Highlights
-
•
The prevalence rate of clinically significant depression, anxiety, and PTSD symptoms for hospital discharged patients are respectively 19%, 10.4%, and 12.4%.
-
•
Disease severity, living with children, death of family member, and perceived discrimination are risk factors for PTSD.
-
•
Disease severity, living with children, death of family member from COVID, higher total number of symptoms after discharge, and perceiving self has having been target of discrimination are risk factors for clinically significant anxiety.
-
•
Higher educational level, living with children, smoking, higher disease severity, higher total number of symptoms after discharge, and perceived discrimination are risk factors for clinically significant depression.
Keywords: COVID-19, Anxiety, Depression, PTSD
Abbreviation: COVID-19, 2019 novel coronavirus; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2; ICU, intensive care unit; PTSD, post-traumatic stress disorder
Abstract
The coronavirus disease 2019 (COVID-19) pandemic can have a profound impact on the mental health of patients who survived the illness. However, little is known about the prevalence rate of mental health disorders among hospital discharged COVID-19 patients and its associated factors. A cross-sectional survey of hospital discharged patients was conducted April 11–22, 2020 in Wuhan, China (where the pandemic began). 675 participants completed the survey, including 90 (13.3%) medical staff (physicians and nurses who had been ill). We used Fisher's exact test and multivariable logistic regression methods to explore the risk factors associated with mental health problems (anxiety, depression, and PTSD symptoms associated with COVID-19 hospitalization). Adverse mental health effects of COVID-19 are evident after discharge from the hospital, with sleep difficulties highlighted as a central issue. As we found that perceived discrimination was a central predictor of mental illness, preventing and addressing social stigma associated with COVID-19 may be crucial for improving mental health for recovered patients.
1. Introduction
In December 2019, a novel coronavirus disease (COVID-19) emerged in Wuhan, China. As of May 18, 2020, the outbreak has resulted in almost 5 million confirmed infections around the world (Dong et al., 2020), and the number of deaths and infections caused by COVID-19 continues to rise. The present study investigated mental illness outcomes among people who had been hospitalized with COVID-19 and then discharged.
Along with the symptoms of the disease itself, patients infected by COVID-19 may experience a variety of stressors and traumatic events, such as difficulty gaining admission to hospital wards, social and physical isolation, and deaths of other patients and/or family members. Furthermore, treatment for COVID-19 may have adverse effects on mental health and contribute to problems such as anxiety and insomnia (Zhao, et al., 2020). Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) might also infect the brain, which could directly cause and have adverse effects on brain function and mental health (Holmes, et al., 2020). Previous studies following severe acute respiratory syndrome (SARS) infected patients in 2003 found that the prevalence of post-traumatic stress disorder (PTSD) in SARS survivors was respectively 46.2% and 38.8% at 3 months and 12 months after discharge (Gao, et al., 2020). A recent study found that 96.2% clinically stable COVID-19 patients reported clinically significant symptoms of PTSD (Bo et al., 2020).
To date, no studies have reported the prevalence of anxiety symptoms, depression symptoms, and PTSD for the hospital discharged COVID-19 patients. There is also limited information regarding risk factors for mental health problems among discharged COVID-19 patients. It is urgent for researchers to clarify these issues to understand the mechanisms of the development of mental health issues for COVID-19 patients (Xiang et al., 2020)
2. Method
2.1. Study design
The study protocol was approved by the research ethics committee of Renmin University of China on March 20, 2020. Nurses described the survey to each participant and obtained their oral consent. The participants completed the survey either online at home or offline at temporary quarantine places. The study is a cross-sectional survey conducted from April 11 to 22, 2020.
2.2. Participants
All participants were recovered COVID-19 patients who had been discharged from hospitals, average time since discharge was 36.75 days. The discharge date ranged from Jan. 27, 2020 to April 21, 2020. According to the treatment guidelines in China, COVID-19 patients had to be isolated in quarantine places for 14 days after hospital discharge and then isolated in their homes for another 14 days afterward. Nurses distributed the questionnaires online or gave them when doing follow-up home visits after discharge. An estimate of 90% of patients visited responded to the survey. This is a very high compliance rate.
2.3. Demographic variables
Demographic data were self-reported by the participants, including occupation (hospital staff or not), gender (male or female), age, marital status, educational level (high school degree or lower, educational specialist diploma, Bachelor's degree, or Master's degree or higher, scores were respectively coded as from 1 to 4), income level (2000 −5000 yuan, 5000–10,000 yuan, 10,000 to 20,000 yuan, and more than 20,000 yuan per month, scores were respectively coded as from 1 to 4). (1000 yuan convert to US$141 as of May 2020.)
2.4. Illness severity, symptoms, treatment
The questionnaire elicited self-reports of key clinical variables, including ICU admission, severity level of COVID-19 pneumonia (mild, moderate, severe, and critically ill), admission date, discharge date, current symptoms after hospital discharge (cough, chest distress, chest pain, dizziness, fatigue, dyspnea etc.), use of invasive mechanical ventilation, use of corticosteroids, presence of chronic underlying disease (e.g. diabetes, hypertension), and positive COVID-19 RNA test after hospital discharge.
