Version Changes
Revised. Amendments from Version 1
In response to comments from the reviewers, the following is our amendments: Abstract
We changed: ‘This species was…’ to be ‘The species was…’
Four activated charcoal sources were tested in this study not include control, in the Methods has been explained with five different charcoal sources including control (no active charcoal source)
All mistake numbers in the Abstract have been adjusted based on reviewer comments
Methods and Experimental
All the mistake numbers in the Methods have been adjusted based on the reviewer comments including type mistake in the Tables and Figures
Discussion
Some sentences have been modified to make easy to understand such as “ Hence, a longer foveola gastrica and larger intestinal villi were able to provide more nutrients to be absorbed due to a larger surface area of digestive organs” and also the contain amount of rice husk charcoal has been revised. Some words have been added to make easier to understand.
Abstract
Background : The giant trevally, Caranx ignobilis, is a commercially important marine fish in Indonesia. This species was initially cultured in Aceh Province. Previous reports showed that charcoal has a positive effect on survival and feed utilization of the giant trevally. However, the effects of adding charcoal to the diet on gut and intestine biometrics has, to our knowledge, never been described.
Methods : Four activated charcoal sources were tested in this study using a completely randomized experimental design; coconut shell charcoal, mangrove wood charcoal, rice husk charcoal, and kernel palm shell charcoal. All treatments were performed with four replications. Juvenile giant trevally (average body weight, 16.52 ± 3.12 g; and average total length, 10.26 ± 0.64 cm) were stocked into the experimental tank at a density of 15 fish per tank. The fish were fed an experimental diet twice daily at 7 AM and 5 PM ad satiation for 42 days.
Results : Analysis of variance showed that adding charcoal to the diet had significant effects on the length and width of the foveola gastrica and villous intestine (P < 0.05). The greatest length and width of the foveola gastrica was recorded in fish fed an experimental diet of rice husk charcoal with average values of 311.811 ± 9.869 µm and 241.786 ± 10.394 µm, respectively. The greatest length of intestinal villous was found in fish fed the mangrove wood charcoal diet, with a value of 135.012 ± 5.147 µm, but this length was not significantly different to that in fish fed rice charcoal and kernel palm shell charcoal. However, the greatest width of intestinal villous was recorded in fish fed the control diet (without charcoal; P < 0.05).
Conclusion: The optimal sizes of the foveola gastrica and villous intestine were found in fish fed an experimental diet with rice husk charcoal.
Keywords: Foveola gastrica, villous intestine, coconut shell, mangrove wood, rice husk, and kernel palm shell
Introduction
Trevally fish are a commercially important group of marine fish in the family Carangidae. A total of 146 species of trevally have been recorded worldwide 1. These fish are distributed in tropical, subtropical, and temperate waters 2– 7. In Indonesia, trevally fish are found in the Aceh waters 8, 9, East Borneo 10, Papua and Wester Nusa Tenggara 11, 12, and Java 13.
Giant trevally, Caranx ignobilis, is among the most popular trevally fish in Indonesia. The population of this species has declined over the years due to overfishing 7, 14– 16. Culture of this fish has been initiated in Aceh Province, Indonesia. However, farmers are faced with a feeding obstacle. Giant trevally in culture systems are currently fed waste fish and a commercial diet (Hi-Pro-Vite, Central Proteina Prima Company). The commercial diet is costly and difficult to obtain in remote areas, and the waste fish supply is very seasonal. Trash fish are limited in nutrients, particularly the essential amino acid composition 17. Therefore, it is crucial to formulate a diet for giant trevally using local raw materials with higher protein, that is inexpensive, easy to find, and digestible.
Activated charcoal is commonly added to the diet to increase digestibility and trigger growth in fish. For example, Jahan et al. 18 successfully used activated charcoal to increase the digestibility and growth performance of river catfish, Pangasiaodon sp. Other researchers have used charcoal in the diets of fish species, such as Nile tilapia, Oreochromis niloticus 19– 21, tiger pufferfish, Takifugu rubripes 22, Japanese flounder, Paralichthys olivaceus 23, African catfish, Clarias gariepinus 24, 25, gilthead seabream, Sparus aurata 26, and sturgeon, Huso huso 27. Firdus et al. 28 added rice husk charcoal to the diet of giant trevally. However, the effect of charcoal on the morphology of the gut and intestine has not been reported.
Organogenesis of the digestive system occurs as fish age, and this process is strongly dependent on the quantity and quality of food 29– 32, which is related to the development of mucosal cells, amplification of apical plasma membranes, and formation of the foveola gastrica and intestinal villi 33, 34. It has been hypothesized that adding activated charcoal to the diet triggers the digestive organogenesis system process 35, 36. In this study, we tested four charcoal sources in the diet to evaluate the morphology of the gut and intestine of giant trevally. Information on the gut and intestinal morphology is important to understand the absorption mechanism of nutrients from the diet.
