Abstract
Objectives
Although effective antiretroviral therapy (ART) has been used for more than two decades, HIV-associated neurocognitive disorder remains prevalent. Thus, whether ART can improve neurocognitive impairment is controversial. This review aims to explore the effects of ART on cognitive impairment in people living with HIV (PLWH).
Methods
A systematic literature search was conducted in eight databases (PubMed, Embase, Web of Science, Cochrane Library, CNKI, VIP, China Biology Medicine disc, and WanFang) to identify studies that compare cognitive function between study groups who are administered and not administered ART. We searched for articles published up to April 2019. Article evaluation and data extraction were independently conducted by two reviewers.
Results
Sixteen articles (6,694 participants)—14 cross-sectional studies and 2 cohort studies—were included in this meta-analysis. The cross-sectional studies demonstrated that ART group did not perform better than the non-ART group (OR = 1.16; 95% CI, 1.03–1.30). However, the cohort studies reported a significant improvement in cognitive function at three months (OR = 4.01; 95% CI, 2.35–6.85) and six months (OR = 9.24; 95% CI, 1.71–49.96) after ART initiation compared with the baseline data. No significant cognitive improvement was found in participants younger than 55 years old, but the two cross-sectional studies showed that ART may improve cognitive function in PLWH under 65 years old with poor physical condition and immune status.
Conclusions
ART could improve cognitive function in PLWH with poor physical condition and immune status, but it does not considerably improve cognition in the entire PLWH population.
Keywords: Anti-retroviral agents, Cognition, Cognitive dysfunction, HIV infections, Neurocognitive disorders
What is known?
-
•
HIV-associated neurocognitive impairment remains prevalent despite effective antiretroviral therapy.
-
•
HIV-associated neurocognitive impairment may be associated with viral factors, neuroinflammation, antiretroviral factors, mental health conditions, comorbid conditions, lifestyle factors, and aging.
-
•
The last meta-analysis showed that antiretroviral therapy was related to improvement in attention, executive function, and motor function, but neurocognitive improvement was not observed in other domains of cognitive function.
What is new?
-
•
This meta-analysis discussed the impact of antiretroviral therapy on overall cognitive function and explored the relationship between antiretroviral therapy and cognitive function in different age groups.
-
•
The meta-analysis of cross-sectional studies showed that people living with HIV who received antiretroviral therapy did not perform better than those who did not receive antiretroviral therapy. However, the meta-analysis of cohort studies revealed that antiretroviral therapy led to neurocognitive improvement in people living with HIV who had a severe HIV infection.
-
•
Antiretroviral therapy has positive impacts on the cognitive function of people living with HIV who are in poor physical condition and have poor immune status, but it does not improve cognitive function in other groups of people living with HIV.
1. Introduction
Since the introduction of antiretroviral therapy (ART), HIV infection has become a treatable chronic illness [1]. Early initiation of ART at an early stage of HIV infection suppresses virus replication, restores a normal level of CD4 cells, reduces the incidence of HIV-related diseases, and delays the progress of the disease [2,3]. However, despite the prolonged life expectancy among people living with HIV (PLWH), management of long-term complications, such as diseases in the cardiovascular, osteological, renal, and central nervous systems, remains a challenge in the healthcare industry [1]. HIV-associated neurocognitive disorder (HAND), a frequently used term for neurocognitive impairment (NCI) in PLWH, is a common disease of the central nervous system in PLWH [4]. It is divided into three sub-types: asymptomatic NCI, mild neurocognitive disorder, and HIV-associated dementia [4]. HAND is often reported even in PLWH who received effective treatment, and it is associated with reduced quality of life and increased mortality rate [5]. The prevalence of HAND varies from 20 to 69% depending on the population and how HAND is defined [[6], [7], [8], [9], [10], [11], [12], [13]]. Since the use of ART became widespread, the prevalence of HIV-associated dementia, along with other neurological complications, has decreased. However, the prevalence of asymptomatic NCI and mild neurocognitive disorder continue to increase, despite long-term virologic suppression [6,14,15].
Several factors may contribute to cognitive impairment, including viral factors, neuroinflammation, antiretroviral factors, mental health conditions, comorbid conditions, lifestyle factors, and aging [16,17]. HIV infects the nervous system [18], and the central nervous system acts as a sanctuary for HIV despite the effectiveness of ART [19]. ART drugs with a high central nervous system penetration effectiveness score (CPE), including zidovudine, nevirapine, indinavir-r, efavirenz, and lopinavir-r, may prevent and reverse HAND [1]. However, the toxicity of ART drugs may contribute to cognitive impairment. The risks of NCI increase with age and may be more notable in PLWH. Moreover, the interaction between aging and the impaired immune response caused by HIV leads to a premature and persistent inflammatory state that could accelerate cognitive changes [16,20,21]. The process of cognitive impairment is reflected by biomarkers, such as CD4 cell count [22,23].
Findings on whether ART improves cognitive function are inconsistent [24]. Most studies indicated that ART enhances cognitive function [[25], [26], [27], [28], [29]], but a few studies have suggested that ART exerts no effect on cognitive function [30,31]. Cysique and colleagues [25] found that 13.5–40.9% of PLWH with cognitive impairment exhibited cognitive improvement from the baseline to 48 weeks after initiating ART. Additionally, cognitive improvement had been found among neuro-asymptomatic PLWH during the first year after initiating ART [26]. A prospective study reported that the cognitive function of PLWH with severe baseline cognitive impairment improved significantly more than those who were less impaired after commencing ART [32]. PLWH who have low CD4 cell counts [33] and severely impaired immune systems [28] and who have been receiving ART for a short time period [29] demonstrated cognitive improvement after initiation of ART, while PLWH with good immune status [22] were less likely to show improvement in cognition after ART initiation. In another study, persistence of neuropsychological deficits was observed despite long-term ART in PLWH with neurocognitive impairment [30]. The reasons for this inconsistency in results are unclear and require further investigation.
Several reviews [17,[34], [35], [36], [37]] have failed to reveal the effects of ART on cognitive function in PLWH. Traditional reviews only support statistically significant results and use a vote-counting strategy as evidence of efficacy [38]. Two meta-analyses have been conducted, but one study focuses on improvements in different cognitive domains [38] while the other discusses only executive dysfunction in the era of modern ART [39]. Therefore, the purpose of this meta-analysis is to determine whether ART improves cognitive function in PLWH. To our knowledge, this work is first to explore whether ART improves cognitive function in general. Our hypotheses are as follows: (1) NCI prevalence is lower among PLWH on ART than among those off ART and (2) assuming that aging may cause damage to cognition, younger PLWH (age <55) in the ART group have better cognitive function than those in the non-ART group.
