Abstract
Objective
To investigate the effects of mirror neuron theory-based visual feedback therapy (VFT) on restoration of upper limb function of stroke patients and motor-related cortical function using functional magnetic resonance imaging (fMRI).
Methods
Hemiplegic stroke patients were randomly divided into two groups: a VFT group and a control (CTL) group. Sixteen patients in the VFT group received conventional rehabilitation (CR) and VFT for 8 weeks, while 15 patients in the CTL group received only CR. The Barthel Index (BI) was used to assess the activities of daily living at baseline and the 8th week of the recovery training period. The Fugl–Meyer assessment (FMA) scale, somatosensory evoked potential (SEP), and fMRI were used to evaluate the recovery effect of the training therapies. The latencies and amplitudes of N9 and N20 were measured. Before recovery training, fMRI was performed for all patients in the VFT and CTL groups. In addition, 17 patients (9 in the VFT group and 8 in the CTL group) underwent fMRI for follow-up 2 months after treatment. Qualitative data were analyzed using the χ2 test. The independent sample t-test was used to compare normally distributed data among different groups, the paired sample t-test was used to compare data between groups, and the non-parametric test was used to comparing data without normal distribution among groups.
Results
There were no significant differences between the VFT and CTL group in all indexes. However, after 8 weeks of recovery training, these indexes were all significantly improved (P < 0.05). As compared with the CTL group, the FMA scores, BI, and N9/N20 latencies and amplitudes of SEP in the VFT group were significantly improved (P < 0.05). Two months after recovery training, fMRI showed that the degree of activation of the bilateral central anterior gyrus, parietal lobe, and auxiliary motor areas was significantly higher in the VFT group than the CTL group (P < 0.05).
Conclusions
VFT based on mirror neuron theory is an effective approach to improve upper extremity motor function and daily activity performance of stroke patients. The therapeutic mechanism promotes motor relearning by activating the mirror neuron system and motor cortex. SEP amplitudes increased only for patients who participated in visual feedback. VFT promotes sensory-motor plasticity and behavioral changes in both the motor and sensory domains.
Keywords: Activities of daily living, Action execution, Mirror neurons, Motor cortex, Rehabilitation, Somatosensory evoked potentials, Stroke, Visual feedback
What is known?
-
•
Upper extremity function loss is a major sequel of stroke. An influential idea in neuroscience is that the sensory-motor system is activated when visual feedback therapy(VFT). This idea has recently been extended to motor learning, in which observation results in sensory-motor plasticity and behavioral changes in both motor and somatosensory domains. However, it is unclear how the brain maps visual information onto motor circuits for learning.
What is new?
-
•
This paper gives a mirror neuron theory-based visual feedback therapy (VFT) is a therapy method for functional restoration of upper extremity. Watching patients movement activates brain networks involved in producing movement. VFT in motor learning provide evidence that the somatosensory system, especially primary somatosensory cortex, is involved in motor learning by observing visual feedback.
1. Introduction
Stroke is a major cause of long-term disability in adults, as more than 50% of surviving stroke patients have left upper limb dysfunction [1]. Over the last decade, visual feedback therapy (VFT) has been used as an optional or add-on therapy for the rehabilitation of patients with neurological disorders. VFT was developed based on the discovery and role of the mirror neuron system in motor learning to enhance the effects of upper limb training [2,3]. During VFT, patients are instructed to observe and imitate the actions portrayed in a video [4]. An increasing number of randomized controlled trials and review studies have reported that VFT improved upper limb function of stroke patients. However, the treatment protocols and outcome measures differed among these studies. Motor cortical activity can be modified by observing and matching the actions performed, which is known as observational learning, for both explicitly and implicitly acquired motor skills. The specific mirror activation of the movement representations included in a motor task is triggered as a result of actions both observed and executed [5,6].
Some studies have identified VFT associations in the human brain. Functional neuroimaging studies have demonstrated that VFT activates a network of cortical regions, incorporating the superior and inferior parietal cortices, the ventral premotor cortex, primary motor cortex, dorsal premotor cortex, inferior frontal gyrus, and supplementary motor areas [7]. The potential mirror mechanisms result in comparable activation of motor or motor-related cortical networks when individuals are observing or conducting an identical action. Activation of this neural system by VFT enhances motor skill acquisition of the observer. A possible mechanism of this skill-enhancing effect of VFT might be long-term potentiation, such as plasticity of the respective regions, which is suggested to be promoted by task-related motor cortex activities and excitability enhancements. However, it is unclear how the brain maps visual information onto motor circuits for learnig. Here, we test the idea that the somatosensory system and, specifically, the primary somatosensory cortex (S1), plays a role in motor learning by VFT [8]. S1 cortical processing was assessed before and after observation by measuring somatosensory evoked potentials (SEPs) associated with median nerve stimulation. SEP amplitudes increased only for participants who observed learning. In this study, the Fugl–Meyer assessment (FMA) scale, Barthel index (BI), SEP, and functional magnetic resonance imaging (fMRI) were employed to assess the effect of VFT on restoration of upper limb function of stroke patients.