Methods
Time and site
The study was conducted at the Center for Brackish Water Aquaculture, Ujung Batee, Aceh, Indonesia from February to July 2018. The activated charcoal was characterized at the Integrated Laboratory of Calibration, Universitas Gajah Mada, Yogyakarta, Indonesia. Histological samples were prepared at the Laboratory of Histology, Faculty of Mathematics and Natural Sciences, Universitas Syiah Kuala, Banda Aceh, Indonesia.
Experimental design
A completely randomized experimental design with five treatments consisting of control and four different charcoal sources was used in this study. The experimental groups were: (A) the experimental diet without charcoal, (B) the experimental diet with 2% charcoal from coconut shell, (C) the experimental diet with 2% charcoal from mangrove wood, (D) the experimental diet with 2% charcoal from rice husk, and (E) the experimental diet with 2% charcoal from kernel palm shell. All treatments were performed with four replications.
Experimental fish
A total of 300 giant trevally juveniles of mixed sex (average body weight, 16.52 ± 3.12 g; total length, 10.28 ± 0.64 cm) were purchased from a local farmer in Lancang Barat Village, Aceh Utara District, Aceh, Indonesia. The fish were acclimatized in ponds (ponds size 2 m x 1.8 m and temperature of around 29°C) at the Center for Brackish Water Aquaculture, Ujung Batee for 2 weeks. The fish were fed an experimental diet containing 50% crude protein twice daily at 7 AM and 5 PM at 3% of body weight per day ( Table 1).
Table 1. The composition of raw materials in the experimental diet (g kg −1) with 50% crude protein.
| Raw materials | Crude
protein (%) |
Composition (g kg −1) | |
|---|---|---|---|
| Diet without charcoal
(Diet A, Control) |
Diet with charcoal
(Diet B, C, D, E) |
||
| Ebi-shrimp meal | 58.80 | 50 | 50 |
| Fish meal | 59.00 | 660 | 660 |
| Rice flour | 7.26 | 180 | 160 |
| Soybean meal | 45.06 | 20 | 20 |
| Bloodmeal | 71.00 | 20 | 20 |
| Corn flour | 6.48 | 10 | 10 |
| Coconut oil | 0 | 5 | 5 |
| CaCO3 | 0 | 5 | 5 |
| Isoleucine | 100 | 10 | 10 |
| L-Tryptophan | 100 | 17.5 | 17.5 |
| DL-Methionine | 100 | 17.5 | 17.5 |
| Mineral mix | 0 | 5 | 5 |
| Active charcoal | 0 | - | 20 |
| Total material | 1000 | 1000 | |
| Total crude
protein |
50% | 50% | |
Note: (A) diet without charcoal, (B) diet with charcoal from coconut shells, (C) diet with charcoal from mangrove wood, (D) diet with charcoal from rice husks, (E) diet with charcoal from kernel palm shells.
Charcoal preparation and activation
The raw coconut shells, mangrove wood, rice husks, and kernel palm shells were chopped and ground. Approximately 500 g of the ground materials were placed on aluminum foil and heated in a furnace at 400°C for 1 hour. Nitrogen gas was flowed into the furnace to remove the oxygen. Then, the temperature was decreased to 30°C gradually and held for 1 hour. After 1 hour, the charcoal was removed from the furnace, sieved through a No. 40 mesh, and held in a jar before activating. A total of 100 g of sieved charcoal was taken and mixed with 400 ml of 0.2 M citric acid. The solution was stirred for 24 hours. After 24 hours, the solution was filtered through filter paper. The filtered charcoal was washed with distilled water and dried in an oven at 110°C for 24 hours.
Diet preparation
The experimental diet was formulated from both plant and animal-based protein sources, such as Ebi-shrimp meal, fish meal, blood meal, soybean meal, rice flour, and corn flour. All raw materials were subjected to a proximate analysis before use in the formulation. Three types of amino acids i.e. isoleucine, L-tryptophan, and DL-methionine were also added ( Table 1). A total of 2% of the tested charcoal sources was added to the formulation ( Table 1). The formulated diets were subjected to a proximate analysis before use in the experiment.
Stocking and feeding
The fish was captured randomly, measured for body weight and total length, and then distributed into 20 plastic containers (48 × 43 × 70 cm) at a stocking density of 15 fish per container. The water volume in the container was 75 L. The fish were fed an experimental diet twice daily at 7 AM and 5 PM to satiation for 42 days.
