Abstract
Since their discovery, Black Phosphorus (BP)-based nanomaterials have received extensive attentions in the fields of electromechanics, optics and biomedicine, due to their remarkable properties and excellent biocompatibility. The most essential feature of BP is that it is composed of a single phosphorus element, which has a high degree of homology with the inorganic components of natural bone, therefore it has a full advantage in the treatment of bone defects. This review will first introduce the source, physicochemical properties, and degradation products of BP, then introduce the remodeling process of bone, and comprehensively summarize the progress of BP-based materials for bone therapy in the form of hydrogels, polymer membranes, microspheres, and three-dimensional (3D) printed scaffolds. Finally, we discuss the challenges and prospects of BP-based implant materials in bone immune regulation and outlook the future clinical application.
Keywords: Tissue engineering, Nanomaterial, Black phosphorus, Bone therapy
Graphical abstract
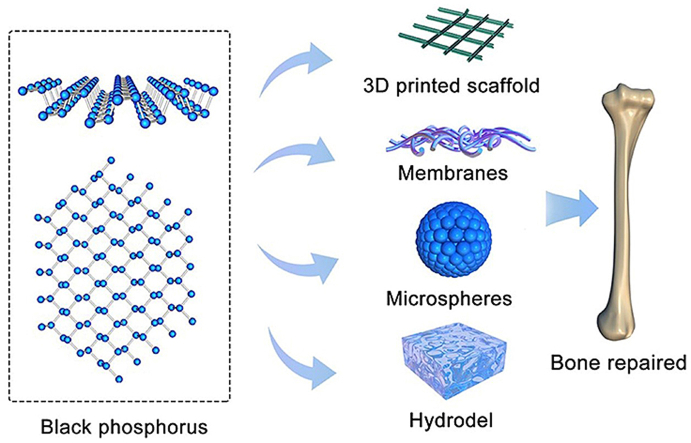
As an emerging 2D nanomaterial, Black phosphorus (BP) has received extensive attentions in the treatment of bone defect, due to their remarkable properties and excellent biocompatibility. Up to now, BP-based materials are mostly used in the form of hydrogels, polymer membranes, microspheres, and 3D printed scaffolds. We expect this review will provide some new ideas for the future design of BP-based materials.
Highlights
-
•
Black phosphorus an emerging 2D nanomaterial having unique physicochemical properties and biocompatibility.
-
•
Intrinsic connection between black phosphorus and bone tissue engineering.
-
•
Black phosphorus-based biomaterials for bone therapy applications.
-
•
Challenges and perspectives of black phosphorus-based materials for future development.
1. Introduction
Phosphorus, atomic number 15, is in the third period of the periodic table, Group VA, which is widely distributed in nature [1,2]. The phosphorus content in the earth's crust reaches 0.12% [3], and the concentration in seawater is as high as 1.2 μM [4]. In addition, phosphorus is also one of the elements with high content in human body, which is ranked sixth after calcium [5]. It accounts for up to 1% of the body's total mass and contains about 660 g in adults [[6], [7], [8], [9]], of which 85% of phosphorus is present in bones and teeth in the form of hydroxyapatite [10,11], which is necessary to maintain the mechanical strength of bones and induce bone regeneration [[12], [13], [14]]. Moreover, phosphorus, as the main component of genetic material such as nucleic acid, also plays an important physiological role in maintaining life, transmitting nerve stimulation, and catalyzing reaction [[15], [16], [17]]. For example, hypophosphatemia caused by hyperparathyroidism [18], vitamin D deficiency [19], and renal tubular acidosis [20] can lead to hypophosphatemic rickets [21] and osteomalacia [22], seriously harming health and normal physiological functions.
The most common elemental phosphorus in nature is mainly white phosphorus [23,24], but its stability is poor, and easy to oxidize spontaneous combustion in humid air, accompanying with odor and highly toxic [[25], [26], [27]]. Therefore, no one has ever associated white phosphorus with tissue engineering. On the contrary, BP is the most stable and the least reactive allotrope in phosphorus [28]. As for the origin of BP, it could be traced back to 1914, Bridgman et al. exposed white phosphorus under high-temperature (200 °C) and high-pressure (1.2 GPa) environment for the first time to produce a more stable block BP [29]. However, there was not a huge wave of research on BP at that time. In the following decades, research on BP was still tepid, until the rapid development of graphene [30], Metal Organic Frameworks (MOFs) [31], Covalent Organic Frameworks (COFs) [32] and other new 2D materials (Xenes) [33] in tissue repair, regeneration and anti-tumor treatment, that the research on thin-layer BP nanomaterials was revived.
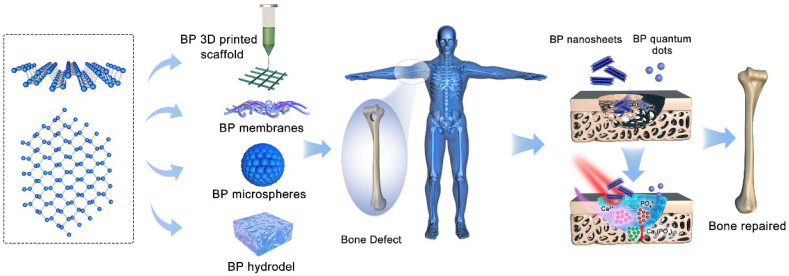
Up to now, there have been a lot of studies on the preparation [27], preservation [34], optimization and application of BP nanomaterials in biomedical applications [35]. Luo et al. and Qiu et al. had reviewed BP-based materials for biomedical applications [36,37]. However, based on the physicochemical properties of BP and the fact that phosphorus is one of the most important inorganic components of bone, this review pays attention to the application of BP-based biomaterials in the field of bone therapy whose scope is wider than bone regeneration, that including osteoarthritis treatment, rheumatoid treatment and bone defect regeneration treatment (Fig. 1, see Table 1) and briefly reviews and discusses the current challenges and future prospects of BP-based biomaterials in bone immune regulation.
Fig. 1.
Atomic structure of BP and the application of BP-based materials for bone regeneration.
Table 1.
A summary of the properties, functionalized modifications and potential applications of BP-based materials for bone therapy.
| Materials | Modification | Property | Therapy mode | Ref |
|---|---|---|---|---|
| BPNSs | BP/PEA/GelMA hydrogel | Sustained supply of calcium-free phosphorus Enhance mechanical performance of hydrogels |
Rabbit model of cranial defects | 128 |
| BPNSs | BP/Gel hydrogel | Reinforce crosslinking networks Promote mineralization NIR photothermal antibacterial |
Rat Model of cranial defects | 155 |
| BPNSs | BP/double network hydrogel | Improve mineralization Tunable mechanical properties |
Rat Model of cranial defects | 156 |
| BPNSs | BP/Chitosan/PRP hydrogel | NIR photothermal conversion Generate ROS to suppress inflammatory Promote osteogenesis Improve cell adhesion and proliferation Reduce arthritis friction |
Rat Model of rheumatoid arthritis | 157 |
| BPNSs | BP/BG 3D printed scaffold | NIR photothermal conversion Promote osteogenesis |
Osteosarcoma model Rat Model of cranial defects |
172 |
| BPNSs | BP/GO 3D printed scaffold | Enhance cell attachment Increased cell proliferation Stimulate cell osteogenesis |
In vitro cell experiment | 173 |
| BPNSs | BP/β-TCP/DOX/Peptide 3D printed scaffold | Sufficient mechanical strength Excellent photothermal effect Controlled release Reduce the long-term toxicity phenomenon of released DOX in vivo Promote osteogenesis |
Bone tumor-bearing nude mice Rat Model of cranial defects |
174 |
| BPQDs | Apt-MVs microspheres | Molecular recognition-guided NIR photothermal effect Promote biomineralization. |
Rat Model of cranial defects | 192 |
| BPNSs | BP-SrCl2/PLGA microspheres | NIR-triggered drug delivery system Photothermal effects improves bone regeneration |
Rat femoral defects | 196 |
| BPNSs | PCL/BP/Col) nanofiber | Promote cell attachment and proliferation Improve osteogenic differentiation |
In vitro cell experiment | 210 |
| BPNSs | BP/PLGA membranes | Heat-induced osteogenesis Highly-efficient NIR photothermal response |
Rat model of tibia defect | 212 |
2. Physicochemical properties of BP
In recent years, with the development of BP nanomaterials, researchers have found that BP, like graphene, is a two-dimensional (2D) crystalline material with a unique layered structure [38], and exhibits a thick-dependent band gap between 0.3 eV (bulk) to 2.0 eV (monolayer) [39], with different layers connected to each other by van der Waals forces [40]. As the van der Waals force between the stacked BP layers is relatively weak, BP can be easily exfoliated into single or fewer-layer nanosheets by mechanical or liquid phase exfoliation [41], and smaller quantum dot structures (BPQDs) can also be prepared [42]. Well, the main differences between BPNSs and BPQDs are that, BPQDs which belong to 0D nanomaterial, have higher band gaps, ultra-small sizes, higher surface-to-volume ratios and more active edge sites per unit mass [42]. At present, liquid phase exfoliation is the most common method for preparing BP nanomaterials, which is based on the equilibrium surface energy between solvent and materials [43]. And N-methyl-2-pyrrolidone (NMP) has been chosen as the most commonly used solvent because it does not contain water or any chemical groups which may react with BP [44]. In addition, BP has an electron mobility of up to 1000 cm2 V−1 s−1 at room temperature [23], which is much higher than that of similar 2D nanomaterials such as Molybdenum sulfide (MoS2), and the current on/off ratio can reach 104–105 [45]. Benefiting from the excellent adjustable band gap and high electron mobility of BP, the initial application of BP mainly focuses on the fields of electronics and optics [46], and the main representative forms are semiconductors [3], transistors [47], photodiodes and so on [48]. In the single-layer BP structure, each phosphorus atom is covalently bonded to three adjacent phosphorus atoms [44]. However, instead of forming a flat plane, they are bound to form a fold-like structure, which creates a prerequisite for BP as a drug delivery nano-carrier [49]. In addition, BP has different degrees of light absorption capacity at all wavelengths, especially in the near-infrared (NIR) region, exhibiting better photothermal conversion efficiency [50]. As a result, BP is also an emerging material in the biomedical field.
Among them, research on cancer therapy is probably the most in-depth. Chen et al. achieved up to 950% load of doxorubicin (DOX) for tumor treatment by using the multi-fold-like structure and negative surface charge of BP nanosheets [49]. Li et al. developed the NIR/ROS (reactive oxygen species)-responsive BPQDs nanovesicles (BPNV) loaded with immune adjuvant CpG oligodeoxynucleotides by self-assembly of polyethylene glycol (PEG) modified BPQD and ROS sensitive polypropylene (PPS), which were used in the multi-mode treatment of tumors through the combination of synergistic photodynamic therapy and immunotherapy [51]. Mei et al. coated BPQDs with erythrocyte membrane, and induced apoptosis of breast cancer tumor cells by using the photothermal therapy (PTT) effect of BP under NIR, so as to achieve the purpose of tumor inhibition and treatment [52]. Without doubt, gold nanoparticles [53] and other emerging Xenes [54], are wildly employed in photothermal anti-tumor therapy [55], due to their NIR light response characteristics, ease of fabrication, and tunability in optical properties [56], but their wild applications are subject to serious limitations such as a weak photothermal conversion efficient, relatively low biosafety and biodegradability, difficulty in metabolizing out of the human body, undegradability, and cytotoxicity [56,57]. However, unlike gold nanoparticles, which have safety concerns that can induce toxicity to cells directly, lead to vascular obstruction resulting from long-term potential nanoparticle aggregation, and cause to immune rejection [58,59], BP is composited by the phosphorus element alone which accounts for up to 1% in the body [60]. Therefore, it has better biocompatibility and biodegradability, and the degradation products will not cause huge damage to the kidneys and liver, which is more suitable for biomedical applications and has more practical value [61,62].
3. Biodegradability and stability of BP
In comparison to other 2D nanomaterials for instance MoS2, Boron nitride (BN) and graphene, the biodegradable properties of BP in vivo make it more promising and safer as a biomedical material [63]. BP is easily degraded after exposure to water or oxygen to produce non-toxic intermediates, such as phosphates, phosphites and other PxOy [64]. Besides, recent studies have shown that fewer-layer BP nanosheets, especially single-layer nanosheets, are more prone to interact with water and oxygen react and are more easily degraded [65,66]. However, in practical application, due to the poor stability of BP material under the conditions of water and oxygen, partial degradation occur at the initial stage of implantation or before implantation, which may affect the final therapeutic effect. This phenomenon is mainly due to the existence of a lone pair of electrons in the phosphorus atom in BP, which can easily adsorb the surrounding oxygen molecules [67,68]. The specific degradation mechanism is that the oxygen molecules on the surface of BP produce superoxide ions (O2−), O2− combine with the lone pairs of electrons in the surface layer of BP to form phosphorus oxide, which rapidly degrades to phosphate under aqueous conditions, after that the remaining unoxidized BP continues to be degraded [[69], [70], [71]]. For BP material, in order to obtain a wide range of applications, it is necessary to improve its stability, because BP structure and function will be greatly reduced or even disappear after oxidative degradation.
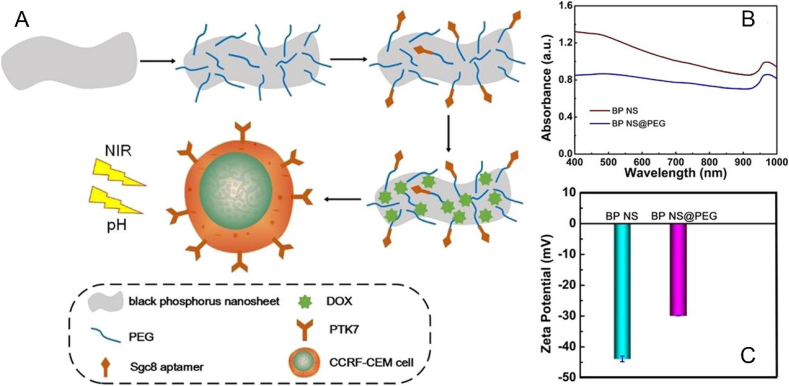
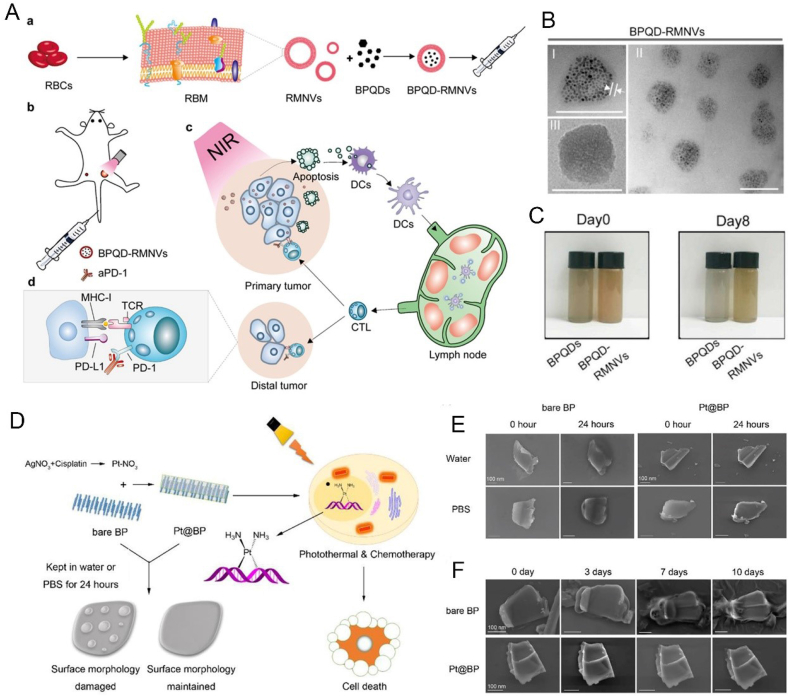
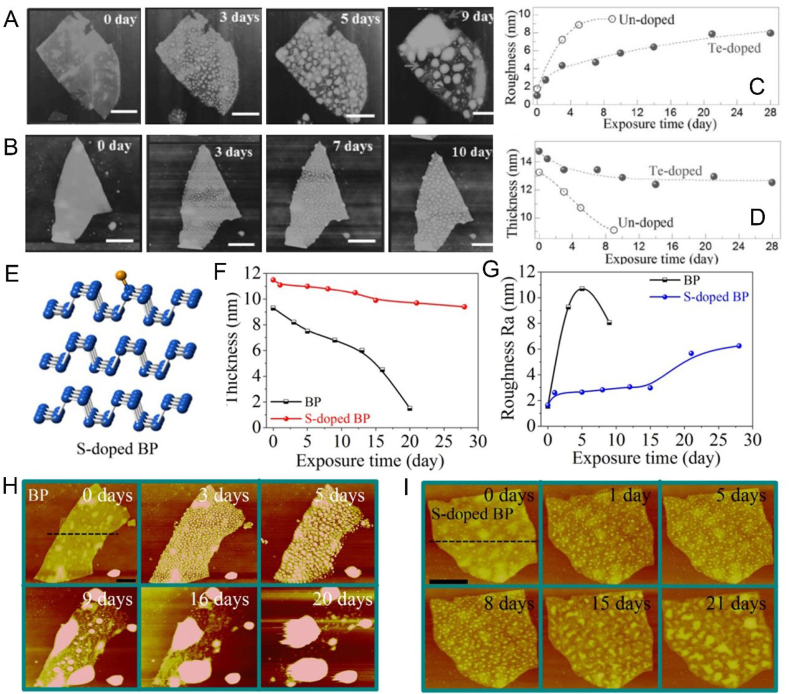
At present, the main ways to improve the stability of BP are adding protective layer [72], surface chemical modification [73], doping with other elements [74] and physical mixture [75]. For example, Zong et al. prepared a drug nanocarrier based on BP nanosheets modified by PEG through electrostatic adsorption, which can enhance the stability of bare BP nanosheets and work as a synergetic and targeted chemophotothermal platform for treatment of acute lymphoblastic leukemia (ALL) (Fig. 2) [76]. Liang et al. designed a vesicular preparation of BPQDs coated with red blood cell membranes (RBCM), which can induce apoptosis of breast cancer cells in situ by NIR laser irradiation, thereby mobilizing the immune system to eliminate residual and metastatic cancer cells (Fig. 3A–C) [52]. Zhang et al. prepared metal ion platinum (Pt)-modified bare BP through cation-π interaction to improve the stability of BP nanosheets (Fig. 3D–F) [77]. Wang et al. customized the tripeptide Fmoc-Lys-Lys-Phe (Fmoc-KKF) for surface modification of BP nanosheets, and the modified BP@FKK complex not only had excellent stability, but also showed good cellular compatibility and enhanced cell uptake capacity [78]. Yang et al. doped tellurium (Te) to improve the stability of the prepared BP (Fig. 4A–D) [79]. Lv et al. achieved a sulfur (S) doped BP by using high-pressure and high-temperature methods, which presented higher stability and more stable transistor performance even after exposing to air for 21 days (Fig. 4E–I) [80]. Tang et al. developed fluorinated BPQDs via one step approach, which possessed superior air stability due to its fluorine adatom‐induced antioxidation and anti-hydration behavior [81]. Xing et al. constructed BP/cellulose hydrogels for effective cancer therapy [75]. There have been a lot of studies on enhancing the stability of BP, however, research on BP is still in the preliminary exploration stage, and further research is needed to obtain a more stable BP without affecting the function of BP.