2.5. Social factors
Participants were asked about whether they currently live with parents or children, whether any of their family members were infected, and whether these family members died from COVID. We constructed a brief 5-item perceived discrimination scale for the current study based upon phone interview with COVID-19 patients and similar scales previously used to assess HIV patients: “my family are unwilling to live with me”, “I'm rejected by my neighbors and community”, “my family members are rejected by relatives and neighbors”, “relatives and friends are afraid of me”, and “I suffer from verbal abuse”. Item responses included: “None of the time” (score 0), “a bit” (score 1), “moderate” (score 2), “severe” (score 3), and “very serious” (score 4). A higher score indicates a higher level of perceived discrimination. The composite scale had a Cronbach's alpha value of 0.834.
2.6. Outcomes
Anxiety, depression, and post-traumatic stress disorder (PTSD) symptoms were evaluated using Chinese versions of validated measurement tools. Specifically, the 7-item Generalized Anxiety Disorder scale (GAD-7; range, 0–21) (Kroenke et al., 2001; He et al., 2010), and the 9-item Patient Health Questionnaire (PHQ-9; range, 0–27) (Spitzer et al., 2006; Zhang et al., 2013) were used to assess the severity of symptoms of anxiety and depression in the past two weeks. The 20-item PTSD Checklist for DSM-5 (PCL-5; range, 0–80) (Blevins et al., 2015; Wang et al., 2015), was used to assess PTSD in the past month. The PCL-5 asks people to report on responses to a “stressful” experience and all participants in this study were asked to consider experiences with COVID-19 as the stressful experience, similar to other studies (Bo et al., 2020). Based on prior work, scores of 5 for both anxiety and depression were considered mild, whereas anxiety scores above 10 (moderate; 15 was severe) and depression scores of 10 (moderately severe; 20 indicated severe) were the cutoffs for clinically significant symptoms.
Significant PTSD related to COVID-19 was assumed to be present among participants who reported all of the following: at least one re-experiencing symptom, one avoidance symptom, two negative alterations in cognition or mood symptoms, and two arousal symptoms, where a symptom was considered present with a score of 2 (moderate) or higher. The reliabilities of GAD-7, PHQ-9 and PCL-5 were respectively 0.890, 0.875, 0.948.
2.7. Analysis strategy
We did analyses on three mental health outcomes: anxiety, depression, and PTSD related to COVID-19. At first, descriptive analysis of demographic variables, clinical features, perceived discrimination, anxiety, depression, and PTSD will be presented. Categorical variables are described as frequency rates and percentages and were compared for the study's outcomes by using Fisher's exact test. The continuous variables are described using median, range, and standard deviation values, and were compared with t-tests.
Next, we tested the association between the predictor variables and presence of clinical outcomes using bivariate logistic regression analyses. The forward selection (likelihood ratio) method and stepwise method were respectively used to select the most significant variables from the 19 initial predicting variables. In the regression analysis, missing data were deleted listwise.
3. Result
3.1. Demographic profile of sample
675 participants completed the survey, of whom 577 (85.5%) completed measures of perceived discrimination. The median age of the sample was 55(ICQ [41,66]). 358(53%) of the participants were female. 90 (13.3%) of the sample was medical staff (physicians and nurses). Only 77 (11.4%) of the participants were unmarried. 482 (63.4%) had a monthly income that was less than 5000 yuan (about 700 US dollars). 529 (78.4%) had an educational level below undergraduate. 140 (20.7%) of the participants lived with their parents and 338 (50.1%) lived with children.
3.2. Illness and symptoms
Most of the participants had been moderately ill with COVID-19 (406, 60.1%). Others were mildly (145, 21.5%), severely (116, 17.2%), or critically ill (8, 1.2%). 251 (37.2%) of the participants reported some chronic underlying disease. 35 (5.2%) of the patients had been in intensive care (ICU). 30 (4.4%) of the patients had re-detectable positive COVID-19 RNA test after hospital discharge. 96 (14.2%) of the participants had been given substantial doses of corticosteroid during hospital treatment. 13 (2.4%) of participants received invasive mechanical ventilation. 216 (32%) of the participants had at least one COVID symptom after discharge. Chest distress (99,14.7% of the full sample), cough (93, 13.8%) and fatigue (86,12.7%) were the most common symptoms after hospital discharge. 221 (32.7%) participants had at least one family member infected, and 47 (7%) had family members who had died because of COVID-19 infection.
3.3. Descriptive analysis of mental health outcomes
About 84 (12.4%) were provisionally diagnosed with clinically significant symptoms of PTSD due to COVID-19. The median score of PCL-5 was 12 (ICQ [4,16]). For anxiety, 70 (10.4%) were categorized as having moderate to severe symptoms, with another 218 (32.3%) reporting mild symptoms. 128 (19%) were categorized as having moderate to severe depression symptoms, with another 315 (46.7%) reporting mild symptoms. The median scores of GAD-7 and PHQ-9 were respectively 4 (ICQ [2, 6]) and 5 (ICQ [3, 8]). Of note, there was significant overlap in symptoms; 41 (6.07%) had severe anxiety and clinically significant PTSD, 57 (8.44%) had clinically significant PTSD and depression, another 57 (8.44%) had both depression and anxiety symptoms, and 37 (5.48%) were categorized with all three.
