We read with great interest Dr. Felsenstein review about SARS-CoV2 available treatment options [1]. It is reasonable to try to stop the ominous cycle established by lymphocytes, macrophages, and NK cells in sHLH-like/SARS-CoV2 patients, using agents targeting interleukins related to the inflammatory process like IL-6 and IL-1β [2].
Many recent case series have shown a moderate clinical benefit of using tocilizumab in SARS-CoV2 pneumonia [3]. Protease inhibitors [4] have also shown variable clinical results but neither proved to be definitive treatment in severe cases.
SARS-CoV2 infection evolves bimodally: During the first 5 to 7 days there is cytopathic damage and corresponding symptoms like cough, fever, and dyspnea [5]; and a second one where inflammatory dysregulation can present as sHLH-like disease [6]. When sHLH-like ensues, previously described treatments, including interleukin blockers, could help but up until this day, mortality rates remain similar to the sHLH syndrome described in rheumatologic diseases.
Our group has been dedicated to the study of circulating monocytes and their phenotyping behavior. Monocytes, with inflammatory and non-inflammatory phenotypes, migrate to the tissue at hours to days cycles, which allows for the renovation of tissue-resident macrophage pool [7].
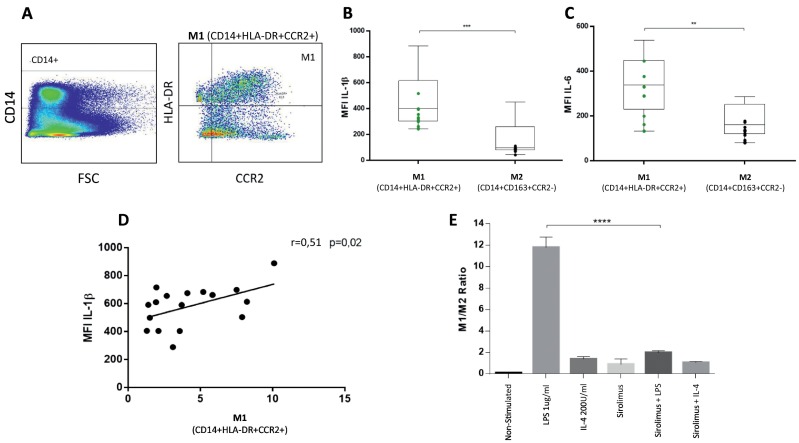
We successfully classified M1 and M2 monocytes according to its membrane protein expression and intracellular interleukin production in healthy subjects. Human M1 monocytes in vitro were able to express higher levels of IL-6 and IL-1β after LPS stimulation [8] (Fig. 1 ). These monocytes also express higher levels of CCL2 receptor, which is crucial to rapid and efficient tissue recruiting (Fig. 1). Interestingly, we have shown that mTORC protein complex acts as an on/off switch in monocyte phenotyping. When monocytes are treated in vitro with sirolimus, and mTORC blocker, they are unable to express M1 proteins even in presence of LPS. (Fig. 1).
Fig. 1.
Human circulating monocytes classified using multiparametric cytometry. (A) Gating strategy using CD14, CCR2 and HLA-DR. (B and C) M1 contains higher levels of inflammatory cytokines after stimulation with 1μg/ml of LPS. In (D), M1 directly correlates with levels of intracellular IL-1β. (E) Sirolimus reverse in vitro LPS-induced M1 phenotype (ratio M1/M2 normalized to non-stimulated). ***p ≤ 0.0008, **p ≤ 0.002, ****p ≤ 0.0001. FACS Fortessa Cytometer. Mann-Whitney U non-parametric analysis. Spearman correlation tests. (n = 16, healthy subjects).
Sirolimus is an mTORC inhibitor used to prevent organ rejection in transplant recipients. Widely available, its administration is simple, and levels can be measured periodically. Immunosuppressive potency is moderate and adverse reactions are dose and time-dependent [9]. In patients with high Hscore and high probability of sHLH-like/SARS-CoV2 disease, the use of this medication could be a suitable option.