Fig. 2.
(A) Schematic illustration of the fabrication process and structure of the BP-based nanocarrier. (B) Absorbance spectra of BP and BP@PEG. (C)) Zeta potential of BP and BP@PEG. Reprinted with permissons from Ref. [76], Copyright 2019, Ameriacan Chemical Society. Abbreviations: CCRF-CEM, ALL cell line; PTK7, tyrosine kinase 7; Sgc8, aptamers.
Fig. 3.
(A) Schematic illustration of preparation of BPQD-RMNVs and their photothermal cancer immunotherapy mechanism. (B) TEM image of BPQD-RMNVs; (C) Images of the BPQDs and BPQD-RMNVs after storage in water for 8 days. Reprinted with permissons from Refs. [52], Copyright 2019, Elsevier. (D) Schematic illustration of Pt@BP and photothermal cancer immunotherapy mechanism. (E,F) SEM images of bare BP and Pt@BP in water and PBS for 0 and 24 h or for 0, 3,7,10 days. Reprinted with permissons from Ref. [77], Copyright 2019, Ameriacan Chemical Society. Abbreviations: RBCs, red blood cell; RMMVs, RM nanovesicles; aPD-1, cell death protein 1 antibody; MHC-1, major histocompatibility complex-1; DCs, dendritic cells; APCs, antigen presenting cells; CTL, cytotoxic Tlymphocyte.
Fig. 4.
(A, B) AFM images of an undoped BP flake or a Te-doped BP flake after ambient exposure for 0, 3, 5, and 9 days or 0, 3, 7, and 10 days. (C,D) Variation of surface roughness and thickness with increased exposure time. Reprinted with permissons from Refs. [179], Copyright 2016, Wiley-VCH. (E) schematic diagram of S-doped BP. (F,G) Variation of thickness and roughness with exposure time. (H,I) AFM images of an undoped BP or S-doped BP flake after exposure times of 0, 3, 5, 9, 16, and 20 days, or 0, 1, 5, 8, 15, and 21 days. Reprinted with permissons from Ref. [80], Copyright 2018, Ameriacan Chemical Society.
4. The destination of BP tissue engineering application-bone therapy
4.1. Bone physiology and bone mineralization
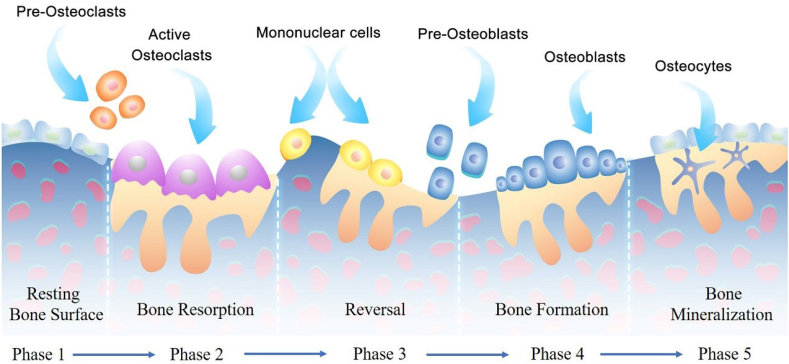
The essence of bone tissue is a biomaterial with a hierarchical structure from micro to macro [82]. It is composed of organic matter, mainly type I collagen [83], to support and tension [84], and mineral salts [85], mostly calcium phosphate, to provide stiffness and pressure to the bone [86]. Bone tissue is in a constant remodeling state (Fig. 5) via the coordinate regulation between osteoclasts derived from differentiation of hematopoietic stem cells absorbing dead bone and osteoblasts derived from differentiation of mesenchymal stem cells promoting the formation of new bone, which is important for maintaining normal bone structure and function [87]. However, when the balance is broken, that is, the function of osteoclasts is stronger than that of osteoblasts or the activity of osteoblasts is higher than osteoclasts, diseases such as osteoporosis and osteosclerosis may occur [88,89]. Moreover, in the process of bone remodeling, in addition to the participation of osteoblasts and osteoclasts [90], bone mineralization caused by calcium and phosphorus metabolism also plays an important role [91].
Fig. 5.
Schematic illustration of the normal bone remodeling process.
Bone mineralization is based on type I collagen as a scaffold, under the synergistic adjustment of mineral salts, non-collagen, proteoglycan, glycoprotein and related enzymes [84,86]. Bone mineralization occurs in two steps [92]. First, calcium ions and phosphate ions accumulate in matrix vesicles (MV) to form hydroxyapatite (HA) crystals, followed by HA proliferation through the cell membrane into the extracellular matrix [93]. Calcium-binding phospholipids, calcium-binding proteins, and bone-binding proteins promote calcium accumulation in MV. The membrane binding proteins in MV form calcium channels and bind calcium into MV. Sodium (Na)/P cooperation protein type III located on the cell membrane and matrix vesicle membrane to provide phosphate [85]. Cytosolic phosphate produces phosphate by hydrolyzing phosphocholine and phosphoethanolamine. When the accumulation of calcium and phosphate exceeds the dissolution point of calcium phosphate (CaPO4), the HA in MV forms the CaPO4 precipitation [94]. The second step of mineralization is that HA penetrates the MV and extends into the extracellular matrix (ECM), connects with collagen, and then continues to grow [95]. The outward extension of HA requires MV to be in an environment with a suitable concentration of calcium and phosphate and PH value. HA exists in clusters around MV and gradually fills the gaps between collagen fibers in the bone matrix to achieve mineralization [96].
4.2. Current status of bone defects
Bone defect caused by infection, trauma, tumor, bone joint disease, bone nonunion or delayed union has always been a difficulty in clinical treatment [97,98]. The essence of the treatment should be to strengthen the bone mineralization ability of the defect area and the capacity of osteoblasts to form new bone [99,100]. Although research on the treatment of bone defects has been continuing for more than a century, the treatment method is still limited and the effect is still unsatisfactory, especially for osteoporotic fractures or bone defects caused by aging [101]. At present, the "gold standard" for bone defect treatment is still autogenous bone transplantation, which is accompanied by a large number of risks, such as anesthesia risk, intraoperative damage to the donor area, limited bone extraction (5–70 cm3) and potential complications, thus limiting the wide application of autogenous bone transplantation [102,103]. The risk of immune rejection and transmission of diseases such as hepatitis B and syphilis unavoidably exist in bone allografts including homologous and allogeneic bone grafts [104]. Therefore, based on the urgent clinical needs, the development of safe and effective bone substitutes may have epoch-making milestone significance.
In recent years, with the rapid development of tissue engineering technology, artificial bone replacement materials have become the main research and development direction of reconstructing the defect bone morphology and function, and have made a lot of achievements [105,106]. Compared with "gold standard" autogenous bone, the advantages of artificial bone replacement materials mainly include the unlimited amount of synthesis [107], the ability to act as drug delivery carrier [108], and the ease of functional modification [109]. Thus, giving the materials new functions such as vascularization [110], bone targeted recognition [111], and recruitment of stem cells [112], are more conducive than the physiological repair of defects. For example, Yan et al. developed a 3D printed polycaprolactone (PCL) scaffold with vascularization capabilities for the repair of critical bone defects [113]. Kim et al. synthesized a pH-responsive and thermo-sensitive hydrogel designed by introducing sulfamethazine oligomer (SMO) to the copolymer of PCL and PEG to release bone morphogenetic protein 2 (BMP2) targeted osteoblasts and promote mineralization [114]. Wang et al. prepared adaptive nanoparticles that recruit stem cells to enhance the repair of bone defects [115]. Liu et al. developed a lithium coated titanium scaffold that promotes bone integration through the Wnt/β-catenin pathway [116]. These artificial bone substitute materials have good bone repair capabilities, however, they are also many defects, for example: the release of deferoxamine (DFO) from vascularized 3D scaffolds is uncontrolled; BMP2 released by pH-responsive hydrogels is easily inactivated and expensive; metal scaffold has inability to degrade, and needs for a secondary surgery to remove it later. Therefore, it is of great significance to develop bone substitutes with controlled release, biodegradability and high biocompatibility.
4.3. Advantages of BP in bone therapy
BP-based biomaterials have significant advantages in bone therapy, the most important one is that BP is composed of a single phosphorus element [66], which has a high degree of homology with the inorganic components of natural bone [117]. Although, antimonene and boron nanosheets are also emerging materials in nanomedicine, they are not the major constitution of bone inorganic substance and it is not clear whether antimonene has an ability to repair bone or how boron regulate the mechanism of bone regeneration [118,119]. In comparison, even though currently widely used HA, β-tricalcium phosphate (β-TCP), and other calcium phosphate-based bone substitute materials also simulate the composition of natural bone minerals, and give them excellent mechanical properties and ability to induce new bone deposition [[120], [121], [122], [123]], the HA structure is stable, not easy to degrade, and unable to provide a microenvironment of 3D micro-nano structures suitable for bone growth, showing the disadvantages of poor cell crawling and cell adhesion, as well as the difficulty of ingrowth [[124], [125], [126]]. As a result, the defect healing rate after material implantation is often slow, the amount of bone formation is limited, and the clinical efficacy is unsatisfactory, especially for the patients with critical bone defect and the population with weak regeneration ability such as multi-basic diseases [127].
Compared with HA, the advantages of BP are as follows: 1) BP can be degraded into non-toxic phosphate after oxidation, then attract surrounding free calcium ions and combine into calcium phosphate mineralization and deposition to promote in-situ bone regeneration and repair [128]; 2) Due to the strong light absorption ability of BP in the NIR region, BP-based nanomaterials have a stable, damage-free light-controlled adjustment release mode, which makes the release of loaded drugs more stable and longer-lasting [49]; 3) In addition, there have been numerous studies showing that BP has good capability of photothermal conversion under NIR region and local hyperthermia treatment has also been confirmed to up-regulate alkaline phosphatase (ALP) [129,130], heat shock protein (HSP) [131], to increase the formation of mineralized crystal, and to achieve longitudinal and concentric growth of bone, thereby accelerating the process of bone repair [132,133]. ALP is the main marker that reflects bone metabolic capacity and its level is related to the differentiation of osteoblasts and greatly affects the process of osteoblast cell matrix mineralization [134]. HSP is essential for the normal formation of subchondral bone [135]. Therefore, we have reason to believe that BP-based nanomaterials will have greater development space in the field of bone repair.
5. Application of BP- based hydrogel in bone regeneration
Hydrogels are a type of natural/synthetic polymer chains interconnected by cross-linking agents to produce hydrophilic biomaterials with a gel macromolecular structure [[136], [137], [138]]. Due to their hydrophilic properties, hydrogels have abilities to swell, hydrate, and mimic the mechanical and lubricating properties of different biological tissues [[139], [140], [141], [142]]. Besides, the injectable and crosslinking properties including physical crosslinking (hydrophobic interaction, hydrogen bonding, electrostatic force, etc.) and chemical crosslinking (covalent bonding) make it easy to design and operate [[143], [144], [145], [146]]. In recent decades, hydrogels have achieved unprecedented development in biomedicine due to their high biocompatibility and adjustable physicochemical properties [147]. The 3D hydrogel network system provides a microstructure of the original extracellular matrix for cell transplantation and differentiation [148], bioremediation [149], wound healing [150], and continuous drug delivery [151]. For example, Cheng et al. used thiol-modified BSA protein and vascular polypeptide to cross-link with Ag ions to construct a peptide-protein injectable hydrogel system for the first time. Taking advantage of the vascularization of vascular peptide, the 3D extracellular matrix characteristics of BSA protein and the rapid antibacterial ability of silver ions, the repair of infected wounds was promoted. In addition, hydrogels are also widely used in critical bone defects [152]. Yan et al. prepared silk fibroin hydrogels with high cell adhesion function to repair bone defects by enhancing the osteogenic differentiation ability of bone marrow mesenchymal stem cells (BMSCs) [153].
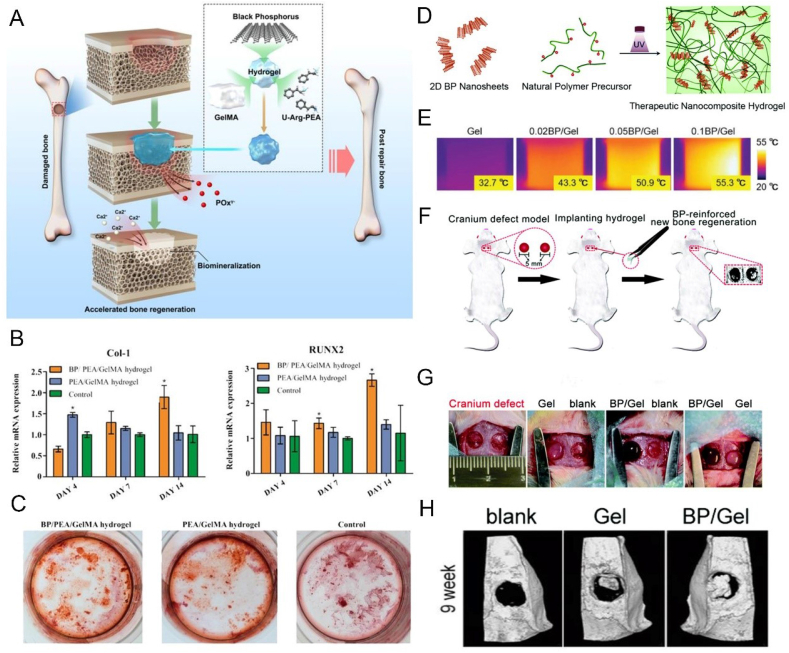
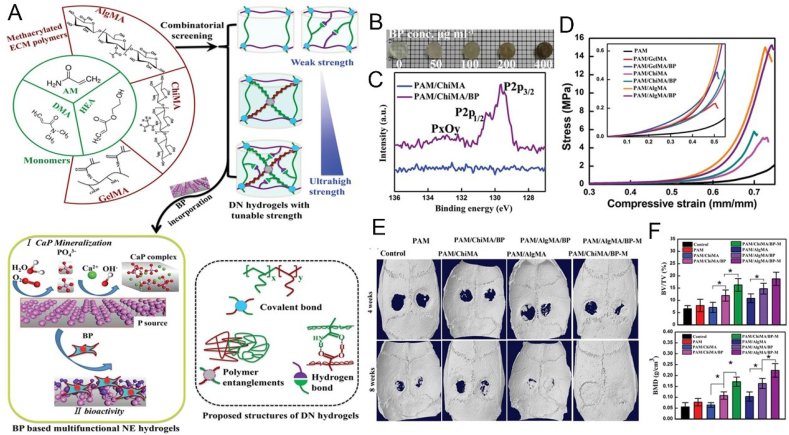
The main reason BP has received extensive attention from researchers is its unique pre-osteogenesis ability in the field of bone repair. Huang et al. developed a BP-based Gelatin methacryloyl (GelMA) hydrogel that continuously and gently supplied phosphate ions, a degradation product of BP, to capture calcium ions, which could improve the osteogenic differentiation of stem cells through the BMP2 pathway and promote in-situ mineralization deposition of calcium phosphate to promote bone regeneration (Fig. 6A–C) [128]. At the same time, they also proved that the introduction of BP nanosheets not only improved the mechanical properties of hydrogels, but also endowed them with the ability of NIR release response. Therefore, in combination with the effect of phosphorus-rich materials for mineralization and bone repair reported in previous studies [154], they believe that this strategy of continuous supply of calcium-free phosphorus achieved by this hydrogel platform containing BP nanosheets provide hope for effective bone regeneration. Miao et al. reported GelMA nanocomposite hydrogels mixed with BP nanosheets (Fig. 6D–H) [155]. They found that the added BP nanosheets confer a variety of functions on the natural matrix, including enhanced networking, photothermal properties, enhanced mineralization, and bone regeneration, thus providing a simple and efficient therapeutic strategy for bone tissue engineering. Wang et al. developed a novel dual network (DN) nanohydrogel containing BP nanosheets with adjustable mechanical properties (Fig. 7) [156]. By introducing BP nanosheets, CaP (calcium phosphate) crystal formation and excellent mechanical properties were induced, and concurrent osteogenic differentiation of osteoblasts provided a favorable ECM microenvironment to promote the repair and regeneration of defective bone. Pan et al. combined BP nanosheets into a platelet rich plasma (PRP) chitosan thermal response hydrogel for the treatment of arthritis and bone defects caused by rheumatoid arthritis (RA) [157]. The local heat generated by BP under NIR and the generated reactive oxygen species (ROS) are transferred to the diseased joint to remove the proliferative synovial tissue, meanwhile, the release of BP degradation products can be precisely controlled, providing sufficient raw materials for osteogenesis, and promoting the repair of bone defects caused by RA.
