. Physiol. Biochem. 2013;32:157–165. doi: 10.1159/000356635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol M.E., Corwin J.T. Regenerative proliferation in organ cultures of the avian cochlea: identification of the initial progenitors and determination of the latency of the proliferative response. J. Neurosci. 1996;16:5466–5477. doi: 10.1523/JNEUROSCI.16-17-05466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warchol M.E., Lambert P.R., Goldstein B.J., Forge A., Corwin J.T. Regenerative proliferation in inner ear sensory epithelia from adult guinea pigs and humans. Science. 1993;259:1619–1622. doi: 10.1126/science.8456285. [DOI] [PubMed] [Google Scholar]
- Xu Y.P., Shan X.D., Liu Y.Y., Pu Y., Wang C.Y., Tao Q.L., Deng Y., Cheng Y., Fan J.P. Olfactory epithelium neural stem cell implantation restores noise-induced hearing loss in rats. Neurosci. Lett. 2016;616:19–25. doi: 10.1016/j.neulet.2016.01.016. [DOI] [PubMed] [Google Scholar]
- Yilmaz A., Benvenisty N. Defining human pluripotency. Cell Stem Cell. 2019;25:9–22. doi: 10.1016/j.stem.2019.06.010. [DOI] [PubMed] [Google Scholar]
- Ying Q.L., Stavridis M., Griffiths D., Li M., Smith A. Conversion of embryonic stem cells into neuroectodermal precursors in adherent monoculture. Nat. Biotechnol. 2003;21:183–186. doi: 10.1038/nbt780. [DOI] [PubMed] [Google Scholar]
- Zhao L.D., Li L., Wu N., Li D.K., Ren L.L., Guo W.W., Sun J.H., Liu H.Z., Chen Z.T., Xing G.Q. Migration and differentiation of mouse embryonic stem cells transplanted into mature cochlea of rats with aminoglycoside-induced hearing loss. Acta Otolaryngol. 2013;133:136–143. doi: 10.3109/00016489.2012.720029. [DOI] [PubMed] [Google Scholar]
- Zhong C., Chen Z., Luo X., Wang C., Jiang H., Shao J., Guan M., Huang L., Huang X., Wang J. Barhl1 is required for the differentiation of inner ear hair cell-like cells from mouse embryonic stem cells. Int. J. Biochem. Cell Biol. 2018;96:79–89. doi: 10.1016/j.biocel.2018.01.013. [DOI] [PubMed] [Google Scholar]