Abstract
Background.
Seasonal patterns in hospitalizations have been observed in various psychiatric disorders, however, it is unclear whether they also exist in schizophrenia. Previous studies found mixed results and those reporting the presence of seasonality differ regarding the characteristics of these patterns. Further, they are inconclusive whether sex is an influencing factor. The aim of this study was therefore to examine if seasonal patterns in hospitalizations can be found in schizophrenia, with special regard to a possible influence of sex, by using a large national dataset.
Methods.
Data on all hospital admissions within Austria due to schizophrenia (F20.0–F20.6) for the time period of 2003–2016 were included. Age standardized monthly variation of hospitalization for women and men was analyzed and the level of significance adjusted for multiple testing.
Results.
The database comprised of 110,735 admissions (59.6% men). Significant seasonal variations were found in the total sample with hospitalization peaks in January and June and a trough in December (p < 0.0001). No significant difference in these patterns was found between women and men with schizophrenia (p < 0.0001).
Conclusion.
Our study shows that schizophrenia-related hospitalizations follow a seasonal pattern in both men and women. The distribution of peaks might be influenced by photoperiod changes which trigger worsening of symptoms and lead to exacerbations in schizophrenia. Further research is necessary to identify underlying factors influencing seasonal patterns and to assess whether a subgroup of patients with schizophrenia is especially vulnerable to the impact of seasonal variations.
Key words: Hospitalization, schizophrenia, seasonality, sex
Introduction
In various psychiatric disorders, especially mood disorders, the presence of seasonal patterns was found to profoundly affect the clinical course and to be a risk factor for increased severity with higher rates of mood episodes, younger age of onset and increased prevalence of psychiatric comorbidities [1–5]. However, inconsistent results exist regarding the influence of seasonality on schizophrenia which is a severe and often chronic mental disorder affecting 20 million people worldwide [6]. Since the end of the 18th century, seasonal patterns in symptom severity as well as in the course of hospitalizations of patients suffering from schizophrenia have been observed [7,8]. Nevertheless, controversy about whether, and to which extent, a seasonality effect in schizophrenia exists continues: While some studies reported a significant increase of admissions due to schizophrenia in spring and summer [9–12], others negated such seasonal variations [2,13]. Further, in an Irish study summer peaks were observed for first time admissions only, but no seasonal effects found for readmissions [14], while results from a community-based hospital in New York City claimed such variation for readmissions only [15]. Variations in the occurrence of psychosis along the course of the year were assumed to underlie climatic conditions [12,14] and their influence on biological mechanisms including melatonergic and serotonergic interactions [16]. Previous studies also suggested an association between hospitalization rates and duration of photoperiod [17,18] and a possible influence of public holidays and religious festivities [18–20]. Besides previous inconsistent results regarding seasonal hospitalization patterns in patients with schizophrenia in general, there has been some evidence for sex-specific seasonal patterns in hospitalizations due to schizophrenia [11,18,21,22].
For a more comprehensive understanding of seasonal hospitalization patterns in patients with schizophrenia, we examined a large national dataset on hospital admissions for schizophrenia over a 14-year time period. With regard to the number of included hospital admission data combined with the length of observation, this is, to the best of our knowledge, the largest study so far, as previous studies consisted of individual, but relatively small patient samples.
Material and Methods
Data source
In this study, hospital discharge data of all counties in Austria were analyzed for individuals diagnosed with schizophrenia (F20.0–F20.6) between 2003 and 2016. After the selection of cases according to the inclusion and exclusion criteria defined by the authors, the dataset was provided in anonymized form by Statistics Austria, the national statistics agency which collects data from the Austrian health system and provides data access for scientific research. The dataset includes sex, age schizophrenia subtype (F20.0–F20.6), month of discharge and length of hospital inpatient stay in days.
