Summary
The subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus in the hippocampus are known as neurogenic niches. We show that the median eminence (ME) of the hypothalamus comprises BrdU+ newly proliferating cells co-expressing NG2 (oligodendrocyte progenitors) and RIP (pre-myelinating oligodendrocytes), suggesting their differentiation toward mature oligodendrocytes (OLs). ME cells can generate neurospheres (NS) in vitro, which differentiate mostly to OLs compared with SVZ-NS that typically generate neurons. Interestingly, this population of oligodendrocyte progenitors is increased in the ME from experimental autoimmune encephalomyelitis (EAE)-affected mice. Notably, the thrombospondin 1 (TSP1) expressed by astrocytes, acts as negative regulator of oligodendrogenesis in vitro and is downregulated in the ME of EAE mice. Importantly, transplanted ME-NS preferentially differentiate to MBP+ OLs compared with SVZ-NS in Shiverer mice. Hence, discovering the ME as a new site for myelin-producing cells has a great importance for advising future therapy for demyelinating diseases and spinal cord injury.
Keywords: median eminence, multiple sclerosis, experimental autoimmune encephalomyelitis, oligodendrocyte progenitors cells, myelin repair, corpus callosum, subventricular zone, neural stem cells
Graphical Abstract
Highlights
-
•
Median eminence of the hypothalamus contains proliferating OPCs
-
•
The number of OPCs in the median eminence is increased under demyelination
-
•
Median eminence cells generate neurospheres in vitro
-
•
Thrombospondin 1 inhibits oligodendrocyte differentiation of median eminence cells
Zilkha-Falb and colleagues show an enrichment of BrdU+ NG2+ cells in the median eminence (ME) of EAE mice. ME cells generate neurospheres differentiating to MBP+ OLs after transplantation to the LV of Shi mice. TSP1 inhibits oligodendrocyte differentiation of ME cells, corroborating its decreased expression in the ME of EAE mice. These findings suggest the ME as oligodendrogenic niche to increase OPCs under demyelination.
Introduction
Adult neural stem cells (NSCs) are major candidates for regenerative therapies in demyelinating diseases. Myelin, provided by oligodendrocytes (OLs), is essential for the proper function of neurons in the central nervous system (CNS). Myelin loss occurs in many demyelinating diseases, such as multiple sclerosis (MS) and causes a variety of neurological disabilities in adults (Duncan and Radcliff, 2016, Lassmann, 2018). In demyelinating disease lesions OLs are lost and to promote functional recovery, lesions must be supplied with endogenous or transplanted myelinating cells (Blakemore et al., 2002, Chari and Blakemore, 2002, Jeffery et al., 1999). Oligodendrocyte progenitor cells (OPCs) marked by NG2, a chondroitin sulfate proteoglycan, and PDGFRα (platelet-derived growth factor receptor α) are recruited to demyelinated lesions (Chang et al., 2000, Redwine and Armstrong, 1998, Reynolds et al., 2001, Sim et al., 2002). Olig2, a basic-helix-loop-helix transcription factor, is strongly upregulated in OPCs under pathological conditions and demyelination in the adult CNS (Buffo et al., 2005, Fancy et al., 2004, Talbott et al., 2005).
NSCs have been found in two main neurogenic regions of the brain (Emsley et al., 2005); the subgranular zone (SGZ) of the dentate gyrus (DG) in the hippocampus (Altman and Das, 1965) and the subventricular zone (SVZ) of the lateral ventricles (LVs) (Gould, 2007, Lim and Alvarez-Buylla, 2016). Neurogenesis may also occur in other areas of the adult brain—albeit at lower levels (Seri et al., 2006, Taupin, 2006). Recently, a novel site of NSCs was reported in the circumventricular organs (CVO) (Bennett et al., 2009). To date, studies focused on neurogenic niches directed mainly to neurogenesis with less attention to characterizing oligodendrogenic niches.
Studies of oligodendrogenesis in white matter regions of adult rodent CNS, revealed spontaneous NG2 cell division and new OLs formation (Cerghet et al., 2006, Dimou et al., 2008, Horner et al., 2000, Komitova et al., 2009, Seri et al., 2006).
In contrast to multiple studies reporting spontaneous neurogenesis in various CNS disorders (Taupin, 2008) as well as in the murine model of MS, experimental autoimmune encephalomyelitis (EAE) (Aharoni et al., 2005), spontaneous oligodendrogenesis was barely reported (Nait-Oumesmar et al., 2008, Picard-Riera et al., 2002).
The generation of new OLs in the SVZ was investigated extensively (Brousse et al., 2015, Kazanis et al., 2017, Serwanski et al., 2018, Xing et al., 2014), however, their ability to participate in myelin repair has remained uncertain. Nevertheless, it is of great importance to characterize niches of OPCs for their regulation in pathological conditions or re-population in vitro for further therapy. In addition, a number of molecules/molecular pathways were reported to regulate neurogenesis; however, the mechanism that preferentially directs oligodendrogenesis versus neurogenesis and lineage commitment is not yet clear.
Here, we looked into brain sites in naive mice that are highly enriched with bromodeoxyuridine (BrdU)+ newly proliferating cells. Of these, the median eminence (ME) was attractive for its exclusive oligodendrogenic fate. Identifying the ME as a site that preferentially hosts NG2 cells, we studied in vitro the self-renewal potential of ME-NSCs and the preferential differentiation to OLs compared with SVZ-NSCs. Indeed, we characterized the oligodendrogenic fate of ME-resident BrdU+ cells in naive and EAE mice. More practically, we studied their ability to differentiate toward mature MBP-producing OLs in Shiverer (Shi) mice, a model for demyelination. Comprehensively, we studied in vitro a potential role for thrombospondin 1 (TSP1) in regulating the oligodendrogenic versus neurogenic fate and compared the expression of TSP1 in SVZ and ME of naive with that of EAE mice.
We show that the ME of naive mice is enriched with BrdU+ NG2+ cells and that their number is increased in EAE mice. In vitro, ME-NSCs generate neurospheres (NSs) that mostly differentiate to OLs while SVZ-NSCs differentiate to neurons. In accordance, ME-NSCs preferentially differentiate to MBP+ OLs after transplantation to the LV of Shi mice, compared with SVZ-NSCs that differentiate toward neurons. Our results show that TSP1 favors neuronal differentiation of ME-NSCs, while an antagonist of TSP1 (LSKL) preferentially directs toward OLs. Finally, analysis of TSP1 in EAE mice shows decreased expression in the ME (versus SVZ) compared with naive mice.
These findings suggest the ME as an exclusive oligodendrogenic niche, an endogenous pool of OPCs, to enhance generation of OPCs under pathological conditions. Indeed, this study suggests that TSP1 plays as negative regulator of oligodendrogenesis in vitro.
Results
Characterization of OPCs in the ME of the Hypothalamus
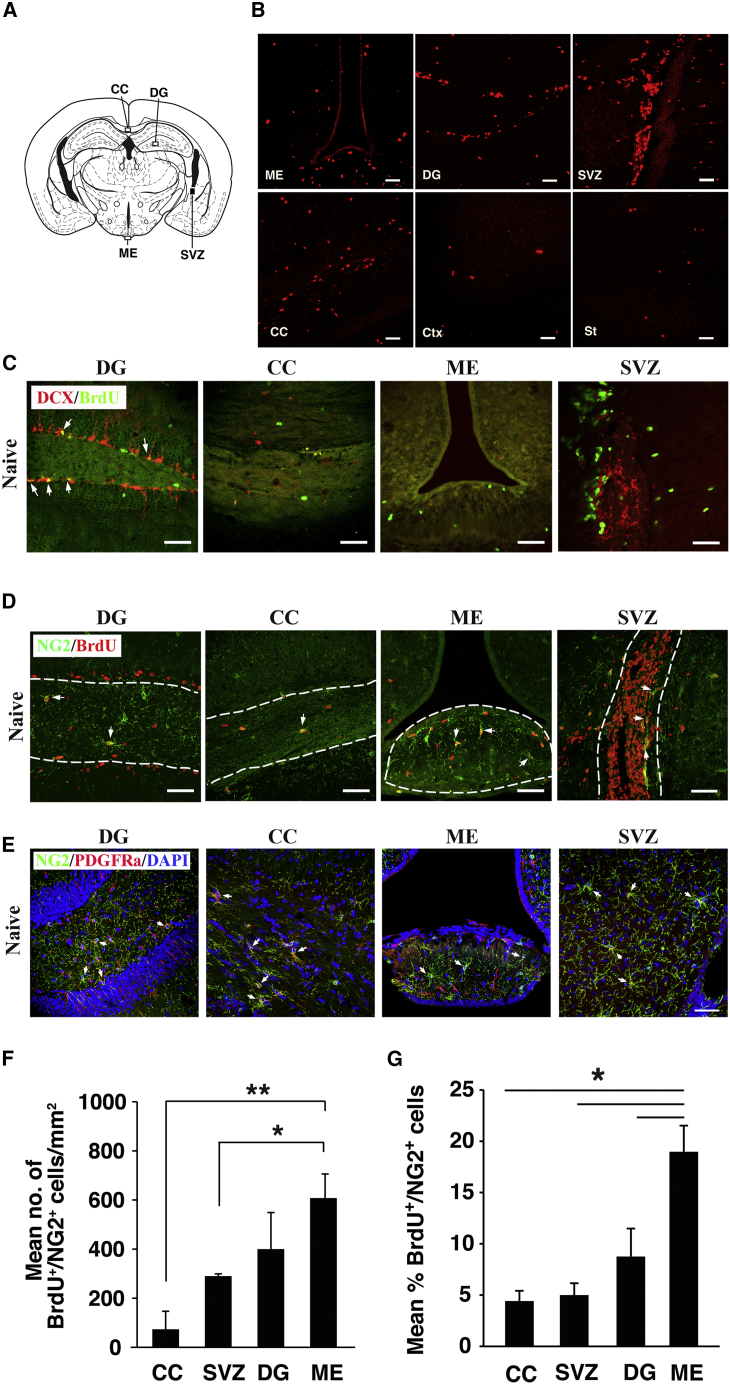
Brains of 8-week-old naive mice were analyzed for regions enriched with newly generated NSCs. C57Bl/6J mice were injected daily with BrdU for 3 days and brains were scanned for BrdU-enriched regions (Figure 1A). Comparative analysis revealed that the cortex (Ctx) and the striatum contain a minor population of BrdU+ cells compared with the CC, DG, and SVZ. Interestingly, another BrdU+-enriched region was identified in the ME (Figure 1B). To characterize the identity of BrdU+ cells in this region, brain sections of naive mice were stained for neuronal and oligodendrocyte progenitor markers. No staining was detected for the early neuronal progenitor marker (DCX) in the ME (Figure 1C), or for NeuN (not shown). Surprisingly, the ME contained a large number and high density of OPCs (NG2+ BrdU+) compared with the CC, DG, and SVZ (Figure 1D). The majority of these NG2+ cells were also PDGFRa+ (Figures 1E and S1). Quantitation of NG2+ BrdU+/mm2 and their percentages indicated that the number and density of OPCs is markedly higher in the ME (608 ± 106 cells in ME compared with 400 ± 149 cells in DG, 290 ± 9.1 cells in SVZ and 73 ± 73 cells in CC; Figures 1F and 1G), suggesting that the ME of naive mice is rich with newly formed OPCs.