2. Methods
2.1. Study approval and design
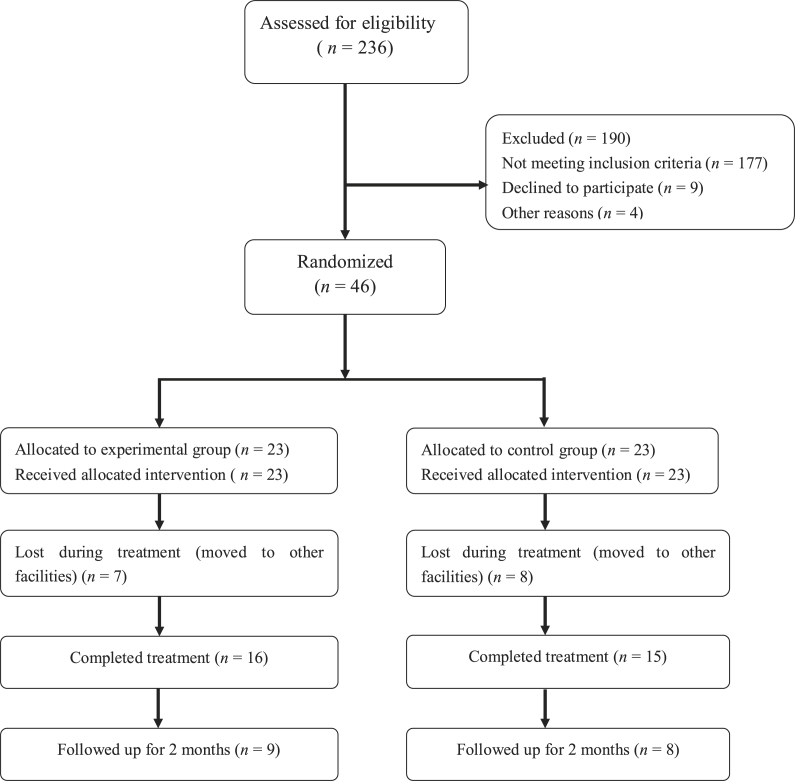
The study cohort consisted of 46 stroke patients admitted to the Rehabilitation Medical Center of the Second Affiliated Hospital of Jiaxing University (Jiaxing City, Zhejiang Province, China) from July 2017 to June 2018 and randomly assigned to the control (CTL) group (n = 23) or the VFT group (n = 23). The subjects and the rehabilitation specialist nurses were blinded to group assignment. Of these 46 patients, the data of 31 individuals (16 in the VFT group and 15 in the CTL group) were included for statistical analysis (Fig. 1). There were no statistically significant differences between the two groups in regard to sex ratio, age, disease course, hemiplegia, and other general clinical data (P > 0.05, Table 1).
Fig. 1.
Flow chart of the cases included in this study.
Table 1.
General information of patients in the two groups.
| Information | VFT (n = 16) | CTL (n = 15) | t | P |
|---|---|---|---|---|
| Gender, n[%] | ||||
| Male | 10(62.50%) | 8(53.33%) | – | 0.722a |
| Female | 6(37.50%) | 7(46.67%) | ||
| Age, years, | 57.75 ± 16.75 | 56.89 ± 17.93 | 0.192 | 0.424 |
| Course, | 30.67 ± 17.85 | 31.54 ± 18.79 | 0.184 | 0.427 |
| Side of hemiplegia, n[%] | ||||
| Left side | 9(56.25%) | 8(53.33%) | – | 1.000a |
| Right side | 7(43.75%) | 7(46.67%) | ||
| Stroke type, n[%] | ||||
| Intracerebral hemorrhage | 5(31.25%) | 4(26.67%) | – | 1.000a |
| Cerebral infarction | 11(68.75%) | 11(73.33%) | ||
Note: VFT, visual feedback therapy; CTL, control; a Fisher’s exact probability value.
The study protocol was approved by the Ethics Committee of the Second Affiliated Hospital of Jiaxing University (approval no. jxey-2017012) and conducted in accordance with the tenets of the Declaration of Helsinki. Before inclusion, the researchers informed all potential patients and their families about the details of this study, including the treatment methods, possible therapeutic effects, and treatment risks. Informed consent was obtained from all patients prior to participation in this study.
2.2. Inclusion and exclusion criteria
Patients who met the following criteria were included for analysis: (1) definitive diagnosis of stroke by cranial computed tomography or fMRI; (2) disease onset of <3 months, sitting balance > grade 1, and hand and upper limb > Brunnstrom stage III; (3) stable condition with unilateral limb hemiplegia; (4) Mini-Mental State Examination score > 27 and ability to execute the treatment instructions; (5) age of 40–75 years; and (6) signed informed consent to voluntary participate in this study.
The exclusion criteria included: (1) transient ischemic attack, venous sinus thrombosis, subarachnoid hemorrhage, progressive stroke, reversible ischemic attack; (2) lesions of the brain stem, bilateral cerebral hemisphere, and/or cerebellum; (3) osteoarthritis and diseases affecting the ability to sit or the upper limbs; (4) severe spasm of the upper limbs; (5) severe diseases of the heart, lung, liver, or kidney; (6) inability to complete the tests; (7) visual spatial disorders; (8) other serious diseases that could possibly complicate the course of treatment; (9) poor compliance or failure to complete the treatment protocol; and (10) self-withdrawal from the study.
2.3. Research methods
All patients received conventional medical therapies for stroke (i.e., nutrient intervention, improvement of microcirculation and cerebral metabolism, control of blood pressure and sugar, etc.) and traditional rehabilitation training (i.e., exercise and occupational therapies). During an 8-week rehabilitation period, training was conducted 3–4 h per day for 6 days per week. Training included the Bobath and Brunnstrom approaches, proprioceptive neuromuscular facilitation techniques, physical exercise, participation in daily activities, physiotherapy, occupational therapy, etc. Additionally, patients received VFT (VFT group) or conventional medical therapies and traditional rehabilitation training (CTL group). The specific training of patients in the VFT group included the following:
-
(1)
Before exercise training, the patient was seated 2 m in front of a color television screen with the hemiparalateral arm resting on a table (Fig. 2, Fig. 3).
-
(2)
Patients were instructed to imitate and practice actions involved in activities of daily living (ADLs) as shown on the television screen.
-
(3)
The following 40 actions for ADLs were assessed in this study: flexion/extension, abduction/adduction, pronation/supination, and shrugging of the shoulders, flexion/extension of the elbows, flexion/extension and radial/ulnar deviation of the wrists, flexion/extension of the thumbs, grasping of larger/small balls, cubes, and cylinders, picking up coins, picking up and setting down identity cards, twisting of wide-mouth and narrow-mouth bottle caps, picking up and setting down keys and pens, operating a computer mouse, typing on a keyboard, dialing a phone, grasping a spoon and chopsticks, closing a zipper, buttoning a shirt, and putting on clothes.
-
(4)
All of the actions were demonstrated by the same rehabilitation specialist nurse. Each action was observed from three different angles (straight ahead, right above, and right inside).
-
(5)
The duration of each action video was about 50 s and consisted of three different angles: forward (20 s), directly above (15 s), and right inside (15 s). Each action at each angle was recorded 2–3 times.
-
(6)
Each action portrayed in the video was scored from 1 to 30 with 1 being the easiest and 30 being the most difficult. The six action videos with similar difficulty levels were grouped together. There were five groups of videos. The first group was the easiest and the fifth group was the most difficult. The duration of each group of videos was about 5 min.
-
(7)
The patients were instructed to watch the videos from the first group and try to imitate the movements with the paralyzed arm. Each patient was required to complete more than four actions in one group independently prior to the next group of actions.
