Version Changes
Revised. Amendments from Version 1
The manuscript has been updated to incorporate the comments from our reviewers as follows. The immunocytochemistry data of APOE during directed differentiation that was presented in Figure 2C is now shown in Figure 3 alongside the quantification data for the percentage of cells positive for SOX2, TBR2, and APOE at the intracellular regions. The information regarding the access of underlying data for Figure 3 is also provided. We have also updated our discussion on the limitations of this study and make appropriate suggestions for further investigations to overcome these problems. In particular, we highlight the importance of (1) using a more quantitative approach for measuring APOE protein levels, (2) an improved imaging technique that will enable observations of densely packed cells at the earliest stage of neural induction, and finally (3) the examination of both differentiation markers and APOE in a time-dependent manner. Other minor updates include the information on PFA fixation time for immunocytochemistry and highlighting the fact that APOE is expressed mainly by glial cells in the Introduction. The authors would like to thank the reviewers for their valuable feedback.
Abstract
Apolipoprotein E (APOE) is a multifunctional protein that plays significant roles in important cellular mechanisms in peripheral tissues and is as well expressed in the central nervous system, notably by adult neural stem cells (NSCs) in the hippocampus. Evidence from animal studies suggest that APOE is critical for adult NSC maintenance. However, whether APOE has the potential to play a similar role in human NSCs has not been directly investigated. To address this question, we conducted a focused study characterising APOE gene and protein expression in an in vitro model of neural differentiation utilising human induced pluripotent stem cells. We found that APOE gene expression was dramatically decreased as the cells became more differentiated, indicating that APOE expression levels reflect the degree of cellular differentiation during neural induction. Furthermore, qualitative analysis results of immunocytochemistry showed that intracellular localisation of APOE protein becomes more pronounced as neural differentiation progresses. Taken together, our findings suggest a potential role for APOE in human NSC maintenance and justify further investigations being carried out to understand whether changes in APOE levels can directly impact the neurogenic capacity of human stem cells.
Keywords: Induced pluripotent stem cells, Neural stem cells, Directed differentiation, Apolipoprotein E
Abbreviations
AD (Alzheimer’s disease); APOE (Apolipoprotein E); iPSCs (induced pluripotent stem cells); NSCs (neural stem cells)
Introduction
Apolipoprotein E (APOE) is a pleiotropic protein that plays an important role in lipid metabolism ( Mahley & Rall, 2000) and is highly expressed in the brain mainly by glial cells ( Boyles et al., 1985; Elshourbagy et al., 1985). Although the primary function of APOE is lipid transport, its expression is also found in other cell types outside the context of lipid metabolism in the brain ( Liao et al., 2017). For example, a recent single-cell RNA sequencing study on human post-mortem Alzheimer’s disease (AD) brains showed that activated microglia (relevant to the disease state) express high levels of APOE unlike naïve microglia (relevant to healthy/homeostatic state) in the prefrontal cortex, indicating that APOE expression is associated with immune function ( Mathys et al., 2019). Furthermore, neuronal APOE can also be expressed at high levels under stress conditions such as brain injury although APOE expression is normally low in healthy neurons ( Mahley & Huang, 2012; Xu et al., 2006). Interestingly, APOE is highly expressed in nestin/glial fibrillary acidic protein (GFAP) double-positive neural stem cells (NSCs) in the adult hippocampus of mice, and one of the phenotypes characterised in APOE-null mice is the premature depletion of NSC pool in the hippocampus, suggesting that NSC maintenance requires APOE expression ( Yang et al., 2011).
Although the existing literature suggest that APOE plays an important role in stem cell maintenance, one should note that the majority of these findings were generated from rodent models. Since NSCs obtained from different species have been shown to behave in fundamentally different ways ( Mertens et al., 2013; Otani et al., 2016; Ray & Gage, 2006), characterisation of APOE expression in ‘human’ NSCs should be done prior to investigating its exact function. However, such evidence has not been reported to this date. To reduce this knowledge gap, we conducted a short study examining the expression pattern of APOE gene and protein in human induced pluripotent stem cells (iPSCs) undergoing neural induction in vitro. We found that gene expression is the highest in cells at the earliest stage of neural induction, whereas protein expression becomes more localised intracellularly, indicating that APOE expression pattern changes according to the differentiation state of cells.
Methods
A list of materials used in this study is presented in Table 1.
Table 1. List of materials used in this study.