2. Methods
2.1. Search strategy
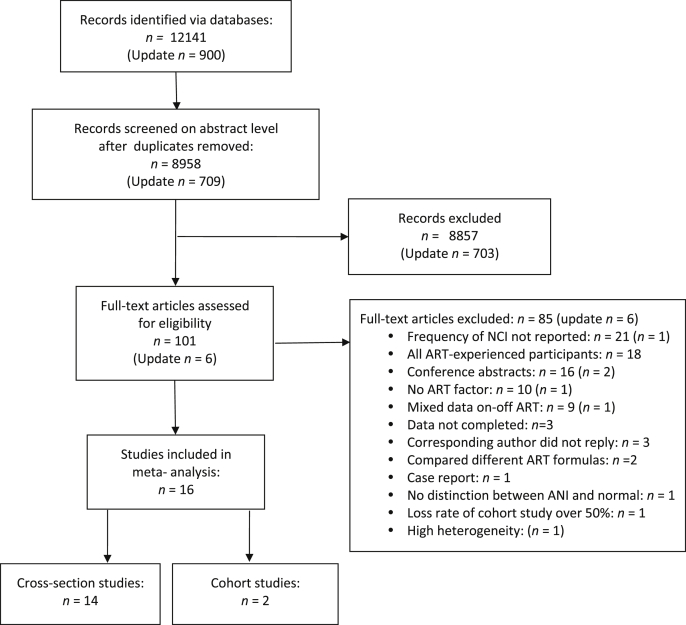
We conducted a systematic review of literature published in the PubMed, Embase, Web of Science, and Cochrane Library databases as well as Chinese databases such as CNKI, VIP, CBM, and WanFang. All records and articles published up to April 2019 were extracted. The following terms were searched in [all fields] with no language restriction: (‘HIV’ OR ‘Human immunodeficiency virus’ OR ‘Acquired immune deficiency syndrome’ OR ‘AIDS’ OR ‘HIV infections’ OR ‘Human immunodeficiency virus infection’) AND (‘highly active antiretroviral therapy’ OR ‘cART’ OR ‘ART’ OR ‘HAART’ OR ‘Antiretroviral therapy’) AND (‘Cognition’ OR ‘Cognition disorders’ OR ‘Cognitive disorders’ OR ‘Neurocognitive disorders’ OR ‘Cognitive impairment’ OR ‘Cognitive function’ OR ‘HIV-associated neurocognitive disorders’ OR ‘Neuropsychological functioning’ OR ‘Cognitive dysfunction’). These terms were adjusted according to the search rules of each database. Case reports, comments, letters, editorials, and animal experiments were excluded. Articles were extracted as text files and then imported into Endnote. After electronically and manually removing duplicate articles, one reviewer (Gao) screened all related titles and abstracts and excluded obviously unrelated articles. Two independent reviewers (Gao and Meng) evaluated the full texts of the related articles to assess their eligibility. Disagreements were solved by a third reviewer (Xiao). In addition, the reference lists of the eligible studies were manually searched to identify articles that might have been missed in the electronic search. We contacted the authors for more information when needed. Flow diagram of the article selection process for this meta-analysis was shown in Fig. 1.
Fig. 1.
Flow diagram of the article selection process.
2.2. Selection criteria
The studies eligible for inclusion were human studies comparing the cognitive function of PLWH before and after initiation of ART or comparing the cognitive function of PLWH receiving ART and those not receiving ART. Studies that used research tools that follow specific standards for NCI and reported the frequency of NCI for each group were included. We did not restrict studies based on the tools they used in the search terms in order to retrieve a broader set of studies. Studies were excluded if they used PLWH with an active or past central nervous system opportunistic infection or neurological comorbidities.
2.3. Data extraction
Data were extracted by one reviewer (Gao) and then checked by another reviewer (Meng) using a data extraction table. The following information were recorded: study characteristics, author/s, publication date, country, study design, setting, inclusion and exclusion of study population, sample size, age (range), sex, ART regimen, cut-off scores for defining NCI, and frequency of NCI in ART-naïve and ART-experienced groups. In addition, the follow-up month and frequency of NCI at each time point were recorded for cohort studies.
2.4. Quality assessment
Given that cohort and cross-sectional studies were selected, two quality assessment tools were used in this meta-analysis. One tool was the Newcastle-Ottawa Scale (NOS), which assesses the risk of bias in cohort and case–control studies [40]. A “star system” was developed to assess three aspects of studies: the selection of the study groups, the comparability of the groups, and ascertainment of either the exposure or outcome of interest for case–control or cohort studies. Studies with four to six stars on the NOS are of moderate quality, whereas studies with seven stars or more are of high quality [41]. The second tool was the Agency for Healthcare Research and Quality’s checklist of quality assessment criteria for cross-sectional studies [42]. Scores of 0–3, 4–7, and 8–11 represent low, moderate, and high quality, respectively. Two reviewers (Gao and Meng) assessed the quality of studies independently, and incongruities were discussed and resolved.
2.5. Statistical analysis
This meta-analysis was performed using Review Manager 5.3.5 for Windows. The dichotomous outcome (neurocognitive impairment: yes/no) was compared based on the odds ratio (OR) with a 95% confidence interval (CI). Heterogeneity was defined by a chi-squared test as P < 0.10 or I2 > 40% [43]. An I2 value of <40% indicates the absence of heterogeneity, in which case the fixed effects model was used [43]. In cases in which I2 > 40%, and thus heterogeneity exists, the random effects model was used. Subgroups were analyzed by age. When heterogeneity was detected, sensitivity analysis was performed by removing each study one by one and then reanalyzing the remaining studies. Funnel plots were used to detect potential publication bias.
3. Results
3.1. Characteristics of the selected studies
A total of 13,041 articles were identified from searching the databases. After removal of duplicates and articles deemed to be irrelevant based on their titles and abstracts, 107 publications were left. The results of the full-text review led to the exclusion of 91 articles. Thus, 16 articles were included in the meta-analysis. No additional articles were included based on the review of their references. Fig. 1 shows the process of selecting articles for this meta-analysis.
