Abstract
Apolipoprotein E4 (APOE4) genotype is a risk factor for poor outcome after traumatic brain injury (TBI), particularly in young patients, but the underlying mechanisms are not known. By analogy to effects of APOE4 on the risk of Alzheimer disease (AD), the APOE genotype may influence β-amyloid (Aβ) and tau deposition after TBI. To test this hypothesis, we crossed 3xTG-AD transgenic mice carrying 3 human familial AD mutations (PS1M146V, tauP301L, and APPSWE) to human ApoE2-, ApoE3-, and ApoE4-targeted replacement mice. Six- to 8-month-old 3xTG-ApoE mice were assayed by quantitative immunohistochemistry for amyloid precursor protein (APP), Aβ1–40 (Aβ40), Aβ1–42 (Aβ42), total human tau, and phosphoserine 199 (pS199) tau at 24 hours after moderate controlled cortical impact. There were increased numbers of APP-immunoreactive axonal varicosities in 3xTG-ApoE4 mice versus the other genotypes. This finding was repeated in a separate cohort of ApoE4-targeted replacement mice without human transgenes compared with ApoE3 and ApoE2 mice. There were no differences between genotypes in the extent of intra-axonal Aβ40 and Aβ42; none of the mice had extracellular Aβ deposition. Regardless of injury status, 3xTG-ApoE4 mice had more total human tau accumulation in both somatodendritic and intra-axonal compartments than other genotypes. These results suggest that the APOE4 genotype may have a primary effect on the severity of axonal injury in acute TBI.
Keywords: Apolipoprotein E, Axon injury, Traumatic brain injury
INTRODUCTION
Clinical studies have revealed an increased risk of poor outcome after traumatic brain injury (TBI) in patients with one or more apolipoprotein E4 (APOE4) alleles (1–6). The largest of these studies demonstrated that the effect of APOE genotype was significant only in younger patients (4). Apolipoprotein E is a major lipid carrier in the brain (7), and there have been multiple mechanisms proposed to account for the effect in TBI and other brain injuries (8). One hypothesis is that APOE genotype affects secondary neurodegenerative processes after TBI. This hypothesis is based on the ideas that 1) APOE4 is a major genetic risk factor for Alzheimer disease (AD) (9, 10); 2) TBI is a major environmental risk factor for AD (11, 12); and 3) ApoE has been shown to interact with two key AD proteins, β-amyloid (Aβ) and tau (13). Supporting this hypothesis, human mutant amyloid precursor protein (APP) transgenic mice with the APOE4 allele show greater accumulation of Aβ chronically after TBI (14). However, the APOE genotype can affect acute outcomes after TBI (3, 15–17), and Aβ and tau pathologies can accumulate rapidly after TBI (18–22). Although ApoE isoform–specific differences have been studied in mouse models of TBI, few studies have addressed interactions between ApoE genotype on acute Aβ deposition after injury (23–25). Furthermore, to our knowledge, the interaction between the APOE genotype and tau pathology has not been investigated.
We recently showed that 3xTG-AD mice carrying 3 human familial AD mutations (PS1M146V, tauP301L, and APPSWE) develop both acute Aβ and tau pathology after injury (26, 27). We hypothesized that, in the presence of the APOE4 allele, both acute Aβ and tau pathology after injury would be exacerbated. To test this, we crossed 3xTG-AD transgenic mice to mice that have the human APOE2, APOE3, and APOE4 alleles knocked-in (28, 29). The results of stereologic analysis suggest that APOE genotype may not affect acute Aβ or tau pathology after injury. Instead, APOE genotype seems to result in increased numbers of injured axons as evident by APP-positive white matter varicosities. Considering this surprising finding, we confirmed that the APOE4 genotype worsens posttraumatic axonal injury in non–3xTG-AD mice expressing human APOE alleles. Altogether, this indicates a primary role for ApoE in acute posttraumatic axonal injury that seems to be unrelated to its interactions with Aβ or tau.
MATERIALS AND METHODS
Animals
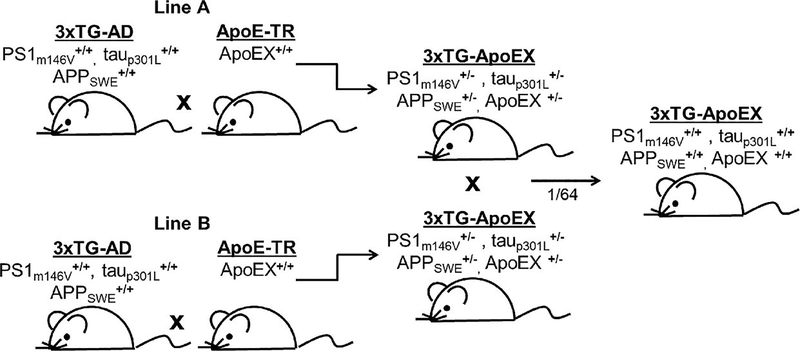
Female 3xTG-AD mice were initially acquired from Frank LaFerla (University of California, Irvine, CA) and were bred to male ApoE-targeted replacement mice (ApoE-TR) of all 3 isoforms (Fig. 1). Heterozygotes from 2 separate crosses were mated to avoid sibling matings. The resulting litters were screened for quadruple homozygotes by polymerase chain reaction using previously published methods (28, 29). Because the tau and APP alleles cosegregate in the 3xTG-AD line whereas the PS1 and ApoE alleles segregate independently, one of 64 mice born were expected to be homozygous for all 4 transgenes (Fig. 1). We produced at least 4 of these quadruple homozygotes for each genotype to establish the 3xTG-ApoE2, -ApoE3, and -ApoE4 lines. Nonsibling quadruple homozygotes were then mated to yield the experimental mice used for the current study. All mice were housed on a 12-hour light–dark cycle with food and water ad libitum in accordance with the Animal Studies Committee at Washington University in St Louis, Mo.