Population
Aiming to examine hospitalization patterns for a homogeneous sample of patients with schizophrenia, individuals with other psychotic disorders including “schizotypal disorder” (F21), “persistent delusional disorder” (F22), “acute and transient psychotic disorders” (F23), “induced delusional disorder” (F24), “schizoaffective disorders” (F25) as well as “other nonorganic psychotic disorders” (F28), and “unspecified nonorganic psychosis” (F29) were excluded from analysis. Due to lacking specificity of categorization within schizophrenia-spectrum disorders, patients with the diagnosis “other schizophrenia” (F20.8) (n = 881) and “unspecified schizophrenia” (F20.9) (n = 2,848) were also excluded. Furthermore, a subsample of patients with a duration of hospitalization ≥400 days (n = 937), representing a group of extremely ill patients, was also excluded from further analysis as well as patients younger than 15 years (n = 184) who were assumed to represent a rather specific cohort of individuals with very early-onset schizophrenia. Inclusion and exclusion criteria were carefully discussed and determined within the research team with the consent of all members.
Statistics
The number of admissions was described by frequency tables and compared between men and women by Chi-squared test. Seasonality patterns were shown by presenting observed and expected percentage of hospitalizations by month within a year. Expected percentage by month was calculated by multiplying the sum of all admissions by proportion of days of a specific month compared to the total sum of days. Age at admission was described by mean and standard deviation. Comparison of age between men and women was done by Wilcoxon signed rank test. The effect of sex was shown by calculating the ratio of admission rate men to women within age groups (10 years) along with 95% confidence intervals. The significance level was adjusted to account for multiple testing (p < 0.001). Analysis was performed using SAS (SAS Institute Inc., Cary, NC).
Results
The database comprised 110,735 schizophrenia-related hospital admissions. The overall admission estimate rate for all schizophrenia subtypes was 107.5 per 100,000 residents per year (see Table 1). For hospital admission rates and estimates for subtypes see Table 1. The mean age of patients in our dataset was 41.2 years (SD = 14.1). On average, men were 5 years younger (mean = 37.5 years; SD = 13) than women (42.5 years; SD = 14.7). In total, 59.6% (n = 65,955) of the admissions were men, which were also significantly more often diagnosed within all subtypes (p < 0.0001) (see Table 2). Mean length of hospital stay was significantly shorter in women (mean = 21.55 days, SD = 30.39) when compared to men (mean = 22.03 days, SD = 34.07) (p < 0.0001).
Table 1.
Mean annual admission rates per 100,000 population with 95% CI for schizophrenia (total sample and subgroups).
| Schizophrenia subgroup | Observed events | Estimated rate per 100,000 person years | Lower CI | Upper CI |
|---|---|---|---|---|
| F20.0 | 89,469 | 86.35 | 85.79 | 86.92 |
| F20.1 | 2,955 | 2.38 | 2.73 | 2.93 |
| F20.2 | 1,676 | 1.64 | 1.56 | 1.72 |
| F20.3 | 1,753 | 1.68 | 1.60 | 1.76 |
| F20.4 | 481 | 0.48 | 0.44 | 0.52 |
| F20.5 | 13,429 | 13.63 | 13.40 | 13.87 |
| F20.6 | 972 | 0.93 | 0.87 | 0.99 |
| F20.0–F20.6 | 110,735 | 107.50 | 106.90 | 108.20 |
Abbreviation: CI, confidence intervals.
Table 2.
Number and percentage of admissions for schizophrenia (total sample and subgroups) according to sex.