The 16 included articles—2 cohort [44,45] and 14 cross-sectional studies [[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]]—were published between 2002 and 2017. Table 1 shows the characteristics of the selected studies. A total of 6,694 PLWH were recruited, 61.03% of whom were males. These studies were conducted in Uganda (n = 3), Italy (n = 1), Malawi (n = 1), China (n = 2), Brazil (n = 2), USA (n = 2), Germany (n = 1), Ethiopia (n = 1), Indonesia (n = 1), Ireland (n = 1), and Western Europe and Canada (n = 1).
Table 1.
Characteristics of the selected studies.
|
Cohort studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, publication year, location |
Sample size, age (SD), sex |
Follow up month | Tools | Cut-off point | Frequency of NCI |
NOS | ||
| Time point | HIV + ART(−) NCI/total | HIV + ART(+) NCI/total | ||||||
| Sacktor 2006 Uganda |
23 32.8 (1.3) 100% male |
0; 3 months; 6 months |
IHDS; NP Karnofsky Performance Scales Memorial Sloan Kettering HIV dementia stage |
MSK HIV dementia stage ≥ 1 | baseline 3 months 6 months |
14/23 |
6/23 1/21 |
High |
| Nakasujja 2010 Uganda |
102 34.2 (6.2) 27.4% male |
0; 3 months; 6 months |
IHDS | IHDS ≤ 10 | baseline 3 months 6 months |
70/102 |
34/95 28/93 |
High |
|
Cross-sectional studies | ||||||||
|---|---|---|---|---|---|---|---|---|
| Author, publication year, location |
Sample size, Age (range) sex |
Tools | Cut-off point | Frequency of NCI between groups |
AHRQ |
|||
| HIV-positive ART(+) NCI/total |
HIV-positive ART(−) NCI/total |
|||||||
| Richardson 2002 USA |
149 36.7 (19–55) 100% female |
NP | Two or more test scores fall at least 1 SD below the mean of the control group | 30/82 | 36/67 | Moderate | ||
| Toozi 2005 Italy |
412 NR 71.1% male |
NP | 1 SD below the normative mean on at least 2 NP tests or 2 SDs below the mean in at least 1 test | 104/181 | 85/166 | Moderate | ||
| Patel 2010 Malawi |
179 36.7 (18–65) 35% male |
IHDS | IHDS ≤ 10 | 18/134 | 7/45 | Moderate | ||
| Zhang 2012 China |
134 37.9 56.7% male |
IHDS NP |
Based on NP and IHDS | 39/98 | 11/36 | Low | ||
| Sérgio Murilo 2012 Brazil |
52 57.6 59% male |
IHDS and two on MMSE (registration memory and attention) | IHDS < 10.5 and at least 1.5 SD below the mean of the external normative group on at least two of the five subscales | 17/49 | 2/3 | Moderate | ||
| Wang 2013 China |
309 34 88% male |
MoCA | MoCA < 26 | 114/236 | 35/73 | Moderate | ||
| Nakku 2013 Uganda |
618 NR 27.3% male |
IHDS | IHDS ≤ 10 | 259/399 | 137/219 | Moderate | ||
| Cross 2013 USA |
507 42 65% male |
IHDS | IHDS ≤ 10 | 160/380 | 49/127 | Moderate | ||
| Robertson 2014 Europe and Canada |
2863 42.9 (19–83) 61.7% male |
BNCS | A z-score of 1 SD below the mean in two tests or 2 SDs below the mean in one test | 836/1969 | 352/894 | Moderate | ||
| Pinheiro 2016 Brazil |
392 42.8 (18–82) 44.6% male |
Grooved Pegboard Test, Color Trails Tests 1 and 2, Finger Tapping Test, MoCA, and IHDS. | Scores in the upper quartile for at least three of the five measures and reach the IHDS cut-off value | 132/350 | 10/42 | Moderate | ||
| Marin-Webb 2016 Germany |
90 43 (23–62) 98.3% male |
IHDS | IHDS ≤ 10 | 28/80 | 2/10 | Moderate | ||
| Belete 2017 Ethiopia |
234 38.3 (18–64) 35.5 male |
IHDS | IHDS ≤ 9.5 | 75/206 | 3/28 | Moderate | ||
| Widyadharma 2017 Indonesia |
96 NR (15–49) 66.7% male |
MMSE | MMSE < 24 | 27/74 | 5/22 | Moderate | ||
| McNamara 2017 Ireland |
604 40.9 (18–77) 78.8% male |
BNCS | 1 SD below the population mean in two tests or 2 SDs below the population mean in one test | 262/504 | 46/95 | Moderate | ||
Note: IHDS, International HIV Dementia Scale; NP, neuropsychological test battery; MMSE, Mini-Mental State Examination; MoCA, Montreal Cognitive Assessment test; BNCS, Brief Neurocognitive Screen; NOS, Newcastle–Ottawa Scale; AHRQ, Agency for Healthcare Research and Quality checklist; NR, not reported.
The 14 cross-sectional studies recruited 6,569 PLWH, 4,742 of whom were PLWH on ART. Regarding the tools used to assess cognitive functions, five studies used the International HIV Dementia Scale (IHDS), three used a neuropsychological battery, two used the Brief Neurocognitive Screen (BNCS), one used the Montreal Cognitive Assessment test (MoCA), one used the Mini-Mental State Examination (MMSE), and two used multiple measures. These screening tools did not cause heterogeneity (I2 = 26%).
The two cohort studies included in this review were carried out in Uganda, and their selection criteria and the characteristics of participants were similar. However, different tools were used to define NCI; one study used IHDS, and the other used the Memorial Sloan Kettering Dementia Stage.