| Name | Company | Catalogue
Number |
||
|---|---|---|---|---|
|
CytoTune-iPS 2.0 Sendai
Reprogramming Kit |
Thermo Fisher | A16517 | ||
| Essential 8™ medium | Thermo Fisher | A1517001 | ||
| NUNC™ plates | Thermo Fisher | 140675 | ||
| Geltrex™ | Thermo Fisher | A1413302 | ||
| Versene (EDTA) solution | Lonza | BE17-711E | ||
| Hank's Balanced Salt Solution (HBSS) | Thermo Fisher | 14170-161 | ||
|
Dulbecco’s Modified Eagle’s Medium/
Nutrient Mixture F-12 Ham |
Sigma Aldrich | D6421 | ||
| GlutaMAX™ | Thermo Fisher | 35050-061 | ||
| N-2 (100X) | Thermo Fisher | 17502-048 | ||
| Neurobasal® medium | Thermo Fisher | 21103-049 | ||
| B-27 minus vitamin A (50X) | Thermo Fisher | 12587-010 | ||
| LDN193189 | Sigma Aldrich | SML0559 | ||
| SB431542 | Sigma Aldrich | S4317 | ||
| XAV939 | Sigma Aldrich | X3004 | ||
| Cyclopamine | LC Laboratories | C-8700 | ||
| Y-27632 | Sigma Aldrich | Y0503 | ||
| DNeasy Blood & Tissue Kit | QIAGEN | 69504 | ||
| Taq DNA Polymerase | QIAGEN | 201203 | ||
| HhaI digestion enzyme | Thermo Fisher | ER1851 | ||
| TRIzol® reagent | Thermo Fisher | 15596026 | ||
|
SuperScript® III First-Strand
Synthesis System |
Thermo Fisher | 18080051 | ||
| HOT FIREPol® EvaGreen® qPCR Mix | Solis Biodyne | 08-24-00001 | ||
| Name | Concentration | Host/Clonality | Company | Catalogue Number |
| Hoechst 33342 | 1:2000 | - | Thermo Fisher | H3570 |
| anti-human SOX2 | 1:1000 | Rabbit monoclonal | Millipore | AB5603 |
| anti-human TBR2 | 1:250 | Rabbit polyclonal | Abcam | AB23345 |
| anti-human ApoE | 1:200 | Goat polyclonal | Millipore | AB947 |
| anti-Rabbit Alexa Fluor 488 | 1:500 | Donkey polyclonal | Thermo Fisher | A21206 |
| anti-Goat Alexa Fluor 594 | 1:500 | Donkey polyclonal | Thermo Fisher | A11058 |
Cell line
CTR_M3_36S human induced pluripotent stem cell (iPSC) line was reprogrammed from keratinocytes obtained from a neurotypical male. Keratinocytes were reprogrammed by introducing a set of Sendai virus encoding human OCT4, SOX2, KLF4, and C-MYC (Yamanaka factors) using the CytoTune-iPS 2.0 Sendai Reprogramming Kit (Thermo Fisher) according to the manufacturer’s instructions. The virus was a gift from Dr. Mahito Nakanishi (AIST, Japan).
Stem cell maintenance
Cells were regularly tested for mycoplasma and certified mycoplasma-free. iPSCs were maintained in Essential 8™ medium (Thermo Fisher) without antibiotics at 37°C, 5% CO 2, 5% O 2 in 6-well NUNC™ plates (Thermo Fisher) coated with Geltrex™ (Thermo Fisher). Passaging of iPSCs lines were done with Versene (EDTA) solution (Lonza) according to the manufacturer's instructions. Passaging ratio for iPSC maintenance was kept between 1:6 and 1:18.
Directed differentiation
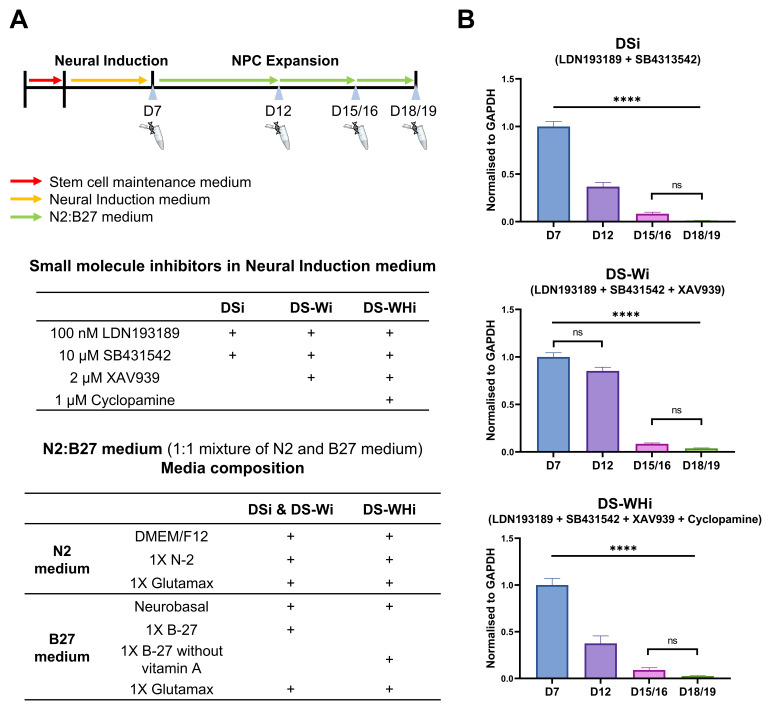
iPSC colonies approaching 80% confluence were passaged at 3:2 ratio on 6-well NUNC™ plates coated with Geltrex™ on D-2/-1 and maintained at 37°C, 5% CO 2, 5% O 2 for 24–48 hrs until they approached 100% confluence. Directed differentiation began on D0 by changing Essential 8™ medium to neural induction medium and incubating the cells at 37°C, 5% CO 2, 20% O 2. Neural induction lasted for 7 days. To prepare neural induction medium, N2:B27 was first prepared by mixing the N2 medium (Dulbecco’s Modified Eagle’s Medium/Nutrient Mixture F-12 Ham (DMEM/F12) (Sigma Aldrich) supplemented with 1X GlutaMAX™ (Thermo Fisher) and 1X N-2 supplement (Thermo Fisher)) and the B27 medium (Neurobasal® medium (Thermo Fisher) supplemented with 1X GlutaMAX™ and 1X B-27 supplement (Thermo Fisher) or 1X B-27 without vitamin A supplement (Thermo Fisher)) at 1:1 ratio. The following small molecule inhibitors were added to N2:B27 to make the neural induction medium: 100 nM LDN193189 (Sigma Aldrich) and 10 µM SB431542 (Sigma Aldrich) for dual SMAD inhibition (DSi); 100 nM LDN193189, 10 µM SB431542, and 2 µM XAV939 (Sigma Aldrich) for dual SMAD inhibition plus Wnt/β-catenin inhibition (DS-Wi); and 100 nM LDN193189, 10 µM SB431542, 2 µM XAV939, and 1 µM Cyclopamine (LC Laboratories) for dual SMAD inhibition plus Wnt/β-catenin plus sonic hedgehog inhibition (DS-WHi). Neural induction medium was used from D0 to D7, and N2:B27 was used from D8 onwards. Medium was changed every 24 hrs throughout the entire directed differentiation period.