Fig. 2.
Patient receiving visual feedback therapy.
Fig. 3.
Patients receiving visual feedback therapy in a group.
The specific training session lasted for about 30 min per day for 6 days per week over a period of 8 weeks.
2.4. Assessment methods
All patients in this study were assessed by two specially trained nurses and the average of two scores was used for analysis. The two nurses who assessed the patients did not participate in the treatment regimen. SEP was used by the staff of the Neuroelectrophysiological Laboratory to detect sensory pathways.
2.4.1. The Fugl–Meyer assessment scale
The Fugl–Meyer assessment (FMA) scale was used to evaluate synergistic movements of the flexors and extensors of the shoulders, elbows, and wrists, wrist stability, joint action (e.g., hand grip, finger pinching, finger pointing, etc.), coordination ability, and speed. The FMA scale consists of 10 major items and 33 minor items that are assigned a score of 0–2 points for a possible total of 66 points [9]. The grading criteria were as follows: a score of less than 50% of the total score (66 points) is considered as a severe movement disorder (grade I); 50%–84% is considered as a significant movement disorder (grade II); 85%–95% is considered as a moderate movement disorder (grade III); and >96% of total score is considered as mild dyskinesia (grade IV) [10,11]. Since this scale has good reliability and validity, it is highly recommended for clinical and scientific evaluation of post-stroke motor function [12].
2.4.2. Barthel Index (BI)
Barthel Index (BI) [13] was used to evaluate the ADLs of patients before and 8 weeks after treatment. In 1989, the Canadian scholars Shah and Vanchay weighted BI on the basis of unchanged evaluation content in order to improve the defects of a few of the rankings, rough classification, and low sensitivity. They divided the 10 evaluation items into five grades (i.e., total dependence, maximum, medium, minimum, and total independence) and assigned each grade a different score. In the same way, the scores of decoration and bathing items ranged from 0 to 5; the scores of eating, dressing, defecation control, urination control, toilet control, and the ability to ascend and descend stairs was assigned a score of 0, 2, 5, 8, or l0; the ability of transferring from a bed or chair and flat walking was assigned a score of 0, 3, 8, 12, or 15. The total possible score of the 10 items was 100 points. Independence was positively correlated with a greater score. Detailed scoring rules were formulated according to the degree of assistance required.
2.4.3. SEP
SEP was assessed using a Viking electromyography evoked potential instrument (Nicolet Instrument Corporation, Madison, WI, USA). The stimulating electrodes were placed on the transverse stripes of the wrist at 2–3 cm and the median nerves were stimulated. A square wave pulse stimulator was used for stimulation (duration, 0.2 ms; frequency, 5 Hz). The stimulation intensity is controlled with the thumb. A needle electrode was used to record the latency and amplitude of the N9 and N20 hemispheres. The N9 wave was recorded at the Erb point and the N20 wave was recorded at the C3 and C4 points. Additionally, a reference electrode was placed at the Fz point. Each stimulus was superimposed about 150 times, the duration of analysis was 100 ms, and each measurement was repeated twice. The values were averaged for analysis. The test was performed in a quiet environment.
2.5. fMRI
All patients underwent fMRI before treatment. After treatment, nine patients in the VFT group and eight in the CTL group were re-examined by fMRI, which was conducted by an experienced technician.
A 1.5T superconducting twin-speed magnetic resonance scanner (SignaHDxt 1.5T; GE Healthcare, Chicago, IL, USA) was used for fMRI examinations. Visual stimulation was performed using a SA-9900 brain function audiovisual stimulation system (Shenzhen Sinorad Medical Electronics, Inc., Shenzhen, Guangdong Province, China). The system can ensure the synchronization of the action observation stimulation task and scanning.
Video Stimulation Task for Motion Training: A healthy adult female was photographed from the side while grabbing and releasing a small cylinder with her right hand. Then, a 30-s video was edited and imported into SA-9900 Brain Function Visual and Audiovisual Stimulation System for use as a stimulus event. The stimulation time and rest time of a single stimulus event were 30 s. Each fMRI test consisted of three stimulus events over a duration of 3 min and 12 s, which included 12 s for preparation. In the resting state, the subjects received no stimulus. In the stimulus state, the subjects received the same stimulus events. During the stimulation time, a 30-s action training video was presented. During the rest time, a white plus sign (+) on a black background was presented for 30 s.
Prior to entering the MRI room, all patients were informed what to observe during the examination. Then, the subjects watched one of the action training video in advance, which featured movements of a healthy female, and then were instructed to attempt a slight grasp with the hemiplegic hand. The subjects were instructed to remain still in order to avoid head movements. After lying down on the examination table, the subjects also practiced several grasp movements to ensure that they fully understood the main points of motion training, synchronized with the motion training video, and were instructed to avoid head movements. During the MRI scan, the subjects were told to lie quietly on the scanning bed with the head fixed on a foam pad and to insert foam earplugs to reduce the influence of noise. A mirror above the scanning coil displayed a motion training video. The angle of the mirror was adjusted to ensure that the participants could view the action training video when lying down. During the MRI scan, the patient was instructed to avoid movement, not speak, and to concentrate on the displayed hand movements. The following parameters were used to obtain T1-weighted sagittal images with three-dimensional sequences: slice thickness, 1.2 mm; inversion angle, 13°; and field of view, 24 × 24. The scanning parameters for functional imaging with a plane echo imaging sequence were as follows: repetition time, 3000 ms; echo time, 40 ms; deflection angle, 90°; layers, 40, image field of view, 22 × 22; and matrix, 64 × 64.
2.6. Statistical analysis
All statistical analyses were performed using SPSS Statistics for Windows, version 17.0. (SPSS, Inc., Chicago, IL, USA). Quantitative data were analyzed using the chi-square test and are presented as the mean ± standard deviation. The independent sample t-test was used to compare normally distributed data among different groups, the paired sample t-test was used to compare data between groups, and the non-parametric test was used to compare data without normal distribution among different groups. A probability (P) value of <0.05 was considered statistically significant. The fMRI images were processed using Statistical Parametric Mapping software (SPM8; The MathWorks, Inc., Natick, MA, USA). Prior to data processing, the orientation of all data was adjusted. Then, head alignment, high-resolution structural imaging, and average functional image registration were performed. After registration, a high-resolution structural image was segmented. All aligned functional images were standardized to the Montreal Institute of Neurology space using standardized parameters generated during the segmentation process. The standardized voxel size was 3 × 3 × 3 mm and the standard voxel size was 3 × 3 mm. The quasi-functional image was smoothed in space. The single-sample t-test was used for intra-group analysis of activated brain regions and voxels, and the double-sample t-test was used for inter-group analysis. Comparisons of the VFT and control groups before and after activation of the brain areas were performed. A P value of <0.001 and cluster of >5 voxels were considered as a significant difference. The Fisher exact test was used to assess the occurrence rate of activated areas in the main functional areas of the brain between the two groups before and after treatment.