3.4. Risk factors of mental health outcomes
Results from Fisher's exact test can be found in Table 1, Table 2, Table 3 . Most COVID-19 symptoms after discharge were risk factors of depression and anxiety, but only cough, fatigue, and chest distress were significant for PTSD due to COVID-19 (p<.05). Treatment by invasive mechanical ventilation and testing positive for COVID-19 RNA after discharge were not significant predictors of any of the three mental illness indicators (p>.05). Treatment with corticosteroid was associated with lower risk of PTSD due to COVID-19 (p=.016) but higher risk of anxiety (p=.022). ICU was associated with higher level of depression (p = 0.003). Not surprisingly, severity of disease consistently acted as a main risk factor for PTSD due to COVID-19 (p<.001), severe depression (p<.001) and severe anxiety (p<.001). Thus, the more severe the disease was, the worse the mental illness outcomes. Perceived discrimination was a strong risk factor for all 3 mental illness indicators (p<.001).
Table 1.
Characteristics of COVID-19 Patients and PTSD outcomes.
| Patients, No. (%) | ||||
|---|---|---|---|---|
| Characteristic | All (n = 675) | No (n = 591) | PTSD (n = 84) | P value |
| Demographic Characteristics | ||||
| Age, average | 53.58 | 53.48 | 54.29 | 0.016 |
| Female | 358 (53.0) | 314 (53.1) | 44 (52.4) | 0.908 |
| Married | 598(88.6( | 520 (88.0) | 78(92.9( | 0.269 |
| Undergraduate or above | 146 (21.6) | 128 (21.7) | 18 (21.4) | 1 |
| Income above 5000¥ | 193(28.6) | 101(29.8) | 17 (20.2) | 0.072 |
| Medical staff | 90 (13.3) | 79 (13.4) | 11 (13.1) | 0.553 |
| Smoking | 84(12.4) | 72(12.2) | 12(14.3) | 0.596 |
| Family situation | ||||
| Live with parents | 140(20.7) | 118(20.0) | 22 (26.2) | 0.196 |
| Live with children | 338(50.1) | 281(47.5( | 57(67.9) | <.001 |
| Family member infected | 221(32.7) | 199 (33.7) | 22 (26.2) | 0.106 |
| Family member died | 47(7) | 41 (6.9) | 6 (7.1) | 1 |
| Clinical features in hospital | ||||
| Underlying illness | 251(37.2) | 226(38.2) | 25(29.8) | 0.148 |
| Severity levels | ||||
| Mild | 145 (21.5) | 136 (23.0) | 9 (10.7) | <.001 |
| Moderate | 406 (60.1) | 346 (58.5) | 60 (71.4) | |
| Severe | 116 (17.2) | 102 (17.3) | 14 (16.7) | |
| Critically ill | 8 (1.2) | 7 (1.2) | 1 (1.2) | |
| Days in hospital, average | 27.87 | 28.05 | 26.62 | 0.004 |
| Hospital treatment | ||||
| Invasive mechanical ventilation | 13(2.4) | 12 (2.5) | 1 (1.6) | 1 |
| Corticosteroids | 96(20.8) | 91(22.2) | 5 (9.6) | 0.016 |
| ICU | 35(5.2) | 30 (5.1) | 5 (6.0) | 0.163 |
| Signs and symptoms after discharge | ||||
| Cough | 93(13.8) | 72 (12.2) | 21 (25.0) | 0.003 |
| Chest pain | 40(5.9) | 35 (5.9) | 5 (6.0) | 1 |
| Chest Distress | 99(14.7) | 76 (12.9) | 23 (27.4) | <.001 |
| Dyspnea | 36(5.3) | 29 (4.9) | 7 (8.3) | 0.194 |
| Dizziness | 38(5.6) | 30 (5.1) | 8 (9.5) | 0.123 |
| Fatigue | 86(12.7) | 64(10.8) | 22 (26.2) | <.001 |
| Other symptoms | 49(7.3) | 38 (6.4) | 11 (13.1) | 0.04 |
| Re-detectable positive COVID-19 RNA test | 30 (5.3) | 26 (5.2) | 4 (6.1) | 0.769 |
| Social factors | ||||
| Discrimination, mean (S.D.) | 7.67(2.94) | 6.34 (2.56) | 8.69 (2.81) | 0.064 |
Table 2.
Characteristics of COVID-19 Patients and anxiety outcomes.