Neural passaging 1, 2, and 3 were performed with Accutase (Thermo Fisher) on D7, D12, and D15/16, respectively. Briefly, cells were washed with room temperature HBSS and treated with Accutase at 37°C, 5% CO 2, 5% O 2 for 3–4 minutes. Cold Accutase was used for neural passagings 1 and 2, and room temperature Accutase was used for neural passaging 3. Cells in Accutase were then collected with a P1000 pipette. Extra care was taken during neural passagings 1 and 2 where P1000 pipetting was done no more than 5 times when cells in Accutase were collected. Collected cells were then mixed with room temperature DMEM/F12 (twice the volume of Accutase used) so that Accutase could be deactivated, and centrifugation was performed twice to wash off the Accutase from cells. Centrifugation was done at 900 revolutions per minute (RPM) for 2 min during neural passaging 1 and 2, and at 1250 RPM for 2 min during neural passaging 3. After centrifugation, cells were plated on new 6-well NUNC™ plates coated with Geltrex™. Passaging ratios were 1:1 for neural passaging 1 and 2, and 2:3 for neural passaging 3. To ensure cell survival 10 µM Y-27632 (Sigma Aldrich), a Rho-associated coiled-coil containing protein kinase (ROCK) inhibitor, was mixed with the plating medium at each neural passaging and then removed after 24 hrs.
Genotyping
Genomic DNA was extracted from iPSCs using the DNeasy Blood & Tissue Kit (QIAGEN) according to the manufacturer’s instructions. The APOE locus containing the rs429358 and rs7412 SNPs was amplified with Taq DNA Polymerase (QIAGEN) according to the manufacturer's instructions. Briefly, the reaction mix containing 1X PCR Buffer, 1X Q-Solution, 10 mM dNTP mix (0.2 mM final concentration), primers (forward and reverse each at 0.4 µM final concentration), 1.25 units Taq polymerase, and 1 µg of genomic DNA was incubated at 95°C for 4 mins to activate the Taq polymerase. Then, 35 cycles of ‘denaturation at 94°C for 30 secs, annealing at 68°C for 30 secs, elongation at 72°C for 1 min’ was performed. Then, final extension was done at 72°C for 10 mins. This polymerase chain reaction (PCR) was done with S1000 Thermal Cycler (Bio-Rad). The primers used for APOE genotyping ( Table 2) were previously designed by Henderson and colleagues ( Henderson et al., 2002), and they are able to generate PCR products that can be visualised easily by gel electrophoresis after HhaI enzyme (Thermo Fisher) digestion. Amplified PCR products were digested with 1 unit of HhaI digestion enzyme and gel electrophoresis was performed using a 3% agarose gel containing 0.5 µg/mL ethidium bromide. Raw dataset is available as an underlying data via Figshare (doi: 10.6084/m9.figshare.12199721.v1).
Table 2. Sequence of primers used in this study.
| Genotyping | Forward | Reverse |
|---|---|---|
| APOE | GAC GCG GGC ACG GCT GTC
CAA GGA GCT GCA GGC GAC GCA GGC CCG GCT GGA CGC GGA CAT GGA GGA |
AGG CCA CGC TCG ACG CCC TCG
CGG GCC CCG GCC TGG TAC ACT |
| Gene
expression |
Forward | Reverse |
| APOE | GTT GCT GGT CAC ATT CCT GG | GCA GGT AAT CCC AAA AGC GAC |
| GAPDH | AGC CTC AAG ATC ATC AGC AA | CTG TGG TCA TGA GTC CTT CC |
Gene expression analysis
Total RNA was extracted from D7, D12, D15/16, and D18/19 cells that were not used for neural passaging with TRIzol® reagent (Thermo Fisher) according to manufacturer’s instructions and eluted in 25–30 µL of diethyl pyrocarbonate (DEPC)-treated water. Reverse transcription of total RNA into complementary DNA was performed using SuperScript® III First-Strand Synthesis System (Thermo Fisher) according to the manufacturer's instructions. Briefly, the random hexamers were annealed to total RNA at 25°C for 10 mins, then the synthesis was performed at 50°C for 50 mins, and then the reaction was terminated at 85°C for 5 mins. The final product was diluted to 5 ng/µL of total RNA converted to cDNA using DEPC-treated water. S1000 Thermal Cycler (Bio-Rad) was used for reverse transcription.
For gene expression analysis, real-time quantitative polymerase chain reaction (qPCR) was performed using the HOT FIREPol® EvaGreen® qPCR Mix (Solis Biodyne) according to the manufacturer’s instructions. Briefly, the reaction mix containing the HOT FIREPol® EvaGreen® qPCR Mix, primers (forward and reverse each at 0.2 µM final concentration), and cDNA was incubated at 95°C for 15 mins to activate the HOT FIREPol® DNA polymerase, then 45 cycles of ‘denaturation at 95°C for 30 secs, annealing at 60°C for 30 secs, elongation at 72°C for 30 secs’ was performed. Melting curve analysis was done on each gene based on the melting profile generated every 1°C increment between 60°C and 95°C. MJ Research PTC-200 Thermal Cycler (Bio-Rad) was used for qPCR. The sequence of primers are presented in Table 2. C T values of APOE were normalised to that of GAPDH, and relative expression of APOE across samples were quantified using the 2 -ΔΔCt method where D7 sample was used as a reference for each differentiation lineage. Raw dataset is available as an underlying data via Figshare (doi: 10.6084/m9.figshare.12136944).