FIGURE 1.
Generation of 3xTG-ApoE mice. For each of the 3 human ApoE alleles, 2 independent lines of 3xTg-ApoE heterozygotes were initially produced from 3xTg-AD and ApoE-targeted replacement homozygotes (ApoE-TR: ApoEX+/+ represents ApoE2, ApoE3, or ApoE4 homozygote mice). Nonsibling heterozygotes were mated, and one of 64 mice was expected to be homozygous at all 4 alleles. Quadruple homozygotes were selected by polymerase chain reaction and used as the initial founders of each 3xTG-ApoE line.
Controlled Cortical Impact
Two- to 3-month-oldmale and female ApoE-TR mice (28) and 6- to 8-month-old male and female 3xTG-ApoE mice were used in the following experiments: 17 3xTG-ApoE2, 13 3xTG-ApoE3, 11 3xTG-ApoE4, 5 ApoE2, 5 ApoE3, and 5 ApoE4 mice were randomly assigned to either injury or sham groups, and a 2-mm controlled cortical impact (CCI) was performed, as previously described (30). Briefly, mice were anesthetized with isoflurane and placed in a stereotaxic frame. A rectal probe and heat pad were used to maintain constant body temperature. A midline incision was made to expose the skull, and a 5-mm circular burr was used to perform craniotomy over the left somatosensory cortex. A 3-mm steel impactor tip was then aligned to +1.5 mm (A/P) relative to lambda and −1.2 mm (M/L) relative to midline. An electromagnetic device delivered an impact to the brain to a depth of 2 mm (5 m per second, 100 milliseconds dwell time). The contusion was irrigated with saline, and a plastic skullcap was affixed with suture glue to cover the craniotomy. Sham mice underwent the same surgical procedure but did not receive an impact. Mice were allowed to recover on a heat pad before being returned to their cage.
Immunohistochemistry
At 24 hours after CCI, mice were deeply anesthetized with isoflurane and perfused with 0.3% heparin in PBS. Brains were dissected and fixed in 4% paraformaldehyde for 24 hours and then equilibrated in 30% sucrose PBS. All sections were sliced 50 μm thick on a freezing microtome. Every sixth section (300 μm) was then immunostained using antibodies to APP (0.25 μg/mL; Zymed, Invitrogen), Aβ1–40 ([Aβ40] 0.5 μg/mL; Invitrogen), Aβ1–42 ([Aβ42] 0.5 μg/mL; Invitrogen), total human tau (1 μg/mL; Thermo Scientific), or pS199 tau (1:1000; Invitrogen). Three minutes of 70% formic acid retrieval was used to unmask epitopes for Aβ40 and Aβ42 and 10 minutes of formic acid for pS199 tau. All other staining methods were followed as published (26).
Stereology
Stereology was performed blinded to genotype using a Nikon Eclipse 80i microscope with a motorized stage. Both the optical fractionator and space ball probes in Stereo Investigator version 8.2 were used for analysis. Regions of interest were drawn under a 4× objective as previously described for fimbria, pericontusional corpus callosum and external capsule, and hippocampal CA1, beginning with the most anterior section containing both blades of the dentate gyrus and including the following 3 to 4 sections per mouse (26, 31). All counts were performed using a 60× lens. For APP and tau, a grid size of 200 × 200 μm and counting frame of 40 × 40 μm were used. For Aβ40 and Aβ42, a 200 × 200–μm grid and 80 × 80–μm counting frame were used. For pS199 tau, a 200 × 200–μm grid and a 50 × 50–μm counting frame were used. For the space ball hemispherical probe, a radius of 17 μm was used. In all cases, a guard depth of 5 μm and a probe height of 17 μm were used. These parameters ensured that the Gunderson coefficient of error was less than 0.15 in all cases.
Statistics
Scatter plots were constructed and Shapiro-Wilk tests performed to assess for evidence of non-normally distributed data. All data were normally distributed, except for APP stereology in ApoE4 mice (p < 0.05). In this case, a Kruskal-Wallis one-way analysis of variance (ANOVA) was performed, followed by Mann-Whitney U tests. All other data were analyzed by 2-way ANOVAs (injury and genotype), with Bonferroni corrections for multiple comparisons. Significance was determined as p < 0.05 for 2-way ANOVA and p < 0.01 for all post hoc comparisons. Planned comparisons included injured versus sham mice for each genotype and comparisons of injured 3xTG-ApoE4 mice with injured 3xTG-ApoE2 and -ApoE3 mice. This resulted in a total of 5 planned comparisons for each analysis.