Histological sample preparation
Gastric and intestinal samples were collected at the end of the study. Three fish from each treatment were taken randomly from the experimental tanks. The fish were anesthetized with 30 mg L −1 clove oil 37, and the abdomen of the fish was gently dissected following the procedure of Purushothaman et al. 38. The stomach and intestines were removed with scalpel scissors and preserved in 4% formalin for 1 week. Histological sampling was carried using the paraffin method based on Osman and Caceci 39. The samples were dehydrated through an alcohol series and cleared in xylol. Subsequently, the gut and intestine samples were embedded in paraffin. The paraffin block was sectioned to 6 µm, and the sections were stained with hematoxylin and eosin. The size (height and width) of villi was determined using a binocular microscope (Zeiss Primo Star, Carl Zeiss Suzhou Co., Ltd., Suzhou, China) which was connected to a CCD camera and computer monitor 19. All efforts were made to lessen harm to the animals by complying to the guidelines of ethics animal use in research of Syiah Kuala University.
Data analysis
The qualitative gut and intestinal morphology data were subjected to one-way analysis of variance followed by Duncan’s multiple range test. The analysis was performed using SPSS ver. 18.0 software. The qualitative (histological) gut and intestinal data were analyzed descriptively. A P-value < 0.05 was considered significant.
Results
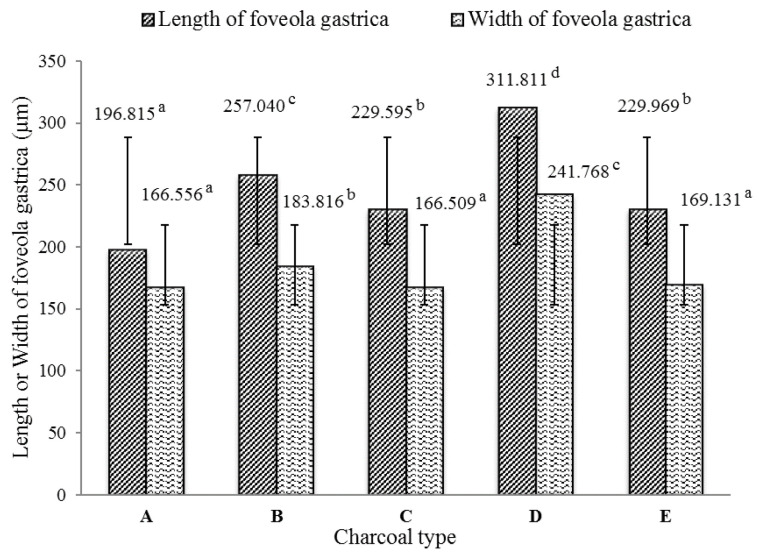
Adding activated charcoal to the diet significantly affected the length and width of the foveola gastrica and intestinal villi ( P < 0.05). In general, fish fed the activated charcoal diets produced better results than those not fed the charcoal ( Figure 1 and Figure 2). The best foveola gastrica morphology was obtained with the rice husk charcoal and the mean length and width of the foveola gastrica were 311.811 µm and 241.786 µm, respectively; followed by coconut shell charcoal (257.040 µm and 183.816 µm), kernel palm charcoal (229.969 µm and 169.131 µm µm), and mangrove wood charcoal (229.595 µm and 166.509 µm).
Figure 1. The average of length and width of the foveola gastrica.
( A) Diet without charcoal, ( B) diet with coconut shell charcoal, ( C) diet with mangrove wood charcoal, ( D) diet with rice husk charcoal, ( E) diet with kernel palm shell charcoal.
Figure 2. Histology of foveola gastrica from a juvenile giant trevally.
( A) Diet without charcoal, ( B) diet with coconut shell charcoal, ( C) diet with mangrove wood charcoal, ( D) diet with rice husk charcoal, ( E) diet with kernel palm shell charcoal. M, tunica mucosa; SM, tunica submucosa; Mc, tunica muscularis; Le, lamina epithelialis; Lp, lamina propria; m, muscle; Lm, longitudinal muscle fibers; Cm, circular muscle fibers (Cm).
The greatest length of the villous intestine was recorded in fish fed a diet with activated charcoal than those not fed the activated charcoal ( Figure 3). The greatest growth of intestinal villi was determined in the mangrove active charcoal (mean, 135.012 µm) group, but this value was not significantly different from the rice husk or kernel palm shell charcoals ( Figure 4). However, the greatest intestinal villi width was obtained in the treatment without activated charcoal (38.341 µm), and this value was significantly different from the other treatments.
Figure 3. The average length and width of intestine villi from juvenile giant trevally.