| Schizophrenia subgroups | Total | |||||||
|---|---|---|---|---|---|---|---|---|
| F20.0 | F20.1 | F20.2 | F20.3 | F20.4 | F20.5 | F20.6 | F20.0–F20.6 | |
| Male | 53,370 | 1,900 | 871 | 1,016 | 291 | 7,925 | 582 | 65,955 |
| 59.7% | 64.3% | 52% | 58% | 60.5% | 59% | 59.9% | 59.6% | |
| Female | 36,099 | 1,055 | 805 | 737 | 190 | 5,504 | 390 | 44,780 |
| 40.3% | 35.7% | 48% | 42% | 39.5% | 41% | 40.1% | 40.4% | |
| Total | 89,469 | 2,955 | 1,676 | 1,753 | 481 | 13,429 | 972 | 110,735 |
Seasonality of hospitalizations
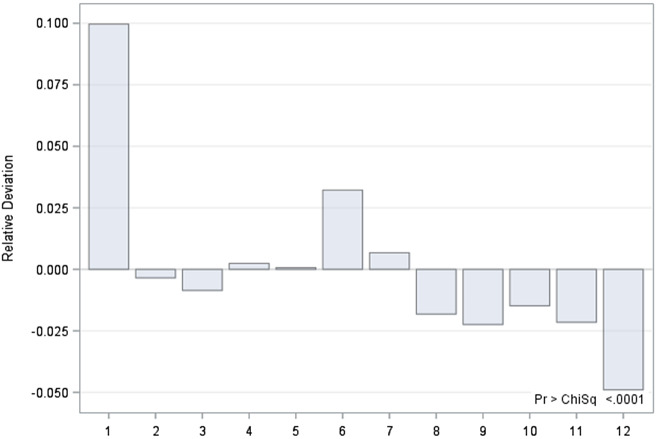
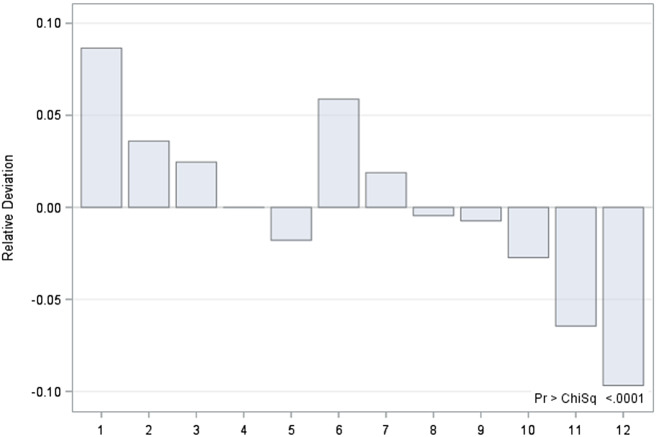
Seasonal patterns for hospitalizations due to schizophrenia were revealed in the total sample (p < 0.0001). When data was examined according to sex, significant deviations in observed hospitalizations were found for both women and men from the expected number of hospitalizations revealing maximum peaks for January and June, with hospitalizations exceeding the expected numbers by up to 10% in both groups (p < 0.0001). The largest negative deviation from the expected rate of hospitalizations was found for December, again in both sexes (p < 0.0001) (Figures 1 and 2). No significant differences between sexes were found regarding seasonality patterns.
Figure 1.
Deviations (in percentage) of expected from observed number of hospitalization of male patients by month.
Figure 2.
Deviations (in percentage) of expected from observed number of hospitalization of female patients by month.
Discussion
Hospital admission peaks were found in January and June with a continuous decline from summer to December for both sexes individually as well as combined. The largest trough in schizophrenia-related hospitalizations was found in December for both sexes. In line with previous data [23,24], men were over-represented in the total sample and all subgroups. Contrary to our hypothesis based on previous studies reporting hospitalization peaks for female patients with schizophrenia in summer [11] and spring [21] and for male patients with schizophrenia in winter with a trough in hospitalization rates in summer [18], no significant sex differences regarding seasonal patterns in hospitalizations due to schizophrenia were found in our study.
As no conclusive result has been reported yet, a multitude of possible factors might be associated with the observed hospitalization patterns in patients with schizophrenia including genetic variations, sociocultural factors as well as meteorological factors [12,17,19,20,25–27].
Addressing the interplay of sunlight, photoperiod time, vitamin D and melatonin, it has been observed that people born in winter and early spring are more likely to develop schizophrenia later on in life [26]. These findings have been hypothesized to be also caused by a shorter photoperiod and thus sunlight exposure at time of birth and the following months, subsequently causing lower vitamin D levels in patients with schizophrenia [25–28]. Examining the effect of sunlight exposure, it is important to note that vitamin D and melatonin are thought to exert inhibitory effects on each other, and literature has described psychotic exacerbations in patients due to increases of melatonin [29]. Furthermore, secretion of melatonin is reported to peak in January and July [29–31]. Yet, the role of melatonin is still unclear since significant changes in melatonin levels and melatonin circadian rhythm were found in patients with schizophrenia. Furthermore, potential benefits of an adjunctive use of melatonin along with vitamin D were suggested for patients with schizophrenia by preventing side effects of typical and atypical antipsychotics and potentially attenuating the development and severity of psychotic disorders. Melatonin’s impact on the tryptophan catabolic pathway was also discussed via its effect on cortisol secretion and stress response, thus influencing cognition, amygdala associated affect and striatal motivational processing [32].