| Patients, No. (%) | ||||
|---|---|---|---|---|
| Characteristic | All (n = 675) | Mild/No (n = 605) | Moderate & Serious (n = 70) | P value |
| Demographic Characteristics | ||||
| Age, average | 53.58 | 53.66 | 52.81 | 0.17 |
| Female | 358 (53.0) | 313 (51.7) | 45 (64.3) | 0.057 |
| Married | 598(88.6( | 532 (87.9) | 66 (94.3) | 0.162 |
| Undergraduate or above | 146 (21.6) | 124 (20.5) | 22 (31.4) | 0.045 |
| Income above 5000¥ | 193(28.6) | 198(32.7) | 23 (32.9) | 0.404 |
| Medical staff | 90 (13.3) | 78 (12.9) | 12(17.1) | 0.352 |
| Smoking | 84(12.4) | 74 (12.2) | 10 (14.3) | 0.57 |
| Family situation | ||||
| Live with parents | 140(20.7) | 124 (20.5) | 16 (22.9) | 0.642 |
| Live with children | 338(50.1) | 292 (48.3) | 46 (65.7) | 0.008 |
| Family member infected | 221(32.7) | 198(32.7) | 23(32.9) | 1 |
| Family member died | 47(7) | 43 (7.1) | 4 (5.7) | 0.808 |
| Clinical features in hospital | ||||
| Underlying illness | 251(37.2) | 222 (36.7) | 29 (41.4) | 0.436 |
| Severity levels | ||||
| Mild | 145 (21.5) | 134 (22.1) | 11 (15.7) | <.001 |
| Moderate | 406 (60.1) | 373 (61.7) | 33 (47.1) | |
| Severe | 116 (17.2) | 92(15.2) | 24 (34.3) | |
| Critically ill | 8 (1.2) | 6 (1.0) | 2 (2.9) | |
| Days in hospital, average | 27.87 | 27.45 | 31.46 | 0.095 |
| Hospital treatment | ||||
| Invasive mechanical ventilation | 13(2.4) | 11(2.2) | 2(3.9) | 0.343 |
| Corticosteroids | 96(20.8) | 82 (19.4) | 14 (35.9) | 0.022 |
| ICU | 35(5.2) | 29 (4.8) | 6 (8.6) | 0.247 |
| Signs and symptoms after discharge | ||||
| Cough | 93(13.8) | 73 (12.1) | 20 (28.6) | <.001 |
| Chest pain | 40(5.9) | 30 (5.0) | 10 (14.3) | 0.005 |
| Chest Distress | 99(14.7) | 77 (12.7) | 22(31.4) | <.001 |
| Dyspnea | 36(5.3) | 27 (4.5) | 9 (12.9) | 0.008 |
| Dizziness | 38(5.6) | 30 (5.0) | 8 (11.4) | 0.048 |
| Fatigue | 86(12.7) | 64 (10.6) | 22 (31.4) | <.001 |
| Other symptoms | 49(7.3) | 36 (6.0) | 13 (18.6) | <.001 |
| Re-detectable positive COVID-19 RNA test | 30 (5.3) | 28(5.4) | 2 (3.8) | 1 |
| Social factors | ||||
| Discrimination, mean (S.D.) | 7.67(2.94) | 7.22(2.36) | 10.36(4.30) | <.001 |
Table 3.
Characteristics of COVID-19 Patients and depression outcomes.
| Patients, No. (%) | ||||
|---|---|---|---|---|
| Characteristic | All (n = 675) | Mild/No (n = 572) | Moderate & Serious (n = 103) | P value |
| Demographic Characteristics | ||||
| Age, average | 53.58 | 53.76 | 52.77 | 0.164 |
| Female | 358 (53.0) | 292(53.4) | 66 (51.6) | 0.768 |
| Married | 598(88.6( | 479 (87.6) | 119 (93.0) | 0.09 |
| Undergraduate or above | 146 (21.6) | 110 (20.1) | 36 (28.1) | 0.056 |
| Income above 5000¥ | 193(28.6) | 147 (26.9) | 46 (35.9) | 0.05 |
| Medical staff | 90 (13.3) | 67 (12.2) | 23 (18.0) | 0.111 |
| Smoking | 84 (12.4) | 58 (10.6) | 26(20.3) | 0.004 |
| Family situation | ||||
| Live with parents | 140(20.7) | 112 (20.5) | 28 (21.9) | 0.718 |
| Live with children | 338(50.1) | 249(45.5) | 89 (69.5) | <.001 |
| Family member infected | 221(32.7) | 168(30.7) | 53(41.4) | 0.022 |
| Family member died | 47(7) | 36 (6.6) | 11 (8.6) | 0.44 |
| Clinical features in hospital | ||||
| Underlying illness | 251(37.2) | 192 (35.1) | 59 (46.1) | 0.025 |
| Severity levels | ||||
| Mild | 145 (21.5) | 128 (23.4) | 17(13.3) | <.001 |
| Moderate | 406 (60.1) | 340 (62.2) | 66(51.6) | |
| Severe | 116 (17.2) | 74 (13.5) | 42(32.8) | |
| Critically ill | 8 (1.2) | 5(0.9) | 3(2.3) | |
| Days in hospital, average | 27.87 | 26.78 | 32.5 | 0.004 |
| Hospital treatment | ||||
| Invasive mechanical ventilation | 13(2.4) | 10(2.2) | 3(3.2) | 0.478 |
| Corticosteroids | 96(20.8) | 78 (20.2) | 18(24.3) | 0.436 |
| ICU | 35(5.2) | 21 (3.8) | 14(10.9) | 0.003 |
| Signs and symptoms after discharge | ||||
| Cough | 93(13.8) | 60 (11.0) | 33(25.8) | <.001 |
| Chest pain | 40(5.9) | 22(4.0) | 18 (14.1) | <.001 |
| Chest distress | 99(14.7) | 54(9.9) | 45(35.2) | <.001 |
| Dyspnea | 36(5.3) | 22(4.0) | 14(10.9) | 0.004 |
| Dizziness | 38(5.6) | 18 (3.3) | 20 (15.6) | <.001 |
| Fatigue | 86(12.7) | 49(9.0) | 37(28.9) | <.001 |
| Other symptoms | 49(7.3) | 25(4.6) | 24(18.8) | <.001 |
| Re-detectable positive COVID-19 RNA test | 30 (5.3) | 22(4.7) | 8(8.1) | 0.213 |
| Social factors | ||||
| Discrimination, mean (S.D.) | 7.67(2.94) | 7.25(2.41) | 10.42 (4.24) | <.001 |
The rates of developing heightened depression, anxiety and PTSD due to COVID-19 were the same among medical staff as among regular patients. Patients who lived with their own children had higher risk of PTSD due to COVID-19 (p<.001), anxiety (p<.001), and depression (p<.001), as compared with patients who did not live with their children. Patients who had family members infected had higher risk of depression than other patients (p=.022 on the Fisher's exact test). Although death of a family member from COVID-19 was not significant for any test (p = 1, 0.808, and 0.440), it was significant in the multivariate analyses.