3. Results
3.1. Effect of VFT on upper limbs physical function score and BI
As shown in Table 2, there was no significant difference in the FMA score of the upper limbs and BI before recovery training between the two groups (P > 0.05). After 8 weeks of recovery training, the FMA score of the upper limbs and BI had been improved, as compared to those before treatment in both groups (P < 0.05). After 8 weeks of treatment, the FMA score of the upper limbs and BI were significantly better in the VFT group than the CTL group (P < 0.05).
Table 2.
Physical function of the upper limbs and BI of the two groups ().
| Group | FMA | BI | |
|---|---|---|---|
| VFT (n = 16) |
Baseline | 21.76 ± 6.89 | 42.75 ± 11.09 |
| 8th week | 43.85 ± 6.42ab | 72.33 ± 11.82ab | |
| t | 7.51 | 9.56 | |
|
P |
0.03 |
<0.001 |
|
| CTL (n = 15) |
Baseline | 22.01 ± 5.67 | 43.78 ± 12.11 |
| 8th week | 38.31 ± 7.36a | 63.75 ± 10.45a | |
| t | 6.42 | 7.22 | |
| P | 0.04 | 0.03 |
Note: BI, Barthel Index; VFT, visual feedback therapy; CTL, control; FMA, Fugl–Meyer assessment; a means P < 0.05 compared with baseline data; b means P < 0.05 compared with data of CTL group.
3.2. SEP results
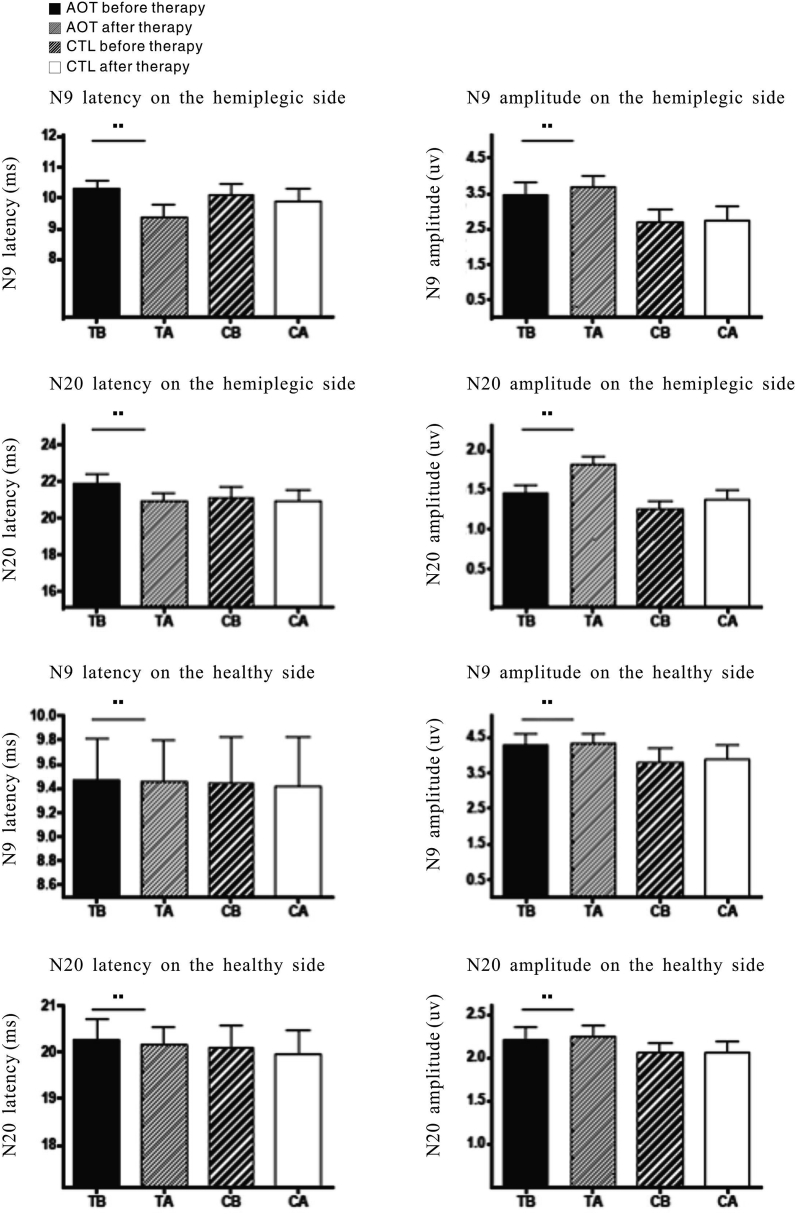
The SEP detection results of patients in the VFT and CTL group are shown in Table 3. After 8 weeks of treatment, the latencies of N9 and N20 of the hemiparetic side of patients in the VFT group were significantly shortened and the amplitudes were significantly increased (P < 0.05) as compared to the baseline levels, although there was no significant change in the N9 amplitude in week 4. Meanwhile, in the CTL group, the latencies of N20 in week 4 and N9 and N20 in week 8 were significantly shorter than the baseline levels, while the amplitude of N20 in week 8 was significantly increased over the baseline level (P < 0.05). The difference in latency between N20 and pretreatment was statistically significant (P < 0.05). In addition, after 8 weeks of treatment, there were no significant differences in the latencies and amplitudes of N9 and N20 of the contralateral side of patients in the VFT group (P > 0.05), as compared with the baseline levels, with the exception of N20 latency in week 8, which was significantly higher than the baseline level. Interestingly, similar changes were observed for the latencies and amplitudes of N9 and N20 of the contralateral side of patients in the CTL group (Fig. 4).
Table 3.