Figure 1.
OPCs in the ME of Naive Mice
(A) Scheme shows regions analyzed for BrdU+ cells.
(B) BrdU staining in the ME DG, SVZ, CC, Ctx, and St.
(C) Absence of neuronal progenitors (DCX) in the ME and CC compared with DG. Arrows indicate DCX+ BrdU+ cells.
(D) Images show OPCs (NG2) in the DG, CC, SVZ, and ME boxed in (A). Arrows indicate NG2+ BrdU+ cells.
(E) Images show NG2+ PDGFRa+ OPCs.
(F and G) Quantitative analysis of NG2+ BrdU+ cells in the DG, CC, SVZ, and ME as (F) mean number of NG2+ BrdU+ cells/mm2. ∗p = 0.013, ∗∗p = 0.00079, and (G) as their mean percentage of BrdU+. ∗p = 0.0007 (n = 4 for each group).
Scale bar, 50 μm.
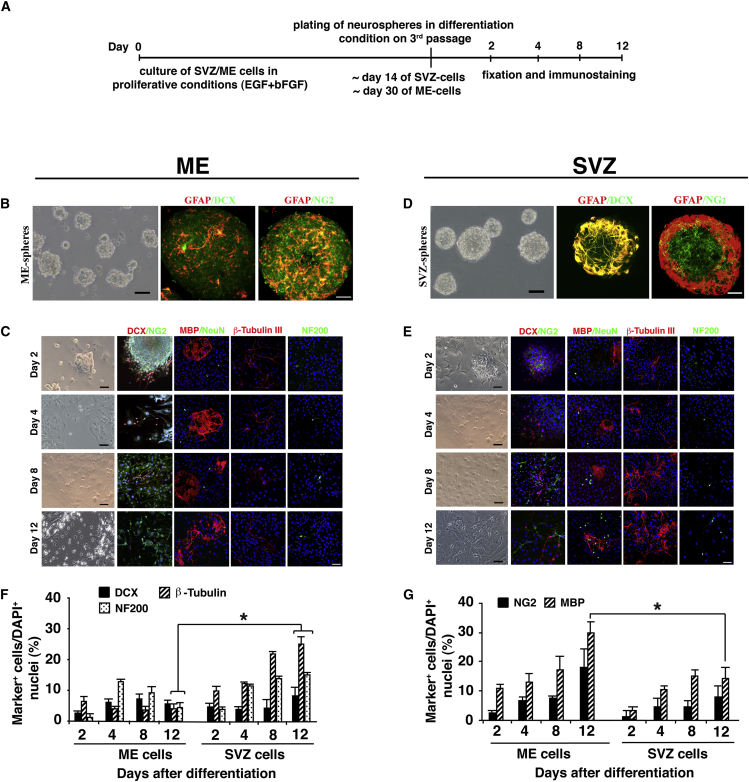
Cells Originated from the ME Generate NS and Differentiate to Neuronal/Oligo Progenitor Cells
ME cells were examined for self-renewal and assessed for their capability to generate NS compared with those from the SVZ (Figure 2A, schematic explanation). Interestingly, ME cells generated NS 3–4 weeks after plating, while SVZ-NS could be detected already 7 days after plating. Clearly, the ME-NS (Figure 2B), which contain many NG2+ OPCs and few DCX+ neuronal progenitors and GFAP+ astrocytes, showed pronounced differentiation to oligodendrocyte lineage (Figure 2C) compared with SVZ-NS (Figures 2D and 2E). Quantitative analysis indicated that ME-NS express DCX 2 days after differentiation (4.2 ± 0.6% of DAPI+ cells in ME-NS compared with 5.32 ± 1.1% in SVZ-NS), but that this neurogenic potential gradually decreases at days 4, 8, and 12 after differentiation and that markers of mature neurons (β-tubulin III and NF200) are poorly expressed as compared with SVZ-NS (Figure 2F). Analysis of cells that were differentiated to OPCs or mature OLs (Figure 2G) indicated that ME-NS preferentially differentiated to NG2+ OPCs 2 days after differentiation (2.67 ± 0.65% compared with 1.58 ± 1.84% in SVZ-NS) and their number gradually increased at days 4, 8, and 12 after differentiation (6.8 ± 1.4%, 7.9 ± 0.7%, and 18.5 ± 6%, respectively) compared with differentiation from SVZ-NS (5.1 ± 2.8%, 4.82 ± 1.8%, and 8.64 ± 3.6%, respectively). Moreover, the number of MBP+ mature OLs increased after 4, 8, and 12 days (13.4 ± 2.5%, 17.4 ± 4.2%, and 29.8 ± 3.4%, respectively) compared with differentiation from SVZ-NS (10.9 ± 0.85%, 16.1 ± 1.9%, and 15.2 ± 3.3%, respectively; Figure 2G). These results are confirmed by western blot (WB) of β-tubulin and MBP (Figure S2).
Figure 2.
ME Cells Generate NS and Differentiate In Vitro to Neuro/Oligo Progenitors
(A) Explanation of NS culture and fixation schedule.
(B and D) Generation of NS from (B) isolated ME compared with (D) SVZ cells. Expended ME-NS express markers of neuronal (DCX), oligodendrocyte progenitors (NG2), and astrocytes (GFAP). Shown are bright field images of NS and immunostaining for neural markers.
(C and E) Temporal analysis of neural markers expressed by ME-NS (C) upon differentiation compared with (E) SVZ-NS and their quantitative analysis of (F) neuronal and (G) oligodendrocyte markers (G).
Scale bar, 50 μm.
These results demonstrate the self-renewal capacity of ME cells and suggest that ME cells preferentially differentiate to OLs.
Increased Number of OPCs in the ME of EAE Mice
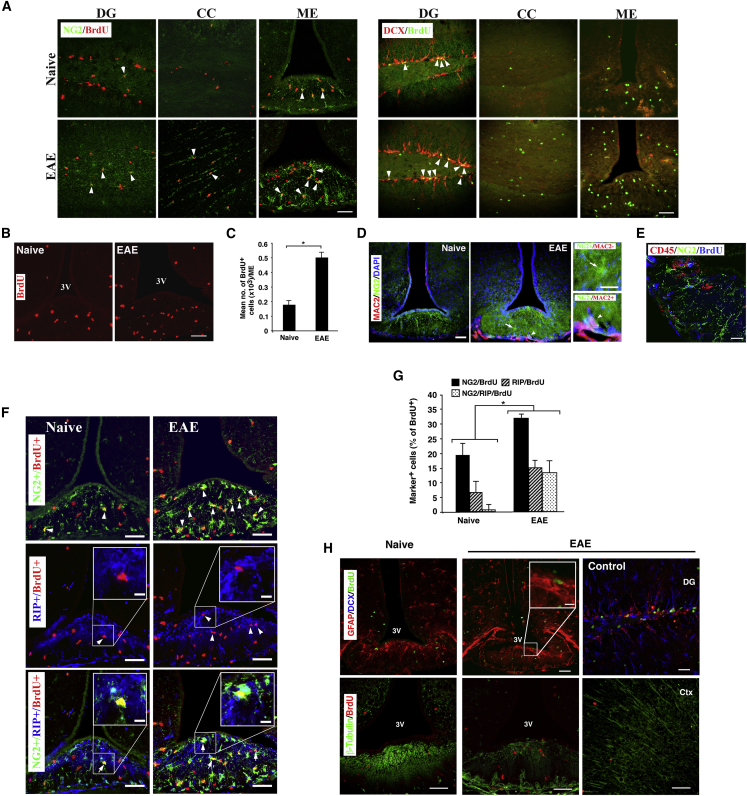
The self-renewal capacity of ME cells and their potential to generate OLs prompted us to study the effect of a pathological condition, such as EAE, on newly generated OLs in the ME. Immunostaining for BrdU+ cells in the ME, CC, and DG clearly showed that EAE induced cell proliferation in these regions and especially in the ME compared with these regions from naive mice, and that a fraction of these BrdU+ cells was also NG2+ (Figure 3A, left panel), which was increased in EAE mice as compared with naive mice. In contrast, there was no evidence of DCX+ cells in the ME and CC compared with DG, although there was an increased number of BrdU+ cells (Figure 3A, right panel). Higher-magnification images of BrdU+ cells in the ME clearly showed that, under EAE, an increased cell proliferation was detected compared with the same region in naive mice (Figure 3B). The mean number of BrdU+ cells per ME was increased (by 2.7-fold; p < 0.01) in EAE mice compared with naive mice (Figure 3C). Staining for macrophages and OPCs (Figure 3D) excluded the possibility that these parenchymal resident BrdU+ cells were immune cells that penetrated the CNS as the MAC2+ cells resided at the edge of ME tissue but not in the parenchyma where the BrdU+ cells were detected (Figure 3D). Moreover, the immune cell marker, CD45 staining, excluded the possibility that the NG2+ BrdU+ cells were infiltrating immune cells (Figure 3E). To characterize these newly generated cells, adjacent sections were immunostained for markers of OLs. Comparison of sections from EAE mice to sections of naive mice showed that BrdU+ cells expressed NG2 (Figure 3F, upper panel) or RIP of pre-myelinating OLs (Figure 3F, middle panel) and that their number was increased in EAE mice, while a comparable intensity of MBP immunoreactivity in the ME of naive and EAE mice was observed (Figure S4A).