Immunocytochemistry
Cells were fixed with 4% paraformaldehyde for 10 mins at room temperature, permeabilized with 0.1% Triton™ X-100 in 1X Tris-buffered saline (TBS) for 15–30 minutes, and then blocked with 5% normal donkey serum in TBS for 30 minutes. Primary antibodies were incubated at 4°C overnight followed by 3 washings with TBS. Secondary antibodies conjugated with fluorescent dyes were incubated at room temperature for 1 hours followed by 2 washings with TBS. Nuclei were stained with 5 µg/mL Hoechst® 33342 solution (Thermo Fisher) for 30 seconds immediately prior to imaging, and cells were washed with TBS 2 times after nuclear staining. All primary antibodies were diluted in 5% normal donkey serum in TBS, secondary antibodies in 1% normal donkey serum in TBS, and Hoechst® 33342 solution in TBS. Imaging was done with IX 70 inverted epifluorescence microscope (Olympus) connected to AxioVision imaging software (version 4.4). Scale bars were inserted on the images using ImageJ software (version 1.49c). CellProfiler (version 3.1.9) was used to quantify the percentage of cells immunopositive for SOX2, TBR2, and APOE at the intracellular regions. Raw dataset for the images is available as an underlying data via Figshare (doi: 10.6084/m9.figshare.12199745.v1). Raw dataset for the quantification is available as an underlying data via Figshare (doi: 10.6084/m9.figshare.12781604.v1).
Statistical analysis
GraphPad Prism (version 8.4.2.679) was used for statistical analysis. The statistical significance of the mean differences between groups were analysed by one-way analysis of variance (ANOVA) followed by Bonferroni correction for multiple testing. The mean, standard error of measurement (SEM), and number of biological replicates are reported. P-value < 0.05 was considered significant to reject the null hypothesis that the differences observed between groups is due to random variation.
Results
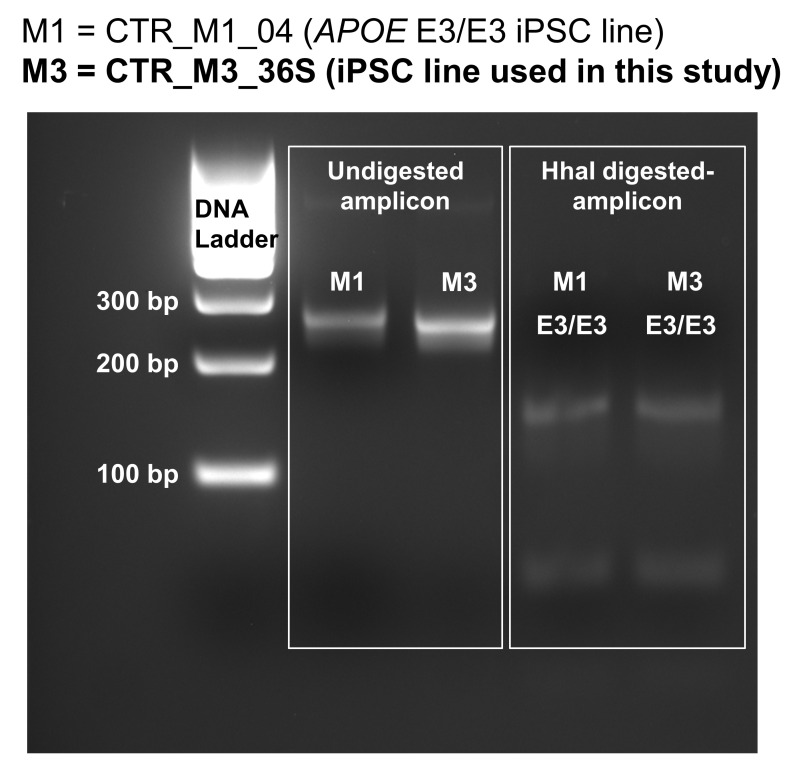
To characterise the expression of APOE in human stem cells undergoing neural induction, an iPSC line derived from a neurotypical male with APOE3 homozygous genotype (CTR_M3_36S cell line) ( Figure 1; ( Lee, 2020b)) ( Deans et al., 2017) were differentiated into neural lineages. Genotyping of CTR_M3_36S was performed using the method developed by Henderson and colleagues ( Henderson et al., 2002), and CTR_M3_36S was confirmed to be homozygous for APOE3 by comparing the data with that of an APOE3 homozygous cell line (CTR_M1_04) that reported by Henderson and colleagues ( Henderson et al., 2002, Figure 1). Neural induction into dorsal forebrain progenitors was performed using modified dual SMAD inhibition protocols ( Cocks et al., 2014; Deans et al., 2017; Kathuria et al., 2018; Yu et al., 2014), where combinations of small molecule inhibitors were used to inhibit bone morphogenetic protein (BMP), transforming growth factor (TGF)-β, Wnt/β-catenin, and sonic hedgehog signalling pathways from D0 to D7 of neural induction ( Figure 2A).
Figure 1. APOE genotyping of the cell line used in this study.
CTR_M3_36S human iPSC line derived from a neurotypical male is homozygous for APOE3 (denoted as M3 in this figure). CTR_M1_04 human iPSC line that was known to be homozygous for APOE3 was used as control (denoted as M1 in this figure). HhaI-digested PCR amplicons were run on a 3% agarose gel, and the band loci were compared with the data previously reported by Henderson and colleagues who developed this genotyping method ( Henderson et al., 2002). The band loci for both CTR_M3_36S and CTR_M1_04 lines match with the homozygous APOE3 data reported by Henderson and colleagues (see Figure 1 of Henderson et al., 2002).
Figure 2. APOE gene expression changes according to the differentiation state of cells during in vitro directed differentiation.