3.2. Association between ART and neurocognitive impairment
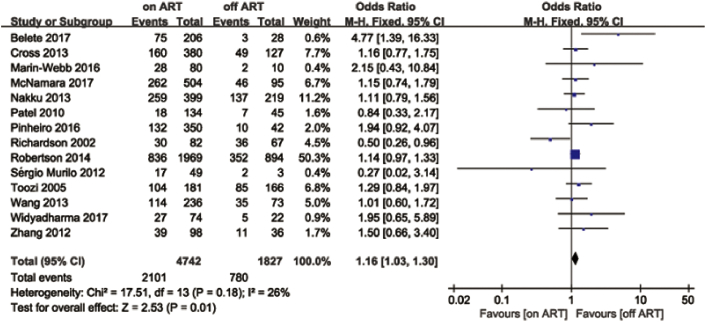
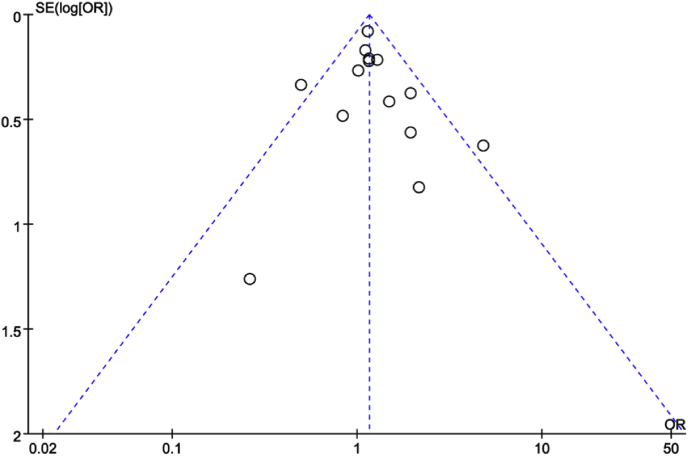
The meta-analysis of 14 cross-sectional studies [[46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59]] reporting the frequency of NCI in ART and non-ART groups revealed that PLWH on ART did not demonstrate better neurocognitive performance than PLWH off ART based on the fixed effects model (OR = 1.16; 95% CI, 1.03–1.30; Fig. 2). Heterogeneity (I2 = 26%) might not be important [43]. Two studies [46,54] were found to lie outside the 95% CI region in a funnel plot (Fig. 3), and I2 decreased from 26% to 0% after these two studies were discarded. However, the results remained similar; that is, no association was demonstrated between ART and the cognitive functions of PLWH (OR = 1.17; 95% CI, 1.04–1.31).
Fig. 2.
Forest plot showing the effects of ART on cognitive impairment compared with the non-ART group.
Fig. 3.
Funnel plot showing the publication bias of the 14 cross-sectional studies.
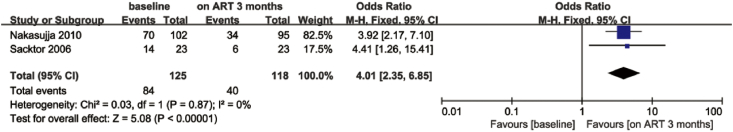
Two cohort studies [44,45] performed assessments at the baseline and at three and six months after ART initiation. Neurocognitive impairment was improved three months after ART initiation compared with the baseline (OR = 4.01; 95% CI, 2.35–6.85; Fig. 4). No heterogeneity (I2 = 0) was found, which may be attributed to the similarity in the inclusion and exclusion criteria of these two cohort studies. However, heterogeneity increased to 60% at 6 months. The cognitive improvement was statistically proved with a random model (OR = 9.24; 95%CI, 1.71–49.96; Fig. 5).
Fig. 4.
Forest plot showing the effects of ART on cognitive impairment before and three months after ART.
Fig. 5.
Forest plot showing the effects of ART on cognitive impairment before and six months after ART.
3.3. Relationship between ART and cognitive impairment in different age groups
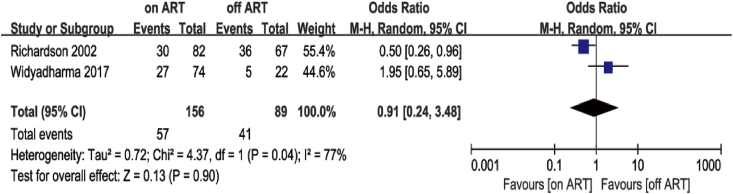
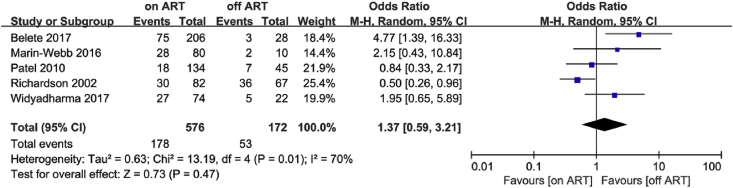
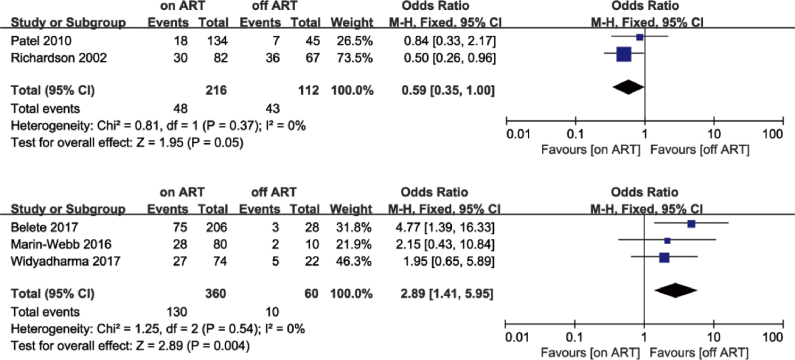
Based on the UNAIDS Report [60] and age stratification reported in the articles, we chose 55 and 65 years of age as the cut-off points. Only two studies met the <55 years of age criterion [54,58]. The meta-analysis showed no difference in cognitive impairment between the ART group and non-ART group in these studies (OR = 0.91; 95% CI, 0.24–3.48), with high heterogeneity (I2 = 77%; Fig. 6). Regarding the <65 years of age criterion, data pooled from five studies [46,49,52,54,58] showed similar results to those of the <55 group (OR = 1.37; 95% CI, 0.59–3.21; Fig. 7). A sensitivity analysis was performed because of the high heterogeneity (I2 = 70%). I2 decreased from 70% to 41% after removing Richardson’s study [54], but it did not change much after removing other studies. In Richardson’s study, 27.5% of PLWH had CD4 cell counts of less than 200, and 65.1% had a medium or high level of prior drug use (marijuana, cocaine, crack cocaine, heroin, or alcohol). We believe that the poor physical condition and immune status might explain the reduction in I2. The PLWH in Patel’s study [52] were also in poor physical condition; most PLWH (89.9%) were in WHO stage 3 or 4. Thus, we separated these two studies from the other three and then conducted separate meta-analyses. The analysis of the three articles [46,49,58] indicated that the ART group did not perform better than the non-ART group (OR = 2.89; 95% CI, 1.41–5.95), whereas the analysis of the two studies [52,54] revealed that the ART group performed better than the non-ART group (OR = 0.59; 95% CI, 0.35–1.00; Fig. 8).