Fig. 6.
BP-based hydrogels can enhance bone regeneration. (A) Schematic illustration of BP-based hydrogels for bone regeneration. (B) Osteogenic gene (Col-1, Runx2) expression in hDPSCs. (C) ARS staining of hDPSCs on day 15. Reprinted with permissons from Refs. [128], Copyright 2019, Ameriacan Chemical Society. (D) Schematic illustration of the preparation of BP-based GelMA hydrogel. (E)) Infrared thermographic photographs of different BP/Gel hydrogels under 808 nm NIR (1 W cm−2). (F) The process of establishing cranium defect model and hydrogel implantation. (G) The defect areas were implanted with different hydrogels. (H) Micro-CT images of the defect at week 9. Reprinted with permissons from Ref. [155], Copyright 2019, Royal Society of Chemistry. Abbreviations: U-Arg-PEAs, unsaturated poly(ester amide)s; Col 1, collagen 1; Runx2, runt-related transcription factor 2; ARS, alizarin S red.
Fig. 7.
BP-based hydrogels can enhance bone regeneration. (A) Schematic illustration of multifunctional BP-based hydrogels for bone regeneration. (B) Digital photographs of BP-based hydrogels with different concentration. (C) The XPS spectra of different hydrogels. (D) Representative compressive stress–strain curves for differnet hydrogels at various applied strains. (E) Micro-CT images of the cranial defect after different treatments for 4 and 8 weeks. (F) BV/TV and BMD of newly regenerated bone in different groups. Reprinted with permissons from Refs. [156], Copyright 2019, Wiley-VCH. Abbreviations: DN, double network; NE, nanoengineered; PAM, polyacrylamide; HEA, 2-hydroxyethylacrylate; PDMA, poly N,N-dimethyl acrylamide; ChiMA, chitosan methacrylate; AlgMA, alginate methacrylate; GelMA, gelatin methacrylate; BV, bone volume; TV, tissue volume; BMD, bone mineral density.
With the progress of bone repair, the timely invasion of new blood vessels into the fiber/chondral callus will be more conducive to the physiological repair of bone [[158], [159], [160]]. The restoration of the effective blood supply maintains and ensures the completion of the bone remodeling/bone remodeling process in an orderly manner, otherwise it will lead to obstacles in multiple links such as intra-membrane ossification, bone replacement of cartilage callus, bone remodeling/shaping, and even the occurrence of bone nonunion or delayed bone healing [161]. However, there is no report about the use of vascularized BP hydrogel for the repair of critical defect bones. We believe that in the design of future BP hydrogels, loading BP with vasoactive drugs, peptides, or siRNA, and using the light responsiveness of BP will help to bring materials closer to physiological bone repair with precisely controlled release system of hydrogels, and will greatly enrich the current design concept of BP hydrogel.
6. Application of BP-based 3D printed scaffold in bone regeneration
The ideal bone tissue engineering repair scaffold needs to have key features such as biocompatibility, biodegradability, bone conductivity and mechanical properties [162], among which, bone conductivity and good mechanical support capabilities may be essentially required for the development of bone repair materials [163]. Traditional hydrogels and other scaffolds tend to have good cell adhesion, proliferation and biodegradation capacity, without the ability of bone conductivity and long-lasting mechanical support [164,165]. With the rapid development of 3D printing technology, this demand has been well met. The 3D printed scaffold with interconnected porous structure, can provide a 3D environment, similar to the ECM, which promotes the adhesion and proliferation of osteoblasts on its surface as well as pores, induces blood vessel formation and transports nutrient [[166], [167], [168], [169]]. Furthermore, before the new bone formation in weight-bearing bones, the 3D printed scaffold can provide a certain amount of mechanical support, and prevent fibrous tissue from forming an encapsulation between the scaffold and the host bone [170]. Therefore, it is considered to be the most effective method for preparing a structurally bionic bone repair scaffold. For example, Walsh et al. described a cell-free biomaterial implant that has been functionalized with a low dose combination of BMP2 and vascular endothelial growth factor (VEGF) on an osteoconductive collagen-hydroxyapatite 3D scaffold, enabling rapid regeneration of rat critical-sized cranial defect [171]. However, the current 3D printed scaffolds do not have good mineralization abilities, and it is difficult to achieve controlled release of loaded drug. Therefore, it is of great practical value to develop 3D scaffolds with good biomechanical properties, bone mineralization and controlled release.
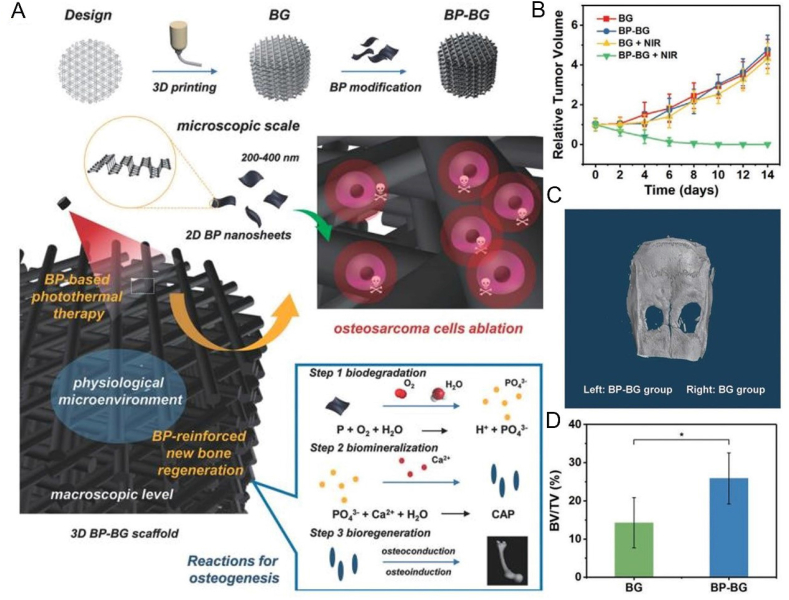
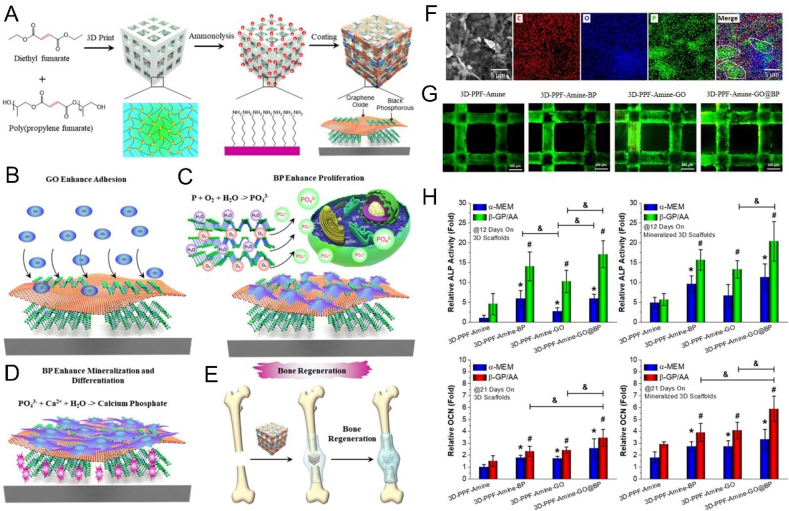
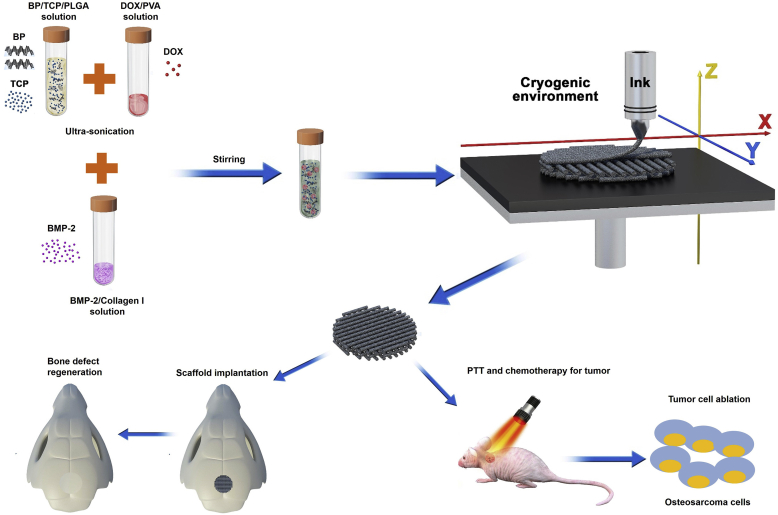
In the past 2 years, the application of BP-based 3D printed scaffolds in the treatment of bone tumors and repair of bone defects has gradually increased. Yang et al. constructed a 3D printed BP-Bioglass (BG) scaffold, which ablated osteosarcoma via the PTT effect of BP under NIR, and drove the binding of calcium ions by the degradation product phosphate to achieve the biological mineralization (Fig. 8) [172]. Meanwhile, the excellent characteristics of the BG scaffold contributed in bone formation, bone conduction and bone induction, as well as the promotion of the in-situ regeneration of the defective bone. Both in vitro and in vivo experiments had shown that the BP-BG scaffold had high osteogenic capacity, indicating that it has excellent potential in the treatment of osteosarcoma. In another case, BP was first wrapped in negatively charged graphene oxide (GO) nanosheets, then these BP@GO sheets were absorbed on a positively charged poly (propylene glycol fumarate) 3D scaffold (Fig. 9) [173]. Due to the huge surface area of GO and the phosphate released by BP's continuous oxidation, the synergistic effect of GO and BP on cell proliferation and osteogenic differentiation was played. According to the concept of "kill first, then repair and regenerate ", based on 3D printing technology at low temperature, Wang et al. printed out a 3D porous nanocomposite scaffold with high mechanical strength, containing poly(lactic-co-glycolic acid) (PLGA), β-TCP, BP, DOX and BMP2 and used this scaffold to reconstruct the defect after bone tumor resection [174] (Fig. 10). The synergy of BP photothermal therapy and DOX chemotherapy is used to achieve the purpose of tumor ablation and long-term suppression of recurrence. Eventually, due to the degradation of β-TCP and BP and the release of BMP2, the defect obtained a better bone regeneration effect.
Fig. 8.
BP-based scaffolds can ablate osteosarcoma and enhance bone formation. (A) Schematic illustration of the preparation process of 3D BP-BG scaffold and step-by-step treatment strategy. (B) Time-dependent tumor-growth curves of the mice after different treatments. (C) Micro-CT images of the cranial defect after treatment for 8 weeks. (D) The BV/TV percentage over the entire defect space (*p < 0.05). Reprinted with permissons from Ref. [172], Copyright 2018, Wiley-VCH.
Fig. 9.
GO@BP-based scaffolds can enhance bone regeneration. (A) Schematic illustration of the fabrication process of 3D GO@BP-based scaffolds. (B–D) The function of GO and BP nanosheets for enhancing cell adhesion, proliferation and differentiation of pre-osteoblasts. (E) Bone regeneration schematic demonstration of 3D GO@BP-based scaffold. (F) The corresponding C, O, P element mappings of GO@BP nanosheets. (G) Live/dead staining of different functioned 3D scaffolds for MC3T3 preosteoblast cells on day 3. (H) Relative ALP activity (14 days) and OCN content (21 days) of MC3T3 pre-osteoblasts on 3D GO@BP-bsaed scaffolds and 3D GO@BP-bsaed scaffolds after mineralization in SBF solution (*, #, & p < 0.05). Reprinted with permissons from Refs. [173], Copyright 2019, Ameriacan Chemical Society. Abbreviations: GO, graphene oxide; PPF, poly(propylene fumarate); C, carbon; ALP, alkaline phosphatase; OCN, osteocalcin; α-MEM, α-minimum essential medium; β-GP, β-glycerophosphate; AA, ascorbic acid.
Fig. 10.
Cryogenic 3D printing of porous scaffolds can enhance bone regeneration. (A) Schematic illustration of the formulation of inks, and the fabrication of 3D multi-functional scaffolds at low temperature. (B) Macroscopic morphology of different scaffolds. (C) Confocal laser scanning microscope (CLSM) piture of expression of vinculin adhesive plaques and F-actin cytoskeleton of rBMSCs on different scaffolds in 24 h. (D) ALP staining (14 days) and ARS staining (21 days) of rBSMCs cultured with extracts of different scaffolds. (E) Micro-CT images of the cranial defect after different treatments for 8 and 12 weeks. Reprinted with permissons from Ref. [174], Copyright 2020, IOP Publisher. Abbreviations: PVA, polyvinyl alcohol; TP, TCP/PLGA scaffold; PTP, P24/TCP/PLGA scaffold; DPTP, DOX/P24/TCP/PLGA scaffold; BPTP, BP/P24/TCP/PLGA scaffold; BDPTP, DOX/P24/BP/TCP/PLGA scaffold.
In the case of bone defects, especially segmental large defects, 3D scaffolds can be biodegraded under the condition of persistent body load to match the formation of new bone tissue [175,176]. In addition, compared with other technologies, 3D scaffolds are able to deliver personalized, customized treatments [177]. The BP-based 3D printed scaffold integrates BP photothermal, osteogenesis and other natural advantages, making use of the good skeleton support of 3D scaffold, which will be better applied in segmental bone repair and treatment, and make up for the shortcomings of other existing materials such as insufficient mechanical properties.
7. Application of BP-based microspheres in bone regeneration
Minimal invasion is one of the key points to evaluate the efficacy of repair. From a clinical perspective, injectable materials can simplify surgeons’ operational difficulty, reduce the risk of infection and scar formation, reduce treatment costs, and improve patient comfort [178,179]. Microspheres may be the best minimally invasive treatment [180,181]. Microspheres are free-flowing microcapsules or microparticles, with a diameter of 1–1000 μm, and can be used for encapsulation of drugs, bioactive molecules, and cells [182]. Compared with ordinary dosage form drugs, microspheres can improve the stability of drugs [183], reduce the stimulation of the gastrointestinal tract [184], enhance the controlled release and delivery to the targeted region [185,186]. For example, Li et al. used tetraethylenepentamine-graphene (rGO-TEPA) to induce CaCO3 mineralization and successfully constructed a CaCO3/rGO-TEPA drug carrier with a hollow structure and rough surface to achieve DOX loading and sustained release, which can regulate release and promote anti-tumor treatment through pH response [187]. In addition to being an excellent controlled-release carrier, microspheres can be packaged individually or combined with other materials to make a porous 3D structure serving as tissue engineering scaffolds [188,189]. For example, Xin et al. developed a 3D-printable clickable PEG microparticles bio-ink, using thiol-ene click chemistry to produce with unreacted norbornene groups of PEG microgels, which is then photochemically annealed with PEG-di-thiol linker via a second thiol-ene click reaction [190]. Besides, they also verified that human mesenchymal stem cells (hMSCs) with high survival rate could spread, proliferate and activate mechanical signaling pathways in response to the physical and chemical properties of PEG microgels after annealing.