Figure 3.
Increased Number of OPCs in the ME of EAE Mice
(A) Images show NG2/BrdU and DCX/BrdU staining in the DG, CC, and ME.
(B) Enhanced BrdU staining of ME in EAE mice compared with naive mice. Arrows indicate double-positive cells.
(C) Increased BrdU+ cells in EAE mice compared with naive mice (n = 5 per group).
(D) Macrophages (MAC2+) are not localized where OPCs are scored.
(E) NG2+ cells are distinctive from CD45+ cells.
(F and G) (F) Images of BrdU+ NG2+, BrdU+ RIP+ (arrowheads), and BrdU+ NG2+ RIP+ cells (arrows) in the ME of EAE mice and (G) their quantitative analysis (n = 4 per group).
(H) Immunostaining for DCX or β-tubulin with BrdU in the ME.
Scale bar, 50 μm. (C) ∗p < 0.01, (G) ∗p = 0.02.
Moreover, numerous BrdU+ cells expressed both NG2 and RIP in sections from EAE mice compared with those from naive mice (Figure 3F, lower panel). The percentage of NG2+ BrdU+ and RIP+ BrdU+ cells increased in the ME of EAE mice by 13% and 8.4%, respectively. Moreover, a marked increase in the percentage of NG2+ RIP+ BrdU+ in EAE mice could be observed (Figure 3F). Of note, ME sections from EAE or naive mice showed neither BrdU+ co-expressing β-tubulin III nor DCX compared with such positive staining in the Ctx (Figure 3H). A small number of GFAP+ BrdU+ cells could be detected in the ME of EAE mice (Figure 3H). Notably, such oligodendrogenesis can be observed in the ventro-medial region (VMH) and the arcuate nucleus (Arc) of the hypothalamus, but to a lesser extent, and obviously the number of NG2+ BrdU+ cells is significantly higher in the ME of naive and EAE mice compared with the Arc and VMH (Figure S3).
These results suggest that EAE extensively promotes oligodendrogenesis in the ME.
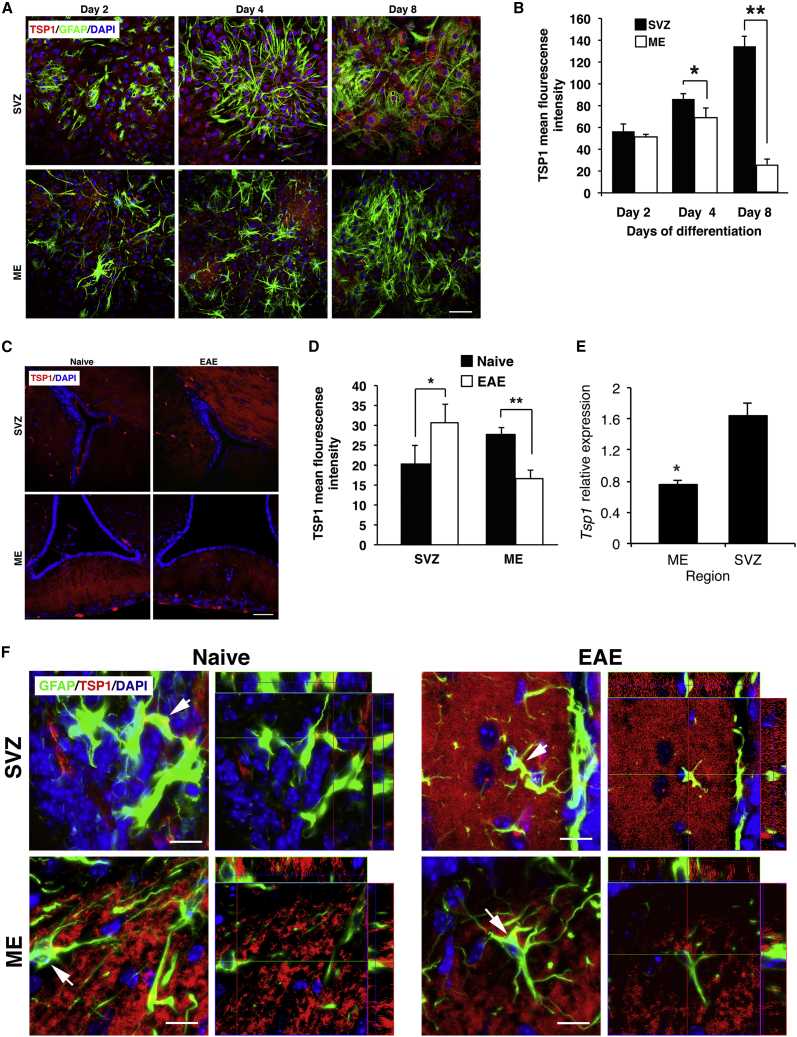
Expression of TSP1 by NSCs In Vitro and Spatial Analysis of TSP1
TSP1 was implicated in the direction of SVZ-NSCs toward neuronal differentiation (Lu and Kipnis, 2010), raising the possibility that TSP1 may act as negative regulator of oligodendrogenesis in ME. Hence, we compared the expression of TSP1 by SVZ-NSCs with that of ME-NSCs. Expression of TSP1 could not be detected in NS of ME or SVZ (data not shown). However, as shown in representative images (Figure 4A) and by quantitative analysis (Figure 4B), its expression in the ME was upregulated 2 and 4 days after differentiation and then significantly declined after 8 days. In contrast, expression of TSP1 in SVZ-NSCs was only slightly increased at days 2 and 4 after differentiation but increased after 8 days. In brain sections, TSP1 expression was decreased in ME of EAE mice compared with naive mice. However, TSP1 level in the SVZ was increased (Figure 4C), and the mean fluorescent intensity (MFI) confirmed these differences in TSP1 expression (Figure 4D). The MFI showed 1.5-fold increase in the SVZ of EAE mice compared with naive mice (p = 0.003), while there was 1.8-fold decrease in the ME of EAE mice compared with naive mice (p < 0.001; Figure 4D). Tsp1 relative expression in EAE mice was decreased by 2-fold in ME (p = 0.04; Figure 4E) compared with naive mice. Moreover, GFAP+ immunostaining indicated decreased TSP1 expression (control staining; Figure S4B) by astrocytes in ME of EAE mice compared with naive mice and increase in the LV of EAE mice (Figures 4F and S4C, low power images).
Figure 4.
Expression of TSP1 by ME- and SVZ-NSCs
(A and B) (A) Immunostaining for GFAP and TSP1 after the indicated time points of differentiation and (B) quantitative analysis of TSP1 fluorescence intensity (n = 6).
(C) Images of immunostaining for TSP1 in SVZ and ME.
(D) Quantitative analysis of TSP1 fluorescence intensity in SVZ and ME (n = 4 per group). ∗p = 0.003, ∗∗p = p < 0.001.
(E) Tsp1 relative expression in the ME and SVZ. ∗p = 0.036.
(F) Orthogonal views of GFAP and TSP1 in ME and SVZ in naive mice compared with EAE mice. Low-power images of TSP1 are shown in Figure S4C. Scale bar, 50 μm.