We selected the effective predictors using forward selection (likelihood ratio) method, see Table 4 . The final logistic regression model showed that higher disease severity (OR, 3.27, 95% C. I. [1.69,6.32]), living with children (OR, 6.71, 95% C. I ., [2.79,16.12]), death of family member (OR, 7.05, 95% C. I ., [1.78,27.88]), and regarding oneself as having been the target of discrimination (OR, 1.67, 95% C. I ., [1.48, 1.90]) were all risk factors for significant PTSD symptoms due to COVID-19. Corticosteroids use in treatment (OR, 0.17, 95% C. I., [.05, 0.58]) was associated with lower risk of PTSD.
Table 4.
Factors Associated with Mental Health Outcomes Identified by Multivariable Logistic Regression Analysis.
| PTSD | |||
|---|---|---|---|
| variables | b coefficient (SE) | Odds ratio (95% CI for expected odds ratio) | p value |
| Live with children | 1.9(0.45) | 6.71[2.79, 16.12] | <.001 |
| Disease severity | 1.19(0.34) | 3.27[1.69, 6.32] | <.001 |
| Family member died | 1.95(0.70) | 7.05[1.78, 27.88] | 0.005 |
| Discrimination | 0.52(0.06) | 1.67[1.48, 1.90] | <.001 |
| Corticosteroids | −1.78(0.63) | 0.17[0.05, 0.58] | 0.005 |
| Moderate to Severe Depression | |||
|---|---|---|---|
| variables | b coefficient (SE) | Odds ratio (95% CI for expected odds ratio) | p value |
| Educational level | 0.43(0.19) | 1.54[1.07, 2.22] | 0.021 |
| Live with children | 1.56(0.39) | 4.75[2.20,10.23] | <.001 |
| Smoking | 1.59(0.44) | 4.89[2.05, 11.66] | <.001 |
| Disease severity | 1.48(0.29) | 4.40[2.51, 7.74] | <.001 |
| No of Symptoms | 0.65(0.14) | 1.92[1.47, 2.50] | <.001 |
| Discrimination | 0.44(0.06) | 1.55[1.37, 1.75] | <.001 |
| Moderate to Severe Anxiety | |||
|---|---|---|---|
| variables | b coefficient (SE) | Odds ratio (95% CI for expected odds ratio) | p value |
| Live with children | 2.08 (0.54) | 8.01[2.79,23.04] | <.001 |
| Disease severity | 1.07(0.32) | 2.91[1.55, 5.48] | <.001 |
| Family member died | 1.46(0.75) | 4.29[0.99, 18.64] | 0.052 |
| No of Symptoms | 0.39(0.13) | 1.48[1.14,1.92] | 0.003 |
| Discrimination | 0.36(0.06) | 1.43[1.27,1.62] | <.001 |
Note: Age, gender, marital status, educational level, income level, medical staff, smoking, live with parents, live with children, family member infected, family member died, underlying illness, severity COVID-19 disease levels, invasive mechanical ventilation, large dose corticosteroids use, ICU, No of Symptoms, re-detectable positive COVID-19 RNA test, perceived discrimination were entered into the model initially.
The odds of reporting moderate to severe anxiety were significantly increased by each of the following factors: higher disease severity (OR, 2.91, 95% C. I ., [1.55,5.48]), living with children (OR, 8.01, 95% C. I ., [2.79,23.04]), death of family member from COVID (OR, 4.29, 95% C. I ., [0.99, 18.64]), higher total number of symptoms after discharge (OR, 1.48, 95% C. I ., [1.14, 1.92]), and perceiving self has having been target of discrimination (OR, 1.43, 95% C. I ., [1.27, 1.62]).
The odds of severe depression were significantly increased by each of the following: higher educational level (OR, 1.54, 95% C. I ., [1.07, 2.22]), living with children(OR, 4.75, 95% C. I ., [2.20, 10.23]), smoking (OR, 4.89, 95% C. I ., [2.05, 11.66]), higher disease severity (OR, 4.40, 95% C. I ., [2.51, 7.74]), higher total number of symptoms after discharge (OR, 1.92, 95% C. I ., [1.47,2.50]), and perceived discrimination (OR, 1.55, 95% C. I ., [1.37,1.75]).