A) Schematic diagram of directed differentiation. CTR_M3_36S iPSCs were maintained in stem cell maintenance medium after replating (D-2/-1). On D0 neural induction began by changing the stem cell maintenance medium to neural induction medium. N2:B27 was used from D8 onwards. Medium was changed every 24 hrs throughout the entire differentiation period. Neural passaging 1, 2, and 3 were carried out on D7, D12, and D15/16, respectively. Total RNA extraction was made on cells that were not used for neural passaging on D7, D12, D15/16, and D18/19. Neural induction medium composition for each differentiation lineage and N2:B27 medium composition are also shown. B) APOE gene expression is reduced along neural induction regardless of lineage. Real-time qPCR was performed on CTR_M3_36S iPSCs undergoing directed differentiation at D7, D12, D15/16, and D18/19. APOE expression was normalised to that of GAPDH. D7 samples were used as reference samples for each lineage. One-way ANOVA with Bonferroni correction. n = 3. Mean (bars) with S.E.M. (error bars) shown. **** ANOVA p-value < 0.0001. ns: non-significant after Bonferroni correction. DSi: dual SMAD inhibition. DS-Wi: DSi plus Wnt/β-catenin inhibition. DS-WHi: DS-Wi plus sonic hedgehog inhibition.
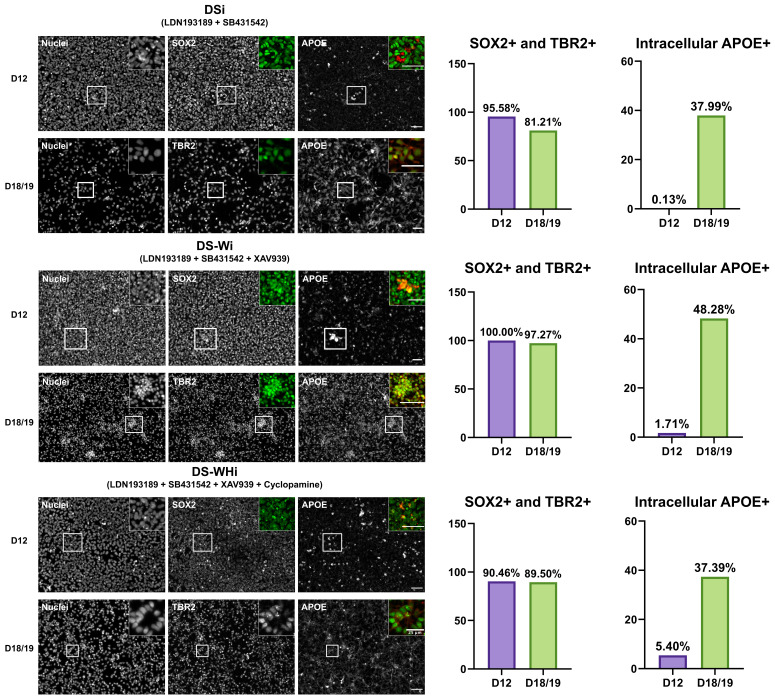
Gene expression analysis revealed that APOE expression was the highest at D7, and the drastic down-regulation of APOE from D7 > to D18/19 was observed regardless of the combination of small molecule inhibitors used from D0 to D7 (p < 0.0001) ( Figure 2B, underlying data ( Lee, 2020a)). Immunocytochemistry showed that D12 and D18/19 cells expressed SRY-box transcription factor 2 (SOX2), a NSC marker, and T-Box Brain Protein 2 (TBR2), a neural progenitor cell (NPC) marker, respectively, for all combinations of small molecule inhibitors used from D0 to D7. Qualitative analysis of immunocytochemistry results revealed that ApoE became more localised to the intracellular region at D18/19 compared to D12 ( Figure 3; ( Lee, 2020c; Lee, 2020d)).
Figure 3. APOE localisation pattern becomes more intracellular with in vitro directed differentiation.
APOE protein is more localised intracellularly in differentiated cells. (Left) Representative images of cells at D12 and D18/19 expressing SOX2 (NSC marker) and TBR2 (NPC marker), respectively. Insets show images of SOX2/TBR2 in green and APOE in red. Scale bars indicate 50 µm unless stated otherwise. (Right) Quantification of SOX2-/TBR2-positive cells and intracellular APOE-positive cells amongst SOX2-TBR2-positive cells. DSi: dual SMAD inhibition. DS-Wi: DSi plus Wnt/β-catenin inhibition. DS-WHi: DS-Wi plus sonic hedgehog inhibition.
Discussion
Unlike the existing animal models of APOE deficiency and humanised APOE expression where genetic modifications were introduced globally (whole body) rather than specifically to NSCs, the in vitro model used in this study allowed us to examine APOE expression pattern exclusively in stem cells that were pushed towards the neural lineage. Our findings demonstrate that in cells at the earliest stage of neurodevelopment, 1) human APOE gene expression is high, and 2) APOE protein is not clearly localised at the intracellular region. Various combinations of small molecule inhibitors did not alter these patterns of expression.