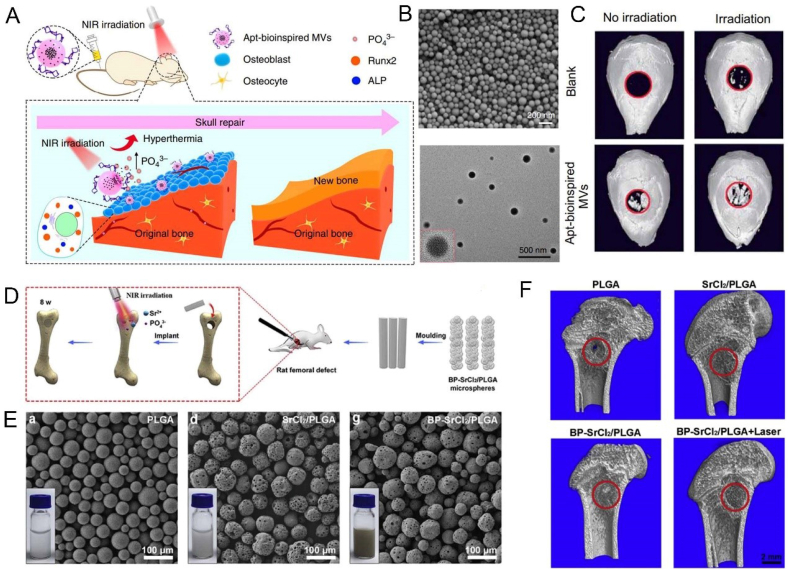
In recent years, based on the requirements of minimally invasive and high drug-loading capabilities, microsphere biomaterials have achieved more applications in bone repair. For example, Hu et al. incorporated interleukin 4 (IL4) into a modified gelatin microsphere with self-assembly heparin nanofibers, through the immune regulation of macrophage polarization (transforming pro-inflammatory M1 macrophages into positive healing M2 phenotypes), this hybrid microsphere was able to eliminate inflammation response, and ultimately enhance the osteoblast differentiation and bone regeneration of diabetes [191]. Because of its unique physicochemical properties and superior osteogenesis ability, BP has also attracted more and more attention in the construction of microsphere materials. Wang et al. reported bio-inspired matrix vesicles that encapsulated BPQDs via PLGA microspheres and functionalized them with specific aptamers that could target osteoblasts, while the phosphate microenvironment caused by BP degradation stimulated cell biomineralization (Fig. 11A–C) [192]. In addition, the microspheres have photothermal effect by introducing BPQDs, and further promote bone regeneration by stimulating the up-regulated expression of HSP and ALP. Although the interconnected pores between granular microspheres are beneficial to bone regeneration, polymer microspheres usually have unstable morphology, lacks of shaping ability, and are prone to loss [[193], [194], [195]]. Wang et al. constructed NIR-trigged microspheres for sustained-release strontium (Sr2+) for repairing distal femoral defects via incorporating SrCl2 and BP into PLGA (Fig. 11D–F) [196]. With the addition of BP, BP-SrCL2/PLGA microspheres had a good photothermal response ability under NIR, and was endowed with the precise release ability of Sr2+ through the thermal destruction of PLGA shell. Besides, these novel microspheres show excellent cell viability and biodegradability.
Fig. 11.
BP-based microspheres can enhance bone regeneration. (A) overview of Apt-bioinspired MVs in cell mineralization and bone regeneration. (B) SEM and TEM images of Apt-bioinspired MVs. (C) Micro-CT images of crancial defect after different treatments. Reprinted with permissons from Refs. [192] This is an open access article distributed under the terms of the Creative Commons CC BY license, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited. (D) BP-SrCl2/PLGA microspheres for bone regeneration. (E) SEM images and solution photograph of different microspheres. (F) Micro-CT images of different treatments for 8 weeks. Reprinted with permissons from Ref. [196], Copyright 2018, Elsevier. Abbreviations: Apt, aptamer; MVs, Matrix vesicles.
In microcapsules, the cargo molecules are encapsulated in a core, and the shell is composed of a matrix material; while in monolithic particles, the cargo molecules are dispersed in the matrix [197]. The density of the non-porous traditional microspheres depends on the density of the matrix material itself, while the specific surface area increases with decreasing particle size [198]. These defects limit the application of traditional microspheres. The multi-compartmental or multi-porous microspheres can greatly improve the specific surface area of the microspheres because of their better classification, packaging capabilities and loading efficiency [199,200]. A microsphere can carry multiple drug molecules or cells, which can achieve better physiological repair. Combined with the advantages of BP, we believe that such multi-spaced microspheres and porous microspheres have more potential value and better therapeutic effects in bone repair applications.
8. Application of BP-based electrospun membrane in bone regeneration
Electrospinning has become a versatile and popular technique for constructing tissue engineering scaffolds because it can produce layered microstructures and highly controllable nanofibers [201]. Electrospun nanofibrous membranes have a porous microstructure that mimics the natural extracellular matrix, making it a temporary platform for cells to migrate, adhere, differentiate and proliferate [202]. Of course, the promotion of cell adhesion, proliferation, and the ability of tissue repair or other behaviors are not the prerequisites for the application of all electrospun nanofibrous membranes. In some cases such as in the prevention of tissue adhesion, less cell adhesion, less cell proliferation, even no cell proliferation is what we expect [203,204]. Inspired by the super lubrication mechanism of articular cartilage, our group successfully constructed hydration super-lubricated electrospun nanofibrous membranes for the prevention of tendon adhesion. This copolymer was synthesized by grafting polydopamine methacrylamide (pDMA) and 2-methacryloyloxyethyl phosphorylcholine (MPC) onto PCL electrospun membranes in-situ via one step method [205].
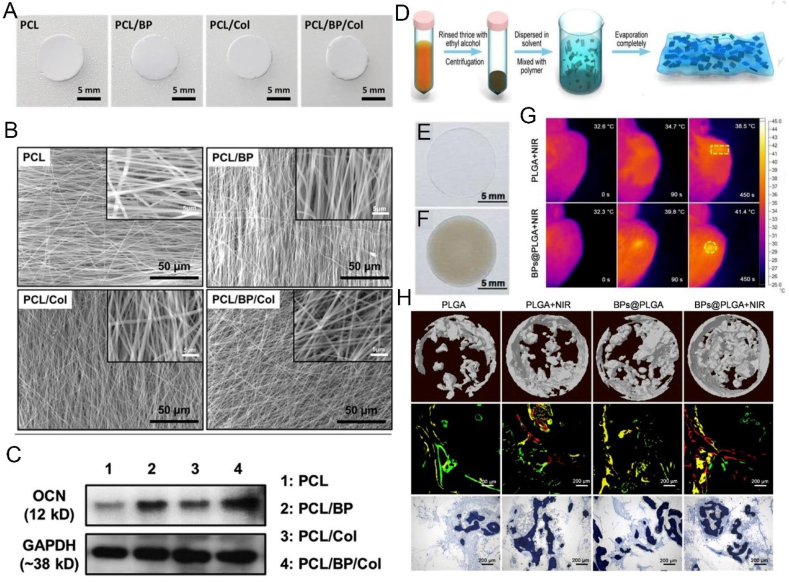
In addition to preventing tissue adhesion, electrospun nanofibrous membranes have also been widely used in repairing bone defects [206,207]. Inspired by the balance between bone resorption and bone formation during bone remodeling, Wang et al. developed an electrospun PCL/gelatin nanofiber scaffold with mesoporous silicate nanoparticles (MSN) to achieve the synergic effect of alendronate (ALN) and silicate in regulating bone remodeling and promoting the regeneration of defective bone [208]. Inspired by the structure of the periosteum, Wu et al. constructed a multi-layer micro/nano fiber membrane with sustained release VEGF, which greatly mimicked both intramembranous and endochondral ossification, where periosteal regeneration was achieved via collagen self-assembly and micro-sol electrospinning technologies [209]. However, there has not been a lot of related reports on the promotion of bone regeneration using BP-based electrospun nanofibrous membranes. Lee et al. successfully prepared PCL/BP/Collagen nanofiber membrane by electrospinning technology, and confirmed that this membrane not only can promote the attachment and proliferation of osteoblasts, but also promote their osteogenic differentiation, which can be used as a potential scaffold material for bone tissue engineering (Fig. 12A–C) [210]. However, the successful construction of electrospun membranes may be limited by equipment, air humidity and temperature [211]. BP nanosheets were blended into trichloromethane (DCM) solution containing PLGA before poured into a specific mold after stirring and sonication to prepare a BP@PLGA membrane (Fig. 12D–H) [212]. This easily prepared biofilm was found to be completely biodegradable, and the degradation products were harmless H2O, CO2 and PO43−, which could be used as essential raw materials for bone regeneration. Also, after being implanted in vivo, even if it was covered by 7 mm thick biological tissue, the BP@PLGA membrane still showed an efficient NIR photothermal response capability. It has been proven that moderate and regular heat induction could effectively up-regulate the expression of HSP, and ultimately promote in vivo and in vitro osteogenesis.
Fig. 12.
BP-based membranes can enhance bone regeneration. (A) Digital photographs of different membranes. (B) SEM images of different membranes. (C) Immunoblotting for OCN expression in MC3T3-E1 preosteoblasts on different membranes. Reprinted with permissons from Ref. [210], Copyright 2019, Elsevier. (D) Preparation and characterizations. (a) Schematic illustration of preparation process of BPs@PLGA membranes. (E, F) Macroscopic images of PLGA and BPs@PLGA membranes. (G) Infrared thermographic maps with the notations of the highest temperature in irradiated areas. (H) Micro-CT images, fluorescent staining, and toluidine blue staining of different treatments. Reprinted with permissons from Ref. [212], Copyright 2019, Elsevier. Abbreviations: Col, collagen; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Peripheral nerve fibers are important for not only normal bone homeostasis and bone growth, but also for post-traumatic bone repair mechanisms (such as fracture healing) [213]. Sympathetic nerves and sensory-derived nerve fibers innervate trabeculae, periosteum, and callus, which are involved in the control of angiogenesis and matrix differentiation during endochondral ossification in embryonic limb development, suggesting a unique role in regulating bone growth and limb formation [214,215]. In bone metabolism and bone remodeling, calocalcin gene-related peptide (CGRP) and vasoactive intestinal peptide (VIP) have anabolic effects, inducing osteoblast activity and inhibiting osteoclast generation [216,217]. The promotion effect of BP on the recovery of nerve regeneration has also been supported by relevant literature, mainly based on two characteristics: the phosphate degradation product and the high electrical conductivity [218]. Phosphate can promote early regeneration of peripheral nerves by reducing scar formation and inducing axon regeneration, while the specific mechanism is that phosphate can promote cell attachment and axon extension [219,220]. Besides, the essence of nerve signal conduction is electrical conduction, therefore, restoring the damaged electrical functions of injured neurons can promote nerve regeneration [221]. Due to the higher electrical conductivity of BP, its potential application in peripheral nerve regeneration become possible. Qian et al. prepared a concentric integrated layer-by-layer biological self-assembled BP-based nanocomposite scaffold [218]. They found that this nanocomposite scaffold had the ability to induce neurogenesis and angiogenesis, and stimulate remyelination and calcium-dependent axonal regeneration, under mild oxidative stress, which provided new sight for nerve regeneration. It is foreseeable that in the future application of BP-based nanomaterials in bone repair, the focus is not solely on the degradation product phosphate to promote in-situ mineralization, but also on the possibility of BP-induced nerve regeneration to regulate bone repair and regeneration.
9. Conclusion and outlook
In summary, this work reviews the source, physicochemical properties of BP, and its unique biological characteristics including excellent biocompatibility, biodegradability, photothermal conversion ability, and comprehensively summarizes the progress of BP-based nanomaterials in bone therapy. From the aspects summarized above, the main approaches of BP-based nanomaterial in bone repair include: 1) BP-based nanomaterials are gradually oxidized and degraded into phosphate after implantation, and then capture the surrounding calcium ions, thereby are able to promote in-situ mineralization and regeneration; 2) After implantation of BP-based nanomaterials, even though they are covered by thick biological tissues, their still have high efficiency of photothermal conversion, which creates a regular and stable local thermal environment can activate multiple osteogenic proteins including HSP and ALP, so as to further promote the process of bone repair; 3) Due to the high conductivity, BP-based nanomaterials can assist the recovery of nerve which plays an important role in the regeneration of bone. Therefore, the application of BP-based nanomaterials in the field of bone therapy has achieved good results, and has provided certain theoretical and practical support for subsequent in-depth research.
Although BP-based nanomaterials have achieved satisfactory results in bone repair treatment, compared with other 2D nanomaterials such as graphene, the research on BP is still in its infancy, and there are difficulties and challenges to be overcome for future clinical applications, such as expensive; cumbersome preparation; poor stability; difficult to store for a long time and unclear immune mechanism. Among them, the most important point is the possibility of in vivo immune rejection of all implants, including BP-based nanomaterials [222]. As is known to all, bone repair is a complicated process, including inflammation hematoma reaction, fiber callus formation, osseous callus formation and bone remodeling stage [223]. In the inflammation hematoma reaction phase, the immune regulatory response of immune cells, including macrophages, is thought to affect osteoblast differentiation, apoptosis and other behaviors [224,225].
In addition, from the perspective of body immunity, all implanted materials are likely to be foreign matters that cause autoimmune reactions [226]. The implantation of bone repair materials not only changes the local microenvironment of bone, but also makes the relationship among immune cells, biomaterials and microenvironment more complicated [227]. With regard to the relationship between bone repair materials and immune defense, the viewpoint has gradually changed from "how to avoid the inflammatory response caused by implanted materials" to "the immune response of the body stimulated after the implantation of materials plays a key role in the participation of materials in bone tissue regeneration" [228]. Macrophages are the major players in this reaction, and their content in bone tissue is about one sixth of the total bone marrow cells [229]. As early as 1980, scientists observed that osteo-macrophages not only exist in static surface of the rest bone tissue, but also exist beside mature osteoblasts in the active site of bone remodeling, which provides first-hand evidence for osteo-macrophages to support bone formation [230,231]. With the deepening of research, some scholars have found that osteo-macrophages can significantly enhance mineralization in vitro experiments, and in vivo animal experiments have also confirmed that mice with macrophage knockout exhibit less bone formation [232]. These data comprehensively demonstrated the great potential of macrophages in regulating bone formation. In addition, macrophages can also respond effectively to signaling molecules in the microenvironment by changing the phenotype [229,233]. For example, M2 macrophages mainly release anti-inflammatory factors to play an immunomodulatory role, and release related growth factors to promote vascularization and extracellular matrix formation, which is beneficial for tissue repair and regeneration [234]. Even though, the phagocytosis of foreign materials by macrophages will trigger an inflammatory reaction to a certain extent [235], which is a normal host response to foreign material, the intensity and persistence of the inflammation will ultimately affect the biocompatibility, stability and implant effectiveness of the material in vivo [236].
In addition, some ions released during the degradation of the implanted material in the body will also change the microenvironment of the immune response [237]. For example, calcium ions (Ca2+) are immunomodulated through the Wnt/5A pathway [238], magnesium ions can be immunomodulated by inhibiting the Toll-like receptor (TLR) pathway [239], Sr2+ can inhibit the secretion of tumor necrosis factor-α (TNF-α) by blocking the nuclear factor-kappa B (NF- kB) pathway [240]. While the effect of BP on the polarization of M1 and M2 macrophages and their immune mechanism has not yet been reported. Recently, some scholars have pointed out that the bare BP may have a potential inflammatory response, and when modified with titanium sulfonate ligand (TiL4), it can effectively escape from the uptake of macrophages and reduce the cytotoxicity and proinflammatory effects [241]. Mo et al. found that after adsorption of plasma proteins on BP nanomaterials, a protein corona structure (protein-nanoparticle interaction) might be formed, which would significantly reshape the BP-corona composite nanomaterials, affecting cell uptake, activating the NF-kB pathway, and inducing immunotoxicity and immune interference in macrophages [242]. Moreover, we notice that the immune response is also affected by the surface charge of materials, which is generally believed that cationic (positively charged) particles can enhance inflammation more than anionic (negatively charged) and neutral particles [243,244]. Most immune cells have a negative surface charge and positively charged particles will drain the negative surface charge of the cell membrane, which may affect the location and confirmation of the protein. That will normally induce signal transduction into the cytoplasm, resulting in obvious biological reactions, including inflammatory reactions [245]. Accordingly, we speculate that decreasing the concentration of BP or forming BP composite particles, may effectively reduce inflammatory reaction. However, the study on how BP nanomaterial regulates bone repair through bone immunity needs to be further studied. With the unremitting efforts of more and more researchers, the application of BP-based nanomaterials in bone therapy will be better developed and reach a higher level.