RESULTS
APOE Genotype Alters the Extent of Axonal Injury
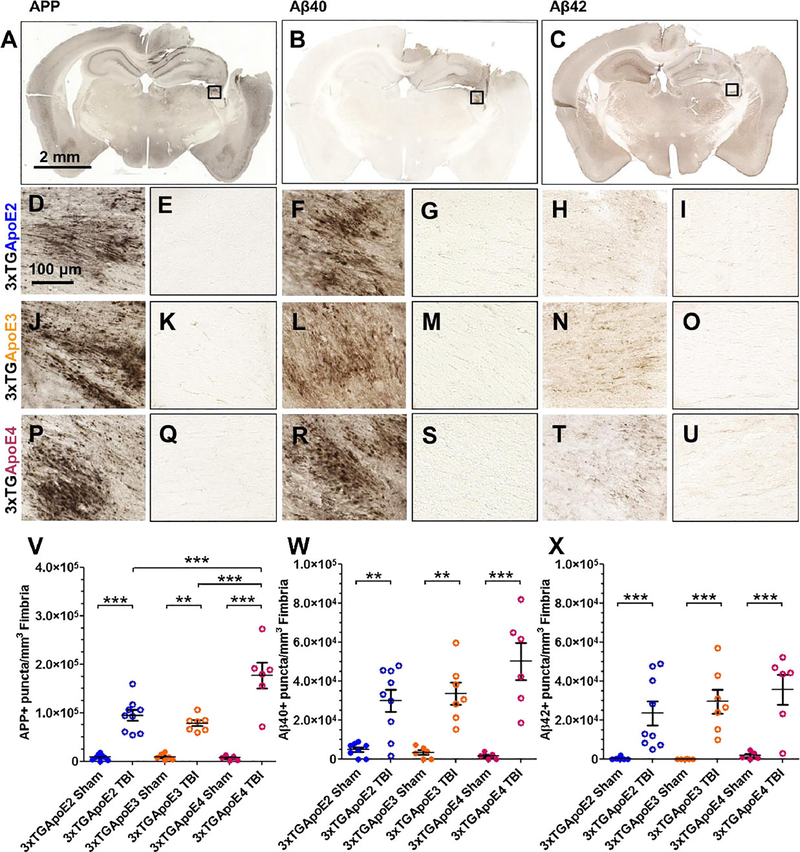
Amyloid precursor protein is a marker of fast axonal transport failure that accumulates in injured axons at the location of disrupted microtubules (32–34). For these experiments, APP was used to assay axonal injury (Figs. 2A, D, E, J, K, P, Q, V; 3A–C). In contrast to CCI-injured mice at 24 hours, 3xTG-ApoE sham-operated mice did not have axons containing APP. At 24 hours after injury, APP-positive axons were visible in corpus callosum, external capsule, and ipsilateral fimbria (Fig. 2A). Stereologic quantification of APP-positive axons in fimbria revealed main effects of genotype (p = 0.001), injury (p < 0.000001), and a genotype × injury interaction (Fig. 2V; p = 0.0006). Planned post hoc comparisons indicated that injured 3xTG-ApoE4 mice have significantly greater numbers of APP-positive axonal varicosities in ipsilateral fimbria compared with those of injured 3xTG-ApoE2 (p = 0.00016) and injured 3xTG-ApoE3 mice (p = 0.00002).
FIGURE 2.
Amyloid precursor protein (APP) and Aβ immunohistochemistry in 3xTG-ApoE mice. (A–C) Representative coronal slice images of APP (A), Aβ1–40 (Aβ40) (B), and Aβ1–42 (Aβ) (C) staining from an injured 3xTG-ApoE2 mouse. (D–I) Higher magnification images of fimbria (box) from injured (D, F, H) and sham (E, G, I) 3xTG-ApoE2 stained for APP (D, E), Aβ40 (F, G), and Aβ42 (H, I). (J–O) 3xTG-ApoE3 injured (J, L, N) and sham (K, M, O) stained for APP (J, K), Aβ40 (L, M), and Aβ42 (N, O). (P–U) 3xTG-ApoE4 injured (P, R, T) and sham (Q, S, U) stained for APP (P, Q), Aβ40 (R, S), and Aβ42 (T, U). Stereologic quantification of axonal varicosities containing APP (V), Aβ40 (W), or Aβ42 (X). Error bars represent SE. ** p < 0.01, *** p < 0.001.
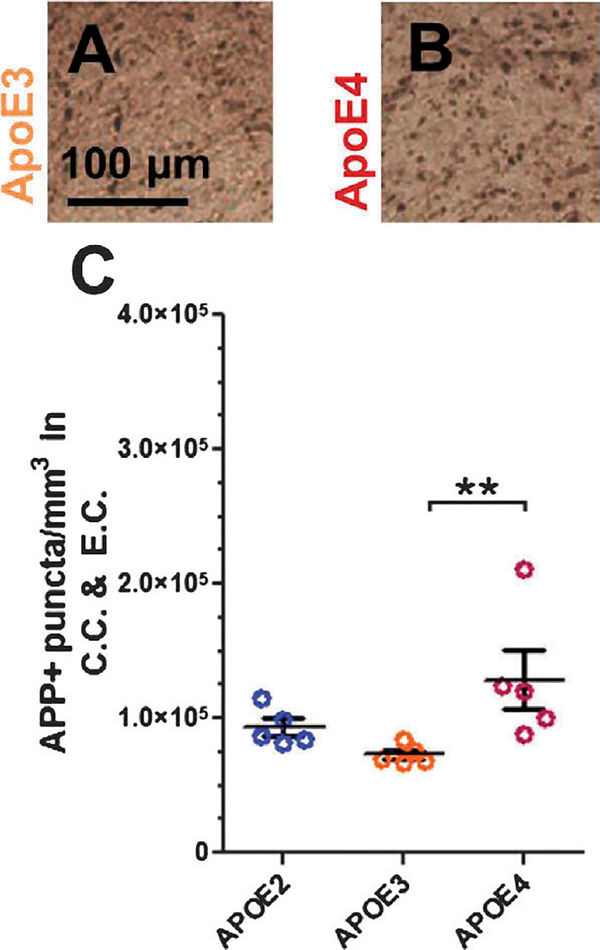
FIGURE 3.