Although further investigations will be needed to understand the exact role of APOE in neurodevelopment, the existing literature seems to suggest that APOE can be both downstream and upstream of stem cell maintenance. For example, several chromatin precipitation studies have shown that POU class 5 homeobox 1 (POU5F1), SOX2, Kruppel like factor 4 (KLF4), MYC proto-oncogene (MYC) and Nanog Homeobox (NANOG) all bind to the promoter region of APOE, suggesting that APOE expression could be directly regulated by such stem cell maintenance factors ( ENCODE Project Consortium, 2004; ENCODE Project Consortium, 2011; Kim et al., 2008; Lachmann et al., 2010; Liu et al., 2008; Marson et al., 2008). However, other evidence suggests that APOE itself could be a direct regulator of cell fate determination. Meyer and colleagues ( Meyer et al., 2019) showed that changing the APOE genotype from ε4 (AD risk factor) to ε3 (neutral genotype) in human NPCs can suppress premature neuronal differentiation and maturation via increasing the transcription repressor activity of RE1 silencing transcription factor (REST). Interestingly, APOE mRNA levels were lower in ε4 NPCs compared to ε3 NPCs, suggesting higher APOE gene expression is indeed likely to be associated with the undifferentiated state of NPCs. As a follow-up to our findings and the existing literature, we propose that further investigations should be carried out to elucidate the role of APOE in stem cell maintenance. For example, one could examine whether prolonged expression and/or overexpression of APOE gene in human NPCs can suppress further differentiation in these cells.
In this study, qualitative analysis was performed on APOE immunocytochemistry results. As the cells became more differentiated from NSCs to NPCs, APOE localisation pattern became more clearly intracellular. To validate this observation, co-localisation analysis with cytoskeletal proteins (such as Tubulin beta-3 chain and Microtubule-associated protein 2) or with plasma membrane proteins (such as N-Cadherin) will be needed in future investigations. Furthermore, APOE has been shown to exist in both secreted and intracellular forms in the existing literature ( Huang & Mahley, 2014). Therefore, it will also be important to examine which form of APOE is produced at each differentiation stage. It is possible that more APOE is secreted in undifferentiated cells compared to differentiated cells, which may not be fully captured using immunocytochemistry techniques performed on fixed cells. Interestingly, Gan and colleagues previously reported that APOE is indeed secreted by NSCs as well as NPCs, and secreted APOE was found to play a vital role in regulating NSC survival and neurosphere formation ( Gan et al., 2011). Further investigations on changes of secreted and intracellular protein levels throughout neural differentiation will be able to clarify whether cells indeed produce different forms and levels of APOE depending on its differentiation state. This will in turn provide more definitive clues to whether APOE plays a stage-dependent role in neurodevelopment.
In this study, qualitative analysis was performed on APOE immunocytochemistry results. As the cells became more differentiated from NSCs to NPCs, APOE localisation pattern became more intracellular. To validate this observation, however, additional experiments with a more direct quantitative approach should be conducted. For example, APOE protein levels in various subcellular compartments could be measured and compared by performing Western Blot. Since APOE has been shown to exist in both secreted and intracellular forms ( Huang & Mahley, 2014), it will be interesting to see which form of APOE is produced at each differentiation stage. It is possible that more APOE is secreted in undifferentiated cells compared to differentiated cells, which may not be fully captured using immunocytochemistry techniques performed on fixed cells. Interestingly, Gan and colleagues previously reported that APOE is indeed secreted by NSCs as well as NPCs, and secreted APOE was found to play a vital role in regulating NSC survival and neurosphere formation ( Gan et al., 2011). Therefore, further investigations on secreted and intracellular APOE using quantitative approaches will be able to clarify whether cells indeed produce different forms and levels of APOE depending on its differentiation state. This will in turn provide more definitive clues to whether APOE plays a stage-dependent role in neurodevelopment.
One limitation of this study is that the time-dependent changes of differentiation markers such as SOX2 and TBR2 were not examined alongside APOE. It is worth noting, however, that TBR2 was shown to be capable of suppressing SOX2 expression during differentiation of NSCs to NPCs ( Hodge et al., 2012). Given this information, it is unlikely that TBR2-positive cells observed in this study at D18/19 will simultaneously express high levels of SOX2. However, time-dependent changes of various markers of differentiation would add further validity to our observations and unequivocally clarify whether APOE expression is indeed correlated with the differentiation state of the cells. Another limitation of this study is that the exact locus of APOE expression could not be examined in detail using a standard epifluorescence microscope in this study. High-resolution microscopy techniques (such as confocal microscopy) would have been more ideal to identify the accurate loci of APOE expression and overcome the challenges of imaging densely packed cells at the earliest stages of neural induction (D0–D7). Further investigations with improved imaging capacity will therefore allow us to characterise APOE during the earlier stages of neural induction and hint at potential mechanisms underlying its role in neurodevelopment.
Since NSCs derived from iPSCs in vitro may not fully resemble the developmental and postnatal NSCs found in vivo, APOE expression should be further investigated in animal models of brain development as well. The most direct evidence of in vivo APOE expression in NSCs to this date comes from a study by Yang and colleagues, where Nestin-positive NSCs in the mouse developing dentate gyrus was isolated using fluorescence-activated cell sorting, and APOE expression was examined from as early as postnatal day 7 (P7) ( Yang et al., 2011). NSCs at P7 had low expression of APOE which increased with the age of mice, and the deletion of APOE had detrimental effects on the maintenance of stem cells in the dentate gyrus. Although these findings clearly demonstrate the importance of APOE in brain development, the study had limitations in that prenatal NSCs were not examined, and functional studies of APOE were based on global rather than conditional knockouts. Furthermore, Yang and colleagues’ data cannot be directly compared with our dataset due to species difference and the lack of detailed characterisation of NSCs in this study. To address this knowledge gap, more data from both in vitro and in vivo samples derived from various species should be generated and compared against each other. We hope that our focused study has laid a strong foundation to such collaborative investigations that may be conducted in the future.
In conclusion, we report that human APOE gene expression levels are highly correlated with the undifferentiated state of cells during directed differentiation in vitro, and ApoE protein is localised more in the intracellular region in cells at later stages of differentiation. Combining our observations and previous evidence reported in the literature, we speculate that APOE has an important role in stem cell maintenance and propose that further investigations should be carried out to validate our findings including methods that were not employed in this study. Moreover, it would be interesting to examine the exact underlying mechanisms such as 1) whether APOE is an upstream or downstream factor of stem cell maintenance, and 2) whether APOE4 genotype and APOE loss-of-function would produce similar phenotypes.