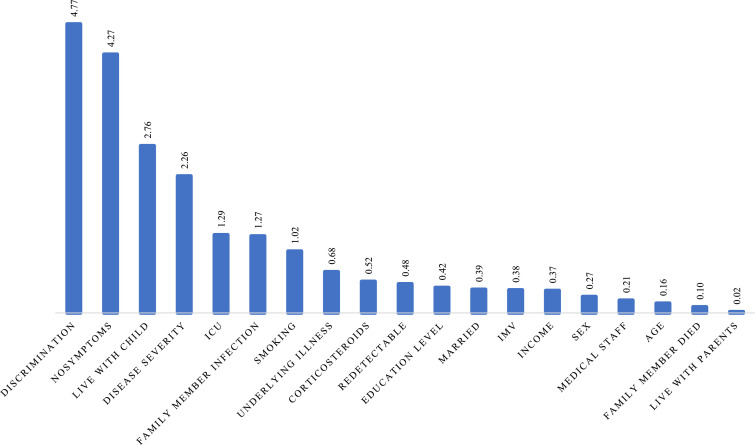
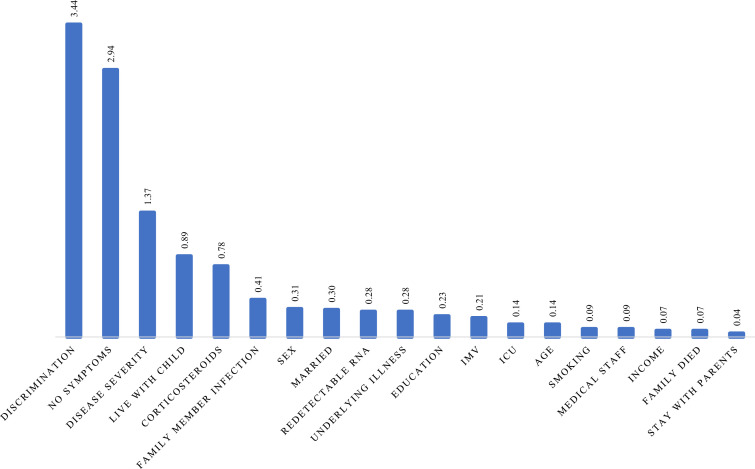
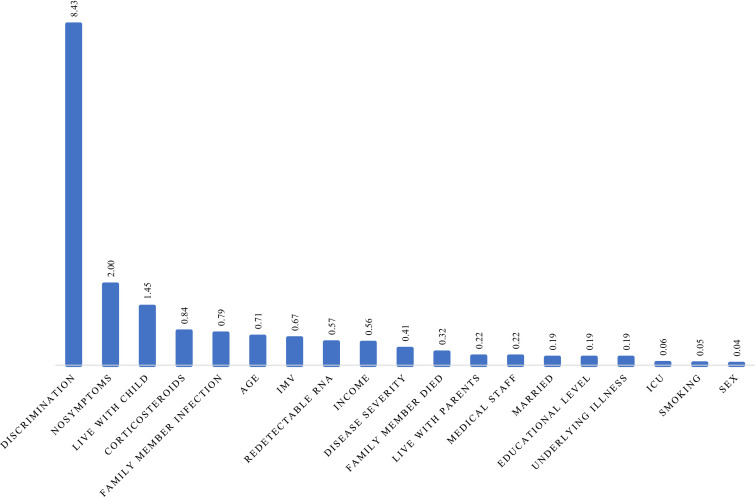
Ranking of importance scores allowed an ordering of the factors in terms of their efficiency to predict the outcome. With GUIDE random forest (Loh et al., 2008), we calculated the importance score to help us select the predictor variables most likely to influence the outcome. Perceived discrimination was always one of the most important variables in predicting anxiety, depression, or PTSD, see Fig. 1, Fig. 2, Fig. 3 .
Fig. 1.
Importance score of predicting variables for moderate to severe depression.
Fig. 2.
Importance score of predicting variables for moderate to severe anxiety.
Fig. 3.
Importance score of predicting variables for PTSD.
3.5. Item analyses
Item analysis further indicated that sleep problems, intrusive thoughts, feeling distant or cut off from people, and inability to concentrate were the most prevalent symptoms of PTSD due to COVID-19. Our analyses of depression items also showed that sleep difficulty was the most frequently reported symptom.
4. Discussion
Our survey indicates that there are substantial mental health problems among patients who have been hospitalized with COVID-19 and discharged. We had a large sample of 675 patients, and relatively few refused to do our survey, so the sample may be considered representative, at least for Chinese individuals.
Half of the sample reported at least mild symptoms of depression and generalized anxiety. Nevertheless, moderate to severe anxiety was reported by 10.4%, and moderate to severe depression was found among 19%. Importantly, these rates are significantly higher than anxiety and depression rates noted in studies of the general adult population in China (12 months generalized anxiety prevalence rates: 0.2%; major depression prevalence rates: 2.1%) (Huang et al., 2019). Clearly, the adverse mental health consequences of COVID-19 do not end with discharge from the hospital, and many continue to report moderate to severe problems a month later.