Fig. 6.
Forest plot comparing the effects of ART on cognitive impairment in the ART group and non-ART group, age <55.
Fig. 7.
Forest plot comparing the effects of ART on cognitive impairment in the ART group and non-ART group, age <65.
Fig. 8.
Forest plot comparing the effects of ART on cognitive impairment in the ART group and non-ART group, age <65, grouping.
4. Discussion
This meta-analysis of cohort studies revealed a significant neurocognitive improvement in individuals with advanced HIV infection who have initiated ART. However, the results for the cross-sectional studies showed that ART groups did not perform better than non-ART groups. Two articles in the <65 years of age group showed that PLWH on ART performed better than PLWH off ART, but in the <55 years of age group, ART had no marked effects on cognitive improvement in either the ART or non-ART groups.
Cognitive improvement generally increased within six months following initiation of ART, which is partly consistent with results of a cohort study that assessed changes in different cognitive domains among PLWH with cognitive impairment [25]. The participants involved in the selected cohort studies were ART-naïve, had poor immune status and physical condition, and had CD4 lymphocyte counts of less than 200 cell/μl. This suggests that initiation of ART had significant benefits on the cognitive function of PLWH with advanced HIV infection. Another cohort study reported that ART enhanced cognitive improvement in neuro-asymptomatic PLWH [26]. Improvements in cognitive function in PLWH who initiated ART may be associated with reduction of HIV viremia [25] and/or recovery of cerebral synaptodendritic injury [61]. However, the CD4 lymphocyte counts of PLWH in all the above-mentioned cohort studies were less than 300 cell/μl. WHO recommended that ART be provided to all PLWH, regardless of their CD4 level, in 2015, and as a result, China started to provide free ART to all PLWH [62]. Recent studies have demonstrated that early initiation of ART might reduce severe AIDS-related and non-AIDS-related events and death while improving the quality of life of PLWH whose CD4 count is more than 500 cell/μl [[63], [64], [65]]. Our results may not be applicable to PLWH with CD4 lymphocyte counts of more than 300 cell/μl. Also, the impacts of early ART initiation on cognitive function remain to be examined. Future studies should pay more attention to the effects of ART on cognition among PLWH who initiate ART and who have a high CD4 cell count. Furthermore, cohort studies should demonstrate how ART affects cognition during a long follow-up period.
The meta-analysis of 14 cross-sectional studies showed that ART had little improvement of the cognitive function among PLWH, consistent with a previous study [38]. There may be several reasons for this result. First, some of the included cross-sectional studies showed that ART was not associated with neurocognitive impairment [47,48]. Although the cross-sectional studies used the same inclusion criteria to recruit PLWH on or off ART, whether the demographic and clinical characteristics of both groups were comparable is unknown. Second, some of the studies were not specifically designed to examine the effects of ART on cognition, and because we extracted certain parts of data for the purpose of this review, we might have introduced some confounding factors, making the results difficult to explain [56,59]. Third, important variables, such as medical adherence and history of ART, that are correlated with the effects of ART on cognitive function were not considered in our analysis, which might have biased our findings. Finally, the different tools used to assess cognitive impairment may have caused some bias. Future cross-sectional studies should discuss the relationship between ART and cognitive impairment and use a rigorous scientific research design to control for confounding variables.
Two articles showed that, of PLWH under 65 years of age, those on ART had better cognitive function than those off ART. However, ART had no significant effect on cognitive function among PLWH younger than 55 years. In studies that showed better performance among the ART group, the examined PLWH had severe HIV infection and were in poor physical condition. In contrast, in the studies showing that ART had no significant benefits, participants had a high mean current CD4 count (554); 89% were on ART (mean CPE score = 7.25) [49]; 86.8% were at WHO stage 1 or 2 [46]; and 77.08% were on ART and did not exhibit risk factors for cognitive impairment, such as hypertension, diabetes, heart diseases, smoking, and dyslipidemia [58]. These findings align with the results of our cohort studies. One previous study suggested that cognitive outcomes were linked to immune system integrity [38]. However, severe HIV infection and poor physical condition are associated with various factors, so we cannot identify which of these factors play a role in cognitive outcomes in this meta-analysis. Given the small sample size and uneven number of participants in each age group, the findings warrant further investigation. In addition, we were unable to show the influence of ART on cognition among different age groups because of the limited data available for this analysis. Future studies may fill this gap. Moreover, Paddick [66] found that neurocognitive impairment is less associated with HIV infection, and its influencing factors are more similar to those associated with neurodegenerative dementia in PLWH aged 50 and above. In addition, HAND is more persistent and severe in older PLWH [67]. In 2013, an estimated 3.6 million PLWH were aged 50 years or older was 3.6 million. Thus, further studies should pay more attention to this population and figure out better ways to meet their needs.
This meta-analysis has some limitations, despite the great efforts made to address them. First, the selected cohort studies were conducted in Uganda, and the participants had poor cognitive and bodily function. Thus, caution must be taken when generalizing our findings to other populations. Second, several confounding factors may influence the cognitive function of PLWH, such as age, comorbidities, ART regimen, and immune status. We attempted to perform subgroup analyses of these factors, but they were limited by insufficient data. Finally, the different tools used in the studies have distinct sensitivities and specificities, which may have caused bias.
5. Conclusion
This meta-analysis showed that ART could improve cognitive function in PLWH in cohort studies, but cross-sectional studies did not find that ART had considerable effects on the cognitive function of PLWH. Since early ART has been strongly recommended in recent years [68,69], more studies should focus on determining whether ART improves cognitive function. In the future, more studies should identify the relationship between ART and cognitive function by considering other risk and confounding factors.
Author statement
Chang Gao: Conceptualization, Methodology, Data curation, Investigation, Writing - Original draft. Jingjing Meng: Data curation, Investigation. Xueling Xiao: Conceptualization, Data curation, Writing - Original draft. Min Wang: Supervision. Ann Williams: Supervision. Honghong Wang: Writing-Review and Editing.