While previous publications have suggested the change in temperature to be responsible for deterioration of mental health status in schizophrenia [33,34], we assume that temperature changes are a proxy-variable for changes in photoperiod. This hypothesis is strengthened by findings revealing hospitalization peaks during periods of lower temperature and shorter photoperiod: For the population of Australia, hospitalization peaks due to schizophrenia for July/August—representing winter and thus the shortest photoperiod per day on the southern hemisphere—were reported [18,22]. Recent studies from China also published agreeing results revealing significantly higher rates of hospitalizations in patients with schizophrenia following shorter photoperiods [17,35]. As Austria is situated in the northern hemisphere, the photoperiod continuously shortens from July onwards until the end of December. Thereafter, following the winter solstice, the photoperiod significantly increases again in January and continues this trend until June, when it again, following the summer solstice, reverses the trend [36]. This data is congruent with our results, revealing hospitalization peaks in patients suffering from schizophrenia in January as well as June and a continuous decline from August to December, which leads us to conclude that the change in relative duration of photoperiod may be an important influencing factor in triggering psychotic exacerbations and hereby leads to increased numbers of hospitalizations.
When investigating genetic factors associated with a seasonal variation in hospitalizations due to schizophrenia, Byrne et al. [27] reported possible risk factors in genome-wide association studies, as in schizophrenia the genetic profile score was associated with the highest global seasonality score compared to bipolar disorder and major depressive disorder. In the last decades, a multitude of risk factors including genetic factors could be identified concerning the etiology and course of schizophrenia [37–39]. It is known from previous studies that specific neuroanatomical markers of genetic liability to psychosis exist, grey matter reductions in the anterior cingulate were shown to be potential markers of genetic liability to psychosis while reductions in the superior temporal gyrus and cerebellum were interpreted as markers of a first onset of psychosis [40]. It should be noted, that a focus on genetic explanations of the etiology of psychotic disorders was found to be more often associated with stigmatizing attitudes [41]. However, until now we have no further knowledge about specific markers regarding the influence of seasonality on the etiology and course of schizophrenia. Thereof, the biopsychosocial model continues as highly relevant approach to the etiology and treatment of schizophrenia by embracing the role of biological, psychological, and social factors contributing to the disorder [42].
Besides genetic factors, neurobiological factors might influence seasonal patterns in schizophrenia-related hospitalizations. Significantly higher cortisol levels and higher rates of dexamethasone test nonsuppression were found in male patients with schizophrenia in winter compared to female patients with schizophrenia and patients with depression [28]. Significant seasonal variations in rates of dexamethasone test nonsuppression were found only in patients with schizophrenia in comparison to healthy controls [29].
In addition to genetic and neurobiological factors, a possible interpretation of the significant decrease in hospitalizations in December may be that during a time of religious festivities in countries with a Christian majority celebrating Christmas, a possible reduction in staff presence may necessitate a reduction of admission rates in December which then may increase thereafter in January [18,20]. Similar results were found in a study examining hospitalization patterns during Passover, the most widely celebrated holiday in Israel, reporting a significant reduction in first admissions for men with schizophrenia, but not for female patients or readmissions [19]. Yet, this interpretation does not explain the peak of hospitalizations due to schizophrenia in June.
Analyzing periods of decreased hospital admissions might help to understand the role of potential protective factors in preventing or delaying hospitalizations due to specific social factors. Addressing the overlap of genetic and neurobiological risk factors between seasonality and schizophrenia, further investigation is warranted at genetic, environmental and clinical levels aiming for the development of specific markers and potential new treatment options of schizophrenia.
Limitations
A study based on national health care data has several limitations, including possible misclassifications and potential over-selection of severely ill patients. Since identification of individuals within this data set is technically impossible, calculations of the number of inpatient stays per individual patient, and assessments of comorbid diagnoses or psychopharmacological treatment were unfortunately not feasible. Nevertheless, administrative discharge data have been shown to be sufficiently robust to support their use in research [43,44]. For psychotic disorders, administrative data has been found to be predictive of true diagnosis with high reliability, especially for the clinical diagnosis of schizophrenia [45,46].