SEP results at baseline and 8th week in the two groups ().
| Group | N9 latency (ms) |
N9 amplitude(uv) | N20 latency(ms) | N20 amplitude(uv) | |
|---|---|---|---|---|---|
| VFT (n = 16) |
Hemiparalysis side | ||||
| Baseline | 10.29 ± 1.20 | 3.51 ± 1.35 | 21.84 ± 2.23 | 1.46 ± 0.37 | |
| 8th week | 9.03 ± 1.68ab | 3.91 ± 1.18ab | 20.32 ± 2.57ab | 2.08 ± 0.51ab | |
| t | 6.51 | 7.49 | 7.64 | 9.27 | |
| P | 0.04 | 0.03 | 0.03 | 0.02 | |
| Contralateral side | |||||
| Baseline | 9.44 ± 1.39 | 4.28 ± 1.38 | 20.25 ± 1.72 | 2.23 ± 0.55 | |
| 8th week | 9.37 ± 1.49 | 4.24 ± 1.16 | 19.85 ± 1.53b | 2.27 ± 0.58 | |
| t | 2.22 | 4.62 | 5.96 | 5.67 | |
|
P |
0.38 |
0.10 |
0.57 |
0.06 |
|
| CTL (n = 15) |
Hemiparalysis side | ||||
| Baseline | 10.08 ± 1.45 | 2.70 ± 1.41 | 21.11 ± 2.31 | 1.26 ± 0.40 | |
| 8th week | 9.52 ± 1.54a | 2.84 ± 1.47 | 20.51 ± 2.69a | 1.51 ± 0.44b | |
| t | 8.43 | 3.42 | 7.35 | 2.17 | |
| P | 0.03 | 0.21 | 0.03 | 0.42 | |
| Contralateral side | |||||
| Baseline | 9.44 ± 1.52 | 3.77 ± 1.64 | 20.10 ± 1.89 | 2.05 ± 0.47 | |
| 8th week | 9.27 ± 1.23 | 3.97 ± 1.52 | 19.77 ± 2.00 | 2.11 ± 0.50 | |
| t | 1.32 | 2.62 | 4.69 | 3.76 | |
| P | 0.83 | 0.23 | 0.47 | 0.36 | |
Note: SEP, somatosensory evoked potential; VFT, visual feedback therapy; CTL, control; a means P < 0.05 compared with baseline data; b means P < 0.05 compared with data of CTL group.
Fig. 4.
Comparison of SEPs before and after recovery training. SEP, somatosensory evoked potential; VFT, visual feedback therapy; CTL, control; TB, VFT group before; TA, VFT group after; CB, CTL group before; CA, CTL group after.
3.3. FAM score and BI at follow-up for 2 months after treatment between 2 groups
In this study, a total of 17 patients (9 in the VFT group and 8 in the CTL group) were followed-up for 2 months after treatment. As shown in Table 4, the FMA score of the upper limbs and BI of both groups at 2 months later were obviously higher than those at the end of recovery training. Moreover, the FMA score and BI of the VFT group were significantly improved at months 1 and 2 as compared to those of the CTL group (P < 0.05).
Table 4.
FMA score and BI in the two groups at the end of treatment and follow-up for two months (.
| Group | FMA | BI | |
|---|---|---|---|
| VFT (n = 9) |
At the end of treatment | 43.85 ± 6.42 | 72.21 ± 11.82 |
| Follow-up for 2 months | 50.11 ± 7.11ab | 82.11 ± 9.93ab | |
| t | 8.31 | 9.41 | |
|
P |
0.01 |
0.01 |
|
| CTL (n = 8) |
At the end of treatment | 38.31 ± 7.36 | 63.75 ± 10.45 |
| Follow-up for 2 months | 45.50 ± 7.52a | 73.25 ± 11.57a | |
| t | 8.50 | 7.60 | |
| P | 0.01 | 0.02 |
Note: FMA, Fugl–Meyer assessment; VFT, visual feedback therapy; CTL, control; a means P < 0.05 compared with the baseline data; b means P < 0.05 compared with the data of CTL group.
3.4. fMRI imaging results
Brain activation of the two groups before and after treatment is shown in Table 5. At followed-up 2 months after treatment, activation of the precentral gyrus, parietal lobe, and supplementary motor area were obviously increased in the VFT group as compared to the CTL group (P < 0.05). However, there were no significant changes in brain activation in the CTL group as compared with the baseline levels (P > 0.05).
Table 5.
Activation areas of brain in the two groups after treatment (n).
| Group | Time | Precentral gyrus | Parietal lobe | SMA | Occipital lobe | Basal ganglia |
|---|---|---|---|---|---|---|
| VFT (n = 9) |
At the end of treatment | 2 | 2 | 2 | 7 | 2 |
| Follow-up for 2 months | 8ab | 8ab | 9ab | 8 | 4 | |
|
P |
0.015 |
0.015 |
0.002 |
1.000 |
0.620 |
|
| CTL (n = 8) |
At the end of treatment | 2 | 2 | 2 | 6 | 0 |
| Follow-up for 2 months | 4 | 4 | 3 | 6 | 2 | |
| P | 0.608 | 0.608 | 1.000 | 1.000 | 0.467 |
Note: VFT, visual feedback therapy; CTL, control; a means P < 0.05 compared with the data at the end of treatment; b means P < 0.05 compared with the data of CTL group; Fisher’s exact was used for data analysis.
4. Discussion
The mirror neuron is a special type of neuron. Human beings can activate the same neurons either by performing certain actions themselves, by observing the same activities of others, or by action intentions [14,15]. The mirror neurons distributed in different brain regions constitute a mirror neuron system, which provides an "observation-execution matching mechanism" for action perception and execution [16,17]. Motion observation therapy based on the mirror neuron theory can promote plasticity change and functional reorganization of the brain through activation of the mirror neuron system and accelerate restoration of upper limb function of stroke patients [18,19]. Nojima et al. [20] reported that this "observation-execution matching mechanism" plays a key role in fundamental neurophysiological processes, such as motor understanding, kinematic learning, motor imagery, and motion imitation, especially in the restoration of upper limb motor function after stroke. A fMRI study of stroke patients conducted by Wang et al. [21] found high signals in the first motor area and auxiliary motor area of the affected cerebral cortex in the motion observation therapy group. Marangon et al. [22] and Liepert et al. [23] used transcranial magnetic stimulation to perform VFT for chronic and acute stroke patients, respectively, and found that VFT can facilitate movement of the hemiparetic limb. Evoked potentials have a positive effect on the recovery of hemiparesis of stroke patients [24]. Brunner et al. [25] showed that the activation of neurons in the insular lobe, infratemporal gyrus, parietal lobule, and inferior parietal lobule increased significantly during the course of observation therapy and task execution of stroke patients using fMRI. Activated clusters were mostly observed in the thalamus, infratemporal gyrus, and motor-related areas. Most activation clusters are observed in the thalamus and motor-related areas, such as the premotor cortex, auxiliary motor area, and motor cortex. Likewise, the results of the present study indicate that activation of the cerebellum and premotor areas is closely related to the recovery of arm motor function [26,27].