Funding
None.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Footnotes
Peer review under responsibility of Chinese Nursing Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2020.03.007.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Elbirt D., Mahlab-Guri K., Bezalel-Rosenberg S., Gill H., Attali M., Asher I. HIV-associated neurocognitive disorders (HAND) Isr Med Assoc J. 2015;17(1):54–59. [PubMed] [Google Scholar]
- 2.Mills E.J., Bakanda C., Birungi J., Chan K., Ford N., Cooper C.L. Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Ann Intern Med. 2011;155(4):209–216. doi: 10.7326/0003-4819-155-4-201108160-00358. [DOI] [PubMed] [Google Scholar]
- 3.Danel C., Moh R., Gabillard D., Badje A., Le Carrou J., Ouassa T. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N Engl J Med. 2015;373(9):808–822. doi: 10.1056/NEJMoa1507198. [DOI] [PubMed] [Google Scholar]
- 4.Antinori A., Arendt G., Becker J.T., Brew B.J., Byrd D.A., Cherner M. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69(18):1789–1799. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mora-Peris B., Stevens E., Ferretti F., Underwood J., Taylor S., Winston A. Evolution of changes in cognitive function after the initiation of antiretroviral therapy. AIDS Res Ther. 2016;13:20. doi: 10.1186/s12981-016-0104-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heaton R.K., Clifford D.B., Franklin D.R., Jr., Woods S.P., Ake C., Vaida F. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simioni S., Cavassini M., Annoni J.M., Rimbault Abraham A., Bourquin I., Schiffer V. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24(9):1243–1250. doi: 10.1097/QAD.0b013e3283354a7b. [DOI] [PubMed] [Google Scholar]
- 8.Cysique L.A., Maruff P., Brew B.J. Prevalence and pattern of neuropsychological impairment in human immunodeficiency virus-infected/acquired immunodeficiency syndrome (HIV/AIDS) patients across pre- and post-highly active antiretroviral therapy eras: a combined study of two cohorts. J Neurovirol. 2004;10(6):350–357. doi: 10.1080/13550280490521078. [DOI] [PubMed] [Google Scholar]
- 9.McArthur J.C., Haughey N., Gartner S., Conant K., Pardo C., Nath A. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9(2):205–221. doi: 10.1080/13550280390194109. [DOI] [PubMed] [Google Scholar]
- 10.Robertson K., Liner J., Hakim J., Sankalé J.L., Grant I., Letendre S. NeuroAIDS in Africa. J Neurovirol. 2010;16(3):189–202. doi: 10.3109/13550284.2010.489597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wright E.J., Nunn M., Joseph J., Robertson K., Lal L., Brew B.J. NeuroAIDS in the Asia Pacific region. J Neurovirol. 2008;14(6):465–473. doi: 10.1080/13550280802235932. [DOI] [PubMed] [Google Scholar]
- 12.Sacktor N., Lyles R.H., Skolasky R., Kleeberger C., Selnes O.A., Miller E.N. HIV-associated neurologic disease incidence changes: multicenter AIDS Cohort Study, 1990-1998. Neurology. 2001;56(2):257–260. doi: 10.1212/wnl.56.2.257. [DOI] [PubMed] [Google Scholar]
- 13.Robertson K.R., Smurzynski M., Parsons T.D., Wu K., Bosch R.J., Wu J. The prevalence and incidence of neurocognitive impairment in the HAART era. AIDS. 2007;21(14):1915–1921. doi: 10.1097/QAD.0b013e32828e4e27. [DOI] [PubMed] [Google Scholar]
- 14.Heaton R.K., Franklin D.R., Ellis R.J., McCutchan J.A., Letendre S.L., Leblanc S. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saylor D., Dickens A.M., Sacktor N., Haughey N., Slusher B., Pletnikov M. HIV-associated neurocognitive disorder – pathogenesis and prospects for treatment. Nat Rev Neurol. 2016;12(4):234–248. doi: 10.1038/nrneurol.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nightingale S., Winston A. Measuring and managing cognitive impairment in HIV. AIDS. 2017;31(Suppl 2) doi: 10.1097/QAD.0000000000001402. S165–72. [DOI] [PubMed] [Google Scholar]
- 17.Winston A., Vera J.H. Can antiretroviral therapy prevent HIV-associated cognitive disorders? Curr Opin HIV AIDS. 2014;9(1):11–16. doi: 10.1097/COH.0000000000000016. [DOI] [PubMed] [Google Scholar]
- 18.Navia B.A., Jordan B.D., Price R.W. The AIDS dementia complex: I. clinical features. Ann Neurol. 1986;19(6):517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 19.Pialoux G., Fournier S., Moulignier A., Poveda J.D., Clavel F., Dupont B. Central nervous system as a sanctuary for HIV-1 infection despite treatment with zidovudine, lamivudine and indinavir. AIDS. 1997;11(10):1302–1303. doi: 10.1097/00002030-199710001-00009. [DOI] [PubMed] [Google Scholar]
- 20.Nasi M., De Biasi S., Gibellini L., Bianchini E., Pecorini S., Bacca V. Ageing and inflammation in patients with HIV infection. Clin Exp Immunol. 2017;187(1):44–52. doi: 10.1111/cei.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kamkwalala A., Newhouse P. Mechanisms of cognitive aging in the HIV-positive adult. Curr Behav Neurosci Rep. 2017;4(3):188–197. doi: 10.1007/s40473-017-0122-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garvey L., Surendrakumar V., Winston A. Low rates of neurocognitive impairment are observed in neuro-asymptomatic HIV-infected subjects on effective antiretroviral therapy. HIV Clin Trials. 2011;12(6):333–338. doi: 10.1310/hct1206-333. [DOI] [PubMed] [Google Scholar]
- 23.Ellis R.J., Badiee J., Vaida F., Letendre S., Heaton R.K., Clifford D. CD4 nadir is a predictor of HIV neurocognitive impairment in the era of combination antiretroviral therapy. AIDS. 2011;25(14):1747–1751. doi: 10.1097/QAD.0b013e32834a40cd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nightingale S., Winston A., Letendre S., Michael B.D., McArthur J.C., Khoo S. Controversies in HIV-associated neurocognitive disorders. Lancet Neurol. 2014;13(11):1139–1151. doi: 10.1016/S1474-4422(14)70137-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cysique L.A., Vaida F., Letendre S., Gibson S., Cherner M., Woods S.P. Dynamics of cognitive change in impaired HIV-positive patients initiating antiretroviral therapy. Neurology. 2009;73(5):342–348. doi: 10.1212/WNL.0b013e3181ab2b3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winston A., Puls R., Kerr S.J., Duncombe C., Li P.C., Gill J.M. Dynamics of cognitive change in HIV-infected individuals commencing three different initial antiretroviral regimens: a randomized, controlled study. HIV Med. 2012;13(4):245–251. doi: 10.1111/j.1468-1293.2011.00962.x. [DOI] [PubMed] [Google Scholar]