Data availability
Underlying data
Figshare: raw data for qPCR. https://doi.org/10.6084/m9.figshare.12136944.v1 ( Lee, 2020a)
This project contains the following underlying data:
-
-
Lee et al. raw data for qPCR.csv (C(t) values, efficiency of amplification, and values calculated for normalised gene expression analysis for APOE.)
Figshare: raw data for Genotyping. https://doi.org/10.6084/m9.figshare.12199721.v1 ( Lee, 2020b)
This project contains the following underlying data:
-
-
Lee et al. raw data for Genotyping.TIF (Gel image used in Figure 1. Genomic DNA from human iPSCs amplified for the APOE locus, then digested with HhaI enzyme. Run on 3% agarose gel.)
Figshare: raw data for D12 and D18/19 immunocytochemistry. https://doi.org/10.6084/m9.figshare.12199745.v1 ( Lee, 2020c)
This project contains the following underlying data:
-
-
01 Lee et al. raw data for DS D12 Hoechst.tif
-
-
02 Lee et al. raw data for DS D12 SOX2.tif
-
-
03 Lee et al. raw data for DS D12 APOE.tif
-
-
04 Lee et al. raw data for DS D18-19 Hoechst.tif
-
-
05 Lee et al. raw data for DS D18-19 TBR2.tif
-
-
06 Lee et al. raw data for DS D18-19 APOE.tif
-
-
07 Lee et al. raw data for DS+Wi D12 Hoechst.tif
-
-
08 Lee et al. raw data for DS+Wi D12 SOX2.tif
-
-
09 Lee et al. raw data for DS+Wi D12 APOE.tif
-
-
10 Lee et al. raw data for DS+Wi D18-19 Hoechst.tif
-
-
11 Lee et al. raw data for DS+Wi D18-19 TBR2.tif
-
-
12 Lee et al. raw data for DS+Wi D18-19 APOE.tif
-
-
13 Lee et al. raw data for DS+WHi D12 Hoechst.tif
-
-
14 Lee et al. raw data for DS+WHi D12 SOX2.tif
-
-
15 Lee et al. raw data for DS+WHi D12 APOE.tif
-
-
16 Lee et al. raw data for DS+WHi D18-19 Hoechst.tif
-
-
17 Lee et al. raw data for DS+WHi D18-19 TBR2.tif
-
-
18 Lee et al. raw data for DS+WHi D18-19 APOE.tif
(Images were taken with IX70 inverted epifluorescence microscope (Olympus) connected to AxioVision imaging software 4.4. ImageJ 1.49c was used to generate TIFF files.)
Figshare: raw data for quantification of D12 and D18/19 immunocytochemistry. https://doi.org/10.6084/m9.figshare.12781064.v1 ( Lee, 2020d)
This project contains the following underlying data:
- pipeline_01_DSi.cppipe
- pipeline_02_DS-Wi.cppipe
- pipeline_03_DS-WHi.cppipe
- quantification.xlsx
(cppipe files: CellProfiler v.3.1.9 was used to generate the pipelines for each image set (one differentiation protocol, 3 channels, 2 timepoints. quantification.xlsx: The raw data generated after running the pipelines were copied to a spreadsheet. 'Cells / Nuclei (%)' column shows the percentage of cells positive for SOX2 at D12 and TBR2 at D18/19. 'Intracellular APOE / Cells (%)' column shows the percentage of intracellular APOE-positive cells amongst SOX2- and TBR2-positive cells. These 2 columns are presented as bar graphs on Figure 3 of the manuscript.)
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
Acknowledgements
The authors would like to thank Dr Graham Cocks for the insightful discussion regarding the project. We also thank Rupert Faraway, Matthew J. Reid, and James Williams, past members of the iPSC technicians’ team of Jack Price group, for reprogramming, performing quality-control, and providing guidance for maintaining the CTR_M3_36S iPSC line used in this study. We also thank Dr. Mahito Nakanishi (AIST, Japan) for providing the Yamanaka factors Sendai virus that was used for reprogramming.
Funding Statement
PhD studentship of HL is sponsored by the King’s College London Graduate School PGR (postgraduate research) International Research Studentship. The lab of ST (Thuret Lab) is partly funded by Medical Research Council (MRC) research grants [MR/N030087/1 and MR/S00484X/1].