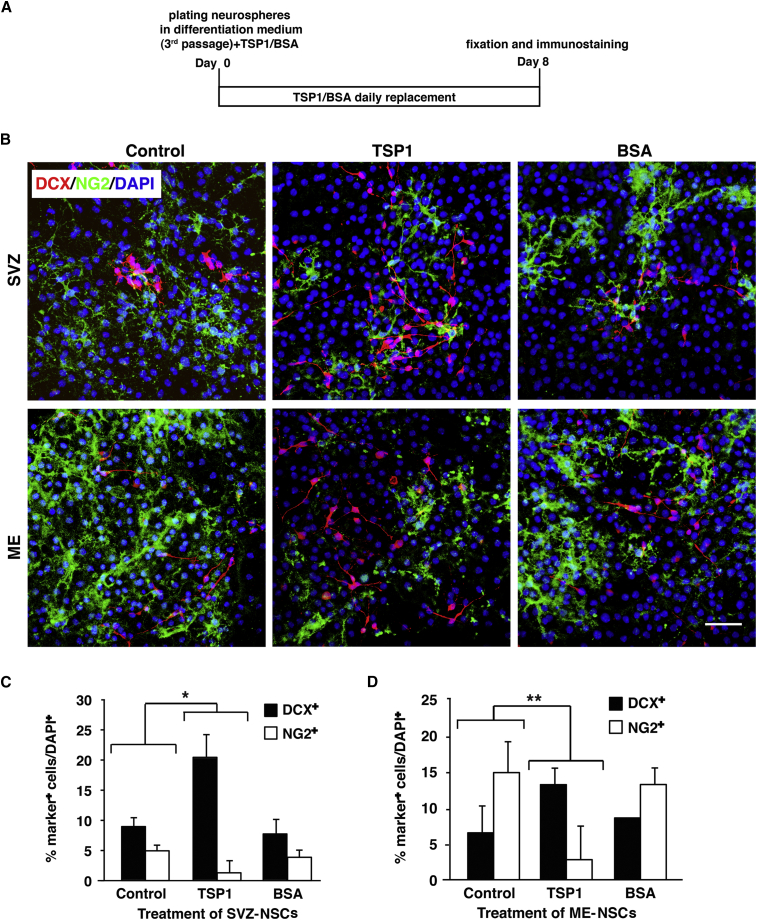
Effect of TSP1 on Differentiation of NSCs from SVZ versus ME and Expression of TSP1 by NSCs
The increased number of OPCs in the ME of EAE mice and the decreased TSP1 expression hints on the role of TSP1 as negative regulator of oligodendrogenesis. To study this possibility, SVZ- or ME-NSCs were differentiated for 8 days in the absence or presence of TSP1 or bovine serum albumin (BSA) as a control (both at 2 μg/mL) and cultures were stained against NG2 and DCX (Figure 5A, schematic explanation). Incubation with TSP1 resulted in significantly reduced number of NG2+ OPCs differentiated from both SVZ- and ME-NSCs, especially in ME-NSCs compared with BSA or vehicle control (Figure 5B). Inversely, addition of TSP1 increased the number of neuronal progenitors in ME-NSCs and dramatically in SVZ-NSCs. Quantitative analysis showed that incubation with TSP1 increased the number of DCX+ neuronal progenitors differentiated from SVZ-NSCs by 12% and decreased NG2+ OPCs by almost 4% (Figure 5C).
Figure 5.
Effect of TSP1 on Differentiation of ME-NSCs Compared with SVZ-NSCs
(A) Explanation of NS culture, differentiation in presence of TSP1, and fixation schedule.
(B) Staining for DCX and NG2 in ME- and SVZ-NSCs cultures treated with TSP1 or BSA. NSCs cultured in differentiation medium served as control. Shown are representative images out of three repeats.
(C and D) Quantitative analysis of (C) SVZ-NSC and (D) ME-NSC differentiation to DCX+ cells and NG2+ cells in the presence of TSP1 (mean ± SD). Scale bar, 50 μm. ∗p ≤ 0.004, ∗∗p ≤ 0.005.
A similar effect could be detected in differentiation of ME-NSCs, where TSP1 increased the number of neuronal progenitors by 8.2% and dramatically decreased the number of OPCs (by ∼13%) relative to control cultures (Figure 5D). A WB for the neuronal β-tubulin and oligodendroglial GST-pI confirmed these results (Figure S5).
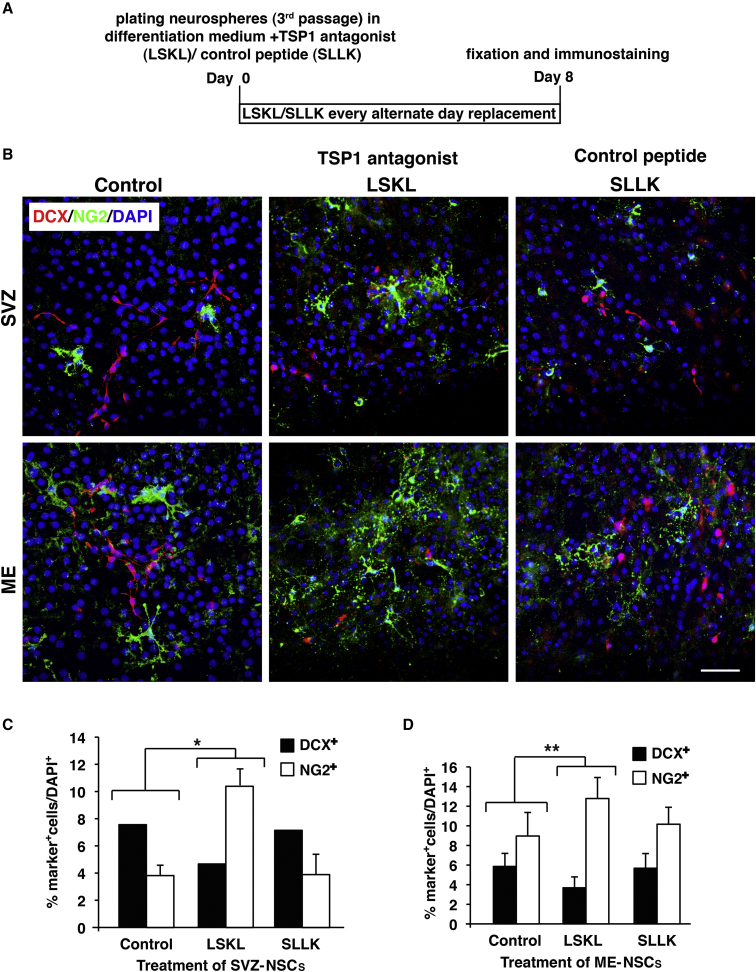
TSP1 Antagonist Shifts NSC Differentiation toward OPCs
To further confirm a role for TSP1 as a negative regulator of oligodendrogenesis in vitro, we studied the differentiation of ME-NSCs compared with SVZ-NSCs in the presence of TSP1 antagonist LSKL, a 4-amino acid peptide that specifically blocks the binding of TSP1 and the large latent transforming growth factor β (TGF-β) complex (Lanz et al., 2010). ME- or SVZ-NSCs were differentiated in the presence of LSKL or its control-scrambled peptide SLLK (50 μg/mL) and labeled for NG2 and DCX (Figure 6A, schematic explanation). Incubation with TSP1 antagonist increased the number of OPCs differentiated from both SVZ- and ME-NS, especially in SVZ-NSCs. Inversely, the TSP1 antagonist reduced the number of neuronal progenitors in ME- and SVZ-NSCs (Figure 6B). Quantitative analysis showed that incubation with the TSP1 antagonist significantly increased the number of NG2+ OPCs differentiated from SVZ-NSCs by 7%, and decreased the number of DCX+ neuronal progenitors by almost 4% (Figure 6C). Similarly, the TSP1 antagonist increased the number of NG2+ OPCs differentiated from ME-NSCs by almost 3%, and decreased the number of DCX+ neuronal progenitors by 2.4%, compared with control cultures (Figure 6D). A WB for the neuronal β-tubulin and oligodendroglial GST-pI confirmed these results (Figure S6). Altogether, these results further suggest that TSP1 negatively regulates oligodendrogenesis in vitro.
Figure 6.
Effect of TSP1 Antagonist on Differentiation of ME-NSCs Compared with SVZ-NSCs
(A) Explanation of NS culture, differentiation in the presence of TSP1 antagonist or control peptide and fixation schedule.
(B) Staining for DCX and NG2 in ME- and SVZ-NSCs cultures treated with TSP1 antagonist (LSKL) or control peptide (SLLK) (50 μg/mL).
(C and D) Quantitative analysis of (C) SVZ-NSCs and (D) ME-NSCs differentiation to DCX+ and NG2+ cells in the presence of TSP1 antagonist (mean ± SD). Scale bar, 50 μm. ∗p ≤ 0.004, ∗∗p ≤ 0.015.
ME-Originated Cells Preferentially Generate MBP+ OLs in the CNS of Shi Mice
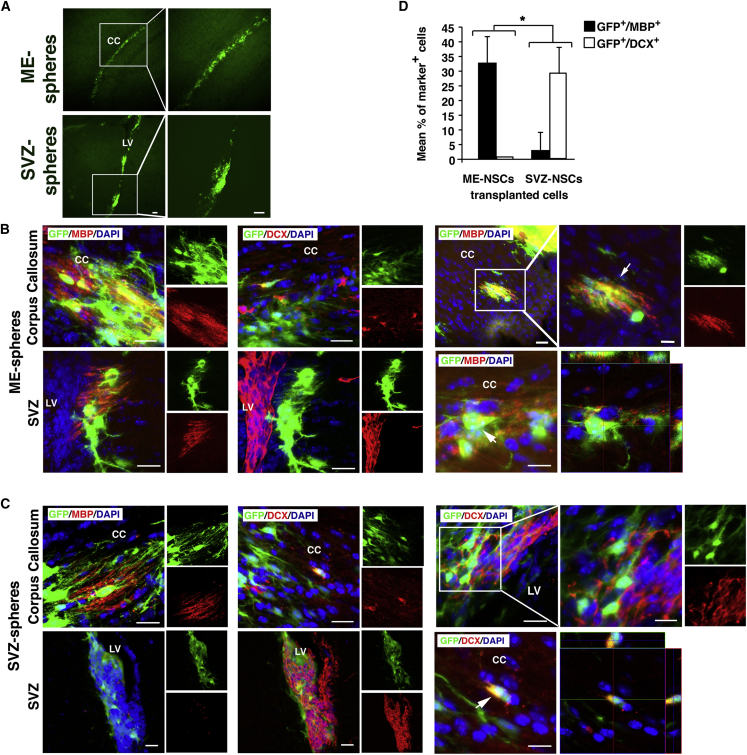
The in vitro results show that ME-NS can differentiate to mature MBP+ OLs (Figure 2), express chemokine receptor for migration, and provide neurotrophic support (Figures S7A and S7B). To unambiguously determine whether grafted ME-NSCs become fully mature myelinating OLs, ME-NSCs derived from GFP mice were grafted into the LV of Shi mice lacking MBP (Warrington et al., 1993). Hence, MBP labeling in their brain can only come from grafted cells.