- 27.Sacktor N., Nakasujja N., Okonkwo O., Skolasky R.L., Robertson K., Musisi S. Longitudinal neuropsychological test performance among HIV seropositive individuals in Uganda. J Neurovirol. 2013;19(1):48–56. doi: 10.1007/s13365-012-0139-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen R.A., Boland R., Paul R., Tashima K.T., Schoenbaum E.E., Celentano D.D. Neurocognitive performance enhanced by highly active antiretroviral therapy in HIV-infected women. AIDS. 2001;15(3):341–345. doi: 10.1097/00002030-200102160-00007. [DOI] [PubMed] [Google Scholar]
- 29.Robertson K.R., Robertson W.T., Ford S., Watson D., Fiscus S., Harp A.G. Highly active antiretroviral therapy improves neurocognitive functioning. J Acquir Immune Defic Syndr. 2004;36(1):562–566. doi: 10.1097/00126334-200405010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Tozzi V., Balestra P., Bellagamba R., Corpolongo A., Salvatori M.F., Visco-Comandini U. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45(2):174–182. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 31.Marra C.M., Zhao Y., Clifford D.B., Letendre S., Evans S., Henry K. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23(11):1359–1366. doi: 10.1097/QAD.0b013e32832c4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joska J.A., Westgarth-Taylor J., Hoare J., Thomas K.G., Paul R., Myer L. Neuropsychological outcomes in adults commencing highly active anti-retroviral treatment in South Africa: a prospective study. BMC Infect Dis. 2012;12:39. doi: 10.1186/1471-2334-12-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.De Ronchi D., Faranca I., Berardi D., Scudellari P., Borderi M., Manfredi R. Risk factors for cognitive impairment in HIV-1-infected persons with different risk behaviors. Arch Neurol. 2002;59(5):812–818. doi: 10.1001/archneur.59.5.812. [DOI] [PubMed] [Google Scholar]
- 34.Liner K.J., II, Hall C.D., Robertson K.R. Effects of antiretroviral therapy on cognitive impairment. Curr HIV AIDS Rep. 2008;5(2):64–71. doi: 10.1007/s11904-008-0011-7. [DOI] [PubMed] [Google Scholar]
- 35.Cysique L.A., Brew B.J. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev. 2009;19(2):169–185. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- 36.Joska J.A., Gouse H., Paul R.H., Stein D.J., Flisher A.J. Does highly active antiretroviral therapy improve neurocognitive function? A systematic review. J Neurovirol. 2010;16(2):101–114. doi: 10.3109/13550281003682513. [DOI] [PubMed] [Google Scholar]
- 37.Liner K.J., II, Ro M.J., Robertson K.R. HIV, antiretroviral therapies, and the brain. Curr HIV AIDS Rep. 2010;7(2):85–91. doi: 10.1007/s11904-010-0042-8. [DOI] [PubMed] [Google Scholar]
- 38.Al-Khindi T., Zakzanis K.K., van Gorp W.G. Does antiretroviral therapy improve HIV-associated cognitive impairment? A quantitative review of the literature. J Int Neuropsychol Soc. 2011;17(6):956–969. doi: 10.1017/S1355617711000968. [DOI] [PubMed] [Google Scholar]
- 39.Walker K.A., Brown G.G. HIV-associated executive dysfunction in the era of modern antiretroviral therapy: a systematic review and meta-analysis. J Clin Exp Neuropsychol. 2018;40(4):357–376. doi: 10.1080/13803395.2017.1349879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wells G., Shea B., O’Connell D., Peterson J., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [Internet]. Ottawa Hospital Research Institute. Available from:
- 41.Dinu M., Abbate R., Gensini G.F., Casini A., Sofi F. Vegetarian, vegan diets and multiple health outcomes: a systematic review with meta-analysis of observational studies. Crit Rev Food Sci Nutr. 2017;57(17):3640–3649. doi: 10.1080/10408398.2016.1138447. [DOI] [PubMed] [Google Scholar]
- 42.Rostom A., Dube C., Cranney A., Saloojee N., Sy R., Garritty C. Agency for Healthcare Research and Quality; 2004 Sep. Celiac disease. Evidence reports/technology assessments, No. 104.http://www.ncbi.nlm.nih.gov/books/NBK35156 Available from: [Google Scholar]
- 43.Higgins J.P., Green S. Wiley Online Library; 2008. Cochrane handbook for systematic reviews of interventions: Cochrane book series.https://onlinelibrary.wiley.com/doi/book/10.1002/9780470712184 [Internet] Available from: [Google Scholar]
- 44.Nakasujja N., Skolasky R.L., Musisi S., Allebeck P., Robertson K., Ronald A. Depression symptoms and cognitive function among individuals with advanced HIV infection initiating HAART in Uganda. BMC Psychiatr. 2010;10:44. doi: 10.1186/1471-244X-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sacktor N., Nakasujja N., Skolasky R., Robertson K., Wong M., Musisi S. Antiretroviral therapy improves cognitive impairment in HIV+ individuals in sub-Saharan Africa. Neurology. 2006;67(2):311–314. doi: 10.1212/01.wnl.0000225183.74521.72. [DOI] [PubMed] [Google Scholar]
- 46.Belete T., Medfu G., Yemiyamrew E. Prevalence of HIV associated neurocognitive deficit among HIV positive people in Ethiopia: a cross sectional study at Ayder Referral Hospital. Ethiop J Health Sci. 2017;27(1):67–76. doi: 10.4314/ejhs.v27i1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cross S., Önen N., Gase A., Overton E.T., Ances B.M. Identifying risk factors for HIV-associated neurocognitive disorders using the international HIV dementia scale. J Neuroimmune Pharmacol. 2013;8(5):1114–1122. doi: 10.1007/s11481-013-9505-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandes Filho S.M., De Melo H.R. Frequency and risk factors for HIV-associated neurocognitive disorder and depression in older individuals with HIV in northeastern Brazil. Int Psychogeriatr. 2012;24(10):1648–1655. doi: 10.1017/S1041610212000944. [DOI] [PubMed] [Google Scholar]