CRediT authorship contribution statement
Liang Cheng: Writing - original draft, Writing - review & editing. Zhengwei Cai: Writing - original draft. Jingwen Zhao: Writing - review & editing. Fei Wang: Writing - review & editing. Min Lu: Writing - review & editing. Lianfu Deng: Project administration. Wenguo Cui: Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
This study was financially supported by the National Key R&D Program of China (2018YFC1106200), Shanghai Municipal Health and Family Planning Commission (201840027), Shanghai Municipal Education Commission-Gaofeng Clinical Medicine Grant Support (20171906), Shanghai Jiao Tong University “Medical and Research” Program (ZH2018ZDA04).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
References
- 1.Nath N.S., Bhattacharya I., Tuck A.G., Schlipalius D.I., Ebert P.R. Mechanisms of phosphine toxicity. J. Toxicol. 2011;2011:494168–494177. doi: 10.1155/2011/494168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elser J.J. Phosphorus: a limiting nutrient for humanity? Curr. Opin. Biotechnol. 2012;23(6):833–838. doi: 10.1016/j.copbio.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 3.Liu H., Du Y., Deng Y., Ye P.D. Semiconducting black phosphorus: synthesis, transport properties and electronic applications. Chem. Soc. Rev. 2015;44(9):2732–2743. doi: 10.1039/c4cs00257a. [DOI] [PubMed] [Google Scholar]
- 4.Wu J., Sunda W., Boyle E.A., Karl D.M. Phosphate depletion in the western North Atlantic ocean. Science. 2000;289(5480):759–762. doi: 10.1126/science.289.5480.759. [DOI] [PubMed] [Google Scholar]
- 5.Calvo M.S., Lamberg-Allardt C.J. Phosphorus. Adv. Nutr. 2015;6(6):860–862. doi: 10.3945/an.115.008516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comber S., Gardner M., Georges K., Blackwood D., Gilmour D. Domestic source of phosphorus to sewage treatment works. Environ. Technol. 2013;34(9–12):1349–1358. doi: 10.1080/09593330.2012.747003. [DOI] [PubMed] [Google Scholar]
- 7.Pravst I. Risking public health by approving some health claims?–The case of phosphorus. Food Pol. 2011;36(5):726–728. [Google Scholar]
- 8.Shao J., Ruan C., Xie H., Li Z., Wang H., Chu P.K. Black‐Phosphorus‐Incorporated hydrogel as a sprayable and biodegradable photothermal platform for postsurgical treatment of cancer. Adv. Sci. 2018;5(5):1700848–1700859. doi: 10.1002/advs.201700848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anju S., Ashtami J., Mohanan P.V. Black phosphorus, a prospective graphene substitute for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;97:978–993. doi: 10.1016/j.msec.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 10.Cui L., Houston D.A., Farquharson C., MacRae V.E. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone. 2016;87:147–158. doi: 10.1016/j.bone.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 11.Huesa C., Yadav M.C., Finnilä M.A., Goodyear S.R., Robins S.P., Tanner K.E. PHOSPHO1 is essential for mechanically competent mineralization and the avoidance of spontaneous fractures. Bone. 2011;48(5):1066–1074. doi: 10.1016/j.bone.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang K., Zhou Y., Xiao C., Zhao W., Wu H., Tang J. Application of hydroxyapatite nanoparticles in tumor-associated bone segmental defect. Sci. Adv. 2019;5(8) doi: 10.1126/sciadv.aax6946. eaax6946- eaax6962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li L., Zuo Y., Zou Q., Yang B., Lin L., Li J. Hierarchical structure and mechanical improvement of an n-HA/GCO-PU composite scaffold for bone regeneration. ACS Appl. Mater. Interfaces. 2015;7(40):22618–22629. doi: 10.1021/acsami.5b07327. [DOI] [PubMed] [Google Scholar]
- 14.Sørensen K.U., Kruger M.C., Hansen-Møller J., Poulsen H.D. Bone biochemical markers for assessment of bone responses to differentiated phosphorus supply in growing-finishing pigs. J. Anim. Sci. 2018;96(11):4693–4703. doi: 10.1093/jas/sky311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H.U., Park S.Y., Lee S.C., Choi S., Seo S., Kim H. Black Phosphorus (BP) nanodots for potential biomedical applications. Small. 2016;12(2):214–219. doi: 10.1002/smll.201502756. [DOI] [PubMed] [Google Scholar]
- 16.Sun C., Wen L., Zeng J., Wang Y., Sun Q., Deng L. One-pot solventless preparation of PEGylated black phosphorus nanoparticles for photoacoustic imaging and photothermal therapy of cancer. Biomaterials. 2016;91:81–89. doi: 10.1016/j.biomaterials.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 17.Heyer C.M., Weiss E., Schmucker S., Rodehutscord M., Hoelzle L.E., Mosenthin R. The impact of phosphorus on the immune system and the intestinal microbiota with special focus on the pig. Nutr. Res. Rev. 2015;28(1):67–82. doi: 10.1017/S0954422415000049. [DOI] [PubMed] [Google Scholar]
- 18.Madeo B., De Vincentis S., Kara E., Vescini F., Trenti T., Guaraldi G. Reliability of calcium-phosphorus (Ca/P) ratio as a new, accurate and inexpensive tool in the diagnosis of some Ca-P disorders. J. Endocrinol. Invest. 2019;42(9):1041–1049. doi: 10.1007/s40618-019-01025-6. [DOI] [PubMed] [Google Scholar]
- 19.Akhtar Ali S., Mathalikunnel A., Bhardwaj V., Braskett M., Pitukcheewanont P. Nutritional hypophosphatemic rickets secondary to Neocate® use. Osteoporos. Int. 2019;30(9):1887–1891. doi: 10.1007/s00198-019-04836-8. [DOI] [PubMed] [Google Scholar]
- 20.Ali Y., Parekh A., Baig M., Ali T., Rafiq T. Renal tubular acidosis type II associated with vitamin D deficiency presenting as chronic weakness. Ther. Adv. Endocrinol. Metabol. 2014;5(4):86–89. doi: 10.1177/2042018814547359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bitzan M., Goodyer P.R. Hypophosphatemic rickets, pediatr. Clin. North. Am. 2019;66(1):179–207. doi: 10.1016/j.pcl.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 22.Schubert L., DeLuca H.F. Hypophosphatemia is responsible for skeletal muscle weakness of vitamin D deficiency. Arch. Biochem. Biophys. 2010;500(2):157–161. doi: 10.1016/j.abb.2010.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Amaral P.E.M., Nieman G.P., Schwenk G.R., Jing H., Zhang R., Cerkez E.B. High electron mobility of amorphous red phosphorus thin films. Angew Chem. Int. Ed. Engl. 2019;58(20):6766–6771. doi: 10.1002/anie.201902534. [DOI] [PubMed] [Google Scholar]
- 24.Peruzzini M., Gonsalvi L., Romerosa A. Coordination chemistry and functionalization of white phosphorus via transition metal complexes. Chem. Soc. Rev. 2005;34(12):1038–1047. doi: 10.1039/b510917e. [DOI] [PubMed] [Google Scholar]
- 25.Al Barqouni L.N., Skaik S.I., Shaban N.R., Barqouni N. White phosphorus burn. Lancet. 2010;376(9734):68. doi: 10.1016/S0140-6736(10)60812-4. [DOI] [PubMed] [Google Scholar]
- 26.Berndtson A.E., Fagin A., Sen S., Greenhalgh D.G., Palmieri T.L. White phosphorus burns and arsenic inhalation: a toxic combination. J. Burn Care Res. 2014;35(2):e128–e131. doi: 10.1097/BCR.0b013e31828c73dd. [DOI] [PubMed] [Google Scholar]
- 27.Gusmão R., Sofer Z., Pumera M. Black Phosphorus rediscovered: from bulk material to monolayers. Angew Chem. Int. Ed. Engl. 2017;56(28):8052–8072. doi: 10.1002/anie.201610512. [DOI] [PubMed] [Google Scholar]
- 28.Zhou Y., Zhang M., Guo Z., Miao L., Han S.-T., Wang Z., Zhang X. Recent advances in black phosphorus-based photonics, electronics, sensors and energy devices. Mater. Horiz. 2017;4(6):997–1019. [Google Scholar]
- 29.Bridgman P.W. Future note on black phosphorus. J. Am. Chem. Soc. 1916;38(3):609–612. [Google Scholar]
- 30.Shin S.R., Li Y.C., Jang H.L., Khoshakhlagh P., Akbari M., Nasajpour A. Graphene-based materials for tissue engineering. Adv. Drug Deliv. Rev. 2016;105(Pt B):255–274. doi: 10.1016/j.addr.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Su Y., Cockerill I., Wang Y., Qin Y.X., Chang L., Zheng Y. Zinc-Based biomaterials for regeneration and therapy, Trends. Biotechnol. 2019;37(4):428–441. doi: 10.1016/j.tibtech.2018.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guan Q., Zhou L.L., Li W.Y., Li Y.A., Dong Y.B. Covalent organic Frameworks (COFs) for cancer therapeutics. Chemistry. 2020;26(25):5583–5591. doi: 10.1002/chem.201905150. [DOI] [PubMed] [Google Scholar]
- 33.Tao W., Kong N., Ji X., Zhang Y., Sharma A., Ouyang J. Emerging two-dimensional monoelemental materials (Xenes) for biomedical applications. Chem. Soc. Rev. 2019;48(11):2891–2912. doi: 10.1039/c8cs00823j. [DOI] [PubMed] [Google Scholar]
- 34.Lei W., Liu G., Zhang J., Liu M. Black phosphorus nanostructures: recent advances in hybridization, doping and functionalization. Chem. Soc. Rev. 2017;46(12):3492–3509. doi: 10.1039/c7cs00021a. [DOI] [PubMed] [Google Scholar]
- 35.Choi J.R., Yong K.W., Choi J.Y., Nilghaz A., Lin Y., Xu J. Black Phosphorus and its biomedical applications. Theranostics. 2018;8(4):1005–1026. doi: 10.7150/thno.22573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo M., Fan T., Zhou Y., Zhang H., Mei L. 2D black phosphorus-based biomedical applications. Adv. Funct. Mater. 2019;29(13):1808306–1808325. [Google Scholar]
- 37.Qiu M., Singh A., Wang D., Qu J., Swihart M., Zhang H. Biocompatible and biodegradable inorganic nanostructures for nanomedicine: silicon and black phophorus. Nano Today. 2019;25:135–155. [Google Scholar]
- 38.Zhang R., Zhou X., Zhang D., Lou W., Zhai F., Chang K. Electronic and magneto-optical properties of monolayer phosphprene quantum dots. 2D Mater. 2015;2(4) 045012-045019. [Google Scholar]
- 39.Liu H., Neal A.T., Zhu Z., Luo Z., Xu X., Tománek D. Phosphorene: an unexplored 2D semiconductor with a high hole mobility. ACS Nano. 2014;8(4):4033–4041. doi: 10.1021/nn501226z. [DOI] [PubMed] [Google Scholar]
- 40.Kou L., Chen C., Smith S.C. Phosphorene: fabrication, properties, and applications. J. Phys. Chem. Lett. 2015;6(14):2794–2805. doi: 10.1021/acs.jpclett.5b01094. [DOI] [PubMed] [Google Scholar]
- 41.Li L., Yu Y., Ye G.J., Ge Q., Ou X., Wu H. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014;9(5):372–377. doi: 10.1038/nnano.2014.35. [DOI] [PubMed] [Google Scholar]
- 42.Gui R., Jin H., Wang Z., Li J. Black phosphorus quantum dots: synthesis, properties, functionalized modification and applications. Chem. Soc. Rev. 2018;47(17):6795–6823. doi: 10.1039/c8cs00387d. [DOI] [PubMed] [Google Scholar]
- 43.Li Q., Zhou Q., Shi L., Chen Q., Wang J. Recent advances in oxidation and degradation mechanisms of ultrathin 2D materials under ambient conditions and their passivation strategies. J. Mater. Chem. 2019;7(9):4291–4312. [Google Scholar]
- 44.Guo Z., Chen S., Wang Z., Yang Z., Liu F., Xu Y. Metal‐ion‐modified black phosphorus with enhanced stability and transistor performance. Adv. Mater. 2017;29(42):1703811–1703819. doi: 10.1002/adma.201703811. [DOI] [PubMed] [Google Scholar]
- 45.Wang L., Zou X., Lin J., Jiang J., Liu Y., Liu X. Perovskite/black phosphorus/MoS2 photogate reversed photodiodes with ultrahigh light on/off ratio and fast response. ACS Nano. 2019;13(4):4804–4813. doi: 10.1021/acsnano.9b01713. [DOI] [PubMed] [Google Scholar]
- 46.Gao X.G., Chen G.X., Li D.K., Li X.K., Liu Z.B., Tian J.G. Modulation of photothermal anisotropy using black phosphorus/rhenium diselenide heterostructures. Nanoscale. 2018;10(23):10844–10849. doi: 10.1039/c8nr02229a. [DOI] [PubMed] [Google Scholar]
- 47.Zhu W., Park S., Yogeesh M.N., McNicholas K.M., Bank S.R., Akinwande D. Black Phosphorus flexible thin film transistors at gighertz frequencies. Nano Lett. 2016;16(4):2301–2306. doi: 10.1021/acs.nanolett.5b04768. [DOI] [PubMed] [Google Scholar]
- 48.Afzal A.M., Dastgeer G., Iqbal M.Z., Gautam P., Faisal M.M. Performance p-BP/n-PdSe(2) near-infrared photodiodes with a fast and gate-tunable photoresponse. ACS Appl. Mater. Interfaces. 2020;12(17):19625–19634. doi: 10.1021/acsami.9b22898. [DOI] [PubMed] [Google Scholar]
- 49.Chen W., Ouyang J., Liu H., Chen M., Zeng K., Sheng J. Black phosphorus nanosheet-based drug delivery system for synergistic photodynamic/photothermal/phemotherapy of cancer. Adv. Mater. 2017;29(5):1603864–1603871. doi: 10.1002/adma.201603864. [DOI] [PubMed] [Google Scholar]
- 50.Chen W., Ouyang J., Yi X., Xu Y., Niu C., Zhang W. Black phosphorus nanosheets as a neuroprotective nanomedicine for neurodegenerative disorder therapy. Adv. Mater. 2018;30(3):1703458–1703465. doi: 10.1002/adma.201703458. [DOI] [PubMed] [Google Scholar]
- 51.Li Z., Hu Y., Fu Q., Liu Y., Wang J., Song J. NIR/ROS‐responsive black phosphorus QD vesicles as immunoadjuvant carrier for specific cancer photodynamic immunotherapy. Adv. Funct. Mater. 2020;30(3):1905758–1905769. [Google Scholar]
- 52.Liang X., Ye X., Wang C., Xing C., Miao Q., Xie Z. Photothermal cancer immunotherapy by erythrocyte membrane-coated black phosphorus formulation. J. Contr. Release. 2019;296:150–161. doi: 10.1016/j.jconrel.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 53.Yata T., Takahashi Y., Tan M., Nakatsuji H., Ohtsuki S., Murakami T. DNA nanotechnology-based composite-type gold nanoparticle-immunostimulatory DNA hydrogel for tumor photothermal immunotherapy. Biomaterials. 2017;146:136–145. doi: 10.1016/j.biomaterials.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 54.Qin S.Y., Feng J., Rong L., Jia H.Z., Chen S., Liu X.J. Theranostic GO-based nanohybrid for tumor induced imaging and potential combinational tumor therapy. Small. 2014;10(3):599–608. doi: 10.1002/smll.201301613. [DOI] [PubMed] [Google Scholar]
- 55.Nam J., Son S., Ochyl L.J., Kuai R., Schwendeman A., Moon J.J. Chemo-photothermal therapy combination elicits anti-tumor immunity against advanced metastatic cancer. Nat. Commun. 2018;9(1):1074–1087. doi: 10.1038/s41467-018-03473-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Qiu M., Wang D., Liang W., Liu L., Zhang Y., Chen X. Novel concept of the smart NIR-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. U.S.A. 2018;115(3):501–506. doi: 10.1073/pnas.1714421115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mao H., Laurent S., Chen W., Akhavan O., Lmani M., Ashkarran A.A. Graphene: promises, facts, opportunities, and challenges in nanomedicine. Chem. Rev. 2013;113(5):3407–3421. doi: 10.1021/cr300335p. [DOI] [PubMed] [Google Scholar]
- 58.Paris J.L., Baeza A., Vallet-Regi M. Overcoming the stability, toxicity and biodegradation challenges of tumor stimuli-responsive inorganic nanoparticles for delivery of cancer therapeutics. Expet Opin. Drug Deliv. 2019;16(10):1095–1112. doi: 10.1080/17425247.2019.1662786. [DOI] [PubMed] [Google Scholar]
- 59.Khlebtsov N., Dykman L. Biodistribution and toxicity of engineered gold nanoparticles: a review of in vitro and in vivo studies. Chem. Soc. Rev. 2011;40(3):1647–1671. doi: 10.1039/c0cs00018c. [DOI] [PubMed] [Google Scholar]
- 60.Kwon H., Seo S.W., Kim T.G., Lee E.S., Lanh P.T., Yang S. Ultrathin and flat layer black phosphorus fabricated by reactive oxygen and water rinse. ACS Nano. 2016;10(9):8723–8731. doi: 10.1021/acsnano.6b04194. [DOI] [PubMed] [Google Scholar]