Amyloid precursor protein immunohistochemistry in ApoE-TR mice. (A, B) Representative images from pericontusional corpus callosum and external capsule of ApoE3 (A) and ApoE4 (B) mice immunostained for APP. (C) Stereologic quantification of axonal varicosities containing APP. Error bars represent SE. **p < 0.01. APP, amyloid precursor protein; CC, corpus callosum; EC, external capsule.
Considering this unexpected result, this experiment was repeated using ApoE-TR mice. Stereologic quantification of APP-positive axons (Fig. 3A–C) in pericontusional corpus callosum and external capsule revealed an effect of genotype (p = 0.005), where ApoE4 mice have more APP-positive axons than ApoE3 mice (p = 0.008), but not ApoE2 mice (p = 0.056). Thus, we confirmed the finding that ApoE4-expressing mice have greater APP-positive axonal injury in 2 separate mouse models.
Injury Results in Increased Intra-Axonal Aβ in All Genotypes
Because APP is cleaved by secretases to produce Aβ in injured axons (20, 26), adjacent sets of serial sections were stained for Aβ40 (Fig. 2B, F, G, L, M, R, S, W) and Aβ42 (Fig. 2C, H, I, N, O, T, U, X). Similar to APP, Aβ40 and Aβ42 were seen in injured mice but not in shams. Analysis of Aβ40 revealed a main effect of injury (Fig. 2W; p < 0.000001) but no effect of genotype (p = 0.26) or genotype × injury interaction (p = .094). There seemed to be a trend toward increased Aβ40 in 3xTg-ApoE4 mice, but this did not reach statistical significance. Similarly, analysis of Aβ42 also revealed a main effect of injury (Fig. 2X; p = 0.000001) but no effect of genotype (p = 0.4192) or genotype × injury interaction (p = 0.5639).
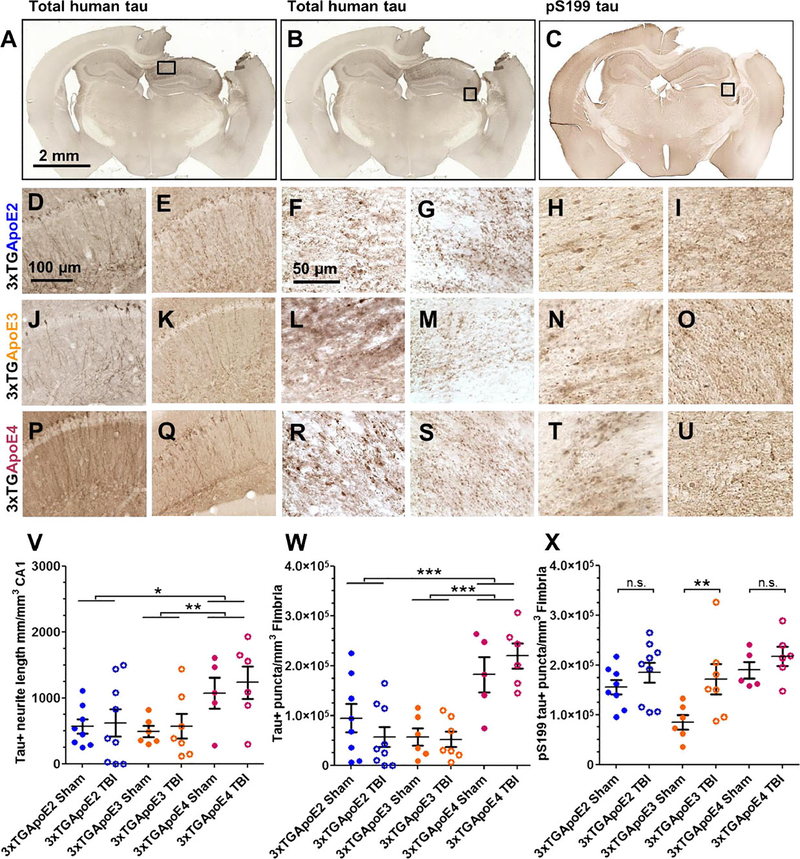
APOE Genotype Alters Somatodendritic and Intra-Axonal Tau in 3xTG-AD Mice
The microtubule-stabilizing protein tau also accumulates in the hippocampal CA1 and in the fimbria after injury in 3xTG-AD mice (26). To image accumulation of tau in 3xTG-ApoE mice, an antibody that recognizes total human tau was used to label a fourth set of serial sections (Fig. 4A, B, D–G, J–M, P–S). Similar to 3xTg-AD mice, tau was observed in the somatodendritic compartment of hippocampal CA1 neurons. The length of these tau-positive neurites was measured using stereologic methods. This revealed a main effect of genotype (Fig. 4V; p = 0.006) but no effect of injury (p = 0.52) or genotype × injury (p = 0.96). 3xTG-ApoE4 sham and injured mice had significantly more accumulated tau than 3xTG-ApoE3 mice (p = 0.009), but the difference with 3xTG-ApoE2 mice was not statistically significant after correction for multiple comparisons (p = 0.013).