Two-week-old Shi mice were transplanted with either ME- or SVZ-NS (n = 4 per group). At 3 weeks post-grafting, GFP+ cells derived from ME-NS had migrated and were found mainly in the CC and rarely in LV, while GFP+ cells derived from SVZ-NS were detected mainly in the LV and in the parenchyma in the vicinity of the LV (Figure 7A). These results confirm the ability of adult SVZ- and ME-NS to survive in the LV and CC of Shi mice, respectively, after migration and differentiation. Higher-magnification images showed GFP+ cells derived from ME-NS in the CC that co-express MBP. Such GFP+ MBP+ cells could be observed also in the parenchyma at the vicinity of LV but to a lower extent. The pattern of MBP labeling indicated that grafted cells had formed myelin patches throughout the CC (Figures 7B, upper set of panels and S7C) with process-bearing ramified GFP+ cells that co-express MBP (Figure 7B, inset in upper right set of panels). As shown in Figure 7B (middle set), only a small number of GFP+ cells derived from ME-NS also expressed DCX in the CC. In addition, although these GFP+ cells were detected at the vicinity of LV, none co-expressed DCX. Analysis the fate of SVZ-NS showed that only few GFP+ cells derived from SVZ-NS co-expressed MBP in the CC (Figure 7C, upper left set of panels), a typical niche of OLs, while no MBP+ GFP+ cells could be detected in the LV (Figure 7C, lower left set of panels). However, while only few GFP+ cells derived from SVZ-NS co-expressed the neuronal marker DCX in CC (Figure 7C lower right set of panels), the frequency of such cells was higher in the parenchyma near the LV with a morphology reminiscent of neurons (Figure 7C, inset in upper right set of panels).
Figure 7.
ME-Derived OLs Express MBP in Shi Mice
Coronal brain sections processed 3 weeks after injection of GFP-labeled NS into the LV.
(A) Images show GFP+ ME-NS (upper panel) or GFP+ SVZ-NS.
(B) Images show transplanted GFP+ ME-NSCs in the ME (upper panel) and SVZ (lower panel) expressing MBP (left column) or DCX (middle column). Upper images in the right column show higher magnification of GFP+ MBP+ in the CC. Scale bars, 20 μm and 10 μm (inset). The lower image is an orthogonal view showing localization of MBP with GFP+ ME-NSCs. Scale bar, 10 μm.
(C) Images show GFP+ SVZ-NSCs in the CC (upper panel) and adjacent LV (lower panel). Shown are immunostaining for MBP (left column) and DCX (middle column). Upper images in the right column show higher magnification of GFP+ DCX+ in the SVZ. Scale bars, 20 μm and 10 μm (inset). The lower image is orthogonal view showing localization of DCX with GFP+ SVZ-NSCs. Scale bar, 10 μm.
(D) Quantitative analysis of GFP+ cells co-express MBP or DCX in Shi mice transplanted with ME-NSCs compared with SVZ-NSCs (n = 5 per group). Scale bars, 50 μm (A–C). ∗p < 0.001.
Quantification 3 weeks after transplantation showed that 32.9 ± 8.8% of GFP+ cells derived from ME-NS co-expressed MBP. Most of them were detected in the myelinogenic area, such as the CC, while only 0.8% co-expressed the neuronal marker DCX and were detected near the LV (Figure 7D). Inversely, only 3.1 ± 6% of GFP+ cells derived from SVZ-NS co-expressed MBP+, while 29 ± 9% of these cells co-expressed DCX and were mostly detected in the vicinity of the LV (Figure 7D). These results suggest that ME-NS preferentially generate OLs in adult subcortical white matter after a heterotopic graft compared with SVZ-NS and that oligodendrogenic or neurogenic fate chosen by NS depended not only on the environment cues encountered in the host regions but also on intrinsic features of the cells origin.
Discussion
Here, we show that the ME of naive mice is enriched with BrdU+ cells identified as NG2+ cells and that the number of these BrdU+ NG2+ cells is increased in the ME of EAE mice. Interestingly, these cells can generate NS in vitro. In line with the in situ observation, ME-NSCs mostly differentiate to OLs upon in vitro differentiation. In accordance, these ME-NSCs preferentially differentiate to MBP+-producing OLs after transplantation to the LV of Shi mice. Mechanistically, our results show that TSP1 favors neuronal differentiation of ME-NSCs, while the TSP1 antagonist preferentially directs differentiation to OLs. Finally, analysis of TSP1 expression in EAE mice shows decreased expression in the ME (versus SVZ) compared with naive mice, which is in accordance with the effect of TSP1 in vitro.
These findings suggest that the ME is an exclusive oligodendrogenic niche that can serve as an endogenous pool of OPCs, and as a target to enhance the generation of OPCs under pathological conditions. Indeed, this study suggests that TSP1 plays a negative regulator of oligodendrogenesis in vitro.
Our study shows that the ME, considered as a CVO (Ganong, 2000), is enriched with BrdU+ cells both in naive and to a greater extent in EAE mice, can generate NS and differentiate to neurons and OLs, as described previously (Bennett et al., 2009, Lin and Iacovitti, 2015, Robins et al., 2013a, Robins et al., 2013b). Interestingly, ME-NSCs preferentially differentiate to OLs, corroborated by the in vivo results showing that BrdU+ cells in the ME are NG2+, while no immunoreactivity for neuronal marker with BrdU+ can be detected. BrdU+ NG2+ cells in the ME may represent a pool of latent progenitors, which are relatively quiescent in the brain of naive mice, yet possess the potential to proliferate and differentiate under specific circumstances. In this regard, like other CVOs, with their abundance of fenestrated capillaries and their proximity to ventricles, the ME may be ideally positioned to response to pathological conditions such as inflammation, stroke (Lin and Iacovitti, 2015), traumatic brain injury (Falnikar et al., 2018), or physiological cues, suggesting that signaling factors present in blood may be relevant to stimulating the ME niche to generate OPCs. In support of this notion, Kokoeva et al. (2005) showed that ciliary neurotrophic factor robustly induces cell proliferation in the hypothalamus.
An interesting issue is the physiological significance of the increased OPCs in the ME, which was mainly studied with respect to energy balance, weight loss, and nutritional signaling (Djogo et al., 2016, Kohnke et al., 2019), but not with demyelinating conditions.
In the pathological context, our findings of increased number of OPCs in the ME of EAE mice, and particularly their differentiation to pre-myelinating OLs and MBP+ cells, suggest these OPCs as a backup pool to replace mature OLs that turnover with time, for OLs that support sensory neurons and essential for balance of body energy and metabolism rather than represent a transient population with myelin-unrelated roles.
Hence, our study suggesting the role of these OPCs during myelin repair further shed light on the pathological context of OPCs pool in the ME.
As a CVO, the ME is exposed to many factors that may maintain stem cells under normal physiological conditions and, like known neurogenic SVZ and SGZ regions, the ME microenvironment may possess factors critical for maintaining progenitors and regulating differentiation (Falnikar et al., 2018). Similarly, numerous studies show that dehydration stress (Virard et al., 2008) or demyelination (Picard-Riera et al., 2002) promote proliferation of OPCs. In line with our findings, recent studies demonstrated enhanced proliferation in the ME in a stroke model, however, and interestingly the proportion of differentiating neurons was significantly increased after focal ischemic stroke (MCAO) compared with OLs and astrocytes, representing a shift toward neurogenesis in the injured brain (Falnikar et al., 2018, Lin and Iacovitti, 2015). This raises the possibility of niche plasticity and differentiation on demand upon injury. Intriguingly, we could not detect neuronal progenitors in the ME as shown previously (Lin and Iacovitti, 2015, Robins et al., 2013a, Robins et al., 2013b). These differences could be attributed to methodological disparities between studies.
Our data suggest that the ME predominantly give rise to OPCs and OLs in healthy brain, which is enhanced in demyelinating condition. Such remyelination capacity of SVZ-NSCs was demonstrated in an EAE model (Pluchino et al., 2003) and of OPCs from human fetal forebrain transplanted in Shi mice (Windrem et al., 2004).
Although we did not track the origin of OPCs lining the ME, recent findings evidently suggest that stem cells in this region do not migrate there from other areas of high stem cell proliferation (i.e., SVZ) and instead the third and fourth ventricles are likely novel niches for stem cell production (Lin and Iacovitti, 2015).