( A) Diet without charcoal, ( B) diet with coconut shell charcoal, ( C) diet with mangrove wood charcoal, ( D) diet with rice husk charcoal, ( E) diet with kernel palm shell charcoal.
Figure 4. Histology of intestinal villi from a giant trevally juvenile.
( A) Diet without charcoal, ( B) diet with coconut shell charcoal, ( C) diet with mangrove wood charcoal, ( D) diet with rice husk charcoal, ( E) diet with kernel palm shell charcoal. M, tunica mucosa; SM, tunica submucosa; Mc, tunica muscularis.
Raw biometic data, in addition to unprocessed imaged, are available as Underlying data 40– 42.
Discussion
The results show that adding activated charcoal to the diet of C. ignobilis significantly affected favoela gastrica and intestinal villi biometrics. According to Pirarat et al. 19, activated charcoal plays a significant role stimulating the development of epithelial cells of the digestive organs. Activated charcoal in the diet functions as a decontaminating agent to eliminate pathogenic organisms and toxic compounds, such as mycotoxins 20. Hence, a longer foveola gastrica and larger intestinal villi were able to provide more nutrients to be absorbed due to a larger surface area of digestive organs 43. Optimal development of the alimentary tract was recorded in giant trevally juveniles fed the experimental diet containing rice husk charcoal. This was presumably due to the high hemicellulose, cellulose, and lignin contents in the rice husk charcoal. A previous report indicated that rice husk charcoal contains 29.3% hemicellulose, 34.4% cellulose, and 19.2% lignin 44, while mangrove wood charcoal has 30% hemicellulose, 36% cellulose, and 28% lignin 45, coconut shell charcoal has 19.27% hemicellulose, 33.61% cellulose, and 36.51% lignin 46, and kernel palm shell charcoal has 26.27% cellulose, 12.61% hemicellulose, and 42.96% lignin 47. Maria and Banu 48 and Jamilatun et al. 49 reported that the concentration and quality of charcoal depend on the composition of hemicellulose, cellulose, and lignin. The quality of the activated charcoal is higher when these three components increase. According to Jasman 50, rice husk contains 85–95% activated charcoal, while mangrove wood has 76% activated charcoal 51, kernel palm shell 65% activated charcoal 47, and coconut shell has 60% activated charcoal 46.
The microscopic observations showed that the intestinal villi of the fish fed the diet with activated rice husk charcoal had a more pointed shape compared to other treatments, in which the villi tended to be round and blunt. According to Guo et al. 52, blunt or rounded villi probably occur due to inflammation in the intestinal mucosa, which is characterized by infiltration of neutrophils into the lamina propria. An increase of intestinal villus size is related to nutrient absorption capacity. According to Nafis et al. 53, long mucosal folds increase nutrient absorption and reduce food flow movement due to reduced peristaltic contractions, which provides sufficient time to optimally absorb nutrients. The increase in intestinal villi size is strongly related to the activities of digestive enzymes, such as lactase, sucrase, alkaline phosphatase, and disaccharidase 54– 57.
The morphology of the intestinal villi of fish fed a diet without activated charcoal was wider and shorter than that of fish fed the diets with activated charcoal. This was probably due to impaired intestinal mucosal integrity, causing interference in nutrient absorption. According to Choct 58, shortening of the intestinal villi is related to the accumulation of intestinal pathogenic bacteria, resulting in increased susceptibility to infection in the intestinal mucosal layer. This causes the digestive organs to form more secretory cells than absorbent cells, which reduces nutrient uptake 59, 60. The active charcoal likely acts as an adsorbent of metabolic pathogens in the intestine in the form of endotoxins and ammonia, therefore, it was able to improve intestinal function 61.
Conclusions
The application of activated charcoal in the diet significantly affected the length and width of the foveola gastrica and intestinal villi of giant trevally, C. ignobilis. The optimal biometrics of the foveola gastrica and intestinal villi were observed in fish fed the experimental diet with activated rice husk charcoal.
Data availability
Figshare: Gut and intestinal biometrics of the giant trevally, Caranx ignobilis, fed an experimental diet with difference sources of activated charcoal. https://doi.org/10.6084/m9.figshare.12203525.v2 40.
This project contains the following underlying data:
DATA BIOMETRIC GUT OF GIANT TREVALLY Caranx ignobilis_Edited (XLSX). (Raw biometric data for the foveola gastrica of all fish examined in this study.)
DATA BIOMETRIC OF INTESTINE OF GIANT TREVALLY Caranx ignobilis_edited (XLSX). (Raw biometric data for the intestinal villi of all fish examined in this study.)
Figshare: Gut and intestinal biometrics of the giant trevally, Caranx ignobilis, fed an experimental diet with difference sources of activated charcoal. https://doi.org/10.6084/m9.figshare.12301124.v2 41.