Conclusion
To the best of our knowledge, covering more than 110,000 hospitalizations over a time period of 14 years, this is the largest population-based study on hospitalization patterns in schizophrenia. By revealing a distinct and significant seasonal variation in hospitalizations due to schizophrenia for both sexes, it can be concluded that seasonality is profoundly influencing the clinical course in men and women with schizophrenia leading to worsening of the mental health status and subsequently causing the necessity of an inpatient stay. The distribution of hospitalization peaks can be hypothesized to be influenced by photoperiod changes which hereby trigger worsening of symptom severity in patients with schizophrenia. A better understanding of the underlying factors influencing these seasonal patterns could help to identify subgroups of individuals with schizophrenia who are more prone to exacerbations due to seasonal variations and the development of specific markers of the disorder. Further research is warranted to identify underlying influencing factors and their mechanisms triggering seasonal patterns in patients with schizophrenia.
Financial Support
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
The authors report no conflict of interest.
Data Availability Statement
The data that support the finding of this study are available from Statistik, Austria (https://www.statistik.at/).
References
- [1].Cassidy F., Carroll B.J. Seasonal variation of mixed and pure episodes of bipolar disorder. J Affect Disord. 2002;68(1):25–31. [DOI] [PubMed] [Google Scholar]
- [2].Amr M., Volpe F.M. Seasonal influences on admissions for mood disorders and schizophrenia in a teaching psychiatric hospital in Egypt. J Affect Disord. 2012;137(1–3):56–60. [DOI] [PubMed] [Google Scholar]
- [3].Medici C.R., Hadzi-Pavlovic D., Munk-Jørgensen P. & Parker G. Seasonal variations in hospital admissions for mania: Examining for associations with weather variables over time. J Affect Disord. 2016;205:81–86. [DOI] [PubMed] [Google Scholar]
- [4].Fellinger M., Waldhoer T., König D., Hinterbuchinger B., Pruckner N., Baumgartner J. et al. Seasonality in bipolar disorder: effect of sex and age. J Affect Disord. 2019;243:322–326. [DOI] [PubMed] [Google Scholar]
- [5].Geoffroy P.A., Bellivier F., Scott J., Boudebesse C., Lajnef M., Gard S. et al. Bipolar disorder with seasonal pattern: clinical characteristics and gender influences. Chronobiol Int. 2013;30(9):1101–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].James S.L,. Abate D., Abate K.H., Abay S.M., Abbafati C., Abbasi N. et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pinel P. A treatise on insanity: In which are contained the principles of a new and more practical nosology of maniacal disorders than has yet been offered to the public. New York, NY: Hafner, 1806. [Google Scholar]
- [8].Esquirol E. Mental maladies. A treatise on insanity. Philadelphia, PA: Lea and Blanchard, 1845. [Google Scholar]
- [9].Tian W.H., Lee H.C., Liu T.C., Chen C.S. & Lin H.C. Seasonal variation in schizophrenia admissions in a Chinese population. Schizophr Res. 2006;86(1–3):333–334. [DOI] [PubMed] [Google Scholar]
- [10].Hare E.H., Walter S.D. Seasonal variation in admissions of psychiatric patients and its relation to seasonal variation in their births. J Epidemiol Community Health. 1978;32(1):47–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Takei N., O’Callaghan E., Sham P., Glover G., Tamura A. & Murray R. Seasonality of admissions in the psychoses: effect of diagnosis, sex, and age at onset. Br J Psychiatry. 1992;161:506–511. [DOI] [PubMed] [Google Scholar]
- [12].Shiloh R., Shapira A., Potchter O., Hermesh H., Popper M. & Weizman A. Effects of climate on admission rates of schizophrenia patients to psychiatric hospitals. Eur Psychiatry. 2005;20(1):61–64. [DOI] [PubMed] [Google Scholar]