Significant PTSD symptoms due to COVID-19 were considerably less common than depression, but 12.4% surpassed the cutoff for provisional PTSD diagnosis. The latter contrasts sharply with Bo et al. (2020) finding that 96% of patients currently hospitalized for COVID-19 had clinically significant symptoms of PTSD due to COVID-19. It seems reasonable to conclude that PTSD symptoms from COVID are quite high among hospitalized individuals, which may be due to the overlap between PTSD symptoms and acute illness (i.e., difficulties sleeping, feeling cut off from others, difficultly concentrating), and diminish substantially after discharge. Even so, clinically significant PTSD symptoms were still indicated a month after discharge in one out of eight respondents. Item analyses for PTSD and depression suggested that sleep difficulties were the most common symptoms, consistent with our personal interview findings with frontline nurses (exploratory interviews). McNally et al. (2015) suggest sleep problems may impair both emotions and attention regulation and cause irritability and concentration problems. Considering that a study of SARS survivors showed about 25% reported significant PTSD symptoms after 30 months (Mak et al., 2009), evaluation of long-term psychiatric outcomes is also warranted.
Given the wide variation in mental health outcomes, we sought to ascertain what predicted the more serious adverse outcomes. Not surprisingly, all three mental illness outcomes were more severe when the physical illness itself had been more severe — and also when more symptoms recurred after discharge. People who lived with their children were also more likely to have severe symptoms of mental illness, and those who experience the death of a family member were at greater risk for anxiety and PTSD symptoms. These findings suggest that post-COVID hospitalization, particularly for those with severe illness and with family stressors, should receive mental health resources directly after hospital discharge.
Our findings do underscore the importance of social stigma and discrimination in exacerbating the emotional impact of COVID-19. Both the importance score analysis and multivariate logistic regression showed that perceived discrimination was associated with clinically significant PTSD symptoms, severe depression, and severe anxiety. Previous studies on SARS suggested that people avoid recovered patients due to fear of infection (Person et al., 2004). Thus, fears of discrimination by others are likely founded for COVID-19 patients, and the sense of feeling isolated and “othered” seems to contribute to symptoms of mental illness. Considering that isolation is central to the quarantine process to diminish COVID-19 transmission, finding methods of helping quarantined patients remain connected to loved ones may be crucial to preventing mental health problems.
High-dose corticosteroids were administered to many critically ill patients in Wuhan (Zhou et al., 2020) and were associated with higher mortality risk. Previous research on SARS patients found that high-dose corticosteroid use could lead to osteonecrosis of the femoral head (OFNH). Many patients worry about the sequelae of corticosteroid treatment and be more anxious. On the other hand, the use of corticosteroids in hospital treatment may be protective against PTSD symptoms (Zohar et al., 2011).
5. Limitations
This study has several limitations. First, it is a cross-sectional study, which limits causal inference. Second, there may be a sample bias. It is possible that non-Chinese samples would have different patterns of mental illness associated with COVID-19. In addition, further work with a larger number of the most critically ill patients would be desirable. Third, our mental health outcomes were cut off based on the sum-score of diagnostic criteria, which may lead to Berkson's bias, presenting a potential threat to the validity our findings (De et al., 2019). Fourth, limited by the length of the questionnaire, we may have missed some important risk factors in our analysis such as waiting time for hospital admission. Fifth, we did not directly examine any medical comorbidities (Mazza et al., 2020). Sixth, we do not have any assessment of mental health issues prior to COVID-19, and we are thus unable to ascertain if mental health symptoms were pre-existing, exacerbated or acutely caused by the stress of having COVID-19. This is a significant limitation, as we recognize that prior depression and anxiety are significant risk factors for symptom recurrence (Burcusa and Iacono, 2007; Scholten et al., 2013). However, because the rates of depression and anxiety in this study are higher than population levels (Huang et al., 2019), and because the PTSD symptoms addressed symptom responses to hospitalization with COVID, it is likely that heightened mental illness symptoms are related to the experience of having COVID-19.
We also recognize that mental illness may be associated with COVID-19 for people not directly infected by the virus, such as front line healthcare workers (Tsmakis et al., 2020). In addition, lockdown and quarantine appears to be exacerbating interpersonal violence (Mazza et al., 2020), as people are home more often and have fewer alternative outlets for emotional outbursts. Financial stressors associated with job losses due to COVID-19 related business shutdowns are also potential contributors to mental health issues (Kawhol and Nordt, 2020), particularly in countries with less government protections Future work should examine mental health problems in these groups in addition to recovered patients.
Conclusions
The effects of COVID-19 extend beyond the (often quite serious) physical affliction. Almost half of the sample reported at least mild levels of depression and anxiety, and substantial minorities reported clinically significant symptoms PTSD and/or moderate to severe levels of anxiety and depression. Some of these were linked to the severity of the physical illness and to recurring symptoms after discharge (as well as illness and death of family members), and at present there is no way to prevent those. However, we also found that feeling oneself to be the target of stigma and discrimination based on one's having COVID was strongly associated with all three negative outcomes. Some efforts toward public education to reduce stigma and discrimination may be warranted to reduce the secondary suffering associated with the pandemic.
Authors statement
This study is supported by fund for building world-class universities (disciplines) of Renmin University of China. Project No. KYGJA2020001
Declaration of Competing Interest
The authors have declared that no competing interests exist.