This project contains uncropped, unprocessed images of the intestinal villi of giant trevally.
Figshare: Gut and intestinal biometrics of the giant trevally, Caranx ignobilis, fed an experimental diet with difference sources of activated charcoaltem. https://doi.org/10.6084/m9.figshare.12269606.v2 42.
This project contains uncropped, unprocessed images of the foveola gastrica of the giant trevally.
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgments
We thank the Kemenristekdikti for supporting this study. All staff at the Center for Brackish Water Aquaculture in Ujung Batee who assisted with this study are acknowledged. Special thanks to Mr. Boihaqi and Maisyarah Rita for their assistance during the study.
Funding Statement
This study was supported by Kemenristekdikti, the Republic of Indonesia through the Doctoral Dissertation Scheme, Contract Number: 17/UN11.2/PP/SP3/2018.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 2 approved]
References
- 1. Fishbase: Family Carangidae - Jacks and pompanos.2020. (Accessed on 9 April 2020). Reference Source [Google Scholar]
- 2. Wetherbee BM, Holland KN, Meyer CG, et al. : Use of a marine reserve in Kaneohe Bay, Hawaii by the giant trevally, Caranx ignobilis. Fish Res. 2004;67(3):253–263. 10.1016/j.fishres.2003.11.004 [DOI] [Google Scholar]
- 3. Ho JS, Lin CL: Three species of Caligus Müller, 1785 (Copepoda: Caligidae) parasitic on Caranx spp. (Teleostei: Carangidae) off Taiwan. Syst Parasitol. 2007;68(1):33–43. 10.1007/s11230-006-9084-0 [DOI] [PubMed] [Google Scholar]
- 4. Meyer CG, Holland KN, Papastamatiou YP: Seasonal and diel movements of giant trevally Caranx ignobilis at remote Hawaiian atolls: implications for the design of marine protected areas. Mar Ecol Prog Ser. 2007;333:13–25. 10.3354/meps333013 [DOI] [Google Scholar]
- 5. Amarasinghe US, Wickramaratne IU, Wijeyaratne MJS: Hook selectivity of giant trevally ( Caranx ignobilis) and nakedbreast trevally ( Carangoides gymnostethus) (Carangidae) caught in the hook-and-line fishery off Negombo, Sri Lanka. Sri Lanka J Aquat Sci. 2011;16:11–26. 10.4038/sljas.v16i0.6712 [DOI] [Google Scholar]
- 6. Acuña-Marrero D, Salinas-De-León P: New record of two Indo-Pacific reef fish, Caranx ignobilis and Naso annulatus, from the Galapagos Islands. Mar Biodivers Rec. 2013;6:1–5. 10.1017/S1755267213000456 [DOI] [Google Scholar]
- 7. Neethiselvan N, Karthy A, Mol CB: Gillnet selectivity on the yellow fin Trevally ( Caranx ignobilis, Forsskal, 1775) along Thoothukudi coast, Southeast coast of India. J Exp Zool India. 2015;18(1):29–37. Reference Source [Google Scholar]
- 8. Timorya Y, Abdullah A, Batubara AS, et al. : Conservation and economic status fishes in the Krueng Sabee River, Aceh Jaya District, Aceh Province, Indonesia. IOP Conf Ser: Earth Environ Sci. 2018;216:012044 10.1088/1755-1315/216/1/012044 [DOI] [Google Scholar]
- 9. Batubara AS, Muchlisin ZA, Thamren MY, et al. : Check list of marine fishes from Simeulue Island waters, Aceh Province, Indonesia. Aceh J Anim Sci. 2017;2(2):77–84. 10.13170/ajas.2.2.9584 [DOI] [Google Scholar]
- 10. Abdusysyahid S, Anggoro S, Bambang AN: The distribution of capture fisheries based small pelagic-mackerel fish species in Balikpapan waters, East Kalimantan. Int J Eng Sci. 2014;6(2):149–153. 10.12777/ijse.6.2.149-153 [DOI] [Google Scholar]
- 11. Purba GY, Haryono E, Manan J, et al. : Jellyfish Lakes at Misool Islands, Raja Ampat, West Papua, Indonesia. Biodiversitas. 2018;19:172–182. 10.13057/biodiv/d190124 [DOI] [Google Scholar]
- 12. Wahyudewantoro G: The fish diversity of mangrove waters in Lombok Island, West Nusa Tenggara, Indonesia. Biodiversitas. 2018;19:71–76. 10.13057/biodiv/d190112 [DOI] [Google Scholar]