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
[version 2; peer review: 3 approved]
References
- Boyles JK, Pitas RE, Wilson E, et al. : Apolipoprotein E associated with astrocytic glia of the central nervous system and with nonmyelinating glia of the peripheral nervous system. J Clin Invest. 1985;76(4):1501–1513. 10.1172/JCI112130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocks G, Curran S, Gami P, et al. : The utility of patient specific induced pluripotent stem cells for the modelling of Autistic Spectrum Disorders. Psychopharmacology (Berl). 2014;231(6):1079–1088. 10.1007/s00213-013-3196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deans PJM, Raval P, Sellers KJ, et al. : Psychosis Risk Candidate ZNF804A Localizes to Synapses and Regulates Neurite Formation and Dendritic Spine Structure. Biol Psychiatry. 2017;82(1):49–61. 10.1016/j.biopsych.2016.08.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elshourbagy NA, Liao WS, Mahley RW, et al. : Apolipoprotein E mRNA is abundant in the brain and adrenals, as well as in the liver, and is present in other peripheral tissues of rats and marmosets. Proc Natl Acad Sci U S A. 1985;82(1):203–207. 10.1073/pnas.82.1.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium: A user’s guide to the encyclopedia of DNA elements (ENCODE). PLoS Biol. 2011;9(4):e1001046. 10.1371/journal.pbio.1001046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENCODE Project Consortium: The ENCODE (ENCyclopedia of DNA elements) project. Science. 2004;306(5696):636–640. 10.1126/science.1105136 [DOI] [PubMed] [Google Scholar]
- Gan HT, Tham M, Hariharan S, et al. : Identification of ApoE as an autocrine/paracrine factor that stimulates neural stem cell survival via MAPK/ERK signaling pathway. J Neurochem. 2011;117(3):565–578. 10.1111/j.1471-4159.2011.07227.x [DOI] [PubMed] [Google Scholar]
- Henderson JN, Crook R, Crook J, et al. : Apolipoprotein E4 and tau allele frequencies among Choctaw Indians. Neurosci Lett. 2002;324(1):77–79. 10.1016/s0304-3940(02)00150-7 [DOI] [PubMed] [Google Scholar]
- Hodge RD, Nelson BR, Kahoud RJ, et al. : Tbr2 is essential for hippocampal lineage progression from neural stem cells to intermediate progenitors and neurons. J Neurosci. 2012;32(18):6275–6287. 10.1523/JNEUROSCI.0532-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Mahley RW: Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. Neurobiol Dis. 2014;72 PtA:3–12. 10.1016/j.nbd.2014.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathuria A, Nowosiad P, Jagasia R, et al. : Stem cell-derived neurons from autistic individuals with SHANK3 mutation show morphogenetic abnormalities during early development. Mol Psychiatry. 2018;23(3):735–746. 10.1038/mp.2017.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Chu J, Shen X, et al. : An extended transcriptional network for pluripotency of embryonic stem cells. Cell. 2008;132(6):1049–1061. 10.1016/j.cell.2008.02.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachmann A, Xu H, Krishnan J, et al. : ChEA: transcription factor regulation inferred from integrating genome-wide ChIP-X experiments. Bioinformatics. 2010;26(19):2438–2444. 10.1093/bioinformatics/btq466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H: raw data for qPCR. figshare. Dataset.2020a. 10.6084/m9.figshare.12136944.v1 [DOI]
- Lee H: raw data for Genotyping. figshare. Dataset.2020b. 10.6084/m9.figshare.12199721.v1 [DOI]
- Lee H: raw data for D12 and D18/19 immunocytochemistry. figshare. Dataset.2020c. 10.6084/m9.figshare.12199745.v1 [DOI]
- Lee H: raw data for quantification of D12 and D18/19 immunocytochemistry. figshare. Dataset.2020d. 10.6084/m9.figshare.12781064.v1 [DOI]
- Liao F, Yoon H, Kim J: Apolipoprotein E metabolism and functions in brain and its role in Alzheimer’s disease. Curr Opin Lipidol. 2017;28(1):60–67. 10.1097/MOL.0000000000000383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Huang J, Chen T, et al. : Yamanaka factors critically regulate the developmental signaling network in mouse embryonic stem cells. Cell Res. 2008;18(12):1177–1189. 10.1038/cr.2008.309 [DOI] [PubMed] [Google Scholar]
- Mahley RW, Huang Y: Apolipoprotein e sets the stage: response to injury triggers neuropathology. Neuron. 2012;76(5):871–885. 10.1016/j.neuron.2012.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahley RW, Rall SC: Apolipoprotein E: far more than a lipid transport protein. Annu Rev Genomics Hum Genet. 2000;1:507–537. 10.1146/annurev.genom.1.1.507 [DOI] [PubMed] [Google Scholar]
- Marson A, Levine SS, Cole MF: Connecting microRNA genes to the core transcriptional regulatory circuitry of embryonic stem cells. Cell. 2008;134(3):521–533. 10.1016/j.cell.2008.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Davila-Velderrain J, Peng Z, et al. : Single-cell transcriptomic analysis of Alzheimer’s disease. Nature. 2019;570(7761):332–337. 10.1038/s41586-019-1329-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J, Stüber K, Wunderlich P, et al. : APP processing in human pluripotent stem cell-derived neurons is resistant to NSAID-based γ-secretase modulation. Stem Cell Reports. 2013;1(6):491–498. 10.1016/j.stemcr.2013.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Feldman HM, Lu T, et al. : REST and Neural Gene Network Dysregulation in iPSC Models of Alzheimer’s Disease. Cell Rep. 2019;26(5):1112–1127.e9. 10.1016/j.celrep.2019.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otani T, Marchetto MC, Gage FH, et al. : 2D and 3D Stem Cell Models of Primate Cortical Development Identify Species-Specific Differences in Progenitor Behavior Contributing to Brain Size. Cell Stem Cell. 2016;18(4):467–480. 10.1016/j.stem.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray J, Gage FH: Differential properties of adult rat and mouse brain-derived neural stem/progenitor cells. Mol Cell Neurosci. 2006;31(3):560–573. 10.1016/j.mcn.2005.11.010 [DOI] [PubMed] [Google Scholar]
- Xu Q, Bernardo A, Walker D, et al. : Profile and regulation of apolipoprotein E (ApoE) expression in the CNS in mice with targeting of green fluorescent protein gene to the ApoE locus. J Neurosci. 2006;26(19):4985–4994. 10.1523/JNEUROSCI.5476-05.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CP, Gilley JA, Zhang G, et al. : ApoE is required for maintenance of the dentate gyrus neural progenitor pool. Development. 2011;138(20):4351–4362. 10.1242/dev.065540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DX, Di Giorgio FP, Yao J, et al. : Modeling hippocampal neurogenesis using human pluripotent stem cells. Stem Cell Reports. 2014;2(3):295–310. 10.1016/j.stemcr.2014.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]