FIGURE 4.
Tau immunohistochemistry. (A–C) Representative coronal slice images of total human tau (A, B) and pS199 tau (C) staining from a 3xTG-ApoE2 mouse. (D–I) Higher magnification images of boxed region from injured (D, F, H) and sham (E, G, I) 3xTG-ApoE2 stained for total tau in CA1 (D, E), or total tau in fimbria (F, G), and pS199 tau (H, I) in fimbria. (J–O) 3xTG-ApoE3 injured (J, L, N) and sham (K, M, O) stained for total tau in CA1 (J, K) or in fimbria (L, M) and pS199 tau in fimbria (N, O). (P–U) 3xTG-ApoE4 injured (P, R, T) and sham (Q, S, U) stained for total tau in CA1 (P, Q) or in fimbria (R, S) and pS199 tau in fimbria (T, U). (V–X) Stereologic quantification of the length of neurites containing tau in the CA1 (V) and axonal varicosities in fimbria containing either tau (W) or pS199 tau (X). Error bars represent SE. *p = 0.013, ** p < 0.01, *** p < 0.001. ns, not significant.
Total human tau was also assessed in the fimbria (Fig. 4B, F, G, L, M, R, S, W). Stereologic analysis again revealed a main effect of genotype (Fig. 4W; p = 0.000003) but no effect of injury (p = 0.94) or genotype × injury (p = 0.31). Notably, both injured and sham 3xTG-ApoE4 mice had greater tau accumulation in axons than either 3xTG-ApoE2 (p = 0.0002) or 3xTG-ApoE3 mice (p = 0.000005).
To test whether APOE genotype affects phosphorylation of tau, a fifth set of adjacent sections was also stained for phospho-serine199 (pS199) tau (Fig. 4C, H, I, N, O, T, U). Similar to total human tau, pS199 tau was observed in the hippocampal CA1 and fimbria at 24 hours after injury (Fig. 4C). Main effects of genotype (Fig. 4X; p = 0.0069) and injury (p = 0.0092) were observed, but there was no genotype × injury interaction effect (p = 0.3097). Only the difference between injured and sham levels of pS199 tau in 3xTG-ApoE3 mice reached statistical significance. No difference was seen between injured and sham 3xTG-ApoE2 and 3xTG-ApoE4 mice or between injured 3xTG-ApoE4 mice and the other genotypes.
DISCUSSION
To summarize, at 24 hours after moderate CCI, 3xTG-AD mice with the APOE4 allele have greater APP accumulation within axons than mice with the APOE2 or APOE3 alleles. Apolipoprotein E4–targeted replacement mice without other human transgenes were also found to have greater APP accumulation versus ApoE3-targeted replacement mice. These observations suggest that modulation of the severity of axonal injury may be a primary contributor to the APOE genotype effect on outcomes after TBI. However, contrary to our hypothesis regarding neurodegenerative pathology after TBI, APOE genotype had no effect on the intra-axonal accumulation of Aβ40 or Aβ42; there was a similar increase in Aβ in all 3 genotypes. Also surprisingly, there was no interaction between injury and APOE genotype on tau pathology; indeed, more total human tau was found in the somatodendritic and axonal compartments of 3xTG-ApoE4 mice regardless of injury status.
Altogether, the 3xTG-ApoE mouse TBI model has similarities with and differences from the 3xTG-AD TBI model previously reported (26, 27). Both recapitulate key aspects of acute human TBI pathology, displaying axonal varicosities containing APP, Aβ, and tau (21). Mice expressing only ApoE or PDAPP-ApoE mice have also been studied in the setting of TBI (14, 25, 35), but neither of these models produced these 3 types of pathologic alterations. Notably, in 3xTG-AD mice, both Aβ and tau pathologies increased acutely after TBI, but in 3xTg-ApoE mice, only Aβ, but not tau, was affected by acute TBI. The absence of a TBI-related exacerbation of tau pathology may be caused by a protective effect of all 3 human ApoE isoforms compared with endogenous mouse ApoE, as has been reported for Aβ pathology in another AD mouse model (36).
One possible explanation for these results is that the axons of 3xTG-ApoE4 mice are more susceptible to injury. This may represent a loss of function of ApoE4 in the setting of TBI, similar to that seen in ApoE-deficient mice (37–39). Alternatively, it may represent a toxic gain of function of ApoE4. Others have proposed that ApoE4 may undergo a cleavage step to produce a toxic fragment that induces mitochondrial dysfunction and neuronal death (40). Studies comparing axonal injury in hemizygous ApoE4+/− mice similar to those conducted by Bien-Ly et al (41) may help differentiate between a loss or gain of function. Interestingly, our group recently showed that wild-type mice treated with COG1410, an ApoE-mimetic, after CCI have fewer APP-positive axons in pericontusional white matter 3 to 7 days after injury compared with saline-treated mice (42). Because COG1410 is a modified peptide sequence from human ApoE, this could indicate that ApoE4 lacks an axon-protective effect found in the other 2 isoforms.