- 13. Piranti AS, Setyaningrum N, Retna UD, et al. : Fish conservation status in eastern part of segara anakan Cilacap Indonesia. IOP Conf Ser: Earth Environ Sci. 2019;406:012029 10.1088/1755-1315/406/1/012029 [DOI] [Google Scholar]
- 14. Abdussamad EM, Kasim HM, Balasubramanian TS: Distribution, biology and behaviour of the giant trevally, Caranx ignobilis-a candidate species for mariculture. Bangladesh J Fish Res. 2008;12:89–94. Reference Source [Google Scholar]
- 15. Santos SR, Xiang Y, Tagawa AW: Population structure and comparative phylogeography of jack species ( Caranx ignobilis and C. melampygus) in the high Hawaiian Islands. J Hered. 2011;102(1):47–54. 10.1093/jhered/esq101 [DOI] [PubMed] [Google Scholar]
- 16. Daly R, Filmalter JD, Daly CA, et al. : Acoustic telemetry reveals multi-seasonal spatiotemporal dynamics of a giant trevally Caranx ignobilis aggregation. Mar Ecol Prog Ser. 2019;621:185–197. 10.3354/meps12975 [DOI] [Google Scholar]
- 17. Muhammadar AA, Mazlan AG, Samat A, et al. : Crude protein and amino acids content in some common feeds of tiger grouper ( Epinephelus fuscoguttatus) juvenile. AACL Bioflux. 2011;4(4):449–504. Reference Source [Google Scholar]
- 18. Jahan R, Abdul MQ, Jahan N, et al. : Dietary added bamboo charcoal can evoke pangasianodon growth and can reduce ammonia from culture medium. Int J Fish Aquac. 2014;6(7):87–93. 10.5897/IJFA2014.0416 [DOI] [Google Scholar]
- 19. Pirarat N, Surinton B, Laddawan K, et al. : Effect of activated charcoal-supplemented diet on growth performance and intestinal morphology of nile tilapia ( Oreochromis niloticus). Thai J Vet Med. 2015;45(1):113–119. Reference Source [Google Scholar]
- 20. Boonanuntanasarn S, Khaomek P, Pitaksong T, et al. : The effects of the supplementation of activated charcoal on the growth, health status and fillet composition-odor of Nile tilapia ( Oreochromis niloticus) before hervesting. Aquacult Int. 2014;22(4):1417–1436. 10.1007/s10499-014-9756-8 [DOI] [Google Scholar]
- 21. Michael FR, Saleh NE, Shalaby SM, et al. : Effect of different dietary levels of commercial wood charcoal on growth, body composition and environmental loading of red tilapia hybrid. Aquac Nutr. 2017;23(1):210–216. 10.1111/anu.12385 [DOI] [Google Scholar]
- 22. Thu M, Koshio S, Ishikawa M, et al. : Effects of dietary bamboo charcoal on growth parameters, apparent digestibility and ammonia nitrogen excretion of tiger puffer fish, Takifugu rubripes. Aquac Sci. 2009;57(1):53–60. 10.11233/aquaculturesci.57.53 [DOI] [Google Scholar]
- 23. Thu M, Koshio S, Ishikawa M, et al. : Effects of supplementation of dietary bamboo charcoal on growth performance and body composition of juvenile Japanese flounder, Paralichthys olivaceus. World Aquac Soc. 2010;41(s2):255–262. 10.1111/j.1749-7345.2010.00365.x [DOI] [Google Scholar]
- 24. Alonge TO, Lawal MO, Aderolu AZ, et al. : Evaluation of soybean meal replacement with sesame seed meal using activated charcoal as an additive in the diet of African catfish juveniles, Clarias gariepinus. Int J Aquat Biol. 2016;4(1):43–50. 10.7508/ijab.2016.01.006 [DOI] [Google Scholar]
- 25. Aderolu AZ, Lawal MO, Adesola TT: Effects of graded activated charcoal in rice husk diets for mud catfish, Clarias gariepinus juveniles (Teleostei: Clariidae). Iran J Ichthyol. 2016;3(3):203–209. Reference Source [Google Scholar]
- 26. Michael FR, Helal AM: Rule of dietary activated wood charcoal on the growth and biochemical composition of Gilthead Seabream ( Sparus aurata) reared under different stocking densities. Life Sci J. 2018;15(4):79–86. 10.7537/marslsj150418.09 [DOI] [Google Scholar]
- 27. Samadaii S, Bahrekazemi M: The effect of diets containing different levels of active charcoal on growth performance, body composition, haematological parameters and possibility of heavy metals detoxification in big sturgeon ( Huso huso). Aquac Res. 2020;51(1):91–101. 10.1111/are.14350 [DOI] [Google Scholar]