- [13].McWilliams S., Kinsella A., O'Callaghan E. The effects of daily weather variables on psychosis admissions to psychiatric hospitals. Int J Biometeorol. 2013;57(4):497–508. [DOI] [PubMed] [Google Scholar]
- [14].Clarke M., Clarke M., Moran P., Keogh F., Morris M., Kinsella A., Larkin C. et al. Seasonal influences on admissions for affective disorder and schizophrenia in Ireland: a comparison of first and readmissions. Eur Psychiatry. 1999;14(5):251–255. [DOI] [PubMed] [Google Scholar]
- [15].Strous R.D., Pollack S., Robinson D., Sheitman B. & Lieberman J. A. Seasonal admission patterns in first episode psychosis, chronic schizophrenia, and nonschizophrenic psychoses. J Nerv Ment Dis. 2001;189(9):642–644. [DOI] [PubMed] [Google Scholar]
- [16].Volpe F.M., Tavares A, Del Porto J.A. Seasonality of three dimensions of mania: psychosis, aggression and suicidality. J Affect Disord. 2008;108(1–2):95–100. [DOI] [PubMed] [Google Scholar]
- [17].Gu S., Huang R., Yang J., Sun S., Xu Y., Zhang R. et al. Exposure-lag-response association between sunlight and schizophrenia in Ningbo, China. Environ Pollut. 2019;247:285–292. [DOI] [PubMed] [Google Scholar]
- [18].Davies G., Davies G., Ahmad F., Chant D., Welham J. & McGrath J. Seasonality of first admissions for schizophrenia in the Southern Hemisphere. Schizophr Res. 2000;41(3):457–462. [DOI] [PubMed] [Google Scholar]
- [19].Aviv A., Aviv A., Bromberg G., Baruch Y., Shapira Y. & Blass D. M. The role of environmental influences on schizophrenia admissions in Israel. Int J Soc Psychiatry. 2011;57(1):57–68. [DOI] [PubMed] [Google Scholar]
- [20].Hillard J.R., Holland J.M., Ramm D. Christmas and psychopathology: data from a psychiatric emergency room population. Arch Gen Psychiatry. 1981;38(12):1377–1381. [DOI] [PubMed] [Google Scholar]
- [21].Takei N., Murray R.M. Gender difference of schizophrenia in seasonal admissions in Scotland. Br J Psychiatry. 1993;162:272–273. [DOI] [PubMed] [Google Scholar]
- [22].Owens N., McGorry P.D. Seasonality of symptom onset in first-episode schizophrenia. Psychol Med. 2003;33(1):163–167. [DOI] [PubMed] [Google Scholar]
- [23].Martinez-Ortega J. M., Jurado D., Gutiérrez-Rojas L., Molero P., Ramos M. A. & Gurpegui, M. Stability of sex differences by diagnosis in psychiatric hospitalizations. Psychiatry Res. 2012;198(1):161–163. [DOI] [PubMed] [Google Scholar]
- [24].Low L.F., Draper B. Hospitalization patterns for psychiatric disorders across the lifespan in Australia from July 1998 to June 2005. Psychiatr Serv. 2009;60(1):113–116. [DOI] [PubMed] [Google Scholar]
- [25].Byrne E.M., Raheja U., Stephens S.H., Heath A.C., Madden P.A., Vaswani D. et al. Seasonality shows evidence for polygenic architecture and genetic correlation with schizophrenia and bipolar disorder. J Clin Psychiatry. 2015;76(2):128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Davies G., Welham J., Chant D., Torrey E.F. & McGrath J. A systematic review and meta-analysis of Northern Hemisphere season of birth studies in schizophrenia. Schizophr Bull. 2003;29(3):587–593. [DOI] [PubMed] [Google Scholar]
- [27].Bogers J., Bostoen T., Broekman T.G. Low levels of vitamin D poorly responsive to daylight exposure in patients with therapy-resistant schizophrenia. Nord J Psychiatry. 2016;70(4):262–266. [DOI] [PubMed] [Google Scholar]
- [28].van der Leeuw C., de Witte L. D., Stellinga A., van der Ley C., Bruggeman R., Kahn R. S.et al. Vitamin D concentration and psychotic disorder: associations with disease status, clinical variables and urbanicity. Psychol Med. 2019;1–7. doi:10.1017/S0033291719001739 [DOI] [PubMed] [Google Scholar]
- [29].Force R.W., Hansen L., Bedell M. Psychotic episode after melatonin. Ann Pharmacother. 1997;31(11):1408. [DOI] [PubMed] [Google Scholar]