In the present study, patients in the VFT group received conventional action therapy based on the paramedic upper extremity supplemented with VFT. After 8 weeks of training and 2 months of follow-up, the FMA score, BI, and SEP indexes were improved, suggesting that VFT can significantly improve upper limb function after stroke. Assessments, such as FMA and the BI, are widely used to assess motor function after stroke, as the reliability and validity of these scales have been confirmed [28]. Our results suggest that motor observation therapy has a significant effect on functional restoration of the upper limbs of hemiplegic patients after stroke. In addition to routine rehabilitation training, action observation therapy was conducted. After 8 weeks of treatment, the results of SEP analysis showed that the latency periods of N9 and N20 of the affected limbs of the observation group were significantly shorter than those of the CTL group, while the amplitudes of N9 and N20 were increased.
As a possible mechanism, increased excitability of the mirror neuron system enhanced the excitability of brain regions related to upper limb motor function and the enhanced excitability of the brain is linked to the mirror neuron system and other brain regions, which leads to stronger plasticity of the motor regions that control the upper limbs in stroke patients. At the same time, because of the abundant visual training and daily life simulation training in the course of treatment, motion observation therapy can fully stimulate imitation, which is of great significance to inhibit the onset of disuse syndrome and accelerate recovery of upper limb motor function, and effectively improve treatment efficiency [29,30]. This possible mechanism has been verified by evoked potential and fMRI examinations, as an improvement in evoked potential indicates restoration of the somatosensory pathway in the VFT group, resulting in a better prognosis [31], [32]. S1 cortical processing before and after observation was assessed by measuring SEPs associated with median nerve stimulation. However, SEP amplitudes increased only for those who participated in observed learning. Moreover, SEPs increased more for participants who exhibited greater motor learning following observation. Taken together, these findings support the idea that motor learning by observation relies on the functional plasticity in S1. We propose that visual signals of the movements of others are mapped onto motor circuits for learning via the somatosensory system [33,34]. Meta-analysis results also revealed that the motor function of the upper limbs at the onset of stroke is the most important factor to predict the motor rehabilitation potential of the upper limbs, as compared with other factors, such as age, sex, lesion location, SEP, and motor evoked potentials [35,36]. The results of the present study showed that 8 weeks of VFT combined with conventional rehabilitation training resulted in shortened latencies and increased amplitudes of N9 and N20 of the hemiparetic side. The results indicated that the sensory pathway of the VFT group was recovered and limb mobility and prognosis were further improved. Therefore, VFT is conducive to exercise learning and has beneficial effects on paraplegic limb function and ADL recovery [37,38].
Further analysis of the fMRI data of the two groups of patients in this study showed that excitability of the anterior central gyrus, parietal lobe, and auxiliary motor areas of the brain increased significantly in the VFT group during the course of action observation therapy. These areas were widely distributed in the mirror neuron system, suggesting that action observation therapy may improve neurological function of stroke patients and increase excitability of the mirror neuron system. The fMRI technique used in this study, as first proposed by Ogawa et al. [39,40], can be used to assess cortical activation by analyzing changes in the levels of oxygenated and deoxygenated hemoglobin in different areas of the brain in real time.
In brief, this study aiming at stroke patients with upper extremity motor dysfunction action concluded that VFT based on mirror neuron theory could improve motor function of the upper extremity of stroke patients due to increased excitability in the distribution of brain neurons in the mirror image [40], [41], [42]. VFT is an effective treatment strategy for patients and a new breakthrough in the recovery of upper extremity function after stroke hemiplegia [[43], [44], [45]]. However, there are also deficiencies in this study. First, the sample size was relatively small, thus larger samples for follow-up observations are needed. The fMRI image data collected and analyzed in this study were limited and must be further confirmed and supplemented in follow-up experiments. Second, in this study, most patients underwent fMRI voluntarily, while some did not. Thus, the patients examined by fMRI were not randomly selected. Third, fMRI and motor evoked potentials analysis should be included in follow-up studies and the underlying mechanisms should be further explored to identify other factors that affect or confirm the role of mirror neurons, which should be supplemented by magnetoencephalography in future studies.
CRediT author statement
Mei-Hong Zhu: Data curation, Writing- Original draft preparation, Writing-Reviewing. Ming Zeng:Conceptualization, Methodology, Software. Mei-Fang Shi: Visualization, Investigation. Xu-Dong Gu: Supervision. Fang Shen: Software, Validation: Ye-Ping Zheng: Writing- Reviewing and Editing. Ya-Ping Jia: Data Curation.
Ethical approval
This study was conducted with the permission from the Ethics Committee of the Second Affiliated Hospital of Jiaxing University (No. jxey-2017012)
Funding
This study was supported in part by grants from Zhejiang province medical and health technology achievement Funding project(2018ZH044). Zhejiang province medical and health science and technology project.(2020KY317), Zhejiang province natural science foundation (LQ19H170001); 2019-2021 period key discipline construction plan funded project of traditional Chinese medicine in Jiaxing city (2019 XK- A07).