- 28. Firdus F, Muhammadar AA, Samadi S, et al. : The effect of feeding with the addition of activated charcoal on feed conversion and survival of Juvenile Giant Trevally ( Caranx ignobilis). IOP Conf Ser: Earth Environ Sci. 2020;425:012051 10.1088/1755-1315/425/1/012051 [DOI] [Google Scholar]
- 29. Infante JZ, Cahu C: Development and response to a diet change of some digestive enzymes in sea bass ( Dicentrarchus labrax) larvae. Fish Physiol Biochem. 1994;12(5):399–408. 10.1007/BF00004304 [DOI] [PubMed] [Google Scholar]
- 30. Pryor GS, Royes JB, Chapman FA, et al. : Mannanoligosaccharides in fish nutrition: effects of dietary supplementation on growth and gastrointestinal villi structure in Gulf of Mexico sturgeon. N Am J Aquac. 2003;65(2):106–111. [DOI] [Google Scholar]
- 31. Liu H, Guo X, Gooneratne R, et al. : The gut microbiome and degradation enzyme activity of wild freshwater fishes influenced by their trophic levels. Sci Rep. 2016;6:24340. 10.1038/srep24340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Özel OT, Coşkun I, Çakmak E: Intestine villi morphology of black sea trout ( Salmo labrax. Pallas, 1814). LimnoFish. 2018;4(1):42–46. 10.17216/limnofish.365434 [DOI] [Google Scholar]
- 33. Infante JZ, Cahu CL: Ontogeny of the gastrointestinal tract of marine fish larvae. Comp Biochem Physiol C Toxicol Pharmacol. 2001;130(4):477–487. 10.1016/s1532-0456(01)00274-5 [DOI] [PubMed] [Google Scholar]
- 34. Wilson JM, Castro LFC: Morphological diversity of the gastrointestinal tract in fishes. Fish physiology. 2010;30:1–55. 10.1016/S1546-5098(10)03001-3 [DOI] [Google Scholar]
- 35. Burr G, Gatlin D, III, Ricke S: Microbial ecology of the gastrointestinal tract of fish and the potential application of prebiotics and probiotics in finfish aquaculture. World Aquaculture society. 2005;36(4):425–436. 10.1111/j.1749-7345.2005.tb00390.x [DOI] [Google Scholar]
- 36. Hoseinifar SH, Roosta Z, Hajimoradloo A, et al. : The effects of Lactobacillus acidophilus. as feed supplement on skin mucosal immune parameters, intestinal microbiota, stress resistance and growth performance of black swordtail ( Xiphophorus helleri). Fish Shellfish Immunol. 2015;42(2):533–538. 10.1016/j.fsi.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 37. Javahery S, Nekoubin H, Moradlu AH: Effect of anaesthesia with clove oil in fish (review). Fish Physiol Biochem. 2012;38(6):1545–1552. 10.1007/s10695-012-9682-5 [DOI] [PubMed] [Google Scholar]
- 38. Purushothaman K, Lau D, Saju JM, et al. : Morpho-histological characterisation of the alimentary canal of an important food fish, Asian seabass ( Lates calcarifer). PeerJ. 2016;4:e2377. 10.7717/peerj.2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Osman AHK, Caceci T: Histology of the stomach of Tilapia nilotica (Linnaeus, 1758) from the River Nile. J Fish Biol. 1991;38(2):211–223. 10.1111/j.1095-8649.1991.tb03107.x [DOI] [Google Scholar]
- 40. Muchlisin ZA, Firdus F, Samadi S, et al. : Gut and intestinal biometrics of the giant trevally, Caranx ignobilis, fed an experimental diet with difference sources of activated charcoal. figshare.Dataset.2020. 10.6084/m9.figshare.12203525.v2 [DOI] [PMC free article] [PubMed]
- 41. Muchlisin ZA, Firdus F, Samadi S, et al. : Gut and intestinal biometrics of the giant trevally, Caranx ignobilis, fed an experimental diet with difference sources of activated charcoal. figshare.Figure.2020. 10.6084/m9.figshare.12301124.v2 [DOI] [PMC free article] [PubMed]
- 42. Muchlisin ZA, Firdus F, Samadi S, et al. : Gut and intestinal biometrics of the giant trevally, Caranx ignobilis, fed an experimental diet with difference sources of activated charcoaltem. figshare.Figure.2020. 10.6084/m9.figshare.12269606.v2 [DOI] [PMC free article] [PubMed]