Another possible interpretation of these results is that ApoE specifically affects the production, processing, or trafficking of APP. For example, ApoE4 has been shown to be more efficient at recycling APP from the cell surface back into the endocytic pathway (43). Additional markers of axonal injury including neurofilament immunohistochemistry, silver staining, electron microscopy, electrophysiology, and diffusion tensor imaging will be of interest to determine whether there is a global effect of APOE genotype on axonal injury or whether the effects are limited to the processing underlying abnormal APP accumulation. Thus, interpretation of the main finding from this study, that ApoE4 mice displayed more APP accumulations after injury than the other genotypes, is limited by our current understanding of the role of ApoE in axon biology.
It is also notable that both sham and injured 3xTG-ApoE4 mice have more total human tau staining but not pS199 tau than mice expressing either ApoE2 or ApoE3. Further characterization of phospho-tau epitopes may contribute to a clearer picture of tau pathology in this model. Increased total human tau in 3xTG-ApoE4 is not unexpected given several previous lines of research. First, ApoE4 fragments have been shown to induce tau accumulation (44, 45). Impaired ApoE4 binding to ApoE receptors in mice may cause dysregulation of tau kinases such as GSK3β and greater accumulation of the protein at baseline (46). Other researchers have reported that ApoE is produced in neurons after injury, and neuronal production of ApoE4 contributes to tau hyperphosphorylation and microtubule instability (47–49). We have not addressed the question of neuronal ApoE production after TBI. Also, ApoE2 and ApoE3 have been shown in vitro to bind tau and prevent hyperphosphorylation, whereas ApoE4 lacks this ability (50). Because the current study did not address the production of ApoE in neurons after TBI, it is unknown whether neuronal ApoE production is the mechanism of tau accumulation occurring in this model. It is unlikely to be a major contributor, however, because no significant difference was detected between sham and injured 3xTG-ApoE4 tau or pS199 tau levels; sham mice are not expected to have neuronal ApoE expression.
Additional studies will be required to understand these phenomena. First, functional tests such as Morris Water Maze will help in understanding the implications of increased APP accumulation within the fimbria. Second, biochemical studies will be necessary to determine the effects of APOE genotype on specific assembly forms of Aβ and tau after TBI. Third, a full characterization of the time course for both Aβ and tau pathology may reveal chronic effects of APOE genotype on Aβ and tau deposition that were not apparent in this initial acute injury analysis. Last, overexpression of the APP and tau transgenes may mask some of the effects of ApoE genotype on neurodegenerative pathologies. Using mice expressing both human APP and tau under endogenous promoters may be an alternative to the 3xTG-AD model for future studies (51, 52).
Altogether, these results demonstrate that it is feasible to produce mouse models for studying interactions between APOE genotype and important aspects of human acute neurodegenerative pathology after TBI. Considering the finding that APOE4 genotype contributes to increased axonal injury, this research has important implications for targeted therapeutics to benefit susceptible APOE4-carrying populations after TBI. Future research will seek to use this model for pharmacogenetic studies and to further understand how APOE genotype modifies axonal injury in both moderate TBI and less severe repetitive closed-skull injury models (53).
ACKNOWLEDGMENTS
The authors thank Frank LaFerla and Patrick Sullivan for providing 3xTG-AD and ApoE founder mice. The authors also thank Hien Tran for methodological assistance and David Holtzman for advice.
This work was funded by NIH NS065069 (David Brody), NIH K08-NS049237 (David Brody), NIH F31-NS076047 (Rachel Bennett), the Burroughs Wellcome Fund (David Brody), the Thrasher Research Fund (David Brody), and a Health South Research Grant (David Brody).
Footnotes
No competing financial interests exist.
Contributor Information
Rachel E. Bennett, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri.
Thomas J. Esparza, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri.
Hal A. Lewis, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri
Eddie Kim, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri; Department of Medicine, University of Alabama, Birmingham, Alabama.
Christine L. Mac Donald, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri.
Patrick M. Sullivan, Department of Medicine, Division of Geriatrics, VAMC, Geriatric Research Education Clinical Center, Duke University, Durham, North Carolina.
David L. Brody, Department of Neurology, Washington University School of Medicine, St. Louis, Missouri; Hope Center for Neurological Disorders, St. Louis, Missouri.