References
- Blevins C.A., Weathers F.W., Davis M.T., Witte T.K., Domino J.L. The Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5): development and initial psychometric evaluation. J. Trauma Stress. 2015;28:489–498. doi: 10.1002/jts.22059. [DOI] [PubMed] [Google Scholar]
- Bo H.X., Li W., Yang Y., Wang Y., Zhang Q., Cheung T., Wu X., Xiang Y.T. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol. Med. 2020:1–2. doi: 10.1017/S0033291720000999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burcusa S.L., Iacono W.G. Risk for recurrence in depression. Clin. Psychol. Rev. 2007;27(8):959–985. doi: 10.1016/j.cpr.2007.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Ron J., Fried E., Epskamp S. Psychological networks in clinical populations: investigating the consequences of Berkson’s bias. Psychol. Med. 2019:1–9. doi: 10.1017/S0033291719003209. [DOI] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020 doi: 10.1016/S1473-3099(20)30120-1. https://coronavirus.jhu.edu/map.html [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X.Y., Li C.B., Qian J., Cui H.S., Wu W.Y. Reliability and validity of a generalized anxiety scale in general hospital outpatients. Shanghai Arch. Psychiatry. 2010;22(4):200–203. doi: 10.3969/j.issn.1002-0829.2010.04.002. [DOI] [Google Scholar]
- Holmes E.A. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020 doi: 10.1016/S2215-0366(20)30168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Wang Y., Wang H., Liu Z., Yu X., Yan J., Yu Y., Kou C., Xu X., Lu J., Wang Z. Prevalence of mental disorders in China: a cross-sectional epidemiological study. Lancet Psychiatry. 2019;6(3):211–224. doi: 10.1016/S2215-0366(18)30511-X. [DOI] [PubMed] [Google Scholar]
- Kawohl W., Nordt C. COVID-19, unemployment, and suicide. Lancet Psychiatry. 2020;7(5):389–390. doi: 10.1016/S2215-0366(20)30141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke K., Spitzer R.L., Williams J.B. The PHQ‐9. J. Gen. Intern. Med. 2001;16:606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh W.Y. Classification and regression tree methods. In: Ruggeri F, Kenett R, Faltin FW, editors. Encyclopedia of Statistics in Quality and Reliability. Wiley; Chichester, UK: 2008. pp. 315–323. [Google Scholar]
- Mak I.W.C., Chu C.M., Pan P.C., Yiu M.G.C., Chan V.L. Long term psychiatric morbidities among SARS survivors. Gen. Hosp. Psychiatry. 2009;31(4):318–326. doi: 10.1016/j.genhosppsych.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazza M., Marano G., Antonazzo B., Cavarretta E., Di Nicola M., Janiri L., Romagnoli E. What about heart and mind in the COID-19 era? Minerva Cardioangiol. 2020 doi: 10.23736/S2724-5683.20.05309-8. [DOI] [PubMed] [Google Scholar]
- Mazza M., Marano G., Lai C., Janiri L., Sani G. Danger in danger: interpersonal violence during COVID-19 quarantine. Psychiatry Res. 2020;289 doi: 10.1016/j.psychres.2020.113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally R.J., Robinaugh D.J., Wu G.W., Wang L., Deserno M.K., Borsboom D. Mental disorders as causal systems: a network approach to posttraumatic stress disorder. Clin. Psychol. Sci. 2015;3(6):836–849. [Google Scholar]
- Person B., Sy F., Holton K., Govert B., Liang A. Fear and stigma: the epidemic within the SARS outbreak. Emerging. Infect. Dis. 2004;10(2):358–363. doi: 10.3201/eid1002.030750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholten W.D., Batelaan N.M., van Balkom A.J., Penninx B.W., Smit J.H., van Oppen P. Recurrence of anxiety disorders and its predictors. J. Affect. Disord. 2013;147(1–3):180–185. doi: 10.1016/j.jad.2012.10.031. [DOI] [PubMed] [Google Scholar]
- Spitzer R.L., Kroenke K., Williams J.B., Löwe B. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch. Intern. Med. 2006;166(10):1092–1097. doi: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- Tsamakis K., Rizos E., Manolis A.J., Chaidou S., Kympouropoulos S., Spartalis E., Triantafyllis A.S. COVID-19 pandemic and its impact on mental health of healthcare professionals. Exp. Ther. Med. 2020;19(6):3451–3453. doi: 10.3892/etm.2020.8646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zhang L., Armour C., Cao C., Qing Y., Zhang J., Liu P., Zhang B., Wu Q., Zhao Z., Fan G. Assessing the underlying dimensionality of DSM-5 PTSD symptoms in Chinese adolescents surviving the 2008 Wenchuan earthquake. J. Anxiety Disord. 2015;1(31):90–97. doi: 10.1016/j.janxdis.2015.02.006. [DOI] [PubMed] [Google Scholar]
- Xiang Y.T., Yang Y., Li W., Zhang L., Zhang Q., Cheung T., Ng C.H. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7(3):228–229. doi: 10.1016/S2215-0366(20)30046-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.L., Liang W., Chen Z.M. Validity and reliability of patient health questionnaire-9 and patient health questionnaire-2 to screen for depression among college students in China. Asia Pac Psychiatry. 2013;5(4):268–275. doi: 10.1111/appy.12103. [DOI] [PubMed] [Google Scholar]