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of Chinese Nursing Association.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijnss.2020.04.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Theeke L., Lucke-Wold A.N., Mallow J., Horstman P. Life after stroke in appalachia. Int J Nurs Sci. 2017;4(2) doi: 10.1016/j.ijnss.2017.02.005. 105-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saposnik G., Cohen L.G., Muhammad Mamdani M., Pooyania S., Ploughman M., Cheung D. Efficacy and safety of non -immersive virtual reality exercising in stroke rehabilitation (EVREST): a randomised, multicentre,single-blind,controlled trial. Lancet Neurol. 2016;15(10):1–9. doi: 10.1016/S1474-4422(16)30121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fu J.M., Zeng M., Shen F., Cui Y., Zhu M.H., Gu X.D. Effects of action observation therapy on upper extremity function, daily activities and motion evoked potential in cerebral infarction patients. Medicine. 2017;96(42) doi: 10.1097/md.0000000000008080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buccino G. Action observation treatment: a novel tool in neurorehabilitation. Biol Sci. 2014;369(1644):1–8. doi: 10.1098/rstb.2013.0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhu M.H., Wang J., Gu X.D., Shi M.F., Zeng Ming, Wang Chun-Yuan. Effect of action observation therapy on daily activities and motor recovery in stroke patients. Int J Nurs Sci. 2015;2(3):279–282. doi: 10.1016/j.ijnss.2015.08.006. [DOI] [Google Scholar]
- 6.Sliwa J., Freiwald W.A. A dedicated network for social interaction processing in the primate brain. Science. 2017;356(5):745–749. doi: 10.1126/science.aam6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel M. Action observation in the modification of postural sway and gait: theory and use in rehabilitation. Gait Posture. 2017;58(10):115–120. doi: 10.1016/j.gaitpost.2017.07.113. [DOI] [PubMed] [Google Scholar]
- 8.Macedo Borges L.R.D., Fernandes A.B.G.S., de Melo L.P., Guerra R.O., Campos T.F. Action observation for rehabilitation after stroke. Stroke. 2019;50(6):1–3. doi: 10.1161/strokeaha.118.024297. [DOI] [Google Scholar]
- 9.Cha Y.J., Yoo E.Y., Jung M.Y., Park S.H., Park J.H., Lee J. Effects of mental practice with action observation training on occupational performance after stroke. J Stroke Cerebrovasc Dis. 2015;24(6):1405–1413. doi: 10.1016/j.jstrokecerebrovasdis.2015.02.022. [DOI] [PubMed] [Google Scholar]
- 10.van Dokkum L., Hauret I., Mottet D., Froger J., Métrot J., Laffont I. The contribution of kinematics in the assessment of upper limb motor recovery early after stroke. Neurorehabilitation Neural Repair. 2014;28(1):4–12. doi: 10.1177/1545968313498514. [DOI] [PubMed] [Google Scholar]
- 11.Saita K., Morishita T., Hyakutake K., Fukuda H., Shiota E., Sankai Y. Combined therapy using botulinum toxin A and single-joint hybrid assistive limb for upper-limb disability due to spastic hemiplegia. J Neurol Sci. 2017;373(1):182–187. doi: 10.1016/j.jns.2016.12.056. [DOI] [PubMed] [Google Scholar]
- 12.Duffy L., Gajree S., Langhorne P., Stott D.J., Quinn T.J. Reliability (inter-rater agreement) of the barthel index for assessment of stroke survivors: systematic review and meta -analysis. Stroke. 2013;44(2):462–468. doi: 10.1161/STROKEAHA.112.678615. [DOI] [PubMed] [Google Scholar]
- 13.Li Y., Zhang Y., Cui C., Liu Y., Lei M., Liu T. The effect of Tai Chi exercise on motor function and sleep quality in patients with stroke: a meta-analysis. Int J Nurs Sci. 2017;4(3):314–321. doi: 10.1016/j.ijnss.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barros Galvão S.C., Borba Costa dos Santos R., Borba dos Santos P., Cabral M.E., Monte-Silva K. Efficacy of coupling repetitive transcranial magnetic stimulation and physical therapy to reduce upper-limb spasticity in patients with stroke: a randomized controlled trial. Arch Phys Med Rehabil. 2014;95(2):222–229. doi: 10.1016/j.apmr.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 15.Lago-Rodriguez A., Lopez-Alonso V., Fernandez-del-Olmo M. Mirror neuron system and observational learning Behavioral and neurophysiological evidence. Behav Brain Res. 2013;248(4):104113. doi: 10.1016/j.bbr.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 16.Fitch W.T. A major blow to primate neonatal imitation and mirror neuron theory. Behav Brain Sci. 2017;40(1) doi: 10.1017/S0140525x16001874. [DOI] [PubMed] [Google Scholar]
- 17.Brunner I.C., Skouen J.S., Ersland L., Grüner R. Plasticity and response to action observation: a longitudinal FMRI study of potential mirror neurons in patients with subacute stroke. Neurorehabilitation Neural Repair. 2014;28(9):874–884. doi: 10.1177/27350. [DOI] [PubMed] [Google Scholar]
- 18.Frenkel-Toledo S., Bentin S., Perry A., Liebermann D.G., Soroker N. Mirror-neuron system recruitment by action observation: effects of focal brain damage on mu suppression. Neuroimage. 2014;87(10):127–137. doi: 10.1016/j.neuroimage.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Winstein C.J., Wolf S.L., Dromerick A.W., Lane C.J., Nelsen M.A., Lewthwaite R. Effect of a task-oriented rehabilitation program on upper extremity recovery following motor stroke the ICARE randomized clinical trial. J Am Med Assoc. 2016;315(6):571–581. doi: 10.1001/jama.2016.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nojima I., Koganemaru S., Kawamata T., Fukuyama H., Mima T. Action observation with kinesthetic illusion can produce human motor plasticity. Eur J Neurosci. 2015;41(4) doi: 10.1111/ejn.12921. 1641-1623. [DOI] [PubMed] [Google Scholar]
- 21.Wang J., Fritzsch C., Bernarding J., Holtze S., Mauritz K., Brunetti M. A comparison of neural mechanisms in mirror therapy and movement observation therapy. J Rehabil Med. 2013;45(4):410–413. doi: 10.2340/16501977-1127. [DOI] [PubMed] [Google Scholar]