REFERENCES
- 1.Mayeux R, Ottman R, Maestre G, et al. Synergistic effects of traumatic head injury and apolipoprotein-epsilon 4 in patients with Alzheimer’s disease. Neurology 1995;45:555–57 [DOI] [PubMed] [Google Scholar]
- 2.Teasdale GM, Nicoll JA, Murray G, et al. Association of apolipoprotein E polymorphism with outcome after head injury. Lancet 1997;350:1069–71 [DOI] [PubMed] [Google Scholar]
- 3.Friedman G, Froom P, Sazbon L, et al. Apolipoprotein E-epsilon4 genotype predicts a poor outcome in survivors of traumatic brain injury. Neurology 1999;52:244–48 [DOI] [PubMed] [Google Scholar]
- 4.Teasdale GM, Murray GD, Nicoll JA. The association between APOE epsilon4, age and outcome after head injury: A prospective cohort study. Brain 2005;128:2556–61 [DOI] [PubMed] [Google Scholar]
- 5.Jordan BD, Relkin NR, Ravdin LD, et al. Apolipoprotein E epsilon4 associated with chronic traumatic brain injury in boxing. JAMA 1997; 278:136–40 [PubMed] [Google Scholar]
- 6.Kutner KC, Erlanger DM, Tsai J, et al. Lower cognitive performance of older football players possessing apolipoprotein E epsilon4. Neurosurgery 2000;47:651–57 [DOI] [PubMed] [Google Scholar]
- 7.Mahley RW. Apolipoprotein E: Cholesterol transport protein with expanding role in cell biology. Science 1988;240:622–30 [DOI] [PubMed] [Google Scholar]
- 8.Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol 2011;10:241–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993;261:921–23 [DOI] [PubMed] [Google Scholar]
- 10.Strittmatter WJ, Saunders AM, Schmechel D, et al. Apolipoprotein E: High-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci USA 1993;90:1977–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jellinger KA. Head injury and dementia. Curr Opin Neurol 2004;17: 719–23 [DOI] [PubMed] [Google Scholar]
- 12.Plassman BL, Havlik RJ, Steffens DC, et al. Documented head injury in early adulthood and risk of Alzheimer’s disease and other dementias. Neurology 2000;55:1158–66 [DOI] [PubMed] [Google Scholar]
- 13.Bu G Apolipoprotein E and its receptors in Alzheimer’s disease: Pathways, pathogenesis and therapy. Nat Rev Neurosci 2009;10:333–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hartman RE, Laurer H, Longhi L, et al. Apolipoprotein E4 influences amyloid deposition but not cell loss after traumatic brain injury in a mouse model of Alzheimer’s disease. J Neurosci 2002;22:10083–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nicoll JA, Roberts GW, Graham DI. Apolipoprotein E epsilon 4 allele is associated with deposition of amyloid beta-protein following head injury. Nat Med 1995;1:135–37 [DOI] [PubMed] [Google Scholar]
- 16.Smith C, Graham DI, Murray LS, et al. Association of APOE e4 and cerebrovascular pathology in traumatic brain injury. J Neurol Neurosurg Psychiatry 2006;77:363–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liaquat I, Dunn LT, Nicoll JA, et al. Effect of apolipoprotein E genotype on hematoma volume after trauma. J Neurosurg 2002;96:90–96 [DOI] [PubMed] [Google Scholar]
- 18.Roberts GW, Gentleman SM, Lynch A, et al. Beta A4 amyloid protein deposition in brain after head trauma. Lancet 1991;338:1422–23 [DOI] [PubMed] [Google Scholar]
- 19.Roberts GW, Gentleman SM, Lynch A, et al. Beta amyloid protein deposition in the brain after severe head injury: Implications for the pathogenesis of Alzheimer’s disease. J Neurol Neurosurg Psychiatry 1994;57: 419–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith DH, Chen XH, Iwata A, et al. Amyloid beta accumulation in axons after traumatic brain injury in humans. J Neurosurg 2003;98:1072–77 [DOI] [PubMed] [Google Scholar]
- 21.Uryu K, Chen XH, Martinez D, et al. Multiple proteins implicated in neurodegenerative diseases accumulate in axons after brain trauma in humans. Exp Neurol 2007;208:185–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ikonomovic MD, Uryu K, Abrahamson EE, et al. Alzheimer’s pathology in human temporal cortex surgically excised after severe brain injury. Exp Neurol 2004;190:192–203 [DOI] [PubMed] [Google Scholar]
- 23.Sabo T, Lomnitski L, Nyska A, et al. Susceptibility of transgenic mice expressing human apolipoprotein E to closed head injury: The allele E3 is neuroprotective whereas E4 increases fatalities. Neuroscience 2000;101: 879–84 [DOI] [PubMed] [Google Scholar]
- 24.Laskowitz DT, Song P, Wang H, et al. Traumatic brain injury exacerbates neurodegenerative pathology: Improvement with an apolipoprotein E–based therapeutic. J Neurotrauma 2010;27:1983–95 [DOI] [PubMed] [Google Scholar]
- 25.Mannix RC, Zhang J, Park J, et al. Age-dependent effect of apolipoprotein E4 on functional outcome after controlled cortical impact in mice. J Cereb Blood Flow Metab 2011;31:351–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tran HT, LaFerla FM, Holtzman DM, et al. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-beta accumulation and independently accelerates the development of tau abnormalities. J Neurosci 2011;31:9513–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tran HT, Sanchez L, Esparza TJ, et al. Distinct temporal and anatomical distributions of amyloid-beta and tau abnormalities following controlled cortical impact in transgenic mice. PLoS One 2011;6:e25475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan PM, Mezdour H, Aratani Y, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J Biol Chem 1997;272:17972–80 [DOI] [PubMed] [Google Scholar]
- 29.Oddo S, Caccamo A, Shepherd JD, et al. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron 2003;39:409–21 [DOI] [PubMed] [Google Scholar]