Beside the SVZ and SGZ, neurogenesis may occur in other areas of the adult brain; CA1 (Rietze et al., 2000), neocortex (Gould et al., 1999, Gould et al., 2001), striatum (Bedard et al., 2002), amygdala (Bernier et al., 2002), substantia nigra (Zhao et al., 2003), third ventricle (Xu et al., 2005), subcortical white matter (Luzzati et al., 2006), caudate nucleus (Takemura, 2005), and leptomeninges (Bifari et al., 2009). However, regions of oligodendrogenesis except for Ctx were purely characterized (Naruse et al., 2017, Winkler et al., 2018). Virard et al. (2008) showed that the adult neurohypophysis contains glial progenitors capable of differentiating into OLs but the structure does not harbor NSCs. Picard-Riera et al. (2002) showed that EAE associated with demyelination enhances proliferation in SVZ and promotes mobilization of SVZ cells to demyelinated white matter structures, generating neurons and OLs. Seri et al. (2006) reported that cells isolated from the SCZ and cultured as NS behave as NSCs in vitro and migrate into the CC to become OLs in vivo.
These studies support our data showing the oligodendrogenic fate of newly generated cells in the ME of naive mice. Moreover, this oligodendrogenesis is enhanced in EAE mice. Our finding that these newly generated cells in the ME can be detected in naive mice (also shown by Robins et al., 2013b) and express the RIP marker of pre-myelinating OLs suggests that these cells do not migrate from other neurogenic regions but represent ME-resident cells with proliferation capacity. It is intriguing if the ME is exclusively oligodendrogenic as shown here, although a low percentage of neuronal progenitors differentiated from ME-NS could be detected in vitro, which may result from the lack of the in vivo environmental cues characterizing the ME. In accordance with this notion, Komitova et al. (2009) shows that fewer than 1% of proliferating cells in the SVZ are NG2 cells which are more abundant in the nonneurogenic parenchyma, suggesting that NG2+ OPCs are distinct from cellular constituents of the SVZ known as type A, B, or C cells (Alvarez-Buylla et al., 2002) and that the SVZ is mostly neuronogenic. Consistently, our study strongly suggests that the ME is an oligodendrogenic rather than an neuronogenic niche, corroborated by the absence of BrdU+ cells co-expressing markers of neuronal progenitors in the ME.
As with neurogenesis (Lin and Iacovitti, 2015), the production of OLs is restricted (Moyse et al., 2008), demonstrated to arise from the tissue microenvironment, which led to the emerging concept of an “oligodendrogenic niche.” However, the possibility that neuro/oligodendrogenesis is directed by combination of both extrinsic and intrinsic factors should be considered as well. The adult neural parenchyma contains a widely distributed potential for oligodendrogenesis that is not expressed in standard conditions, but which may be recruited for brain repair (Buffo, 2007). It could be speculated that niche-dependent differences are mediated by adaptations of OPCs to their environment by reacting to local signaling (van Tilborg et al., 2018).
Astrocytes induce formation of excitatory synapses by secreting TSP1/2 (Christopherson et al., 2005). Our results showing that SVZ-residing astrocytes of EAE mice express high level of TSP1 are consistent with the evidence that reactive astrocytes showing increased expression of TSP1, suggesting that these astrocytes are likely A2 type.
TSP1 was proposed to play a role in neuronal differentiation but inhibit differentiation of SVZ-NS toward OLs (Lu and Kipnis, 2010). Consistently, our in vitro and in vivo data suggest that astrocytic TSP1 plays a negative regulator of the oligodendrogenic fate of ME-NSCs both as an intrinsic and an extrinsic factor. Notably, TSP1 staining in the brain appears widely and diffusely, suggesting that there might be another source than just the astrocytes, likely neurons (Tyzack et al., 2014) and activated microglia (Rivera et al., 2017), which are highly abundant under neuroinflammation. In this study, LSKL, which blocks the binding of TSP1 and the large latent TGF-β complex, antagonized the effect of TSP1, suggesting that the effect of TSP1 is mediated by TGF-β. This notion is further sustained by the decreased expression of TSP1 in the ME of EAE mice associated with increased number of OPCs.
While the SVZ-NSCs mostly generate neuronal lineage and only a few OLs with some lineage plasticity in pathological conditions (Armada-Moreira et al., 2015), our study demonstrates the oligodendrogenic potential of the ME both in vitro and in vivo and the role of TSP1 as a negative regulator of oligodendrogenesis in vitro. Our findings suggest the ME niche as a pool of “backup” OPCs that can differentiate in the case of injury to surrounding OLs and as a target for enhancement of oligodendrogenesis while devising therapies for demyelination diseases.
Experimental Procedures
Animals
C57Bl/6J and SJL/J mice were purchased from Jackson Laboratory (Bar Harbor, ME, USA) or obtained from the Weizmann Institute colony. (C57Bl/6J x SLJ/J)F1 mice were bred at the Weizmann Institute Animal Facility. Shi mice were purchased from Jackson Laboratory. All animals were handled according to the regulations formulated by the Institutional Animal Care and Use committee of the Weizmann Institute (permit no. 02820711-3) and were performed in compliance with its relevant guidelines and regulations.
Reagents and Antibodies
The mouse PLP139-151 peptide was synthesized in the laboratory of Prof. M. Fridkin (Department of Organic Chemistry, The Weizmann Institute of Science), using the Fmoc technique with an automated peptide synthesizer (AMS422; Abimed, Langenfeld, Germany). For list of antibodies see Table S1.
Active Induction of EAE
(C57Bl/6J × SJL/J)F1 mice (6- to 8-week-old female) were immunized, observed, and scored as described previously (Zilkha-Falb et al., 2016).
Administration of BrdU and Tissue Processing
When reached a maximal clinical score (3) usually at day 20 post-immunization, mice were injected i.p. with BrdU (Sigma-Aldrich; 50 mg kg−1 body weight) daily for 3 days and then were sacrificed. Animals were deeply anesthetized with a xylazine/ketamine mixture and perfused intracardially with cold 4% paraformaldehyde (PFA). Brains were postfixed in 2% PFA and cryoprotected in a 15% sucrose solution. Free-floating sections (30 μm thick) were cut coronally with a sliding microtome (Leica SM, 2000r; Leica, Nussloch, Germany).
NSCs Culture from SVZ and ME
NSCs were generated from the SVZ of the LV or the ME of the hypothalamus from brains of C57Bl/6J mice (6–8 weeks old) as described (Zilkha-Falb et al., 2016). For fate tracking, ME- and SVZ-NSCs were generated from GFP mice (kindly provided by the lab of Michal Schwartz, Department of Neurobiology, The Weizmann Institute of Science). NS were differentiated by plating on poly-D-lysine (PDL) (Sigma-Aldrich, Rehovot, Israel), in growth factor-free NS medium containing 3% serum (differentiation medium). For immunocytochemistry, cells were plated on PDL-coated coverslips. To study the effect of TSP1, NS were differentiated in the presence of 2 μg/mL TSP1 (kindly provided by the lab of Prof. Jonathan Kipnis, University of Virginia, Charlottesville, VA, USA) or BSA, and replaced everyday with fresh TSP1 and BSA for 8 days. To study the effect of TSP1 antagonist LSKL and its control-scrambled peptide SLLK, NS were differentiated in the presence of 50 μg/mL of either of the peptides (GenScript) and replaced every alternate day for 8 days.
qRT-PCR Analysis
cDNA (see Supplemental Experimental Procedures) was subjected for qRT-PCR in triplicates using the 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA), and TaqMan Universal PCR Master Mix (Applied Biosystems) with TaqMan gene expression assays of mouse target genes. Relative expression was calculated by Relative Quantification software (Roche Diagnostics) using β-actin for normalization.
Primers sequences used for amplification were as follows:
TSP1: TaqMan Real-Time ready assay ID: Mm00449020_g1
β-Actin: TaqMan Real-Time ready assay ID: Mm00607939_s1
Immuno Labeling
Immunocytochemistry
Coverslips were washed with PBS, fixed with 2% PFA, and immune labeled as described previously (Zilkha-Falb et al., 2016), except for the additional primary antibodies used, monoclonal anti-NeuN (1:400) and monoclonal anti-TSP1 (1:200). Nuclei were visualized by DAPI counterstaining. Slides were examined using an LSM 510 laser scanning confocal microscope (Carl Zeiss, Jena, Germany). Digital images were acquired using the Zeiss LSM 510 software (magnifications, ×40 or ×63).
Immunoflourescent Staining of Tissue Sections
Free-floating sections of brain were washed twice in PBS followed by blocking in either 3% rabbit (for goat anti-DCX, mouse anti-RIP) or goat serum (for rabbit anti-NG2) depending on primary antibodies used and 0.1% Triton X-100 in PBS for 1 h at room temperature (RT). Sections were stained as described previously (Zilkha-Falb et al., 2016), except for the additional primary antibodies used, mouse anti-TSP1 (1:200), rat anti-BrdU (1:200), mouse anti-RIP (1:100) rat anti-MAC2 (100), and rat anti-CD45 (1:100). For BrdU labeling, sections were primarily incubated with 2N HCl at 37°C for 30 min, and rinsed in 0.1 M (pH 8.5) borate buffer at RT for 10 min, rinsed twice in PBS, blocked and immune labeled. Images were acquired as described above. Confocal Z-stacks were taken, and cell numbers were counted manually.
Quantification
Immunofluorescence data that were collected from three independent repeats of experiment (n = 6 from each) provided an independent sampling. Analysis of qPCR was from three independent repeats. For morphometric analysis, we used repeats from at least three independent experiments with data obtained from six images (of randomly chosen field of view under ×20 objective) for each time point per condition. Statistical significance was assessed by unpaired two-tailed Student's t test (Excel software). p values < 0.05 were considered significant. All data are presented as mean ± standard error. ImageJ densitometry software (version 1.36, NIH, Bethesda, MD, USA) was used for quantification of TSP1 intensity.