- 43. Quaiyum MA, Jahan R, Jahan N, et al. : Effects of bamboo charcoal added feed on reduction of ammonia and growth of Pangasius hypophthalmus. J Aquac Res Dev. 2014;5(6):1–5. 10.4172/2155-9546.1000269 [DOI] [Google Scholar]
- 44. Mahvi AH, Maleki A, Elslami A: Potential of rice husk and rice husk ash for phenol removal from aqueous systems. Am J Appl Sci. 2004;1(4):321–326. Reference Source [Google Scholar]
- 45. Verheyden A, Roggeman M, Bouillon S, et al. : Comparison between δ 13C of α-cellulose and bulk wood in the mangrove tree Rhizophora mucronata: implications for dendrochemistry. Chemical Geology. 2005;219(1-4):275–282. 10.1016/j.chemgeo.2005.02.015 [DOI] [Google Scholar]
- 46. Maryono M, Sudding S, Rahmawati R: Preparation and quality analisis of coconut shell charcoal briquette observed by starch concentration. Jurnal Chemica. 2013;14:74–83. [Google Scholar]
- 47. Sunardi S, Nurliani N: Pemanfaatan arang aktif sekam padi dengan aktivator natrium karbonat (Na2CO3) 5% untuk mengurangi kadar besi (Fe) dalam air ledeng. Jurnal Teknologi Hasil Hutan. 2008;23:99–104. Reference Source [Google Scholar]
- 48. Maria NSL, Banu ASS: Activated carbon from rice husk for treating dye waste water. Internasional Journal of Green Chemistry. 2015;1(1):1–9. Reference Source [Google Scholar]
- 49. Jamilatun S, Setyawan M, Salamah S, et al. : Pembuatan arang aktif dari tempurung kelapa dengan aktivasi sebelum dan sesudah pilorisis.2015. Reference Source [Google Scholar]
- 50. Jasman J: Uji coba arang aktif sekam padi sebagai media filtrasi dalam menurunkan kadar Fe pada air sumur bor di Asrama Jurusan Kesehatan Lingkungan Manado. Jurnal Kesehatan Lingkungan. 2011;1(1):49–53. Reference Source [Google Scholar]
- 51. Jauhari A: Penanggulangan kadar besi (Fe) air sumur menggunakan arang aktif kayu bakau (Rhizophora mucronata.Lamck) dengan aktivator natrium karbonat. Jurnal Hutan Tropis Borneo. 2009;28:321–331. [Google Scholar]
- 52. Guo J, Luo Y, Lua AC, et al. : Adsorption of hydrogen sulphide (H 2S) by activated carbons derived from oil-palm shell. Carbon. 2007;45(2):330–336. 10.1016/j.carbon.2006.09.016 [DOI] [Google Scholar]
- 53. Nafis M, Zainuddin Z, Masyitha D: Gambaran histologi saluran pencernaan ikan gabus (Channa striata). Jimvet. 2017;1(2):196–202. Reference Source [Google Scholar]
- 54. Samanya M, Yamauchi K: Morphological changes of the intestinal villi in chickens fed the dietary charcoal powder including wood vinegar compounds. J Poult Sci. 2001;38(4):289–301. 10.2141/jpsa.38.289 [DOI] [Google Scholar]
- 55. Gilmore MS, Ferretti JJ: The thin line between gut commensal and pathogen. Science. 2003;299(5615):1999–2002. 10.1126/science.1083534 [DOI] [PubMed] [Google Scholar]
- 56. Yamauchi K: Review of a histological intestinal approach to assessing the intestinal function in chickens and pigs. Anim Sci J. 2007;78(4):356–370. 10.1111/j.1740-0929.2007.00448.x [DOI] [Google Scholar]
- 57. Šabatková J, Kumprecht I, Zobač P, et al. : The probiotic BioPlus 2B as an alternative to antibiotics in diets for broiler chickens. Acta Veterinaria Brno. 2008;77:569–574. 10.2754/avb200877040569 [DOI] [Google Scholar]
- 58. Choct M: Managing gut health through nutrition. Br Poult Sci. 2009;50(1):9–15. 10.1080/00071660802538632 [DOI] [PubMed] [Google Scholar]
- 59. McLean E, Donaldson EM: Absorption of bioactive proteins by the gastrointestinal tract of fish: a review. J Aquat Anim Health. 1990;2(1):1–11. [DOI] [Google Scholar]
- 60. Ringø E, Olsen RE, Mayhew TM, et al. : Electron microscopy of the intestinal microflora of fish. Aquaculture. 2003;227(1-4):395–415. 10.1016/j.aquaculture.2003.05.001 [DOI] [Google Scholar]
- 61. Mulyono P, Wibisono W: Kinetika adsorpsi amonia dalam air dengan karbon aktif. Jurnal Media Teknik. 2007;2:26–42. Reference Source [Google Scholar]