- [30].Touitou Y., Fèvre M., Bogdan A., Reinberg A., De Prins J., Beck H., & Touitou C. Patterns of plasma melatonin with ageing and mental condition: stability of nyctohemeral rhythms and differences in seasonal variations. Eur J Endocrinol. 1984;106(2):145–151. [DOI] [PubMed] [Google Scholar]
- [31].Arendt J., Wirz-Justice A., Bradtke J. Annual rhythm of serum melatonin in man. Neurosci Lett. 1978;7(4):327–330. [DOI] [PubMed] [Google Scholar]
- [32].Anderson G., Maes M. Melatonin: an overlooked factor in schizophrenia and in the inhibition of anti-psychotic side effects. Metabol Brain Dis. 2012;27(2):113–119. [DOI] [PubMed] [Google Scholar]
- [33].Wang S, Zhang X., Xie M., Zhao D., Zhang H., Zhang Y. et al. Effect of increasing temperature on daily hospital admissions for schizophrenia in Hefei, China: a time-series analysis. Public Health. 2018;159:70–77. [DOI] [PubMed] [Google Scholar]
- [34].Wang X, Lavigne E., Ouellette-Kuntz H. & Chen B.E. Acute impacts of extreme temperature exposure on emergency room admissions related to mental and behavior disorders in Toronto, Canada. J Affect Disord. 2014;155:154–161. [DOI] [PubMed] [Google Scholar]
- [35].Tapak L, Maryanaji Z., Hamidi O., Abbasi H. & Najafi-Vosough R. Investigating the effect of climatic parameters on mental disorder admissions. Int J Biometeorol. 2018;62(12):2109–2118. [DOI] [PubMed] [Google Scholar]
- [36].ZAMG. Ephemeriden in Wien für das Jahr 2020. Vienna, Austria: Zentralanstalt für Meteorologie und Geodynamik, 2020. [Google Scholar]
- [37].Walker E, Kestler L., Bollini A., & Hochman K.M. Schizophrenia: etiology and course. Annu Rev Psychol. 2004;55:401–430. [DOI] [PubMed] [Google Scholar]
- [38].Kavanagh D., Tansey K.E., O’Donovan M. C. & Owen, M. J. Schizophrenia genetics: emerging themes for a complex disorder. Mol Psychiatry. 2015;20(1):72–76. [DOI] [PubMed] [Google Scholar]
- [39].Hall J, Hall J., Trent S., Thomas K.L., O’Donovan M. C. & Owen M.J. Genetic risk for schizophrenia: convergence on synaptic pathways involved in plasticity. Biol Psychiatry. 2015;77(1):52–58. [DOI] [PubMed] [Google Scholar]
- [40].Fusar-Poli P, Smieskova R., Serafini G., Politi P., & Borgwardt S. Neuroanatomical markers of genetic liability to psychosis and first episode psychosis: a voxelwise meta-analytical comparison. World J Biol Psychiatry. 2014;15(3):219–228. [DOI] [PubMed] [Google Scholar]
- [41].Serafini G, Pompili M., Haghighat R., Pucci D., Pastina M., Lester D. et al. Stigmatization of schizophrenia as perceived by nurses, medical doctors, medical students and patients. J Psychiatr Ment Health Nursing. 2011;18(7):576–585. [DOI] [PubMed] [Google Scholar]
- [42].Borrell-Carrió F., Suchman A.L., Epstein R.M. The biopsychosocial model 25 years later: principles, practice, and scientific inquiry. Ann Family Med. 2004;2(6):576–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Burns E.M., Rigby E., Mamidanna R., Bottle A., Aylin P., Ziprin P. & Faiz O.D. Systematic review of discharge coding accuracy. J Public Health (Oxf). 2012;34(1):138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Campbell SE, Campbell M. K., Grimshaw J. M. & Walker A. E. A systematic review of discharge coding accuracy. J Public Health Med. 2001;23(3):205–211. [DOI] [PubMed] [Google Scholar]
- [45].Davis K.A., Sudlow C.L., Hotopf M. Can mental health diagnoses in administrative data be used for research? A systematic review of the accuracy of routinely collected diagnoses. BMC Psychiatry. 2016;16:263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jakobsen K.D., Frederiksen, J.N., Hansen T., Jansson L.B., Parnas J. & Werge T. Reliability of clinical ICD-10 schizophrenia diagnoses. Nord J Psychiatry. 2005;59(3):209–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the finding of this study are available from Statistik, Austria (https://www.statistik.at/).