Author Contributions
R.Z-.F., N.K., and A.B.-N. conceived the study and designed the experiments. R.Z-.F. and N.K. performed the experiments. R.Z-.F. conducted the confocal imaging and analysis. R.Z-.F. and A.B.-N. wrote the manuscript. All authors discussed the results and conclusions and reviewed the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This paper is dedicated to the memory of A.B.-N., who recently passed away. Ben-Nun was a strong-minded, vigorous, and inspiring scientist in the field of immunology and autoimmunity research. He was a real friend, a very kind and gentle person.
Published: May 14, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.stemcr.2020.04.005.
Supplemental Information
References
- Aharoni R., Arnon R., Eilam R. Neurogenesis and neuroprotection induced by peripheral immunomodulatory treatment of experimental autoimmune encephalomyelitis. J. Neurosci. 2005;25:8217–8228. doi: 10.1523/JNEUROSCI.1859-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman J., Das G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A., Seri B., Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res. Bull. 2002;57:751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- Armada-Moreira A., Ribeiro F.F., Sebastião A.M., Xapelli S. Neuroinflammatory modulators of oligodendrogenesis. Neuroimmunol. Neuroinflam. 2015;2:263–273. [Google Scholar]
- Bedard A., Cossette M., Levesque M., Parent A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci. Lett. 2002;328:213–216. doi: 10.1016/s0304-3940(02)00530-x. [DOI] [PubMed] [Google Scholar]
- Bennett L., Yang M., Enikolopov G., Iacovitti L. Circumventricular organs: a novel site of neural stem cells in the adult brain. Mol. Cell. Neurosci. 2009;41:337–347. doi: 10.1016/j.mcn.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier P.J., Bedard A., Vinet J., Levesque M., Parent A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl. Acad. Sci. U S A. 2002;99:11464–11469. doi: 10.1073/pnas.172403999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bifari F., Decimo I., Chiamulera C., Bersan E., Malpeli G., Johansson J., Lisi V., Bonetti B., Fumagalli G., Pizzolo G. Novel stem/progenitor cells with neuronal differentiation potential reside in the leptomeningeal niche. J. Cell Mol. Med. 2009;13:3195–3208. doi: 10.1111/j.1582-4934.2009.00706.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore W.F., Chari D.M., Gilson J.M., Crang A.J. Modelling large areas of demyelination in the rat reveals the potential and possible limitations of transplanted glial cells for remyelination in the CNS. Glia. 2002;38:155–168. doi: 10.1002/glia.10067. [DOI] [PubMed] [Google Scholar]
- Brousse B., Magalon K., Durbec P., Cayre M. Region and dynamic specificities of adult neural stem cells and oligodendrocyte precursors in myelin regeneration in the mouse brain. Biol. Open. 2015;4:980–992. doi: 10.1242/bio.012773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffo A. Fate determinant expression in the lesioned brain: Olig2 induction and its implications for neuronal repair. Neurodegener Dis. 2007;4:328–332. doi: 10.1159/000101890. [DOI] [PubMed] [Google Scholar]
- Buffo A., Vosko M.R., Erturk D., Hamann G.F., Jucker M., Rowitch D., Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc. Natl. Acad. Sci. U S A. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerghet M., Skoff R.P., Bessert D., Zhang Z., Mullins C., Ghandour M.S. Proliferation and death of oligodendrocytes and myelin proteins are differentially regulated in male and female rodents. J. Neurosci. 2006;26:1439–1447. doi: 10.1523/JNEUROSCI.2219-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A., Nishiyama A., Peterson J., Prineas J., Trapp B.D. NG2-positive oligodendrocyte progenitor cells in adult human brain and multiple sclerosis lesions. J. Neurosci. 2000;20:6404–6412. doi: 10.1523/JNEUROSCI.20-17-06404.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chari D.M., Blakemore W.F. Efficient recolonisation of progenitor-depleted areas of the CNS by adult oligodendrocyte progenitor cells. Glia. 2002;37:307–313. [PubMed] [Google Scholar]
- Christopherson K.S., Ullian E.M., Stokes C.C., Mullowney C.E., Hell J.W., Agah A., Lawler J., Mosher D.F., Bornstein P., Barres B.A. Thrombospondins are astrocyte-secreted proteins that promote CNS synaptogenesis. Cell. 2005;120:421–433. doi: 10.1016/j.cell.2004.12.020. [DOI] [PubMed] [Google Scholar]
- Dimou L., Simon C., Kirchhoff F., Takebayashi H., Gotz M. Progeny of Olig2-expressing progenitors in the gray and white matter of the adult mouse cerebral cortex. J. Neurosci. 2008;28:10434–10442. doi: 10.1523/JNEUROSCI.2831-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djogo T., Robins S.C., Schneider S., Kryzskaya D., Liu X., Mingay A., Gillon C.J., Kim J.H., Storch K.F., Boehm U. Adult NG2-glia are required for median eminence-mediated leptin sensing and body weight control. Cell Metab. 2016;23:797–810. doi: 10.1016/j.cmet.2016.04.013. [DOI] [PubMed] [Google Scholar]
- Duncan I.D., Radcliff A.B. Inherited and acquired disorders of myelin: the underlying myelin pathology. Exp. Neurol. 2016;283:452–475. doi: 10.1016/j.expneurol.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emsley J.G., Mitchell B.D., Kempermann G., Macklis J.D. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog. Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Falnikar A., Stratton J., Lin R., Andrews C.E., Tyburski A., Trovillion V.A., Gottschalk C., Ghosh B., Iacovitti L., Elliott M.B. Differential response in novel stem cell niches of the brain after cervical spinal cord injury and traumatic brain injury. J. Neurotrauma. 2018;35:2195–2207. doi: 10.1089/neu.2017.5497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fancy S.P., Zhao C., Franklin R.J. Increased expression of Nkx2.2 and Olig2 identifies reactive oligodendrocyte progenitor cells responding to demyelination in the adult CNS. Mol. Cell Neurosci. 2004;27:247–254. doi: 10.1016/j.mcn.2004.06.015. [DOI] [PubMed] [Google Scholar]
- Ganong W.F. Circumventricular organs: definition and role in the regulation of endocrine and autonomic function. Clin. Exp. Pharmacol. Physiol. 2000;27:422–427. doi: 10.1046/j.1440-1681.2000.03259.x. [DOI] [PubMed] [Google Scholar]
- Gould E. How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 2007;8:481–488. doi: 10.1038/nrn2147. [DOI] [PubMed] [Google Scholar]
- Gould E., Reeves A.J., Graziano M.S., Gross C.G. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- Gould E., Vail N., Wagers M., Gross C.G. Adult-generated hippocampal and neocortical neurons in macaques have a transient existence. Proc. Natl. Acad. Sci. U S A. 2001;98:10910–10917. doi: 10.1073/pnas.181354698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner P.J., Power A.E., Kempermann G., Kuhn H.G., Palmer T.D., Winkler J., Thal L.J., Gage F.H. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery N.D., Crang A.J., O'Leary M.,T., Hodge S.J., Blakemore W.F. Behavioural consequences of oligodendrocyte progenitor cell transplantation into experimental demyelinating lesions in the rat spinal cord. Eur. J. Neurosci. 1999;11:1508–1514. doi: 10.1046/j.1460-9568.1999.00564.x. [DOI] [PubMed] [Google Scholar]
- Kazanis I., Evans K.A., Andreopoulou E., Dimitriou C., Koutsakis C., Karadottir R.T., Franklin R.J.M. Subependymal zone-derived oligodendroblasts respond to focal demyelination but fail to generate myelin in young and aged mice. Stem Cell Rep. 2017;8:685–700. doi: 10.1016/j.stemcr.2017.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohnke S., Lam B., Buller S., Zhao C., Nuzzaci D., Tadross J., Holmqvist S., Ridley K., Hathaway H., Macklin W. Nutritional signals rapidly activate oligodendrocyte differentiation in the adult hypothalamic median eminence. bioRxiv. 2019:751198. doi: 10.1101/751198. [DOI] [Google Scholar]
- Kokoeva M.V., Yin H., Flier J.S. Neurogenesis in the hypothalamus of adult mice: potential role in energy balance. Science. 2005;310:679–683. doi: 10.1126/science.1115360. [DOI] [PubMed] [Google Scholar]
- Komitova M., Zhu X., Serwanski D.R., Nishiyama A. NG2 cells are distinct from neurogenic cells in the postnatal mouse subventricular zone. J. Comp. Neurol. 2009;512:702–716. doi: 10.1002/cne.21917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanz T.V., Ding Z., Ho P.P., Luo J., Agrawal A.N., Srinagesh H., Axtell R., Zhang H., Platten M., Wyss-Coray T. Angiotensin II sustains brain inflammation in mice via TGF-beta. J. Clin. Invest. 2010;120:2782–2794. doi: 10.1172/JCI41709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann H. Pathogenic mechanisms associated with different clinical courses of multiple sclerosis. Front. Immunol. 2018;9:3116. doi: 10.3389/fimmu.2018.03116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim D.A., Alvarez-Buylla A. The adult ventricular-subventricular zone (V-SVZ) and olfactory bulb (OB) neurogenesis. Cold Spring Harb. Perspect. Biol. 2016;8 doi: 10.1101/cshperspect.a018820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin R., Iacovitti L. Classic and novel stem cell niches in brain homeostasis and repair. Brain Res. 2015;1628:327–342. doi: 10.1016/j.brainres.2015.04.029. [DOI] [PubMed] [Google Scholar]