- 61.Shao J., Xie H., Huang H., Li Z., Sun Z., Xu Y. Biodegradable black phosphorus-based nanospheres for in vivo photothermal cancer therapy. Nat. Commun. 2016;7:12967–12980. doi: 10.1038/ncomms12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zeng X., Luo M., Liu G., Wang X., Tao W., Lin Y. Polydopamine-Modified black phosphorous nanocapsule with enhanced stability and photothermal performance for tumor multimodal treatments. Adv. Sci. 2018;5(10):1800510–1800518. doi: 10.1002/advs.201800510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu Q., Chen G., Gong K., Wang J., Ge X., Liu X., Guo S. MnO2-laden black phosphorus for MRI-guided synergistic PDT, PTT, and chemotherapy. Matter. 2019;1(2):496–512. [Google Scholar]
- 64.Doganov R.A., O'Farrell E.C., Koenig S.P., Yeo Y., Ziletti A., Carvalho A., Campbell D.K. Transport properties of pristine few-layer black phosphorus by van der Waals passivation in an inert atmosphere. Nat. Commun. 2015;10(6):6647–6654. doi: 10.1038/ncomms7647. [DOI] [PubMed] [Google Scholar]
- 65.Gusmão R., Sofer Z., Pumera M. Functional protection of exfoliated black phosphorus by noncovalent modification with anthraquinone. ACS Nano. 2018;12(6):5666–5673. doi: 10.1021/acsnano.8b01474. [DOI] [PubMed] [Google Scholar]
- 66.Wu S., He F., Xie G., Bian Z., Luo J., Wen S. Black phosphorus: degradation favors lubrication. Nano Lett. 2018;18(9):5618–5627. doi: 10.1021/acs.nanolett.8b02092. [DOI] [PubMed] [Google Scholar]
- 67.Utt K.L., Rivero P., Mehboudi M., Harriss E.O., Borunda M.F., Pacheco SanJuan A.A. Fluctuations of the local shape, and the photo-oxidation of black phosphorus. ACS Cent. Sci. 2015;1(6):320–327. doi: 10.1021/acscentsci.5b00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziletti A., Carvalho A., Trevisanutto P., Campbell D., Coker D., Neto A.C. Phosphorene oxides: bandgap engineering of phosphorene by oxidation. Phys. Rev. B. 2015;91(8) 085407-085417. [Google Scholar]
- 69.Ziletti A., Carvalho A., Campbell D.K., Coker D.F., Castro Neto A.H. Oxygen defects in phosphorene. Phys. Rev. Lett. 2015;114(4) doi: 10.1103/PhysRevLett.114.046801. 046801-046806. [DOI] [PubMed] [Google Scholar]
- 70.Favron A., Gaufrès E., Fossard F., Phaneuf-L'Heureux A.L., Tang N.Y., Lévesque P.L. Photooxidation and quantum confinement effects in exfoliated black phosphorus. Nat. Mater. 2015;14(8):826–832. doi: 10.1038/nmat4299. [DOI] [PubMed] [Google Scholar]
- 71.Zhang T., Wan Y., Xie H., Mu Y., Du P., Wang D. Degradation chemistry and stabilization of exfoliated few-layer black phosphorus in water. J. Am. Chem. Soc. 2018;140(24):7561–7567. doi: 10.1021/jacs.8b02156. [DOI] [PubMed] [Google Scholar]
- 72.Wan B., Yang B., Wang Y., Zhang J., Zeng Z., Liu Z. Enhanced stability of black phosphorus field-effect transistors with SiO₂ passivation. Nanotechnology. 2015;26(43):435702–435708. doi: 10.1088/0957-4484/26/43/435702. [DOI] [PubMed] [Google Scholar]
- 73.Ryder C.R., Wood J.D., Wells S.A., Yang Y., Jariwala D., Marks T.J. Covalent functionalization and passivation of exfoliated black phosphorus via aryl diazonium chemistry. Nat. Chem. 2016;8(6):597–602. doi: 10.1038/nchem.2505. [DOI] [PubMed] [Google Scholar]
- 74.Koenig S.P., Doganov R.A., Seixas L., Carvalho A., Tan J.Y., Watanabe K. Electron doping of ultrathin black phosphorus with Cu Adatoms. Nano Lett. 2016;16(4):2145–2151. doi: 10.1021/acs.nanolett.5b03278. [DOI] [PubMed] [Google Scholar]
- 75.Xing C., Chen S., Qiu M., Liang X., Liu Q., Zhou Q. Black phosphorus hydrogels: conceptually novel black phosphorus/cellulose hydrogels as promising photothermal agents for effective cancer therapy. Adv. Healthc. Mater. 2018;7(7):1870030–1870041. doi: 10.1002/adhm.201701510. [DOI] [PubMed] [Google Scholar]
- 76.Zong S., Wang L., Yang Z., Wang H., Wang Z., Cui Y. Black phosphorus-based drug nanocarrier for targeted and synergetic chemophotothermal therapy of acute lymphoblastic leukemia. ACS Appl. Mater. Interfaces. 2019;11(6):5896–5902. doi: 10.1021/acsami.8b22563. [DOI] [PubMed] [Google Scholar]
- 77.Zhang J., Ma Y., Hu K., Feng Y., Chen S., Yang X. Surface coordination of black phosphorus with modified cisplatin. Bioconjugate Chem. 2019;30(6):1658–1664. doi: 10.1021/acs.bioconjchem.9b00128. [DOI] [PubMed] [Google Scholar]
- 78.Wang H., Hu K., Li Z., Wang C., Yu M., Li Z. Black phosphorus nanosheets passivation using a tripeptide. Small. 2018;14(35):1801701–1801708. doi: 10.1002/smll.201801701. [DOI] [PubMed] [Google Scholar]
- 79.Yang B., Wan B., Zhou Q., Wang Y., Hu W., Lv W. Te-Doped black phosphorus field-Effect transistors. Adv. Mater. 2016;28(42):9408–9415. doi: 10.1002/adma.201603723. [DOI] [PubMed] [Google Scholar]
- 80.Lv W., Yang B., Wang B., Wan W., Ge Y., Yang R. Sulfur-Doped black phosphorus field-effect transistors with enhanced stability. ACS Appl. Mater. Interfaces. 2018;10(11):9663–9668. doi: 10.1021/acsami.7b19169. [DOI] [PubMed] [Google Scholar]
- 81.Tang X., Chen H., Ponraj J.S., Dhanabalan S.C., Xiao Q., Fan D. Fluoriantion-enhanced ambient stability and electronic tolerance of black phosphorus quantum dots. Adv. Sci. 2018;5(9):1800420–1800429. doi: 10.1002/advs.201800420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rho J.Y., Kuhn-Spearing L., Zioupos P. Mechanical properties and the hierarchical structure of bone. Med. Eng. Phys. 1998;20(2):92–102. doi: 10.1016/s1350-4533(98)00007-1. [DOI] [PubMed] [Google Scholar]
- 83.Boskey A. Bone mineral crystal size. Osteoporos. Int. 2003;14(Suppl 5):S16–S20. doi: 10.1007/s00198-003-1468-2. [DOI] [PubMed] [Google Scholar]
- 84.Zimmermann E.A., Ritchie R.O. Bone as a structural material. Adv. Healthc. Mater. 2015;4(9):1287–1304. doi: 10.1002/adhm.201500070. [DOI] [PubMed] [Google Scholar]
- 85.Murshed M., McKee M.D. Molecular determinants of extracellular matrix mineralization in bone and blood vessels. Curr. Opin. Nephrol. Hypertens. 2010;19(4):359–365. doi: 10.1097/MNH.0b013e3283393a2b. [DOI] [PubMed] [Google Scholar]
- 86.Bonucci E. Bone mineralization. Front. Biosci. 2012;17:100–128. doi: 10.2741/3918. [DOI] [PubMed] [Google Scholar]
- 87.Chen X., Wang Z., Duan N., Zhu G., Schwarz E.M., Xie C. Osteoblast-osteoclast interactions. Connect. Tissue Res. 2018;59(2):99–107. doi: 10.1080/03008207.2017.1290085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Teti A., Econs M.J. Osteopetroses, emphasizing potential approaches to treatment. Bone. 2017;102:50–59. doi: 10.1016/j.bone.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 89.D'Amelio P., Grimaldi A., Di Bella S., Brianza S.Z., Cristofaro M.A., Tamone C. Estrogen deficiency increases osteoclastogenesis up-regulating T cells activity: a key mechanism in osteoporosis. Bone. 2008;43(1):92–100. doi: 10.1016/j.bone.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 90.Matsuoka K., Park K.a., Ito M., Ikeda K., Takeshita S. Osteoclast‐derived complement component 3a stimulates osteoblast differentiation. J. Bone Miner. Res. 2014;29(7):1522–1530. doi: 10.1002/jbmr.2187. [DOI] [PubMed] [Google Scholar]
- 91.Bala Y., Farlay D., Boivin G. Bone mineralization: from tissue to crystal in normal and pathological contexts. Osteoporos. Int. 2013;24(8):2153–2166. doi: 10.1007/s00198-012-2228-y. [DOI] [PubMed] [Google Scholar]
- 92.Hasegawa T. Ultrastructure and biological function of matrix vesicles in bone mineralization. Histochem. Cell Biol. 2018;149(4):289–304. doi: 10.1007/s00418-018-1646-0. [DOI] [PubMed] [Google Scholar]
- 93.Landis W.J., Jacquet R. Association of calcium and phosphate ions with collagen in the mineralization of vertebrate tissues. Calcif. Tissue Int. 2013;93(4):329–337. doi: 10.1007/s00223-013-9725-7. [DOI] [PubMed] [Google Scholar]
- 94.Anderson H.C., Garimella R., Tague S.E. The role of matrix vesicles in growth plate development and biomineralization. Front. Biosci. 2005;10:822–837. doi: 10.2741/1576. [DOI] [PubMed] [Google Scholar]
- 95.van Driel M., van Leeuwen J. Vitamin D endocrinology of bone mineralization. Mol. Cell. Endocrinol. 2017;453:46–51. doi: 10.1016/j.mce.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 96.Anderson H.C. Matrix vesicles and calcification. Curr. Rheumatol. Rep. 2003;5(3):222–226. doi: 10.1007/s11926-003-0071-z. [DOI] [PubMed] [Google Scholar]
- 97.Yu X., Tang X., Gohil S.V., Laurencin C.T. Biomaterials for bone regenerative engineering. Adv. Healthc. Mater. 2015;4(9):1268–1285. doi: 10.1002/adhm.201400760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Calori G.M., Mazza E., Colombo M., Ripamonti C. The use of bone-graft substitutes in large bone defects: any specific needs? Injury. 2011;42(Suppl 2):S56–S63. doi: 10.1016/j.injury.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 99.Wang T., Zhang X., Bikle D.D. Osteogenic differentiation of periosteal cells during fracture healing. J. Cell. Physiol. 2017;232(5):913–921. doi: 10.1002/jcp.25641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Li L., Lu H., Zhao Y., Luo J., Yang L., Liu W. Functionalized cell-free scaffolds for bone defect repair inspired by self-healing of bone fractures: a review and new perspectives. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;98:1241–1251. doi: 10.1016/j.msec.2019.01.075. [DOI] [PubMed] [Google Scholar]
- 101.Black D.M., Rosen C.J. Clinical practice. Postmenopausal osteoporosis. N. Engl. J. Med. 2016;374(3):254–262. doi: 10.1056/NEJMcp1513724. [DOI] [PubMed] [Google Scholar]
- 102.Sakkas A., Schramm A., Winter K., Wilde F. Risk factors for post-operative complications after procedures for autologous bone augmentation from different donor sites. J. Cranio-Maxillo-Fac. Surg. 2018;46(2):312–322. doi: 10.1016/j.jcms.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 103.Sakkas A., Wilde F., Heufelder M., Winter K., Schramm A. Autogenous bone grafts in oral implantology-is it still a "gold standard"? A consecutive review of 279 patients with 456 clinical procedures. Int. J. Implant. Dent. 2017;3(1):23–40. doi: 10.1186/s40729-017-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Windhager R., Hobusch G.M., Matzner M. [Allogeneic transplants for biological reconstruction of bone defects] Orthopä. 2017;46(8):656–664. doi: 10.1007/s00132-017-3452-0. [DOI] [PubMed] [Google Scholar]
- 105.Emara K.M., Diab R.A., Emara A.K. Recent biological trends in management of fracture non-union. World. J. Orthop. 2015;6(8):623–628. doi: 10.5312/wjo.v6.i8.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lopes D., Martins-Cruz C., Oliveira M.B., Mano J.F. Bone physiology as inspiration for tissue regenerative therapies. Biomaterials. 2018;185:240–275. doi: 10.1016/j.biomaterials.2018.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang T., Zhai Y., Nuzzo M., Yang X., Yang Y., Zhang X. Layer-by-layer nanofiber-enabled engineering of biomimetic periosteum for bone repair and reconstruction. Biomaterials. 2018;182:279–288. doi: 10.1016/j.biomaterials.2018.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Oshiro J.A., Sato M.R., Scardueli C.R., Lopes de Oliveira G.J.P., Abucafy M.P., Chorilli M. Bioactive molecule-loaded drug delivery systems to optimize bone tissue repair. Curr. Protein Pept. Sci. 2017;18(8):850–863. doi: 10.2174/1389203718666170328111605. [DOI] [PubMed] [Google Scholar]
- 109.Xia Y., Sun J., Zhao L., Zhang F., Liang X.J., Guo Y. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials. 2018;183:151–170. doi: 10.1016/j.biomaterials.2018.08.040. [DOI] [PubMed] [Google Scholar]
- 110.Fan H., Zeng X., Wang X., Zhu R., Pei G. Efficacy of prevascularization for segmental bone defect repair using β-tricalcium phosphate scaffold in rhesus monkey. Biomaterials. 2014;35(26):7407–7415. doi: 10.1016/j.biomaterials.2014.05.035. [DOI] [PubMed] [Google Scholar]
- 111.Zhang B., Li H., He L., Han Z., Zhou T., Zhi W. Surface-decorated hydroxyapatite scaffold with on-demand delivery of dexamethasone and stromal cell derived factor-1 for enhanced osteogenesis. Mater. Sci. Eng. C Mater. Biol. Appl. 2018;89:355–370. doi: 10.1016/j.msec.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 112.Cui Z.K., Kim S., Baljon J.J., Wu B.M., Aghaloo T., Lee M. Microporous methacrylated glycol chitosan-montmorillonite nanocomposite hydrogel for bone tissue engineering. Nat. Commun. 2019;10(1):3523–3533. doi: 10.1038/s41467-019-11511-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Yan Y., Chen H., Zhang H., Guo C., Yang K., Chen K., Cheng R. Vascularized 3D printed scaffolds for promoting bone regeneration. Biomaterials. 2019;190–191:97–110. doi: 10.1016/j.biomaterials.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 114.Kim H.K., Shim W.S., Kim S.E., Lee K.H., Kang E., Kim J.H. Injectable in situ-forming pH/thermo-sensitive hydrogel for bone tissue engineering. Tissue Eng. A. 2009;15(4):923–933. doi: 10.1089/ten.tea.2007.0407. [DOI] [PubMed] [Google Scholar]
- 115.Wang M., Wu H., Li Q., Yang Y., Che F., Wang G. Novel aptamer-functionalized nanoparticles enhances bone defect repair by improving stem cell recruitment. Int. J. Nanomed. 2019;14:8707–8724. doi: 10.2147/IJN.S223164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Liu W., Chen D., Jiang G., Li Q., Wang Q., Cheng M. A lithium-containing nanoporous coating on entangled titanium scaffold can enhance osseointegration through Wnt/β-catenin pathway. Nanomedicine. 2018;14(1):153–164. doi: 10.1016/j.nano.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 117.Gaharwar A.K., Cross L.M., Peak C.W., Gold K., Carrow J.K., Brokesh A. 2D Nanoclay for biomedical applications: regenerative medicine, therapeutic delivery, and additive manufacturing, and Additive Manufacturing. Adv. Mater. 2019;31(23):1900332–1900360. doi: 10.1002/adma.201900332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tao W., Ji X., Zhu X., Li L., Wang J., Zhang Y. Two-dimensional antimonene-based phonic nanomedicine for cancer theranostics. Adv. Mater. 2018;30(38):1802061–1803072. doi: 10.1002/adma.201802061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ji X., Kong N., Wang J., Li W., Xiao Y., Gan S.T. A novel top-down synthesis of ultrathin 2D boron nanosheets for multimodal imaging-guided cancer therapy. Adv. Mater. 2018;30(36):1803031–1803042. doi: 10.1002/adma.201803031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shao N., Guo J., Guan Y., Zhang H., Li X., Chen X. Development of organic/inorganic compatible and sustainably bioactive composites for effective bone regeneration. Biomacromolecules. 2018;19(9):3637–3648. doi: 10.1021/acs.biomac.8b00707. [DOI] [PubMed] [Google Scholar]
- 121.Das A., Pamu D. A comprehensive review on electrical properties of hydroxyapatite based ceramic composites. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;101:539–563. doi: 10.1016/j.msec.2019.03.077. [DOI] [PubMed] [Google Scholar]
- 122.Pereira R.C., Benelli R., Canciani B., Scaranari M., Daculsi G., Cancedda R. Beta-tricalcium phosphate ceramic triggers fast and robust bone formation by human mesenchymal stem cells. J. Tissue. Eng. Regen. Med. 2019;13(6):1007–1018. doi: 10.1002/term.2848. [DOI] [PubMed] [Google Scholar]
- 123.Sai Y., Shiwaku Y., Anada T., Tsuchiya K., Takahashi T., Suzuki O. Capacity of octacalcium phosphate to promote osteoblastic differentiation toward osteocytes in vitro. Acta Biomater. 2018;69:362–371. doi: 10.1016/j.actbio.2018.01.026. [DOI] [PubMed] [Google Scholar]