Recently, elevated titers of antiphospholipid antibodies in COVID-19 patients has been associated with high thrombotic risk in severe disease [10]. In vivo studies have shown that Lupus patients treated with sirolimus was associated with diminished production of these autoantibodies even with just 1 month of use [11]. Also, in catastrophic antiphospholipid syndrome that underwent kidney transplantation, sirolimus was able to assure functionality of the allograft, inhibiting vascular dysfunction [12].
With this data and previous reports of its use in influenza pneumonia [13], it seems reasonable to think that the SARS-CoV2 induced sHLH-Like syndrome could be successfully slowed or terminated by sirolimus, due to its action blocking the migration of monocytes to lung tissue. It would also be feasible to adventure a role in prevention of thrombotic events in severely ill patients due to its effect on antiphospholipid antibodies production.
We propose at least compassionate use of sirolimus in SARS-CoV2 patients who are classified as high risk of ominous progression or are currently using tocilizumab, corticosteroids and/or protease inhibitors and Hscore shows high probability of sHLH. Sirolimus is a non-expensive, widely available drug that causes moderate immunosuppression, and mostly associated with chronic use in anti-organ rejection therapy. We do not expect any major complications related to its use in an acute setting even when we encourage close monitoring to detect and timely treat thrombocytopenia and anemia associated both to the drug and SARS-CoV2 severe presentation [14].
References
- 1.Felsenstein S., Herbert J.A., McNamara P.S., Hedrich C.M. COVID-19: immunology and treatment options. Clin. Immunol. 2020;215 doi: 10.1016/j.clim.2020.108448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: a single center experience. J. Med. Virol. 2020 Jul;92(7):814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of Lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020 May 7;382(19):1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zheng Y., Xu H., Yang M., Zeng Y., Chen H., Liu R. Epidemiological characteristics and clinical features of 32 critical and 67 noncritical cases of COVID-19 in Chengdu. J. Clin. Virol. 2020;127 doi: 10.1016/j.jcv.2020.104366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guilliams M., Mildner A., Yona S. Developmental and functional heterogeneity of monocytes. Immunity. 2018;49(4):595–613. doi: 10.1016/j.immuni.2018.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Karsulovic C., Tempio F., Lopez M., Guerrero J., Goecke A. Pro-inflammatory response of non-inflammatory Classical monocytes stimulated with LPS in vitro. bioRxiv. 2020 doi: 10.1101/2020.05.04.077537. [DOI] [Google Scholar]
- 9.Shigeta K., Kikuchi M., Tanaka M., Takasaki S., Oishi H., Sado T. Development of a precise quantitative method for monitoring sirolimus in whole blood using LC/ESI-MS/MS. Biomed. Chromatogr. 2020 Aug;34(8):e4853. doi: 10.1002/bmc.4853. [DOI] [PubMed] [Google Scholar]
- 10.Harzallah I., Debliquis A., Drenou B. Lupus anticoagulant is frequent in patients with Covid-19. J. Thromb. Haemost. 2020 Apr 23 doi: 10.1111/jth.14867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Winans mTORC1 blockade with rapamycin and N-Acetylcysteine reduces anti-phospholipid antibody levels in controlled clinical trials of patients with SLE [abstract] Arthritis Rheumatol. 2017;69 [Google Scholar]
- 12.Canaud G., Bienaime F., Tabarin F., Bataillon G., Seilhean D., Noel L.H. Inhibition of the mTORC pathway in the antiphospholipid syndrome. N. Engl. J. Med. 2014;371(4):303–312. doi: 10.1056/NEJMoa1312890. [DOI] [PubMed] [Google Scholar]
- 13.Wang C.H., Chung F.T., Lin S.M., Huang S.Y., Chou C.L., Lee K.Y. Adjuvant treatment with a mammalian target of rapamycin inhibitor, sirolimus, and steroids improves outcomes in patients with severe H1N1 pneumonia and acute respiratory failure. Crit. Care Med. 2014;42(2):313–321. doi: 10.1097/CCM.0b013e3182a2727d. [DOI] [PubMed] [Google Scholar]
- 14.Mendy A., Apewokin S., Wells A.A., Morrow A.L. Factors Associated with Hospitalization and Disease Severity in a Racially and Ethnically Diverse Population of COVID-19 Patients. medRxiv. 2020 Jun 27 doi: 10.1101/2020.06.25.20137323. Preprint. [DOI] [Google Scholar]