- Lu Z., Kipnis J. Thrombospondin 1—a key astrocyte-derived neurogenic factor. FASEB J. 2010;24:1925–1934. doi: 10.1096/fj.09-150573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzati F., De Marchis S., Fasolo A., Peretto P. Neurogenesis in the caudate nucleus of the adult rabbit. J. Neurosci. 2006;26:609–621. doi: 10.1523/JNEUROSCI.4371-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moyse E., Segura S., Liard O., Mahaut S., Mechawar N. Microenvironmental determinants of adult neural stem cell proliferation and lineage commitment in the healthy and injured central nervous system. Curr. Stem Cell Res. Ther. 2008;3:163–184. doi: 10.2174/157488808785740334. [DOI] [PubMed] [Google Scholar]
- Nait-Oumesmar B., Picard-Riera N., Kerninon C., Baron-Van Evercooren A. The role of SVZ-derived neural precursors in demyelinating diseases: from animal models to multiple sclerosis. J. Neurol. Sci. 2008;265:26–31. doi: 10.1016/j.jns.2007.09.032. [DOI] [PubMed] [Google Scholar]
- Naruse M., Ishizaki Y., Ikenaka K., Tanaka A., Hitoshi S. Origin of oligodendrocytes in mammalian forebrains: a revised perspective. J. Physiol. Sci. 2017;67:63–70. doi: 10.1007/s12576-016-0479-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard-Riera N., Decker L., Delarasse C., Goude K., Nait-Oumesmar B., Liblau R., Pham-Dinh D., Evercooren A.B. Experimental autoimmune encephalomyelitis mobilizes neural progenitors from the subventricular zone to undergo oligodendrogenesis in adult mice. Proc. Natl. Acad. Sci. U S A. 2002;99:13211–13216. doi: 10.1073/pnas.192314199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pluchino S., Quattrini A., Brambilla E., Gritti A., Salani G., Dina G., Galli R., Del Carro U., Amadio S., Bergami A. Injection of adult neurospheres induces recovery in a chronic model of multiple sclerosis. Nature. 2003;422:688–694. doi: 10.1038/nature01552. [DOI] [PubMed] [Google Scholar]
- Redwine J.M., Armstrong R.C. In vivo proliferation of oligodendrocyte progenitors expressing PDGFalphaR during early remyelination. J. Neurobiol. 1998;37:413–428. doi: 10.1002/(sici)1097-4695(19981115)37:3<413::aid-neu7>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Reynolds R., Cenci di Bello I., Dawson M., Levine J. The response of adult oligodendrocyte progenitors to demyelination in EAE. Prog. Brain Res. 2001;132:165–174. doi: 10.1016/s0079-6123(01)32073-3. [DOI] [PubMed] [Google Scholar]
- Rietze R., Poulin P., Weiss S. Mitotically active cells that generate neurons and astrocytes are present in multiple regions of the adult mouse hippocampus. J. Comp. Neurol. 2000;424:397–408. [PubMed] [Google Scholar]
- Rivera J.C., Noueihed B., Madaan A., Lahaie I., Pan J., Belik J., Chemtob S. Tetrahydrobiopterin (BH4) deficiency is associated with augmented inflammation and microvascular degeneration in the retina. J. Neuroinflam. 2017;14:181. doi: 10.1186/s12974-017-0955-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins S.C., Stewart I., McNay D.E., Taylor V., Giachino C., Goetz M., Ninkovic J., Briancon N., Maratos-Flier E., Flier J.S. alpha-Tanycytes of the adult hypothalamic third ventricle include distinct populations of FGF-responsive neural progenitors. Nat. Commun. 2013;4:2049. doi: 10.1038/ncomms3049. [DOI] [PubMed] [Google Scholar]
- Robins S.C., Trudel E., Rotondi O., Liu X., Djogo T., Kryzskaya D., Bourque C.W., Kokoeva M.V. Evidence for NG2-glia derived, adult-born functional neurons in the hypothalamus. PLoS One. 2013;8:e78236. doi: 10.1371/journal.pone.0078236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seri B., Herrera D.G., Gritti A., Ferron S., Collado L., Vescovi A., Garcia-Verdugo J.M., Alvarez-Buylla A. Composition and organization of the SCZ: a large germinal layer containing neural stem cells in the adult mammalian brain. Cereb. Cortex. 2006;16(Suppl 1) doi: 10.1093/cercor/bhk027. i103–111. [DOI] [PubMed] [Google Scholar]
- Serwanski D.R., Rasmussen A.L., Brunquell C.B., Perkins S.S., Nishiyama A. Sequential contribution of parenchymal and neural stem cell-derived oligodendrocyte precursor cells toward remyelination. Neuroglia. 2018;1:91–105. doi: 10.3390/neuroglia1010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim F.J., Zhao C., Penderis J., Franklin R.J. The age-related decrease in CNS remyelination efficiency is attributable to an impairment of both oligodendrocyte progenitor recruitment and differentiation. J. Neurosci. 2002;22:2451–2459. doi: 10.1523/JNEUROSCI.22-07-02451.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemura N.U. Evidence for neurogenesis within the white matter beneath the temporal neocortex of the adult rat brain. Neuroscience. 2005;134:121–132. doi: 10.1016/j.neuroscience.2005.04.033. [DOI] [PubMed] [Google Scholar]
- Talbott J.F., Loy D.N., Liu Y., Qiu M.S., Bunge M.B., Rao M.S., Whittemore S.R. Endogenous Nkx2.2+/Olig2+ oligodendrocyte precursor cells fail to remyelinate the demyelinated adult rat spinal cord in the absence of astrocytes. Exp. Neurol. 2005;192:11–24. doi: 10.1016/j.expneurol.2004.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. Adult neurogenesis, neuroinflammation and therapeutic potential of adult neural stem cells. Int. J. Med. Sci. 2008;5:127–132. doi: 10.7150/ijms.5.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. Neurogenesis in the adult central nervous system. C R. Biol. 2006;329:465–475. doi: 10.1016/j.crvi.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Tyzack G.E., Sitnikov S., Barson D., Adams-Carr K.L., Lau N.K., Kwok J.C., Zhao C., Franklin R.J., Karadottir R.T., Fawcett J.W. Astrocyte response to motor neuron injury promotes structural synaptic plasticity via STAT3-regulated TSP-1 expression. Nat. Commun. 2014;5:4294. doi: 10.1038/ncomms5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Tilborg E., de Theije C.G.M., van Hal M., Wagenaar N., de Vries L.S., Benders M.J., Rowitch D.H., Nijboer C.H. Origin and dynamics of oligodendrocytes in the developing brain: implications for perinatal white matter injury. Glia. 2018;66:221–238. doi: 10.1002/glia.23256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virard I., Gubkina O., Alfonsi F., Durbec P. Characterization of heterogeneous glial cell populations involved in dehydration-induced proliferation in the adult rat neurohypophysis. Neuroscience. 2008;151:82–91. doi: 10.1016/j.neuroscience.2007.10.035. [DOI] [PubMed] [Google Scholar]
- Warrington A.E., Barbarese E., Pfeiffer S.E. Differential myelinogenic capacity of specific developmental stages of the oligodendrocyte lineage upon transplantation into hypomyelinating hosts. J. Neurosci. Res. 1993;34:1–13. doi: 10.1002/jnr.490340102. [DOI] [PubMed] [Google Scholar]
- Windrem M.S., Nunes M.C., Rashbaum W.K., Schwartz T.H., Goodman R.A., McKhann G., 2nd, Roy N.S., Goldman S.A. Fetal and adult human oligodendrocyte progenitor cell isolates myelinate the congenitally dysmyelinated brain. Nat. Med. 2004;10:93–97. doi: 10.1038/nm974. [DOI] [PubMed] [Google Scholar]
- Winkler C.C., Yabut O.R., Fregoso S.P., Gomez H.G., Dwyer B.E., Pleasure S.J., Franco S.J. The dorsal wave of neocortical oligodendrogenesis begins embryonically and requires multiple sources of sonic hedgehog. J. Neurosci. 2018;38:5237–5250. doi: 10.1523/JNEUROSCI.3392-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y.L., Roth P.T., Stratton J.A., Chuang B.H., Danne J., Ellis S.L., Ng S.W., Kilpatrick T.J., Merson T.D. Adult neural precursor cells from the subventricular zone contribute significantly to oligodendrocyte regeneration and remyelination. J. Neurosci. 2014;34:14128–14146. doi: 10.1523/JNEUROSCI.3491-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Tamamaki N., Noda T., Kimura K., Itokazu Y., Matsumoto N., Dezawa M., Ide C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp. Neurol. 2005;192:251–264. doi: 10.1016/j.expneurol.2004.12.021. [DOI] [PubMed] [Google Scholar]
- Zhao M., Momma S., Delfani K., Carlen M., Cassidy R.M., Johansson C.B., Brismar H., Shupliakov O., Frisen J., Janson A.M. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc. Natl. Acad. Sci. U S A. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilkha-Falb R., Kaushansky N., Kawakami N., Ben-Nun A. Post-CNS-inflammation expression of CXCL12 promotes the endogenous myelin/neuronal repair capacity following spontaneous recovery from multiple sclerosis-like disease. J. Neuroinflam. 2016;13:7. doi: 10.1186/s12974-015-0468-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.