- 124.Oliveira H.L., Da Rosa W.L.O., Cuevas-Suárez C.E., Carreño N.L.V., da Silva A.F., Guim T.N. Histological evaluation of bone repair with hydroxyapatite: a systematic review. Calcif. Tissue Int. 2017;101(4):341–354. doi: 10.1007/s00223-017-0294-z. [DOI] [PubMed] [Google Scholar]
- 125.Hao Y., Yan H., Wang X., Zhu B., Ning C., Ge S. Evaluation of osteoinduction and proliferation on nano-Sr-HAP: a novel orthopedic biomaterial for bone tissue regeneration. J. Nanosci. Nanotechnol. 2012;12(1):207–212. doi: 10.1166/jnn.2012.5125. [DOI] [PubMed] [Google Scholar]
- 126.Mao L., Liu J., Zhao J., Chang J., Xia L., Jiang L. Effect of micro-nano-hybrid structured hydroxyapatite bioceramics on osteogenic and cementogenic differentiation of human periodontal ligament stem cell via Wnt signaling pathway. Int. J. Nanomed. 2015;10:7031–7044. doi: 10.2147/IJN.S90343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhou K., Yu P., Shi X., Ling T., Zeng W., Chen A. Hierarchically porous hydroxyapatite hybrid scaffold incorporated with reduced graphene oxide for rapid bone ingrowth and repai. ACS Nano. 2019;13(8):9595–9606. doi: 10.1021/acsnano.9b04723. [DOI] [PubMed] [Google Scholar]
- 128.Huang K., Wu J., Gu Z. Black phosphorus hydrogel scaffolds enhance bone regeneration via a sustained supply of calcium-free phosphorus. ACS Appl. Mater. Interfaces. 2019;11(3):2908–2916. doi: 10.1021/acsami.8b21179. [DOI] [PubMed] [Google Scholar]
- 129.Sun Z., Xie H., Tang S., Yu X., Guo Z., Shao J. Ultrasmall black phosphorus quantum dots: synthesis and use as photothermal agents. Angew Chem. Int. Ed. Engl. 2015;54(39):11526–11530. doi: 10.1002/anie.201506154. [DOI] [PubMed] [Google Scholar]
- 130.Shui C., Scutt A. Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J. Bone Miner. Res. 2001;16(4):731–741. doi: 10.1359/jbmr.2001.16.4.731. [DOI] [PubMed] [Google Scholar]
- 131.Nørgaard R., Kassem M., Rattan S.I. Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells. Ann. N. Y. Acad. Sci. 2006;1067:443–447. doi: 10.1196/annals.1354.063. [DOI] [PubMed] [Google Scholar]
- 132.Rau L.R., Huang W.Y., Liaw J.W., Tsai S.W. Photothermal effects of laser-activated surface plasmonic gold nanoparticles on the apoptosis and osteogenesis of osteoblast-like cells. Int. J. Nanomed. 2016;11:3461–3473. doi: 10.2147/IJN.S108152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yanagi T., Kajiya H., Kawaguchi M., Kido H., Fukushima T. Photothermal stress triggered by near infrared-irradiated carbon nanotubes promotes bone deposition in rat calvarial defects. J. Biomater. Appl. 2015;29(8):1109–1118. doi: 10.1177/0885328214556913. [DOI] [PubMed] [Google Scholar]
- 134.Yamada T., Ezura Y., Hayata T., Moriya S., Shirakawa J., Notomi T. β₂ adrenergic receptor activation suppresses bone morphogenetic protein (BMP)-induced alkaline phosphatase expression in osteoblast-like MC3T3E1 cells. J. Cell. Biochem. 2015;116(6):1144–1152. doi: 10.1002/jcb.25071. [DOI] [PubMed] [Google Scholar]
- 135.Li S., Chien S., Brånemark P.I. Heat shock-induced necrosis and apoptosis in osteoblasts. J. Orthop. Res. 1999;17(6):891–899. doi: 10.1002/jor.1100170614. [DOI] [PubMed] [Google Scholar]
- 136.Hoffman A.S. Hydrogels for biomedical applications. Adv. Drug Deliv. Rev. 2002;54(1):3–12. doi: 10.1016/s0169-409x(01)00239-3. [DOI] [PubMed] [Google Scholar]
- 137.Burdick J.A., Murphy W.L. Moving from static to dynamic complexity in hydrogel design. Nat. Commun. 2012;3:1269–1277. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 138.Seliktar D. Designing cell-compatible hydrogels for biomedical applications. Science. 2012;336(6085):1124–1128. doi: 10.1126/science.1214804. [DOI] [PubMed] [Google Scholar]
- 139.Lyu D., Chen S., Guo W. Liposome crosslinked polyacrylamide/DNA hydrogel: a smart controlled-release system for small molecular payloads. Small. 2018;14(15):1704039–1704047. doi: 10.1002/smll.201704039. [DOI] [PubMed] [Google Scholar]
- 140.Huang Q., Zou Y., Arno M.C., Chen S., Wang T., Gao J. Hydrogel scaffolds for differentiation of adipose-derived stem cells. Chem. Soc. Rev. 2017;46(20):6255–6275. doi: 10.1039/c6cs00052e. [DOI] [PubMed] [Google Scholar]
- 141.Zhu Y., Zhang Q., Shi X., Han D. Hierarchical hydrogel composite interfaces with robust mechanical properties for biomedical applications. Adv. Mater. 2019;31(45):1804950–1804955. doi: 10.1002/adma.201804950. [DOI] [PubMed] [Google Scholar]
- 142.Milner P.E., Parkes M., Puetzer J.L., Chapman R., Stevens M.M., Cann P. A low friction, biphasic and boundary lubricating hydrogel for cartilage replacement. Acta Biomater. 2018;65:102–111. doi: 10.1016/j.actbio.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 143.Hou S., Niu X., Li L., Zhou J., Qian Z., Yao D. Simultaneous nano- and microscale structural control of injectable hydrogels via the assembly of nanofibrous protein microparticles for tissue regeneration. Biomaterials. 2019;223:119458. doi: 10.1016/j.biomaterials.2019.119458. [DOI] [PubMed] [Google Scholar]
- 144.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nat. Biotechnol. 2003;21(10):1171–1178. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 145.Rowley J.A., Madlambayan G., Mooney D.J. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20(1):45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 146.Hennink W.E., van Nostrum C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2002;54(1):13–36. doi: 10.1016/s0169-409x(01)00240-x. [DOI] [PubMed] [Google Scholar]
- 147.Zhang Y.S., Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356(6337) doi: 10.1126/science.aaf3627. eaaf3627- eaaf3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Cai Z., Huang K., Bao C., Wang X., Sun X., Xia H. Precise construction of cell-Instructive 3D microenvironments by photopatterning a biodegradable hydrogel. Chem. Mater. 2019;31(13):4710–4719. [Google Scholar]
- 149.Cai Z., Gan Y., Bao C., Wu W., Wang X., Zhang Z. Photosensitive hydrogel creates favorable biologic niches to promote spinal cord injury repair. Adv. Healthc. Mater. 2019;8(13):1900013–1900023. doi: 10.1002/adhm.201900013. [DOI] [PubMed] [Google Scholar]
- 150.Chen H., Cheng R., Zhao X., Zhang Y., Tam A., Yan Y. An injectable self-healing coordinative hydrogel with antibacterial and angiogenic properties for diabetic skin wound repair. NPG Asia Mater. 2019;11(1):3–15. [Google Scholar]
- 151.Hamidi M., Azadi A., Rafiei P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008;60(15):1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 152.Cheng L., Cai Z., Ye T., Yu X., Chen Z., Yun Y. Injectable polypeptide-protein hydrogels for promoting infected wound healing. Adv. Funct. Mater. 2020:2001196–2001208. [Google Scholar]
- 153.Yan Y., Cheng B., Chen K., Cui W., Qi J., Li X. Enhanced osteogenesis of bone marrow-derived mesenchymal stem cells by a functionalized silk fibroin hydrogel for bone defect repair. Adv. Healthc. Mater. 2019;8(3):1801043–1801054. doi: 10.1002/adhm.201801043. [DOI] [PubMed] [Google Scholar]
- 154.Goretti Penido M., Alon U.S. Phosphate homeostasis and its role in bone health. Pediatr. Nephrol. 2012;27(11):2039–2048. doi: 10.1007/s00467-012-2175-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Miao Y., Shi X., Li Q., Hao L., Liu L., Liu X. Engineering natural matrices with black phosphorus nanosheets to generate multi-functional therapeutic nanocomposite hydrogels. Biomater. Sci. 2019;7(10):4046–4059. doi: 10.1039/c9bm01072f. [DOI] [PubMed] [Google Scholar]
- 156.Wang Z., Zhao J., Tang W., Hu L., Chen X., Su Y. Multifunctional nanoengineered hydrogels consisting of black phosphorus nanosheets upregulate bone formation. Small. 2019;15(41):1901560–1901575. doi: 10.1002/smll.201901560. [DOI] [PubMed] [Google Scholar]
- 157.Pan W., Dai C., Li Y., Yin Y., Gong L., Machuki J.O. PRP-chitosan thermoresponsive hydrogel combined with black phosphorus nanosheets as injectable biomaterial for biotherapy and phototherapy treatment of rheumatoid arthritis. Biomaterials. 2020;239:119851–119865. doi: 10.1016/j.biomaterials.2020.119851. [DOI] [PubMed] [Google Scholar]
- 158.Stegen S., van Gastel N., Carmeliet G. Bringing new life to damaged bone: the importance of angiogenesis in bone repair and regeneration. Bone. 2015;70:19–27. doi: 10.1016/j.bone.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 159.Carmeliet P., Ferreira V., Breier G., Pollefeyt S., Kieckens L., Gertsenstein M. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380(6573):435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- 160.Ferrara N., Gerber H.P., LeCouter J. The biology of VEGF and its receptors. Nat. Med. 2003;9(6):669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 161.Berendsen A.D., Olsen B.R. Regulation of adipogenesis and osteogenesis in mesenchymal stem cells by vascular endothelial growth factor A. J. Intern. Med. 2015;277(6):674–680. doi: 10.1111/joim.12364. [DOI] [PubMed] [Google Scholar]
- 162.Wu T., Yu S., Chen D., Wang Y. Bionic design, materials and performance of bone tissue scaffolds. Materials. 2017;10(10):1187–1201. doi: 10.3390/ma10101187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Janicki P., Schmidmaier G. What should be the characteristics of the ideal bone graft substitute? Combining scaffolds with growth factors and/or stem cells. Injury. 2011;Suppl 2:S77–S81. doi: 10.1016/j.injury.2011.06.014. [DOI] [PubMed] [Google Scholar]
- 164.Wang Z., An G., Zhu Y., Liu X., Chen Y., Wu H. 3D-printable self-healing and mechanically reinforced hydrogels with host–guest non-covalent interactions integrated into covalently linked networks. Mater. Horiz. 2019;6(4):733–742. doi: 10.1039/C8MH01208C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gao F., Xu Z., Liang Q., Li H., Peng L., Wu M. Osteochondral regeneration with 3D-printed biodegradable high-strength supramolecular polymer reinforced-gelatin hydrogel scaffolds. Adv. Sci. 2019;6(15):1900867–1900879. doi: 10.1002/advs.201900867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Barba A., Diez-Escudero A., Maazouz Y., Rappe K., Espanol M., Montufar E.B. Osteoinduction by foamed and 3D-printed calcium phosphate scaffolds: effect of nanostructure and pore architecture. ACS Appl. Mater. Interfaces. 2017;9(48):41722–41736. doi: 10.1021/acsami.7b14175. [DOI] [PubMed] [Google Scholar]
- 167.Sanzana E.S., Navarro M., Ginebra M.P., Planell J.A., Ojeda A.C., Montecinos H.A. Role of porosity and pore architecture in the in vivo bone regeneration capacity of biodegradable glass scaffolds. J. Biomed. Mater. Res. A. 2014;102(6):1767–1773. doi: 10.1002/jbm.a.34845. [DOI] [PubMed] [Google Scholar]
- 168.Wei G., Ma P.X. Nanostructured biomaterials for regeneration. Adv. Funct. Mater. 2008;18(22):3566–3582. doi: 10.1002/adfm.200800662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Sarkar N., Bose S. Liposome-encapsulated curcumin-loaded 3D printed scaffold for bone tissue engineering. ACS Appl. Mater. Interfaces. 2019;11(19):17184–17192. doi: 10.1021/acsami.9b01218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Murphy S.V., Atala A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014;32(8):773–785. doi: 10.1038/nbt.2958. [DOI] [PubMed] [Google Scholar]
- 171.Walsh D.P., Raftery R.M., Chen G., Heise A., O'Brien F.J., Cryan S.A. Rapid healing of a critical‐sized bone defect using a collagen‐hydroxyapatite scaffold to facilitate low dose, combinatorial growth factor delivery. Tissue. Eng. B. Rev. 2019;13(10):1843–1853. doi: 10.1002/term.2934. [DOI] [PubMed] [Google Scholar]
- 172.Yang B., Yin J., Chen Y., Pan S., Yao H., Gao Y. 2D-Black-Phosphorus-Reinforced 3D-Printed Scaffolds:A stepwise countermeasure for osteosarcoma. Adv. Mater. 2018;30(10):1705611–1705623. doi: 10.1002/adma.201705611. [DOI] [PubMed] [Google Scholar]
- 173.Liu X., Miller A.L., Park S., George M.N., Waletzki B.E., Xu H. Two-Dimensional black phosphorus and graphene oxide nanosheets synergistically enhance cell proliferation and osteogenesis on 3D printed scaffolds. ACS Appl. Mater. Interfaces. 2019;11(26):23558–23572. doi: 10.1021/acsami.9b04121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Wang C., Ye X., Zhao Y., Bai L., He Z., Tong Q. Cryogenic 3D printing of porous scaffolds for in situ delivery of 2D black phosphorus nanosheets, doxorubicin hydrochloride and osteogenic peptide for treating tumor resection-induced bone defects. Biofabrication. 2020;12(3) doi: 10.1088/1758-5090/ab6d35. 035004-035019. [DOI] [PubMed] [Google Scholar]
- 175.Zhang L., Yang G., Johnson B.N., Jia X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019;84:16–33. doi: 10.1016/j.actbio.2018.11.039. [DOI] [PubMed] [Google Scholar]
- 176.Jones N. Science in three dimensions: the print revolution. Nature. 2012;487(7405):22–23. doi: 10.1038/487022a. [DOI] [PubMed] [Google Scholar]
- 177.Ma L., Wang X., Zhao N., Zhu Y., Qiu Z., Li Q. Integrating 3D printing and biomimetic mineralization for personalized enhanced osteogenesis, angiogenesis, and osteointegration. ACS Appl. Mater. Interfaces. 2018;10(49):42146–42154. doi: 10.1021/acsami.8b17495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jahangiri M., Hussain A., Akowuah E. Minimally invasive surgical aortic valve replacement. Heart. 2019;105(Suppl 2):S10–S15. doi: 10.1136/heartjnl-2018-313512. [DOI] [PubMed] [Google Scholar]
- 179.Lykissas M.G., Giannoulis D. Minimally invasive spine surgery for degenerative spine disease and deformity correction: a literature review. Ann. Transl. Med. 2018;6(6):99–107. doi: 10.21037/atm.2018.03.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu X., Jin X., Ma P.X. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat. Mater. 2011;10(5):398–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Fisher M.B., Mauck R.L. Tissue engineering and regenerative medicine: recent innovations and the transition to translation. Tissue. Eng. B. Rev. 2013;19(1):1–13. doi: 10.1089/ten.teb.2012.0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Zdravkov B.D., Čermák J.J., Šefara M., Janků J. Pore classification in the characterization of porous materials: a perspective. Cent. Eur. J. Chem. 2007;5(2):385–395. [Google Scholar]
- 183.Wong C.Y., Al-Salami H., Dass C.R. Microparticles, microcapsules and microspheres: a review of recent developments and prospects for oral delivery of insulin. Int. J. Pharm. 2018;537(1–2):223–244. doi: 10.1016/j.ijpharm.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 184.Khafagy el S., Morishita M., Onuki Y., Takayama K. Current challenges in non-invasive insulin delivery systems: a comparative review. Adv. Drug Deliv. Rev. 2007;59(15):1521–1546. doi: 10.1016/j.addr.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 185.Singh M.N., Hemant K.S., Ram M., Shivakumar H.G. Microencapsulation: a promising technique for controlled drug delivery. Res. Pharm. Sci. 2010;5(2):65–77. [PMC free article] [PubMed] [Google Scholar]