- 49.Marin-Webb V., Jessen H., Kopp U., Jessen A.B., Hahn K. Validation of the International HIV Dementia Scale as a screening tool for HIV-associated neurocognitive disorders in a German-speaking HIV outpatient clinic. PloS One. 2016;11(12) doi: 10.1371/journal.pone.0168225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McNamara P.H., Coen R., Redmond J., Doherty C.P., Bergin C. A high prevalence rate of a positive screen for cognitive impairment in patients with human immunodeficiency virus attending an Irish clinic. Open Forum Infect Dis. 2017;4(1):ofw242. doi: 10.1093/ofid/ofw242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nakku J., Kinyanda E., Hoskins S. Prevalence and factors associated with probable HIV dementia in an African population: a cross-sectional study of an HIV/AIDS clinic population. BMC Psychiatr. 2013;13:126. doi: 10.1186/1471-244X-13-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel V.N., Mungwira R.G., Tarumbiswa T.F., Heikinheimo T., van Oosterhout J.J. High prevalence of suspected HIV-associated dementia in adult Malawian HIV patients. Int J STD AIDS. 2010;21(5):356–358. doi: 10.1258/ijsa.2010.009554. [DOI] [PubMed] [Google Scholar]
- 53.Pinheiro C.A., Souza L.D., Motta J.V., Kelbert E.F., Souza M.S., Martins C.S. Depression and diagnosis of neurocognitive impairment in HIV-positive patients. Braz J Med Biol Res. 2016;49(10):e5344. doi: 10.1590/1414-431X20165344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Richardson J.L., Martin E.M., Jimenez N., Danley K., Cohen M., Carson V.L. Neuropsychological functioning in a cohort of HIV infected women: importance of antiretroviral therapy. J Int Neuropsychol Soc. 2002;8(6):781–793. doi: 10.1017/S1355617702860064. [DOI] [PubMed] [Google Scholar]
- 55.Robertson K., Bayon C., Molina J.M., McNamara P., Resch C., Muñoz-Moreno J.A. Screening for neurocognitive impairment, depression, and anxiety in HIV-infected patients in Western Europe and Canada. AIDS Care. 2014;26(12):1555–1561. doi: 10.1080/09540121.2014.936813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tozzi V., Balestra P., Serraino D., Bellagamba R., Corpolongo A., Piselli P. Neurocognitive impairment and survival in a cohort of HIV-infected patients treated with HAART. AIDS Res Hum Retrovir. 2005;21(8):706–713. doi: 10.1089/aid.2005.21.706. [DOI] [PubMed] [Google Scholar]
- 57.Wang Z., Zheng Y., Liu L., Shen Y., Zhang R., Wang J. High prevalence of HIV-associated neurocognitive disorder in HIV-infected patients with a baseline CD4 count ≤350 cells/μl in Shanghai, China. Biosci Trends. 2013;7(6):284–289. doi: 10.5582/bst.2013.v7.6.284. [DOI] [PubMed] [Google Scholar]
- 58.Widyadharma E., Satiti S., Nuradyo D., Setyopranoto I., Wijayanti Y. The difference of CD4 count between HIV positive patients with cognitive decline and without cognitive decline. Biomed Pharmacol J. 2017;10(2):969–978. doi: 10.13005/bpj/1192. [DOI] [Google Scholar]
- 59.Zhang Y., Qiao L., Ding W., Wei F., Zhao Q., Wang X. An initial screening for HIV-associated neurocognitive disorders of HIV-1 infected patients in China. J Neurovirol. 2012;18(2):120–126. doi: 10.1007/s13365-012-0089-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.UNAIDS HIV and aging: a special supplement to the UNAIDS report on the global AIDS epidemic. 2013. http://www.unaids.org/en/resources/documents/2013/20131101_JC2563_hiv-and-aging [Internet]. Available from:
- 61.Everall I.P., Heaton R.K., Marcotte T.D., Ellis R.J., McCutchan J.A., Atkinson J.H. Cortical synaptic density is reduced in mild to moderate human immunodeficiency virus neurocognitive disorder. HNRC Group. HIV Neurobehavioral Research Center. Brain Pathol. 1999;9(2):209–217. doi: 10.1111/j.1750-3639.1999.tb00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang X., Wang Z., Harrison S., Lau J. Coverage and adherence of antiretroviral therapy among Chinese HIV-positive men who have sex with men with high CD4 counts in the era of “treat all”. Trop Med Int Health. 2019 doi: 10.1111/tmi.13353. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 63.Rodger A.J., Sabin C.A. How have guidelines on when to start antiretroviral therapy affected survival of people living with HIV infection? Curr Opin HIV AIDS. 2016;11(5):487–491. doi: 10.1097/COH.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 64.Lundgren J.D., Babiker A.G., Gordin F., Emery S., Grund B., Sharma S. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. 2015;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lifson A.R., Grund B., Gardner E.M., Kaplan R., Denning E., Engen N. Improved quality of life with immediate versus deferred initiation of antiretroviral therapy in early asymptomatic HIV infection. AIDS. 2017;31(7):953–963. doi: 10.1097/QAD.0000000000001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paddick S., Flatt A., Eaton P., Kellet-Wright J., Duijinmaijer A., Urasa S. Prevalence and risk factors for HIV-associated neurocognitive impairment (HAND) amongst adults aged 50 and over attending a HIV clinic in north Tanzania. J Neurol Neurosurg Psychiatry. 2017;88:A19. doi: 10.1136/jnnp-2017-BNPA.40. [DOI] [Google Scholar]
- 67.Mackiewicz M.M., Overk C., Achim C.L., Masliah E. Pathogenesis of age-related HIV neurodegeneration. J Neurovirol. 2019;25(5):622–633. doi: 10.1007/s13365-019-00728-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ford N., Migone C., Calmy A., Kerschberger B., Kanters S., Nsanzimana S. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS. 2018;32(1):17–23. doi: 10.1097/QAD.0000000000001671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.WHO Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2013. https://www.who.int/hiv/pub/guidelines/arv2013/download/en/ [Internet], Available from: [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.