- 22.Marangon M., Priftis K., Fedeli M., Masiero S., Tonin P., Piccione F. Lateralization of motor cortex excitability in stroke patients during action observation: a TMS study[J] BioMed Res Int. 2014;2014:1–7. doi: 10.1155/2014/251041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liepert J., Greiner J., Dettmers C. Motor excitability changes during action observation in stroke patients. J Rehabil Med : Off J Uems Eur Board Phys Rehabil Med. 2014;46(5):400–405. doi: 10.2340/16501977-1276. [DOI] [PubMed] [Google Scholar]
- 24.D’Innocenzo G., Gonzalez C.C., Nowicky A.V., Williams A.M., Bishop D.T. Motor resonance during action observation is gaze-contingent:A TMS study. Neuropsychologia. 2017;103(8):1–10. doi: 10.1016/j.neuropsychologia.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 25.Brunner I.C., Skouen J.S., Ersland L., Grüner R. Plasticity and response to action observation: a longitudinal fMRI study of potential mirror neurons in patients with subacute stroke. Neurorehabilitation Neural Repair. 2014;28(9):874–884. doi: 10.1177/1545968314527350. [DOI] [PubMed] [Google Scholar]
- 26.Sugg K., Müller S., Winstein C., Hathorn D., Dempsey A. Does action observation training with immediate physical practice improve hemiparetic upper-limb function in chronic stroke? Neurorehabilitation Neural Repair. 2015;29(9):1–11. doi: 10.1177/1545968314565512. [DOI] [PubMed] [Google Scholar]
- 27.Perry A., Saunders S.N., Stiso J., Dewar C., Lubell J., Meling T.R. Effects of prefrontal cortex damage on emotion understanding:EEG and behavioural evidence. Brain. 2017;140(4):1086–1099. doi: 10.1093/brain/awx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Y., Zhao Q., Zhang Y., Wu Q., Jiang X., Cheng G. Effect of mirror therapy on recovery of stroke survivors: a systematic review and network meta-analysis. Neuroscience. 2018;10(390):318–336. doi: 10.1016/j.neuroscience.2018.06.044. [DOI] [PubMed] [Google Scholar]
- 29.Wright David J., Williams Jacqueline, Holmes PaulS. Combined action observation and imagery facilitates corticospinal excitability. Front Hum Neurosci. 2014;8(11):1–9. doi: 10.3389/fnhum.2014.00951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nilsen D.M., Gillen G., Geller D., Hreha K., Osei E., Saleem G.T. Effectiveness of interventions to improve occupational performance of people with motor impairments after stroke:an evidence-based review. Am J Occup Ther. 2015;69(1):1–9. doi: 10.5014/ajot.2015.011965. [DOI] [PubMed] [Google Scholar]
- 31.Zhu M.H., Wang J., Gu X.D., Shi M.F., Zeng M., Wang C.Y. Effect of visual feedback therapy on activities of daily living in patients with stroke. Chin J Nurs. 2015;50(5):577–580. doi: 10.3761/j.issn.0254-1769.2015.05.014. [DOI] [Google Scholar]
- 32.Brunner I.C., Skouen J.S., Ersland L., Grüner R. Plasticity and response to action observation :A longitudinal fMRI study of potential mirror neurons in patients WithSubacute stroke. Neurorehabilitation Neural Repair. 2014;28(9):874–884. doi: 10.1177/1545968314527350. [DOI] [PubMed] [Google Scholar]
- 33.Bhasin A., Bhatia R., Kumaran S., Mohanty S., Padma Srivastava M. Neural interface of mirror therapy in chronic stroke patients: a functional magnetic resonance imaging study. Neurol India. 2012;60(6):570–576. doi: 10.4103/0028-3886.105188. [DOI] [PubMed] [Google Scholar]
- 34.McGregor H.R., Cashaback J.G., Gribble P.L. Functional plasticity in somatosensory cortex supports motor learning by observing. Curr Biol. 2016;26(7):921–927. doi: 10.1016/j.cub.2016.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stadler W., Springer A., Parkinson J., Prinz W. Movement kinematics affect action Prediction: comparing human to nonhuman point-light actions. Psychol Res. 2012;76(3):395–406. doi: 10.1007/s00426-012-0431-2. [DOI] [PubMed] [Google Scholar]
- 36.D’Innocenzo G., Gonzalez C.C., Nowicky A.V., Williams A.M., Bishop D.T.l. Motor resonance during action observation is gaze-contingent:A TMS study. Neuropsychologia. 2017;103(8):1–10. doi: 10.1016/j.neuropsychologia.2017.07.017. [DOI] [PubMed] [Google Scholar]
- 37.Peng T.-H., Zhu J.-D., Chen C.-C., Tai R.-Y., Lee C.-Y., Hsieh Y.-W. Action observation therapy for improving arm function, walking ability, and daily activity performance after stroke: a systematic review and meta-analysis. Clin Rehabil. 2019;12(4):1–9. doi: 10.1177/0269215519839108. [DOI] [PubMed] [Google Scholar]
- 38.Brunner I.C., Skouen J.S., Ersland L., Grüner R. Plasticity and response to action observation:A longitudinal fMRI study of potential mirror neurons in patients with subacute stroke. Neurorehabilitation Neural Repair. 2014;28(9):874–884. doi: 10.1177/1545968314527350. [DOI] [PubMed] [Google Scholar]
- 39.Ogawa S., Lee T.M., Nayak A.S., Glynn P. Oxygenation-sensitive contrast in magnetic resonance image of rodent brain at high magnetic fields. Magn Reson Med. 1990;14(1):68–78. doi: 10.1002/mrm.1910140108. [DOI] [PubMed] [Google Scholar]
- 40.Ogawa S., Lee T.M., Kay A.R., Tank D.W. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Rosa J.J., Natali F., Tettamanti A., Cursi M., Velikova S., Comi G. Action observation and motor imagery in performance of complex movements: evidence from EEG and kinematics analysis. Behav Brain Res. 2015;281(2):290–300. doi: 10.1016/j.bbr.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Press C., Cook R. Beyond action-specific simulation: domain-general motor contributions to perception. Trends Cogn Sci (Regul. Ed.) 2015;19(4) doi: 10.1016/j.tics.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 43.Holt L.L., Lotto A.J. Mirror neurons: from origin to function. Behav Brain Sci. 2014;37(2):177–241. doi: 10.1017/S0140525X13000903. [DOI] [PubMed] [Google Scholar]
- 44.Meyer S., Karttunen A.H., Thijs V., Feys H., Verheyden G. How do somatosensory deficits in the arm and hand relate to upper limb impairment, activity, and participation problems after stroke? A systematic review. Phys Ther. 2014;94(9) doi: 10.2522/ptj.20130271. 1220-l231. [DOI] [PubMed] [Google Scholar]
- 45.Caramazza A., Anzellotti S., Strnad L., Strnad L., Lingnau A. Embodied cognition and mirror neurons:a critical assessment. AnnuRevNeurosci. 2014;37(1):1–15. doi: 10.1146/annurev-neuro-071013-013950. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.