- 186.Prajapati V.D., Jani G.K., Kapadia J.R. Current knowledge on biodegradable microspheres in drug delivery. Expet Opin. Drug Deliv. 2015;12(8):1283–1299. doi: 10.1517/17425247.2015.1015985. [DOI] [PubMed] [Google Scholar]
- 187.Li J., Jiang H., Ouyang X., Han S., Wang J., Xie R. CaCO(3)/Tetraethylenepentamine-Graphene hollow microspheres as biocompatible bone drug carriers for controlled release. ACS Appl. Mater. Interfaces. 2016;8(44):30027–30036. doi: 10.1021/acsami.6b10697. [DOI] [PubMed] [Google Scholar]
- 188.Xu Y., Peng J., Richards G., Lu S., Eglin D. Optimization of electrospray fabrication of stem cell-embedded alginate-gelatin microspheres and their assembly in 3D-printed poly(ε-caprolactone) scaffold for cartilage tissue engineering. J. Orthop. Translat. 2019;18:128–141. doi: 10.1016/j.jot.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nugroho R.W.N., Odelius K., Höglund A., Albertsson A.C. Highlighting the importance of surface grafting in combination with a Layer-by-Layer approach for fabricating advanced 3D poly(l-lactide) microsphere scaffolds. Chem. Mater. 2016;28(10):3298–3307. doi: 10.1021/acs.chemmater.6b00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Xin S., Chimene D., Garza J.E., Gaharwar A.K., Alge D.L. Clickable PEG hydrogel microspheres as building blocks for 3D bioprinting. Biomater. Sci. 2019;7(3):1179–1187. doi: 10.1039/c8bm01286e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hu Z., Ma C., Rong X., Zou S., Liu X. Immunomodulatory ECM-like microspheres for accelerated bone regeneration in diabetes mellitus. ACS Appl. Mater. Interfaces. 2018;10(3):2377–2390. doi: 10.1021/acsami.7b18458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Y., Hu X., Zhang L., Zhu C., Wang J., Li Y. Bioinspired extracellular vesicles embedded with black phosphorus for molecular recognition-guided biomineralization. Nat. Commun. 2019;10(1):1–10. doi: 10.1038/s41467-019-10761-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.França D., Medina  F., Messa L.L., Souza C.F., Faez R. Chitosan spray-dried microcapsule and microsphere as fertilizer host for swellable - controlled release materials. Carbohydr. Polym. 2018;196:47–55. doi: 10.1016/j.carbpol.2018.05.014. [DOI] [PubMed] [Google Scholar]
- 194.Zhang J., Miao Z., Yan J., Zhang X., Li X., Zhang Q. Synthesis of negative-charged metal-containing cyclomatrix polyphosphazene microspheres based on polyoxometalates and application in charge-selective dye adsorption. Rapid. Commun. 2019;40(17) doi: 10.1002/marc.201800730. e1800730- e1800736. [DOI] [PubMed] [Google Scholar]
- 195.Daly A.C., Riley L., Segura T., Burdick J.A. Hydrogel microparticles for biomedical applications. Nat. Rev. Mater. 2020;5:20–43. doi: 10.1038/s41578-019-0148-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wang X., Shao J., El Raouf M.A., Xie H., Huang H. Near-infrared light-triggered drug delivery system based on black phosphorus for in vivo bone regeneration. Biomaterials. 2018;179:164–174. doi: 10.1016/j.biomaterials.2018.06.039. [DOI] [PubMed] [Google Scholar]
- 197.Dastidar D.G., Saha S., Chowdhury M. Porous microspheres: synthesis, characterisation and applications in pharmaceutical & medical fields. Int. J. Pharm. 2018;548(1):34–48. doi: 10.1016/j.ijpharm.2018.06.015. [DOI] [PubMed] [Google Scholar]
- 198.Cai Y., Chen Y., Hong X., Liu Z., Yuan W. Porous microsphere and its applications. Int. J. Nanomed. 2013;8:1111–1120. doi: 10.2147/IJN.S41271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Zhang W., Wang X.C., Li X.Y., Zhang L.L., Jiang F. A 3D porous microsphere with multistage structure and component based on bacterial cellulose and collagen for bone tissue engineering. Carbohydr. Polym. 2020;236:116043–116052. doi: 10.1016/j.carbpol.2020.116043. [DOI] [PubMed] [Google Scholar]
- 200.Kriegel C., Attarwala H., Amiji M. Multi-compartmental oral delivery systems for nucleic acid therapy in the gastrointestinal tract. Adv. Drug Deliv. Rev. 2013;65(6):891–901. doi: 10.1016/j.addr.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 201.Yang G., Li X., He Y., Ma J., Ni G., Zhou S. From nano to micro to macro: electrospun hierarchically structured polymeric fibers for biomedical applications. Prog. Polym. Sci. 2018;81:80–113. [Google Scholar]
- 202.Shimomura K., Rothrauff B.B., Hart D.A., Hamamoto S., Kobayashi M., Yoshikawa H. Enhanced repair of meniscal hoop structure injuries using an aligned electrospun nanofibrous scaffold combined with a mesenchymal stem cell-derived tissue engineered construct. Biomaterials. 2019;192:346–354. doi: 10.1016/j.biomaterials.2018.11.009. [DOI] [PubMed] [Google Scholar]
- 203.Yang J., Chen M., Wu M., Chao K., Ho H., Yang Y. Office hysteroscopic early lysis of intrauterine adhesion after transcervical resection of multiple apposing submucous myomas. Fertil. Steril. 2008;89(5):1254–1259. doi: 10.1016/j.fertnstert.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 204.Özoğul Y., Turkey T., Baykal A., Onat D., Renda N., Sayek I. An experimental study of the effect of aprotinin on intestinal adhesion formation. Am. J. Surg. 1998;175(2):137–141. doi: 10.1016/s0002-9610(97)00273-0. [DOI] [PubMed] [Google Scholar]
- 205.Cheng L., Wang Y., Sun G., Wen S., Deng L., Zhang H. Hydration-Enhanced lubricating electrospun nanofibrous membranes prevent tissue adhesion. Research. 2020;2020:4907185–4907197. doi: 10.34133/2020/4907185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Yao Q., Cosme J.G., Xu T., Miszuk J.M., Picciani P.H., Fong H. Three dimensional electrospun PCL/PLA blend nanofibrous scaffolds with significantly improved stem cells osteogenic differentiation and cranial bone formation. Biomaterials. 2017;115:115–127. doi: 10.1016/j.biomaterials.2016.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jang J., Castano O., Kim H.W. Electrospun materials as potential platforms for bone tissue engineering. Adv. Drug Deliv. Rev. 2009;61(12):1065–1083. doi: 10.1016/j.addr.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 208.Wang Y., Cui W., Zhao X., Wen S., Sun Y., Han J. Bone remodeling-inspired dual delivery electrospun nanofibers for promoting bone regeneration. Nanoscale. 2019;11(1):60–71. doi: 10.1039/c8nr07329e. [DOI] [PubMed] [Google Scholar]
- 209.Wu L., Gu Y., Liu L., Tang J., Mao J., Xi K. Hierarchical micro/nanofibrous membranes of sustained releasing VEGF for periosteal regeneration. Biomaterials. 2020;227:119555–119567. doi: 10.1016/j.biomaterials.2019.119555. [DOI] [PubMed] [Google Scholar]
- 210.Lee Y.B., Song S.-J., Shin Y.C., Jung Y.J., Kim B., Kang M.S. Ternary nanofiber matrices composed of PCL/black phosphorus/collagen to enhance osteodifferentiation. J. Ind. Eng. Chem. 2019;80:802–810. [Google Scholar]
- 211.Miguel S.P., Figueira D.R., Simões D., Ribeiro M.P., Coutinho P., Ferreira P. Electrospun polymeric nanofibres as wound dressings: a review. Colloids Surf. B Biointerfaces. 2018;169:60–71. doi: 10.1016/j.colsurfb.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 212.Tong L., Liao Q., Zhao Y., Huang H., Gao A., Zhang W. Near-infrared light control of bone regeneration with biodegradable photothermal osteoimplant. Biomaterials. 2019;193:1–11. doi: 10.1016/j.biomaterials.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 213.Grässel S.G. The role of peripheral nerve fibers and their neurotransmitters in cartilage and bone physiology and pathophysiology. Arthritis Res. Ther. 2014;16(6):485–498. doi: 10.1186/s13075-014-0485-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Madsen J.E., Hukkanen M., Aune A.K., Basran I., Møller J.F., Polak J.M. Fracture healing and callus innervation after peripheral nerve resection in rats. Clin. Orthop. Relat. Res. 1998;351:230–240. [PubMed] [Google Scholar]
- 215.Gajda M., Adriaensen D., Cichocki T. Development of the innervation of long bones: expression of the growth-associated protein 43. Folia Histochem. Cytobiol. 2000;38(3):103–110. [PubMed] [Google Scholar]
- 216.Niedermair T., Kuhn V., Doranehgard F., Stange R., Wieskötter B., Beckmann J. Absence of substance P and the sympathetic nervous system impact on bone structure and chondrocyte differentiation in an adult model of endochondral ossification. Matrix Biol. 2014;38:22–35. doi: 10.1016/j.matbio.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 217.Schinke T., Liese S., Priemel M., Haberland M., Schilling A.F., Catala-Lehnen P. Decreased bone formation and osteopenia in mice lacking alpha-calcitonin gene-related peptide. J. Bone Miner. Res. 2004;19(12):2049–2056. doi: 10.1359/JBMR.040915. [DOI] [PubMed] [Google Scholar]
- 218.Qian Y., Yuan W.E., Cheng Y., Yang Y., Qu X., Fan C. Concentrically integrative bioassembly of a three-dimensional black phosphorus nanoscaffold for restoring neurogenesis, angiogenesis, and immune homeostasis. Nano Lett. 2019;19(12):8990–9001. doi: 10.1021/acs.nanolett.9b03980. [DOI] [PubMed] [Google Scholar]
- 219.Kim Y.P., Lee G.S., Kim J.W., Kim M.S., Ahn H.S., Lim J.Y. Phosphate glass fibres promote neurite outgrowth and early regeneration in a peripheral nerve injury model. J. Tissue. Eng. Regen. Med. 2015;9(3):236–246. doi: 10.1002/term.1626. [DOI] [PubMed] [Google Scholar]
- 220.Zhang Z., Li X., Li Z., Bai Y., Liao G., Pan J. Collagen/nano-sized β-tricalcium phosphate conduits combined with collagen filaments and nerve growth factor promote facial nerve regeneration in miniature swine: an in vivo study, Oral. Surg. Oral. Med. Oral. Pathol. Oral. Radiol. 2019;128(5):472–478. doi: 10.1016/j.oooo.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 221.Qian Y., Zhao X., Han Q., Chen W., Li H., Yuan W. An integrated multi-layer 3D-fabrication of PDA/RGD coated graphene loaded PCL nanoscaffold for peripheral nerve restoration. Nat. Commun. 2018;9(1):323–339. doi: 10.1038/s41467-017-02598-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Ong S.M., Biswas S.K., Wong S.C. MicroRNA-mediated immune modulation as a therapeutic strategy in host-implant integration. Adv. Drug Deliv. Rev. 2015;88:92–107. doi: 10.1016/j.addr.2015.05.013. [DOI] [PubMed] [Google Scholar]
- 223.Katsimbri P. The biology of normal bone remodelling. Eur. J. Canc. Care. 2017;26(6) doi: 10.1111/ecc.12740. e12740- e12745. [DOI] [PubMed] [Google Scholar]
- 224.Olefsky J.M., Glass C.K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 225.Vi L., Baht G.S., Whetstone H., Ng A., Wei Q., Poon R. Macrophages promote osteoblastic differentiation in-vivo: implications in fracture repair and bone homeostasis. J. Bone Miner. Res. 2015;30(6):1090–1102. doi: 10.1002/jbmr.2422. [DOI] [PubMed] [Google Scholar]
- 226.Chung L., Maestas D.R., Jr., Housseau F., Elisseeff J.H. Key players in the immune response to biomaterial scaffolds for regenerative medicine. Adv. Drug Deliv. Rev. 2017;114:184–192. doi: 10.1016/j.addr.2017.07.006. [DOI] [PubMed] [Google Scholar]
- 227.Loi F., Córdova L.A., Pajarinen J., Lin T.-h., Yao Z., Goodman S.B. Inflammation, fracture and bone repair. Bone. 2016;86:119–130. doi: 10.1016/j.bone.2016.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li Y., Xiao Y., Liu C. The horizon of materiobiology: a perspective on material-guided cell behaviors and tissue engineering. Chem. Rev. 2017;117(5):4376–4421. doi: 10.1021/acs.chemrev.6b00654. [DOI] [PubMed] [Google Scholar]
- 229.Miron R.J., Bosshardt D.D. OsteoMacs: key players around bone biomaterials. Biomaterials. 2016;82:1–19. doi: 10.1016/j.biomaterials.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 230.Chang M.K., Raggatt L.J., Alexander K.A., Kuliwaba J.S., Fazzalari N.L. Osteal tissue macrophages are intercalated throughout human and mouse bone lining tissues and regulate osteoblast function in vitro and in vivo. J. Immunol. 2008;181(2):1232–1244. doi: 10.4049/jimmunol.181.2.1232. [DOI] [PubMed] [Google Scholar]
- 231.Hume D.A., Loutit J.F., Gordon S. The mononuclear phagocyte system of the mouse defined by immunohistochemical localization of antigen F4/80: macrophages of bone and associated connective tissue. J. Cell Sci. 1984;66:189–194. doi: 10.1242/jcs.66.1.189. [DOI] [PubMed] [Google Scholar]
- 232.Cho S.W., Soki F.N., Koh A.J., Eber M.R., Entezami P., Park S.I. Osteal macrophages support physiologic skeletal remodeling and anabolic actions of parathyroid hormone in bone. Proc. Natl. Acad. Sci. U.S.A. 2014;111(4):1545–1550. doi: 10.1073/pnas.1315153111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Stout R.D., Jiang C., Matta B., Tietzel I., Watkins S.K., Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J. Immunol. 2005;175(1):342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 234.Mosser D.M., Edwards J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008;8(12):958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Anderson J.M., Rodriguez A., Chang D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008;20(2):86–100. doi: 10.1016/j.smim.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Miron R.J., Bosshardt D.D. Multinucleated giant cells: good guys or bad guys? Tissue. Eng. B. Rev. 2018;24(1):53–65. doi: 10.1089/ten.TEB.2017.0242. [DOI] [PubMed] [Google Scholar]
- 237.Chen Z., Klein T., Murray R.Z., Crawford R., Chang J., Wu C. Osteoimmunomodulation for the development of advanced bone biomaterials. Mater. Today. 2016;19(6):304–321. [Google Scholar]
- 238.De A. Wnt/Ca2+ signaling pathway: a brief overview. Acta Biochim. Biophys. Sin. 2011;43(10):745–756. doi: 10.1093/abbs/gmr079. [DOI] [PubMed] [Google Scholar]
- 239.Sugimoto J., Romani A.M., Valentin-Torres A.M., Luciano A.A., Ramirez Kitchen C.M., Funderburg N. Magnesium decreases inflammatory cytokine production: a novel innate immunomodulatory mechanism. J. Immunol. 2012;188(12):6338–6346. doi: 10.4049/jimmunol.1101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Yamaguchi M., Weitzmann M.N. The intact strontium ranelate complex stimulates osteoblastogenesis and suppresses osteoclastogenesis by antagonizing NF-κB activation. Mol. Cell. Biochem. 2012;359(1–2):399–407. doi: 10.1007/s11010-011-1034-8. [DOI] [PubMed] [Google Scholar]
- 241.Qu G., Liu W., Zhao Y., Gao J., Xia T., Shi J. Improved biocompatibility of black phosphorus nanosheets by chemical modification. Angew Chem. Int. Ed. Engl. 2017;56(46):14488–14493. doi: 10.1002/anie.201706228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Mo J., Xie Q., Wei W., Zhao J. Revealing the immune perturbation of black phosphorus nanomaterials to macrophages by understanding the protein corona. Nat. Commun. 2018;9(1):2480–2491. doi: 10.1038/s41467-018-04873-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Dobrovolskaia M.A., McNeil S.E. Immunological properties of engineered nanomaterials. Nat. Nanotechnol. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 244.Tan Y., Li S., Pitt B.R., Huang L. The inhibitory role of CpG immunostimulatory motifs in cationic lipid vector-mediated transgene expression in vivo. Hum. Gene Ther. 1999;10(13):2153–2161. doi: 10.1089/10430349950017149. [DOI] [PubMed] [Google Scholar]
- 245.Weiss L. The cell periphery. Int. Rev. Cytol. 1969;26:63–105. doi: 10.1016/s0074-7696(08)61634-4. [DOI] [PubMed] [Google Scholar]