- 30.Brody DL, Mac Donald C, Kessens CC, et al. Electromagnetic controlled cortical impact device for precise, graded experimental traumatic brain injury. J Neurotrauma 2007;24:657–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mac Donald CL, Dikranian K, Song SK, et al. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol 2007;205:116–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tang-Schomer MD, Johnson VE, Baas PW, et al. Partial interruption of axonal transport due to microtubule breakage accounts for the formation of periodic varicosities after traumatic axonal injury. Exp Neurol 2012; 233:364–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Koo EH, Sisodia SS, Archer DR, et al. Precursor of amyloid protein in Alzheimer disease undergoes fast anterograde axonal transport. Proc Natl Acad Sci USA 1990;87:1561–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gentleman SM, Nash MJ, Sweeting CJ, et al. Beta-amyloid precursor protein (beta APP) as a marker for axonal injury after head injury. Neurosci Lett 1993;160:139–44 [DOI] [PubMed] [Google Scholar]
- 35.Crawford F, Wood M, Ferguson S, et al. Apolipoprotein E-genotype dependent hippocampal and cortical responses to traumatic brain injury. Neuroscience 2009;159:1349–62 [DOI] [PubMed] [Google Scholar]
- 36.Holtzman DM, Bales KR, Wu S, et al. Expression of human apolipoprotein E reduces amyloid-beta deposition in a mouse model of Alzheimer’s disease. J Clin Invest 1999;103:R15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Y, Lomnitski L, Michaelson DM, et al. Motor and cognitive deficits in apolipoprotein E–deficient mice after closed head injury. Neuroscience 1997;80:1255–62 [DOI] [PubMed] [Google Scholar]
- 38.Lynch JR, Pineda JA, Morgan D, et al. Apolipoprotein E affects the central nervous system response to injury and the development of cerebral edema. Ann Neurol 2002;51:113–17 [DOI] [PubMed] [Google Scholar]
- 39.Namjoshi DR, Martin G, Donkin J, et al. The liver X receptor agonist GW3965 improves recovery from mild repetitive traumatic brain injury in mice partly through apolipoprotein E. PLoS One 2013;8:e53529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang S, ran Ma T, Miranda RD, et al. Lipid- and receptor-binding regions of apolipoprotein E4 fragments act in concert to cause mitochondrial dysfunction and neurotoxicity. Proc Natl Acad Sci USA 2005;102: 18694–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bien-Ly N, Gillespie AK, Walker D, et al. Reducing human apolipoprotein E levels attenuates age-dependent Abeta accumulation in mutant human amyloid precursor protein transgenic mice. J Neurosci 2012;32: 4803–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang Y, Brody DL. Administration of COG1410 reduces axonal amyloid precursor protein immunoreactivity and microglial activation after controlled cortical impact in mice. J Neurotrauma 2012;29:2332–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ye S, Huang Y, Mullendorff K, et al. Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: ApoE structure as a potential therapeutic target. Proc Natl Acad Sci USA 2005;102: 18700–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang Y, Liu XQ,Wyss-Coray T, et al. Apolipoprotein E fragments present in Alzheimer’s disease brains induce neurofibrillary tangle-like intracellular inclusions in neurons. Proc Natl Acad Sci USA 2001;98:8838–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brecht WJ, Harris FM, Chang S, et al. Neuron-specific apolipoprotein e4 proteolysis is associated with increased tau phosphorylation in brains of transgenic mice. J Neurosci 2004;24:2527–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ohkubo N, Lee YD, Morishima A, et al. Apolipoprotein E and Reelin ligands modulate tau phosphorylation through an apolipoprotein E receptor/disabled-1/glycogen synthase kinase-3beta cascade. FASEB J 2003;17:295–97 [DOI] [PubMed] [Google Scholar]
- 47.Tesseur I, Van Dorpe J, Spittaels K, et al. Expression of human apolipoprotein E4 in neurons causes hyperphosphorylation of protein tau in the brains of transgenic mice. Am J Pathol 2000;156:951–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tesseur I, Van Dorpe J, Bruynseels K, et al. Prominent axonopathy and disruption of axonal transport in transgenic mice expressing human apolipoprotein E4 in neurons of brain and spinal cord. Am J Pathol 2000; 157:1495–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Horsburgh K, Nicoll JA. Selective alterations in the cellular distribution of apolipoprotein E immunoreactivity following transient cerebral ischaemia in the rat. Neuropathol Appl Neurobiol 1996;22:342–49 [DOI] [PubMed] [Google Scholar]
- 50.Strittmatter WJ, Saunders AM, Goedert M, et al. Isoform-specific interactions of apolipoprotein E with microtubule-associated protein tau: Implications for Alzheimer disease. Proc Natl Acad Sci USA 1994;91: 11183–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Guo Q, Wang Z, Li H, et al. APP physiological and pathophysiological functions: Insights from animal models. Cell Res 2012;22:78–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Andorfer C, Kress Y, Espinoza M, et al. Hyperphosphorylation and aggregation of tau in mice expressing normal human tau isoforms. J Neurochem 2003;86:582–90 [DOI] [PubMed] [Google Scholar]
- 53.Shitaka Y, Tran HT, Bennett RE, et al. Repetitive closed-skull traumatic brain injury in mice causes persistent multifocal axonal injury and microglial reactivity. J Neuropathol Exp Neurol 2012;70:551–67 [DOI] [PMC free article] [PubMed] [Google Scholar]